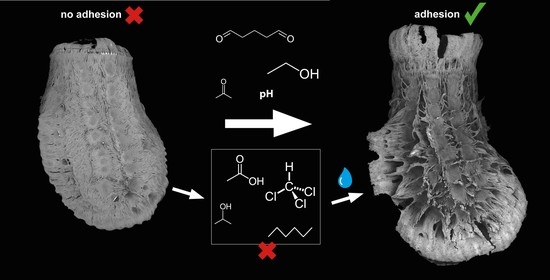
Multifunctional Adhesives on the Eggs of the Leaf Insect Phyllium philippinicum (Phasmatodea: Phylliidae): Solvent Influence and Biomimetic Implications
Abstract
1. Introduction
- Repetitive measurements of a sequence of attachment and detachment cycles;
- Comparative series of different pH ranges and different standard solvents (ethanol, acetone, chloroform, toluol, hexane, etc.);
- Repetitive measurement cycles of cross-observations between the solvent and water.
- Does the solvent influence pinnae reaction and glue liquefaction and consequently attachment performance?
- Do the attachment forces of eggs differ among solvent treatments?
- Do different solvents affect the interaction of the glue in its subsequent contact with water?
2. Materials and Methods
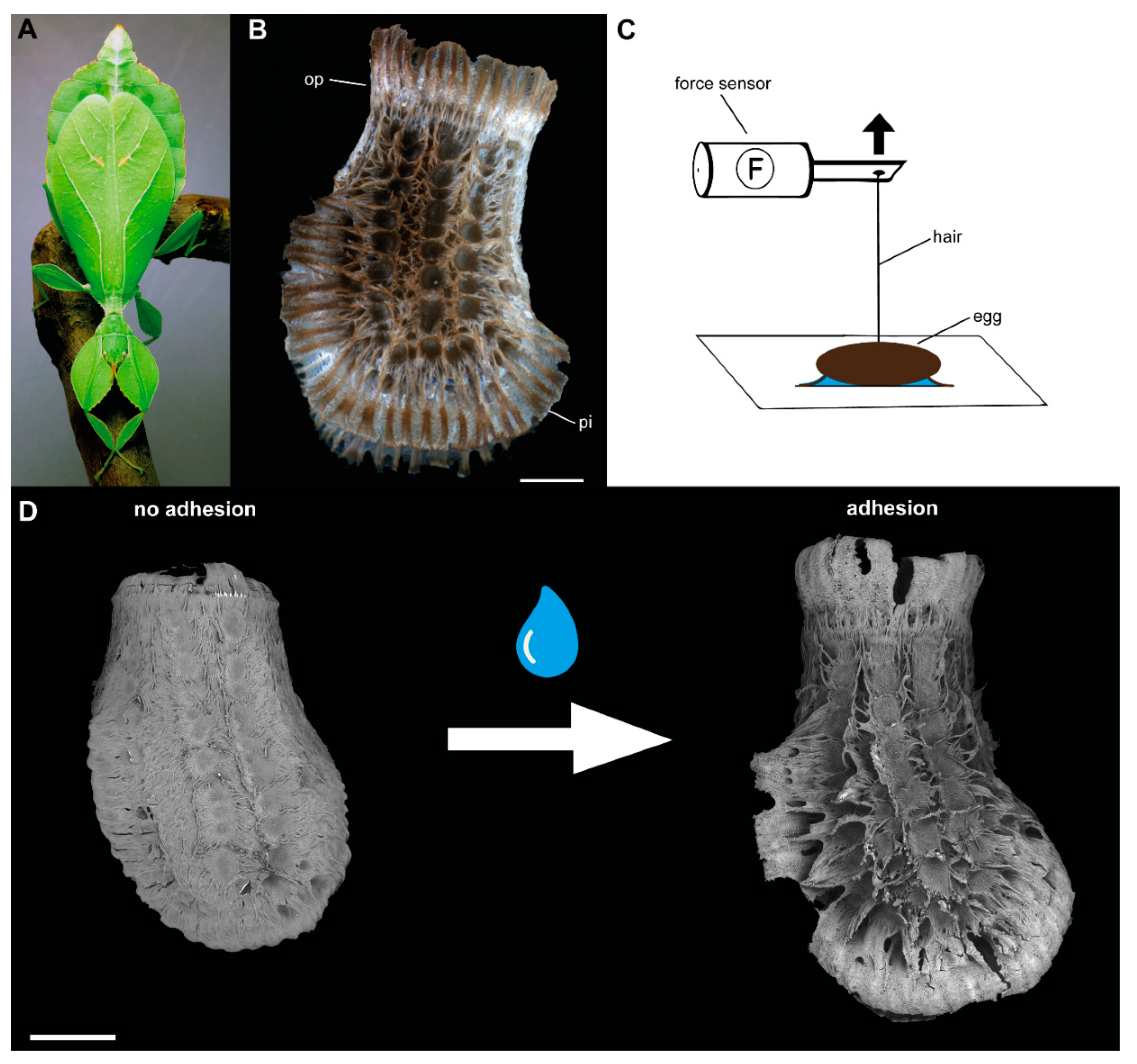
2.1. Specimens
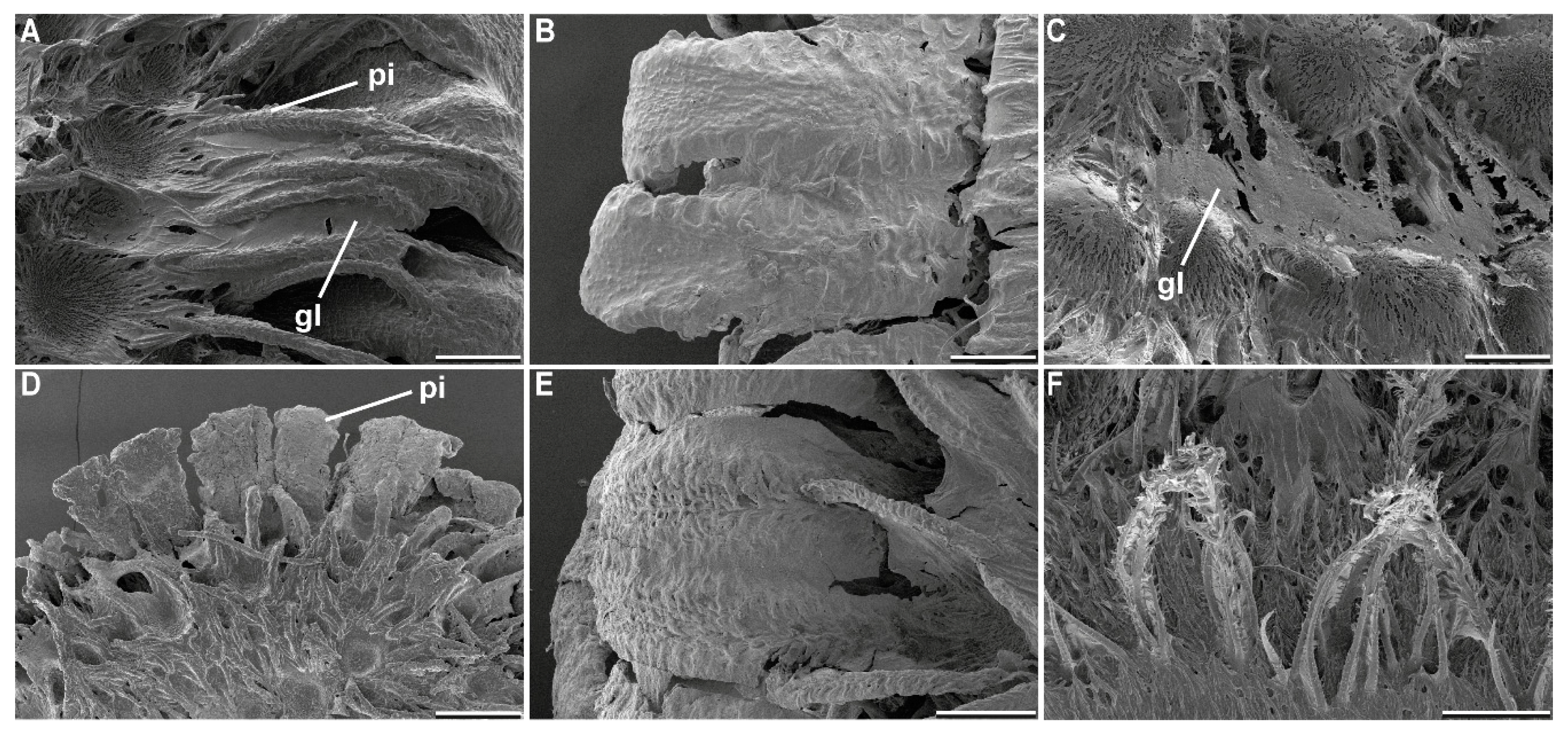
2.2. Morphology
2.3. Detachment Force Measurements
2.3.1. Sequential Detachment
2.3.2. Solvent Cross Treatment
2.4. Statistical Analysis
3. Results
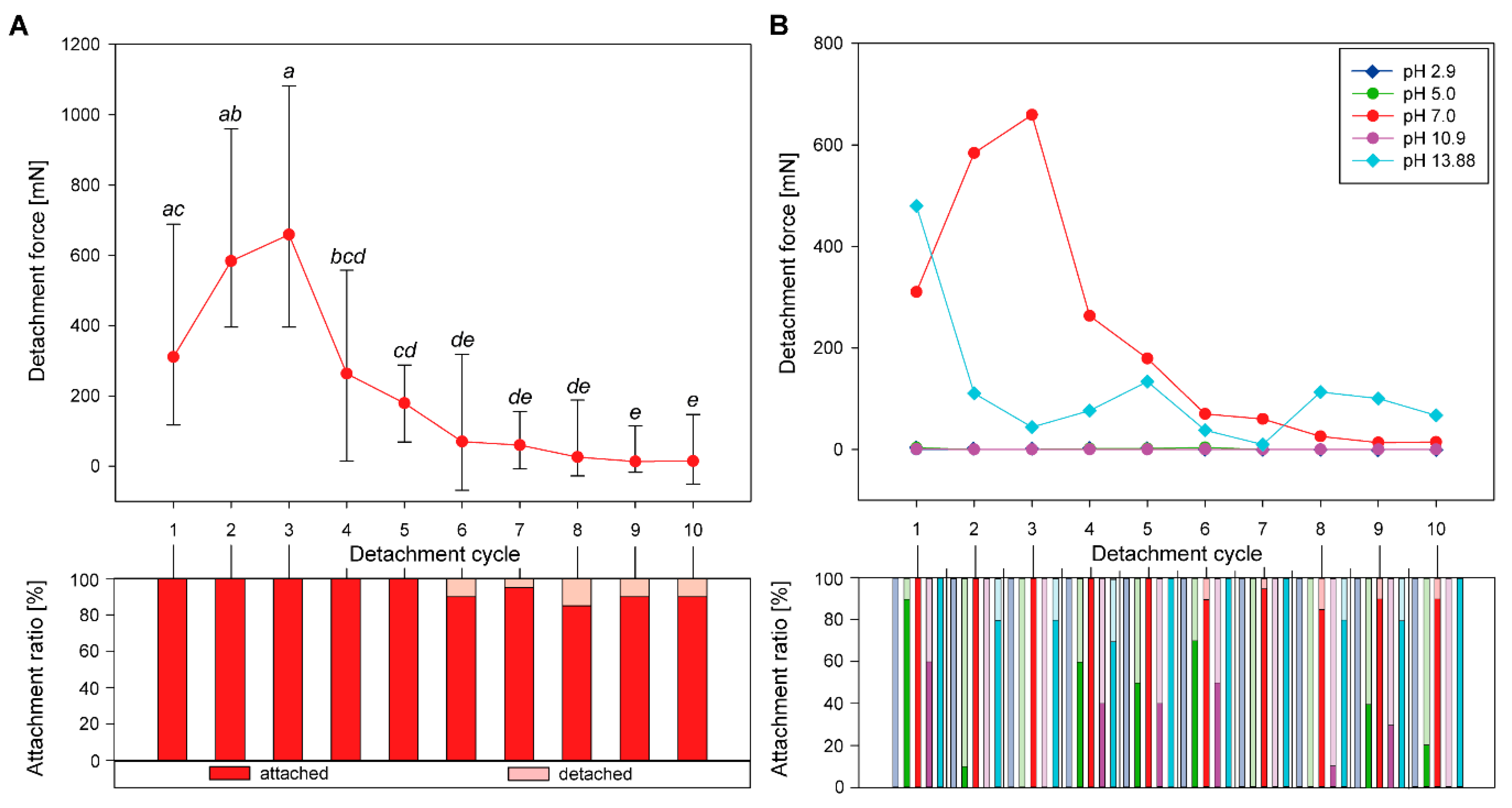
3.1. Sequential Attachment
3.1.1. Detachment Force Progression
3.1.2. Comparison of Initial Detachment Forces
3.1.3. Egg Responses to Solvents
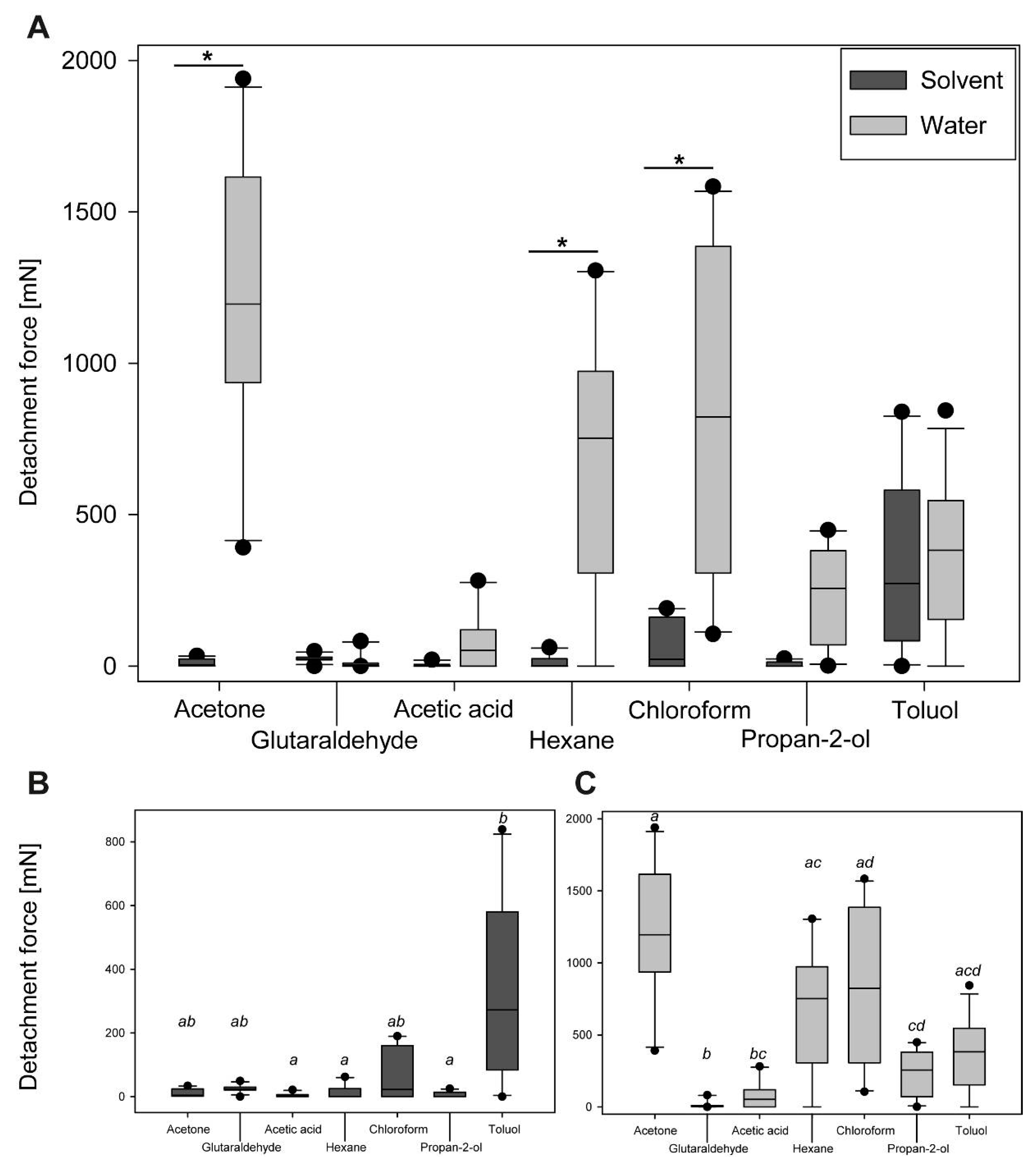
3.2. Sequential Solvent Cross-Treatment
3.2.1. Progression of Sequential Detachment Forces
3.2.2. Comparison of Maximum Detachment Forces
4. Discussion
4.1. Morphological Responses
4.2. Adhesion Performance during Sequential Testing
4.3. Adhesion Performance during Cross-Testing
4.4. Biomimetic Considerations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bedford, G.O. Biology and ecology of the Phasmatodea. Annu. Rev. Entomol. 1978, 23, 125–149. [Google Scholar] [CrossRef]
- Robertson, J.A.; Bradler, S.; Whiting, M.F. Evolution of oviposition techniques in stick and leaf insects (Phasmatodea). Front. Ecol. Evol. 2018, 6, 216. [Google Scholar] [CrossRef]
- Goldberg, J.; Bresseel, J.; Constant, J.; Kneubühler, B.; Leubner, F.; Michalik, P.; Bradler, S. Extreme convergence in egg-laying strategy across insect orders. Sci. Rep. 2015, 5, 7825. [Google Scholar] [CrossRef]
- Sellick, J. The range of egg capsule morphology within the phasmatodea and its relevance to the taxonomy of the order. Ital. J. Zool. 1997, 64, 97–104. [Google Scholar] [CrossRef]
- Sellick, J.T.C. Descriptive terminology of the phasmid egg capsule, with an extended key to the phasmid genera based on egg structure. Syst. Entomol. 1997, 22, 97–122. [Google Scholar] [CrossRef]
- Carlberg, U. A review of the different types of egglaying in the Phasmida in relation to the shape of the eggs and with a discussion on their taxonomic importance (Insecta). Biol. Zent. 1983, 102, 587–602. [Google Scholar]
- Büscher, T.H.; Gorb, S.N. Subdivision of the neotropical Prisopodinae Brunner von Wattenwyl, 1893 based on features of tarsal attachment pads (Insecta, Phasmatodea). ZooKeys 2017, 645, 1–11. [Google Scholar] [CrossRef]
- Büscher, T.H.; Buckley, T.R.; Grohmann, C.; Gorb, S.N.; Bradler, S. The evolution of tarsal adhesive microstructures in stick and leaf insects (Phasmatodea). Front. Ecol. Evol. 2018, 6, 69. [Google Scholar] [CrossRef]
- Büscher, T.H.; Kryuchkov, M.; Katanaev, V.L.; Gorb, S.N. Versatility of Turing patterns potentiates rapid evolution in tarsal attachment microstructures of stick and leaf insects (Phasmatodea). J. R. Soc. Interface 2018, 15, 20180281. [Google Scholar] [CrossRef]
- Büscher, T.H.; Grohmann, C.; Bradler, S.; Gorb, S.N. Tarsal attachment pads in Phasmatodea (Hexapoda: Insecta). Zoologica 2019, 164, 1–94. [Google Scholar]
- Hennemann, F.H.; Conle, O.V.; Gottardo, M.; Bresseel, J. On certain species of the genus Phyllium Illiger, 1798, with proposals for an intra-generic systematization and the descriptions of five new species from the Philippines and Palawan (Phasmatodea: Phylliidae: Phylliinae: Phylliini). Zootaxa 2009, 2322, 1–83. [Google Scholar] [CrossRef]
- Cumming, R.T.; Bank, S.; Le Tirant, S.; Bradler, S. Notes on the leaf insects of the genus Phyllium of Sumatra and Java, Indonesia, including the description of two new species with purple coxae (Phasmatodea, Phylliidae). ZooKeys 2020, 913, 89. [Google Scholar] [CrossRef]
- Cumming, R.T.; Le Tirant, S.; Teemsma, S.N.; Hennemann, F.H.; Willemse, L.; Büscher, T.H. Lost lovers linked at long last: Elusive female Nanophyllium mystery solved after a century of being placed in a different genus (Phasmatodea, Phylliidae). ZooKeys 2020, 969, 43–84. [Google Scholar] [CrossRef]
- Bradler, S.; Buckley, T.R. Biodiversity of Phasmatodea. In Insect Biodiversity: Science and Society II; Foottit, R.G., Adler, P.H., Eds.; Wiley: Hoboken, NJ, USA, 2018; pp. 281–313. [Google Scholar] [CrossRef]
- Cumming, R.T.; Leong, J.V.; Lohman, D.J. Leaf insects from Luzon, Philippines, with descriptions of four new species, the new genus Pseudomicrophyllium, and redescription of Phyllium (Phyllium) geryon Gray, 1843, (Phasmida: Phylliidae). Zootaxa 2017, 4365, 101–131. [Google Scholar] [CrossRef]
- Cumming, R.T. A new species of Phyllium (Phyllium) Illiger, 1798 from Mindanao, Philippines (Phasmida, Phylliidae). Zootaxa 2017, 4303, 297–300. [Google Scholar] [CrossRef]
- Cumming, R.T.; Le Tirant, S.; Hennemann, F.H. A new leaf insect from Obi Island (Wallacea, Indonesia) and description of a new subgenus within Phyllium Illiger, 1798 (Phasmatodea: Phylliidae: Phylliinae). Faunitaxys 2019, 7, 1–9. [Google Scholar]
- Van de Kamp, T.; Hennemann, F.H. A tiny new species of leaf insect (Phasmatodea, Phylliidae) from New Guinea. Zootaxa 2014, 3869, 397–408. [Google Scholar] [CrossRef]
- Wang, M.; Béthoux, O.; Bradler, S.; Jacques, F.M.B.; Cui, Y.; Ren, D. Under cover at pre-angiosperm times: A cloaked phasmatodean insect from the Early Cretaceous Jehol biota. PLoS ONE 2014, 9, e91290. [Google Scholar] [CrossRef]
- Bradler, S. The Phasmatodea Tree of Life: Surprising facts and open questions in the evolution of stick and leaf insects. Entomol. Heute 2015, 27, 1–23. [Google Scholar]
- O’Hanlon, J.C.; Jones, B.R.; Bulbert, M.W. The dynamic eggs of the Phasmatodea and their apparent convergence with plants. Sci. Nat. 2020, 107, 34. [Google Scholar] [CrossRef]
- Büscher, T.H.; Quigley, E.; Gorb, S.N. Adhesion Performance in the Eggs of the Philippine Leaf Insect Phyllium philippinicum (Phasmatodea: Phylliidae). Insects 2020, 11, 400. [Google Scholar] [CrossRef]
- Traveset, A.; Robertson, A.W.; Rodríguez-Pérez, J. A review on the role of endozoochory in seed germination. In Seed Dispersal: Theory and Its Application in a Changing World; Dennis, A.J., Schupp, E.W., Green, R.J., Westcott, D.A., Eds.; CABI: Wallingford, UK, 2007; pp. 78–103. [Google Scholar] [CrossRef]
- Kreitschitz, A.; Kovalev, A.E.; Gorb, S.N. Slipping vs sticking: Water-dependent adhesive and frictional properties of Linum usitatissimum L. seed mucilaginous envelope and its biological significance. Acta Biomater. 2015, 17, 152–159. [Google Scholar] [CrossRef]
- Shelomi, M. Phasmid eggs do not survive digestion by Quails and Chickens. J. Orthoptera Res. 2011, 20, 159–162. [Google Scholar] [CrossRef]
- Suetsugu, K.; Funaki, S.; Takahashi, A.; Ito, K.; Yokoyama, T. Potential role of bird predation in the dispersal of otherwise flightless stick insects. Ecology 2018, 99, 1504–1506. [Google Scholar] [CrossRef]
- Wang, C.-H.; Chu, Y.-I. The morphological study of the egg shell of the Tsuda’s giant stick insect Megacrania alpheus Westwood. NTU Phytopathol. Entomol. 1982, 9, 98–109. [Google Scholar]
- Ushirokita, M. Eggs of stick insect drifting in the wake of screw pine’s seed. Insectarium 1998, 35, 108–115. [Google Scholar]
- Kobayashi, S.; Usui, R.; Nomoto, K.; Ushirokita, M.; Denda, T.; Izawa, M. Does egg dispersal occur via the ocean in the stick insect Megacrania tsudai (Phasmida: Phasmatidae)? Ecol. Res. 2014, 29, 1025–1032. [Google Scholar] [CrossRef]
- Kobayashi, S.; Usui, R.; Nomoto, K.; Ushirokita, M.; Denda, T.; Izawa, M. Population dynamics and the effects of temperature on the eggs of the seawater-dispersed stick insect Megacrania tsudai (Phasmida: Phasmatidae). Zool. Stud. 2016, 55, 20. [Google Scholar] [CrossRef]
- Stanton, A.O.; Dias, D.A.; O’Hanlon, J.C. Egg dispersal in the Phasmatodea: Convergence in chemical signalling strategies between plants and animals? J. Chem. Ecol. 2015, 41, 689–695. [Google Scholar] [CrossRef]
- Compton, S.G.; Ware, A.B. Ants disperse the elaiosome-bearing eggs of an african stick insect. Psyche 1991, 98, 207–213. [Google Scholar] [CrossRef]
- Hughes, L.; Westoby, M. Capitula on stick insects and elaiosomes on seeds: Convergent adaptations for burial by ants. Funct. Ecol. 1992, 6, 642–648. [Google Scholar] [CrossRef]
- Windsor, D.M.; Trapnell, D.W.; Amat, G. The egg capitulum of a Neotropical walkingstick, Calynda biscuspis, induces aboveground egg dispersal by the ponerine ant, Ectatomma ruidum. J. Inst. Behav. 1996, 9, 353–367. [Google Scholar] [CrossRef]
- Kreitschitz, A.; Kovalev, A.; Gorb, S.N. “Sticky invasion”–the physical properties of Plantago lanceolata L. seed mucilage. Beilstein J. Nanotechnol. 2016, 7, 1918–1927. [Google Scholar] [CrossRef]
- Kreitschitz, A.; Gorb, S.N. How does the cell wall ‘stick’in the mucilage? A detailed microstructural analysis of the seed coat mucilaginous cell wall. Flora 2017, 229, 9–22. [Google Scholar] [CrossRef]
- Kreitschitz, A.; Gorb, S.N. The micro- and nanoscale spatial architecture of the seed mucilage—Comparative study of selected plant species. PLoS ONE 2018, 13, e0200522. [Google Scholar] [CrossRef]
- Grubert, M. Studies on the distribution of myxospermy among seeds and fruits of Angiospermae and its ecological importance. Acta Biol. Venez. 1974, 8, 315–551. [Google Scholar]
- Ryding, O. Myxocarpy in the Nepetoideae (Lamiaceae) with notes on myxodiaspory in general. Syst. Geogr. Plants 2001, 71, 503–514. [Google Scholar] [CrossRef]
- Yang, X.; Baskin, J.M.; Baskin, C.C.; Huang, Z. More than just a coating: Ecological importance, taxonomic occurrence and phylogenetic relationships of seed coat mucilage. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 434–442. [Google Scholar] [CrossRef]
- Al Bitar, L.; Gorb, S.N.; Zebitz, C.P.W.; Voigt, D. Egg adhesion of the codling moth Cydia pomonella L. (Lepidoptera, Tortricidae) to various substrates: I. Leaf surfaces of different apple cultivars. Arthropod-Plant Interact. 2012, 6, 471–488. [Google Scholar] [CrossRef]
- Al Bitar, L.; Gorb, S.N.; Zebitz, C.P.W.; Voigt, D. Egg adhesion of the codling moth Cydia pomonella L. (Lepidoptera, Tortricidae) to various substrates: II. Fruit surfaces of different apple cultivars. Arthropod-Plant Interact. 2014, 8, 57–77. [Google Scholar] [CrossRef]
- Voigt, D.; Gorb, S.N. Egg attachment of the asparagus beetle Crioceris asparagi to the crystalline waxy surface of Asparagus officinalis. Proc. R. Soc. B Biol. Sci. 2010, 277, 895–903. [Google Scholar] [CrossRef]
- Fordyce, J.A.; Nice, C.C. Variation in butterfly egg adhesion: Adaptation to local host plant senescence characteristics? Ecol. Lett. 2003, 6, 23–27. [Google Scholar] [CrossRef]
- England, M.W.; Sato, T.; Yagihashi, M.; Hozumi, A.; Gorb, S.N.; Gorb, E.V. Surface roughness rather than surface chemistry essentially affects insect adhesion. Beilstein J. Nanotechnol. 2016, 7, 1471–1479. [Google Scholar] [CrossRef]
- Cogley, T.P.; Anderson, J.R.; Weintraub, J. Ultrastructure and function of the attachment organ of warble fly eggs (Diptera: Oestridae: Hypodermatinae). Int. J. Insect Morphol. Embryol. 1981, 10, 7–18. [Google Scholar] [CrossRef]
- Cogley, T.P.; Cogley, M.C. Morphology of the eggs of the human bot fly, Dermatobia hominis (L. Jr.) (Diptera: Cuterebridae) and their adherence to the transport carrier. Int. J. Insect Morph. Embryol. 1989, 18, 239–248. [Google Scholar] [CrossRef]
- Hinton, H.E. Biology of Insect Eggs; Pergamon Press: Oxford, UK, 1981. [Google Scholar] [CrossRef]
- Margaritis, L.H. Structure and physiology of eggshell. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: Oxford, UK, 1985; pp. 153–230. [Google Scholar]
- Miller, P.L. Oviposition behaviour and eggshell structure in some libellulid dragonflies, with particular reference to Brachythemis lacustris (Kirby) and Orthetrum coerulescens (Fabricius)(Anisoptera). Odonatologica 1987, 16, 361–374. [Google Scholar]
- Ivey, R.K.; Bailey, J.C.; Stark, B.P.; Lentz, D.L. A preliminary report of egg chorion features in dragonflies (Anisoptera). Odonatologica 1988, 17, 393–399. [Google Scholar]
- Trueman, J.W.H. Egg chorionic structures in Corduliidae and Libellulidae (Anisoptera). Odonatologica 1991, 20, 441–452. [Google Scholar]
- Sahlén, G. Ultrastructure of the eggshell and micropylar apparatus in Somatochlora metallica (Vander L.), Orthetrum cancellatum (L.) and Sympetrum sanguineum (Müll.) (Anisoptera: Corduliidae, Libellulidae). Odonatologica 1994, 23, 255–269. [Google Scholar]
- Sahlén, G. Eggshell ultrastructure in Onychogomphus forcipatus unguiculatus (Vander Linden) (Odonata: Gomphidae). Int. J. Insect Morphol. Embryol. 1995, 24, 281–286. [Google Scholar] [CrossRef]
- Andrew, R.J.; Tembhare, D.B. Ultrastructural post-oviposition changes in the egg chorion of the dragon-fly, Zyxomma petiolatum Rambur (Odonata: Libellulidae). Int. J. Insect Morphol. Embryol. 1995, 24, 235–238. [Google Scholar] [CrossRef]
- Andrew, R.J.; Tembhare, D.B. Surface ultrastructure of the egg chorion of Bradinopyga geminata (Rambur) and Rhyothemis variegata variegata (Linn.). Fraseria 1996, 3, 1–5. [Google Scholar]
- Andrew, R.J. Egg chorionic ultrastructure of the dragonfly Tramea virginia (Rambur) (Anisoptera: Libellulidae). Odonatologica 2002, 31, 171–175. [Google Scholar]
- Gaino, E.; Piersanti, S.; Rebora, M. Egg envelope synthesis and chorion modification after oviposition in the dragonfly Libellula depressa (Odonata, Libellulidae). Tissue Cell 2008, 40, 317–324. [Google Scholar] [CrossRef]
- Gaino, E.; Rebora, M. Synthesis and function of the fibrous layers covering the eggs of Siphlonurus lacustris (Ephemeroptera, Siphlonuridae). Acta Zool. 2001, 82, 41–48. [Google Scholar] [CrossRef]
- Li, D.; Huson, M.G.; Graham, L.D. Proteinaceous adhesive secretions from insects; in particular the egg attachment glue of Opodiphthera sp. moths. Arch. Insect Biochem. 2008, 69, 85–105. [Google Scholar] [CrossRef]
- Yoshida, K.; Nagata, M. Adhesive strength of the glue substances in the colleterial glands of the silkmoth, Bombyx mori. J. Seric. Sci. Jpn. 1997, 66, 453–456. [Google Scholar] [CrossRef]
- Yago, M.; Mitamura, T.; Abe, S.; Hashimoto, S. Adhesive strength of glue-like substances from the colleterial glands of Antheraea yamamai and Rhodinia fugax. Int. J. Wild Silkmoth Silk 2001, 6, 11–15. [Google Scholar]
- Beament, J.W.L.; Lal, R. Penetration through the Egg-shell of Pieris brassicae (L.). Bull. Entomol. Res. 1957, 48, 109–125. [Google Scholar] [CrossRef]
- Riley, R.C.; Forgash, A.J. Drosophila melanogaster eggshell adhesive. J. Insect Physiol. 1967, 13, 509–517. [Google Scholar] [CrossRef]
- Amornsak, W.; Noda, T.; Yamashita, O. Accumulation of glue proteins in the developing colleterial glands of the silkworm, Bombyx mori. J. Seric. Sci. Jpn. 1992, 61, 123–130. [Google Scholar] [CrossRef]
- Burkhart, C.N.; Stankiewicz, B.A.; Pchalek, I.; Kruge, M.A.; Burkhart, C.G. Molecular composition of the louse sheath. J. Parasitol. 1999, 85, 559–561. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, Y.; Jiang, Y.; Xu, M. Proteome analysis of the silkworm (Bombyx mori L.) colleterial gland during different development stages. Arch. Insect Biochem. Physiol. 2006, 61, 42–50. [Google Scholar] [CrossRef]
- Betz, O. Adhesive Exocrine Glands in Insects: Morphology, Ultrastructure; Adhesive Secretion. In Biological Adhesive Systems; von Byern, J., Grunwald, I., Eds.; Springer: Vienna, Austria, 2010; pp. 111–152. [Google Scholar] [CrossRef]
- Burgess, I.F. Do nit removal formulations and other treatments loosen head louse eggs and nits from hair? Med. Vet. Entomol. 2010, 24, 55–61. [Google Scholar] [CrossRef]
- Graham, L.D. Biological adhesives from nature. In Encyclopedia of Biomaterials and Biomedical Engineering; Wnek, G.E., Bowlin, G.L., Eds.; Taylor & Francis, CRC Press: Abingdon, UK, 2008; pp. 236–253. [Google Scholar]
- Dalziel, M.; Crispin, M.; Scanlan, C.N.; Zitzmann, N.; Dwek, R.A. Emerging principles for the therapeutic exploitation of glycosylation. Science 2014, 343, 1235681. [Google Scholar] [CrossRef] [PubMed]
- Subramani, K.; Ahmed, W. Self-assembly of proteins and peptides and their applications in bionanotechnology and dentistry. In Emerging Nanotechnologies in Dentistry 2; Elsevier: Amsterdam, The Netherlands, 2018; pp. 231–249. [Google Scholar] [CrossRef]
- Xi, E.; Venkateshwaran, V.; Li, L.; Rego, N.; Patel, A.J.; Garde, S. Hydrophobicity of proteins and nanostructured solutes is governed by topographical and chemical context. Proc. Natl. Acad. Sci. USA 2017, 114, 13345–13350. [Google Scholar] [CrossRef]
- Cheremisinoff, N.P. Industrial Solvents Handbook, Revised and Expanded; CRC Press: Boca Raton, FL, USA, 2003; ISBN 978-0824740337. [Google Scholar]
- Gorb, S.N. Biological attachment devices: Exploring nature’s diversity for biomimetics. Philos. Trans. R. Soc. A 2008, 366, 1557–1574. [Google Scholar] [CrossRef]
- Meng, H.; Liu, Y.; Cencer, M.M.; Lee, B.P. Adhesives and coatings inspired by mussel adhesive proteins. In Bioadhesion and Biomimetics: From Nature to Applications; Bianco-Peled, H., Davidovich-Pinhas, M., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 131–166. [Google Scholar]
- Cerullo, A.R.; Lai, T.Y.; Allam, B.; Baer, A.; Barnes, W.J.P.; Barrientos, Z.; Deheyn, D.D.; Fudge, D.S.; Gould, J.; Harrington, M.J.; et al. Comparative Animal Mucomics: Inspiration for Functional Materials from Ubiquitous and Understudied Biopolymers. ACS. Biomater. Sci. Eng. 2020, 6, 5377–5398. [Google Scholar] [CrossRef]
- Büscher, T.H.; Gorb, S.N. Complementary effect of attachment devices in stick insects (Phasmatodea). J. Exp. Biol. 2019, 222, jeb209833. [Google Scholar] [CrossRef]
- Büscher, T.H.; Becker, M.; Gorb, S.N. Attachment performance of stick insects (Phasmatodea) on convex substrates. J. Exp. Biol. 2020, 223, jeb226514. [Google Scholar] [CrossRef]
- Corbet, P.S. Dragonflies: Behaviour and Ecology of Odonata; Harley Books: Colchester, UK, 1999; ISBN 978-0946589777. [Google Scholar]
- Vincent, J.F.V.; Hillerton, J.E. The tanning of insect cuticle—a critical review and a revised mechanism. J. Insect Physiol. 1979, 25, 653–658. [Google Scholar] [CrossRef]
- Vincent, J.F.; Ablett, S. Hydration and tanning in insect cuticle. J. Insect Physiol. 1987, 33, 973–979. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry, 4th ed.; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar] [CrossRef]
- Habeeb, A.F.S.A.; Hiramoto, R. Reaction of proteins with glutaraldehyde. Arch. Biochem. Biophys. 1968, 126, 16–26. [Google Scholar] [CrossRef]
- Bjerke, J.M.; Freeman, T.P.; Anderson, A.W. A new method of preparing insects for scanning electron microscopy. Stain Technol. 1979, 54, 29–31. [Google Scholar] [CrossRef]
- Brini, E.; Fennell, C.J.; Fernandez-Serra, M.; Hribar-Lee, B.; Lukšič, M.; Dill, K.A. How water’s properties are encoded in its molecular structure and energies. Chem. Rev. 2017, 117, 12385–12414. [Google Scholar] [CrossRef]
- Talley, K.; Alexov, E. On the pH-optimum of activity and stability of proteins. Proteins 2010, 78, 2699–2706. [Google Scholar] [CrossRef]
- Santos, R.; Gorb, S.N.; Jamar, V.; Flammang, P. Adhesion of echinoderm tube feet to rough surfaces. J. Exp. Biol. 2005, 208, 2555–2567. [Google Scholar] [CrossRef]
- Scherge, M.; Gorb, S.N. Biological Micro- and Nanotribology; Springer: Heidelberg/Berlin, Germany, 2001; ISBN 3-540-41188-7. [Google Scholar]
- O’Brien, E.P.; Brooks, B.R.; Thirumalai, D. Effects of pH on proteins: Predictions for ensemble and single-molecule pulling experiments. J. Am. Chem. Soc. 2012, 134, 979–987. [Google Scholar] [CrossRef]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena; Wiley: Hoboken, NJ, USA, 2012; ISBN 978-0-470-54194-4. [Google Scholar]
- Habenicht, G. Kleben: Grundlagen, Technologien, Anwendung, 4th ed.; Springer: Berlin, Germany, 2002; ISBN 978-3-662-08089-4. [Google Scholar]
- Carlberg, U.; Lindquist, B.A.R.; Palmheden, A.-K. Mineral contents of the egg shell of stick insects (Phasmida). Zool. Anz. 1982, 208, 68–73. [Google Scholar]
- Van de Kamp, T.; Greven, H. Structure of the specialised and unspecialised chorion of the egg in the stick insect Malacomorpha cyllarum (Phasmatodea). Entomol. Gen. 2008, 31, 64–74. [Google Scholar] [CrossRef]
- Malhi, Y.; Wright, J. Spatial patterns and recent trends in the climate of tropical rainforest regions. Philos. Trans. R. Soc. B 2004, 359, 311–329. [Google Scholar] [CrossRef]
- Brock, P.D.; Büscher, T.H.; Baker, E. SF Phasmida: Phasmida Species File (version 5.0, Jun 2018). In Species 2000 & ITIS Catalogue of Life, 2020-09-01 Beta; Roskov, Y., Ower, G., Orrell, T., Nicolson, D., Bailly, N., Kirk, P.M., Bourgoin, T., DeWalt, R.E., Decock, W., Nieukerken, E., et al., Eds.; Species 2000; Naturalis: Leiden, The Netherlands, 2020; ISBN 2405-8858. [Google Scholar]
- Hennemann, O.-D. Kleben von Kunststoffen Anwendung, Ausbildung. Trend. Kunststoffe 2000, 90, 184–188. [Google Scholar]
- Jain, D.; Sahni, V.; Dhinojwala, A. Synthetic adhesive attachment discs inspired by spider’s pyriform silk architecture. J. Polym. Sci. B Polym. Phys. 2014, 52, 553–560. [Google Scholar] [CrossRef]
- Wang, L.; Culha, U.; Iida, F. A dragline-forming mobile robot inspired by spiders. Bioinspir. Biomim. 2014, 9, 016006. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.O. Structural Effects of Glue Application in Spiders—What Can We Learn from Silk Anchors? In Bio-inspired Structured Adhesives. Biologically-Inspired Systems, Vol. 9; Heepe, L., Xue, L., Gorb, S.N., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Krohmer, S.D.; Wenning, D.L. Glue Brush. U.S. Patent USD776939S1, 24 January 2017. [Google Scholar]
- Shao, H.; Stewart, R.J. Biomimetic underwater adhesives with environmentally triggered setting mechanisms. Adv. Mater. 2010, 22, 729–733. [Google Scholar] [CrossRef]
- Borne, F.; Kovalev, A.E.; Gorb, S.N.; Courtier-Orgogozo, V. The glue produced by Drosophila melanogaster for pupa adhesion is universal. J. Exp. Biol. 2020, 223, jeb220608. [Google Scholar] [CrossRef]
- Lee, B.P.; Messersmith, P.B.; Israelachvili, J.N.; Waite, J.H. Mussel-inspired adhesives and coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef]
- Karak, N. 15—Biopolymers for paints and surface coatings. In Biopolymers and Biotech Admixtures for Eco-Efficient Construction Materials; Pacheco-Torgal, F., Ivanov, V., Karak, N., Jonkers, H., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 333–368. [Google Scholar] [CrossRef]




| Solvent | Solvent Group | Cycles | Sequential (neggs, Weight 1) | Cross (neggs, Weight 1) |
|---|---|---|---|---|
| Acetic acid (pH 2.9) | pH (protic) | 10/8 2 | 10, 13.45 ± 1.03 | 10, 13.00 ± 1.77 |
| pH 5.0 3 | pH (protic) | 10 | 10, 13.5 ± 1.0 | no |
| pH 7.0 3 | pH (protic) | 10 | 20, 14.45 ± 1.14 | no |
| pH 10.9 3 | pH | 10 | 10, 14.25 ± 1.12 | no |
| KOH (pH 13.9) | pH | 10 | 10, 13.70 ± 0.79 | no |
| Acetone | polar-aprotic | 10/6 2 | 10, 13.95 ± 0.89 | 10, 14.35 ± 0.93 |
| Chloroform | nonpolar-aprotic | 10/6 2 | no | 10, 14.70 ± 0.92 |
| Ethanol | polar-protic | 10 | 10, 14.30 ± 1.14 | no |
| Glutaraldehyde (25%) 4 | fixiative | 2 | no | 12, 13.85 ± 0.95 |
| Hexane | nonpolar-aprotic | 6 | no | 10, 13.45 ± 0.89 |
| Propan-2-ol | polar-protic | 8 | no | 10, 13.45 ± 1.18 |
| Toluol | nonpolar-aprotic | 6 | no | 12, 15.30 ± 1.88 |
| Solvent | Pinnae Expansion Behavior 1 | Glue Behaviour 2 | Initial Detachment Force (mN, Median ± s.d.) | Effect of Replication | Effect of H2O Cross Treatment |
|---|---|---|---|---|---|
| Acetic acid (pH 2.9) | expansion | denaturated | - | no change | activation |
| pH 5.0 | expansion | denaturated | 3.18 ± 9.39 | de-, then increase | n.a. |
| pH 7.0 | expansion | liquefied | 310.48 ± 278.33 | in-, then decrease | n.a. |
| pH 10.9 | inhibited 3 | denaturated | 0.477 ± 3.67 | no change | n.a. |
| KOH (pH 13.9) | expansion | denaturated | 479.78 ± 310.43 | decrease | n.a. |
| Acetone | expansion | solved | 1.80 ± 0.69 | de-, then increase | activation |
| Chloroform | inhibited 3 | solved | - | no adhesion | activation |
| Ethanol | moderate expansion | liquefied | 1.80 ± 52.74 | increase | n.a. |
| Glutaraldehyde | inhibited 3 | no change | 23.43 ± 11.40 | n.a. | activation |
| Hexane | inhibited 3 | no change | - | no adhesion | activation |
| Propan-2-ol | inhibited 3 | no change | - | no adhesion | activation |
| Toluol | inhibited 3 | solved | - | no adhesion | initiation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Büscher, T.H.; Lohar, R.; Kaul, M.-C.; Gorb, S.N. Multifunctional Adhesives on the Eggs of the Leaf Insect Phyllium philippinicum (Phasmatodea: Phylliidae): Solvent Influence and Biomimetic Implications. Biomimetics 2020, 5, 66. https://doi.org/10.3390/biomimetics5040066
Büscher TH, Lohar R, Kaul M-C, Gorb SN. Multifunctional Adhesives on the Eggs of the Leaf Insect Phyllium philippinicum (Phasmatodea: Phylliidae): Solvent Influence and Biomimetic Implications. Biomimetics. 2020; 5(4):66. https://doi.org/10.3390/biomimetics5040066
Chicago/Turabian StyleBüscher, Thies H., Raunak Lohar, Marie-Christin Kaul, and Stanislav N. Gorb. 2020. "Multifunctional Adhesives on the Eggs of the Leaf Insect Phyllium philippinicum (Phasmatodea: Phylliidae): Solvent Influence and Biomimetic Implications" Biomimetics 5, no. 4: 66. https://doi.org/10.3390/biomimetics5040066
APA StyleBüscher, T. H., Lohar, R., Kaul, M.-C., & Gorb, S. N. (2020). Multifunctional Adhesives on the Eggs of the Leaf Insect Phyllium philippinicum (Phasmatodea: Phylliidae): Solvent Influence and Biomimetic Implications. Biomimetics, 5(4), 66. https://doi.org/10.3390/biomimetics5040066