The Molecular and Mechanical Characteristics of Biomimetic Composite Dental Materials Composed of Nanocrystalline Hydroxyapatite and Light-Cured Adhesive
Abstract
:1. Introduction
2. Methods of Production and Study of the Samples
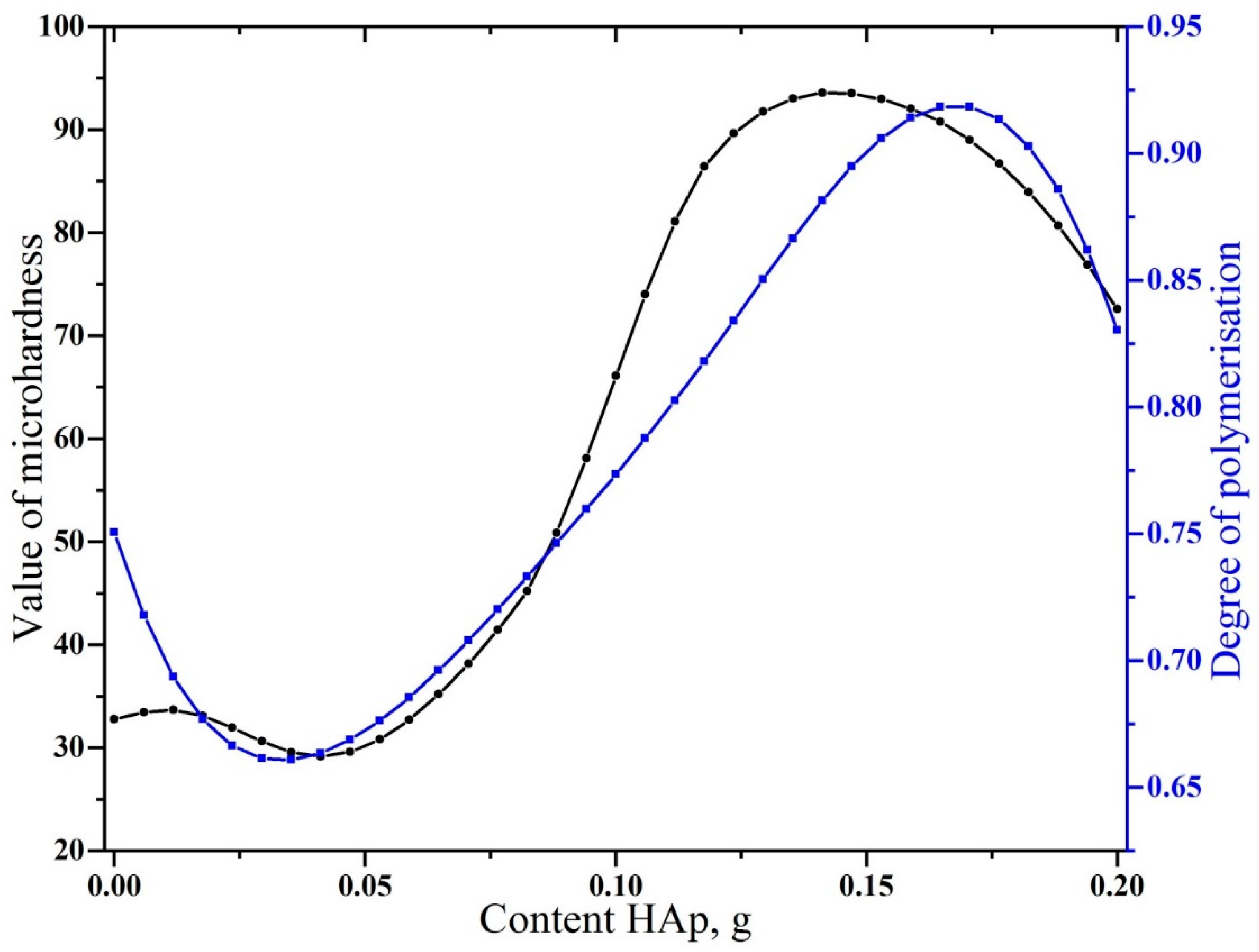
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perdigão, J. Current perspectives on dental adhesion: (1) Dentin adhesion—Not there yet. Jpn. Dent. Sci. Rev. 2020, 56, 190–207. [Google Scholar] [CrossRef]
- Goswami, S. Biomimetic dentistry. J. Oral Res. Rev. 2018, 10, 28–32. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Y.; Gao, L.; Wu, C.; Chang, J. Synthesis of artificial dental enamel by an elastin-like polypeptide assisted biomimetic approach. J. Mater. Chem. B 2018, 6, 844–853. [Google Scholar] [CrossRef]
- Chun, H.J.; Park, K.; Kim, C.-H.; Khang, G. Novel Biomaterials for Regenerative Medicine; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 9789811309472. [Google Scholar]
- Comeau, P.; Willett, T. Impact of Side Chain Polarity on Non-Stoichiometric Nano-Hydroxyapatite Surface Functionalization with Amino Acids. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Tavafoghi, M.; Cerruti, M. The role of amino acids in hydroxyapatite mineralization. J. R. Soc. Interface 2016, 13, 20160462. [Google Scholar] [CrossRef] [Green Version]
- Libonati, F.; Nair, A.K.; Vergani, L.; Buehler, M.J. Mechanics of collagen–hydroxyapatite model nanocomposites. Mech. Res. Commun. 2014, 58, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Delgado, A.H.; Young, A.M. Modelling ATR-FTIR Spectra of Dental Bonding Systems to Investigate Composition and Polymerisation Kinetics. Materials 2021, 14, 760. [Google Scholar] [CrossRef]
- Zafar, M.S.; Amin, F.; Fareed, M.A.; Ghabbani, H.; Riaz, S.; Khurshid, Z.; Kumar, N. Biomimetic Aspects of Restorative Dentistry Biomaterials. Biomimetics 2020, 5, 34. [Google Scholar] [CrossRef]
- Alhenaki, A.M.; Attar, E.A.; Alshahrani, A.; Farooq, I.; Vohra, F.; Abduljabbar, T. Dentin Bond Integrity of Filled and Unfilled Resin Adhesive Enhanced with Silica Nanoparticles—An SEM, EDX, Micro-Raman, FTIR and Micro-Tensile Bond Strength Study. Polymers 2021, 13, 1093. [Google Scholar] [CrossRef]
- Timpe, N.; Fullriede, H.; Borchers, L.; Stiesch, M.; Behrens, P.; Menzel, H. Nanoporous silica nanoparticles with spherical and anisotropic shape as fillers in dental composite materials. BioNanoMaterials 2014, 15, 89–99. [Google Scholar] [CrossRef]
- Mirică, I.-C.; Furtos, G.; Bâldea, B.; Lucaciu, O.; Ilea, A.; Moldovan, M.; Câmpian, R.-S. Influence of Filler Loading on the Mechanical Properties of Flowable Resin Composites. Materials 2020, 13, 1477. [Google Scholar] [CrossRef] [Green Version]
- Furtos, G.; Naghiu, M.-A.; Declercq, H.; Gorea, M.; Prejmerean, C.; Pana, O.; Tomoaia-Cotisel, M. Nano forsterite biocomposites for medical applications: Mechanical properties and bioactivity. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1290–1301. [Google Scholar] [CrossRef]
- Lagazzo, A.; Barberis, F.; Carbone, C.; Ramis, G.; Finocchio, E. Molecular level interactions in brushite-aminoacids composites. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 721–727. [Google Scholar] [CrossRef]
- Kwak, S.Y.; Litman, A.; Margolis, H.C.; Yamakoshi, Y.; Simmer, J.P. Biomimetic Enamel Regeneration Mediated by Leucine-Rich Amelogenin Peptide. J. Dent. Res. 2017, 96, 524–530. [Google Scholar] [CrossRef]
- Provenzi, C.; Leitune, V.C.; Collares, F.M.; Trommer, R.; Bergmann, C.P.; Samuel, S.M. Interface evaluation of experimental dental adhesives with nanostructured hydroxyapatite incorporation. Appl. Adhes. Sci. 2014, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Furtos, G.; Tomoaia-Cotisel, M.; Garbo, C.; Şenilă, M.; Jumate, N.; Vida-Simiti, I.; Prejmerean, C. New Composite Bone Cement Based on Hydroxyapatite and Nanosilver. Part. Sci. Technol. 2013, 31, 392–398. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphate-Containing Biocomposites and Hybrid Biomaterials for Biomedical Applications. J. Funct. Biomater. 2015, 6, 708–832. [Google Scholar] [CrossRef] [Green Version]
- Lezaja, M.; Jokic, B.M.; Veljovic, D.N.; Miletic, V. Shear bond strength to dentine of dental adhesives containing hydroxyapatite nano-fillers. J. Adhes. Sci. Technol. 2016, 30, 2678–2689. [Google Scholar] [CrossRef]
- Al-Hamdan, R.S.; Almutairi, B.; Kattan, H.F.; Alresayes, S.; Abduljabbar, T.; Vohra, F. Assessment of Hydroxyapatite Nanospheres Incorporated Dentin Adhesive. A SEM/EDX, Micro-Raman, Microtensile and Micro-Indentation Study. Coatings 2020, 10, 1181. [Google Scholar] [CrossRef]
- Leitune, V.C.B.; Collares, F.M.; Trommer, R.M.; Andrioli, D.G.; Bergmann, C.P.; Samuel, S.M.W. The addition of nanostructured hydroxyapatite to an experimental adhesive resin. J. Dent. 2013, 41, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Seredin, P.V.; Goloshchapov, D.L.; Prutskij, T.; Ippolitov, Y.A. Fabrication and characterisation of composites materials similar optically and in composition to native dental tissues. Results Phys. 2017, 7, 1086–1094. [Google Scholar] [CrossRef]
- Goloshchapov, D.L.; Gushchin, M.S.; Kashkarov, V.M.; Seredin, P.V.; Ippolitov, Y.A.; Khmelevsky, N.O.; Aksenenko, A.Y. XPS and XANES studies of biomimetic composites based on B-type nano-hydroxyapatite. Results Phys. 2018, 9, 1386–1387. [Google Scholar] [CrossRef]
- Goloshchapov, D.; Buylov, N.; Emelyanova, A.; Ippolitov, I.; Ippolitov, Y.; Kashkarov, V.; Khudyakov, Y.; Nikitkov, K.; Seredin, P. Raman and XANES Spectroscopic Study of the Influence of Coordination Atomic and Molecular Environments in Biomimetic Composite Materials Integrated with Dental Tissue. Nanomaterials 2021, 11, 3099. [Google Scholar] [CrossRef]
- Khan, A.S.; Khalid, H.; Sarfraz, Z.; Khan, M.; Iqbal, J.; Muhammad, N.; Fareed, M.A.; Rehman, I.U. Vibrational spectroscopy of selective dental restorative materials. Appl. Spectrosc. Rev. 2017, 52, 507–540. [Google Scholar] [CrossRef]
- Ye, Q.; Parthasarathy, R.; Abedin, F.; Laurence, J.S.; Misra, A.; Spencer, P. Multivariate Analysis of Attenuated Total Reflection Fourier Transform Infrared (ATR FT-IR) Spectroscopic Data to Confirm Phase Partitioning in Methacrylate-Based Dentin Adhesive. Appl. Spectrosc. 2013, 67, 1473–1478. [Google Scholar] [CrossRef] [Green Version]
- Dal Sasso, G.; Asscher, Y.; Angelini, I.; Nodari, L.; Artioli, G. A universal curve of apatite crystallinity for the assessment of bone integrity and preservation. Sci. Rep. 2018, 8, 12025. [Google Scholar] [CrossRef]
- Bērziņš, K.; Sutton, J.J.; Loch, C.; Beckett, D.; Wheeler, B.J.; Drummond, B.K.; Fraser-Miller, S.J.; Gordon, K.C. Application of low-wavenumber Raman spectroscopy to the analysis of human teeth. J. Raman Spectrosc. 2019, 50, 1375–1387. [Google Scholar] [CrossRef]
- Pezzotti, G.; Zhu, W.; Boffelli, M.; Adachi, T.; Ichioka, H.; Yamamoto, T.; Marunaka, Y.; Kanamura, N. Vibrational algorithms for quantitative crystallographic analyses of hydroxyapatite-based biomaterials: I, theoretical foundations. Anal. Bioanal. Chem. 2015, 407, 3325–3342. [Google Scholar] [CrossRef]
- Goloshchapov, D.; Kashkarov, V.; Nikitkov, K.; Seredin, P. Investigation of the Effect of Nanocrystalline Calcium Carbonate-Substituted Hydroxyapatite and L-Lysine and L-Arginine Surface Interactions on the Molecular Properties of Dental Biomimetic Composites. Biomimetics 2021, 6, 70. [Google Scholar] [CrossRef]
- Daood, U.; Swee Heng, C.; Neo Chiew Lian, J.; Fawzy, A.S. In vitro analysis of riboflavin-modified, experimental, two-step etch-and-rinse dentin adhesive: Fourier transform infrared spectroscopy and micro-Raman studies. Int. J. Oral Sci. 2015, 7, 110–124. [Google Scholar] [CrossRef]
- Kasraei, S.; Khamverdi, Z. Effect of Nanofiller Addition to an Experimental Dentin Adhesive on Microtensile Bond Strength to Human Dentin. J. Dent. Tehran Univ. Med. Sci. 2009, 6, 1–5. [Google Scholar]
- Mikhail, S.S.; Azer, S.S.; Schricker, S.R. Nanofillers in Restorative Dental Materials. In Handbook of Nanomaterials Properties; Bhushan, B., Luo, D., Schricker, S.R., Sigmund, W., Zauscher, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1377–1442. ISBN 978-3-642-31106-2. [Google Scholar]
- Souza, G.M.D. Nanoparticles in Restorative Materials. In Nanotechnology in Endodontics; Kishen, A., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 139–171. ISBN 978-3-319-13574-8. [Google Scholar]
- Del Gutiérrez-Salazar, M.P.; Reyes-Gasga, J. Microhardness and chemical composition of human tooth. Mater. Res. 2003, 6, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Aydın, B.; Pamir, T.; Baltaci, A.; Orman, M.N.; Turk, T. Effect of storage solutions on microhardness of crown enamel and dentin. Eur. J. Dent. 2015, 9, 262–266. [Google Scholar] [CrossRef] [Green Version]
- Seredin, P.; Goloshchapov, D.; Ippolitov, Y.; Vongsvivut, J. Engineering of a Biomimetic Interface between a Native Dental Tissue and Restorative Composite and Its Study Using Synchrotron FTIR Microscopic Mapping. Int. J. Mol. Sci. 2021, 22, 6510. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Ippolitov, Y.; Ippolitov, I.; Vongsvivut, J. To the Question on the Use of Multivariate Analysis and 2D Visualisation of Synchrotron ATR-FTIR Chemical Imaging Spectral Data in the Diagnostics of Biomimetic Sound Dentin/Dental Composite Interface. Diagnostics 2021, 11, 1294. [Google Scholar] [CrossRef]
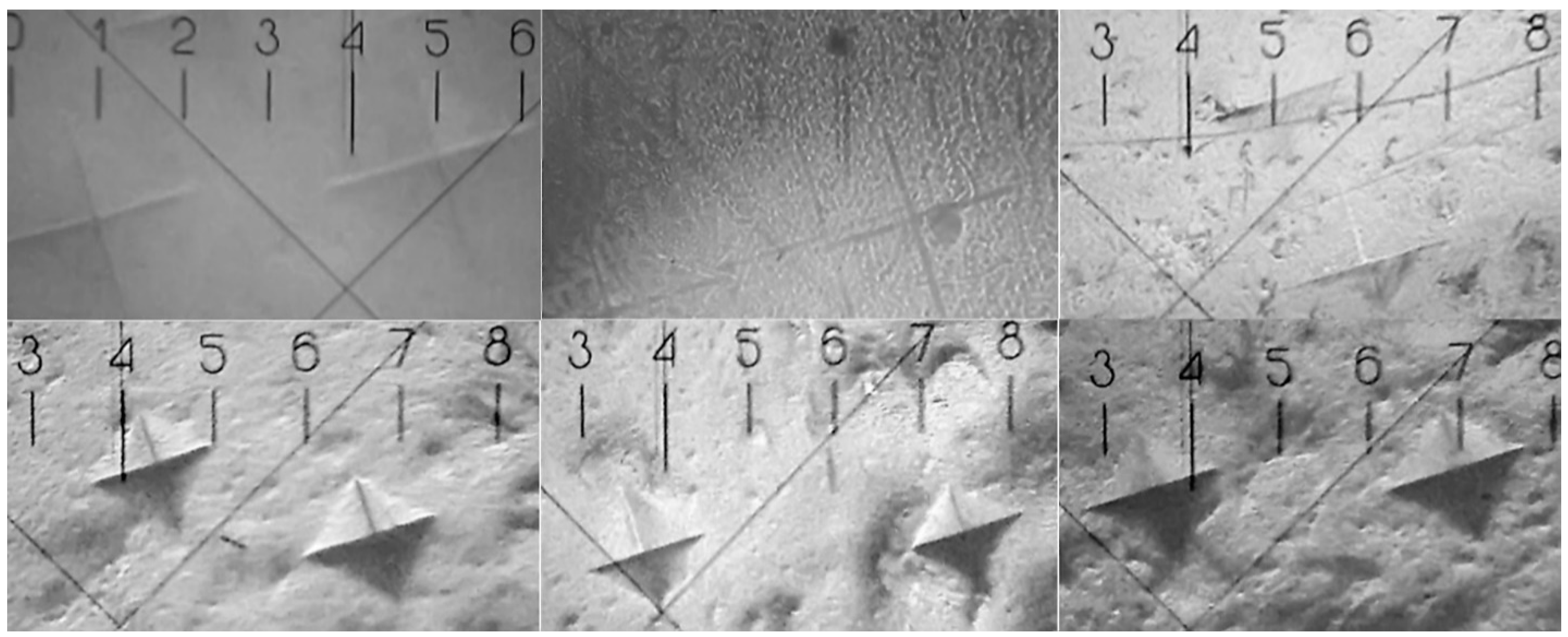
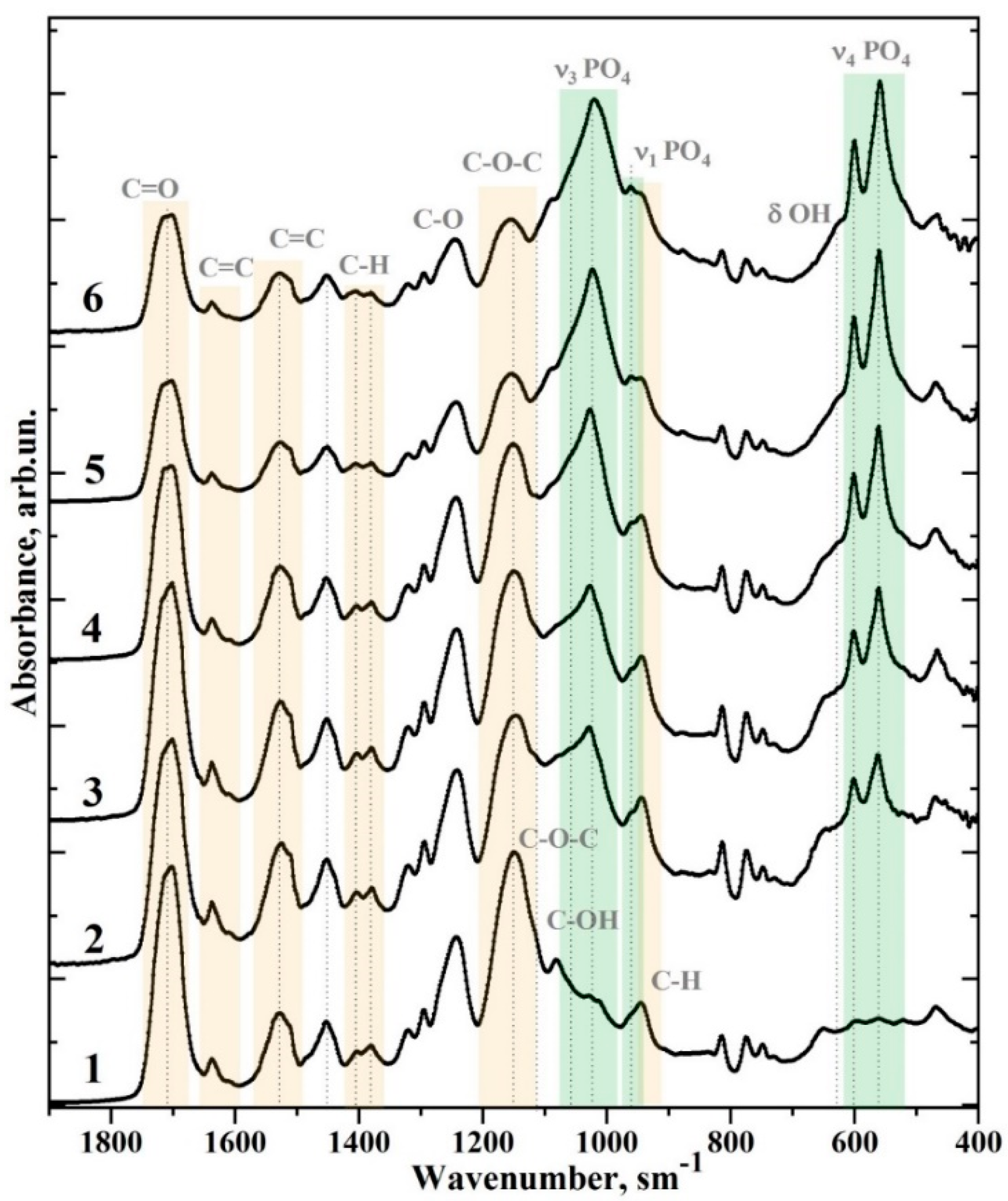
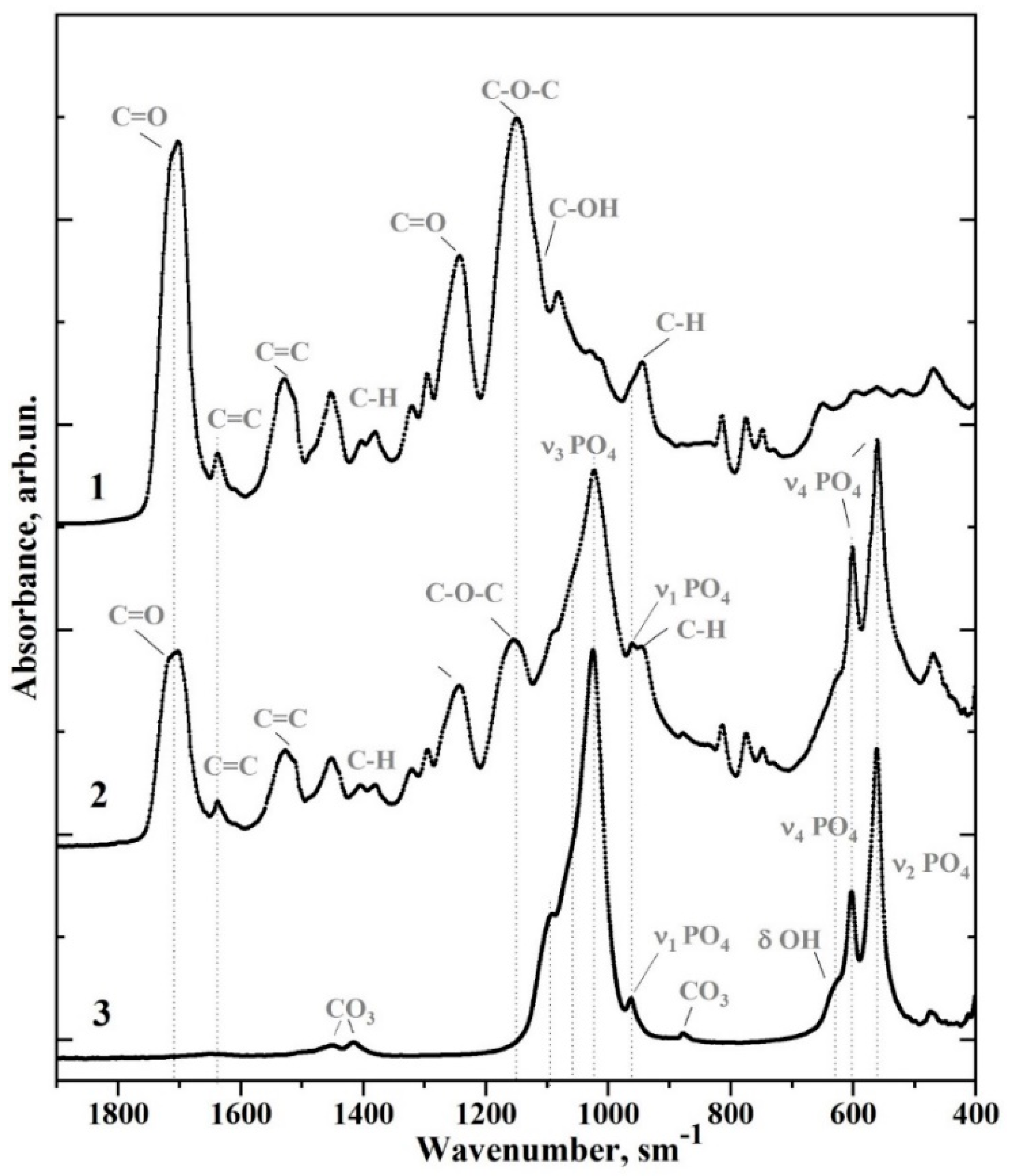
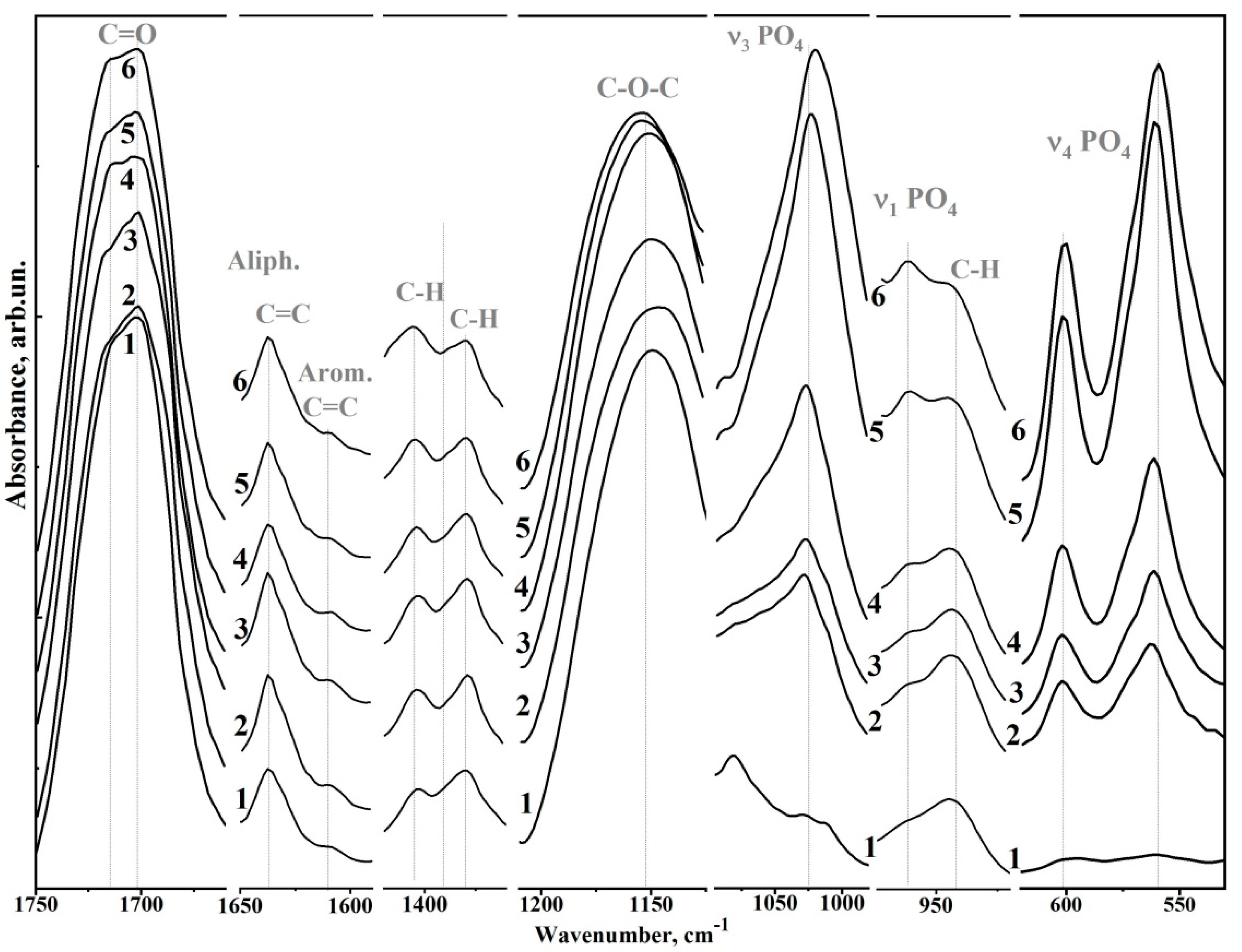
| Sample | Bis-GMA, mL | nano-cHAp, g | Hμ (HV) | Degree of Conversion |
|---|---|---|---|---|
| #1 | 250 | 0.2 | 33.68 | 0.827 ± 0.012 |
| #2 | 250 | 0.16 | 29.16 | 0.93 ± 0.016 |
| #3 | 250 | 0.12 | 43.56 | 0.80 ± 0.014 |
| #4 | 250 | 0.08 | 87.90 | 0.74 ± 0.015 |
| #5 | 250 | 0.04 | 91.82 | 0.68 ± 0.015 |
| #6 | 250 | 0.01 | 72.60 | 0.654 ± 0.016 |
| Wavenumber (cm−1) | Assignment | Compound | References |
|---|---|---|---|
| 1750–1665 | C=O stretch, (–COOCH3) ether | Bis-GMA* | [8,25,26] |
| 1637 | Aliphatic C=C | Bis-GMA | [8,25,26] |
| 1610 | Aromatic C=C | Bis-GMA | [8,25,26] |
| 1528, 1510 | Aromatic C=C | Bis-GMA | [8,25,26] |
| 1451 | C–H bending, υ3 CO32− in HAp lattice | Bis-GMA, nano-cHAp* | [22] |
| 1414 | υ3 CO32− in HAp lattice | nano-cHAp | [8,25,26] |
| 1403, 1380 | C–H bending | Bis-GMA | [8,25,26] |
| 1320, 1295 | C–O stretch doublet | Bis-GMA | [8,25,26] |
| 1243 | Aromatic C–O | Bis-GMA | [8,25,26] |
| 1150 | C–O–C stretch | Bis-GMA | [8,25,26] |
| 1120 | C–O–C stretch | Bis-GMA | [8,25,26] |
| 1090 | υ3 PO43− | nano-cHAp | [22] |
| 1081 | C–OH stretch | Bis-GMA | [8,25,26] |
| 962 | υ1 PO43− (stretching mode of the P–O bond) | nano-cHAp | [22] |
| 960, 945 | C-H | Bis-GMA | [8,25,26] |
| 878, 870 | CO32− in HAP lattice | nano-cHAp | [22] |
| 815 | C–C–O stretch | Bis-GMA | [8,25,26] |
| 630 | δ OH | nano-cHAp | [22] |
| 602, 597 | υ4 PO43− O–P–O bending modes | nano-cHAp | [22] |
| 562.560 | nano-cHAp | [22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Ippolitov, Y.; Vongsvivut, J. The Molecular and Mechanical Characteristics of Biomimetic Composite Dental Materials Composed of Nanocrystalline Hydroxyapatite and Light-Cured Adhesive. Biomimetics 2022, 7, 35. https://doi.org/10.3390/biomimetics7020035
Seredin P, Goloshchapov D, Kashkarov V, Ippolitov Y, Vongsvivut J. The Molecular and Mechanical Characteristics of Biomimetic Composite Dental Materials Composed of Nanocrystalline Hydroxyapatite and Light-Cured Adhesive. Biomimetics. 2022; 7(2):35. https://doi.org/10.3390/biomimetics7020035
Chicago/Turabian StyleSeredin, Pavel, Dmitry Goloshchapov, Vladimir Kashkarov, Yuri Ippolitov, and Jitraporn Vongsvivut. 2022. "The Molecular and Mechanical Characteristics of Biomimetic Composite Dental Materials Composed of Nanocrystalline Hydroxyapatite and Light-Cured Adhesive" Biomimetics 7, no. 2: 35. https://doi.org/10.3390/biomimetics7020035
APA StyleSeredin, P., Goloshchapov, D., Kashkarov, V., Ippolitov, Y., & Vongsvivut, J. (2022). The Molecular and Mechanical Characteristics of Biomimetic Composite Dental Materials Composed of Nanocrystalline Hydroxyapatite and Light-Cured Adhesive. Biomimetics, 7(2), 35. https://doi.org/10.3390/biomimetics7020035








