Osteoblast-like Cell Differentiation on 3D-Printed Scaffolds Using Various Concentrations of Tetra-Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Composite Gel Preparation
2.2. Scaffold Preparation
2.3. Cell Adhesion
2.4. Ion Complements
2.5. Calcium Release Analysis
2.6. Cell Culture
2.7. Alkaline Phosphatase (ALP) Activity
2.8. Total Protein Synthesis
2.9. Osteocalcin (OCN) Analysis
2.10. Calcium Content
2.11. Mineralization Analysis
2.12. Statistical Analysis
3. Results
3.1. Scaffold Morphology and Cell Attachment
3.2. Ion Complements and EDS-SEM Mapping
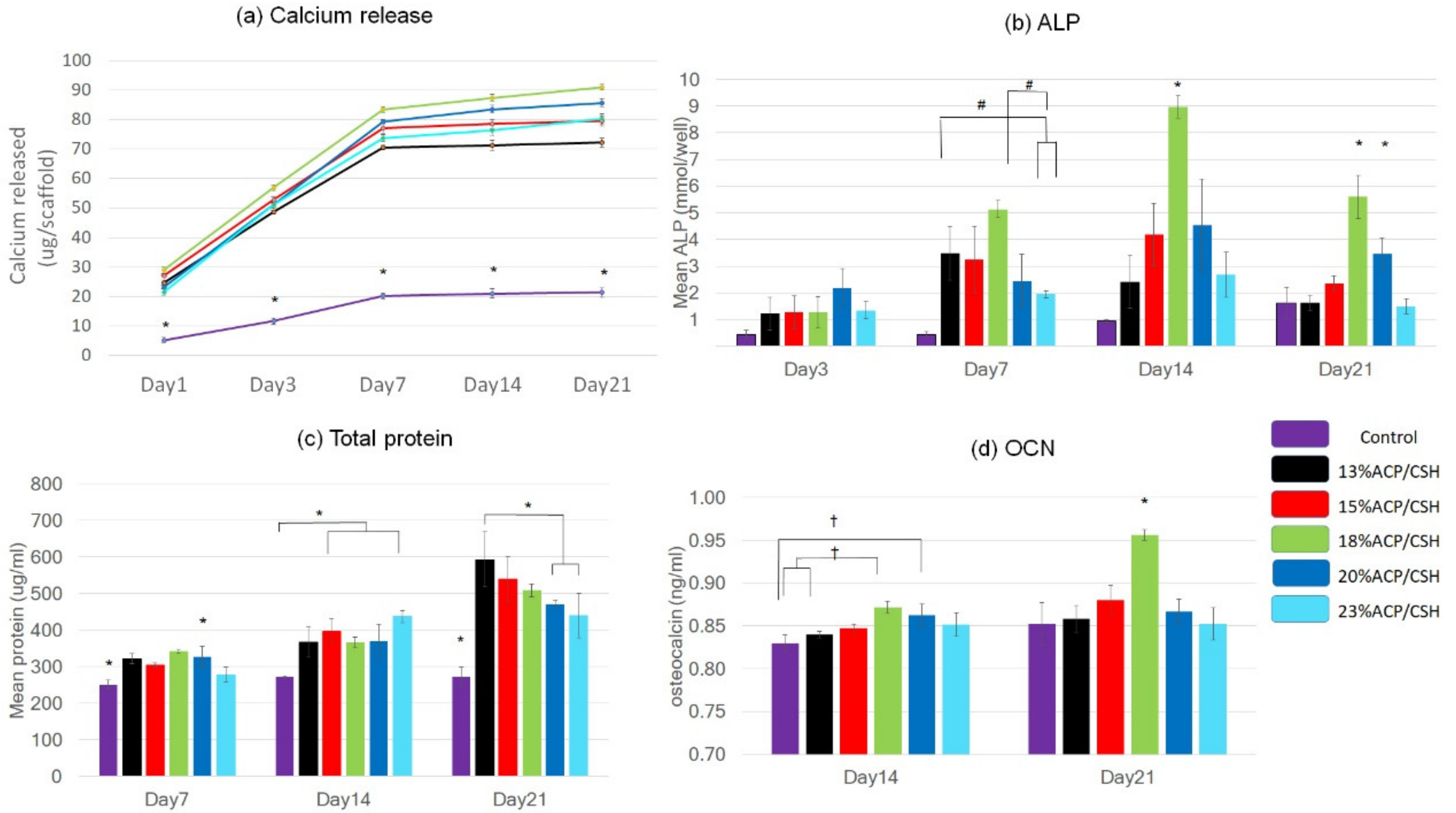
3.3. Calcium Release
3.4. Alkaline Phosphatase (ALP) Analysis
3.5. Total Protein Synthesis
3.6. Osteocalcin Assay (OCN)
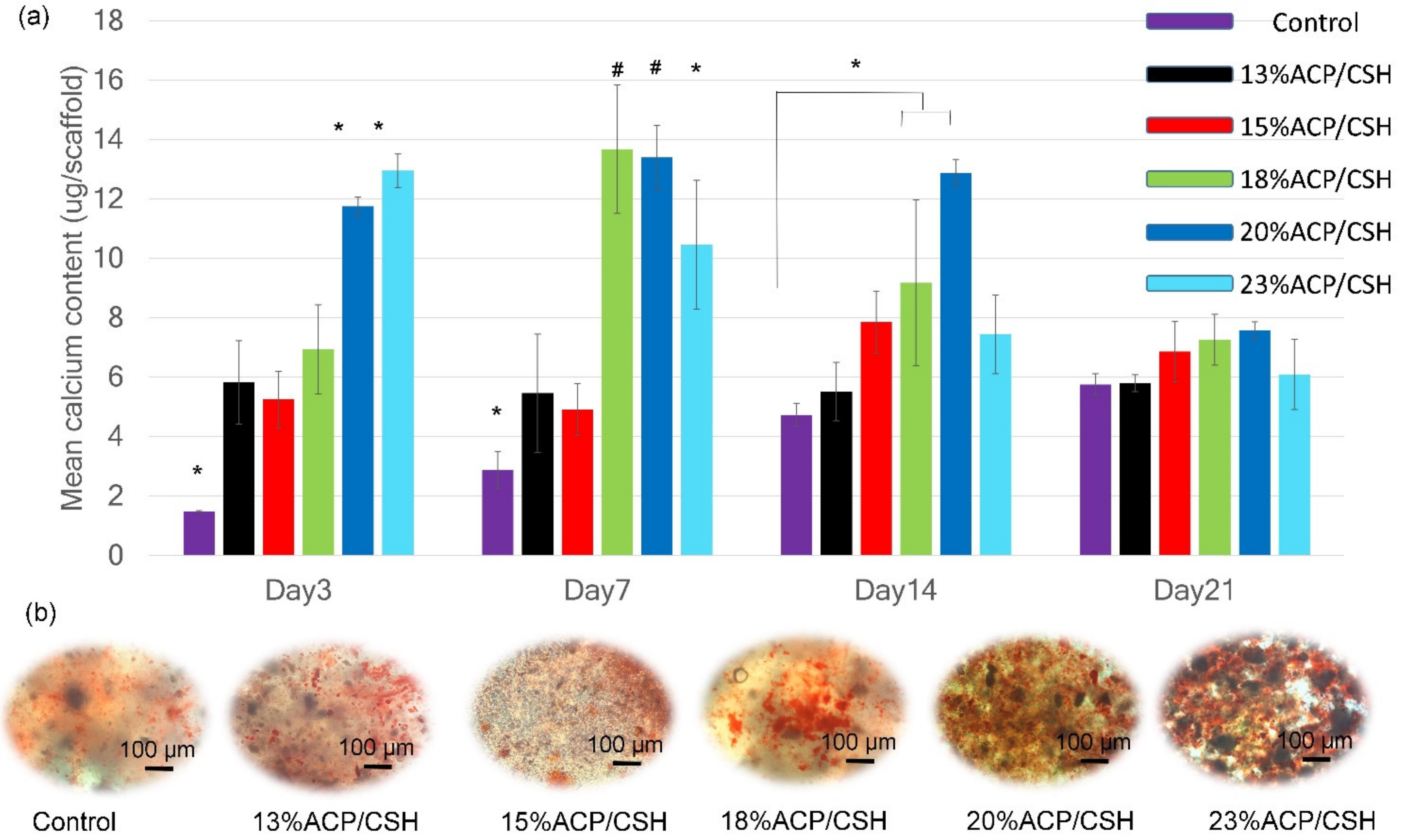
3.7. Calcium Deposition and Mineralization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poh, P.S.P.; Hutmacher, D.W.; Holzapfel, B.M.; Solanki, A.K.; Stevens, M.M.; Woodruff, M.A. In vitro and in vivo bone formation potential of surface calcium phosphate-coated polycaprolactone and polycaprolactone/bioactive glass composite scaffolds. Acta Biomater. 2016, 30, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Ito, S.; Huang, Z.; Hayashi, T.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Ca, P-rich layer formed on high-strength bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 331–343. [Google Scholar] [CrossRef]
- Gleadall, A.; Visscher, D.; Yang, J.; Thomas, D.; Segal, J. Review of additive manufactured tissue engineering scaffolds: Relationship between geometry and performance. Burns Trauma 2018, 6, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, S.; Liu, W.; Liu, S.; Zhao, S.; Zhu, Y. 3D printed porous β-Ca2SiO4 scaffolds derived from preceramic resin and their physicochemical and biological properties. Sci. Technol. Adv. Mater. 2018, 19, 495–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rumiński, S.; Ostrowska, B.; Jaroszewicz, J.; Skirecki, T.; Włodarski, K.; Święszkowski, W.; Lewandowska-Szumieł, M. Three-dimensional printed polycaprolactone-based scaffolds provide an advantageous environment for osteogenic differentiation of human adipose-derived stem cells. J. Tissue Eng. Regen. Med. 2018, 12, e473–e485. [Google Scholar] [CrossRef]
- Brunello, G.; Panda, S.; Schiavon, L.; Sivolella, S.; Biasetto, L.; Del Fabbro, M. The impact of bioceramic scaffolds on bone regeneration in preclinical in vivo studies: A systematic review. Materials 2020, 13, 1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahn, S.L. Plaster: A bone substitute. Oral Surg. Oral Med. Oral Pathol. 1966, 21, 672–681. [Google Scholar] [CrossRef]
- Li, Y.; Kong, F.; Weng, W. Preparation and characterization of novel biphasic calcium phosphate powders (alpha-TCP/HA) derived from carbonated amorphous calcium phosphates. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 89, 508–517. [Google Scholar] [CrossRef]
- Skrtic, D.; Antonucci, J.M.; Eanes, E.D. Amorphous Calcium Phosphate-Based Bioactive Polymeric Composites for Mineralized Tissue Regeneration. J. Res. Natl. Inst. 2003, 108, 167–182. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium phosphate bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [Green Version]
- Bohner, M.; Galea, L.; Doebelin, N. Calcium phosphate bone graft substitutes: Failures and hopes. J. Eur. Ceram. Soc. 2012, 32, 2663–2671. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Properties of osteoconductive biomaterials: Calcium phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, A.; Niemeyer, P.; Salzmann, G.; Südkamp, N.; Hube, R.; Klehm, J.; Menzel, M.; von Eisenhart-Rothe, R.; Bohner, M.; Görz, L. Microporous calcium phosphate ceramics as tissue engineering scaffolds for the repair of osteochondral defects: Histological results. Acta Biomater. 2013, 9, 7490–7505. [Google Scholar] [CrossRef]
- Wu, H.-D.; Lee, S.-Y.; Poma, M.; Wu, J.-Y.; Wang, D.-C.; Yang, J.-C. A novel resorbable α-calcium sulfate hemihydrate/amorphous calcium phosphate bone substitute for dental implantation surgery. Mater. Sci. Eng. C 2012, 32, 440–446. [Google Scholar] [CrossRef]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Jang, J.; Seol, Y.-J.; Kim, H.J.; Kundu, J.; Kim, S.W.; Cho, D.-W. Effects of alginate hydrogel cross-linking density on mechanical and biological behaviors for tissue engineering. J. Mech. Behav. Biomed. Mater. 2014, 37, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Nithya, R.; Sudha, P.N.; Kim, S.-K. Chapter Four—Role of Alginate in Bone Tissue Engineering. Adv. Food Nutr. Res. 2014, 73, 45–57. [Google Scholar]
- Urruela-Barrios, R.; Ramírez-Cedillo, E.; Díaz de León, A.; Alvarez, A.J.; Ortega-Lara, W. Alginate/Gelatin Hydrogels Reinforced with TiO2 and β-TCP Fabricated by Microextrusion-based Printing for Tissue Regeneration. Polymers 2019, 11, 457. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, H.; Ali, M.; Zeeshan, R.; Mutahir, Z.; Iqbal, F.; Nawaz, M.A.H.; Shahzadi, L.; Chaudhry, A.A.; Yar, M.; Luan, S. Chitosan/hydroxyapatite (HA)/hydroxypropylmethyl cellulose (HPMC) spongy scaffolds-synthesis and evaluation as potential alveolar bone substitutes. Colloids Surf. B 2017, 160, 553–563. [Google Scholar] [CrossRef]
- Sun, J.Y.; Zhao, X.; Illeperuma, W.R.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef]
- Darnell, M.C.; Sun, J.Y.; Mehta, M.; Johnson, C.; Arany, P.R.; Suo, Z.; Mooney, D.J. Performance and biocompatibility of extremely tough alginate/polyacrylamide hydrogels. Biomaterials 2013, 34, 8042–8048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wattanaanek, N.; Suttapreyasri, S.; Samruajbenjakun, B. 3D Printing of Calcium Phosphate/Calcium Sulfate with Alginate/Cellulose-Based Scaffolds for Bone Regeneration: Multilayer Fabrication and Characterization. J. Funct. Biomater. 2022, 13, 47. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Ahsaie, M.G.; Rad, M.R.; taghi Baghani, M.; Motamedian, S.R.; Khojasteh, A. Application of selected scaffolds for bone tissue engineering: A systematic review. Oral Maxillofac. Surg. 2017, 21, 109–129. [Google Scholar] [CrossRef] [PubMed]
- Alshaaer, M.; Kailani, M.H.; Ababneh, N.; Abu, M.S.A.; Sweileh, B.; Awidi, A. Fabrication of porous bioceramics for bone tissue applications using luffa cylindrical fibres (LCF) as template. Process. Appl. Ceram. 2017, 11, 13–20. [Google Scholar] [CrossRef]
- Mi, X.; Gupte, M.J.; Zhang, Z.; Swanson, W.B.; McCauley, L.K.; Ma, P.X. Three-Dimensional Electrodeposition of Calcium Phosphates on Porous Nanofibrous Scaffolds and Their Controlled Release of Calcium for Bone Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 32503–32513. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Jinkang, Z.; Zhen, W.; Jianxi, L.; Jiang, C.; Jian, L.; Guolin, M.; Xin, D. The effect of pore size on tissue ingrowth and neovascularization in porous bioceramics of controlled architecturein vivo. Biomed. Mater. 2011, 6, 015007. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Lee, S.S.; Tsai, C.H.; Lu, K.H.; Zhao, J.H.; Chang, Y.C. Platelet-rich fibrin increases cell attachment, proliferation and collagen-related protein expression of human osteoblasts. Aust. Dent. J. 2012, 57, 207–212. [Google Scholar] [CrossRef]
- Zhang, C. Transcriptional regulation of bone formation by the osteoblast-specific transcription factor Osx. J. Orthop. Surg. Res. 2010, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Yao, M.; Zheng, X.; Liang, X.; Su, X.; Zhang, Y.; Lu, A.; Zhang, L. Effects of chitin whiskers on physical properties and osteoblast culture of alginate based nanocomposite hydrogels. Biomacromolecules 2015, 16, 3499–3507. [Google Scholar] [CrossRef]
- Luo, Y.; Lode, A.; Wu, C.; Chang, J.; Gelinsky, M. Alginate/nanohydroxyapatite scaffolds with designed core/shell structures fabricated by 3D plotting and in situ mineralization for bone tissue engineering. ACS Appl. Mater. Interfaces 2015, 7, 6541–6549. [Google Scholar] [CrossRef]
- Li, Y.; Weng, W. In vitro synthesis and characterization of amorphous calcium phosphates with various Ca/P atomic ratios. J. Mater. Sci. Mater. Med. 2007, 18, 2303–2308. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R.; Pashley, D.H.; Rueggeberg, F.A.; Loushine, R.J.; Weller, R.N. Calcium phosphate phase transformation produced by the interaction of the Portland cement component of white mineral trioxide aggregate with a phosphate-containing fluid. J. Endod. 2007, 33, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Kilian, D.; Ahlfeld, T.; Akkineni, A.R.; Bernhardt, A.; Gelinsky, M.; Lode, A. 3D Bioprinting of osteochondral tissue substitutes—In vitro-chondrogenesis in multi-layered mineralized constructs. Sci. Rep. 2020, 10, 8277. [Google Scholar] [CrossRef] [PubMed]
- Pei, P.; Wei, D.; Zhu, M.; Du, X.; Zhu, Y. The effect of calcium sulfate incorporation on physiochemical and biological properties of 3D-printed mesoporous calcium silicate cement scaffolds. Microporous Mesoporous Mater. 2017, 241, 11–20. [Google Scholar] [CrossRef]
- He, W.; Wu, Z.; Wu, Y.; Zhong, Z.; Hong, Y. Construction of the Gypsum-Coated Scaffolds for In Situ Bone Regeneration. ACS Appl. Mater. 2021, 13, 31527–31541. [Google Scholar] [CrossRef]


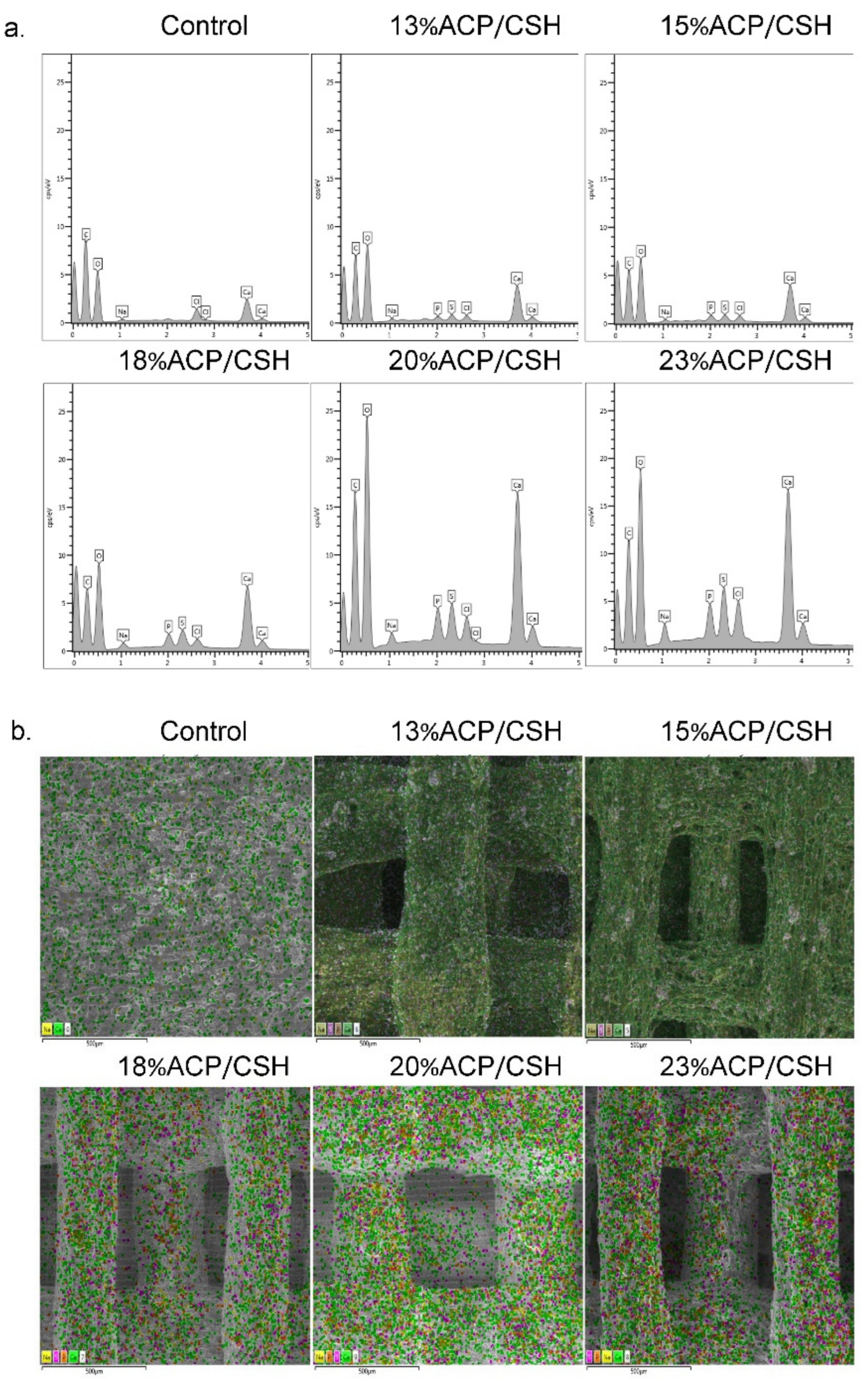
| Groups | C | O | Ca | P | Na | S | Cl | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Control | 50.21 | 1.57 | 41.88 | 1.38 | 5.65 | 0.27 | 0 | 0.00 | 0.41 | 0.06 | 0.00 | 0.00 | 1.86 | 0.12 |
| 13%ACP/CSH | 38.49 | 0.47 | 49.86 | 0.69 | 8.74 | 0.34 | 0.63 | 0.02 | 0.51 | 0.06 | 0.84 | 0.08 | 0.94 | 0.09 |
| 15%ACP/CSH | 38.30 | 0.54 | 49.25 | 0.40 | 9.25 | 0.64 | 0.84 | 0.13 | 0.52 | 0.03 | 0.88 | 0.21 | 0.95 | 0.06 |
| 18%ACP/CSH | 37.02 | 1.63 | 47.53 | 0.75 | 10.26 | 0.43 | 1.23 | 0.15 | 0.74 | 0.05 | 1.49 | 0.17 | 1.73 | 1.05 |
| 20%ACP/CSH | 37.18 | 1.49 | 46.82 | 1.26 | 10.23 | 0.57 | 1.44 | 0.10 | 1.10 | 0.07 | 1.95 | 0.07 | 1.28 | 0.17 |
| 23%ACP/CSH | 34.14 | 0.55 | 45.60 | 0.14 | 12.38 | 0.37 | 1.83 | 0.12 | 1.52 | 0.08 | 2.20 | 0.42 | 2.31 | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wattanaanek, N.; Suttapreyasri, S.; Samruajbenjakun, B. Osteoblast-like Cell Differentiation on 3D-Printed Scaffolds Using Various Concentrations of Tetra-Polymers. Biomimetics 2022, 7, 70. https://doi.org/10.3390/biomimetics7020070
Wattanaanek N, Suttapreyasri S, Samruajbenjakun B. Osteoblast-like Cell Differentiation on 3D-Printed Scaffolds Using Various Concentrations of Tetra-Polymers. Biomimetics. 2022; 7(2):70. https://doi.org/10.3390/biomimetics7020070
Chicago/Turabian StyleWattanaanek, Nattanan, Srisurang Suttapreyasri, and Bancha Samruajbenjakun. 2022. "Osteoblast-like Cell Differentiation on 3D-Printed Scaffolds Using Various Concentrations of Tetra-Polymers" Biomimetics 7, no. 2: 70. https://doi.org/10.3390/biomimetics7020070
APA StyleWattanaanek, N., Suttapreyasri, S., & Samruajbenjakun, B. (2022). Osteoblast-like Cell Differentiation on 3D-Printed Scaffolds Using Various Concentrations of Tetra-Polymers. Biomimetics, 7(2), 70. https://doi.org/10.3390/biomimetics7020070






