A Review of Biomimetic Topographies and Their Role in Promoting Bone Formation and Osseointegration: Implications for Clinical Use
Abstract
:1. Introduction
2. Spine Fusion Devices as a Subset of Bone-Facing Implants
3. Methodology of Literature Search
4. Biomimetic Nature of Titanium and Its Alloys
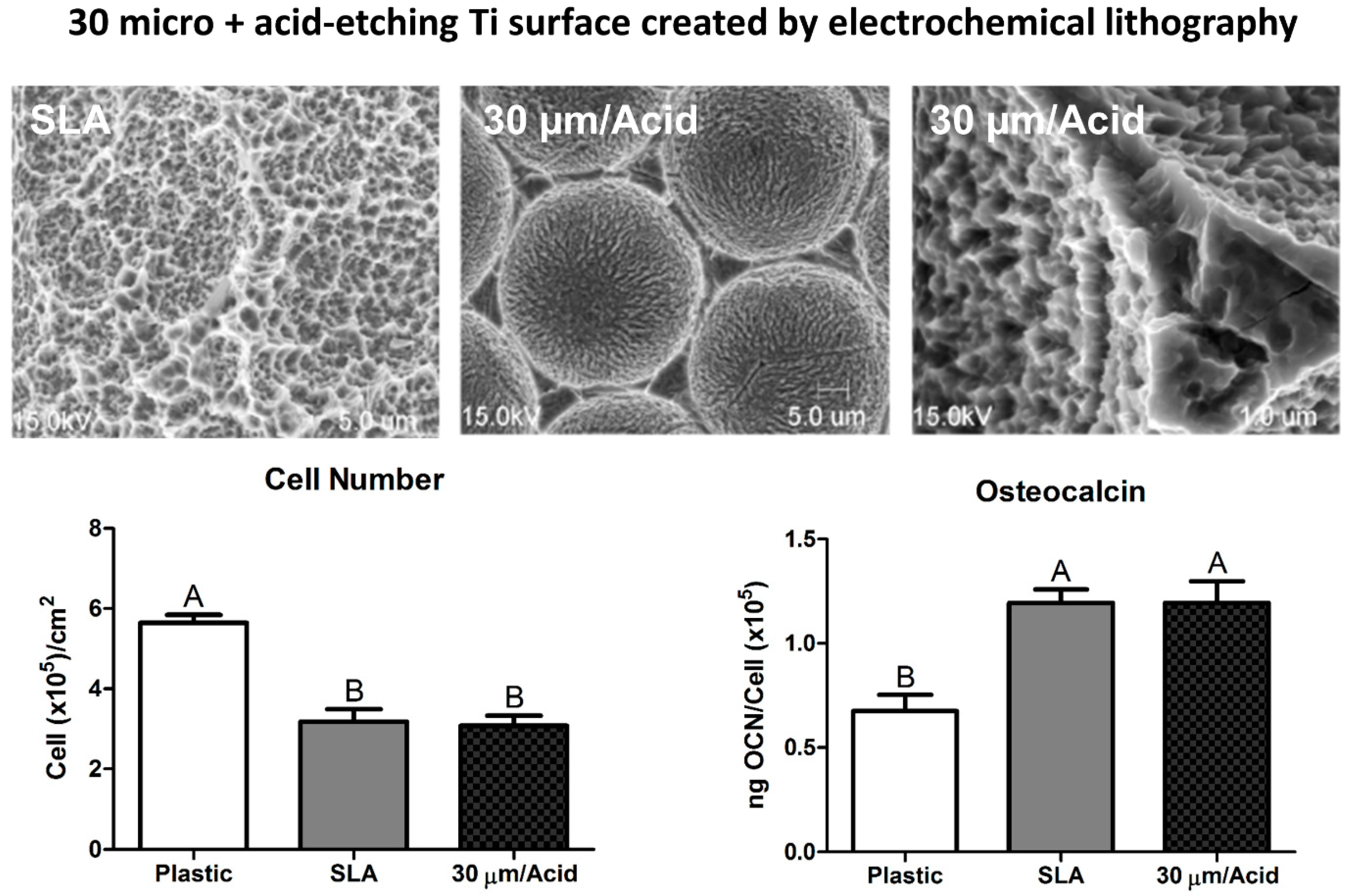
5. Biomimicry: Nanotopography as a Critical Variable in Surface Topography
6. Biomimetic Surface Topography and Immune Modulation
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Terheyden, H.; Lang, N.P.; Bierbaum, S.; Stadlinger, B. Osseointegration—Communication of cells. Clin. Oral Implant. Res. 2012, 23, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Holt, D.J.; Grainger, D.W. Demineralized bone matrix as a vehicle for delivering endogenous and exogenous therapeutics in bone repair. Adv. Drug Deliv. Rev. 2012, 64, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Trindade, R.; Albrektsson, T.; Wennerberg, A. Current Concepts for the Biological Basis of Dental Implants: Foreign Body Equilibrium and Osseointegration Dynamics. Oral Maxillofac. Surg. Clin. N. Am 2015, 27, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Olivares-Navarrete, R.; Schwartz, Z.; Boyan, B.D. Implant osseointegration and the role of microroughness and nanostructures: Lessons for spine implants. Acta Biomater. 2014, 10, 3363–3371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makanji, H.S.; Schroeder, G.D.; Vaccaro, A.R.; Hoffman, E.G. What is the Best Material for an Interbody Cage? Clin. Spine Surg. 2020, 33, 137–139. Available online: https://journals.lww.com/jspinaldisorders/Fulltext/2020/05000/What_is_the_Best_Material_for_an_Interbody_Cage_.4.aspx (accessed on 8 March 2022). [CrossRef] [PubMed]
- Zdeblick, T.A.; Phillips, F.M. Interbody Cage Devices. Spine (Phila Pa 1976) 2003, 28, S2–S7. Available online: https://journals.lww.com/spinejournal/Fulltext/2003/08011/Interbody_Cage_Devices.2.aspx (accessed on 8 March 2022).
- Olivares-Navarrete, R.; Hyzy, S.L.; Berg, M.E.; Schneider, J.M.; Hotchkiss, K.; Schwartz, Z.; Boyan, B.D. Osteoblast lineage cells can discriminate microscale topographic features on titanium-aluminum-vanadium surfaces. Ann. Biomed. Eng. 2014, 42, 2551–2561. [Google Scholar] [CrossRef] [Green Version]
- Elkazaz, M.K.; Koptan, M.W.; Alshatoury, H.A.; Alkosha, H.M.; Abou-Madawi, A. Cervical Intervertebral Cages: Past, Present, Innovations, and Future Trends with Review of the Literature. Egypt. Spine J. 2020, 35, 2–29. [Google Scholar] [CrossRef]
- Trindade, R.; Albrektsson, T.; Galli, S.; Prgomet, Z.; Tengvall, P.; Wennerberg, A. Bone Immune Response to Materials, Part I: Titanium, PEEK and Copper in Comparison to Sham at 10 Days in Rabbit Tibia. J. Clin. Med. 2018, 7, 526. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, Y.; Naito, K.; Yamagata, T.; Yoshimura, M.; Shimokawa, N.; Nishikawa, M.; Ohata, K.; Takami, T. Safety of anterior cervical discectomy and fusion using titanium-coated polyetheretherketone stand-alone cages: Multicenter prospective study of incidence of cage subsidence. J. Clin. Neurosci. 2020, 74, 47–54. [Google Scholar] [CrossRef]
- Kashii, M.; Kitaguchi, K.; Makino, T.; Kaito, T. Comparison in the same intervertebral space between titanium-coated and uncoated PEEK cages in lumbar interbody fusion surgery. J. Orthop. Sci. 2020, 25, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Suh, P.B.; Puttlitz, C.; Lewis, C.; Bal, B.S.; McGilvray, K. The Effect of Cervical Interbody Cage Morphology, Material Composition, and Substrate Density on Cage Subsidence. JAAOS—J. Am. Acad. Orthop. Surg. 2017, 25, 160–168. Available online: https://journals.lww.com/jaaos/Fulltext/2017/02000/The_Effect_of_Cervical_Interbody_Cage_Morphology,.10.aspx (accessed on 8 March 2022). [CrossRef] [PubMed]
- Campbell, P.G.; Cavanaugh, D.A.; Nunley, P.; Utter, P.A.; Kerr, E.; Wadhwa, R.; Stone, M. PEEK versus titanium cages in lateral lumbar interbody fusion: A comparative analysis of subsidence. J. Neurosurg. Spine 2020, 49, E10. [Google Scholar] [CrossRef] [PubMed]
- Massaad, E.; Fatima, N.; Kiapour, A.; Hadzipasic, M.; Shankar, G.M.; Shin, J.H. Polyetheretherketone Versus Titanium Cages for Posterior Lumbar Interbody Fusion: Meta-Analysis and Review of the Literature. Neurospine 2020, 17, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Canseco, J.A.; Karamian, B.A.; Patel, P.D.; Divi, S.N.; Timmons, T.; Hallman, H.; Nachwalter, R.; Lee, J.K.; Kurd, M.F.; Anderson, D.G.; et al. PEEK Versus Titanium Static Interbody Cages: A Comparison of 1-Year Clinical and Radiographic Outcomes for 1-Level TLIFs. Clin. Spine Surg. 2021, 34, E483–E493. Available online: https://journals.lww.com/jspinaldisorders/Fulltext/2021/10000/PEEK_Versus_Titanium_Static_Interbody_Cages__A.12.aspx (accessed on 8 March 2022). [CrossRef] [PubMed]
- Theron, A.J.; Tintinger, G.R.; Anderson, R. Harmful interactions of non-essential heavy metals with cells of the innate immune system. J. Clin. Toxicol. 2011, s3, 5. [Google Scholar] [CrossRef] [Green Version]
- Hallab, N.; JJacobs, J.; Black, J. Hypersensitivity to metallic biomaterials: A review of leukocyte migration inhibition assays. Biomaterials 2000, 21, 1301–1314. [Google Scholar] [CrossRef]
- Yang, J.J.; Yu, C.H.; Chang, B.; Yoem, J.S.; Lee, J.H.; Lee, C.-K. Subsidence and Nonunion after Anterior Cervical Interbody Fusion Using a Stand-Alone Polyetheretherketone (PEEK) Cage. Clin. Orthop. Surg. 2011, 3, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Jiya, T.U.; Smit, T.; Van Royen, B.J.; Mullender, M. Posterior lumbar interbody fusion using non resorbable poly-ether-ether-ketone versus resorbable poly-L-lactide-co-D, L-lactide fusion devices. Clinical outcome at a minimum of 2-year follow-up. Eur. Spine J. 2011, 20, 618–622. [Google Scholar] [CrossRef] [Green Version]
- Olivares-Navarrete, R.; Hyzy, S.L.; Slosar, P.J.; Schneider, J.M.; Schwartz, Z.; Boyan, B.D. Implant materials generate different peri-implant inflammatory factors: Poly-ether-ether-ketone promotes fibrosis and microtextured titanium promotes osteogenic factors. Spine (Phila Pa 1976) 2015, 40, 399–404. [Google Scholar] [CrossRef]
- Evers, M. Spine Market: Data & Infographics—Orthoworld. 2020. Available online: https://www.orthoworld.com/reports-downloads/ (accessed on 8 March 2022).
- Girasole, G.; Muro, G.; Mintz, A.; Chertoff, J. Transforaminal lumbar interbody fusion rates in patients using a novel titanium implant and demineralized cancellous allograft bone sponge. Int. J. Spine Surg. 2013, 7, e95–e100. [Google Scholar] [CrossRef] [PubMed]
- Brånemark, P.I. Osseointegration and its experimental background. J. Prosthet. Dent. 1983, 50, 399–410. [Google Scholar] [CrossRef]
- Branemark, P.I.; Adell, R.; Albrektsson, T.; Lekholm, U.; Lundkvist, S.; Rockler, B. Osseointegrated titanium fixtures in the treatment of edentulousness. Biomaterials 1983, 4, 25–28. [Google Scholar] [CrossRef]
- Salvi, G.E.; Bosshardt, D.D.; Lang, N.P.; Abrahamsson, I.; Berglundh, T.; Lindhe, J.; Ivanovski, S.; Donos, N. Temporal sequence of hard and soft tissue healing around titanium dental implants. Periodontol. 2000 2015, 68, 135–152. [Google Scholar] [CrossRef]
- Ripamonti, U.; Roden, L.C.; Renton, L.F. Osteoinductive hydroxyapatite-coated titanium implants. Biomaterials 2012, 33, 3813–3823. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Rautray, T.R.; Narayanan, R.; Kim, K.-H. Ion implantation of titanium based biomaterials. Prog Mater Sci 2011, 56, 1137–1177. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.Y.; Schwartz, Z.; Hummert, T.W.; Schraub, D.M.; Simpson, J.; Lankford, J., Jr.; Dean, D.D.; Cochran, D.L.; Boyan, B.D. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63). J. Biomed. Mater. Res. 1995, 29, 389–401. [Google Scholar] [CrossRef]
- Zhao, G.; Zinger, O.; Schwartz, Z.; Wieland, M.; Landolt, D.; Boyan, B.D. Osteoblast-like cells are sensitive to submicron-scale surface structure. Clin. Oral Implant. Res. 2006, 17, 258–264. [Google Scholar] [CrossRef]
- Zinger, O.; Zhao, G.; Schwartz, Z.; Simpson, J.; Wieland, M. Differential regulation of osteoblasts by substrate microstructural features. Biomaterials 2005, 26, 1837–1847. [Google Scholar] [CrossRef]
- Mulari, M.T.K.; Qu, Q.; Härkönen, P.L.; Väänänen, H.K. Osteoblast-like cells complete osteoclastic bone resorption and form new mineralized bone matrix in vitro. Calcif. Tissue Int. 2004, 75, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Hefti, T.; Frischherz, M.; Spencer, N.D.; Hall, H.; Schlottig, F. A comparison of osteoclast resorption pits on bone with titanium and zirconia surfaces. Biomaterials 2010, 31, 7321–7331. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Schwartz, Z.; Lohmann, C.H.; Sylvia, V.L.; Cochran, D.L.; Dean, D.D.; Puzas, J.E. Pretreatment of bone with osteoclasts affects phenotypic expression of osteoblast-like cells. J. Orthop. Res. 2003, 21, 638–647. [Google Scholar] [CrossRef]
- Schaffner, P.; Dard, M.M. Structure and function of RGD peptides involved in bone biology. Cell. Mol. Life Sci. 2003, 60, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Turlybekuly, A.; Pogrebnjak, A.D.; Sukhodub, L.F.; Sukhodub, L.B.; Kistaubayeva, A.S.; Savitskaya, I.S.; Shokatayeva, D.H.; Bondar, O.V.; Shaimardanov, Z.K.; Plotnikov, S.V.; et al. Synthesis, characterization, in vitro biocompatibility and antibacterial properties study of nanocomposite materials based on hydroxyapatite-biphasic ZnO micro- and nanoparticles embedded in Alginate matrix. Mater. Sci. Eng. C 2019, 104, 109965. [Google Scholar] [CrossRef]
- Brammer, K.S.; Oh, S.; Gallagher, J.O.; Jin, S. Enhanced cellular mobility guided by TiO2 nanotube surfaces. Nano Lett. 2008, 8, 786–793. [Google Scholar] [CrossRef]
- Jang, T.S.; Jung, H.D.; Kim, S.; Moon, B.S.; Baek, J.; Park, C.; Song, J.; Kim, H.E. Multiscale porous titanium surfaces via a two-step etching process for improved mechanical and biological performance. Biomed Mater. 2017, 12, 025008. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface modifications and their effects on titanium dental implants. Biomed. Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef] [Green Version]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.; Goodwin, W.B.; DeGlee, B.M.; Gittens, R.A.; Vernon, J.P.; Hyzy, S.L.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. Surface modification of bulk titanium substrates for biomedical applications via low-temperature microwave hydrothermal oxidation. J. Biomed. Mater. Res. A 2017, 106, 782–796. [Google Scholar] [CrossRef]
- Lotz, E.M.; Olivares-Navarrete, R.; Hyzy, S.L.; Berner, S.; Schwartz, Z.; Boyan, B.D. Comparable responses of osteoblast lineage cells to microstructured hydrophilic titanium–zirconium and microstructured hydrophilic titanium. Clin. Oral Implant. Res. 2017, 28, e51–e59. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Zhang, T.; Xiu, P.; Cai, H.; Wei, Q.; Fan, D.; Lin, X.; Song, C.; Liu, Z. Functionalization of 3D-printed titanium alloy orthopedic implants: A literature review. Biomed. Mater. 2020, 15, 052003. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Bonewald, L.F.; Paschalis, E.P.; Lohmann, C.H.; Rosser, J.; Cochran, D.L.; Dean, D.D.; Schwartz, Z.; Boskey, A.L. Osteoblast-mediated mineral deposition in culture is dependent on surface microtopography. Calcif. Tissue Int. 2002, 71, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, Z.; Lohmann, C.H.; Oefinger, J.; Bonewald, L.F.; Dean, D.D.; Boyan, B.D. Implant surface characteristics modulate differentiation behavior of cells in the osteoblastic lineage. Adv. Dent. Res. 1999, 13, 38–48. [Google Scholar] [CrossRef]
- Bonewald, L.F.; Harris, S.E.; Rosser, J.; Dallas, M.R.; Dallas, S.L.; Camacho, N.P.; Boyan, B.; Boskey, A. Von Kossa Staining Alone Is Not Sufficient to Confirm that Mineralization In Vitro Represents Bone Formation. Calcif. Tissue Int. 2003, 72, 537–547. [Google Scholar] [CrossRef]
- Lotz, E.M.; Olivares-Navarrete, R.; Berner, S.; Boyan, B.D.; Schwartz, Z. Osteogenic response of human MSCs and osteoblasts to hydrophilic and hydrophobic nanostructured titanium implant surfaces. J. Biomed. Mater. Res.—Part A 2016, 104, 3137–3148. [Google Scholar] [CrossRef]
- Buser, D.; Janner, S.F.M.; Wittneben, J.; Brägger, U.; Ramseier, C.A.; Salvi, G.E. 10-Year survival and success rates of 511 titanium implants with a sandblasted and acid-etched surface: A retrospective study in 303 partially edentulous patients. Clin. Oral Implant. Res. 2012, 26, 1121–1128. [Google Scholar] [CrossRef]
- Lotz, E.M.; Cohen, D.J.; Schwartz, Z.; Boyan, B.D. Titanium implant surface properties enhance osseointegration in ovariectomy induced osteoporotic rats without pharmacologic intervention. Clin. Oral Implant. Res. 2020, 31, 374–387. [Google Scholar] [CrossRef]
- Hyzy, S.L.; Cheng, A.; Cohen, D.J.; Yatzkaier, G.; Whitehead, A.J.; Clohessy, R.M.; Gittens, R.A.; Boyan, B.D.; Schwartz, Z. Novel hydrophilic nanostructured microtexture on direct metal laser sintered Ti–6Al–4V surfaces enhances osteoblast response in vitro and osseointegration in a rabbit model. J. Biomed. Mater. Res.—Part A 2016, 104, 2086–2098. [Google Scholar] [CrossRef]
- Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin. Oral Implant. Res. 2011, 22, 349–356. [Google Scholar] [CrossRef]
- Kou, P.M.; Schwartz, Z.; Boyan, B.D.; Babensee, J.E. Dendritic cell responses to surface properties of clinical titanium surfaces. Acta Biomater. 2011, 7, 1354–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abaricia, J.O.; Shah, A.H.; Olivares-Navarrete, R. Substrate stiffness induces neutrophil extracellular trap (NET) formation through focal adhesion kinase activation. Biomaterials 2021, 271, 120715. [Google Scholar] [CrossRef] [PubMed]
- Abaricia, J.O.; Farzad, N.; Heath, T.J.; Simmons, J.; Morandini, L.; Olivares-Navarrete, R. Control of innate immune response by biomaterial surface topography, energy, and stiffness. Acta Biomater. 2021, 133, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016, 31, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Hotchkiss, K.M.; Clark, N.M.; Olivares-Navarrete, R. Macrophage response to hydrophilic biomaterials regulates MSC recruitment and T-helper cell populations. Biomaterials 2018, 182, 202–215. [Google Scholar] [CrossRef]
- Abaricia, O.; Shah, A.H.; Chaubal, M.; Hotchkiss, K.M.; Olivares-Navarrete, R. Wnt signaling modulates macrophage polarization and is regulated by biomaterial surface properties. Biomaterials 2020, 243, 119920. [Google Scholar] [CrossRef]
- Refai, A.K.; Textor, M.; Brunette, D.M.; Waterfield, J.D. Effect of titanium surface topography on macrophage activation and secretion of proinflammatory cytokines and chemokines. J. Biomed. Mater. Res. 2004, 70, 194–205. [Google Scholar] [CrossRef]
- Hyzy, S.L.; Olivares-Navarrete, R.; Ortman, S.; Boyan, B.D.; Schwartz, Z. Bone morphogenetic protein 2 alters osteogenesis and anti-inflammatory profiles of mesenchymal stem cells induced by microtextured titanium in vitro. Tissue Eng. Part A 2017, 23, 1132–1141. [Google Scholar] [CrossRef]
- El Awadly, T.; Wu, G.; Ayad, M.; Radi, I.; Wismeijer, D.; Abo El Fetouh, H.; Osman, R.B. A histomorphometric study on treated and untreated ceramic filled PEEK implants versus titanium implants: Preclinical in vivo study. Clin. Oral Implant. Res. 2019, 31, 246–254. [Google Scholar] [CrossRef]
- Najeeb, S.; Khurshid, Z.; Matinlinna, J.P.; Siddiqui, F.; Nassani, M.Z.; Baroudi, K. Nanomodified Peek Dental Implants: Bioactive Composites and Surface Modification—A Review. Int. J. Dent. 2015, 2015, 381759. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.M.B.; Ng, S.H.; Yoon, Y.J. A review on 3D printed bioimplants. Int. J. Precis. Eng. Manuf. 2015, 16, 1035–1046. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berger, M.B.; Slosar, P.; Schwartz, Z.; Cohen, D.J.; Goodman, S.B.; Anderson, P.A.; Boyan, B.D. A Review of Biomimetic Topographies and Their Role in Promoting Bone Formation and Osseointegration: Implications for Clinical Use. Biomimetics 2022, 7, 46. https://doi.org/10.3390/biomimetics7020046
Berger MB, Slosar P, Schwartz Z, Cohen DJ, Goodman SB, Anderson PA, Boyan BD. A Review of Biomimetic Topographies and Their Role in Promoting Bone Formation and Osseointegration: Implications for Clinical Use. Biomimetics. 2022; 7(2):46. https://doi.org/10.3390/biomimetics7020046
Chicago/Turabian StyleBerger, Michael B., Paul Slosar, Zvi Schwartz, David J. Cohen, Stuart B. Goodman, Paul A. Anderson, and Barbara D. Boyan. 2022. "A Review of Biomimetic Topographies and Their Role in Promoting Bone Formation and Osseointegration: Implications for Clinical Use" Biomimetics 7, no. 2: 46. https://doi.org/10.3390/biomimetics7020046
APA StyleBerger, M. B., Slosar, P., Schwartz, Z., Cohen, D. J., Goodman, S. B., Anderson, P. A., & Boyan, B. D. (2022). A Review of Biomimetic Topographies and Their Role in Promoting Bone Formation and Osseointegration: Implications for Clinical Use. Biomimetics, 7(2), 46. https://doi.org/10.3390/biomimetics7020046








