Strengthening Structures in the Petiole–Lamina Junction of Peltate Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Screening
2.2. Plant Material and Sampling
2.3. Anatomy and Morphology
3. Results
3.1. Distribution of Peltate Species in the Plant Kingdom
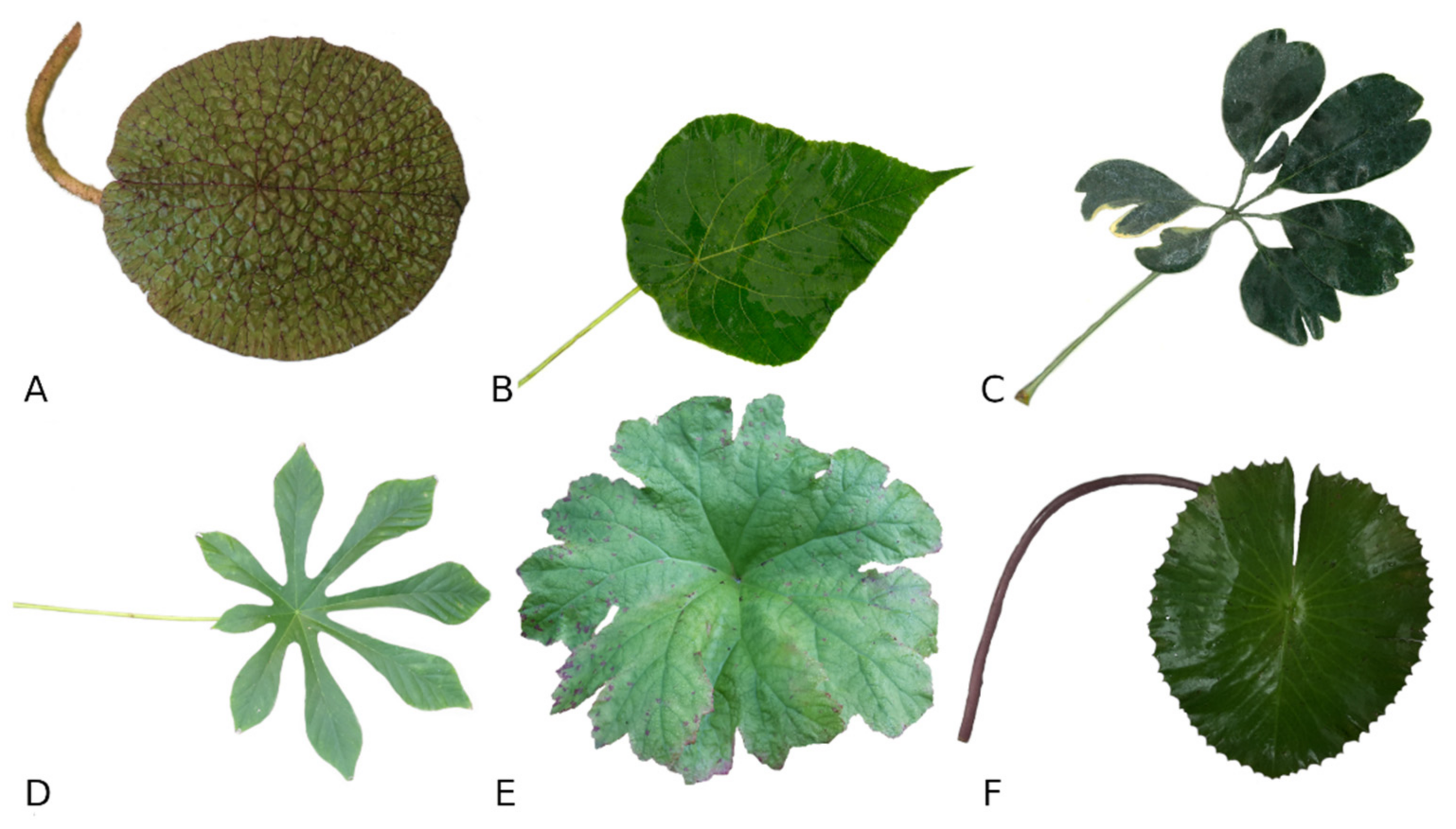
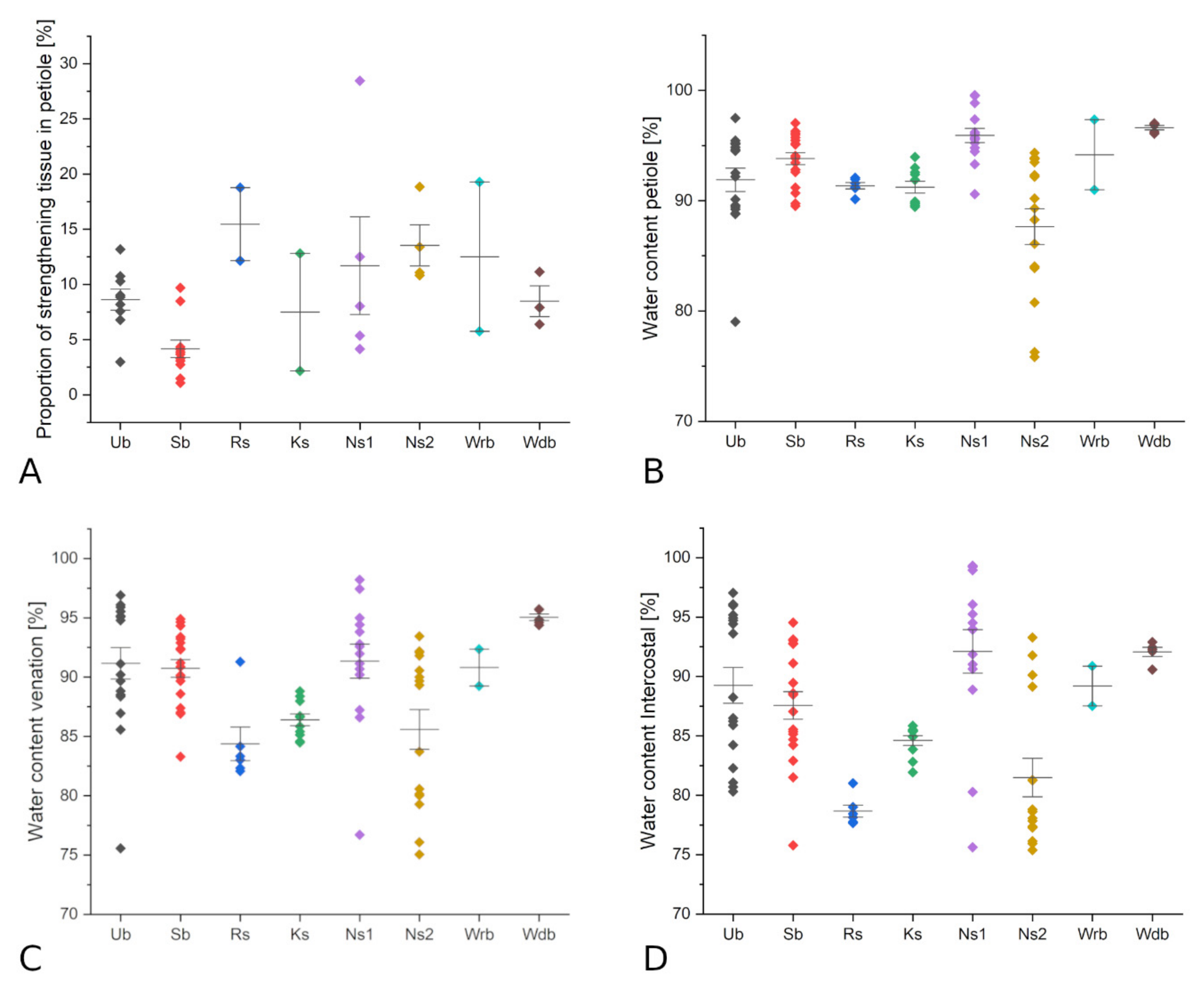
3.2. Leaf Anatomy, Morphology and Characteristics of Selected Peltate Plant Species
3.2.1. ANA Grade
Nymphaeaceae Salisbury
| Species | Number of v.b. | Proportion of Strength. Tissue (in Petiole, %) | Strengthening Structure | Sample Size (n) | Petiole Area/Lamina Area (%) | Water Content Petiole (%) | Water Content Lamina (%) | Water Content Intercostal (%) | Water Content Venation (%) |
|---|---|---|---|---|---|---|---|---|---|
| Alocasia longiloba | 53 | 2.7 | Sb | ||||||
| Amorphophallus konjac | 180 | 10.3 | Ub | ||||||
| Anthurium forgetii | 60 | 9.7 | Sb | ||||||
| Begonia kellermanii | 11 | 5.4 | Ns1 | 5 | 0.14 | 99.3 | 98.7 | 98.9 | 95.9 |
| Begonia nelumbiifolia | 34 | 8.0 | Ns1 | 4 | 0.06 | 95.2 | 89.7 | 89.7 | 89.4 |
| Begonia peltata | 24 | 4.2 | Ns1 | ||||||
| Begonia sudjanae | 4 | 0.08 | 94.8 | 92.3 | 92.4 | 92.2 | |||
| Cabomba aquatica | 2 | 1.1 | ? | 5 | 0.81 | 93.4 | 85.4 | 85.1 | 88.3 |
| Caladium hybrid | 50 | 3.3 | Sb | 3 | 0.04 | 94.5 | 87.6 | 86.4 | 91.8 |
| Cecropia peltata | 23 | 11.1 | Ns2 | 8 | 0.03 | 89.6 | 83.6 | 78.7 | 90.6 |
| Colocasia esculenta | 90 | 4.1 | Sb | 2 | 0.05 | 95.6 | 89.8 | 88.8 | 92.2 |
| Darmera peltata | 24 | 8.8 | Ub | 2 | 0.12 | 88.9 | 83.7 | 82.6 | 87.1 |
| Euryale ferox | 42 | 6.4 | Wdb | 5 | 0.28 | 96.8 | 93.8 | 92.0 | 95.3 |
| Hernandia nymphaeifolia | 11 | 8.5 | Sb | 6 | 0.07 | 88.5 | 81.1 | 80.2 | 88.5 |
| Hydrocotyle vulgaris | 3 | 2.2 | Ks | 4 | 0.30 | 89.7 | 84.9 | 84.7 | 85.3 |
| Jatropha podagrica | 10 | 10.8 | Ns2 | 2 | 0.08 | ||||
| Kalanchoe nyikae | 3 | 3.1 | Sb | ||||||
| Macaranga tanarius | 27 | 18.8 | Ns2 | 3 | 0.04 | 77.5 | 76.9 | 76.8 | 77.0 |
| Marsilea strigosa | 1 | 3.9 | Sb | ||||||
| Nelumbo nucifera | 63 | 19.3 | Wrb | 5 | 0.09 | 91.0 | 88.0 | 87.5 | 89.2 |
| Nymphaea colorata | 21 | 5.7 | Wrb | ||||||
| Nymphaea lotus | 1 | 0.10 | 97.3 | 91.5 | 90.9 | 92.4 | |||
| Oxalis bowiei | 7 | 3.0 | Ub | ||||||
| Passiflora coriacea | 6 | 13.2 | Ub | 2 | 0.08 | 85.6 | 86.2 | 86.1 | 86.6 |
| Peperomia argyreia | 8 | 1.5 | Sb | ||||||
| Peperomia cyclaminoides | 6 | 1.1 | Sb | 2 | 0.79 | 96.1 | 94.1 | 94.1 | 94.1 |
| Peperomia sodiroi | 7 | 4.3 | Sb | 3 | 0.25 | 93.5 | 92.9 | 92.6 | 89.8 |
| Perichasma laetificata | 8 | 18.8 | Rs | ||||||
| Pilea peperomioides | 6 | 10.7 | Ub | 7 | 0.16 | 95.0 | 95.1 | 95.1 | 95.5 |
| Piper peltatum | 13 | 8.2 | Ub | 3 | 0.03 | 91.7 | 85.0 | 83.8 | 87.8 |
| Podophyllum hybrid | 15 | 12.5 | Ns1 | ||||||
| Remusatia vivipara | 28 | 3.7 | Sb | 5 | 0.08 | 95.4 | 87.7 | 86.7 | 90.6 |
| Ricinus communis | 9 | 13.4 | Ns2 | 4 | 0.05 | 86.4 | 77.6 | 76.7 | 81.0 |
| Rodgersia podophylla | 63 | 7.6 | Ub | 2 | 0.04 | 89.1 | 85.9 | 80.6 | 88.4 |
| Schefflera arboricola | 39 | 28.5 | Ns1 | 1 | 0.05 | 90.6 | 75.8 | 75.6 | 76.7 |
| Stephania delavayi | 8 | 12.1 | Rs | 6 | 0.05 | 91.3 | 79.9 | 78.7 | 84.6 |
| Syneilesis palmata | 30 | 9.0 | Sb | 1 | 0.08 | ||||
| Tropaeolum tuberosum | 7 | 12.8 | Ks | 3 | 0.08 | 92.2 | 85.8 | 85.4 | 87.7 |
| Umbilicus rupestris | 8 | 6.8 | Ub | 1 | 0.44 | 97.5 | 97.0 | 97.0 | 96.9 |
| Victoria amazonica | 90 | 11.1 | Wdb | ||||||
| Victoria cruziana | 12 | 7.9 | Wdb | 1 | 0.08 |
3.2.2. Magnoliids
Hernandiaceae Blume
Piperaceae Giseke
3.2.3. Monocotyledons
Araceae Jussieu
3.2.4. Eudicotyledons
Araliaceae Jussieu
Asteraceae Bercht. and J. Presl
Begoniaceae C. Agardh
Berberidaceae Jussieu
Crassulaceae J. Saint-Hilaire
Eurphorbiaceae Jussieu
Menispermaceae Jussieu
Nelumbonaceae A. Richard
Oxalidaceae R. Brown
Passifloraceae Juss. ex Roussel
Saxifragaceae Jussieu
Tropaeolaceae Juss. ex de Candolle
Urticaceae Jussieu
3.2.5. Polypodiopsida
Marsileaceae Mirbel
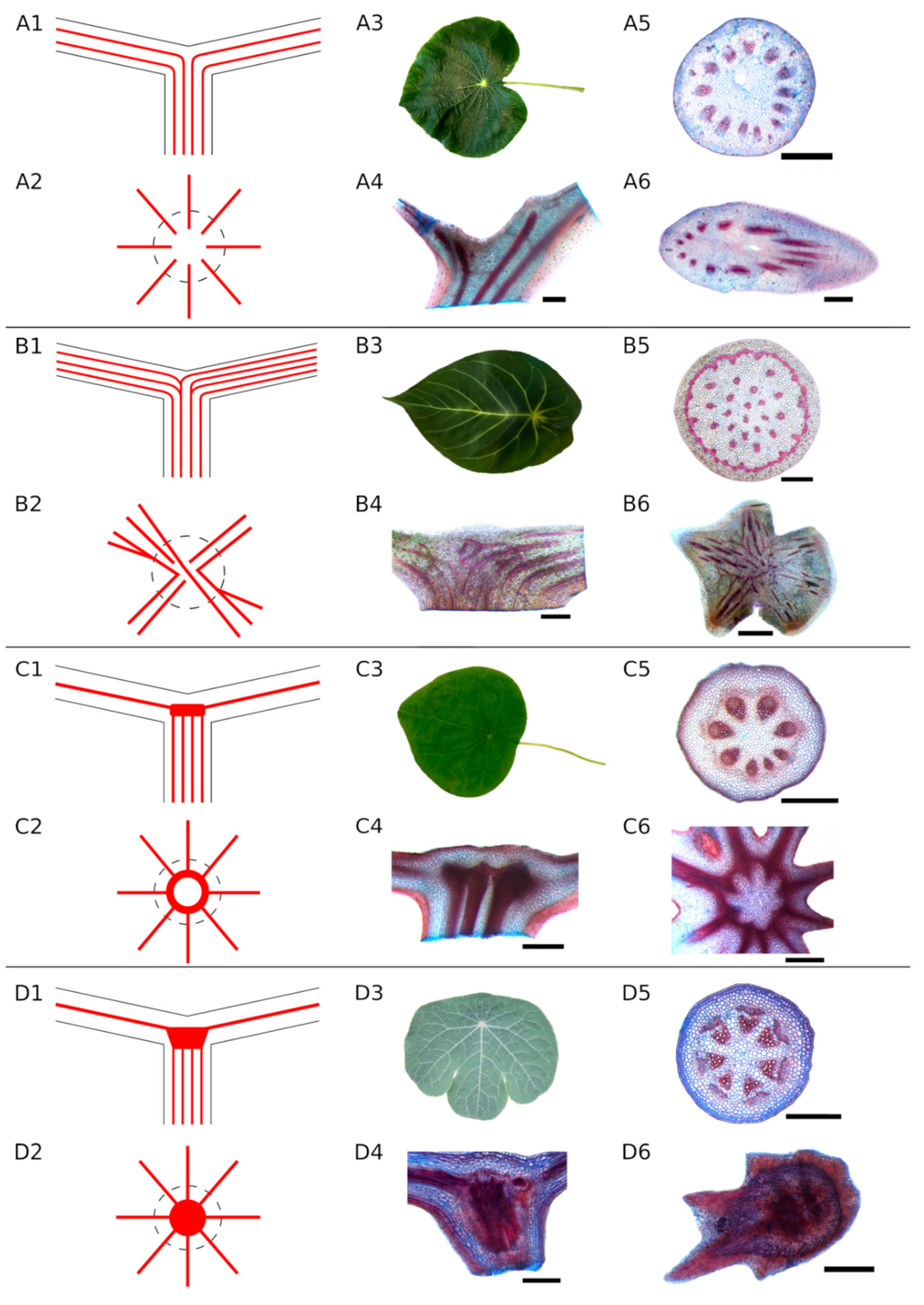
3.3. Classification of Strengthening Structures in the Petiole–Lamina Transition Zone
- Unbranched type (A): The fibre strands in the petiole continue unbranched through the petiole–lamina junction into the lamina (Figure 2A). The strands follow the leaf veins.
- Simple branching type (B): Some fibre strands in the petiole branch in the transition zone and then extend into the leaf veins while others proceed unbranched into the lamina (Figure 2B). The fibre strands branching in the transition zone can either run in into the same leaf vein of the lamina or follow a different direction into different leaf veins, forming a connection between, e.g., opposite sides of the lamina. All species with at least one visible branching fibre strand were assigned to this category.
- Ring-like structure (Type C): The fibre strands from the petiole run parallel towards the petiole–lamina junction, then merge into a fibre ring without any visible branching. This ring is oriented parallel to the lamina and connects all the strands from the petiole with each other. Starting from the ring, the strands spread into the leaf veins (Figure 2C). This structure was found only in Stephania and Perichasma.
- Knot-like structure (Type D): Isolated fibre strands from the petiole assemble to a dense knot-like structure in the transition zone. It is not possible to distinguish between individual fibre strands in this knot. A few stronger fibre strands spread out from these structures and continue into the lamina (Figure 2D).
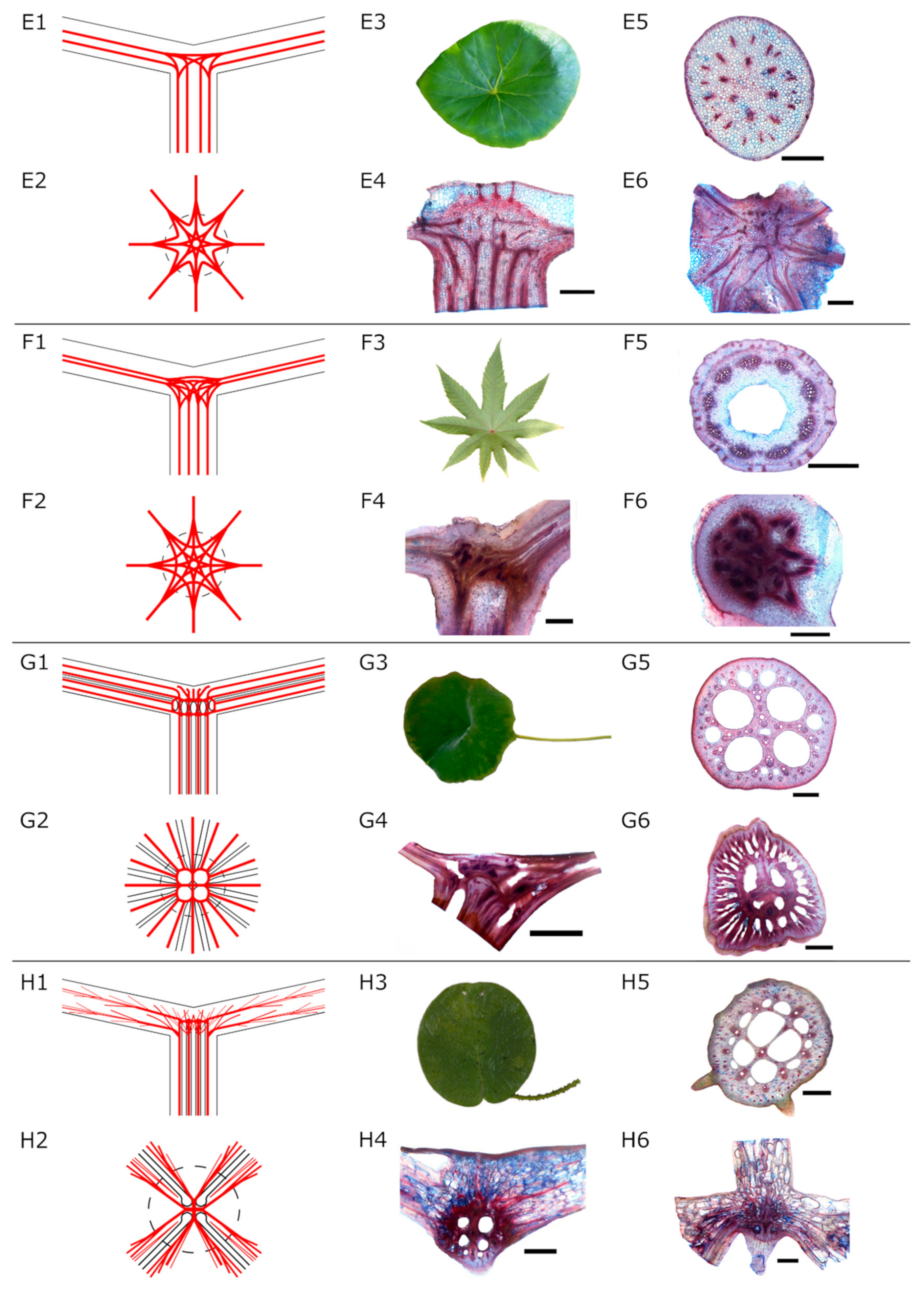
- Net-like structure (Type E and F): The individual fibre strands the petiole form a net-like structure in the transition zone. Individual fibre strands are still visible. Fibres branch and merge in different intensities, forming nets of different densities. Two subtypes were identified here: (1) slight to moderate branching (Figure 2E) and (2) intensive branching (Figure 2F) of the fibre strands. From this net, several fibre bundles spread out into the lamina following the larger veins.
- Radially branching type (water plants, Type G): The fibre strands from the petiole are united in a dense structure with large fibre strands and big intercellular cavities for gas exchange. The fibre strands run parallel to the air channels branch in the transition zone and extend into the lamina in a radial pattern (Figure 2G).
- Diffuse branching type (water plants, Type H): Parallel fibre strands from the petiole branch in the transition zone forming a diffuse net of fibres of different sizes (Figure 2H). Air channels are visible.
4. Discussion
4.1. The Peltate Leaf Shape and Its Representatives in the Plant Kingdom
4.2. Supporting a Peltate Lamina—Different Adaptations in the Petiole–Lamina Junction
4.2.1. Simple Structures in the Petiole–Lamina Junction—The Unbranched and Simple Branching Structure
4.2.2. The Net-Like Branching Structure
4.2.3. Dense Structures in the Petiole–Lamina Junction—The Ring- and Knot-Like Structures
4.2.4. Specifics of Water Plants
4.2.5. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faisal, T.R.; Abad, E.M.K.; Hristozov, N.; Pasini, D. The impact of tissue morphology, cross-section and turgor pressure on the mechanical properties of the leaf petiole in plants. J. Bionic Eng. 2010, 7, S11–S23. [Google Scholar] [CrossRef]
- Adams, W.W., III; Terashima, I. The Leaf: A Platform for Performing Photosynthesis; Springer: Berlin, Germany, 2018. [Google Scholar]
- Sitte, P.; Ziegler, H.; Ehrendorfer, F.; Bresinsky, A. Strasburger Lehrbuch Der Botanik, 34th ed.; Gustav Fischer Verlag: Stuttgart/Jena/Lübeck/Ulm, Germany, 1998. [Google Scholar]
- Raven, P.H.; Evert, R.F.; Eichhorn, S.E. Biologie Der Pflanzen, 3rd ed.; Walter de Gruyter: Berlin, Germany; New York, NY, USA, 2000. [Google Scholar]
- Vogel, S. Drag and reconfiguration of broad leaves in high winds. J. Exp. Bot. 1989, 40, 941–948. [Google Scholar] [CrossRef]
- Niklas, K.J. Petiole mechanics, light interception by lamina, and “Economy in Design”. Oecologia 1992, 90, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Raupach, M.R.; Thom, A.S. Turbulence in and above plant canopies. Annu. Rev. Fluid Mech. 1981, 13, 97–129. [Google Scholar] [CrossRef]
- Gosselin, F.P.; De Langre, E. Drag reduction by reconfiguration of a poroelastic system. J. Fluids Struct. 2011, 27, 1111–1123. [Google Scholar] [CrossRef]
- Niklas, K.J. A mechanical perspective on foliage leaf form and function. New Phytol. 1999, 143, 19–31. [Google Scholar] [CrossRef]
- Niklas, K.J. Plant Biomechanics: An Engineering Approach to Plant Form and Function; University of Chicago Press: Chicago, IL, USA, 1992. [Google Scholar]
- Levionnois, S.; Coste, S.; Nicolini, E.; Stahl, C.; Morel, H.; Heuret, P. Scaling of petiole anatomies, mechanics and vasculatures with leaf size in the widespread neotropical pioneer tree species cecropia obtusa trécul (Urticaceae). Tree Physiol. 2020, 40, 245–258. [Google Scholar] [CrossRef]
- Niklas, K.J. Flexural Stiffness allometries of angiosperm and fern petioles and rachises: Evidence for biomechanical convergence. Evolution 1991, 45, 734–750. [Google Scholar] [CrossRef] [PubMed]
- Sacher, M.; Lautenschläger, T.; Kempe, A.; Neinhuis, C. Umbrella leaves—Biomechanics of transition zone from lamina to petiole of peltate leaves. Bioinspir. Biomim. 2019, 14, 046011. [Google Scholar] [CrossRef]
- Leroux, O. Collenchyma: A versatile mechanical tissue with dynamic cell walls. Ann. Bot. 2012, 110, 1083–1098. [Google Scholar] [CrossRef]
- Keating, R.C. Collenchyma in Araceae: Trends and relation to classification. Bot. J. Linn. Soc. 2000, 134, 203–214. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Fleck, S. Petiole mechanics, leaf inclination, morphology, and investment in support in relation to light availability in the canopy of liriodendron tulipifera. Oecologia 2002, 132, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Evert, R.F. Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Ebel, F. Die schildblättrigkeit krautiger angiospermen-sippen in ihrer beziehung zu standort und verbreitung. Flora 1998, 193, 203–224. [Google Scholar] [CrossRef]
- Troll, W. Morphologie der schildförmigen blätter. Planta 1932, 17, 153–230. [Google Scholar] [CrossRef]
- Troll, W. Vergleichende Morphologie Der Höheren Pflanzen, Vegetationsorgane; Gebrüder Bornträger: Berlin, Germany, 1939; Volume 1. [Google Scholar]
- Troll, W.; Meyer, H.-J. Entwicklungsgeschichtliche untersuchungen über das zustandekommen unifazialer blattstrukturen. Planta 1955, 46, 286–360. [Google Scholar] [CrossRef]
- Roth, I. Beiträge zur entwicklungsgeschichte der schildblätter. Planta 1952, 40, 350–376. [Google Scholar] [CrossRef]
- Givnish, T.J.; Vermeij, G.J. Sizes and shapes of liane leaves. Am. Nat. 1976, 110, 743–778. [Google Scholar] [CrossRef]
- De Candolle, C. Sur les feuilles peltées. Bull. Trav. Soc. Bot. Genève 1899, 9, 1–51. [Google Scholar]
- Uittien, H. Ueber den zusammenhang zwischen blattnervatur und sprossverzweigung. Recl. Trav. Bot. Néerl. 1929, 25, 390–483. [Google Scholar]
- Chase, M.W.; Christenhusz, M.J.M.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; Stevens, P.F. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar]
- Roth, I. Zur entwicklungsgeschichte des blattes, mit besonderer berücksichtigung von stipular-und ligularbildungen. Planta 1949, 37, 299–336. [Google Scholar] [CrossRef]
- Leinfellner, W. Über die peltaten kronblätter der sapindaceen. Österr. Bot. Z. 1958, 105, 443–514. [Google Scholar] [CrossRef]
- Missouri Botanical Garden EFloras. Available online: http://www.efloras.org (accessed on 29 September 2020).
- Royal Botanic Garden, Kew POWO, Plants of the World Online. Available online: http://www.plantsoftheworldonline.org/ (accessed on 29 September 2020).
- GBIF Secretariat GBIF.Org (Global Biodiversity Information Facility). Available online: http://www.gbif.org (accessed on 29 September 2020).
- Missouri Botanical Garden Tropicos.Org. Available online: http://www.tropicos.org/ (accessed on 29 September 2020).
- The Plant List, Version 1.1. Available online: http://www.theplantlist.org/ (accessed on 29 September 2020).
- Cole, T.C.; Hilger, H.H. Angiosperm Phylogeny Poster–Flowering Plant Systematics; Freie Universität: Berlin, Germany; Missouri Botanical Garden: St. Louis, MO, USA, 2019. [Google Scholar]
- Ellis, B.; Daly, D.C.; Hickey, L.J.; Mitchell, J.D. Manual of Leaf Architecture: Morphological Description and Categorization of Dicotyledonous and Net-Veined Monocotyledonous Angiosperms; Leaf Architecture Working Group: Washington, DC, USA, 1999. [Google Scholar]
- Jäger, E. Die Pflanzengeographische ozeanitätsgliederung der holarktis und die ozeanitätsbindung der Pflanzenareale. Feddes Repert. 1968, 79, 157–335. [Google Scholar] [CrossRef]
- Pott, R. Allgemeine Geobotanik: Biogeosysteme Und Biodiversität; Springer: Berlin, Germany, 2014. [Google Scholar]
- Wu, Z.; Raven, P.H.; Chen, J. Flora of China: Caryophyllaceae through Lardizabalaceae; Science Press: Beijing, China, 2001; Volume 6. [Google Scholar]
- Jain, A.; Singh, H.B.; Kanjilal, P.B. Economics of Foxnut (Euryale Ferox Salisb.) Cultivation: A Case Study from Manipur in North Eastern India. Indian J. Nat. Prod. Resour. 2010, 1, 63–67. [Google Scholar]
- Wu, Z.; Raven, P.H.; Hong, D. Flora of China: Menispermaceae through Capparaceae; Science Press: Beijing, China, 2008; Volume 7. [Google Scholar]
- De Candolle, C. Piperaceae sodiroanae. In Bulletin de l’Herbier Boissier; Imprimerie Romet: Geneva, Switzerland, 1898; Volume 6, p. 506. [Google Scholar]
- Trelease, W. The Piperaceae of Northern South America; University of Illinois Press: Urbana, IL, USA, 1950. [Google Scholar]
- Tebbs, M. Revision of piper (Piperaceae) in the new world 3. The taxonomy of piper sections lepianthes and radula. Bull. Br. Mus. Nat. Hist. Bot. 1993, 23, 1–50. [Google Scholar]
- Wu, Z.; Raven, P.H.; Hong, D. Flora of China: Acoraceae through Cyperaceae; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 1994; Volume 23. [Google Scholar]
- Ebel, F.; Mühlberg, H.; Hagen, A. Ökomorphologische Studien an Schwingmoorpflanzen. Hercynia-Ökol. Umw. Mitteleur. 1989, 26, 432–444. [Google Scholar]
- Jäger, E. Rothmaler-Exkursionsflora von Deutschland. Gefäßpflanzen: Grundband; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Lansdown, R.V. Hydrocotyle Vulgaris. The IUCN Red List of Threatened Species 2014: E.T164201A42415437. Available online: https://www.iucnredlist.org/species/164201/42415437 (accessed on 8 October 2020).
- Wu, Z.; Raven, P.H.; Hong, D. Flora of China: Clusiaceae through Araliaceae; Science Press: Beijing, China, 2007; Volume 13. [Google Scholar]
- Missouri Botanical Garden Gardnening Help, Plant Finder: Syneilesis Palmata. Available online: https://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=277696&isprofile=0& (accessed on 27 November 2020).
- Wu, Z.; Raven, P.H.; Hong, D. Flora of China: Asteraceae; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2011; Volume 20. [Google Scholar]
- Doorenbos, J.; Sosef, M.S.M.; De Wilde, J.J.F.E. The Sections of Begonia Including Descriptions, Keys and Species Lists (Studies in Begoniaceae VI); Wageningen Agricultural University: Wageningen, The Netherlands, 1998. [Google Scholar]
- Otto, C.F.; Dietrich, A.G. Begonia peltata. Allg. Gartenzeitung 1841, 9, 58–59. [Google Scholar]
- Twyford, A. The Evolution of Begonia Section Gireoudia in Central America; University of Edinburgh, Royal Botanic Gardens Edinburgh: Edinburgh, UK, 2000. [Google Scholar]
- Bailey, L.H. Manual of Cultivated Plants / Most Commonly Grown in the Continental United States and Canada; MacMillan: New York, NY, USA, 1954. [Google Scholar]
- Burt-Utley, K. A Revision of Central American Species of Begonia Section Gireoudia (Begoniaceae); Tulane University: New Orleans, LA, USA, 1985. [Google Scholar]
- Hoover, W.S. Notes on spatial distribution patterns for three Mexican species of Begonia. Phytologia 1979, 43, 107–132. [Google Scholar] [CrossRef]
- Flora of North America Editorial Committee. Flora of North America-Magnoliophyta: Magnoliidae and Hamamelidae; Oxford University Press: New York, NY, USA, 1997; Volume 3. [Google Scholar]
- Eggli, U. Crassulaceae (Dickblattgewächse); Ulmer: Stuttgart, Germany, 2003; Volume 4. [Google Scholar]
- Castroviejo, S.; Aedo, C.; Laínz, M.; Morales, R.; Munoz Garmendia, F.; Nieto Feliner, G.; Paiva, J. Flora Iberica, Ebenaceae-Saxifragaceae; Real Jardín Botánico, CSIC: Madrid, Spain, 1997; Volume 5. [Google Scholar]
- Lauber, K.; Wagner, G.; Gygax, A. Flora Helvetica: Illustrierte Flora Der Schweiz: Mit Artbeschreibungen Und Verbreitungskarten von 3200 Wild Wachsenden Farn- Und Blütenpflanzen, Einschliesslich Wichtiger Kulturpflanzen; Haupt Verlag: Bern, Switzerland, 2018. [Google Scholar]
- CABI; Quiroz, D. Macaranga Tanarius. Available online: https://www.cabi.org/isc/datasheet/32763 (accessed on 9 October 2020).
- Wu, Z.; Raven, P.H.; Hong, D. Flora of China: Oxalidaceae through Aceraceae; Science Press: Beijing, China, 2008; Volume 11. [Google Scholar]
- Flora of North America Editorial Committee. Flora of North America-Magnoliophyta: Vitaceae to Garryaceae; Oxford University Press: New York, NY, USA, 2016; Volume 12. [Google Scholar]
- Welzen, P.C. Revisions and phylogenies of Malesian Euphorbiaceae: Subtribe Lasiococcinae (Homonoia, Lasiococca, Spathiostemon) and clonostylis, ricinus, and wetria. Blumea Biodivers. Evol. Biogeogr. Plants 1998, 43, 131–164. [Google Scholar]
- Kundu, B.C.; Guha, S. The genus perichasma (Menispermaceae). Adansonia 1977, 17, 221–234. [Google Scholar]
- Aiton, W. Oxalis. In A General History of the Dichlamydeous Plants; Gilbert & Rivington: London, UK, 1831; Volume 1, pp. 753–768. [Google Scholar]
- Groom, Q. Typification of Oxalisbowiei WT Aiton Ex, G. Don (Oxalidaceae). PhytoKeys 2019, 119, 23. [Google Scholar] [CrossRef] [PubMed]
- Porter-Utley, K. A Revision of Passiflora, L. Subgenus Decaloba (DC.) Rchb. Supersection Cieca (Medik.) JM MacDougal & Feuillet (Passifloraceae). PhytoKeys 2014, 43, 1. [Google Scholar]
- Flora of North America Editorial Committee Flora of North America-Magnoliophyta: Paeoniacaea to Ericaceae; Oxford University Press: New York, NY, USA, 2009; Volume 8.
- Wu, Z.; Raven, P.H. Flora of China: Brassicaceae through Saxifragaceae; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2001; Volume 8. [Google Scholar]
- Grau, A. ; Mashua, Tropaeolum Tubrosum Ruíz & Pav; International Potato Center: Lima, Peru, 2003; Volume 25. [Google Scholar]
- Hodge, W.H. Three native tuber foods of the high andes. Econ. Bot. 1951, 5, 185–201. [Google Scholar] [CrossRef]
- National Research Council. Lost Crops of the Incas: Little-Known Plants of the Andes with Promise for Worldwide Cultivation; National Academy Press: Washington, DC, USA, 1989. [Google Scholar]
- Berg, C.C.; Rosselli, P.F.; Davidson, D.W. Cecropia. Flora Neotropica 2005, 94, 1–230. [Google Scholar]
- Radcliffe-Smith, A. Pilea Peperomioides: Urticaceae. Kew Mag. 1984, 1, 14–19. [Google Scholar] [CrossRef]
- Wu, Z.; Raven, P.H.; Hong, D. Flora of China: Ulmaceae through Basellaceae; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2003; Volume 5. [Google Scholar]
- Ernandes, P.; Marchiori, S. The rare water fern marsilea strigosa willd: Morphological and anatomical observations concerning a small population in a mediterranean temporary pond in puglia. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2012, 146, 131–136. [Google Scholar] [CrossRef]
- Samain, M.-S.; Vanderschaeve, L.; Chaerle, P.; Goetghebeur, P.; Neinhuis, C.; Wanke, S. Is morphology telling the truth about the evolution of the species rich genus peperomia (Piperaceae)? Plant Syst. Evol. 2009, 278, 1. [Google Scholar] [CrossRef]
- Sack, L.; Scoffoni, C. Leaf venation: Structure, function, development, evolution, ecology and applications in the past, present and future. New Phytol. 2013, 198, 983–1000. [Google Scholar] [CrossRef] [PubMed]
- Tadrist, L.; Saudreau, M.; de Langre, E. Wind and gravity mechanical effects on leaf inclination angles. J. Theor. Biol. 2014, 341, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Niklas, K.J.; Paolillo, D.J., Jr. The role of the epidermis as a stiffening agent in tulipa (Liliaceae) stems. Am. J. Bot. 1997, 84, 735–744. [Google Scholar] [CrossRef]
- Moonlight, P.W.; Ardi, W.H.; Padilla, L.A.; Chung, K.-F.; Fuller, D.; Girmansyah, D.; Hollands, R.; Jara-Muñoz, A.; Kiew, R.; Leong, W.-C. Dividing and conquering the fastest–growing genus: Towards a natural sectional classification of the mega–diverse genus begonia (Begoniaceae). Taxon 2018, 67, 267–323. [Google Scholar] [CrossRef]
- Kaul, R.B. Anatomical observations on floating leaves. Aquat. Bot. 1976, 2, 215–234. [Google Scholar] [CrossRef]

| Clade | Order | Family | Species | Continent | Distribution | Habit | Habitat | Floral Zone | Floristic Kingdom | Peltation | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F | Salviniales | Marsileaceae | Marsilea ancylopoda A. Braun | North America, South America | Tropical America, Subtropical America | h | p | a | boreostrop, trop, austrostrop, austr | Neo | c |
| F | Salviniales | Marsileaceae | Marsilea batardae Launert | Europe | Portugal, Spain | h | p | a | sm, m | Hol | c |
| F | Salviniales | Marsileaceae | Marsilea macrocarpa C. Presl | Africa | C Africa, S Africa, Madagascar | h | p | a | trop, austrostrop | Pal | c |
| F | Salviniales | Marsileaceae | Marsilea oligospora Goodd. | North America | USA | h | p | a | t, sm, m | Hol | c |
| F | Salviniales | Marsileaceae | Marsilea strigosa Willd. | Europe | Mediterranean region, Russia, Kazakhstan | h | - | a | sm | Hol | c |
| ANA | Nymphaeales | Cabombaceae | Brasenia schreberi J.F.Gmel. | Africa, South America, Asia | worldwide | h | p | a | austr, strop, trop, m, sm, temp | Hol, Pal, Neo, Aus | e |
| ANA | Nymphaeales | Cabombaceae | Cabomba aquatica Aubl. | South America | S America | h | p | a | strop, trop | Neo | c |
| ANA | Nymphaeales | Nymphaeaceae | Euryale ferox Salisb. ex K.D.Koenig and Sims | Asia | Asia | h | a,p | a | boreostrop, m | Pal | c |
| ANA | Nymphaeales | Nymphaeaceae | Nymphaea colorata Peter | Africa | Tanzania | h | p | a | trop | Pal | e |
| ANA | Nymphaeales | Nymphaeaceae | Nymphaea gigantea Hook. | Australia | Australia | h | p | a | austrostrop | Aus | e |
| ANA | Nymphaeales | Nymphaeaceae | Nymphaea lotus L. | Africa | Africa | h | p | a | strop, trop | Pal | e |
| ANA | Nymphaeales | Nymphaeaceae | Victoria amazonica (Poepp.) J.C. Sowerby | South America | S America | h | p | a | trop | Neo | c |
| ANA | Nymphaeales | Nymphaeaceae | Victoria cruziana Orb. | South America | S America | h | p | a | austrostrop | Neo | c |
| MAG | Laurales | Hernandiaceae | Hernandia nymphaeifolia (C.Presl) Kubitzki | Asia, Africa | SE Asia, Madagascar | l | p | t | trop | Pal | e |
| MAG | Laurales | Hernandiaceae | Hernandia sonora L. | North America | Mexico, Caribbean | l | p | t | boreostrop | Neo | e |
| MAG | Piperales | Piperaceae | Peperomia argyreia E.Morr. | South America | Brazil | h | p | t | austrostrop | Neo | e |
| MAG | Piperales | Piperaceae | Peperomia bracteata A.W.Hill | North America | Guatemala, Mexico | h | p | t | boreostrop | Neo | c |
| MAG | Piperales | Piperaceae | Peperomia cyclaminoides A.W. Hill | South America | Bolivia | h | - | t | boreostrop | Neo | c |
| MAG | Piperales | Piperaceae | Peperomia monticola Miq. | North America | Mexico | h | p | t | boreostrop | Neo | e |
| MAG | Piperales | Piperaceae | Peperomia sodiroi C.DC. | South America | Ecuador | h | p | t | trop | Neo | e |
| MAG | Piperales | Piperaceae | Piper peltatum L. | North America, South America | S America, C America | h,l | p | t | strop, trop | Neo | e |
| MAG | Piperales | Piperaceae | Piper fragile Benth. | Asia | C Malesia, W Pacific | - | - | t | trop | Pal, Aus | e |
| MAG | Piperales | Piperaceae | Piper peltatifolium C.Y. Hao, H.S. Wu, Y.H. Tan | Asia | China | l | p | t | boreostrop | Pal | e |
| MON | Alismatales | Araceae | Alocasia cuprea K.Koch | Asia | Borneo | h | p | t | trop | Pal | e |
| MON | Alismatales | Araceae | Alocasia fallax Schott | Asia | Bangladesh, India | h | p | t | boreostrop | Pal | e |
| MON | Alismatales | Araceae | Alocasia longiloba Miq. | Asia | SE Asia | h | p | t | boreostrop, trop | Pal | e |
| MON | Alismatales | Araceae | Alocasia peltata M. Hotta | Asia | Borneo | h | p | t | trop | Pal | e |
| MON | Alismatales | Araceae | Alocasia reversa N.E. Br. | Asia | Borneo | h | p | t | trop | Pal | e |
| MON | Alismatales | Araceae | Amorphophallus konjac K.Koch | Asia | China, SE Asia | h | p | t | m | Hol | e |
| MON | Alismatales | Araceae | Anthurium forgetii N.E.Br. | South America | Colombia | h | p | t | trop | Neo | e |
| MON | Alismatales | Araceae | Anthurium jureianum Cath. and Olaio | South America | Brazil | h | p | t | austrostrop | Neo | e |
| MON | Alismatales | Araceae | Ariopsis peltata Nimmo | Asia | India | h | p | t | boreostrop, trop | Pal | e |
| MON | Alismatales | Araceae | Ariopsis proanthera N.E. Br. | Asia | Bangladesh, India, Myanmar, Nepal, Thailand | h | p | t | boreostrop, trop | Pal | e |
| MON | Alismatales | Araceae | Arisaema caudatum Engl. | Asia | India | h | p | t | boreostrop, trop | Pal | c |
| MON | Alismatales | Araceae | Arisaema ciliatum H.Li | Asia | China | h | p | t | m | Hol | c |
| MON | Alismatales | Araceae | Arisaema fischeri Manudev and Nampy | Asia | India | h | p | t | boreostrop, trop | Pal | c |
| MON | Alismatales | Araceae | Arisaema peltatum C.E.C. Fisch. | Asia | India | h | p | t | boreostrop, trop | Pal | e |
| MON | Alismatales | Araceae | Arisaema subulatum Manudev and Nampy | Asia | India | h | p | t | boreostrop, trop | Pal | c |
| MON | Alismatales | Araceae | Caladium bicolor Vent. | North America, South America | C America, S America | h | p | t | boreostrop, trop | Neo | e |
| MON | Alismatales | Araceae | Caladium clavatum Hett., Bogner and J. Boos | South America | Ecuador | h | p | t | trop | Neo | e |
| MON | Alismatales | Araceae | Caladium humboldtii (Raf.) Schott | South America | Brazil, Venezuela | h | p | t | trop | Neo | e |
| MON | Alismatales | Araceae | Caladium smaragdinum K. Koch and C.D. Bouché | South America | Colombia, Venezuela | h | p | t | trop | Neo | e |
| MON | Alismatales | Araceae | Caladium steudnerifolium Engl. | South America | Bolivia, Colombia, Ecuador, Peru | h | p | t | trop, austrostrop | Neo | e |
| MON | Alismatales | Araceae | Colocasia boyceana Gogoi and Borah | Asia | India | h | p | t | boreostrop, trop | Pal | e |
| MON | Alismatales | Araceae | Colocasia esculenta (L.) Schott | Asia | SE Asia | h | p | t | trop, boreostrop, m | Pal | e |
| MON | Alismatales | Araceae | Colocasia fallax Schott | Asia | SE Asia | h | p | t | m, boreostrop | Pal | e |
| MON | Alismatales | Araceae | Colocasia hassanii H. Ara | Asia | Bangladesh | h | p | t | boreostrop | Pal | e |
| MON | Alismatales | Araceae | Colocasia mannii Hook. f. | Asia | Bangladesh, India | h | p | t | boreostrop | Pal | e |
| MON | Alismatales | Araceae | Remusatia hookeriana Schott | Asia | China, India, Myanmar, Nepal, Thailand | h | p | t | m, boreostrop, trop | Hol, Pal | e |
| MON | Alismatales | Araceae | Remusatia pumila (D.Don) H.Li and A.Hay | Asia | China, Bangladesh, Nepal, Thailand | h | p | t | boreostrop | Pal | e |
| MON | Alismatales | Araceae | Remusatia vivipara (Roxb.) Schott | Africa, Asia | Africa, Australia, SE Asia | h | p | t | strop, trop | Pal, Aus | e |
| MON | Alismatales | Araceae | Remusatia yunnanensis (H. Li and A. Hay) A. Hay | Asia | China, Taiwan | h | p | t | m, boreostrop | Hol, Pal | e |
| MON | Alismatales | Araceae | Steudnera assamica Hook. f. | Asia | India | h | p | t | boreostrop | Pal | e |
| MON | Alismatales | Araceae | Steudnera colocasiifolia K.Koch | Asia | SE Asia, China | h | p | t | m, boreostrop | Hol, Pal | e |
| MON | Alismatales | Araceae | Steudnera discolor W. Bull | Asia | India, Bangladesh, Myanmar, Thailand | h | p | t | boreostrop | Pal | e |
| MON | Alismatales | Araceae | Steudnera kerrii Gagnep. | Asia | China, Laos, Thailand, Vietnam | h | p | t | boreostrop | Pal | e |
| MON | Alismatales | Araceae | Steudnera virosa (Roxb.) Prain | Asia | Bangladesh, India | h | p | t | boreostrop, trop | Pal | e |
| MON | Alismatales | Araceae | Xanthosoma peltatum G.S. Bunting | South America | Venezuela | h | p | t | trop | Neo | e |
| EUD | Proteales | Nelumbonaceae | Nelumbo lutea Willd. | North America | S USA | h | p | a | boreostrop, m | Hol | c |
| EUD | Proteales | Nelumbonaceae | Nelumbo nucifera Gaertn. | Asia | India | h | p | a | boreostrop | Pal | c |
| EUD | Ranunculales | Berberidaceae | Podophyllum delavayi Franch. | Asia | SC China | h | p | t | m | Hol | e |
| EUD | Ranunculales | Berberidaceae | Podophyllum glaucescens J.M.H.Shaw | Asia | SE China | h | p | t | m | Hol | e |
| EUD | Ranunculales | Berberidaceae | Podophyllum peltatum L. | North America | N America | h | p | t | m, sm, temp, b | Hol | c |
| EUD | Ranunculales | Berberidaceae | Podophyllum pleianthum Hance | Asia | SE China, Taiwan | h | p | t | m | Hol | c |
| EUD | Ranunculales | Berberidaceae | Podophyllum versipelle Hance | Asia | China, Vietnam | h | p | t | boreostrop | Pal | c |
| EUD | Ranunculales | Menispermaceae | Cissampelos glaberrima A. St.-Hil. | South America | Tropical South America | h,l | p | t | trop, austrostrop | Neo | e |
| EUD | Ranunculales | Menispermaceae | Cissampelos grandifolia Triana and Planch. | North America, South America | C America, Tropical S America | h,l | p | t | boreostrop, trop, austrostrop | Neo | e |
| EUD | Ranunculales | Menispermaceae | Cissampelos hispida Forman | Asia | Thailand | l | p | t | boreostrop, trop | Pal | e |
| EUD | Ranunculales | Menispermaceae | Cissampelos owariensis P. Beauv. ex DC. | Africa | Tropical Africa | h,l | p | t | boreostrop, trop | Pal | e |
| EUD | Ranunculales | Menispermaceae | Cissampelos sympodialis Eichler | South America | Brazil | h,l | p | t | trop, austrostrop | Neo | e |
| EUD | Ranunculales | Menispermaceae | Coscinium blumeanum Miers ex Hook.f. and Thomson | Asia | Malaysia, Thailand, Vietnam | l | p | t | boreostrop, trop | Pal | e |
| EUD | Ranunculales | Menispermaceae | Cyclea cauliflora Merr. | Asia | Philippines, Sulawesi | h,l | p | t | boreostrop, trop | Pal | e |
| EUD | Ranunculales | Menispermaceae | Cyclea debiliflora Miers | Asia | China, India, Vietnam | h,l | p | t | m, boreostrop, trop | Pal | e |
| EUD | Ranunculales | Menispermaceae | Cyclea fissicalyx Dunn | Asia | India | h,l | p | t | boreostrop, trop | Pal | e |
| EUD | Ranunculales | Menispermaceae | Cyclea hypoglauca (Schauer) Diels | Asia | China, Vietnam | h,l | p | t | m, boreostrop, trop | Pal | e |
| EUD | Ranunculales | Menispermaceae | Cyclea peltata (Lam.) Hook. f. and Thomson | Asia | SE Asia, India | h,l | p | t | boreostrop | Pal | e |
| EUD | Ranunculales | Menispermaceae | Disciphania calocarpa Standl. | North America | C America | l | p | t | boreostrop | Neo | e |
| EUD | Ranunculales | Menispermaceae | Disciphania contraversa Barneby | South America | Brazil | - | p | t | austrostrop | Neo | e |
| EUD | Ranunculales | Menispermaceae | Disciphania hernandia (Vell.) Barneby | South America | Brazil | h,l | p | t | trop, austrostrop | Neo | e |
| EUD | Ranunculales | Menispermaceae | Menispermum dauricum DC. | Asia | China, Mongolia, Russia, Japan, Korea | h,l | p | t | b, temp, sm, m, boreostrop | Hol, Pal | e |
| EUD | Ranunculales | Menispermaceae | Perichasma laetificata Miers | Africa | WC Africa | - | - | t | trop | Pal | e |
| EUD | Ranunculales | Menispermaceae | Stephania abyssinica (Quart.-Dill. and A.Rich.) Walp. | Africa | Africa | h | p | t | strop, trop | Pal | e |
| EUD | Ranunculales | Menispermaceae | Stephania brevipes Craib | Asia | Thailand, Vietnam | h | p | t | boreostrop, trop | Pal | e |
| EUD | Ranunculales | Menispermaceae | Stephania delavayi Diels | Asia | China, Myanmar | h | p | t | boreostrop | Pal | e |
| EUD | Ranunculales | Menispermaceae | Stephania japonica (Thunb.) Miers | Asia, Australia and Oceania | Tropical Asia, Subtropical Asia, Australia | h | p | t | boreostrop, trop, austrostrop | Pal, Aus | e |
| EUD | Ranunculales | Menispermaceae | Stephania venosa Spreng. | Asia | SE Asia | h | p | t | boreostrop, trop | Pal | e |
| EUD | Ranunculales | Ranunculaceae | Asteropyrum cavaleriei H.Lév. and Vaniot | Asia | China | h | p | t | m | Hol | e |
| EUD | Ranunculales | Ranunculaceae | Asteropyrum peltatum (Franch.) J.R.Drumm. and Hutch. | Asia | China, Myanmar | h | p | t | boreostrop | Pal | e |
| EUD | Ranunculales | Ranunculaceae | Peltocalathos baurii (MacOwan) Tamura | Africa | South Africa, Lesotho, Eswatini | h | p | t | austr | Cap | c |
| EUD | Ranunculales | Ranunculaceae | Ranunculus clypeatus (Ulbr.) Lourteig | South America | Peru | h | p | t | trop | Neo | e |
| EUD | Ranunculales | Ranunculaceae | Ranunculus lyallii Hook.f. | Australia | New Zealand | h | p | t | austr | Ant | c |
| EUD | Ranunculales | Ranunculaceae | Thalictrum ichangense Lecoy. ex Oliv. | Asia | China, Korea, Vietnam | h | p | t | boreostrop, m | Hol, Pal | e |
| EUD | Ranunculales | Ranunculaceae | Thalictrum pringlei S. Watson | North America | Mexico | h | p | t | boreostrop | Neo | e |
| EUD | Ranunculales | Ranunculaceae | Thalictrum pseudoichangense Q.E. Yang and G.H. Zhu | Asia | China | h | p | t | m, boreostrop | Hol, Pal | e |
| EUD | Ranunculales | Ranunculaceae | Thalictrum roseanum B.Boivin | North America | Mexico | h | p | t | boreostrop | Neo | e |
| EUD-C | Apiales | Apiaceae | Klotzschia brasiliensis Cham. | South America | Brazil | h | - | t | austrostrop | Neo | e |
| EUD-C | Apiales | Apiaceae | Klotzschia glaziovii Urb. | South America | Brazil | l | p | t | austrostrop | Neo | e |
| EUD-C | Apiales | Apiaceae | Klotzschia rhizophylla Urb. | South America | Brazil | h | - | t | austrostrop | Neo | e |
| EUD-C | Apiales | Apiaceae | Petagnaea gussonei (Spreng.) Rauschert | Europe | Sicilia | h | p | t | m | Hol | c |
| EUD-C | Apiales | Araliaceae | Hydrocotyle bonariensis Lam. | North America, South America | C America, S America | h | p | t | boreostrop, trop | Neo | c |
| EUD-C | Apiales | Araliaceae | Hydrocotyle pusilla R.Br. ex Rich. | North America, South America | C America, S America | h | p | t | boreostrop, trop | Neo | c |
| EUD-C | Apiales | Araliaceae | Hydrocotyle umbellata L. | North America, South America | C America, S America | h | p | t | boreostrop, trop | Neo | c |
| EUD-C | Apiales | Araliaceae | Hydrocotyle vulgaris L. | Europe | Europe | h | p | a | m, sm, temp, b | Hol | c |
| EUD-C | Apiales | Araliaceae | Hydrocotyle yanghuangensis (Hieron.) Mathias | South America | Ecuador | h | p | t | trop | Neo | e |
| EUD-C | Apiales | Araliaceae | Oplopanax japonicus Nakai | Asia | Japan | l | p | t | t, sm | Hol | e |
| EUD-C | Apiales | Araliaceae | Schefflera actinophylla (Endl.) Harms | Australia and Oceania | Australia, New Guinea | l | p | t | trop, austrostrop | Pal, Aus | e |
| EUD-C | Apiales | Araliaceae | Schefflera arboricola (Hayata) Merr. | Asia | Taiwan | l | p | t | m | Hol | e |
| EUD-C | Apiales | Araliaceae | Schefflera digitata J.R. Forst. and G. Forst. | Australia and Oceania | New Zealand | l | p | t | austrostrop, austr | Aus | e |
| EUD-C | Asterales | Asteraceae | Ligularia nelumbifolia Hand.-Mazz. | Asia | China | h | p | t | sm, m, boreostrop | Hol, Pal | e |
| EUD-C | Asterales | Asteraceae | Prenanthes subpeltata Stebbins | Africa | Ethiopia, Kenya, Rwanda, Democratic Republic of Congo | h | p | t | boreostrop, trop | Pal | e |
| EUD-C | Asterales | Asteraceae | Psacalium laxiflorum Benth. | North America | Mexico | h | p | t | boreostrop | Neo | c |
| EUD-C | Asterales | Asteraceae | Psacalium megaphyllum Rydb. | North America | Mexico | h | p | t | boreostrop | Neo | e |
| EUD-C | Asterales | Asteraceae | Psacalium peltatum Cass. | North America | Mexico | h | p | t | boreostrop | Neo | c |
| EUD-C | Asterales | Asteraceae | Psacalium pinetorum (Standl. and Steyerm.) Cuatrec. | North America | Guatemala | h | p | t | boreostrop | Neo | e |
| EUD-C | Asterales | Asteraceae | Psacalium putlanum B.L. Turner | North America | Mexico | h | p | t | boreostrop | Neo | e |
| EUD-C | Asterales | Asteraceae | Roldana chapalensis (S. Watson) H. Rob. and Bretell | North America | Mexico | l | p | t | boreostrop | Neo | e |
| EUD-C | Asterales | Asteraceae | Roldana heterogama (Benth.) H.Rob. and Brettell | North America | Costa Rica, Guatemala, Mexico, Panama | h,l | p | t | boreostrop | Neo | e |
| EUD-C | Asterales | Asteraceae | Roldana subpeltata (Sch. Bip.) H. Rob and Brettell | North America | Mexico | h,l | p | t | boreostrop | Neo | e |
| EUD-C | Asterales | Asteraceae | Senecio oxyriifolius DC. | Africa | C Africa, S Africa | h | p | t | austr, austrostrop, trop | Cap, Pal | e |
| EUD-C | Asterales | Asteraceae | Syneilesis aconitifolia (Bunge) Maxim. | Asia | China, Japan, Korea, Russia | h | p | t | t, sm, m, boreostrop | Hol, Pal | c |
| EUD-C | Asterales | Asteraceae | Syneilesis palmata (Thunb.) Maxim. | Asia | Korea, Japan | h | p | t | sm | Hol | c |
| EUD-C | Brassicales | Caricaceae | Jacaratia digitata (Poepp. and Endl.) Solms | South America | Bolivia, Brazil, Ecuador, Venezuela | l | p | t | strop, trop | Neo | e |
| EUD-C | Brassicales | Caricaceae | Jacaratia spinosa (Aubl.) A.DC. | South America | C America, S America | l | p | t | strop, trop | Neo | e |
| EUD-C | Brassicales | Tropaeolaceae | Tropaeolum ciliatum Ruiz and Pav. | South America | Chile | h | p | t | austrostrop | Neo, Ant | e |
| EUD-C | Brassicales | Tropaeolaceae | Tropaeolum majus L. | South America | Peru | h | p | t | austrostrop | Neo | e |
| EUD-C | Brassicales | Tropaeolaceae | Tropaeolum minus L. | South America | Ecuador, Peru | h | a | t | austrostrop, trop | Neo | e |
| EUD-C | Brassicales | Tropaeolaceae | Tropaeolum pentaphyllum Lam. | South America | Argentina, Brazil, Paraguay, Uruguay | h | p | t | austrostrop | Neo | e |
| EUD-C | Brassicales | Tropaeolaceae | Tropaeolum tuberosum Ruiz and Pav. | South America | Bolivia, Colombia, Ecuador, Peru | h | p | t | austrostrop, trop | Neo | e |
| EUD-C | Caryophyllales | Polygonaceae | Coccoloba acapulcensis Standl. | North America | C America | l | p | t | boreostrop | Neo | e |
| EUD-C | Caryophyllales | Polygonaceae | Coccoloba tiliacea Lindau | South America | Argentina, Bolivia | l | p | t | trop, austrostrop, austr | Neo | e |
| EUD-C | Caryophyllales | Polygonaceae | Persicaria perfoliata (L.) Gross | Europe, Asia | Turkey, India, E Asia, SE Asia | h | a | t | b, temp, sm, m, boreostrop, trop | Hol, Pal | e |
| EUD-C | Cornales | Loasaceae | Nasa peltata (Spruce ex Urb. and Gilg) Weigend | South America | Ecuador, Peru | h | - | t | trop | Neo | e |
| EUD-C | Cornales | Loasaceae | Nasa peltiphylla (Weigend) Weigend | South America | Colombia, Ecuador | - | - | t | trop | Neo | e |
| EUD-C | Cucurbitales | Begoniaceae | Begonia caroliniifolia Regel | North America | Mexico, Guatemala, Honduras | h | p | t | boreostrop | Neo | e |
| EUD-C | Cucurbitales | Begoniaceae | Begonia goegoensis N.E.Br. | Asia | Sumatra | h | p | t | trop | Pal | e |
| EUD-C | Cucurbitales | Begoniaceae | Begonia kellermannii C.DC. | South America | S America | h | p | t | trop | Neo | e |
| EUD-C | Cucurbitales | Begoniaceae | Begonia nelumbiifolia Cham. and Schltdl. | South America | S America | h | p | t | austrostrop | Neo | e |
| EUD-C | Cucurbitales | Begoniaceae | Begonia sudjanae C.-A. Jansson | Asia | Sumatra | h | p | t | trop | Pal | e |
| EUD-C | Ericales | Balsaminaceae | Impatiens begonioides Eb. Fisch. and Raheliv. | Africa | Madagascar | h | - | t | trop, austrostrop | Pal | e |
| EUD-C | Fabales | Fabaceae | Lupinus angustifolius L. | Europe, Africa, Asia | Mediterranean area, China | h | a | t | sm, m | Hol, Pal | e |
| EUD-C | Fabales | Fabaceae | Lupinus arboreus Sims | North America | W USA | h,l | p | t | sm, m | Hol | e |
| EUD-C | Fabales | Fabaceae | Lupinus digitatus Forssk. | Africa | N Africa, Senegal | h | a | t | m, boreostrop | Hol, Pal | e |
| EUD-C | Fabales | Fabaceae | Lupinus gussoneanus J. Agardh | Europe | Mediterranean area | h | a | t | sm, m | Hol | e |
| EUD-C | Fabales | Fabaceae | Lupinus mutabilis Sweet | South America | Bolivia, Colombia, Ecuador, Peru, Venezuela | h,l | p | t | trop | Neo | e |
| EUD-C | Gentianales | Apocynaceae | Hoya imbricata Decne. | Asia | Philippines, Sulawesi | h | p | t | boreostrop, trop | Pal | c |
| EUD-C | Gentianales | Apocynaceae | Macropharynx abnorma J.F. Morales, M.E. Endress and Liede | South America | Peru | h,l | p | t | trop | Neo | e |
| EUD-C | Gentianales | Apocynaceae | Macropharynx conflictiva (J.F. Morales) J.F. Morales, M.E. Endress and Liede | South America | Peru | h,l | p | t | trop | Neo | e |
| EUD-C | Gentianales | Apocynaceae | Macropharynx gigantea (Woodson) J.F. Morales, M.E. Endress and Liede | South America | Peru, Bolivia | h,l | p | t | trop, austrostrop | Neo | e |
| EUD-C | Gentianales | Apocynaceae | Macropharynx macrocalyx (Müll. Arg.) J.F. Morales, M.E. Endress and Liede | South America | Brazil, Paraguay | h,l | p | t | trop, austrostrop | Neo | e |
| EUD-C | Gentianales | Apocynaceae | Macropharynx peltata (Vell.) J.F. Morales, M.E. Endress and Liede | South America | Argentina, Brazil, Paraguay | h,l | p | t | trop, austrostrop | Neo | e |
| EUD-C | Gunnerales | Gunneraceae | Gunnera antioquensis L.E. Mora | South America | Colombia | h | p | t | trop | Neo | e |
| EUD-C | Gunnerales | Gunneraceae | Gunnera brephogea Linden and André | South America | Colombia, Ecuador, Peru | h | p | t | trop | Neo | c |
| EUD-C | Gunnerales | Gunneraceae | Gunnera peltata Phil. | South America | Juan Fernández Is. | h | p | t | austr | Ant | c |
| EUD-C | Gunnerales | Gunneraceae | Gunnera quitoensis L.E. Mora | South America | Ecuador | h | p | t | trop | Neo | e |
| EUD-C | Gunnerales | Gunneraceae | Gunnera silvioana L.E. Mora | South America | Colombia, Ecuador | h | p | t | trop | Neo | e |
| EUD-C | Lamiales | Gesneriaceae | Cyrtandra toviana F. Br. | Australia and Oceania | Marquesas Islands | l | p | t | trop | Pal | e |
| EUD-C | Lamiales | Gesneriaceae | Cyrtandra wawrae C.B. Clarke | Australia and Oceania | Hawaii | - | - | t | trop | Pal | e |
| EUD-C | Lamiales | Gesneriaceae | Drymonia peltata (Oliv.) H.E. Moore | North America | Costa Rica | - | - | t | boreostrop | Neo | e |
| EUD-C | Lamiales | Gesneriaceae | Metapetrocosmea peltata (Merr. and Chun) W.T. Wand | Asia | China | h | p | t | boreostrop | Pal | e |
| EUD-C | Lamiales | Gesneriaceae | Paraboea peltifolia D. Fang and L. Zeng | Asia | China | h | p | t | m, boreostrop | Hol, Pal | e |
| EUD-C | Lamiales | Gesneriaceae | Paraboea yunfuensis F. Wen and Y.G. Wie | Asia | China | h | p | t | m, boreostrop | Hol, Pal | e |
| EUD-C | Lamiales | Gesneriaceae | Petrocosmea huanjiangensis Yan Liu and W.B. Xu | Asia | China | h | p | t | m, boreostrop | Hol, Pal | e |
| EUD-C | Lamiales | Gesneriaceae | Petrocosmea pubescens D.J. Middleton and Triboun | Asia | Thailand | h | p | t | boreostrop, trop | Pal | e |
| EUD-C | Lamiales | Gesneriaceae | Sinningia tuberosa (Mart.) H.E. Moore | South America | Brazil | h | p | t | austrostrop | Neo | e |
| EUD-C | Lamiales | Gesneriaceae | Streptocarpus mandrerensis Humbert | Africa | Madagascar | h | - | t | trop, austrostrop | Pal | e |
| EUD-C | Lamiales | Gesneriaceae | Streptocarpus peltatus Randrian., Phillipson, Lowry and Mich. Möller | Africa | Madagascar | h | p | t | trop, austrostrop | Pal | e |
| EUD-C | Lamiales | Gesneriaceae | Trichodrymonia peltatifolia (J.L. Clark and M.M. More) M.M. Mora and J.L. Clark | North America | Panama | h | - | t | boreostrop | Neo | e |
| EUD-C | Lamiales | Lentibulariaceae | Utricularia nelumbifolia Gardn. | South America | Brazil | h | p | a | austrostrop | Neo | c |
| EUD-C | Lamiales | Lentibulariaceae | Utricularia pubescens Sm. | Africa, South America, Asia | S America, Africa, India | h | a | t | strop, trop | Neo, Pal | c |
| EUD-C | Malpighiales | Euphorbiaceae | Endospermum moluccanum (Teijsm. and Binn.) Kurz | Asia, Australia and Oceania | Indonesia, New Guinea, Solomon Islands | l | p | t | trop | Pal | e |
| EUD-C | Malpighiales | Euphorbiaceae | Endospermum peltatum Merr. | Asia | SE Asia | l | p | t | trop | Pal | e |
| EUD-C | Malpighiales | Euphorbiaceae | Homalanthus grandifolius Ridl. | Asia | Borneo, Sumatra | l | p | t | trop | Pal | e |
| EUD-C | Malpighiales | Euphorbiaceae | Homalanthus macradenius Pax and K. Hoffm. | Asia | Philippines | l | p | t | boreostrop, trop | Pal | e |
| EUD-C | Malpighiales | Euphorbiaceae | Jatropha hernandiifolia Vent. | North America | Dominican Republic, Haiti, Puerto Rico | l | p | t | boreostrop | Neo | e |
| EUD-C | Malpighiales | Euphorbiaceae | Jatropha nudicaulis Benth. | South America | Colombia, Ecuador | l | p | t | trop | Neo | e |
| EUD-C | Malpighiales | Euphorbiaceae | Jatropha peltata Sessé | North America | Mexico | h,l | p | t | boreostrop | Neo | e |
| EUD-C | Malpighiales | Euphorbiaceae | Jatropha podagrica Hook. | North America | C America | h,l | p | t | trop | Neo | e |
| EUD-C | Malpighiales | Euphorbiaceae | Jatropha weberbaueri Pax and K. Hoffm. | South America | Peru | - | t | trop | Neo | e | |
| EUD-C | Malpighiales | Euphorbiaceae | Macaranga bancana (Miq.) Müll.Arg. | Asia | Thailand, Malesia | l | p | t | boreostrop, trop | Pal | e |
| EUD-C | Malpighiales | Euphorbiaceae | Macaranga cuspidata Boivin ex Baill. | Africa | Madagascar | l | p | t | trop, austrostrop | Pal | e |
| EUD-C | Malpighiales | Euphorbiaceae | Macaranga magna Turrill | Australia and Oceania | Fiji | l | p | t | austrostrop | Aus | e |
| EUD-C | Malpighiales | Euphorbiaceae | Macaranga tanarius (L.) Mull.Arg. | Asia, Australia and Oceania | SE Asia, Australia | l | p | t | strop, trop | Pal, Aus | e |
| EUD-C | Malpighiales | Euphorbiaceae | Macaranga thompsonii Merr. | Australia and Oceania | Mariana Islands | l | p | t | trop | Pal | e |
| EUD-C | Malpighiales | Euphorbiaceae | Mallotus floribundus (Blume) Müll. Arg. | Asia | SE Asia | l | p | t | boreostrop, trop | Pal | e |
| EUD-C | Malpighiales | Euphorbiaceae | Mallotus lackeyi Elmer | Asia | Borneo, Philippines | l | p | t | trop | Pal | e |
| EUD-C | Malpighiales | Euphorbiaceae | Mallotus peltatus (Geiseler) Müll. Arg. | Asia | China, India, SE Asia | l | p | t | m, boreostrop, trop | Pal | e |
| EUD-C | Malpighiales | Euphorbiaceae | Mallotus surculosus P.I. Forst. | Australia and Oceania | Australia | l | p | t | austrostrop | Aus | e |
| EUD-C | Malpighiales | Euphorbiaceae | Mallotus thorelii Gagnep. | Asia | Cambodia, China, Laos, Thailand, Vietnam | l | p | t | m, boreostrop, trop | Pal | e |
| EUD-C | Malpighiales | Euphorbiaceae | Manihot fabianae M. Mend. | South America | Bolivia | l | p | t | trop, austrostrop | Neo | e |
| EUD-C | Malpighiales | Euphorbiaceae | Manihot mirabilis Pax | South America | Paraguay | l | p | t | trop, austrostrop | Neo | e |
| EUD-C | Malpighiales | Euphorbiaceae | Manihot peltata Pohl | South America | Brazil | l | p | t | trop, austrostrop | Neo | e |
| EUD-C | Malpighiales | Euphorbiaceae | Megistostigma peltatum (J.J. Sm.) Croizat | Asia | Java, Sumatra | l | p | t | trop | Pal | e |
| EUD-C | Malpighiales | Euphorbiaceae | Meineckia peltata (Hutch.) G.L. Webster | Africa | Madagascar | l | p | t | trop, austrostrop | Pal | e |
| EUD-C | Malpighiales | Euphorbiaceae | Ricinus communis L. | Africa | Eritrea, Ethiopia, Somalia | h,l | a,p | t | boreostrop | Pal | e |
| EUD-C | Malpighiales | Passifloraceae | Adenia penangiana (Wall. ex G. Don) W.J. de Wilde | Asia | SE Asia | h | p | t | boreostrop, trop | Pal | e |
| EUD-C | Malpighiales | Passifloraceae | Passiflora coriacea Juss. | South America | C America, S America | h | p | t | boreostrop, trop | Neo | e |
| EUD-C | Malpighiales | Passifloraceae | Passiflora guatemalensis S. Watson | North America, South America | C America, Colombia, Venezuela | h | p | t | boreostrop, trop | Neo | e |
| EUD-C | Malpighiales | Passifloraceae | Passiflora rubrotincta Killip | South America | Bolivia | h | p | t | trop, austrostrop | Neo | e |
| EUD-C | Malpighiales | Passifloraceae | Passiflora spectabilis Killip | South America | Peru | h | p | t | trop | Neo | e |
| EUD-C | Malpighiales | Phyllanthaceae | Astrocasia peltata Standl. | North America | Costa Rica, Mexico | l | p | t | boreostrop | Neo | e |
| EUD-C | Malpighiales | Salicaceae | Xylosma peltata (Sleumer) Lescot | Australia and Oceania | New Caledonia | l | p | t | austrostrop | Aus | e |
| EUD-C | Malvales | Dipterocarpaceae | Shorea peltata Symington | Asia | Borneo, Malaysia, Sumatra | l | p | t | trop | Pal | e |
| EUD-C | Malvales | Malvaceae | Brownlowia ferruginea Kosterm. | Asia | Borneo | l | p | t | trop | Pal | e |
| EUD-C | Malvales | Malvaceae | Brownlowia havilandii Stapf | Asia | Borneo | l | p | t | trop | Pal | e |
| EUD-C | Malvales | Malvaceae | Brownlowia helferiana Pierre | Asia | Malaysia, Myanmar, Thailand | l | p | t | boreostrop, trop | Pal | e |
| EUD-C | Malvales | Malvaceae | Brownlowia peltata Benth. | Asia | Borneo, Myanmar, Thailand, Vietnam | l | p | t | boreostrop, trop | Pal | e |
| EUD-C | Malvales | Malvaceae | Brownlowia stipulata Kosterm. | Asia | Borneo | l | p | t | trop | Pal | e |
| EUD-C | Malvales | Malvaceae | Pterospermum acerifolium (L.) Willd. | Asia | India, SE Asia | h | p | t | boreostrop, trop | Pal | e |
| EUD-C | Myrtales | Melastomataceae | Catanthera peltata M.P. Nayar | Asia | Borneo | - | t | trop | Pal | e | |
| EUD-C | Myrtales | Melastomataceae | Conostegia peltata (Almeda) Kriebel | North America | Panama | l | p | t | trop | Neo | e |
| EUD-C | Myrtales | Melastomataceae | Gravesia peltata H. Perrier | Africa | Madagascar | h | p | t | trop, austrostrop | Pal | e |
| EUD-C | Myrtales | Melastomataceae | Leandra peltata Wurdack | South America | Peru | l | p | t | trop | Neo | e |
| EUD-C | Myrtales | Melastomataceae | Phyllagathis beccariana (Cogn.) M.P. Nayar | Asia | Borneo | h | p | t | trop | Pal | e |
| EUD-C | Myrtales | Melastomataceae | Phyllagathis peltata Stapf ex Ridl. | Asia | Borneo | h | p | t | trop | Pal | e |
| EUD-C | Oxalidales | Oxalidaceae | Oxalis articulata Savigny | South America | Argentina, Brazil, Uruguay | h | p | t | austrostrop | Neo | c |
| EUD-C | Oxalidales | Oxalidaceae | Oxalis bowiei W.T.Aiton ex G.Don | Africa | South Africa | h | p | t | austr | Cap | c |
| EUD-C | Oxalidales | Oxalidaceae | Oxalis decaphylla Kunth | North America | Mexico | h | p | t | boreostrop | Neo | c |
| EUD-C | Oxalidales | Oxalidaceae | Oxalis leucolepis Diels | Asia | China, Nepal | h | p | t | boreostrop, m | Pal | c |
| EUD-C | Oxalidales | Oxalidaceae | Oxalis triangularis A.St.-Hil. | South America | South America | h | p | t | austrostrop, trop | Neo | c |
| EUD-C | Rosales | Moraceae | Dorstenia belizensis C.C. Berg | North America | Belize | h | p | t | boreostrop | Neo | e |
| EUD-C | Rosales | Moraceae | Dorstenia erythrandra C. Wright ex Griseb. | North America | Cuba, Dominican Republic, Haiti | h | p | t | boreostrop | Neo | e |
| EUD-C | Rosales | Moraceae | Dorstenia jamaicensis Britton | North America | Jamaica | h | p | t | boreostrop | Neo | e |
| EUD-C | Rosales | Moraceae | Dorstenia nummularia Urb. and Ekman | North America | Cuba | h | p | t | boreostrop | Neo | e |
| EUD-C | Rosales | Moraceae | Dorstenia peltata Spreng. | North America | Cuba, Dominican Republic | h | p | t | boreostrop | Neo | e |
| EUD-C | Rosales | Urticaceae | Cecropia albicans Trécil | South America | Peru | l | p | t | trop | Neo | e |
| EUD-C | Rosales | Urticaceae | Cecropia distachya Huber | South America | N South America | l | p | t | trop, austrostrop | Neo | e |
| EUD-C | Rosales | Urticaceae | Cecropia elongata Rusby | South America | Bolivia | l | p | t | trop, austrostrop | Neo | e |
| EUD-C | Rosales | Urticaceae | Cecropia latiloba Miq. | South America | N South America | l | p | t | trop, austrostrop | Neo | e |
| EUD-C | Rosales | Urticaceae | Cecropia peltata L. | South America | C America | l | p | t | boreostrop, trop | Neo | e |
| EUD-C | Rosales | Urticaceae | Dendrocnide moroides (Wedd.) Chew | Asia, Australia and Oceania | Australia, Lesser Sunda Islands, Vanuatu | l | p | t | trop, austrostrop, austr | Pal, Aus | e |
| EUD-C | Rosales | Urticaceae | Dendrocnide peltata (Blume) Miq. | Asia, Australia and Oceania | New Guinea, Malesia | l | p | t | trop | Pal | e |
| EUD-C | Rosales | Urticaceae | Elatostema muluense Rodda and A.K. Monro | Asia | Borneo | h | p | t | trop | Pal | e |
| EUD-C | Rosales | Urticaceae | Elatostema peltifolium (Ridl.) H.J.P. Winkl. | Australia and Oceania | New Guinea | h | - | t | trop | Pal | e |
| EUD-C | Rosales | Urticaceae | Musanga cecropioides R. Br. ex Tedlie | Africa | W and C Africa | l | p | t | trop | Pal | c |
| EUD-C | Rosales | Urticaceae | Musanga leo-errerae Hauman and J. Léonard | Africa | C Africa | l | p | t | trop | Pal | c |
| EUD-C | Rosales | Urticaceae | Pilea nonggangensis Y.G. Wei, L.F. Fu and A.K. Monro | Asia | China | h | p | t | m, boreostrop | Hol, Pal | e |
| EUD-C | Rosales | Urticaceae | Pilea panzhihuaensis C.J. Chen, A.K. Monro and L. Chen | Asia | China | h | p | t | m, boreostrop | Hol, Pal | e |
| EUD-C | Rosales | Urticaceae | Pilea peltata Hance | Asia | China, Vietnam | h | p | t | m, boreostrop, trop | Hol, Pal | e |
| EUD-C | Rosales | Urticaceae | Pilea peperomioides Diels | Asia | China | h | p | t | m | Hol | e |
| EUD-C | Rosales | Rosaceae | Rubus peltatus Maxim. | Asia | China, Japan | h | p | t | sm | Hol | e |
| EUD-C | Saxifragales | Crassulaceae | Kalanchoe beharensis Drake | Africa | Madagascar | h | p | t | trop, austrostrop | Pal | e |
| EUD-C | Saxifragales | Crassulaceae | Kalanchoe nyikae Engl. | Africa | Kenya, Tanzania | h | p | t | trop | Pal | e |
| EUD-C | Saxifragales | Crassulaceae | Umbilicus botryoides Hochst. ex A.Rich. | Africa | Africa | h | p | t | boreostrop, trop | Pal | c |
| EUD-C | Saxifragales | Crassulaceae | Umbilicus horizontalis DC. | Africa, Europe | S Europe, N Africa | h | p | t | m | Hol | c |
| EUD-C | Saxifragales | Crassulaceae | Umbilicus luteus Webb and Berthel. | Europe | Mediterranean region | h | p | t | m, sm | Hol | c |
| EUD-C | Saxifragales | Crassulaceae | Umbilicus rupestris (Salisb.) Dandy | Africa, Europe | Europe, Africa | h | p | t | m, sm | Hol | c |
| EUD-C | Saxifragales | Saxifragaceae | Astilboides tabularis Engl. | Asia | China | h | p | t | sm, temp | Hol | c |
| EUD-C | Saxifragales | Saxifragaceae | Chrysosplenium peltatum Turcz. | Asia | Mongolia, Russia | h | p | t | t, sm, m | Hol | e |
| EUD-C | Saxifragales | Saxifragaceae | Darmera peltata (Torr.) Voss | North America | USA | h | p | t | m | Hol | c |
| EUD-C | Saxifragales | Saxifragaceae | Peltoboykinia tellimoides (Maxim.) H. Hara | Asia | China, Japan | h | p | t | t, sm, m | Hol, Pal | e |
| EUD-C | Saxifragales | Saxifragaceae | Peltoboykinia watanabei (Yatabe) H. Hara | Asia | Japan | h | p | t | t, sm, m | Hol | e |
| EUD-C | Saxifragales | Saxifragaceae | Rodgersia aesculifolia Batalin | Asia | China, Mongolia | h | p | t | sm, m, boreostrop | Hol, Pal | c |
| EUD-C | Saxifragales | Saxifragaceae | Rodgersia podophylla A.Gray | Asia | Japan, Korea | h | p | t | sm | Hol | c |
| EUD-C | Solanales | Convolvulaceae | Decalobanthus elmeri (Merr.) A.R. Simoes and Staples | Asia | Borneo | l | p | t | trop | Neo | e |
| EUD-C | Solanales | Convolvulaceae | Decalobanthus peltatus (L.) A.R. Simoes and Staples | Asia, Australia and Oceania | SE Asia, Australia, Madagascar | l | p | t | trop, austrostrop | Pal, Aus | e |
| EUD-C | Solanales | Solanaceae | Nothocestrum peltatum Skottsb. | Australia and Oceania | Hawaii | l | p | t | trop | Pal | e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wunnenberg, J.; Rjosk, A.; Neinhuis, C.; Lautenschläger, T. Strengthening Structures in the Petiole–Lamina Junction of Peltate Leaves. Biomimetics 2021, 6, 25. https://doi.org/10.3390/biomimetics6020025
Wunnenberg J, Rjosk A, Neinhuis C, Lautenschläger T. Strengthening Structures in the Petiole–Lamina Junction of Peltate Leaves. Biomimetics. 2021; 6(2):25. https://doi.org/10.3390/biomimetics6020025
Chicago/Turabian StyleWunnenberg, Julian, Annabell Rjosk, Christoph Neinhuis, and Thea Lautenschläger. 2021. "Strengthening Structures in the Petiole–Lamina Junction of Peltate Leaves" Biomimetics 6, no. 2: 25. https://doi.org/10.3390/biomimetics6020025
APA StyleWunnenberg, J., Rjosk, A., Neinhuis, C., & Lautenschläger, T. (2021). Strengthening Structures in the Petiole–Lamina Junction of Peltate Leaves. Biomimetics, 6(2), 25. https://doi.org/10.3390/biomimetics6020025






