Underwater Attachment of the Water-Lily Leaf Beetle Galerucella nymphaeae (Coleoptera, Chrysomelidae)
Abstract
1. Introduction
2. Materials and Methods
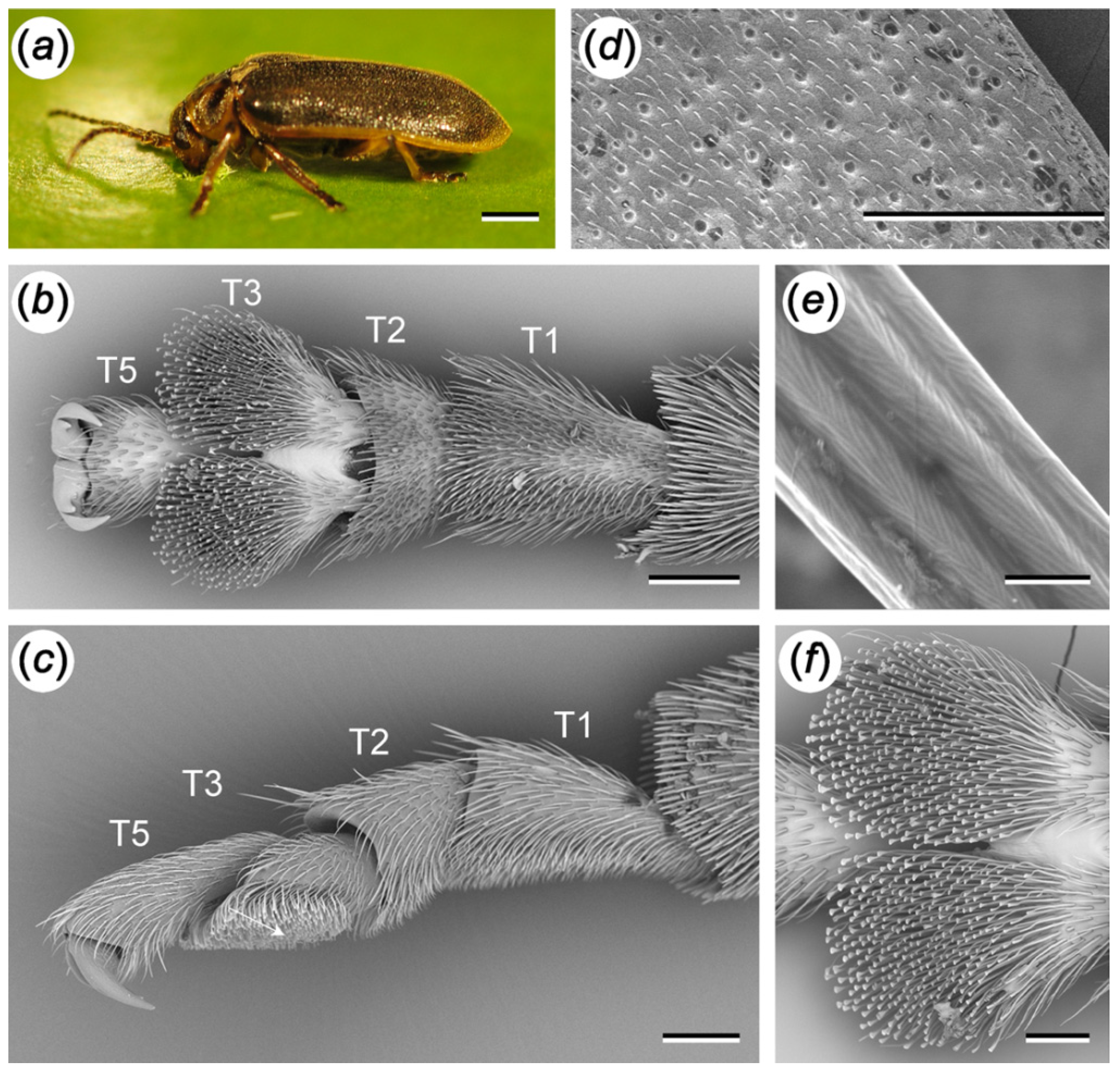
2.1. Tarsal Morphology
2.2. Behaviour Underwater
2.2.1. Horizontal Leaf
2.2.2. Sloped Leaf
2.3. Experiment 1: The Formation of the Subtarsal Air Bubbles on Different Surfaces
2.4. Experiment 2: Traction Force Measurements of the Beetles Walking on Different Surfaces
2.5. Experiment 3: Measurement of the Buoyancy Force
3. Results
3.1. Tarsal Morphology
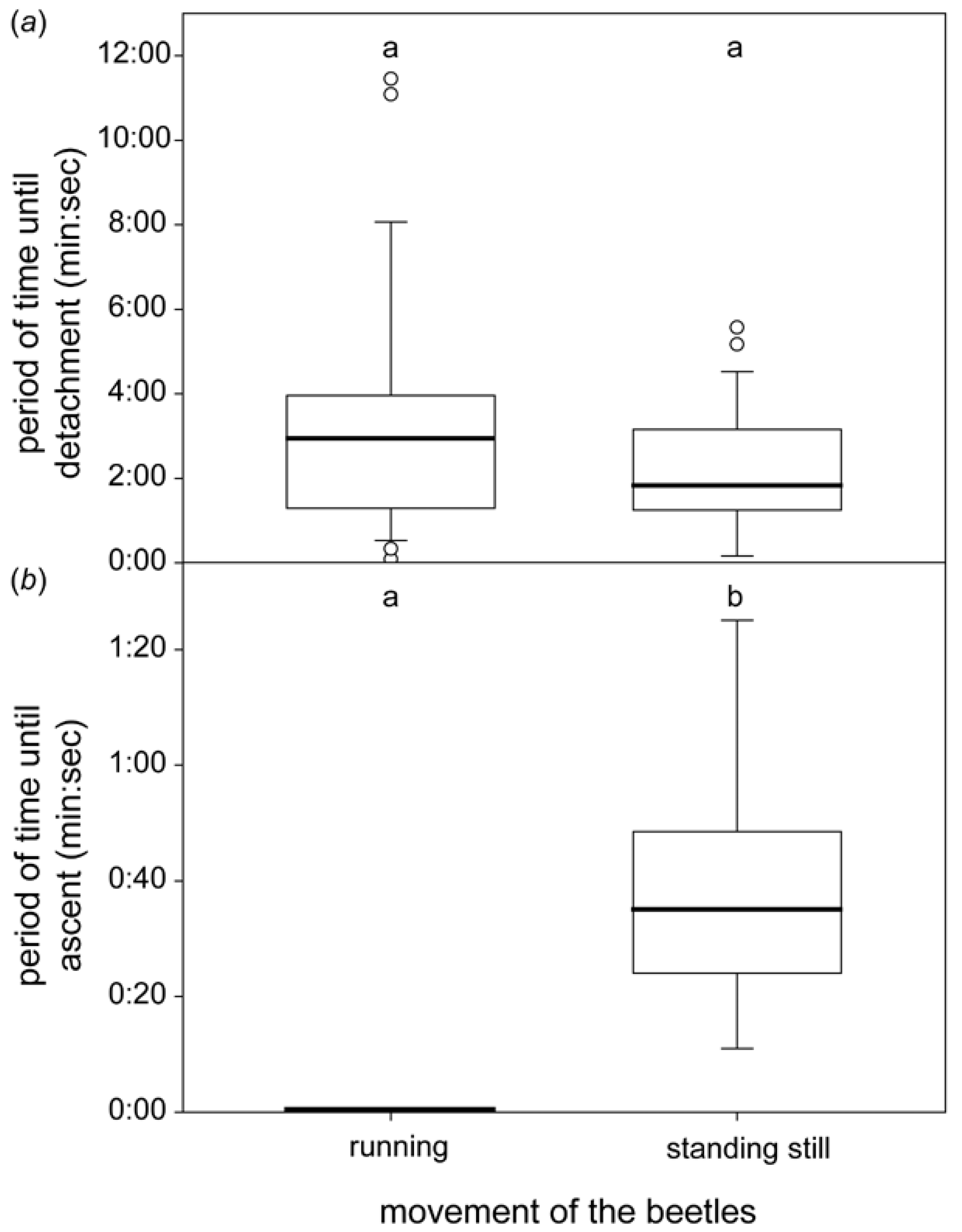
3.2. Behaviour Underwater
3.2.1. Horizontal Leaf
3.2.2. Sloped Leaf
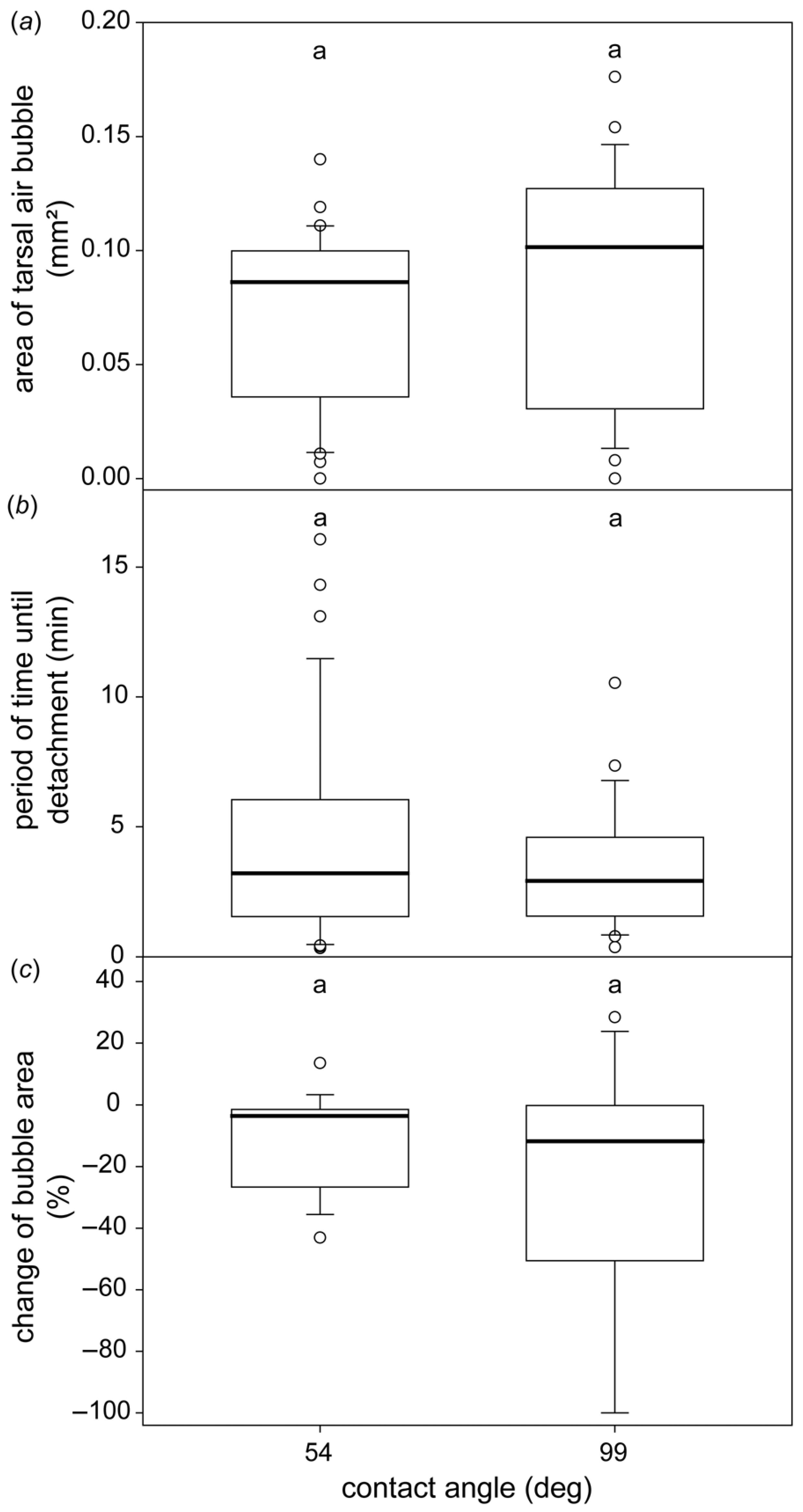
3.3. The Formation of the Subtarsal Air Bubbles on Different Surfaces
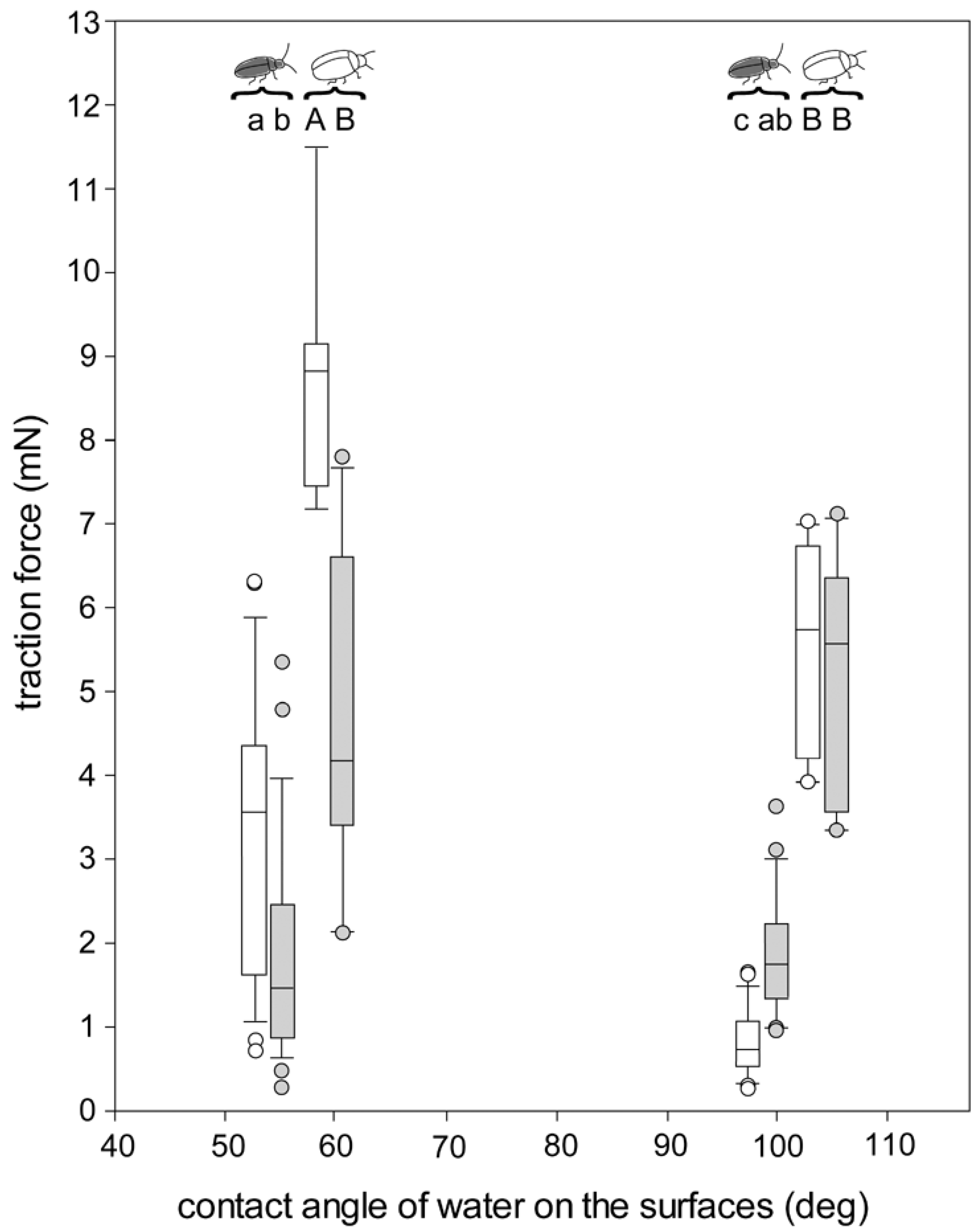
3.4. Traction Force Measurements of the Beetles Walking on Different Surfaces
3.5. Measurement of the Buoyancy Force
4. Discussion
4.1. Tarsal Morphology
4.2. Behaviour Underwater
4.3. Subtarsal Air Bubbles on Different Surfaces
4.4. Traction Forces on Different Surfaces and Buoyancy
4.4.1. Traction Forces on Hydrophilic and Hydrophobic Surfaces in Air
4.4.2. Traction Forces on the Hydrophilic Surface in Air and Underwater
4.4.3. Traction Forces on the Hydrophobic Surface in Air and Underwater
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Danner, E.W.; Kan, Y.; Hammer, M.U.; Israelachvili, J.N.; Waite, J.H. Adhesion of mussel foot protein Mefp-5 to mica: An underwater superglue. Biochemistry 2012, 51, 6511–6518. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Kamino, K. Amyloid-like conformation and interaction for the self-assembly in barnacle underwater cement. Biochemistry 2015, 54, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.J. Protein-based underwater adhesives and the prospects for their biotechnological production. Appl. Microbiol. Biotechnol. 2011, 98, 27–33. [Google Scholar] [CrossRef] [PubMed]
- White, J.D.; Wilker, J.J. Underwater bonding with charged polymer mimics of marine mussel adhesive proteins. Macromolecules 2011, 44, 5085–5088. [Google Scholar] [CrossRef]
- Zhong, C.; Gurry, T.; Cheng, A.A.; Downey, J.; Deng, Z.; Stultz, C.M.; Lu, T.K. Strong underwater adhesives made by self-assembling multi-protein nanofibres. Nat. Nanotech. 2014, 9, 858–866. [Google Scholar] [CrossRef]
- Nishida, J.; Higaki, Y.; Takahara, A. Synthesis and characterization of barnacle adhesive mimetic towards the underwater adhesion. Chem. Lett. 2015, 44, 1047–1049. [Google Scholar] [CrossRef]
- Heepe, L.; Kovalev, A.E.; Gorb, S.N. Direct observation of microcavitation in underwater adhesion of mushroom-shaped adhesive microstructure. Beilstein J. Nanotechnol. 2014, 5, 903–909. [Google Scholar] [CrossRef]
- Stark, A.Y.; McClung, B.; Niewiarowski, P.H.; Dhinojwala, A. Reduction of water surface tension significantly impacts gecko adhesion underwater. Integr. Comp. Biol. 2014, 54, 1026–1033. [Google Scholar] [CrossRef][Green Version]
- Tian, D.; Guo, Z.; Wang, Y.; Li, W.; Zhang, X.; Zhai, J.; Jiang, L. Phototunable underwater oil adhesion of micro/nanoscale hierarchical-structured ZnO mesh films with switchable contact mode. Adv. Funct. Mater. 2014, 24, 536–542. [Google Scholar] [CrossRef]
- Hosoda, N.; Gorb, S.N. Underwater locomotion in a terrestrial beetle: Combination of surface de-wetting and capillary forces. Proc. R. Soc. Lond. B 2012, 279, 4236–4242. [Google Scholar] [CrossRef]
- Chen, Y.; Shih, M.-C.; Wu, M.-H.; Yang, E.-C.; Chi, K.-J. Underwater attachment using hairs: The functioning of spatula and sucker setae from male diving beetles. J. R. Soc. Interface 2014, 11, 20140273. [Google Scholar] [CrossRef] [PubMed]
- Andrade, G.R.; de Araújo, J.L.F.; Nakamura Filho, A.; Guañabens, A.C.P.; de Carvalho, M.D.; Cardoso, A.V. Functional surface of the golden musse’s foot: Morphology, structures and the role of cilia on underwater adhesion. Mater. Sci. Eng. C 2015, 54, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.Y.; Klittich, M.R.; Sitti, M.; Niewiarowski, P.H.; Dhinojwala, A. The effect of temperature and humidity on adhesion of a gecko-inspired adhesive: Implications for the natural system. Sci. Rep. 2016, 6, 30936. [Google Scholar] [CrossRef] [PubMed]
- Beutel, R.G.; Gorb, S.N. Ultrastructure of attachment specializations of hexapods (Arthropoda): Evolutionary patterns inferred from a revised ordinal phylogeny. J. Zool. Syst. Evol. Res. 2001, 39, 177–207. [Google Scholar] [CrossRef]
- Grohmann, C.; Blankenstein, A.; Koops, S.; Gorb, S.N. Attachment of Galerucella nymphaeae (Coleoptera, Chrysomelidae) to surfaces with different surface energy. J. Exp. Biol. 2014, 217, 4213–4220. [Google Scholar] [CrossRef]
- Stork, N.E. A scanning electron microscope study of tarsal adhesive setae in the Coleoptera. Zool. J. Linn. Soc. 1980, 68, 173–306. [Google Scholar] [CrossRef]
- Ishii, S. Adhesion of a leaf feeding ladybird Epilachna vigintioctomaculata (Coleoptera: Coccinellidae) on a vertically smooth surface. Appl. Entomol. Zool. 1987, 22, 222–228. [Google Scholar] [CrossRef]
- Geiselhardt, S.F.; Federle, W.; Prüm, B.; Geiselhardt, S.; Lamm, S.; Peschke, K. Impact of chemical manipulation of tarsal liquids on attachment in the Colorado potato beetle, Leptinotarsa decemlineata. J. Insect Physiol. 2010, 56, 398–404. [Google Scholar] [CrossRef]
- Smirnov, N.N. Nutrition of Galerucella nymphaeae L. (Chrysomelidae), mass consumer of water-lily. Hydrobiologia 1960, 15, 208–224. [Google Scholar] [CrossRef]
- Stork, N.E. Experimental analysis of adhesion of Chrysolina polita (Chrysomelidae, Coleoptera) on a variety of surfaces. J. Exp. Biol. 1980, 88, 91–107. [Google Scholar] [CrossRef]
- Scherge, M.; Li, X.; Schaefer, J.A. The effect of water on friction of MEMS. Tribol. Lett. 1999, 6, 215–220. [Google Scholar] [CrossRef]
- Opitz, A.; Scherge, M.; Ahmed, S.I.-U.; Schaefer, J.A. A comparative investigation of thickness measurements of ultra-thin water films by scanning probe techniques. J. Appl. Phys. 2007, 101, 064310. [Google Scholar] [CrossRef]
- Huber, G.; Mantz, H.; Spolenak, R.; Mecke, K.; Jacobs, K.; Gorb, S.N.; Arzt, E. Evidence for capillarity contributions to gecko adhesion from single spatula nanomechanical measurements. Proc. Natl. Acad. Sci. USA 2005, 102, 16293–16296. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.L.; Wang, C.; Chen, S.H. Effects of surface wettability on gecko adhesion under water. Colloids Surf. B 2014, 122, 662–668. [Google Scholar] [CrossRef]
- Bauer, U.; Scharmann, M.; Skepper, J.; Federle, W. ‘Insect aquaplaning’on a superhydrophilic hairy surface: How Heliamphora nutans Benth. pitcher plants capture prey. Proc. R. Soc. B 2013, 280, 20122569. [Google Scholar] [CrossRef]
- Stark, A.Y.; Badge, I.; Wucinich, N.A.; Sullivan, T.W.; Niewiarowski, P.H.; Dhinojwala, A. Surface wettability plays a significant role in gecko adhesion underwater. Proc. Natl. Acad. Sci. USA 2013, 110, 6340–6345. [Google Scholar] [CrossRef]
- Pushkarova, R.A.; Horn, R.G. Surface forces measured between an air bubble and a solid surface in water. Colloids Surf. A 2005, 261, 147–152. [Google Scholar] [CrossRef]
- England, M.W.; Sato, T.; Yagihashi, M.; Hozumi, A.; Gorb, S.N.; Gorb, E.V. Surface roughness rather than surface chemistry essentially affects insect adhesion. Beilstein J. Nanotechnol. 2016, 7, 1471–1479. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Guo, Y.; Gan, Y.; Liu, G.; Zhang, D.; Chen, H. Air bubble bridge-based bioinspired underwater adhesion. Small 2021, 17, 2103423. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, H.; Guo, Y.; Wang, Y.; Jiang, Y.; Zhang, D.; Ma, M.; Luo, J.; Jiang, L. Wet surface attachment: Micro-nano hierarchical structure enhanced strong wet friction surface inspired by tree frogs. Adv. Sci. 2020, 7, 2070116. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, S.; Wu, Y.; Pei, X.; Gorb, S.N.; Wang, Z.; Liu, W.; Zhou, F. Remote control over underwater dynamic attachment/detachment and locomotion. Adv. Mater. 2018, 30, e1801595. [Google Scholar] [CrossRef] [PubMed]
- Voigt, D.; Konrad, W.; Gorb, S.N. A universal glue: Underwater adhesion of the secretion of the carnivorous flypaper plant Roridula gorgonias. Interface Focus 2015, 5, 20140053. [Google Scholar] [CrossRef] [PubMed]
- Kizilkan, E.; Heepe, L.; Gorb, S.N. Underwater adhesion of mushroom-shaped adhesive microstructure: An air-entrapment-effect. In Biological and Biomimetic Adhesives: Challenges and Opportunities; Santos, R., Aldred, A., Gorb, S.N., Flammang, P., Eds.; RSC Publishing: Cambridge, UK, 2013; pp. 65–71. [Google Scholar]
- Ditsche-Kuru, P.; Koop, J.H.E.; Gorb, S.N. Underwater attachment in current: The role of setose attachment structures on the gills of the mayfly larvae Epeorus assimillis (Ephemeroptera, Heptageniidae). J. Exp. Biol. 2010, 213, 1950–1959. [Google Scholar] [CrossRef] [PubMed]
- Varenberg, M.; Gorb, S.N. A beetle-inspired solution for underwater adhesion. J. R. Soc. Interface 2008, 5, 383–385. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grohmann, C.; Cohrs, A.-L.; Gorb, S.N. Underwater Attachment of the Water-Lily Leaf Beetle Galerucella nymphaeae (Coleoptera, Chrysomelidae). Biomimetics 2022, 7, 26. https://doi.org/10.3390/biomimetics7010026
Grohmann C, Cohrs A-L, Gorb SN. Underwater Attachment of the Water-Lily Leaf Beetle Galerucella nymphaeae (Coleoptera, Chrysomelidae). Biomimetics. 2022; 7(1):26. https://doi.org/10.3390/biomimetics7010026
Chicago/Turabian StyleGrohmann, Constanze, Anna-Lisa Cohrs, and Stanislav N. Gorb. 2022. "Underwater Attachment of the Water-Lily Leaf Beetle Galerucella nymphaeae (Coleoptera, Chrysomelidae)" Biomimetics 7, no. 1: 26. https://doi.org/10.3390/biomimetics7010026
APA StyleGrohmann, C., Cohrs, A.-L., & Gorb, S. N. (2022). Underwater Attachment of the Water-Lily Leaf Beetle Galerucella nymphaeae (Coleoptera, Chrysomelidae). Biomimetics, 7(1), 26. https://doi.org/10.3390/biomimetics7010026






