Collagen-Based Biomaterials in Periodontal Regeneration: Current Applications and Future Perspectives of Plant-Based Collagen
Abstract
1. Introduction
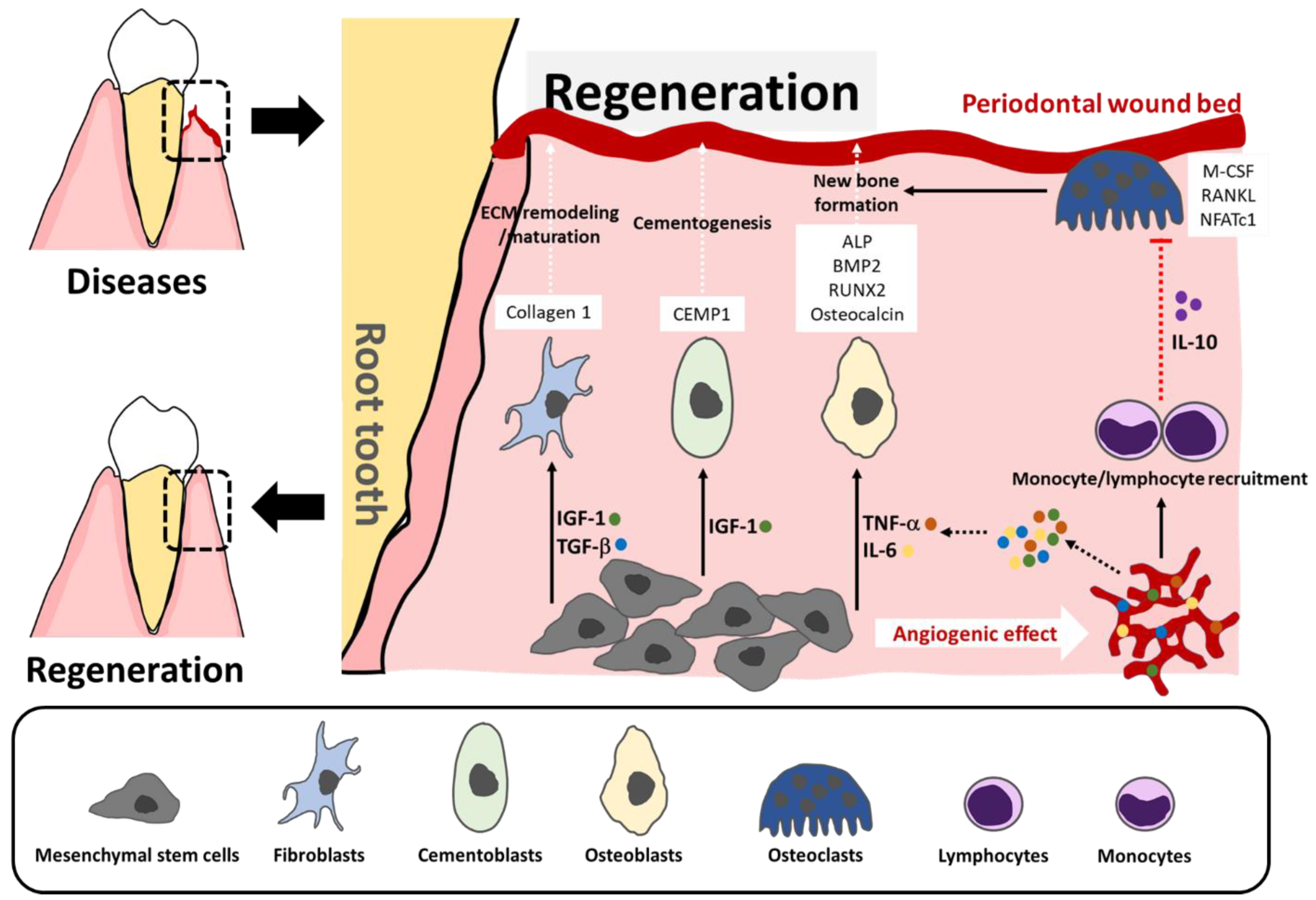
2. Concept of Regeneration in Periodontics
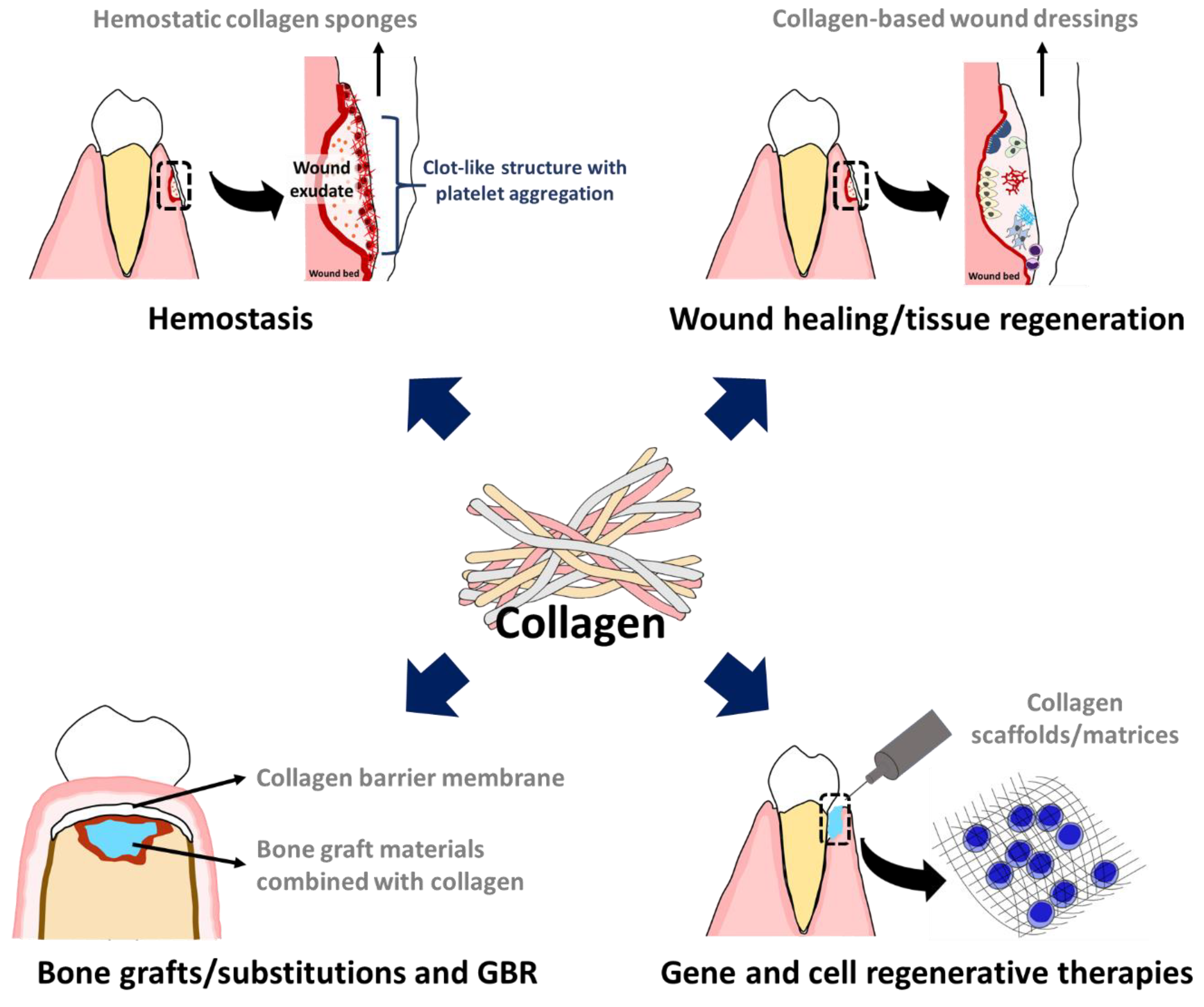
3. Current Applications, Mechanism of Action, and Limitations of Collagen-Based Biomaterials in Periodontal Regeneration
3.1. Hemostasis and Wound Healing
3.2. Guided Bone Regeneration:(GBR)
3.3. Being Carrier and Transport Vehicle of Collagen in Scaffold/Matrix Forms
3.3.1. Bone Graft and Substitution
3.3.2. Gene and Cell Regenerative Therapies
3.4. Limitations of Collagen-Based Biomaterials in Periodontal Regeneration
4. Plant-Based Collagen and Its Perspective
4.1. History
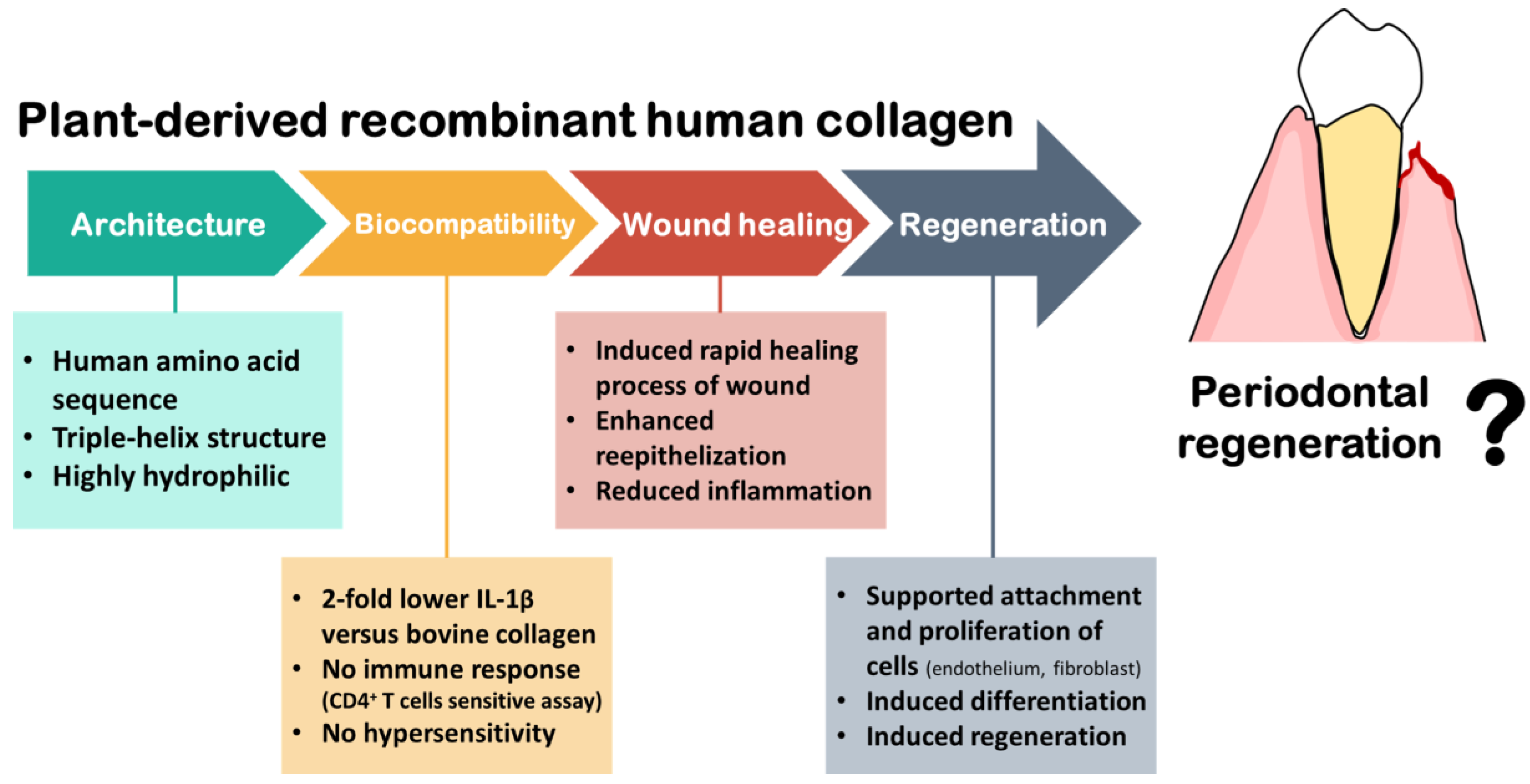
4.2. Plant Recombinant Human Collage
4.3. Medical Studies of Using Plant Recombinant Human Collagen
4.4. Perspective in Periodontal Treatment
5. Summary and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hernández-Monjaraz, B.; Santiago-Osorio, E.; Monroy-García, A.; Ledesma-Martínez, E.; Mendoza-Núñez, V.M. Mesenchymal stem cells of dental origin for inducing tissue regeneration in periodontitis: A mini-review. Int. J. Mol. Sci. 2018, 19, 944. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007, 25, 2648–2659. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Decker, A.; Nibali, L.; Pilipchuk, S.; Berglundh, T.; Giannobile, W. Regenerative medicine for periodontal and peri-implant diseases. J. Dent. Res. 2016, 95, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Christ, G.J.; Saul, J.M.; Furth, M.E.; Andersson, K.-E. The pharmacology of regenerative medicine. Pharmacol. Rev. 2013, 65, 1091–1133. [Google Scholar] [CrossRef]
- Onizuka, S.; Iwata, T. Application of periodontal ligament-derived multipotent mesenchymal stromal cell sheets for periodontal regeneration. Int. J. Mol. Sci. 2019, 20, 2796. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-D.; Kim, K.-H.; Lee, Y.-M.; Ku, Y.; Seol, Y.-J. Periodontal Wound Healing and Tissue Regeneration: A Narrative Review. Pharmaceuticals 2021, 14, 456. [Google Scholar] [CrossRef]
- Vanden Berg-Foels, W.S. In situ tissue regeneration: Chemoattractants for endogenous stem cell recruitment. Tissue Eng. Part B Rev. 2014, 20, 28–39. [Google Scholar] [CrossRef]
- Pankajakshan, D.; Agrawal, D.K. Mesenchymal stem cell paracrine factors in vascular repair and regeneration. J. Biomed. Technol. Res. 2014, 1. [Google Scholar] [CrossRef]
- Silva, G.V.; Litovsky, S.; Assad, J.A.; Sousa, A.L.; Martin, B.J.; Vela, D.; Coulter, S.C.; Lin, J.; Ober, J.; Vaughn, W.K. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation 2005, 111, 150–156. [Google Scholar] [CrossRef]
- Feng, X.; Feng, G.; Xing, J.; Shen, B.; Li, L.; Tan, W.; Xu, Y.; Liu, S.; Liu, H.; Jiang, J. TNF-α triggers osteogenic differentiation of human dental pulp stem cells via the NF-κB signalling pathway. Cell Biol. Int. 2013, 37, 1267–1275. [Google Scholar] [CrossRef]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Sima, C.; Viniegra, A.; Glogauer, M. Macrophage immunomodulation in chronic osteolytic diseases—The case of periodontitis. J. Leukoc. Biol. 2019, 105, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Wikesjö, U.; Sigurdsson, T.; Lee, M.; Tatakis, D.; Selvig, K. Dynamics of wound healing in periodontal regenerative therapy. J. Calif. Dent. Assoc. 1995, 23, 30–35. [Google Scholar] [PubMed]
- Kato, A.; Miyaji, H.; Ishizuka, R.; Tokunaga, K.; Inoue, K.; Kosen, Y.; Yokoyama, H.; Sugaya, T.; Tanaka, S.; Sakagami, R. Combination of root surface modification with BMP-2 and collagen hydrogel scaffold implantation for periodontal healing in beagle dogs. Open Dent. J. 2015, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- d’Aquino, R.; De Rosa, A.; Lanza, V.; Tirino, V.; Laino, L.; Graziano, A.; Desiderio, V.; Laino, G.; Papaccio, G. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur. Cells Mater. 2009, 18, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.A.; Jin, Q.; Taba, M., Jr.; Franceschi, R.T.; Rutherford, R.B.; Giannobile, W.V. BMP gene delivery for alveolar bone engineering at dental implant defects. Mol. Ther. 2005, 11, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Anusaksathien, O.; Webb, S.A.; Printz, M.A.; Giannobile, W.V. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol. Ther. 2004, 9, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Allan, B.; Ruan, R.; Landao-Bassonga, E.; Gillman, N.; Wang, T.; Gao, J.; Ruan, Y.; Xu, Y.; Lee, C.; Goonewardene, M. Collagen Membrane for Guided Bone Regeneration in Dental and Orthopedic Applications. Tissue Eng. Part A 2021, 27, 372–381. [Google Scholar] [CrossRef]
- Sabitha Sudarsan, K.; Priya, M.; Arun, R. Clinical and histological evaluation of alloderm GBR and BioOss in the treatment of Siebert’s class I ridge deficiency. J. Indian Soc. Periodontol. 2008, 12, 73. [Google Scholar]
- Boymuradov, S. Management of maxillary alveolar process fractures by combination of Osteon and Colla Guide resorbable membrane. Med. Health Sci. J. 2011, 5, 73–75. [Google Scholar] [CrossRef]
- Wessing, B.; Emmerich, M.; Bozkurt, A. Horizontal ridge augmentation with a novel resorbable collagen membrane: A retrospective analysis of 36 consecutive patients. Int. J. Periodontics Restor. Dent. 2016, 36, 179–187. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sheikh, Z.; Hamdan, N.; Ikeda, Y.; Grynpas, M.; Ganss, B.; Glogauer, M. Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: A review. Biomater. Res. 2017, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Abuelnaga, M.; Elbokle, N.; Khashaba, M. Evaluation of custom made xenogenic bone grafts in mandibular alveolar ridge augmentation versus particulate bone graft with titanium mesh. Egypt. J. Oral Maxillofac. Surg. 2018, 9, 62–73. [Google Scholar] [CrossRef]
- Mandelli, F.; Perale, G.; Danti, S.; D’Alessandro, D.; Ghensi, P. Clinical and histological evaluation of socket preservation using SmartBone®, a novel heterologous bone substitute: A case series study. Oral Implant. 2018, 2, 87–94. [Google Scholar]
- Wang, E.; Han, J.; Zhang, X.; Wu, Y.; Deng, X.-L. Efficacy of a mineralized collagen bone-grafting material for peri-implant bone defect reconstruction in mini pigs. Regen. Biomater. 2019, 6, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, M.; Shaaban, R.; Abdelhalim, S.; Sadaka, M. Effect of collaplug® on the healing of extraction sockets in patients under oral anticoagulant therapy (clinical study). Alex. Dent. J. 2015, 40, 166–172. [Google Scholar] [CrossRef]
- Sowjanya, N.P.; Rao, N.; Bhushan, N.S.; Krishnan, G. Versitality of the use of collagen membrane in oral cavity. J. Clin. Diagn. Res. JCDR 2016, 10, ZC30. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Jung, H.-D.; Kim, B.-J.; Kim, C.-H.; Jung, Y.-S. Complication rates in patients using absorbable collagen sponges in third molar extraction sockets: A retrospective study. J. Korean Assoc. Oral Maxillofac. Surg. 2015, 41, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R.; Khinda, P.; Shewale, A.; Ghotra, K.; Bhasin, M.T.; Bhasin, P. Comparative efficacy of placental membrane and Healiguide™ in treatment of gingival recession using guided tissue regeneration. J. Indian Soc. Periodontol. 2018, 22, 513. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Hickman, D.A.; Pawlowski, C.L.; Sekhon, U.D.; Marks, J.; Gupta, A.S. Biomaterials and advanced technologies for hemostatic management of bleeding. Adv. Mater. 2018, 30, 1700859. [Google Scholar] [CrossRef] [PubMed]
- Simionescu, B.; Ivanov, D. Natural and synthetic polymers for designing composite materials. In Handbook of Bioceramics and Biocomposites; Springer International Publishing: Cham, Switzerland, 2016; pp. 233–286. [Google Scholar]
- Sionkowska, A. Current research on the blends of natural and synthetic polymers as new biomaterials. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Mahesh, L.; Kurtzman, G.M.; Shukla, S. Regeneration in Periodontics: Collagen-A Review of Its Properties and Applications in Dentistry. Compend. Contin. Educ. Dent. 2015, 36, 358–363. [Google Scholar] [PubMed]
- Ogle, O. Perioperative hemorrhage. In Atlas of Minor Oral Surgery; Dym, H., Ogle, O.E., Eds.; Saunders: Philadelphia, PA, USA, 2000; pp. 62–63. [Google Scholar]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Jackson, C.J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef]
- El Masry, M.S.; Chaffee, S.; Das Ghatak, P.; Mathew-Steiner, S.S.; Das, A.; Higuita-Castro, N.; Roy, S.; Anani, R.A.; Sen, C.K. Stabilized collagen matrix dressing improves wound macrophage function and epithelialization. FASEB J. 2019, 33, 2144–2155. [Google Scholar] [CrossRef]
- Kwon, H.M.; Hur, S.-M.; Park, K.-Y.; Kim, C.-K.; Kim, Y.-M.; Kim, H.-S.; Shin, H.-C.; Won, M.-H.; Ha, K.-S.; Kwon, Y.-G. Multiple paracrine factors secreted by mesenchymal stem cells contribute to angiogenesis. Vasc. Pharmacol. 2014, 63, 19–28. [Google Scholar] [CrossRef]
- Rezaie, J.; Heidarzadeh, M.; Hassanpour, M.; Amini, H.; Shokrollahi, E.; Ahmadi, M.; Rahbarghazi, R. The angiogenic paracrine potential of mesenchymal stem cells. In Update on Mesenchymal and Induced Pluripotent Stem Cells; IntechOpen: London, UK, 2019. [Google Scholar]
- Wessing, B.; Lettner, S.; Zechner, W. Guided bone regeneration with collagen membranes and particulate graft materials: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 87–100. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.; Nastri, L. Selection of collagen membranes for bone regeneration: A literature review. Materials 2020, 13, 786. [Google Scholar] [CrossRef]
- Rodriguez, I.; Selders, G.; Fetz, A.; Gehrmann, C.; Stein, S.; Evensky, J.; Green, M.; Bowlin, G. Barrier membranes for dental applications: A review and sweet advancement in membrane developments. Mouth Teeth 2018, 2, 1–9. [Google Scholar]
- Zellin, G.; Linde, A. Effects of different osteopromotive membrane porosities on experimental bone neogenesis in rats. Biomaterials 1996, 17, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Ratiu, C.; Brocks, M.; Costea, T.; Moldovan, L.; Cavalu, S. PRGF-modified collagen membranes for guided bone regeneration: Spectroscopic, microscopic and nano-mechanical investigations. Appl. Sci. 2019, 9, 1035. [Google Scholar] [CrossRef]
- Ghavimi, M.A.; Bani Shahabadi, A.; Jarolmasjed, S.; Memar, M.Y.; Maleki Dizaj, S.; Sharifi, S. Nanofibrous asymmetric collagen/curcumin membrane containing aspirin-loaded PLGA nanoparticles for guided bone regeneration. Sci. Rep. 2020, 10, 18200. [Google Scholar] [CrossRef] [PubMed]
- Cavalu, S.; Roiu, G.; Pop, O.; Heredea, D.A.P.; Costea, T.O.; Costea, C.F. Nano-Scale Modifications of Amniotic Membrane Induced by UV and Antibiotic Treatment: Histological, AFM and FTIR Spectroscopy Evidence. Materials 2021, 14, 863. [Google Scholar] [CrossRef] [PubMed]
- Vogel, W.; Gish, G.D.; Alves, F.; Pawson, T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol. Cell 1997, 1, 13–23. [Google Scholar] [CrossRef]
- Trentham, D.E. Adverse reactions to bovine collagen implants: Additional evidence for immune response gene control of collagen reactivity in humans. Arch. Dermatol. 1986, 122, 643–644. [Google Scholar] [CrossRef] [PubMed]
- Rios, H.F.; Lin, Z.; Oh, B.; Park, C.H.; Giannobile, W.V. Cell- and gene-based therapeutic strategies for periodontal regenerative medicine. J. Periodontol. 2011, 82, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Turri, A.; Elgali, I.; Vazirisani, F.; Johansson, A.; Emanuelsson, L.; Dahlin, C.; Thomsen, P.; Omar, O. Guided bone regeneration is promoted by the molecular events in the membrane compartment. Biomaterials 2016, 84, 167–183. [Google Scholar] [CrossRef]
- Itoh, K.; Udagawa, N.; Katagiri, T.; Iemura, S.; Ueno, N.; Yasuda, H.; Higashio, K.; Quinn, J.M.; Gillespie, M.T.; Martin, T.J. Bone morphogenetic protein 2 stimulates osteoclast differentiation and survival supported by receptor activator of nuclear factor-κB ligand. Endocrinology 2001, 142, 3656–3662. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.D.; Pham, L.; Billington, C.J., Jr.; Espe, K.; Carlson, A.E.; Westendorf, J.J.; Petryk, A.; Gopalakrishnan, R.; Mansky, K. Bone morphogenic protein 2 directly enhances differentiation of murine osteoclast precursors. J. Cell. Biochem. 2010, 109, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A crucial target for anti-and pro-angiogenic therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.T.; Scott, D.D. A review of bone substitutes. Oral Maxillofac. Surg. Clin. N. Am. 2007, 19, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.W.; Rabie, A.B.M. Effect of gusuibu graft on bone formation. J. Oral Maxillofac. Surg. 2006, 64, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Granz, C.L.; Gorji, A. Dental stem cells: The role of biomaterials and scaffolds in developing novel therapeutic strategies. World J. Stem Cells 2020, 12, 897. [Google Scholar] [CrossRef] [PubMed]
- Iohara, K.; Nakashima, M.; Ito, M.; Ishikawa, M.; Nakasima, A.; Akamine, A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J. Dent. Res. 2004, 83, 590–595. [Google Scholar] [CrossRef]
- Heino, J. The collagen family members as cell adhesion proteins. Bioessays 2007, 29, 1001–1010. [Google Scholar] [CrossRef]
- Lebbink, R.J.; de Ruiter, T.; Adelmeijer, J.; Brenkman, A.B.; van Helvoort, J.M.; Koch, M.; Farndale, R.W.; Lisman, T.; Sonnenberg, A.; Lenting, P.J. Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1. J. Exp. Med. 2006, 203, 1419–1425. [Google Scholar] [CrossRef]
- Kirsch, T.; Harrison, G.; Golub, E.E.; Nah, H.-D. The roles of annexins and types II and X collagen in matrix vesicle-mediated mineralization of growth plate cartilage. J. Biol. Chem. 2000, 275, 35577–35583. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of collagen scaffold in tissue engineering: Recent advances and new perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef]
- Martínez, A.; Blanco, M.; Davidenko, N.; Cameron, R. Tailoring chitosan/collagen scaffolds for tissue engineering: Effect of composition and different crosslinking agents on scaffold properties. Carbohydr. Polym. 2015, 132, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Deng, J.; Sun, X.; Qu, Y.; Man, Y. Collagen membrane and immune response in guided bone regeneration: Recent progress and perspectives. Tissue Eng. Part B Rev. 2017, 23, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Samiei, M.; Alipour, M.; Khezri, K.; Saadat, Y.R.; Forouhandeh, H.; Abdolahinia, E.D.; Vahed, S.Z.; Sharifi, S.; Dizaj, S.M. Application of collagen and mesenchymal stem cells in regenerative dentistry. Curr. Stem Cell Res. Ther. 2021. [Google Scholar] [CrossRef] [PubMed]
- Shoseyov, O.; Posen, Y.; Grynspan, F. Human collagen produced in plants: More than just another molecule. Bioengineered 2014, 5, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Davison-Kotler, E.; Marshall, W.S.; García-Gareta, E. Sources of collagen for biomaterials in skin wound healing. Bioengineering 2019, 6, 56. [Google Scholar] [CrossRef]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish collagen: Extraction, characterization, and applications for biomaterials engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef]
- Subhan, F.; Ikram, M.; Shehzad, A.; Ghafoor, A. Marine collagen: An emerging player in biomedical applications. J. Food Sci. Technol. 2015, 52, 4703–4707. [Google Scholar] [CrossRef]
- Addad, S.; Exposito, J.-Y.; Faye, C.; Ricard-Blum, S.; Lethias, C. Isolation, characterization and biological evaluation of jellyfish collagen for use in biomedical applications. Mar. Drugs 2011, 9, 967–983. [Google Scholar] [CrossRef]
- Setina, C.M.; Haase, J.P.; Glatz, C.E. Process integration for recovery of recombinant collagen type I α1 from corn seed. Biotechnol. Prog. 2016, 32, 98–107. [Google Scholar] [CrossRef]
- Xu, X.; Gan, Q.; Clough, R.C.; Pappu, K.M.; Howard, J.A.; Baez, J.A.; Wang, K. Hydroxylation of recombinant human collagen type I alpha 1 in transgenic maize co-expressed with a recombinant human prolyl 4-hydroxylase. BMC Biotechnol. 2011, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Stein, H.; Wilensky, M.; Tsafrir, Y.; Rosenthal, M.; Amir, R.; Avraham, T.; Ofir, K.; Dgany, O.; Yayon, A.; Shoseyov, O. Production of bioactive, post-translationally modified, heterotrimeric, human recombinant type-I collagen in transgenic tobacco. Biomacromolecules 2009, 10, 2640–2645. [Google Scholar] [CrossRef] [PubMed]
- Gallo, N.; Natali, M.L.; Sannino, A.; Salvatore, L. An overview of the use of equine collagen as emerging material for biomedical applications. J. Funct. Biomater. 2020, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Hu, K.; Xiao, Y.; Zhang, F.; Han, L.; Pan, S.; Li, L.; Wei, Y.; Cui, F. Preparation of recombinant human-like collagen/fibroin scaffold and its promoting effect on vascular cells biocompatibility. J. Bioact. Compat. Polym. 2018, 33, 416–425. [Google Scholar] [CrossRef]
- Benayahu, D.; Pomeraniec, L.; Shemesh, S.; Heller, S.; Rosenthal, Y.; Rath-Wolfson, L.; Benayahu, Y. Biocompatibility of a marine collagen-based scaffold in vitro and in vivo. Mar. Drugs 2020, 18, 420. [Google Scholar] [CrossRef]
- Alves, A.; Costa-Gouveia, J.; de Castro, J.V.; Sotelo, C.; Vázquez, J.; Pérez-Martín, R.; Torrado, E.; Neves, N.; Reis, R.; Castro, A. Study of the immunologic response of marine-derived collagen and gelatin extracts for tissue engineering applications. Acta Biomater. 2022, 141, 123–131. [Google Scholar] [CrossRef]
- Seror, J.; Stern, M.; Zarka, R.; Orr, N. The Potential Use of Novel Plant-Derived Recombinant Human Collagen in Aesthetic Medicine. Plast. Reconstr. Surg. 2021, 148, 32S–38S. [Google Scholar] [CrossRef]
- Willard, J.J.; Drexler, J.W.; Das, A.; Roy, S.; Shilo, S.; Shoseyov, O.; Powell, H.M. Plant-derived human collagen scaffolds for skin tissue engineering. Tissue Eng. Part A 2013, 19, 1507–1518. [Google Scholar] [CrossRef]
- Geahchan, S.; Baharlouei, P.; Rahman, A. Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration. Mar. Drugs 2022, 20, 61. [Google Scholar] [CrossRef]
- Liu, C. Application of marine collagen for stem-cell-based therapy and tissue regeneration. Med. Int. 2021, 1, 6. [Google Scholar] [CrossRef]
- Shilo, S.; Roth, S.; Amzel, T.; Harel-Adar, T.; Tamir, E.; Grynspan, F.; Shoseyov, O. Cutaneous wound healing after treatment with plant-derived human recombinant collagen flowable gel. Tissue Eng. Part A 2013, 19, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Haagdorens, M.; Edin, E.; Fagerholm, P.; Groleau, M.; Shtein, Z.; Ulčinas, A.; Yaari, A.; Samanta, A.; Cepla, V.; Liszka, A. Plant Recombinant Human Collagen Type I Hydrogels for Corneal Regeneration. Regen. Eng. Transl. Med. 2021, 7, 1–15. [Google Scholar] [CrossRef]
- Nollet, L.M.; Toldrá, F. Handbook of Analysis of Edible Animal By-Products; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Baltzer, A.W.; Ostapczuk, M.S. Magnetic resonance imaging and clinically controlled improvement of a combined autologous conditioned plasma combined with rh collagen type I injections in lateral epicondylitis. Orthop. Rev. 2021, 13, 9018. [Google Scholar] [CrossRef]
- Iacopi, E.; Banchellini, E.; Riitano, N.; Abbruzzese, L.; Goretti, C.; Piaggesi, A. Pilot trial of plant-derived recombinant type I human collagen in healing post-surgical diabetic foot wounds. Diabet. Foot J. 2020, 23, 30–36. [Google Scholar]
| Therapies | Collagen Applications | Outcomes | References |
|---|---|---|---|
| Gene and cell regenerative therapies | Collagen hydrogel scaffold (prepare from atelocollagen: type I collagen) as scaffold/matrix for recombinant human BMP2 | Reconstruction of cementum-like tissue, periodontal ligament and alveolar bone, and prevention of ankylosis in one wall intra-bony defect | Kato et al., 2015 [14] |
| Gingistat® collagen sponge scaffold with dental pulp stem cells (DPCs) | Optimal vertical repair of alveolar bone, and complete restoration back to the second molar of periodontal tissue in the model of injury site by extraction of mandibular third extraction | d’Aquina et al., 2009 [15] | |
| 2.6% collagen as matrix for Ad-BMP7 gene delivery | Promotion of alveolar bone defect, and enhancement of new bone-to-implant contacts in edentulous ridge defect followed by dental implantation | Dunn et al., 2005 [16] | |
| 2.6% collagen matrix containing Ad-PDGF-B gene delivery | Increase in bridging bone and tooth-lining cemental regeneration in periodontal defects (large tooth-associated alveolar bone defect) | Jin et al., 2004 [17] | |
| Guided bone regeneration | Porcine-derived collagen barrier membrane (CelGroTM, Bio-Gide®) | Restoration of bone defect in both horizontal and vertical dimension, and sufficient support to the implants with no adverse effects in the GBR for dental implants | Allan et al., 2021 [18] |
| AlloDerm® GBR as barrier membrane with bone grafting materials (BioOss) | Significant induction of ridge growth in both horizontal and vertical dimension in soft and hard tissues in class I ridge defect | Sabitha Sudarsan et al., 2008 [19] | |
| Colla-Guide resorbable barrier membrane in combination with Osteon | Preservation of alveolar crest shape and height by restoring bone tissue via secondary osseous tissue formation in maxillary alveolar process fracture | Boymuradov and Shukhrat, 2011 [20] | |
| CreosTM Xenoprotect in combination with bone grafting materials (BioOss) | Enhancement of bone augmentation of alveolar ridge with accelerated healing time in horizontal alveolar ridge defects | Wessing et al., 2016 [21] | |
| Bone graft and substitution | BioMend® type I bovine collagen | Serving as barrier in the prevention of epithelial cell migration/invasion in allowing GBR and GTR regenerations | Sheikh et al., 2017 [22] |
| Demineralized bone combined with collagen matrix (SmartBoneTM) | Successful osseointegration and new bone formation appeared with vascular connective tissue surrounding periodontal osseous defect | Mandelli et al., 2018, Abuelnaga et al., 2018 [23,24] | |
| Mineralized collagen bone grafting material | Stimulation of new bone formation to reconstruct the deficient alveolar ridge around the dental implant | Wang et al., 2019 [25] | |
| Hemostasis and wound healing | CollaPlug® Bovine-derived collagen | Exerting effective local hemostasis, acceleration of healing in soft tissues, and reduction of post-operative pain | Abdelaziz et al., 2015 [26] |
| Bovine-derived collagen membrane (Dressing products from EUCARE pharmaceuticals) | Increase in the scores of hemostasis, granulation tissue formation, and epithelization with reduced pain score in various intra-oral lesions | Sowjanya et al., 2016 [27] | |
| Absorbable type I collagen sponge (Ateoplug) | Enhancement of tissue regeneration by promoting proliferation and differentiation of MSCs in periodontal tissue, facilitation of endogenous healing of wound, and prevention of post-operative complications in third molar extraction socket | Cho et al., 2015 [28] | |
| Bio-resorbable type I bovine collagen (HealiguideTM) | Significant reduction in gingival recession defects with higher clinical assessment values (recession depth, root coverage percentage, probing depth, clinical attachment level, gingival tissue thickness, and others) | Mahajan et al., 2018 [29] |
| Features | Animal Collagen | Marine Collagen | Plant Collagen |
|---|---|---|---|
| Architecture/structure |
|
|
|
| Biocompatibility |
| ||
| Regenerative ability | |||
| Restriction | Religion restriction | No religion restriction | No religion restriction |
| %Yield | 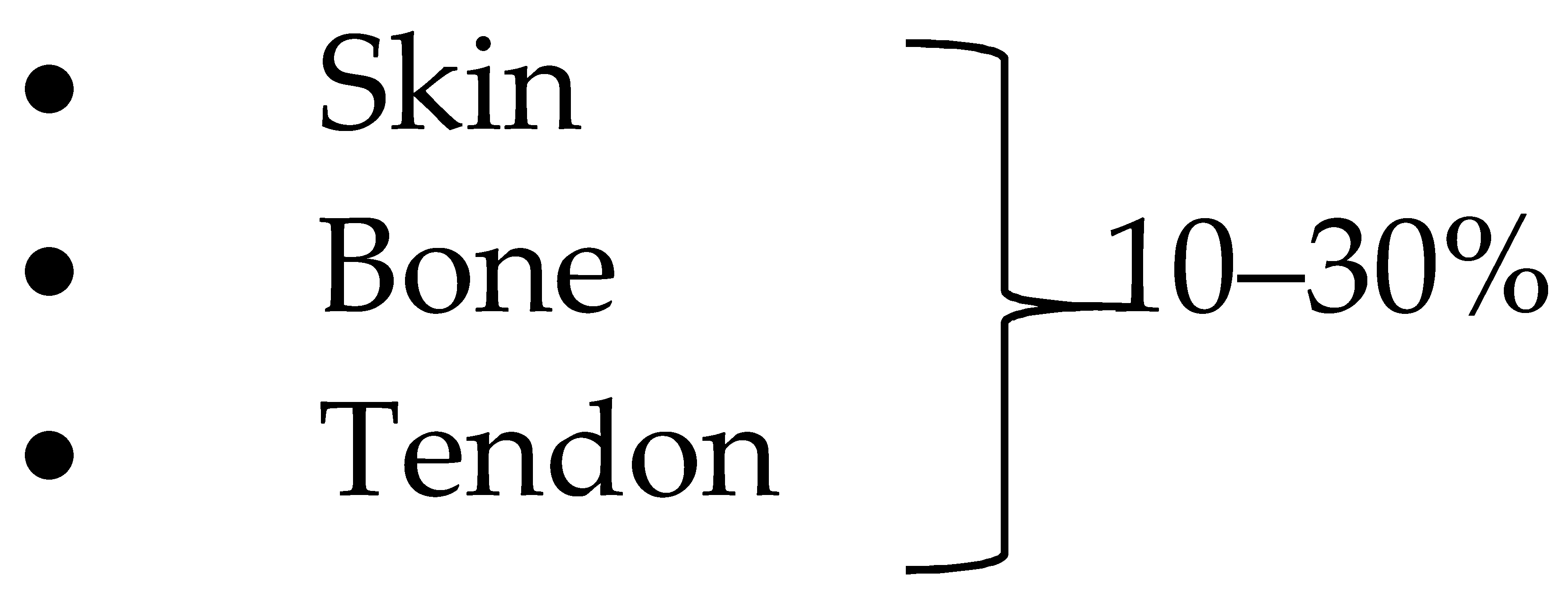 Depending on the species, sex, age and body weight of the animal [86] |
| 1 g/kg (0.1%) [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binlateh, T.; Thammanichanon, P.; Rittipakorn, P.; Thinsathid, N.; Jitprasertwong, P. Collagen-Based Biomaterials in Periodontal Regeneration: Current Applications and Future Perspectives of Plant-Based Collagen. Biomimetics 2022, 7, 34. https://doi.org/10.3390/biomimetics7020034
Binlateh T, Thammanichanon P, Rittipakorn P, Thinsathid N, Jitprasertwong P. Collagen-Based Biomaterials in Periodontal Regeneration: Current Applications and Future Perspectives of Plant-Based Collagen. Biomimetics. 2022; 7(2):34. https://doi.org/10.3390/biomimetics7020034
Chicago/Turabian StyleBinlateh, Thunwa, Peungchaleoy Thammanichanon, Pawornwan Rittipakorn, Natthapol Thinsathid, and Paiboon Jitprasertwong. 2022. "Collagen-Based Biomaterials in Periodontal Regeneration: Current Applications and Future Perspectives of Plant-Based Collagen" Biomimetics 7, no. 2: 34. https://doi.org/10.3390/biomimetics7020034
APA StyleBinlateh, T., Thammanichanon, P., Rittipakorn, P., Thinsathid, N., & Jitprasertwong, P. (2022). Collagen-Based Biomaterials in Periodontal Regeneration: Current Applications and Future Perspectives of Plant-Based Collagen. Biomimetics, 7(2), 34. https://doi.org/10.3390/biomimetics7020034






