Stannous Fluoride Effects on Enamel: A Systematic Review
Abstract
1. Introduction
1.1. Rationale
- Stannous fluoride (SnF2);
- Sodium fluoride (NaF);
- Sodium monofluorophosphate (Na2PFO3 or SMFP).
1.2. Objectives
- In dental patients, what is the effect of stannous fluoride compositions on oral health compared to other dental healthcare products?
- On enamel and other hard tooth tissue, what is the effect of stannous fluoride composition on their structure compared to other dental healthcare products?
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
- Study about stannous fluoride dentifrice/toothpaste/mouth rinse.
- Study of patient side effects of stannous fluoride.
- Study about stannous fluoride chemo-physical interaction.
- Clinical studies on stannous fluoride use and control groups.
- Articles published in the last 10 years.
- Studies involving subjects with other specific diseases, immunological disorders, oncological patients, osteoporosis, and genetic diseases.
- Not enough information regarding the selected topic.
- No access to the title and abstract in English language or letters, commentary, PhD thesis and editorials.
- Not Randomized Controlled Trial (RCT) studies.
2.3. Information Sources
2.4. Search
2.5. Study Selection
2.6. Data Collection Process
2.7. Data Items
2.8. Risk of Bias in Individual Studies
2.9. Summary Measures
- Authors and Year—Authors and year of publication
- Sample size—Information about sample size and sample type
- Groups—Information about number of groups and type of group (each group is separate by “vs.”)
- Time and/or Follow up—Information about timing and follow up of the study
- Main results—Main outcomes and results of the analyzed study
- Statistic results—Statistical results (if performed)
- Main outcome—Classifications of the outcomes obtained by search
- N. of results—Number of obtained results in that outcome set
- Authors and Year—Authors and year of publication
- Sample—Information about sample size and sample type
- Measured at—Obtaining date of the median value
- Mean value—Mean value of groups (SnF2 group first vs. other)
2.10. Risk of Bias Across Studies
2.11. Additional Analyses
3. Results
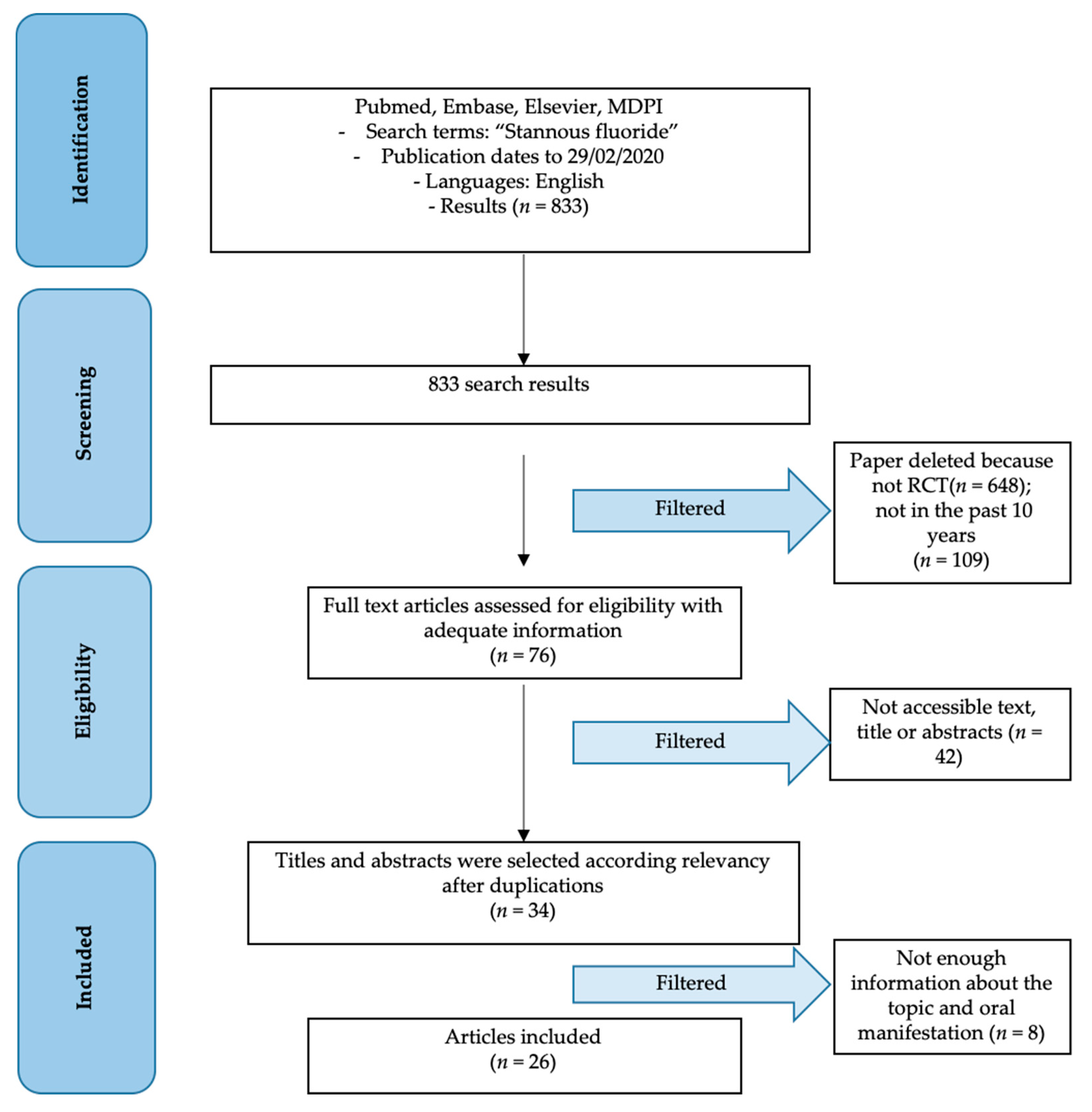
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias within Studies
3.4. Results of Individual Studies
3.5. Additional Analysis
3.6. Risk of Bias Across Studies
4. Discussion
4.1. Summary of Evidence
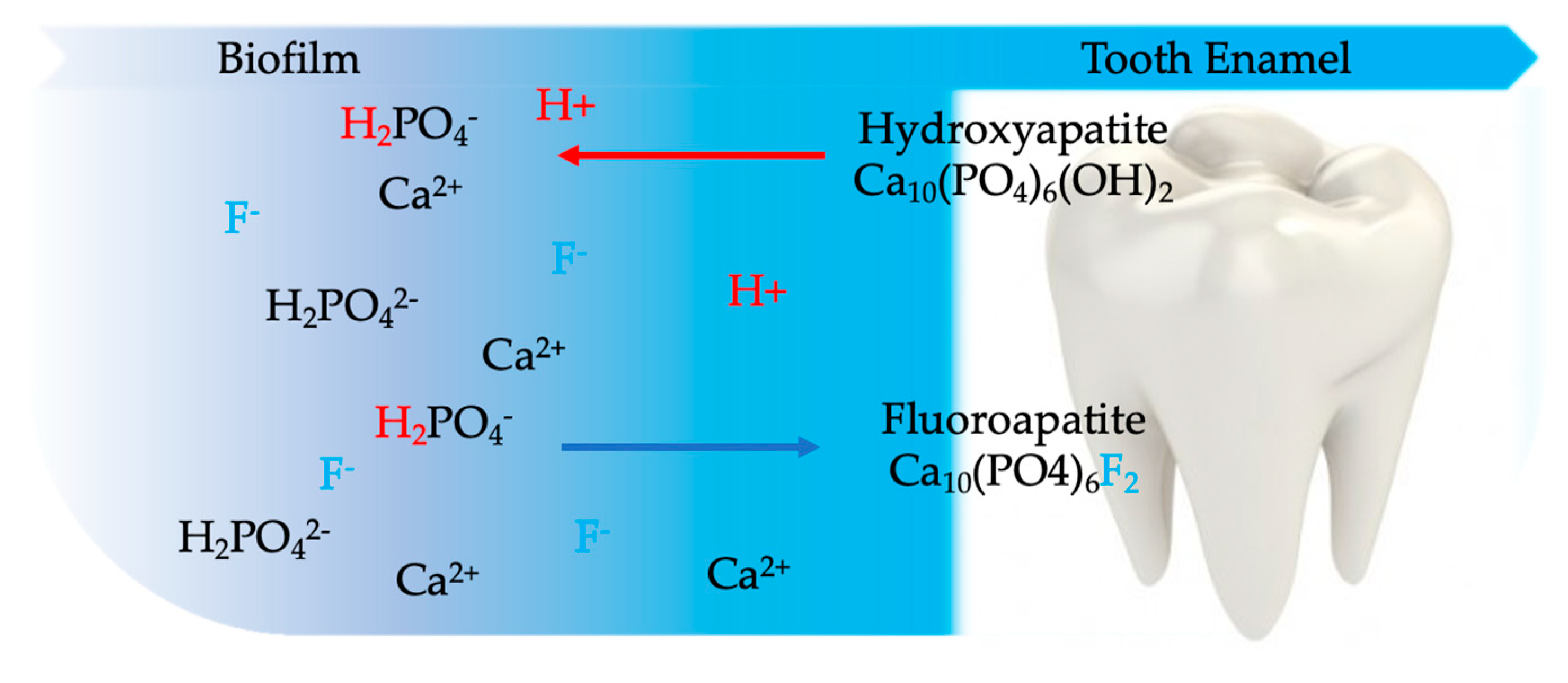
4.1.1. Hard Tissue Effects of Stannous Fluoride Compounds
4.1.2. Biological and Plaque Effects of Stannous Fluoride Compounds
4.1.3. Oral Health Related Quality of Life Effects of Stannous Fluoride Compounds
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Diesendorf, M. The mystery of declining tooth decay. Nature 1986, 322, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C. Fluoride given the all clear. Nature 1991, 349, 732. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jones, D. Dentistry’s Leading Edge. Available online: https://web.archive.org/web/20100826062103/http://www.drdavidjones.com/qa.htm (accessed on 9 August 2020).
- Horowitz, H.S. Proper use of fluoride products in fluoridated communities. Lancet 1999, 353, 1462. [Google Scholar] [CrossRef]
- Hargreaves, J.A. Fluoride toothpaste. Lancet 1971, 2, 929. [Google Scholar] [CrossRef]
- Dahl, A.R.; Hodgson, E. Complexes of stannous fluoride and other group IVB dihalides with mammalian hemoproteins. Science 1977, 197, 1376–1378. [Google Scholar] [CrossRef]
- Dental Association: Toothpaste “Recognition” Subject of Controversy. Science 1961, 134, 1349. [CrossRef]
- Šket, T.; Kukec, A.; Kosem, R.; Artnik, B. The history of public health use of fluorides in caries prevention. Zdr Varst 2017, 56, 140–146. [Google Scholar] [CrossRef]
- Horowitz, H.S. Fluoride and enamel defects. Adv. Dent. Res. 1989, 3, 143–146. [Google Scholar] [CrossRef]
- Cury, J.A.; Ricomini-Filho, A.P.; Berti, F.L.P.; Tabchoury, C.P. Systemic Effects (Risks) of Water Fluoridation. Braz. Dent. J. 2019, 30, 421–428. [Google Scholar] [CrossRef]
- Arora, S.; Kumar, J.V.; Moss, M.E. Does water fluoridation affect the prevalence of enamel fluorosis differently among racial and ethnic groups? J. Public Health Dent. 2018, 78, 95–99. [Google Scholar] [CrossRef]
- Oliveira, M.R.C.; Oliveira, P.H.C.; Oliveira, L.H.C.; Horliana, A.; César, P.F.; Moura, S.K.; Bussadori, S.K. Microhardness of bovine enamel after different fluoride application protocols. Dent. Mater. J. 2019, 38, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.C.; Wiegand, A.; Rios, D.; Buzalaf, M.A.R.; Lussi, A. Fluoride in dental erosion. Monogr. Oral Sci. 2011, 22, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Limeback, H. Enamel formation and the effects of fluoride. Community Dent. Oral Epidemiol. 1994, 22, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Kalsbeek, H. Series: Caries prevention in historical perspective. Fluoride. Ned. Tijdschr. Tandheelkd. 2018, 125, 257–261. [Google Scholar] [CrossRef]
- Koenigs, P.M.; Faller, R.V. Fluoride’s Mechanism of Action. Available online: https://www.dentalcare.com/en-us/professional-education/ce-courses/ce410/fluoride-s-mechanism-of-action (accessed on 11 February 2020).
- Riva, M.A. Advertising: Giacomo Puccini in dental history. Br. Dent. J. 2018, 225, 684. [Google Scholar] [CrossRef]
- Matthews, D.C. Prevention and treatment of periodontal diseases in primary care. Evid. Based Dent. 2014, 15, 68–69. [Google Scholar] [CrossRef][Green Version]
- Katanec, T.; Majstorovic, M.; Negovetic Vranic, D.; Ivic Kardum, M.; Marks, L.A. New toothpaste to deal with dentine hypersensitivity: Double-blind randomized controlled clinical trial. Int. J. Dent. Hyg. 2018, 16, 78–84. [Google Scholar] [CrossRef]
- Stookey, G.K.; Stahlman, D.B. Enhanced fluoride uptake in enamel with a fluoride-impregnated prophylactic cup. J. Dent. Res. 1976, 55, 333–341. [Google Scholar] [CrossRef]
- Richards, A.; Fejerskov, O.; Baelum, V. Enamel fluoride in relation to severity of human dental fluorosis. Adv. Dent. Res. 1989, 3, 147–153. [Google Scholar] [CrossRef]
- Pendrys, D.G.; Stamm, J.W. Relationship of total fluoride intake to beneficial effects and enamel fluorosis. J. Dent. Res. 1990, 69, 5295–5338. [Google Scholar] [CrossRef]
- Pai, N.; McIntyre, J.; Tadic, N.; Laparidis, C. Comparative uptake of fluoride ion into enamel from various topical fluorides in vitro. Aust. Dent. J. 2007, 52, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Muntean, A.; Sava, S.; Delean, A.G.; Mihailescu, A.M.; Dumitrescu, L.S.; Moldovan, M.; Festila, D.G. Toothpaste composition effect on enamel chromatic and morphological characteristics: In vitro analysis. Materials 2019, 12, 2610. [Google Scholar] [CrossRef]
- Scribante, A.; Dermenaki Farahani, M.R.; Marino, G.; Matera, C.; Rodriguez y Baena, R.; Lanteri, V.; Butera, A. Biomimetic Effect of nano-hydroxyapatite in demineralized enamel before orthodontic bonding of brackets and attachments: Visual, adhesion strength, and hardness in in vitro tests. BioMed Res. Int. 2020, 2020, 6747498. [Google Scholar] [CrossRef]
- Kim, H.-N.; Kong, W.-S.; Lee, J.-H.; Kim, J.-B. Reduction of dental caries among children and adolescents from a 15-year community water fluoridation program in a township area, Korea. Int. J. Environ. Res. Public Health 2019, 16, 1306. [Google Scholar] [CrossRef] [PubMed]
- Cervino, G.; Fiorillo, L.; Herford, A.S.; Laino, L.; Troiano, G.; Amoroso, G.; Crimi, S.; Matarese, M.; D’Amico, C.; Nastro Siniscalchi, E.; et al. Alginate Materials and Dental Impression Technique: A Current State of the Art and Application to Dental Practice. Mar. Drugs 2018, 17, 18. [Google Scholar] [CrossRef] [PubMed]
- EzEldeen, M.; Gizani, S.; Declerck, D. Long-term outcome of oral health in patients with early childhood caries treated under general anaesthesia. Eur. Arch. Paediatr. Dent. 2015, 16, 333–340. [Google Scholar] [CrossRef]
- Cortelli, S.C.; Costa, F.O.; Rode Sde, M.; Haas, A.N.; Andrade, A.K.; Pannuti, C.M.; Escobar, E.C.; Almeida, E.R.; Cortelli, J.R.; Pedrazzi, V. Mouthrinse recommendation for prosthodontic patients. Braz. Oral Res. 2014, 28, 1–9. [Google Scholar] [CrossRef]
- Savovic, J.; Turner, R.M.; Mawdsley, D.; Jones, H.E.; Beynon, R.; Higgins, J.P.T.; Sterne, J.A.C. Association between risk-of-bias assessments and results of randomized trials in cochrane reviews: The ROBES meta-epidemiologic study. Am. J. Epidemiol. 2018, 187, 1113–1122. [Google Scholar] [CrossRef]
- Mansournia, M.A.; Higgins, J.P.; Sterne, J.A.; Hernan, M.A. Biases in randomized trials: A conversation between trialists and epidemiologists. Epidemiology 2017, 28, 54–59. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- West, N.X.; He, T.; Hellin, N.; Claydon, N.; Seong, J.; Macdonald, E.; Farrell, S.; Eusebio, R.; Wilberg, A. Randomized in situ clinical trial evaluating erosion protection efficacy of a 0.454% stannous fluoride dentifrice. Int. J. Dent. Hyg. 2019, 17, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Seriwatanachai, D.; Triratana, T.; Kraivaphan, P.; Amaornchat, C.; Mateo, L.R.; Sabharwal, A.; Delgado, E.; Szewczyk, G.; Ryan, M.; Zhang, Y.P. Effect of stannous fluoride and zinc phosphate dentifrice on dental plaque and gingivitis: A randomized clinical trial with 6-month follow-up. J. Am. Dent. Assoc. 2019, 150, S25–S31. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.Q.; Zhang, Y.; Tang, L.; Liu, Y.H. Clinical evaluation of the effect of reducing tooth sensitivity caused by in office bleaching using dentifrices. Beijing Da Xue Xue Bao Yi Xue Ban 2019, 51, 340–344. [Google Scholar]
- Li, Y.; Suprono, M.; Mateo, L.R.; Zhang, Y.P.; Denis, J.; D’Ambrogio, R.; Sullivan, R.; Thomson, P. Solving the problem with stannous fluoride: Extrinsic stain. J. Am. Dent. Assoc. 2019, 150, S38–S46. [Google Scholar] [CrossRef] [PubMed]
- Ionta, F.Q.; Dos Santos, N.M.; Mesquita, I.M.; Dionísio, E.J.; Cruvinel, T.; Honório, H.M.; Rios, D. Is the dentifrice containing calcium silicate, sodium phosphate, and fluoride able to protect enamel against chemical mechanical wear? An in situ/ex vivo study. Clin. Oral Investig. 2019, 23, 3713–3720. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Li, X.; Liu, H.; Mateo, L.R.; Sabharwal, A.; Xu, G.; Szewczyk, G.; Ryan, M.; Zhang, Y.P. Evaluation of a stabilized stannous fluoride dentifrice on dental plaque and gingivitis in a randomized controlled trial with 6-month follow-up. J. Am. Dent. Assoc. 2019, 150, S32–S37. [Google Scholar] [CrossRef]
- Haraszthy, V.I.; Raylae, C.C.; Sreenivasan, P.K. Antimicrobial effects of a stannous fluoride toothpaste in distinct oral microenvironments. J. Am. Dent. Assoc. 2019, 150, S14–S24. [Google Scholar] [CrossRef]
- Hagenfeld, D.; Prior, K.; Harks, I.; Jockel-Schneider, Y.; May, T.W.; Harmsen, D.; Schlagenhauf, U.; Ehmke, B. No differences in microbiome changes between anti-adhesive and antibacterial ingredients in toothpastes during periodontal therapy. J. Periodontal Res. 2019, 54, 435–443. [Google Scholar] [CrossRef]
- Creeth, J.; Gallob, J.; Sufi, F.; Qaqish, J.; Gomez-Pereira, P.; Budhawant, C.; Goyal, C. Randomised clinical studies investigating immediate and short-term efficacy of an occluding toothpaste in providing dentine hypersensitivity relief. BMC Oral Health 2019, 19, 98. [Google Scholar] [CrossRef]
- Zero, D.T.; Lippert, F.; Hara, A.T.; Creeth, J.E.; Newby, E.E.; Butler, A.; Constantin, P.; Bosma, M.L. In situ anticaries efficacy of dentifrices with different formulations—A pooled analysis of results from three randomized clinical trials. J. Dent. 2018, 77, 93–105. [Google Scholar] [CrossRef]
- West, N.X.; Seong, J.; Hellin, N.; Macdonald, E.L.; Jones, S.B.; Creeth, J.E. Assessment of tubule occlusion properties of an experimental stannous fluoride toothpaste: A randomised clinical in situ study. J. Dent. 2018, 76, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Frese, C.; Wohlrab, T.; Sheng, L.; Kieser, M.; Krisam, J.; Frese, F.; Wolff, D. Clinical management and prevention of dental caries in athletes: A four-year randomized controlled clinical trial. Sci. Rep. 2018, 8, 16991. [Google Scholar] [CrossRef] [PubMed]
- West, N.X.; Seong, J.; Hellin, N.; Eynon, H.; Barker, M.L.; He, T. A clinical study to measure anti-erosion properties of a stabilized stannous fluoride dentifrice relative to a sodium fluoride/triclosan dentifrice. Int. J. Dent. Hyg. 2017, 15, 113–119. [Google Scholar] [CrossRef] [PubMed]
- West, N.X.; He, T.; Macdonald, E.L.; Seong, J.; Hellin, N.; Barker, M.L.; Eversole, S.L. Erosion protection benefits of stabilized SnF(2) dentifrice versus an arginine-sodium monofluorophosphate dentifrice: Results from in vitro and in situ clinical studies. Clin. Oral Investig. 2017, 21, 533–540. [Google Scholar] [CrossRef]
- Marchetti, E.; Casalena, F.; Capestro, A.; Tecco, S.; Mattei, A.; Marzo, G. Efficacy of two mouthwashes on 3-day supragingival plaque regrowth: A randomized crossover clinical trial. Int. J. Dent. Hyg. 2017, 15, 73–80. [Google Scholar] [CrossRef]
- Geidel, A.; Krüger, M.; Schrödl, W.; Jentsch, H. Control of Plaque and Gingivitis by an Herbal Toothpaste - A Randomised Controlled Study. Oral Health Prev. Dent. 2017, 15, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, K.; Noack, B.; Herrmann, N.; Hoffmann, T. Tooth staining potential of experimental amine fluoride/stannous fluoride mouth rinse formulations-a randomized crossover forced staining study. Clin. Oral Investig. 2015, 19, 1039–1045. [Google Scholar] [CrossRef]
- Hove, L.H.; Stenhagen, K.R.; Holme, B.; Tveit, A.B. The protective effect of SnF2 containing toothpastes and solution on enamel surfaces subjected to erosion and abrasion in situ. Eur. Arch. Paediatr. Dent. 2014, 15, 237–243. [Google Scholar] [CrossRef]
- Bellamy, P.G.; Harris, R.; Date, R.F.; Mussett, A.J.; Manley, A.; Barker, M.L.; Hellin, N.; West, N.X. In situ clinical evaluation of a stabilised, stannous fluoride dentifrice. Int. Dent. J. 2014, 64 (Suppl. 1), 43–50. [Google Scholar] [CrossRef]
- Bellamy, P.G.; Boulding, A.; Farmer, S.; Day, T.N.; Barker, M.L.; Harris, R.; Mussett, A.J. Randomized in vivo trial evaluating plaque inhibition benefits of an advanced stannous-containing sodium fluoride dentifrice used in conjunction with power brush technology. Int. J. Dent. Hyg. 2014, 12, 89–95. [Google Scholar] [CrossRef]
- Stenhagen, K.R.; Hove, L.H.; Holme, B.; Tveit, A.B. The effect of daily fluoride mouth rinsing on enamel erosive/abrasive wear in situ. Caries Res. 2013, 47, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, H.; Mozaffari, E.; Jonas, L. Scanning electron microscopy of growing dental plaque: A quantitative study with different mouth rinses. Ultrastruct. Pathol. 2013, 37, 233–240. [Google Scholar] [CrossRef] [PubMed]
- West, N.X.; Addy, M.; Newcombe, R.; Macdonald, E.; Chapman, A.; Davies, M.; Moran, J.; Claydon, N. A randomised crossover trial to compare the potential of stannous fluoride and essential oil mouth rinses to induce tooth and tongue staining. Clin. Oral Investig. 2012, 16, 821–826. [Google Scholar] [CrossRef]
- Fine, D.H.; Sreenivasan, P.K.; McKiernan, M.; Tischio-Bereski, D.; Furgang, D. Whole mouth antimicrobial effects after oral hygiene: Comparison of three dentifrice formulations. J. Clin. Periodontol. 2012, 39, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Huysmans, M.C.; Jager, D.H.; Ruben, J.L.; Unk, D.E.; Klijn, C.P.; Vieira, A.M. Reduction of erosive wear in situ by stannous fluoride-containing toothpaste. Caries Res. 2011, 45, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Wigger-Alberti, W.; Gysen, K.; Axmann, E.M.; Wilhelm, K.P. Efficacy of a new mouthrinse formulation on the reduction of oral malodour in vivo. A randomized, double-blind, placebo-controlled, 3 week clinical study. J. Breath Res. 2010, 4, 017102. [Google Scholar] [CrossRef] [PubMed]
- Anil, S. Plasma cell gingivitis among herbal toothpaste users: A report of three cases. J. Contemp. Dent. Pract. 2007, 8, 60–66. [Google Scholar] [CrossRef]
- Seymour, D.G.; Day, J.J.; Pathy, M.S. Sodium fluoride: Too late and too toxic for the elderly osteoporotic patient? Additional comments. Age Ageing 1990, 19, 425–426. [Google Scholar] [CrossRef]
- Zohoori, F.V.; Innerd, A.; Azevedo, L.B.; Whitford, G.M.; Maguire, A. Effect of exercise on fluoride metabolism in adult humans: A pilot study. Sci. Rep. 2015, 5, 16905. [Google Scholar] [CrossRef][Green Version]
- Durbakula, K.; Prabhu, V.; Jose, M. Genotoxicity of non-alcoholic mouth rinses: A micronucleus and nuclear abnormalities study with fluorescent microscopy. J. Investig. Clin. Dent. 2018, 9, e12309. [Google Scholar] [CrossRef]
- Shepherd, N.A.; Levison, D.A.; Heatley, R.V.; Braegger, C.P.; Corrigan, C.J.; Macdonald, T.T.; Ghoda, M.K. Toothpaste and Crohn’s disease. Lancet 1990, 336, 1382. [Google Scholar] [CrossRef]
- Ley, C.; Pischel, L.; Parsonnet, J. Triclosan and triclocarban exposure and thyroid function during pregnancy—A randomized intervention. Reprod. Toxicol. 2017, 74, 143–149. [Google Scholar] [CrossRef]
- Herlofson, B.B.; Barkvoll, P. Oral mucosal desquamation caused by two toothpaste detergents in an experimental model. Eur. J. Oral Sci. 1996, 104, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Cicciù, M.; Fiorillo, L.; Cervino, G. Chitosan Use in Dentistry: A Systematic Review of Recent Clinical Studies. Mar. Drugs 2019, 17, 417. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wegehaupt, F.J.; Zaruba, M.; Becker, K.; Roos, M.; Attin, T.; Wiegand, A. Erosion-inhibiting potential of a stannous chloride-containing fluoride solution under acid flow conditions in vitro. Arch. Oral Biol. 2010, 55, 702–705. [Google Scholar] [CrossRef][Green Version]
- Wang, X.Y.; Miyazaki, K.; Itoh, Y.; Motokawa, W. Effect of experimental fluoride-releasing tooth separator on acid resistance of human enamel in vitro. Dent. Mater. J. 2001, 20, 275–285. [Google Scholar] [CrossRef]
- Stookey, G.K.; Mau, M.S.; Isaacs, R.L.; Gonzalez-Gierbolini, C.; Bartizek, R.D.; Biesbrock, A.R. The relative anticaries effectiveness of three fluoride-containing dentifrices in Puerto Rico. Caries Res. 2004, 38, 542–550. [Google Scholar] [CrossRef]
- Orbak, R.; Canakçi, V.; Tezel, A. Clinical evaluation of an electron-ionizing toothbrush with a tooth paste containing stannous fluoride in treatment of dentine hypersensitivity following periodontal surgery. Dent. Mater. J. 2001, 20, 164–171. [Google Scholar] [CrossRef]
- Seltzer, J.D.; Flynn-Lurie, A.K.; Marsella, R.; Brennan, M.M. Investigation of the clinical efficacy of 0.2% topical stannous fluoride for the treatment of canine superficial pyoderma: A prospective, randomized, double-blinded, placebo-controlled trial. Vet. Dermatol. 2010, 21, 249–258. [Google Scholar] [CrossRef]
- Fiorillo, L.; De Stefano, R.; Cervino, G.; Crimi, S.; Bianchi, A.; Campagna, P.; Herford, A.S.; Laino, L.; Cicciù, M. Oral and Psychological Alterations in Haemophiliac Patients. Biomedicines 2019, 7, 33. [Google Scholar] [CrossRef]
- Cicciù, M.; Herford, A.S.; Cervino, G.; Troiano, G.; Lauritano, F.; Laino, L. Tissue fluorescence imaging (VELscope) for quick non-invasive diagnosis in oral pathology. J. Craniofac. Surg. 2017, 28, e112–e115. [Google Scholar] [CrossRef]
- Nastro, E.; Musolino, C.; Allegra, A.; Oteri, G.; Cicciù, M.; Alonci, A.; Quartarone, E.; Alati, C.; De Ponte, F.S. Bisphosphonate-associated osteonecrosis of the jaw in patients with multiple myeloma and breast cancer. Acta Haematol. 2007, 117, 181–187. [Google Scholar] [CrossRef]
- Isola, G.; Matarese, M.; Ramaglia, L.; Cicciù, M.; Matarese, G. Evaluation of the efficacy of celecoxib and ibuprofen on postoperative pain, swelling, and mouth opening after surgical removal of impacted third molars: A randomized, controlled clinical trial. Int. J. Oral Maxillofac. Surg. 2019, 48, 1348–1354. [Google Scholar] [CrossRef]
- Cicciù, M.; Risitano, G.; Lo Giudice, G.; Bramanti, E. Periodontal health and caries prevalence evaluation in patients affected by Parkinson’s disease. Parkinson’s Dis. 2012. [Google Scholar] [CrossRef]
| Authors and Year | Sample Size | Groups | Time and/or Follow up | Main Results | Statistic Results |
|---|---|---|---|---|---|
| West et al. [33] 2019 | 36 | 2 Groups: 0.454% stannous fluoride dentifrice vs. market dentifrice NaF/triclosan (0.24% sodium fluoride and 0.3% triclosan) | 10 days trial | Stannous fluoride dentifrice demonstrated 93.5% less enamel loss than control | p < 0.001 |
| Seriwatanachai et al. [34] 2019 | 135 | 3 Groups: Stabilized SnF2 dentifrice vs. SnF2 with zinc lactate dentifrice vs. a fluoride dentifrice | 6 months | Both SnF2 dentifrice showed a statistically significant reduction of gingival inflammation and plaque. With no statistical differences between themselves | p < 0.001 |
| Luo et al. [35] 2019 | 150 | 3 Groups: (48) Potassium nitrate vs. (45) stannous fluoride vs. (46) placebo | 30 days | Authors demonstrated how Potassium nitrate toothpaste could reduce sensitivity after an in-office bleaching treatment, with no differences between stannous fluoride and placebo. | p < 0.05 |
| Li et al. [36] 2019 | 18 bovine enamel sample | 3 Groups: 0.454% SnF2 and 1% zinc phosphate vs. Crest Pro-Health Whitening Power vs. non-abrasive SnF2 gel | 6 weeks | In this in vitro study SnF2 and 1% zinc paste performed better results than competitor and non-abrasive gel. It showed a better tooth stain reduction with no adverse effect. | p < 0.01 at 3 weeks |
| Ionta et al. [37] 2019 | 256 bovine enamel sample | 4 Groups: calcium silicate, sodium phosphate, and 1450 ppm sodium monofluorophosphate vs. dentifrice with 3500 ppm stannous chloride, 700 ppm amine fluoride, and 700 ppm sodium fluoride vs. conventional dentifrice, with 1450 ppm sodium monofluorophosphate vs. control (deionized water) | 20 days | The group 1 promoted less enamel loss than water (group 4) but it did not differ from group 2 or 3. But group 1 dentifrice promoted a higher wear after erosion than other groups. | p < 0.05 |
| Hu et al. [38] 2019 | 100 | 2 Groups: SnF2 dentifrice vs. fluoride dentifrice | 6 months | Both groups had a significant reduction in gingival inflammation and a plaque control improvement. SnF2 dentifrice showed a reduction of all indexed compared to control dentifrice | p < 0.001 |
| Haraszthy et al. [39] 2019 | 129 | 2 Groups: Stannous fluoride toothpaste vs. sodium monofluorophosphate toothpaste | 8 weeks | Stannous fluoride group showed a greater reduction of bacteria. From 14% at time zero to 27% at 4 weeks, and 41% at 8-week time. | p < 0.05 |
| Hagenfield et al. [40] 2019 | 41 | 2 Groups: anti-adhesive zinc-substituted carbonated hydroxyapatite (HA) vs. with antimicrobial and anti-adhesive amine fluoride/stannous fluoride (AmF/SnF2) | 12 weeks | There were no differences between groups in microbiome changes. | p > 0.05 |
| Creeth et al. [41] 2019 | 656 | 2 Groups: (329) experimental anhydrous 0.454% SnF2/polyphosphate toothpaste vs. (327) toothpaste containing 0.76% sodium monofluorophosphate | 3 days | Experimental toothpaste reduced dentine hypersensitivity (DH) after 3 days treatment better than test group. | p < 0.0001 |
| Zero et al. [42] 2018 | 168 | 4 Groups: sodium fluoride (NaF)/Carb/silica, NaF/silica, NaF + monofluorophosphate (MFP)/chalk vs. NaF/Carb/silica, NaF + MFP/dical, amine fluoride (AmF)/silica vs. NaF/Carb/silica, NaF + stannous fluoride (SnF2)/silica/hexametaphosphate (HMP) vs. Placebo (0 ppm F) and/or dose-response controls (675 ppm F as NaF [675F-NaF]) ±Carb | 14 days | All 1400–1450 ppm F dentifrices except NaF + SnF2/silica/HMP provided significantly greater lesion remineralization than Placebo. Carb addition did not alter fluoride efficacy. | p < 0.0001 |
| West et al. [43] 2018 | 21 samples | 3 Groups: toothpaste containing 0.454% stannous fluoride vs. Control fluoride toothpaste containing 0.76% sodium monofluorophosphate vs. mineral water | 10 days | After 4 days of treatment the degree of tubule occlusion increased in the dentine samples in the groups 1 and 2 than in water. | p < 0.01 |
| Frese et al. [44] 2018 | 54 | 2 Groups: special stannous fluoride-containing [(AmF)/NaF/SnCl ] mouth rinse (500 ppm F−, 800 ppm Sn2+), 1 × 30 s and a special toothpaste containing NaF/Sn2+ and the biopolymer chitosan (elmex EROSIONSSCHUTZ, CPGABA GmbH, Hamburg, Germany) vs. fluoridated toothpaste (1500 ppm) | 4 years | Two groups showed similar caries prevalence. There was a decrease of caries superficialis and media. | \ |
| West et al. [45] 2017 | 33 human enamel sample | 2 Groups: 0.454% SnF2/0.077% NaF vs. 0.32% NaF/0.3% triclosan. | 15 days | SnF2 group provided a reduction of enamel loss at day 10 and again at day 15. | p < 0.0001 |
| West et al. [46] 2017 | 33 | 2 Groups: SnF2 + 0.77% sodium fluoride (NaF) vs. sodium monofluorophosphate/arginine dentifrice | 10 day | Group 1 provided better enamel protection against erosive acid challenge than group 2 | p < 0.0001 |
| Marchetti et al. [47] 2017 | 20 | 3 Groups: Alcohol free essential oil mouthwash vs. Amine fluoride/stannous fluoride with zinc lactate mouthwash vs. chlorhexidine (CHX) mouthwash | 3 days | Group 1 showed better results on plaque regrowth compared to alcohol-free essential oil mouthwash. But there was a less impact if compared to CHX. | p < 0.001 |
| Geidel et al. [48] 2017 | 76 | 3 Groups: Herbal toothpaste vs. triclosan/copolymer toothpaste vs. amine/stannous fluoride toothpaste | 24 weeks | Approximal plaque index (API) and Oral hygiene index (OHI) changed in all groups with a significantly lower API e OHI in group 1. Sulcus bleeding index (SBI) was improved in all groups after 12 weeks. Bleeding on Probing (BoP) was unchanged. | p = 0.001 |
| Lorenz et al. [49] 2015 | 28 | 5 Groups: amine fluoride/stannous fluoride (AmF/SnF2), 250 ppm F−; low concentration of film-forming agents; low concentration of humectants vs. amine fluoride/stannous fluoride, 250 ppm F−; low concentration of film-forming agents, high concentration of humectants vs. amine fluoride/stannous fluoride, 250 ppm F−; high concentration of film-forming agents; high concentration of humectants vs. Phenolic/essential oil mouth rinse vs. Volvic Still Water, Danone Waters | 10 days | All mouth rinses led to tooth and tongue staining, statistically significant differences existed between groups 1, 3, 4 and 5 on tooth staining | \ |
| Hove et al. [50] 2014 | 64 human teeth sample | 4 Groups: Fluoride-free toothpaste vs. toothpaste 0.4% SnF2 vs. toothpaste 0.454% SnF2 vs. fluoride free toothpaste and a 0.4% SnF2 solution (1000 ppm F) | 9 days | The SnF2 groups showed significantly lower enamel wear than the group 1 | p < 0.05 |
| Bellamy et al. [51] 2014 | 12 | 3 Groups: sodium fluoride dentifrice vs. Stannous fluoride dentifrice vs. water | 15 days | Enamel loss was significantly lower for treatment in group 2 versus 1 or 3 | p < 0.005 |
| Bellamy et al. [52] 2014 | 27 | 2 Groups: SnF2/sodium fluoride (NaF) dentifrice vs. anticavity dentifrice | 17 days | Group 1 showed better results on 17 days usage period, it demonstrated a statistically significant a lower mean plaque area at each timepoint. | p < 0.0001 |
| Stenhagen et al. [53] 2013 | 16 molars sample | 4 Groups: NaF vs. SnF2 vs. TiF4 vs. control | 9 days | The mean surface loss in the NaF, SnF2 and TiF4 groups was significantly lower than in the control group | p < 0.05 |
| Jentsch et al. [54] 2013 | 24 | 3 Groups: Essential oil mouth rinse vs. amine/stannous fluoride mouth rinse vs. chlorhexidine digluconate 0.12% mouth rinse | 96h | The counts of cocci and bacilli and plaque thickness are statistically different only in chlorhexidine digluconate 0.12% group, with positive results | p ≤ 0.05 |
| West et al. [55] 2012 | 20 | 4 Groups: AmF/SnF2 mouthrinse 250 ppm, F— 430 ppm Sn vs. AmF/SnF2—mouthrinse 250 ppm, F—430 ppm Sn vs. essential oil vs. water | 4 days | Rinse 2 produced less stain than rinse 1, but the difference was not significant. Rinse 2 produced significantly more stain than rinse 3 and 4. For tongue staining, rinse 2 produced significantly more staining than 4 but not 1 or 3. | p < 0.05 |
| Fine et al. [56] 2012 | 35 | 3 Groups: Sodium fluoride/triclosan/copolymer dentifrice vs. Stannous fluoride/sodium hexametaphosphate/zinc lactate dentifrice (SnF2/SHMP) vs. sodium fluoride dentifrice | 13 days | Group 1 demonstrated significant reduction on plaque compared to other groups. | p < 0.01 |
| Huysmans et al. [57] 2011 | 20 enamel samples | 3 Groups: SnF2 toothpaste (1050 ppm fluoride from stan- nous fluoride and 350 ppm from amine fluoride) vs. SnF2 toothpaste (containing 1100 ppm fluoride from stannous fluoride and 350 ppm from sodium fluoride) vs. sodium fluoride toothpaste | 5 days | SnF2 toothpastes significantly reduced erosive wear. | p < 0.05 |
| Wigger-Alberti et al. [58] 2010 | 174 | 4 Groups: Amine fluoride/stannous fluoride 0.2% zinc lactate mouthrinse + malodour counteractives vs. 0.05% CHX, 0.05% cetylpyridinium chloride, 0.14% zinc lactate mouthrinse vs. (0.12% CHX mouthrinse vs. tap water. | 21 days | Group 1 showed efficacy to teeth discoloration, a significant reduction of organoleptxic ratings and volatile sulfur compounds was achieved after single application and after days 7 and 21. | p < 0.001 |
| Author | Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Participants and Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | Selective Reporting (Reporting Bias) |
|---|---|---|---|---|---|---|
| West et al. [33] 2019 | + | + | + | + | + | + |
| Seriwatanachai et al. [34] 2019 | + | + | + | + | + | + |
| Luo et al. [35] 2019 | + | + | - | - | + | + |
| Li et al. [36] 2019 | + | + | + | + | + | + |
| Ionta et al. [37] 2019 | + | - | + | - | + | + |
| Hu et al. [38] 2019 | + | + | + | - | + | + |
| Haraszthy et al. [39] 2019 | + | + | - | - | + | + |
| Hagenfield et al. [40] 2019 | + | + | + | + | + | + |
| Creeth et al. [41] 2019 | - | - | + | + | + | + |
| Zero et al. [42] 2018 | + | + | - | - | + | + |
| West et al. [43] 2018 | + | + | + | - | + | + |
| Frese et al. [44] 2018 | + | + | - | - | + | + |
| West et al. [45] 2017 | + | + | + | + | + | + |
| West et al. [46] 2017 | + | + | + | + | + | + |
| Marchetti et al. [47] 2017 | + | - | + | + | + | + |
| Geidel et al. [48] 2017 | - | + | - | - | + | + |
| Lorenz et al. [49] 2015 | + | - | + | - | + | + |
| Hove et al. [50] 2014 | - | - | - | - | + | + |
| Bellamy et al. [51] 2014 | + | - | - | - | + | + |
| Bellamy et al. [52] 2014 | + | - | + | + | + | + |
| Stenhagen et al. [53] 2013 | - | - | - | - | + | + |
| Jentsch et al. [54] 2013 | - | - | - | - | + | + |
| West et al. [55] 2012 | + | - | + | - | + | + |
| Fine et al. [56] 2012 | + | + | + | + | + | + |
| Huysmans et al. [57] 2011 | + | + | - | - | - | + |
| Wigger-Alberti et al. [58] 2010 | + | - | + | + | + | + |
| Main Outcome | No. of Results |
|---|---|
| Enamel loss reduction [17,21,29,30,33,34,36,40] | 9 |
| Bacteria and others microorganisms reduction [23,24,31,32,35,37,39] | 7 |
| tooth stain reduction [20,38,49] | 4 |
| Gingival inflammation reduction [18,22,32] | 2 |
| Dentinal hypersensitivity reduction [19,25] | 2 |
| Carious lesion remineralization [26,28] | 2 |
| Dentin tubule occlusion [27] | 1 |
| organoleptic ratings and volatile sulfur compounds [41] | 1 |
| Author and Year | Sample | Measured at | Mean Value |
|---|---|---|---|
| West et al. 2019 [33] | 36 Intraoral appliances with human enamel sample | Day 10 | 0.097 µm vs. 1.495 µm |
| Ionta et al. 2019 [37] | 256 Bovine enamel blocks | Day 5 | 4.8 ± 2.5 µm vs. 4.8 ± 1.4 µm |
| West et al. 2017 [43] | 33 Intraoral appliances with human enamel sample | Day 15 | 1.60 µm vs. 5.03 µm |
| West et al. 2017 [44] | 33 Human enamel specimens | Day 15 | 5.75 µm vs. 23.75 µm |
| Hove et al. 2014 [50] | 16 Intraoral appliances with human molar | Day 9 | 14.5 ± 9.3 µm and 33.3 ± 7.4 µm and 0.4 ± 1.3 µm vs. 29.2 ± 10.5 µm |
| Bellamy et al. 2014 [51] | 12 intraoral appliances with human enamel sample | Day 15 | 2.03 µm vs. 15.53 µm |
| Stenhagen et al. 2013 [53] | 16 intraoral appliances with molar sample | Day 9 | 1.8 ± 1.9 µm vs. 26.3 ± 4.7 µm vs. 3.1 ± 4.8 |
| Huysmans et al. 2011 [57] | 20 intraoral appliances with human enamel sample | Day 5 | data not clear or incomplete |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorillo, L.; Cervino, G.; Herford, A.S.; Laino, L.; Cicciù, M. Stannous Fluoride Effects on Enamel: A Systematic Review. Biomimetics 2020, 5, 41. https://doi.org/10.3390/biomimetics5030041
Fiorillo L, Cervino G, Herford AS, Laino L, Cicciù M. Stannous Fluoride Effects on Enamel: A Systematic Review. Biomimetics. 2020; 5(3):41. https://doi.org/10.3390/biomimetics5030041
Chicago/Turabian StyleFiorillo, Luca, Gabriele Cervino, Alan Scott Herford, Luigi Laino, and Marco Cicciù. 2020. "Stannous Fluoride Effects on Enamel: A Systematic Review" Biomimetics 5, no. 3: 41. https://doi.org/10.3390/biomimetics5030041
APA StyleFiorillo, L., Cervino, G., Herford, A. S., Laino, L., & Cicciù, M. (2020). Stannous Fluoride Effects on Enamel: A Systematic Review. Biomimetics, 5(3), 41. https://doi.org/10.3390/biomimetics5030041









