Finite Element Analysis Investigate Pulmonary Autograft Root and Leaflet Stresses to Understand Late Durability of Ross Operation
Abstract
1. Introduction
The “Ross Experimental Project”
2. Methods
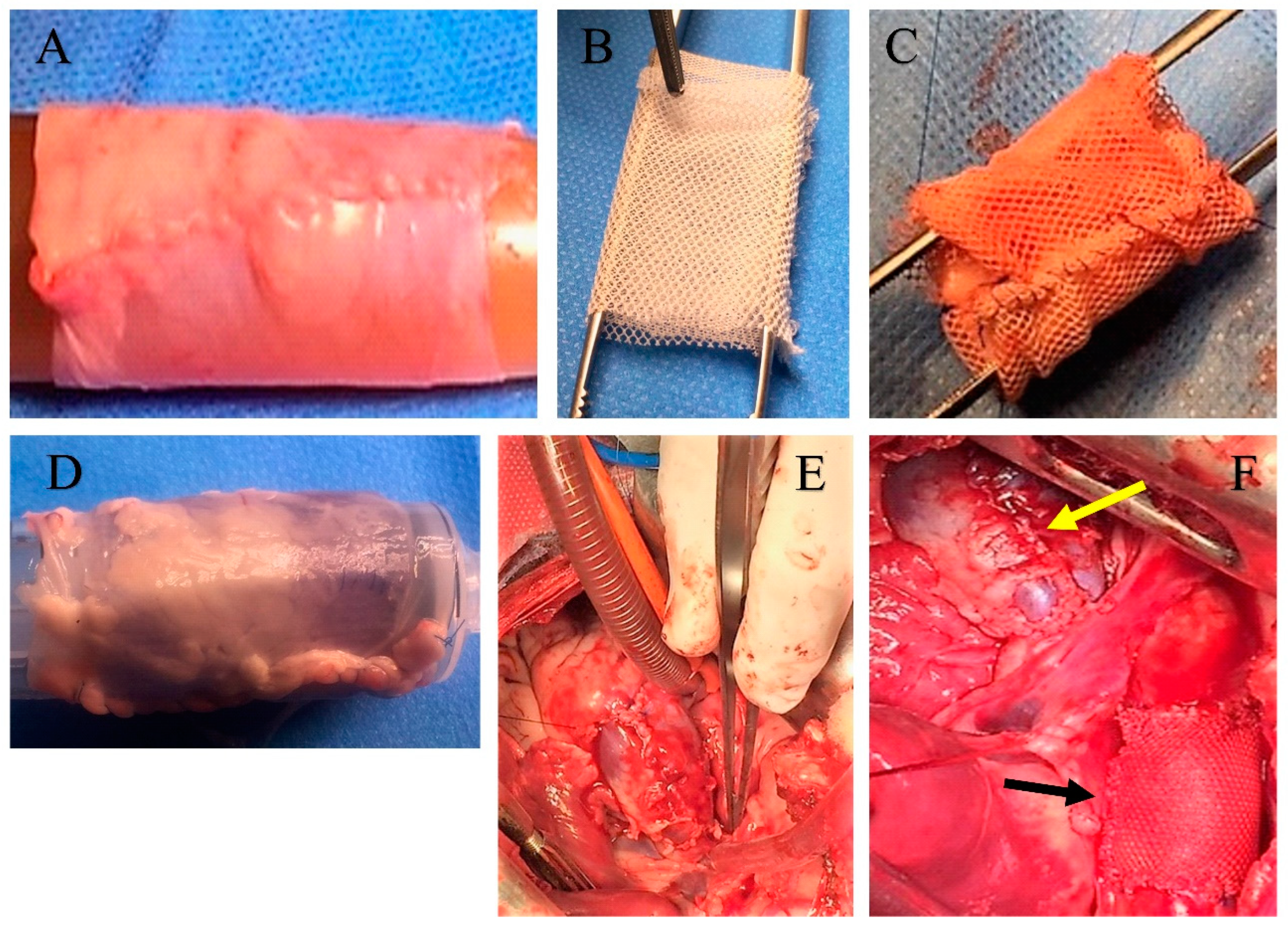
2.1. Experimental Animal Model
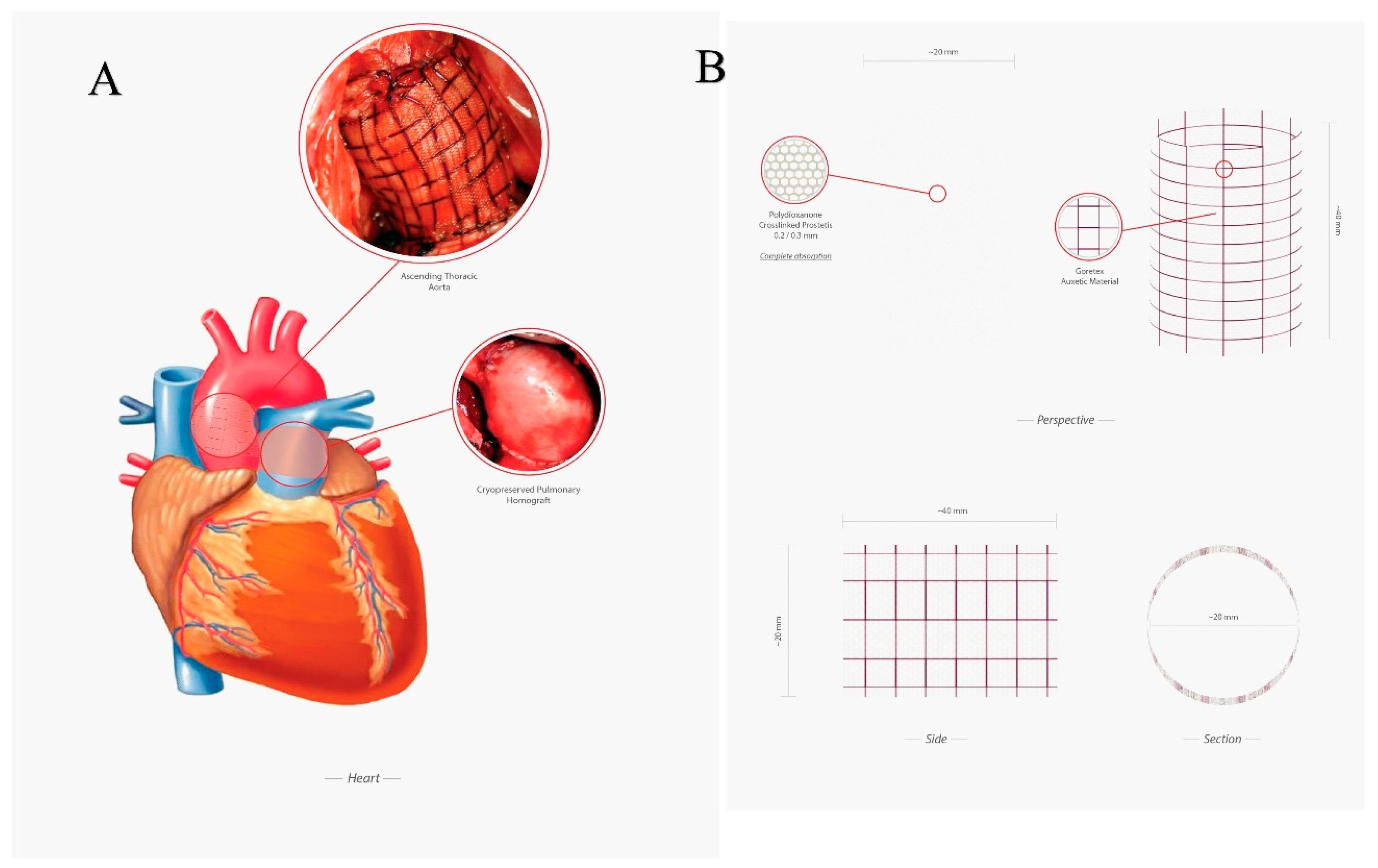
2.1.1. Implantation
2.1.2. Preparation of the Reinforcing Material
2.1.3. Specimen Collection and Characteristics
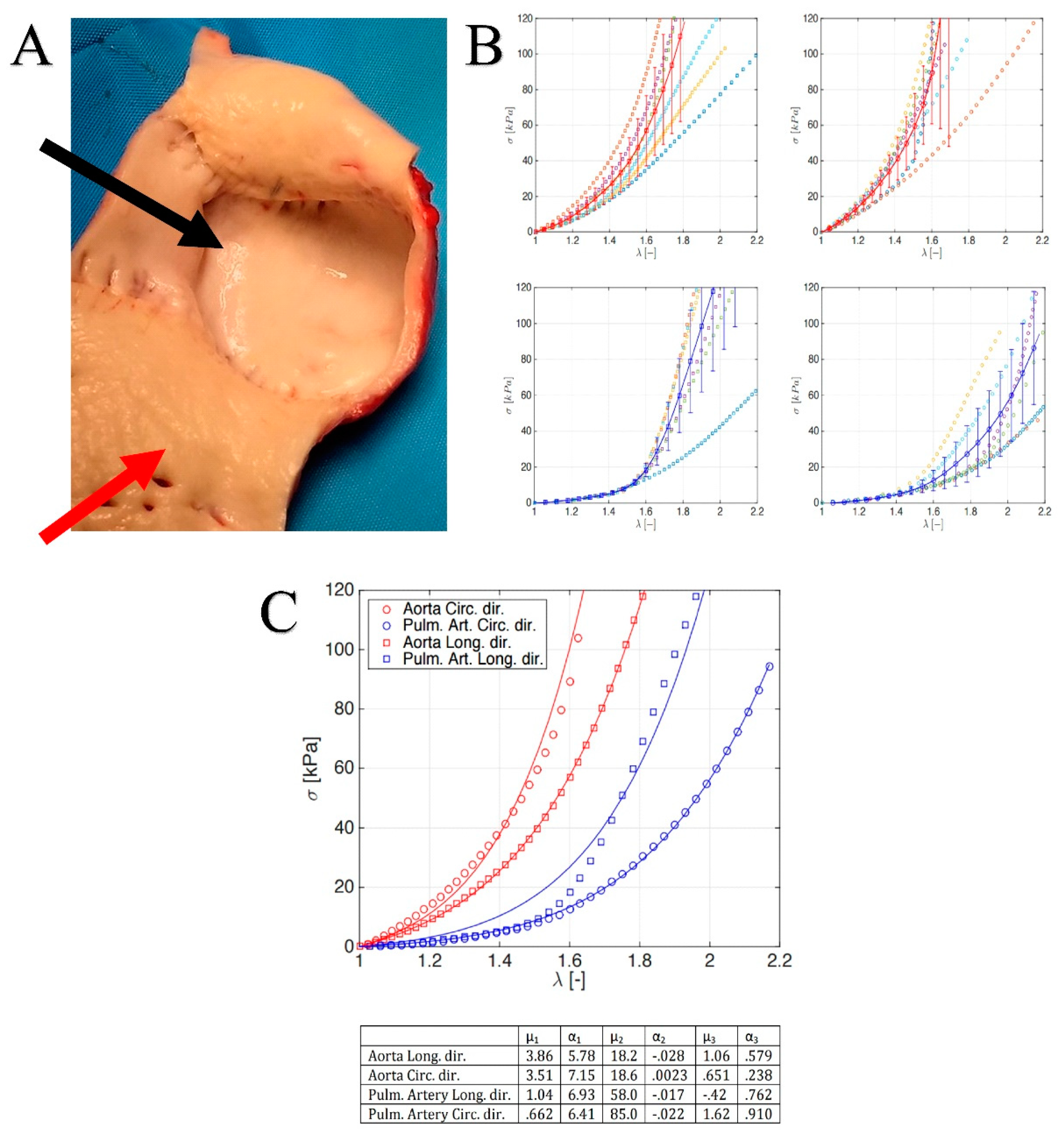
2.2. Constitutive Model and Material Properties
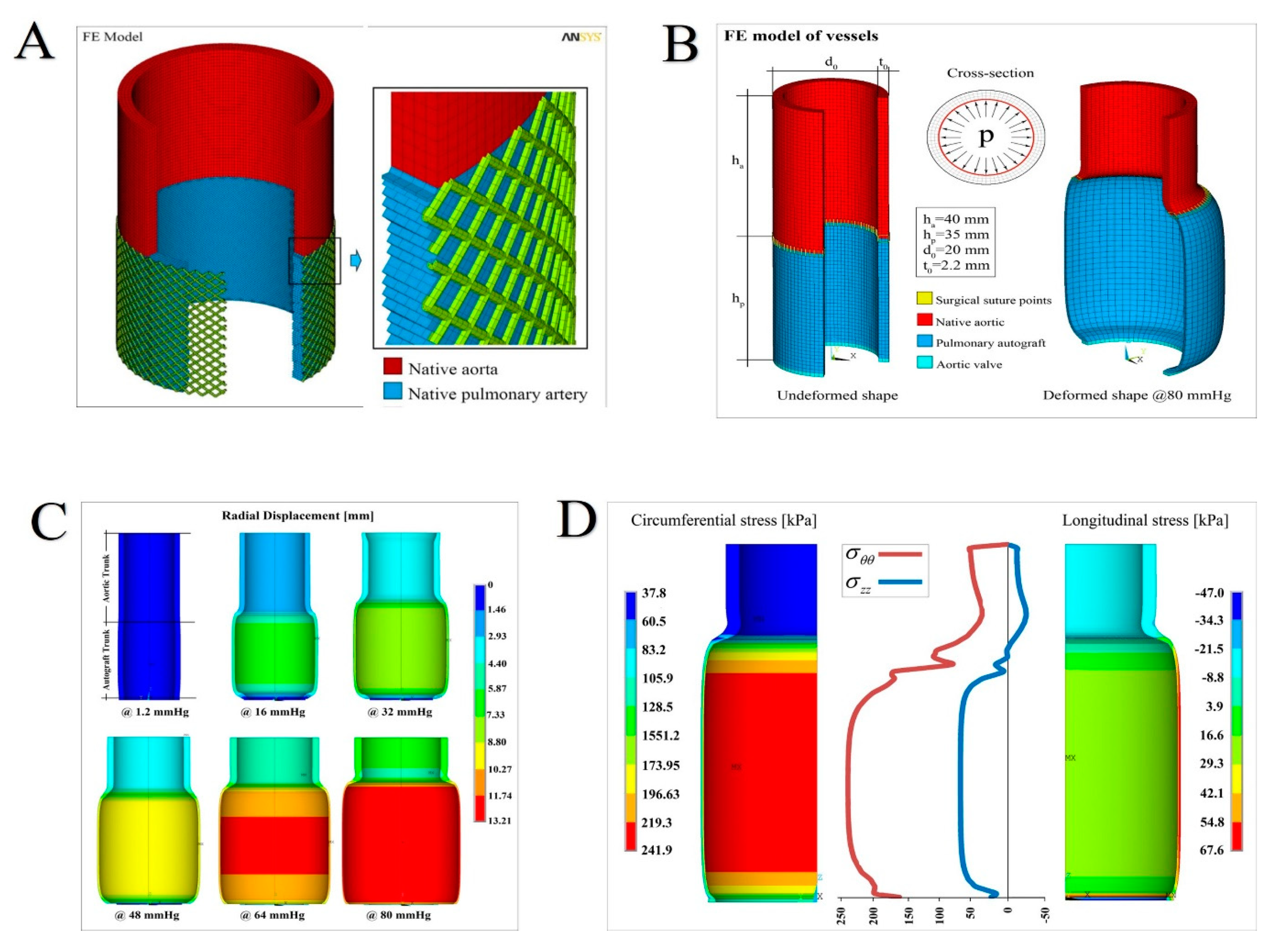
2.2.1. FE Simulations
2.2.2. Comparative Physical-Mathematical Model
- Reference Aorta, regarding the modeling of an aortic tract subjected to internal systemic pressure, say pi, of 120 mmHg (16 kPa, assumed constant). This case establishes for the reference aorta selected benchmark quantities, say the physiological growth over a six-month period (represented by the evolution of both diameter and thickness of the vessel layers) and the wall mechanical stresses (Figure 3A).
- No Reinforcement, analyzing the case of a not reinforced pulmonary artery transposed into aortic position at the pressure pi and subjected to growth and remodeling processes. The results of this simulation are directly compared with the outcome of the control group of the animal model (Figure 3B).
- Composite Reinforcement, concerning the mechanical analysis of the reinforced PA system undergoing growth and remodeling. The presence of the prosthesis is simulated by integrating the mechanical properties of the adventitia with those of the PDS biodegradable structure, by thus additionally providing an external variable pressure po, accounting for the e-PTFE armor elastic confinement whose value depends on the armor constitutive properties and evolves as a function of the pulmonary artery dilatation and growth (Figure 2, Figure 5A,B).
3. Results
3.1. Reinforcement of Pulmonary Autograft
3.2. Histology of Remodeling of Pulmonary Autograft
3.3. Leaflet and Root Stress
4. Discussion
4.1. Biomechanics and Finite Element Analysis of Pulmonary Autograft Leaflet and Root
4.2. Clinical Application
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2017, 70, 252–289. [Google Scholar] [CrossRef]
- Yacoub, M.H.; El-Hamamsy, I.; Sievers, H.H.; Carabello, B.A.; Bonow, R.O.; Stelzer, P.; da Costa, F.D.A.; Schäfers, H.J.; Skillington, P.; Charitos, E.I.; et al. Under-use of the Ross operation—A lost opportunity. Lancet 2014, 384, 559–560. [Google Scholar] [CrossRef]
- Nappi, F.; Avtaar Singh, S.S.; Spadaccio, C.; Acar, C. Ross operation 23 years after surgery: It should not be a “forgotten” option. J. Card. Surg. 2020, 35, 952–956. [Google Scholar] [CrossRef]
- Nappi, F.; Spadaccio, C.; Acar, C.; El-Hamamsy, I. Lights and Shadows on the Ross Procedure: Biological Solutions for Biological Problems. In Seminars in Thoracic and Cardiovascular Surgery; WB Saunders: Philadelphia, PA, USA, 2020. [Google Scholar] [CrossRef]
- Sievers, H.H.; Stierle, U.; Petersen, M.; Klotz, S.; Richardt, D.; Diwoky, M.; Charitos, E.I. Valve performance classification in 630 subcoronary Ross patients over 22 years. J. Thorac. Cardiovasc. Surg. 2018, 156, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Mazine, A.; David, T.E.; Rao, V.; Hickey, E.J.; Christie, S.; Manlhiot, C.; Ouzounian, M. Long-Term Outcomes of the Ross Procedure Versus Mechanical Aortic Valve Replacement: Propensity-Matched Cohort Study. Circulation 2016, 134, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Mazine, A.; El-Hamamsy, I.; Verma, S.; Peterson, M.D.; Bonow, R.O.; Yacoub, M.H.; David, T.E.; Bhatt, D.L. Ross Procedure in Adults for Cardiologists and Cardiac Surgeons: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2761–2777. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Spadaccio, C.; Chello, M.; Acar, C. The Ross procedure: Underuse or under-comprehension? J. Thorac. Cardiovasc. Surg. 2015, 149, 1463–1464. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kouchoukos, N.T.; Dávila-Román, V.G.; Spray, T.L.; Murphy, S.F.; Perrillo, J.B. Replacement of the aortic root with a pulmonary autograft in children and young adults with aortic-valve disease. N. Engl. J. Med. 1994, 330, 1–6. [Google Scholar] [CrossRef]
- Chambers, J.C.; Somerville, J.; Stone, S.; Ross, D.N. Pulmonary autograft procedure for aortic valve disease: Long-term results of the pioneer series. Circulation 1997, 96, 2206–2214. [Google Scholar] [CrossRef]
- Elkins, R.C.; Santangelo, K.; Randolph, J.D.; Knott-Craig, C.J.; Stelzer, P.; Thompson, W.M., Jr.; Razook, J.D.; Ward, K.E.; Overholt, E.D. Pulmonary autograft replacement in children. The ideal solution? Ann. Surg. 1992, 216, 363–370. [Google Scholar] [CrossRef]
- Schoof, P.H.; Cromme-Dijkhuis, A.H.; Bogers, J.J.; Thijssen, E.J.; Witsenburg, M.; Hess, J.; Bos, E. Aortic root replacement with pulmonary autograft in children. J. Thorac. Cardiovasc. Surg. 1994, 107, 367–373. [Google Scholar] [CrossRef]
- Brown, J.W.; Ruzmetov, M.; Shahriari, A.; Rodefeld, M.D.; Mahomed, Y.; Turrentine, M.W. Midterm results of Ross aortic valve replacement: A single-institution experience. Ann. Thorac. Surg. 2009, 88, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Hörer, J.; Hanke, T.; Stierle, U.; Takkenberg, J.J.; Bogers, A.J.; Hemmer, W.; Rein, J.G.; Hetzer, R.; Hübler, M.; Robinson, D.R.; et al. Neoaortic root diameters and aortic regurgitation in children after the Ross operation. Ann. Thorac. Surg. 2009, 88, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Charitos, E.I.; Hanke, T.; Stierle, U.; Robinson, D.R.; Bogers, A.J.; Hemmer, W.; Bechtel, M.; Misfeld, M.; Gorski, A.; Boehm, J.O.; et al. Autograft reinforcement to preserve autograft function after the ross procedure: A report from the german-dutch ross registry. Circulation 2009, 120, S146–S154. [Google Scholar] [CrossRef] [PubMed]
- Sievers, H.H.; Stierle, U.; Charitos, E.I.; Takkenberg, J.J.; Hörer, J.; Lange, R.; Franke, U.; Albert, M.; Gorski, A.; Leyh, R.G.; et al. A multicentre evaluation of the autograft procedure for young patients undergoing aortic valve replacement: Update on the German Ross Registry†. Eur. J. Cardio Thorac. Surg. 2016, 49, 212–218. [Google Scholar] [CrossRef]
- Charitos, E.I.; Takkenberg, J.J.; Hanke, T.; Gorski, A.; Botha, C.; Franke, U.; Dodge-Khatami, A.; Hoerer, J.; Lange, R.; Moritz, A.; et al. Reoperations on the pulmonary autograft and pulmonary homograft after the Ross procedure: An update on the German Dutch Ross Registry. J. Thorac. Cardiovasc. Surg. 2012, 144, 813–821. [Google Scholar] [CrossRef]
- Sievers, H.H.; Stierle, U.; Charitos, E.I.; Hanke, T.; Misfeld, M.; Matthias Bechtel, J.F.; Gorski, A.; Franke, U.F.; Graf, B.; Robinson, D.R.; et al. Major adverse cardiac and cerebrovascular events after the Ross procedure: A report from the German-Dutch Ross Registry. Circulation 2010, 122, S216–S223. [Google Scholar] [CrossRef]
- Mokhles, M.M.; Rizopoulos, D.; Andrinopoulou, E.R.; Bekkers, J.A.; Roos-Hesselink, J.W.; Lesaffre, E.; Bogers, A.J.; Takkenberg, J.J. Autograft and pulmonary allograft performance in the second post-operative decade after the Ross procedure: Insights from the Rotterdam Prospective Cohort Study. Eur. Heart J. 2012, 33, 2213–2224. [Google Scholar] [CrossRef]
- Stulak, J.M.; Burkhart, H.M.; Sundt, T.M., III; Connolly, H.M.; Suri, R.M.; Schaff, H.V.; Dearani, J.A. Spectrum and outcome of reoperations after the Ross procedure. Circulation 2010, 122, 1153–1158. [Google Scholar] [CrossRef]
- Sharabiani, M.T.; Dorobantu, D.M.; Mahani, A.S.; Turner, M.; Peter Tometzki, A.J.; Angelini, G.D.; Parry, A.J.; Caputo, M.; Stoica, S.C. Aortic Valve Replacement and the Ross Operation in Children and Young Adults. J. Am. Coll. Cardiol. 2016, 67, 2858–2870. [Google Scholar] [CrossRef]
- Martin, E.; Mohammadi, S.; Jacques, F.; Kalavrouziotis, D.; Voisine, P.; Doyle, D.; Perron, J. Clinical Outcomes Following the Ross Procedure in Adults: A 25-Year Longitudinal Study. J. Am. Coll. Cardiol. 2017, 70, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Mastrobuoni, S.; de Kerchove, L.; Solari, S.; Astarci, P.; Poncelet, A.; Noirhomme, P.; Rubay, J.; El Khoury, G. The Ross procedure in young adults: Over 20 years of experience in our Institution. Eur. J. Cardio Thorac. Surg. 2016, 49, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.W.; Putter, H.; Klautz, R.J.M.; Bruggemans, E.F.; Holman, E.R.; Bökenkamp, R.; Hazekamp, M.G. Long-Term Follow-Up After the Ross Procedure: A Single Center 22-Year Experience. Ann. Thorac. Surg. 2017, 103, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Skillington, P.D.; Mokhles, M.M.; Takkenberg, J.J.; Larobina, M.; O’Keefe, M.; Wynne, R.; Tatoulis, J. The Ross procedure using autologous support of the pulmonary autograft: Techniques and late results. J. Thorac. Cardiovasc. Surg. 2015, 149, S46–S52. [Google Scholar] [CrossRef]
- Da Costa, F.D.; Takkenberg, J.J.; Fornazari, D.; Balbi Filho, E.M.; Colatusso, C.; Mokhles, M.M.; da Costa, A.B.; Sagrado, A.G.; Ferreira, A.D.; Fernandes, T.; et al. Long-term results of the Ross operation: An 18-year single institutional experience. Eur. J. Cardio Thorac. Surg. 2014, 46, 415–422. [Google Scholar] [CrossRef]
- Andreas, M.; Seebacher, G.; Reida, E.; Wiedemann, D.; Pees, C.; Rosenhek, R.; Heinze, G.; Moritz, A.; Kocher, A.; Laufer, G. A single-center experience with the ross procedure over 20 years. Ann. Thorac. Surg. 2014, 97, 182–188. [Google Scholar] [CrossRef]
- El-Hamamsy, I.; Eryigit, Z.; Stevens, L.M.; Sarang, Z.; George, R.; Clark, L.; Melina, G.; Takkenberg, J.J.; Yacoub, M.H. Long-term outcomes after autograft versus homograft aortic root replacement in adults with aortic valve disease: A randomised controlled trial. Lancet 2010, 376, 524–531. [Google Scholar] [CrossRef]
- Buratto, E.; Shi, W.Y.; Wynne, R.; Poh, C.L.; Larobina, M.; O’Keefe, M.; Goldblatt, J.; Tatoulis, J.; Skillington, P.D. Improved Survival After the Ross Procedure Compared With Mechanical Aortic Valve Replacement. J. Am. Coll. Cardiol. 2018, 71, 1337–1344. [Google Scholar] [CrossRef]
- David, T.E.; David, C.; Woo, A.; Manlhiot, C. The Ross procedure: Outcomes at 20 years. J. Thorac. Cardiovasc. Surg. 2014, 147, 85–93. [Google Scholar] [CrossRef]
- Nappi, F.; Spadaccio, C.; Al-Attar, N.; Acar, C. The Ross procedure at the crossroads: Lessons from biology: Is Dr Ross’s dream concluded? Int. J. Cardiol. 2015, 178, 37–39. [Google Scholar] [CrossRef]
- Spadaccio, C.; Montagnani, S.; Acar, C.; Nappi, F. Introducing bioresorbable scaffolds into the show. A potential adjunct to resuscitate Ross procedure. Int. J. Cardiol. 2015, 190, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Spadaccio, C.; Fraldi, M.; Montagnani, S.; Fouret, P.; Chachques, J.C.; Acar, C. A composite semiresorbable armoured scaffold stabilizes pulmonary autograft after the Ross operation: Mr Ross’s dream fulfilled. J. Thorac. Cardiovasc. Surg. 2016, 151, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Spadaccio, C.; Fouret, P.; Hammoudi, N.; Chachques, J.C.; Chello, M.; Acar, C. An experimental model of the Ross operation: Development of resorbable reinforcements for pulmonary autografts. J. Thorac. Cardiovasc. Surg. 2015, 149, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Carotenuto, A.R.; Di Vito, D.; Spadaccio, C.; Acar, C.; Fraldi, M. Stress-shielding, growth and remodeling of pulmonary artery reinforced with copolymer scaffold and transposed into aortic position. Biomech. Model. Mechanobiol. 2016, 15, 1141–1157. [Google Scholar] [CrossRef]
- Nappi, F.; Fraldi, M.; Spadaccio, C.; Carotenuto, A.R.; Montagnani, S.; Castaldo, C.; Chachques, J.C.; Acar, C. Biomechanics drive histological wall remodeling of neoaortic root: A mathematical model to study the expression levels of ki 67, metalloprotease, and apoptosis transition. J. Biomed. Mater. Res. Part A 2016, 104, 2785–2793. [Google Scholar] [CrossRef]
- Nappi, F.; Spadaccio, C.; Castaldo, C.; Di Meglio, F.; Nurzynska, D.; Montagnani, S.; Chello, M.; Acar, C. Reinforcement of the pulmonary artery autograft with a polyglactin and polydioxanone mesh in the Ross operation: Experimental study in growing lamb. J. Heart Valve Dis. 2014, 23, 145–148. [Google Scholar]
- Nappi, F.; Nenna, A.; Larobina, D.; Carotenuto, A.R.; Jarraya, M.; Spadaccio, C.; Fraldi, M.; Chello, M.; Acar, C.; Carrel, T. Simulating the ideal geometrical and biomechanical parameters of the pulmonary autograft to prevent failure in the Ross operation. Interact. Cardiovasc. Thorac. Surg. 2018, 27, 269–276. [Google Scholar] [CrossRef]
- Nappi, F.; Carotenuto, A.R.; Cutolo, A.; Fouret, P.; Acar, C.; Chachques, J.C.; Fraldi, M. Compliance mismatch and compressive wall stresses drive anomalous remodelling of pulmonary trunks reinforced with Dacron grafts. J. Mech. Behav. Biomed. Mater. 2016, 63, 287–302. [Google Scholar] [CrossRef]
- Carr-White, G.S.; Afoke, A.; Birks, E.J.; Hughes, S.; O’Halloran, A.; Glennen, S.; Edwards, S.; Eastwood, M.; Yacoub, M.H. Aortic root characteristics of human pulmonary autografts. Circulation 2000, 102, Iii15. [Google Scholar] [CrossRef]
- Elkins, R.C.; Thompson, D.M.; Lane, M.M.; Elkins, C.C.; Peyton, M.D. Ross operation: 16-year experience. J. Thorac. Cardiovasc. Surg. 2008, 136, 623–630. [Google Scholar] [CrossRef]
- Mookhoek, A.; Krishnan, K.; Chitsaz, S.; Kuang, H.; Ge, L.; Schoof, P.H.; Bogers, A.; Takkenberg, J.J.M.; Tseng, E.E. Biomechanics of Failed Pulmonary Autografts Compared to Native Aortic Roots. Ann. Thorac. Surg. 2017, 103, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Mookhoek, A.; Krishnan, K.; Chitsaz, S.; Kuang, H.; Ge, L.; Schoof, P.H.; Bogers, A.J.; Takkenberg, J.J.; Tseng, E.E. Biomechanics of Failed Pulmonary Autografts Compared with Normal Pulmonary Roots. Ann. Thorac. Surg. 2016, 102, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Avtaar Singh, S.S.; Acar, C. Biomechanical future of the growing pulmonary autograft in Ross operation. Transl. Pediatri. 2020, 9, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Nenna, A.; Spadaccio, C.; Chello, M. Pulmonary autograft in aortic position: Is everything known? Transl. Pediatri. 2017, 6, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Spadaccio, C.; Nappi, F.; De Marco, F.; Sedati, P.; Taffon, C.; Nenna, A.; Crescenzi, A.; Chello, M.; Trombetta, M.; Gambardella, I.; et al. Implantation of a Poly-L-Lactide GCSF-Functionalized Scaffold in a Model of Chronic Myocardial Infarction. J. Cardiovasc. Transl. Res. 2017, 10, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Spadaccio, C.; Nappi, F.; Al-Attar, N.; Sutherland, F.W.; Acar, C.; Nenna, A.; Trombetta, M.; Chello, M.; Rainer, A. Old Myths, New Concerns: The Long-Term Effects of Ascending Aorta Replacement with Dacron Grafts. Not All That Glitters Is Gold. J. Cardiovasc. Transl. Res. 2016, 9, 334–342. [Google Scholar] [CrossRef]
- Fraldi, M.; Spadaccio, C.; Acar, C.; Nappi, F. Best to Clarify to Avoid Misunderstandings in the Biomechanics of Ross Operation: Parentheses Matter. Ann. Thorac. Surg. 2018, 106, 641–642. [Google Scholar] [CrossRef]
- Ross, D.N. Replacement of aortic and mitral valves with a pulmonary autograft. Lancet 1967, 2, 956–958. [Google Scholar] [CrossRef]
- Gula, G.; Wain, W.H.; Ross, D.N. Ten years’ experience with pulmonary autograft replacements for aortic valve disease. Ann. Thorac. Surg. 1979, 28, 392–396. [Google Scholar] [CrossRef]
- Somerville, J.; Saravalli, O.; Ross, D.; Stone, S. Long-term results of pulmonary autograft for aortic valve replacement. Br. Heart J. 1979, 42, 533–540. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nappi, F.; Nenna, A.; Lemmo, F.; Chello, M.; Chachques, J.C.; Acar, C.; Larobina, D. Finite Element Analysis Investigate Pulmonary Autograft Root and Leaflet Stresses to Understand Late Durability of Ross Operation. Biomimetics 2020, 5, 37. https://doi.org/10.3390/biomimetics5030037
Nappi F, Nenna A, Lemmo F, Chello M, Chachques JC, Acar C, Larobina D. Finite Element Analysis Investigate Pulmonary Autograft Root and Leaflet Stresses to Understand Late Durability of Ross Operation. Biomimetics. 2020; 5(3):37. https://doi.org/10.3390/biomimetics5030037
Chicago/Turabian StyleNappi, Francesco, Antonio Nenna, Francesca Lemmo, Massimo Chello, Juan Carlos Chachques, Christophe Acar, and Domenico Larobina. 2020. "Finite Element Analysis Investigate Pulmonary Autograft Root and Leaflet Stresses to Understand Late Durability of Ross Operation" Biomimetics 5, no. 3: 37. https://doi.org/10.3390/biomimetics5030037
APA StyleNappi, F., Nenna, A., Lemmo, F., Chello, M., Chachques, J. C., Acar, C., & Larobina, D. (2020). Finite Element Analysis Investigate Pulmonary Autograft Root and Leaflet Stresses to Understand Late Durability of Ross Operation. Biomimetics, 5(3), 37. https://doi.org/10.3390/biomimetics5030037






