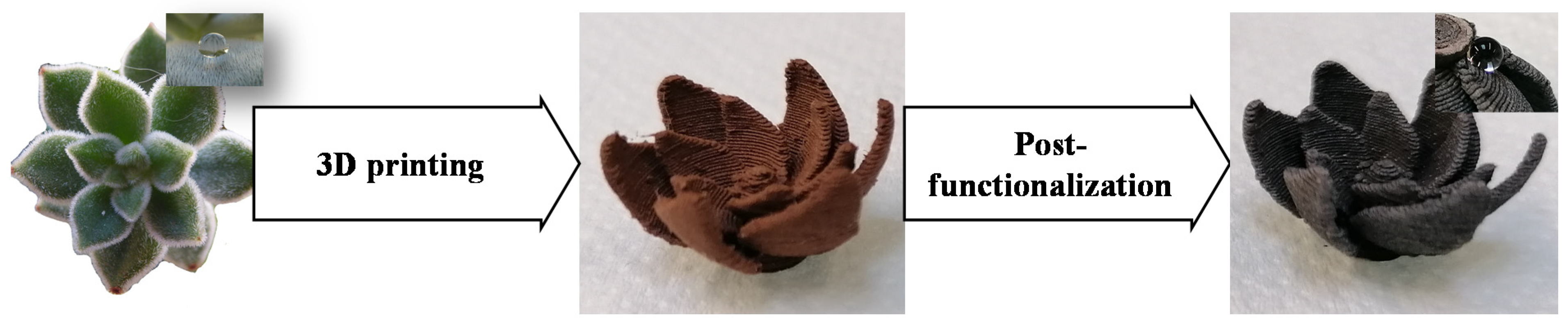
Bioinspired and Post-Functionalized 3D-Printed Surfaces with Parahydrophobic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surface Elaboration
2.1.1. Surface Printing
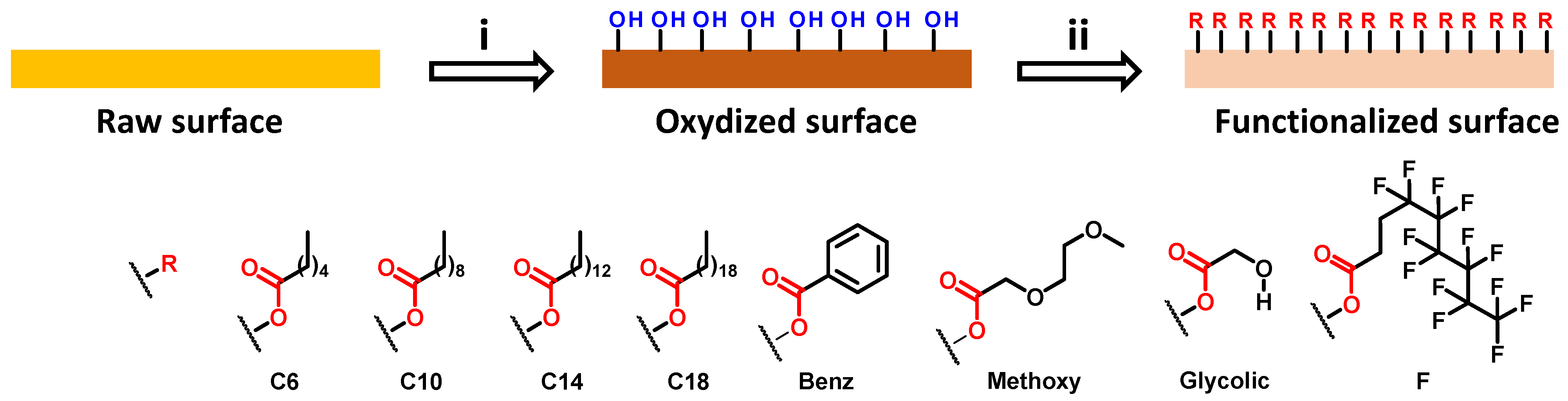
2.1.2. Surface Oxidation
2.1.3. Surface Functionalization
2.2. Surface Characterization
2.2.1. Electronic Microscopy
2.2.2. FT-IR Characterization
2.2.3. Contact Angle Measurement
2.2.4. Roughness Measurement
3. Results
3.1. Surface Elaboration and Functionalization
3.2. Infrared Measurements
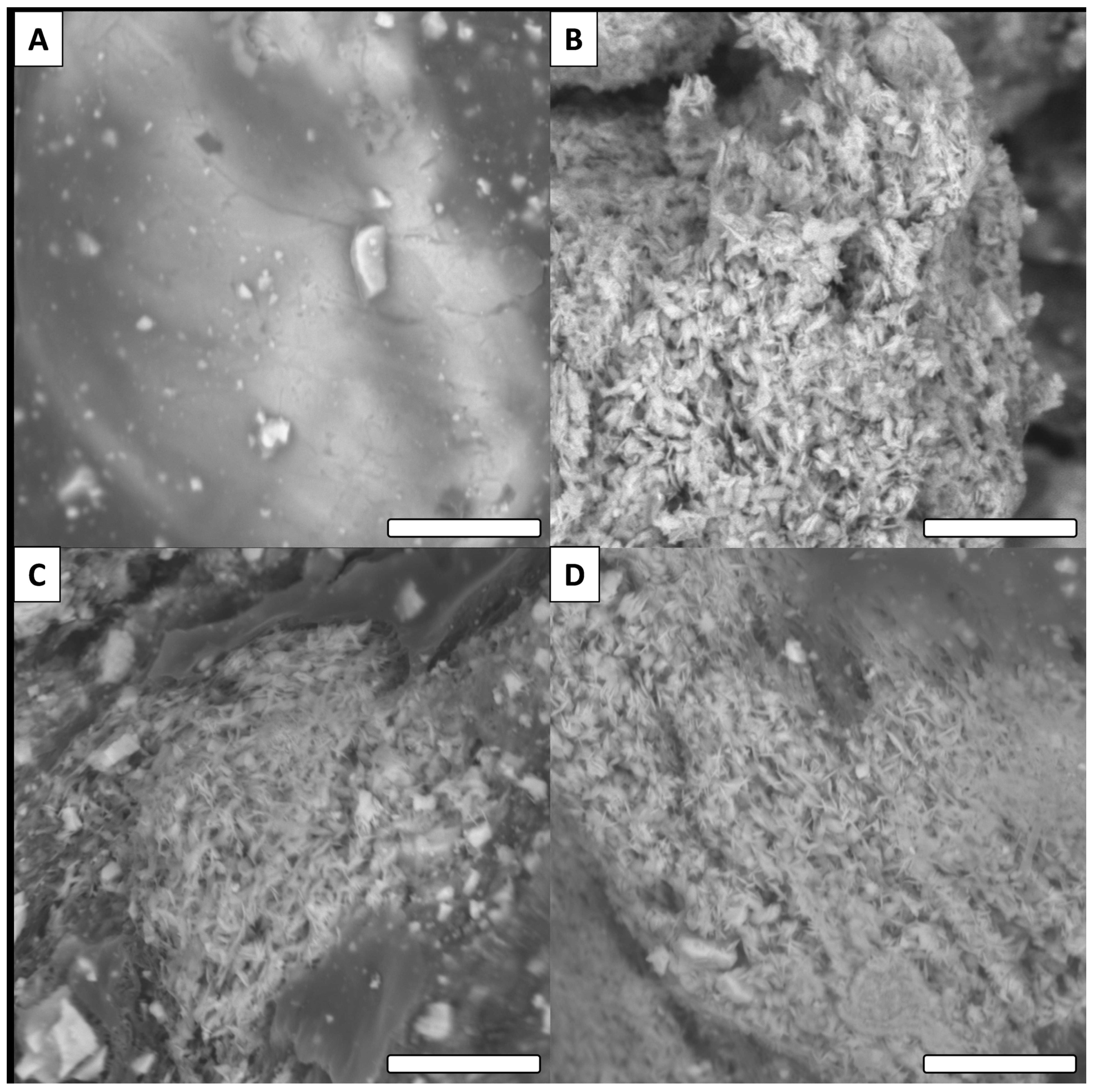
3.3. SEM Observations
3.4. Surfaces Roughness
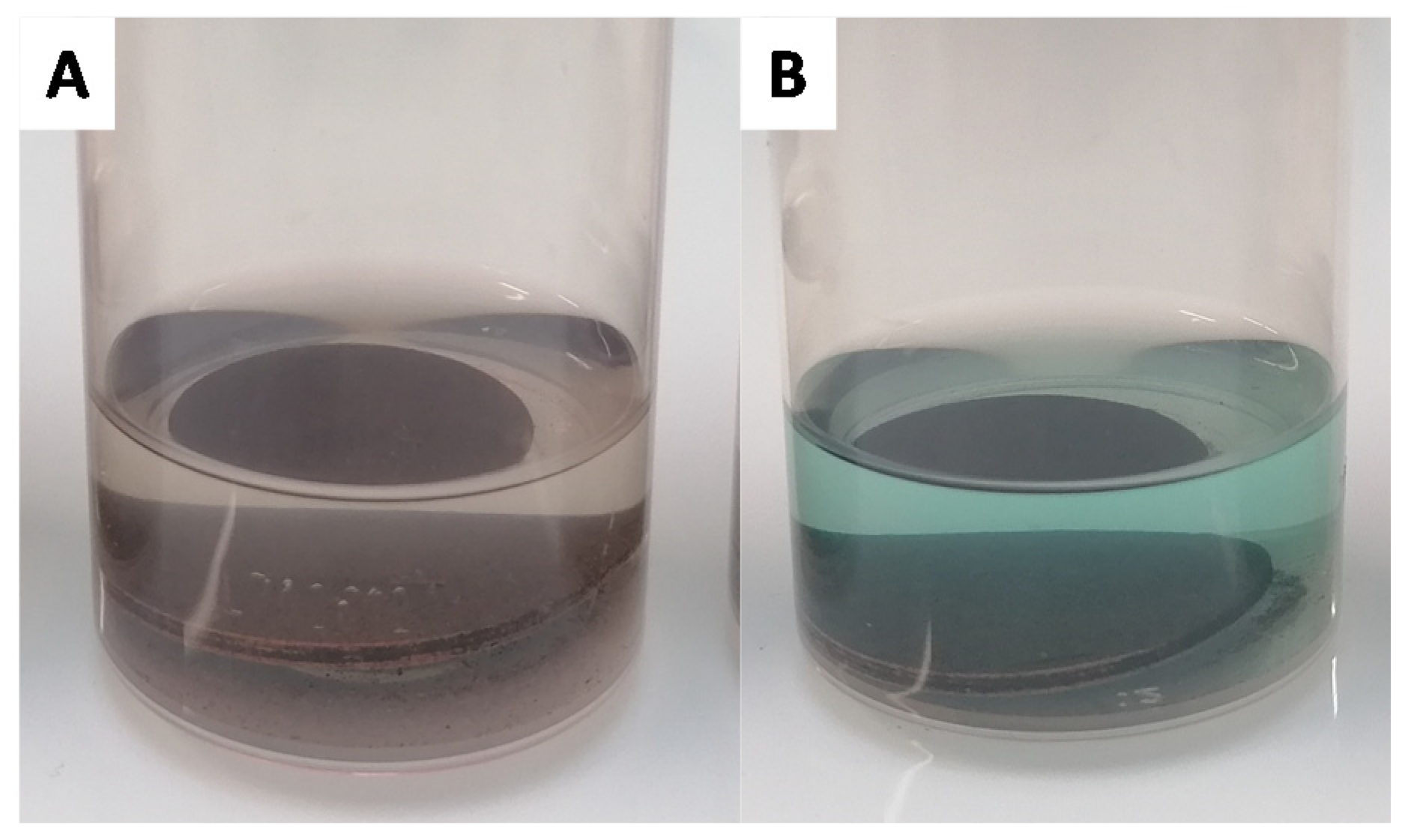
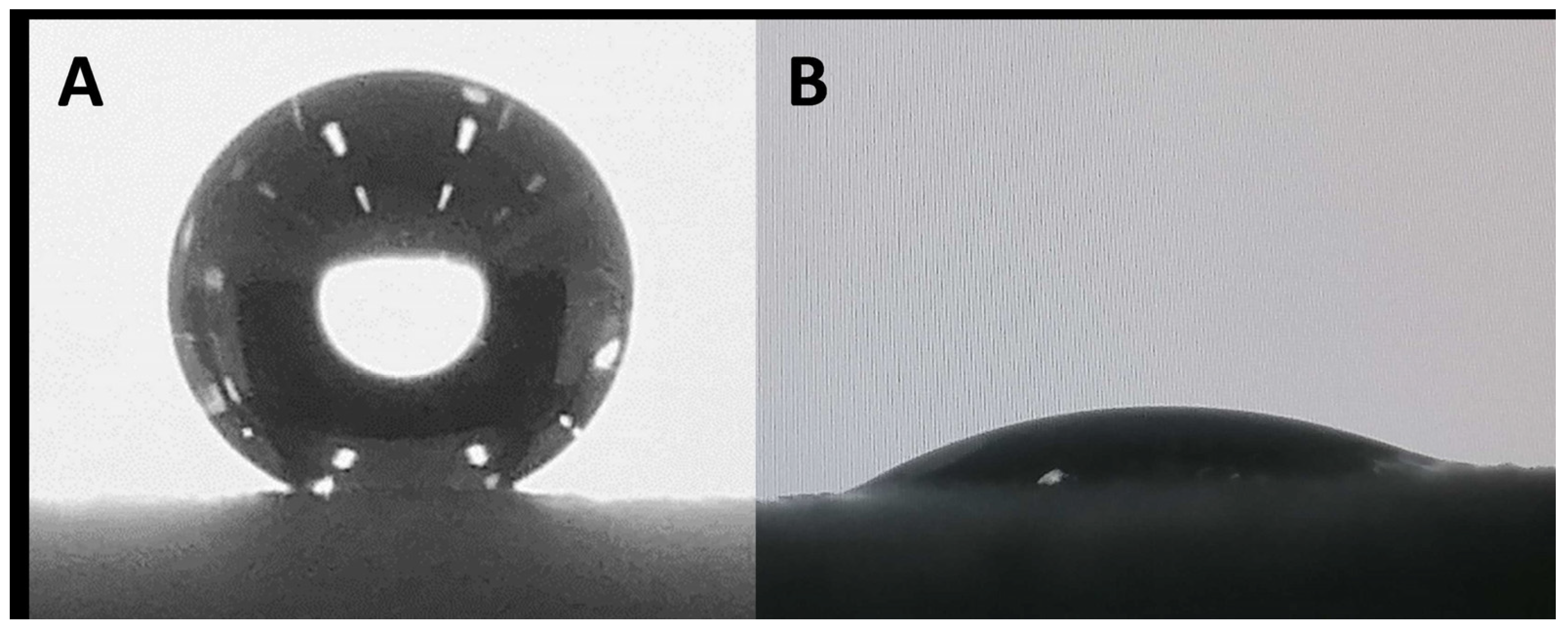
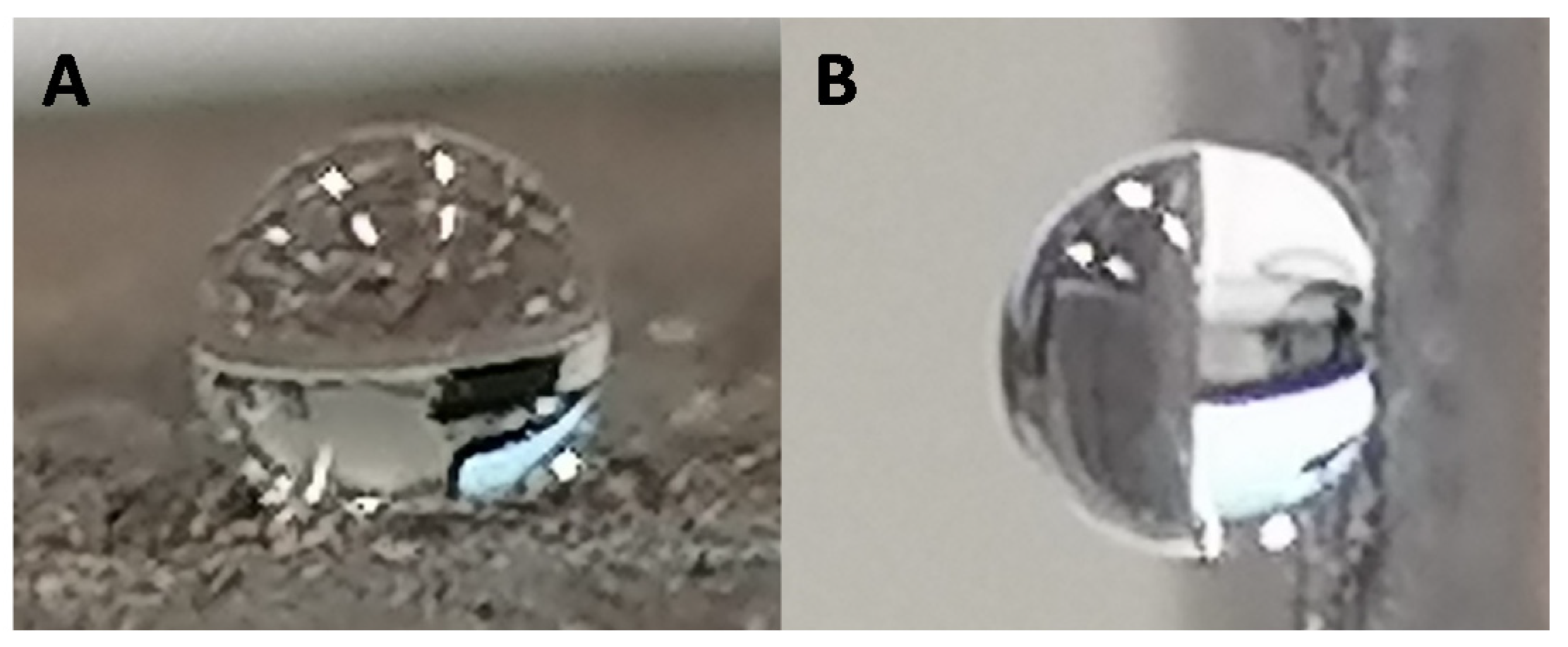
3.5. Surface Wettability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, H.; Zhao, S.; Zhang, X.; Wen, B.; Su, Z. The Future of Freshwater Access: Functional Material-Based Nano-Membranes for Desalination. Mater. Today Energy 2021, 22, 100856. [Google Scholar] [CrossRef]
- Khalil, A.; Ahmed, F.E.; Hilal, N. The Emerging Role of 3D Printing in Water Desalination. Sci. Total Environ. 2021, 790, 148238. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Dommati, H.; Wang, J.-C.; Lee, H.K.; Park, Y.-I.; Park, H.; Kim, I.-C.; Chen, S.-S.; Kwon, Y.-N. Facile Approach for Designing a Novel Micropatterned Antiwetting Membrane by Utilizing 3D Printed Molds for Improved Desalination Performance. J. Membr. Sci. 2021, 637, 119641. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Pal, K.; Sagadevan, S.; Yehye, W.A.; Johan, R.B.; Shah, S.T.; Adebesi, A.; Ali, M.E.; Islam, M.S.; Rafique, R.F. Electrochemically Active Carbon Nanotube (CNT) Membrane Filter for Desalination and Water Purification. In Emerging Technologies for Sustainable Desalination Handbook; Elsevier: Amsterdam, The Netherlands, 2018; pp. 333–363. ISBN 978-0-12-815818-0. [Google Scholar]
- Yasin, A.S.; Mohamed, I.M.A.; Mousa, H.M.; Park, C.H.; Kim, C.S. Facile Synthesis of TiO2/ZrO2 Nanofibers/Nitrogen Co-Doped Activated Carbon to Enhance the Desalination and Bacterial Inactivation via Capacitive Deionization. Sci. Rep. 2018, 8, 541. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hsieh, M.-L.; Bur, J.A.; Lin, S.-Y.; Narayanan, S. Capillary-Driven Solar-Thermal Water Desalination Using a Porous Selective Absorber. Mater. Today Energy 2020, 17, 100453. [Google Scholar] [CrossRef]
- Al-Ibrahim, A.M. Seawater Desalination: The Strategic Choice for Saudi Arabia. Desalin. Water Treat. 2013, 51, 1–4. [Google Scholar] [CrossRef]
- Al-Sahlawi, M.A. Seawater Desalination in Saudi Arabia: Economic Review and Demand Projections. Desalination 1999, 123, 143–147. [Google Scholar] [CrossRef]
- Uddin, S.; Al Ghadban, A.N.; Khabbaz, A. Localized Hyper Saline Waters in Arabian Gulf from Desalination Activity—An Example from South Kuwait. Environ. Monit Assess 2011, 181, 587–594. [Google Scholar] [CrossRef]
- Einav, R.; Harussi, K.; Perry, D. The Footprint of the Desalination Processes on the Environment. Desalination 2003, 152, 141–154. [Google Scholar] [CrossRef]
- Sun, H.; Song, Y.; Zhang, B.; Huan, Y.; Jiang, C.; Liu, H.; Bao, T.; Yu, S.; Wang, H. Bioinspired Micro- and Nanostructures Used for Fog Harvesting. Appl. Phys. A 2021, 127, 461. [Google Scholar] [CrossRef]
- Zhang, F.; Guo, Z. Bioinspired Materials for Water-Harvesting: Focusing on Microstructure Designs and the Improvement of Sustainability. Mater. Adv. 2020, 1, 2592–2613. [Google Scholar] [CrossRef]
- Lu, J.; Ngo, C.-V.; Singh, S.C.; Yang, J.; Xin, W.; Yu, Z.; Guo, C. Bioinspired Hierarchical Surfaces Fabricated by Femtosecond Laser and Hydrothermal Method for Water Harvesting. Langmuir 2019, 35, 3562–3567. [Google Scholar] [CrossRef]
- Bitonto, M.G.D.; Angelotti, A.; Zanelli, A. Fog and Dew Harvesting: Italy and Chile in Comparison. Int. J. Innov. Res. Dev. 2020, 9. [Google Scholar] [CrossRef]
- Suau, D.C. Fog Collection and Sustainable Architecture in Atacama Coast. In Proceedings of the 5th International Conference on Fog, Fog Collection and Dew, Münster, Germany, 25–30 July 2010; pp. 25–30. [Google Scholar]
- Chakrabarti, U.; Paoli, R.; Chatterjee, S.; Megaridis, C.M. Importance of Body Stance in Fog Droplet Collection by the Namib Desert Beetle. Biomimetics 2019, 4, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nørgaard, T.; Dacke, M. Fog-Basking Behaviour and Water Collection Efficiency in Namib Desert Darkling Beetles. Front. Zool. 2010, 7, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guadarrama-Cetina, J.; Mongruel, A.; Medici, M.-G.; Baquero, E.; Parker, A.R.; Milimouk-Melnytchuk, I.; González-Viñas, W.; Beysens, D. Dew Condensation on Desert Beetle Skin. Eur. Phys. J. E 2014, 37, 109. [Google Scholar] [CrossRef]
- Luo, C. Theoretical Exploration of Barrel-Shaped Drops on Cactus Spines. Langmuir 2015, 31, 11809–11813. [Google Scholar] [CrossRef] [Green Version]
- Malik, F.T.; Clement, R.M.; Gethin, D.T.; Beysens, D.; Cohen, R.E.; Krawszik, W.; Parker, A.R. Dew Harvesting Efficiency of Four Species of Cacti. Bioinspir. Biomim. 2015, 10, 036005. [Google Scholar] [CrossRef]
- Godeau, G.; Laugier, J.-P.; Orange, F.; Godeau, R.-P.; Guittard, F.; Darmanin, T. A Travel in the Echeveria Genus Wettability’s World. Appl. Surf. Sci. 2017, 411, 291–302. [Google Scholar] [CrossRef]
- Jiang, Y.; Pan, X.; Yao, M.; Han, L.; Zhang, X.; Jia, Z.; Weng, J.; Chen, W.; Fang, L.; Wang, X.; et al. Bioinspired Adhesive and Tumor Microenvironment Responsive NanoMOFs Assembled 3D-Printed Scaffold for Anti-Tumor Therapy and Bone Regeneration. Nano Today 2021, 39, 101182. [Google Scholar] [CrossRef]
- Wangpraseurt, D.; You, S.; Azam, F.; Jacucci, G.; Gaidarenko, O.; Hildebrand, M.; Kühl, M.; Smith, A.G.; Davey, M.P.; Smith, A.; et al. Bionic 3D Printed Corals. Nat. Commun. 2020, 11, 1748. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Li, H.; Liu, G.; He, Y.; Wang, Y.; Tan, Y.E.; Wang, D.; Peng, X.; Yang, G.; Tsubaki, N. Metal 3D Printing Technology for Functional Integration of Catalytic System. Nat. Commun. 2020, 11, 4098. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Lu, B.; Lin, S.; Qu, K.; Xu, J.; Luo, J.; Zhao, X. 3D Printing of Conducting Polymers. Nat. Commun. 2020, 11, 1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosravani, M.R.; Reinicke, T. On the Environmental Impacts of 3D Printing Technology. Appl. Mater. Today 2020, 20, 100689. [Google Scholar] [CrossRef]
- Yamamoto, M.; Nishikawa, N.; Mayama, H.; Nonomura, Y.; Yokojima, S.; Nakamura, S.; Uchida, K. Theoretical Explanation of the Lotus Effect: Superhydrophobic Property Changes by Removal of Nanostructures from the Surface of a Lotus Leaf. Langmuir 2015, 31, 7355–7363. [Google Scholar] [CrossRef] [PubMed]
- Marmur, A. Hydro- Hygro- Oleo- Omni-Phobic? Terminology of Wettability Classification. Soft Matter 2012, 8, 6867. [Google Scholar] [CrossRef]
- Bellanger, H.; Darmanin, T.; Taffin de Givenchy, E.; Guittard, F. Chemical and Physical Pathways for the Preparation of Superoleophobic Surfaces and Related Wetting Theories. Chem. Rev. 2014, 114, 2694–2716. [Google Scholar] [CrossRef] [PubMed]
- Godeau, G.; Darmanin, T.; Guittard, F. Staudinger Vilarassa Reaction: A Powerful Tool for Surface Modification and Superhydrophobic Properties. J. Colloid Interface Sci. 2015, 457, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Godeau, G.; N’Na, J.; Darmanin, T.; Guittard, F. Poly(3,4-Propylenedioxythiophene) Mono-Azide and Di-Azide as Platforms for Surface Post -Functionalization. Eur. Polym. J. 2016, 78, 38–45. [Google Scholar] [CrossRef]
- Szczepanski, C.R.; M’Jid, I.; Darmanin, T.; Godeau, G.; Guittard, F. A Template-Free Approach to Nanotube-Decorated Polymer Surfaces Using 3,4-Phenylenedioxythiophene (PhEDOT) Monomers. J. Mater. Chem. A 2016, 4, 17308–17323. [Google Scholar] [CrossRef]
- Godeau, G.; N’na, J.; Boutet, K.; Darmanin, T.; Guittard, F. Postfunctionalization of Azido or Alkyne Poly(3,4-Ethylenedioxythiophene) Surfaces: Superhydrophobic and Parahydrophobic Surfaces. Macromol. Chem. Phys. 2016, 217, 554–561. [Google Scholar] [CrossRef]
- Chen, H.-H.; Anbarasan, R.; Kuo, L.-S.; Tsai, M.-Y.; Chen, P.-H.; Chiang, K.-F. Synthesis, Characterizations and Hydrophobicity of Micro/Nano Scaled Heptadecafluorononanoic Acid Decorated Copper Nanoparticle. Nano-Micro Lett. 2010, 2, 101–105. [Google Scholar] [CrossRef]
- Yin, S.; Wu, D.; Yang, J.; Lei, S.; Kuang, T.; Zhu, B. Fabrication and Surface Characterization of Biomimic Superhydrophobic Copper Surface by Solution-Immersion and Self-Assembly. Appl. Surf. Sci. 2011, 257, 8481–8485. [Google Scholar] [CrossRef]
- Sasmal, A.K.; Mondal, C.; Sinha, A.K.; Gauri, S.S.; Pal, J.; Aditya, T.; Ganguly, M.; Dey, S.; Pal, T. Fabrication of Superhydrophobic Copper Surface on Various Substrates for Roll-off, Self-Cleaning, and Water/Oil Separation. ACS Appl. Mater. Interfaces 2014, 6, 22034–22043. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, Y.; Wang, W.; Xu, C.; Ren, L. Superhydrophobic Copper Surface Textured by Laser for Delayed Icing Phenomenon. Langmuir 2020, 36, 1075–1082. [Google Scholar] [CrossRef]
- Grdadolnik, J. Atr-ftir spectroscopy: Its advantages and limitations. Acta Chim. Slov. 2002, 49, 631–642. [Google Scholar]
- Chaudhary, A.; Barshilia, H.C. Nanometric Multiscale Rough CuO/Cu(OH)2 Superhydrophobic Surfaces Prepared by a Facile One-Step Solution-Immersion Process: Transition to Superhydrophilicity with Oxygen Plasma Treatment. J. Phys. Chem. C 2011, 115, 18213–18220. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Y.; Guo, Z. Superhydrophobic Sand Grains Structured with Aligned Cu(OH)2 Nano-Needles for Efficient Oily Water Treatment. Mater. Des. 2017, 135, 377–384. [Google Scholar] [CrossRef]
- Bormashenko, E.; Stein, T.; Pogreb, R.; Aurbach, D. “Petal Effect” on Surfaces Based on Lycopodium: High-Stick Surfaces Demonstrating High Apparent Contact Angles. J. Phys. Chem. C 2009, 113, 5568–5572. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.; Xi, J.; Zhu, Y.; Wang, N.; Xia, F.; Jiang, L. Petal Effect: A Superhydrophobic State with High Adhesive Force. Langmuir 2008, 24, 4114–4119. [Google Scholar] [CrossRef] [PubMed]





| Sample | Ra (µm) |
|---|---|
| Cu (0) | 19.5 ± 2.8 |
| Cu(OH)2 | 23.5 ± 0.8 |
| C6 | 24.4 ± 8.7 |
| C10 | 30.4 ± 10.3 |
| C14 | 25.8 ± 8.8 |
| C18 | 33.7 ± 14.8 |
| Glycolic | 21.7 ± 9.2 |
| Methoxy | 33.2 ± 16.5 |
| Benz | 29.0 ± 13.8 |
| F | 20.9 ± 4.1 |
| Surface | Accuracy (%) |
|---|---|
| Cu | 99.2 ± 0.9 |
| Cu(OH)2 | 97.9 ± 1.2 |
| C6 | 96.1 ± 1.1 |
| C10 | 96.1 ± 0.7 |
| C14 | 96.4 ± 0.4 |
| C18 | 96.7 ± 1.3 |
| Gly | 96.7 ± 1.6 |
| Methoxy | 96.6 ± 1.0 |
| Benz | 95.8 ± 1.3 |
| F | 96.6 ± 0.8 |
| Scheme 101 | Apparent Contact Angle (°) |
|---|---|
| Cu | 101.1 ± 10.8 |
| Cu(OH)2 | 30.0 ± 7.0 |
| C6 | 134.1 ± 3.2 |
| C10 | 147.9 ± 4.8 |
| C14 | 145.4 ± 1.8 |
| C18 | 145.3 ± 2.8 |
| Glycolic | 31.5 ± 5.2 |
| Methoxy | 66.8 ± 6.3 |
| Benz | 132.3 ± 3.3 |
| F | 136.3 ± 4.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciffréo, L.; Marchand, C.; Szczepanski, C.R.; Medici, M.-G.; Godeau, G. Bioinspired and Post-Functionalized 3D-Printed Surfaces with Parahydrophobic Properties. Biomimetics 2021, 6, 71. https://doi.org/10.3390/biomimetics6040071
Ciffréo L, Marchand C, Szczepanski CR, Medici M-G, Godeau G. Bioinspired and Post-Functionalized 3D-Printed Surfaces with Parahydrophobic Properties. Biomimetics. 2021; 6(4):71. https://doi.org/10.3390/biomimetics6040071
Chicago/Turabian StyleCiffréo, Léna, Claire Marchand, Caroline R. Szczepanski, Marie-Gabrielle Medici, and Guilhem Godeau. 2021. "Bioinspired and Post-Functionalized 3D-Printed Surfaces with Parahydrophobic Properties" Biomimetics 6, no. 4: 71. https://doi.org/10.3390/biomimetics6040071
APA StyleCiffréo, L., Marchand, C., Szczepanski, C. R., Medici, M.-G., & Godeau, G. (2021). Bioinspired and Post-Functionalized 3D-Printed Surfaces with Parahydrophobic Properties. Biomimetics, 6(4), 71. https://doi.org/10.3390/biomimetics6040071








