Metal Oxide Nanoparticles as Biomedical Materials
Abstract
1. Introduction
2. Physical-Chemical Properties of Nano-Oxides
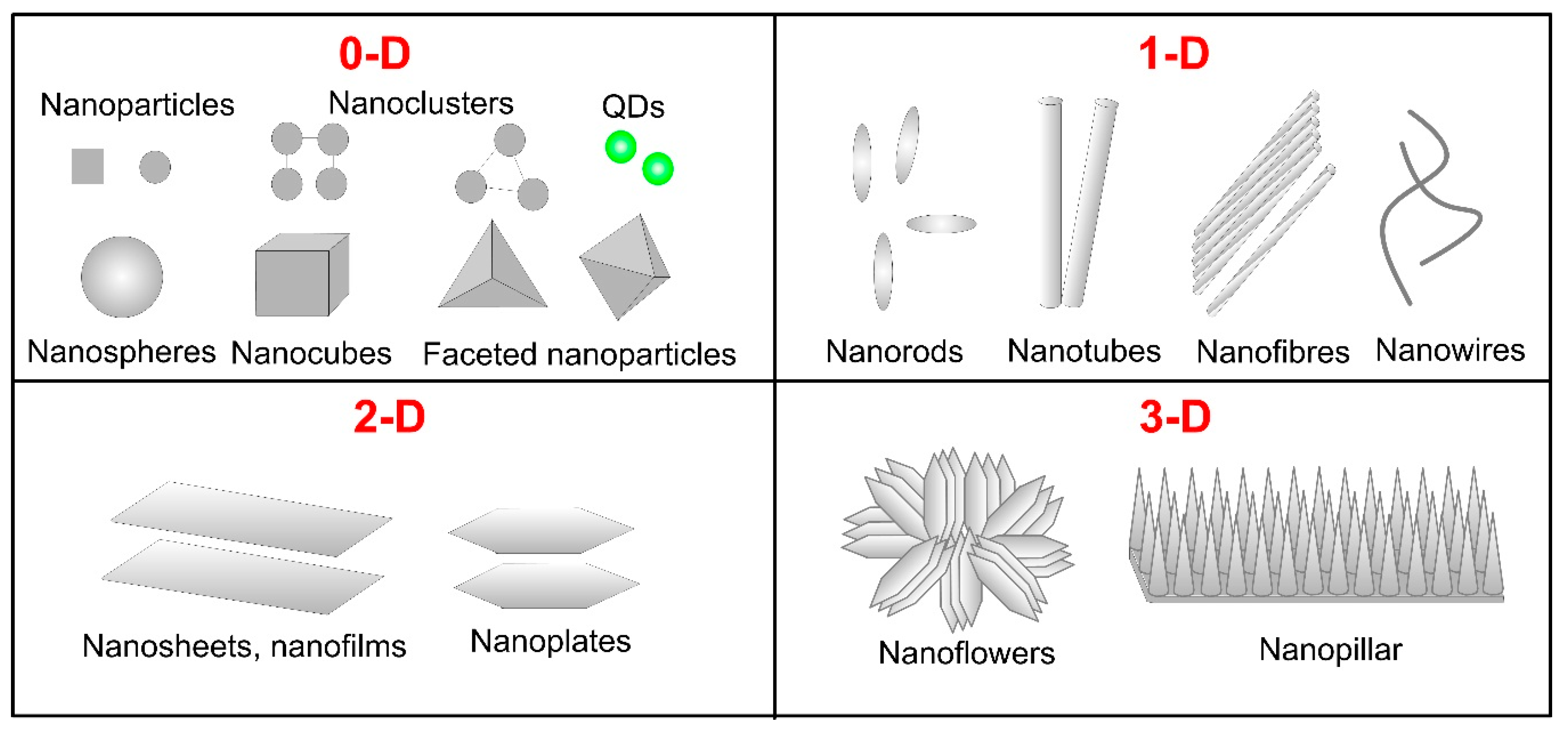
2.1. Shape and Size
2.2. Surface Area and Surface Energy
2.3. Crystal Structure
2.4. Dispersibility and Aggregation
2.5. Surface Properties
2.6. Photocatalytic Activity
2.7. Chemical Composition
2.8. Target Cell Type
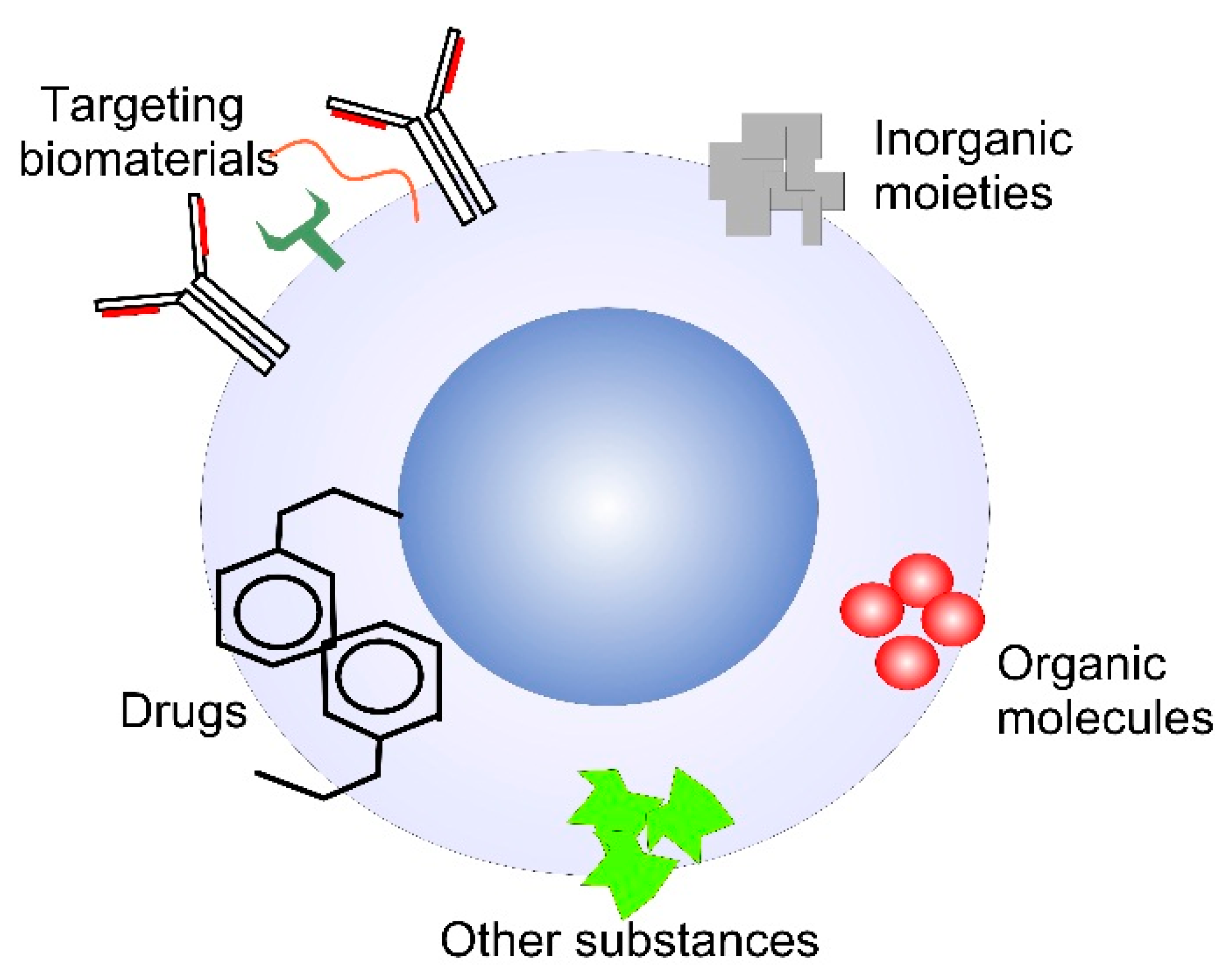
3. Applications of MONPs in Biomedicine
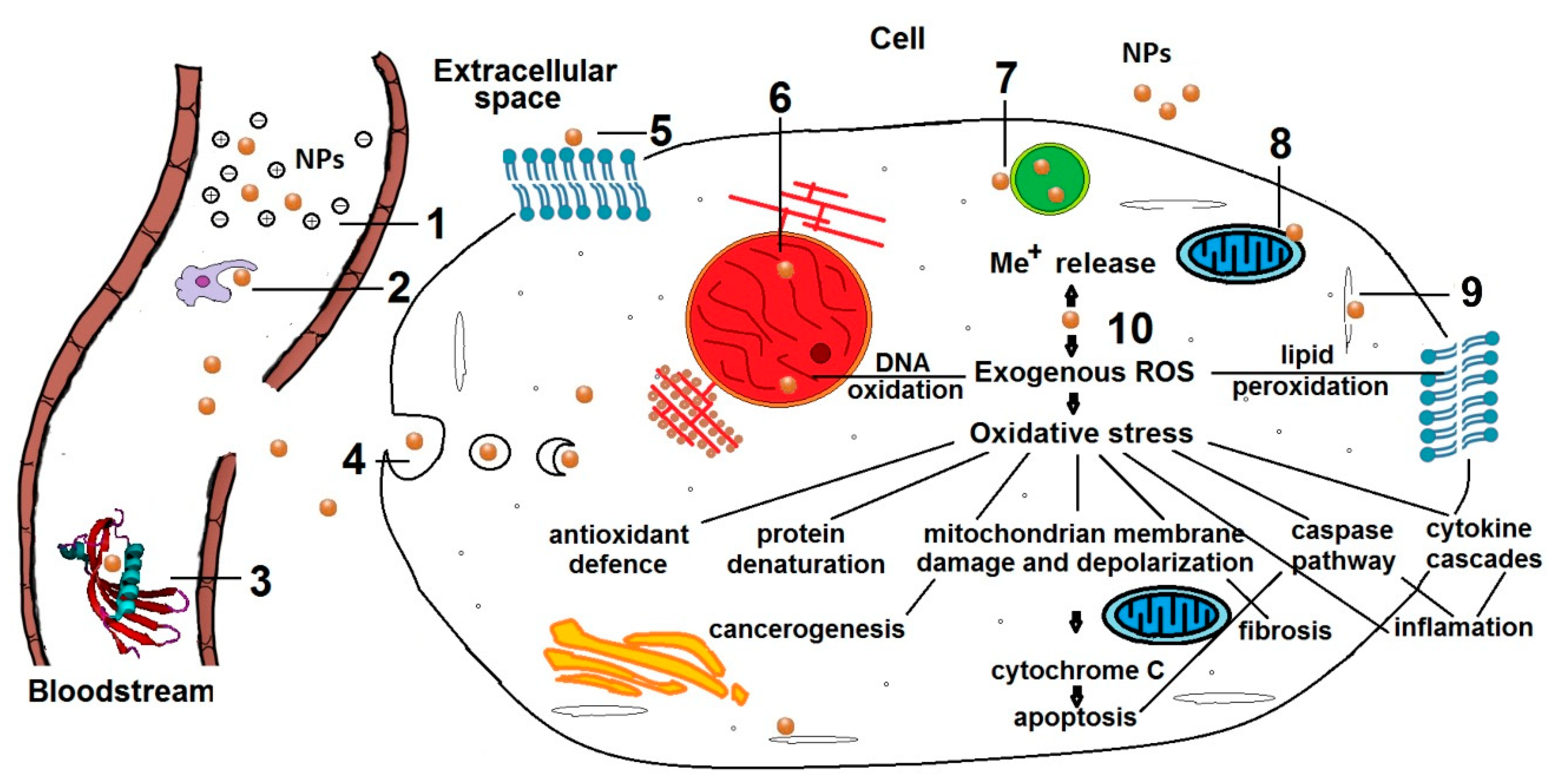
3.1. Internal Tissue Therapy
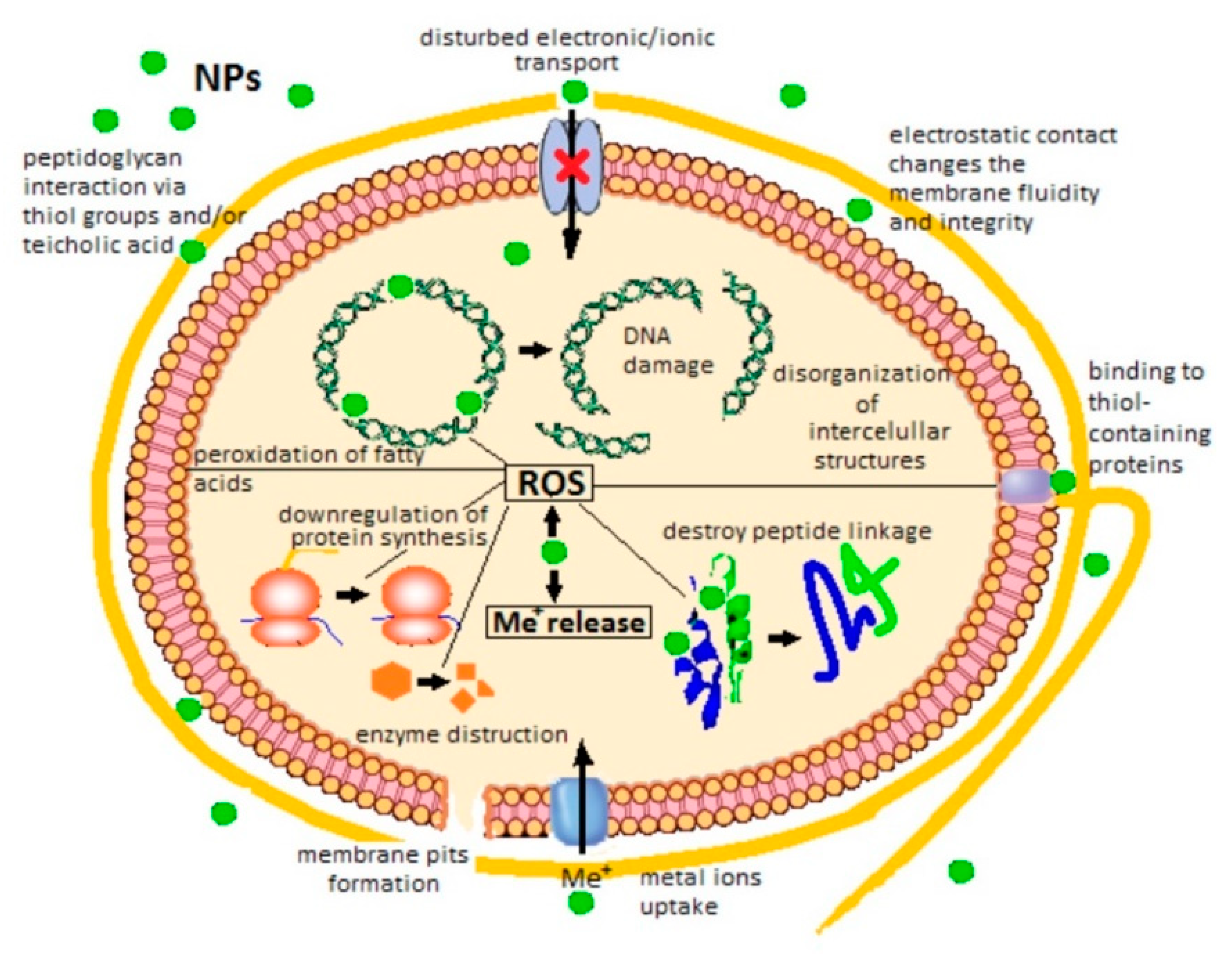
- opsonization [45] or enzymic degradation;
- hindered autophagy and macromolecules metabolization because of ruptured lysosomes which could activate apoptotic caspase pathways [48];
- disturbed production of energy through respiration and cellular metabolism by mitochondria damage [49];
- production of ROS likely leads to protein denaturation, DNA oxidation and membrane lipid peroxidation which damages the cell integrity and influences the respiratory activity causing eventually cell death. Besides, in physiological conditions, ROS produced mainly by mitochondria mediate the intracellular signal transduction, regulate the protein phosphorylation and control intracellular Ca2+ homeostasis [54].
3.1.1. Iron Oxide Nanoparticles
3.1.2. Zinc Oxide Nanoparticles
3.1.3. Titanium Dioxide Nanoparticles
3.2. Immuno-Therapy
3.2.1. Iron Oxides Nanoparticles
3.2.2. Zinc Oxide Nanoparticles
3.3. Diagnosis
3.3.1. Quantum Dots for Labeling
3.3.2. Contrast Agents for Magnetic Resonance Imaging
3.4. Nano-Oxides in Dentistry
3.5. Nano-Oxides in Hard Tissue Regeneration
3.6. Nano-Oxides for Wound Healing
3.7. Nano-Oxides Used as Biosensors
3.8. Antimicrobial Nano-Oxides
3.8.1. Titanium Dioxide Nanoparticles
3.8.2. Zinc Oxide Nanoparticles
3.8.3. Copper Oxide Nanoparticles
3.8.4. Silver Oxide Nanoparticles
3.8.5. Magnesium Oxide Nanoparticles
3.8.6. Calcium Oxide Nanoparticles
3.8.7. Aluminum Oxide Nanoparticles
3.8.8. Iron Oxide Nanoparticles
3.8.9. Nickel Oxide Nanoparticles
3.8.10. Cerium Dioxide Nanoparticles
4. Nanotoxicology
5. Summary and Future Prospectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A549 | Adenocarcinomic human alveolar basal epithelial cells |
| AB | Antibiotic |
| ADMA | Exogenous asymmetric dimethylarginine |
| Ag | Silver |
| APTES | 3-aminopropyl triethoxy saline |
| ATP | Adenosine triphosphate |
| Au | Gold |
| BBB | Blood-brain barrier |
| BC | Bacterial cellulose |
| BSA | Bovine serum albumin |
| CMX | Corona of the fusion protein |
| CPR | Cardiopulmonary resuscitation |
| Cr | Chromium |
| DMF | Dimethylformamide |
| DNA | Deoxyribonucleic acid |
| FTL | Ferritin light chain (a biomarker for ageing) |
| G(-) | Gram-negative |
| G(+) | Gram-positive |
| HeLa | Henrietta Lacks (human cell line) |
| HFL1 | Human fetal lung fibroblast-1 |
| HIV | Human immunodeficiency virus |
| HMSCs | Human mesenchymal stem cells |
| IC50 | Half-maximal inhibitory concentration |
| IL-1α | Interleukin 1 alpha (hematopoietin) |
| IL-4 | Interleukin 4 |
| ITO | Indium tin oxide |
| Ka | Association constant |
| Kmapp | Michaelis–Menten constant |
| L-132 | Human lung epithelial cell |
| MCF-7 | Michigan cancer foundation-7 (cancer cell line) |
| MO | Metal oxide |
| MONPs | Metal oxides nanoparticles |
| MRI | Magnetic resonance imaging |
| MTT | Methylthiazolyldiphenyl-tetrazolium bromide salt |
| nm | nanometer |
| NO | Nitrogen monoxide |
| NP | Nanoparticle |
| O2 | Oxygen |
| PAMAM | Poly (amidoamine) |
| PMMA-AA | Polymethyl methacrylate–acrylic acid |
| PBS | Phosphate-buffered saline |
| PCL | Polycaprolactone |
| PEG | Polyethylene glycol |
| PEI | Polyethylenimine |
| pH | Power of hydrogen |
| ppm | Parts per million |
| QDs | Quantum dots |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| RT | Room temperature |
| SDS | Sodium dodecyl sulphate |
| -SH | Thiol groups |
| SH-SY5Y | Human neuroblast cells |
| siRNA | Small (or short) interfering RNA |
| SPAN | Self-doped polyaniline nanofibers |
| SPR | Surface plasmon resonance |
| T-cell | T-lymphocyte |
| THP-1 | Human monocytes |
| TNF-α | Tumor necrosis factor-α (inflammatory cytokines) |
| U mL-1 | Units per millilitre |
| UV | Ultra-violet |
| VLP | Virus-like particles |
References
- Chung, E.J.; Leon, L.; Rinaldi, C. Nanoparticles for Biomedical Applications, Fundamental Concepts, Biological Interactions and Clinical Applications; Elsevier: Amsterdam, The Netherlands, 2019; p. 440. [Google Scholar]
- Biswas, A.K.; Islam, M.R.; Choudhury, Z.S.; Mostafa, A.; Kadir, M.F. Nanotechnology based approaches in cancer therapeutics. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 043001. [Google Scholar] [CrossRef]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Sánchez-Moreno, P.; Ortega-Vinuesa, J.L.; Peula-García, J.M.; Marchal, J.A.; Boulaiz, H. Smart Drug-Delivery Systems for Cancer Nanotherapy. Curr. Drug Targets 2016, 17, 339–359. [Google Scholar] [CrossRef]
- Rafiei-Sarmazdeh, Z.; Zahedi-Dizaji, S.M.; Kang, A.K. Two-Dimensional Nanomaterials. In Nanostructures; Ameen, S., Shaheer Akhtar, M., Shin, H.-S., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Chavali, M.S.; Nikolova, M.P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Res. 2019, 1, 607. [Google Scholar] [CrossRef]
- Sung, H.; Choi, M. Assembly of Nanoparticles: Towards Multiscale Three-Dimensional Architecturing. KONA Powder Part J. 2013, 30, 31–46. [Google Scholar] [CrossRef][Green Version]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 1–21. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Ankamwar, B. Size and Shape Effect on Biomedical Applications of Nanomaterials. In Biomedical Engineering—Technical Applications in Medicine; Hudak, R., Penhaker, M., Majernik, J., Eds.; IntechOpen: London, UK, 2012. [Google Scholar]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, distribution, clearance and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Yue, Z.; Wei, W.; You, Z.; Yang, Q.; Yue, H.; Su, Z.; Ma, G. Iron Oxide Nanotubes for magnetically guided delivery and pH-activated release of insoluble anticancer drugs. Adv. Funct. Mater. 2011, 21, 3446–3453. [Google Scholar] [CrossRef]
- Andrade, R.G.D.; Veloso, S.R.S.; Castanheira, E.M.S. Shape Anisotropic Iron Oxide-Based Magnetic Nanoparticles: Synthesis and Biomedical Applications. Int. J. Mol. Sci. 2020, 21, 2455. [Google Scholar] [CrossRef]
- Wyss, P.P.; Lamichhane, S.; Rauber, M.; Thomann, R.; Krämer, K.W.; Shastri, V.P. Tripod USPIONs with high aspect ratio show enhanced T2 relaxation and cytocompatibility. Nanomedicine 2016, 11, 1017–1030. [Google Scholar] [CrossRef]
- Hemery, G.; Genevois, C.; Couillaud, F.; Lacomme, S.; Gontier, E.; Ibarboure, E.; Lecommandoux, S.; Garanger, E.; Sandre, O. Monocore vs. multicore magnetic iron oxide nanoparticles: Uptake by glioblastoma cells and efficiency for magnetic hyperthermia. Mol. Syst. Des. Eng. 2017, 2, 629–639. [Google Scholar] [CrossRef]
- Agarwal, R.A.; Gupta, N.K.; Singh, R.; Nigam, S.; Ateeq, B. Ag/AgO Nanoparticles Grown via Time-Dependent Double Mechanism in a 2D Layered Ni-PCP and Their Antibacterial Efficacy. Sci. Rep. 2017, 7, 44852. [Google Scholar] [CrossRef]
- Baun, A.; Sayre, P.; Steinhäuser, K.G.; Rose, J. Regulatory relevant and reliable methods and data for determining the environmental fate of manufactured nanomaterials. NanoImpact 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Utembe, W.; Potgieter, K.; Stefaniak, A.B.; Gulumian, M. Dissolution and biodurability: Important parameters needed for risk assessment of nanomaterials. Part Fibre Toxicol. 2015, 12, 11. [Google Scholar] [CrossRef]
- Michaelis, M.; Fisher, C.; Ciacchi, L.C.; Luttge, A. Variability of zinc oxide dissolution rates. Environ. Sci. Technol. 2017, 51, 4297–4305. [Google Scholar] [CrossRef]
- He, H.; Cao, J.; Fei, X.; Duan, N. High-temperature annealing of ZnO nanoparticles increases the dissolution magnitude and rate in water by altering O vacancy distribution. Environ. Int. 2019, 130, 104930. [Google Scholar] [CrossRef]
- Schmidt, J.; Vogelsberger, W. Aqueous long-term solubility of titania nanoparticles and titanium(IV) hydrolysis in a sodium chloride system studied by adsorptive stripping voltammetry. J. Solut. Chem. 2009, 38, 1267–1282. [Google Scholar] [CrossRef]
- Avramescu, M.-L.; Rasmussen, P.E.; Chénier, M.; Gardner, H.D. Influence of pH, particle size and crystal form on dissolution behaviour of engineered nanomaterials. Environ. Sci. Pollut. Res. 2017, 24, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Gurr, J.R.; Wang, A.S.; Chen, C.H.; Jan, K.Y. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology 2005, 213, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanopart. Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Lousinian, S.; Missopolinou, D.; Panayiotou, C. Fibrinogen adsorption on zinc oxide nanoparticles: A micro-differential scanning calorimetry analysis. J. Colloid Interface Sci. 2013, 395, 294–299. [Google Scholar] [CrossRef]
- He, Y.; Ingudam, S.; Reed, S.; Gehring, A.; Strobaugh, T.P., Jr.; Irwin, P. Study on the mechanism of antibacterial action of magnesium oxide nanoparticles against foodborne pathogens. J. Nanobiotechnology 2016, 14, 54–63. [Google Scholar] [CrossRef]
- Pham, B.T.T.; Colvin, E.K.; Pham, N.T.H.; Kim, B.J.; Fuller, E.S.; Moon, E.A.; Barbey, R.; Yuen, S.; Rickman, B.H.; Bryce, N.S.; et al. Biodistribution and Clearance of Stable Superparamagnetic Maghemite Iron Oxide Nanoparticles in Mice Following Intraperitoneal Administration. Int. J. Mol. Sci. 2018, 19, 205. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Osaka, T.; Nakanishi, T.; Shanmugam, S.; Takahama, S.; Zhang, H. Effect of surface charge of magnetite nanoparticles on their internalization into breast cancer and umbilical vein endothelial cells. Colloids Surf. B 2009, 71, 325–330. [Google Scholar] [CrossRef]
- Xiao, K.; Li, Y.; Luo, J.; Lee, J.S.; Xiao, W.; Gonik, A.M.; Agarwal, R.G.; Lam, K.S. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials 2011, 32, 3435–3446. [Google Scholar] [CrossRef]
- Giammar, D.E.; Maus, C.J.; Xie, L.Y. Effects of particle size and crystalline phase on lead adsorption to titanium dioxide nanoparticles. Environ. Eng. Sci. 2007, 24, 85–95. [Google Scholar] [CrossRef]
- Zimbone, M.; Buccheri, M.A.; Cacciato, G.; Sanz, R.; Rappazzo, G.; Boninelli, S.; Reitano, R.; Romano, L.; Privitera, V.; Grimaldi, M.G. Photocatalytic and antibacterial activity of TiO2 nanoparticles obtained by laser ablation in water. Appl. Catal. B 2015, 165, 487–494. [Google Scholar] [CrossRef]
- Podporska-Carroll, J.; Panaitescu, E.; Quilty, B.; Wang, L.; Menon, L.; Pillai, S.C. Antimicrobial properties of highly efficient photocatalytic TiO2 nanotubes. Appl. Catal. B 2015, 176, 70–75. [Google Scholar] [CrossRef]
- Augustine, R.; Mathew, A.P.; Sosnik, A. Metal Oxide Nanoparticles as Versatile Therapeutic Agents Modulating Cell Signaling Pathways: Linking Nanotechnology with Molecular Medicine. Appl. Mater. Today 2017, 7, 91–103. [Google Scholar] [CrossRef]
- Schanen, B.C.; Das, S.; Reilly, C.M.; Warren, W.L.; Self, W.T.; Seal, S.; Drake, D.R. Immunomodulation and T Helper TH1/TH2 Response Polarization by CeO2 and TiO2 Nanoparticles. PLoS ONE 2013, 8, e62816. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Yin, L.H.; Tang, M.; Pu, Y.P. ZnO, TiO2, SiO2 and Al2O3 nanoparticles-induced toxic effects on human fetal lung fibroblasts. Biomed. Environ. Sci. 2011, 24, 661–669. [Google Scholar]
- Lanone, S.; Rogerieux, F.; Guys, J.; Dupont, A.; Maillot-Marechal, E.; Boczkowski, J.; Lacroix, G.; Hoet, P. Comparative toxicity of 24 manufactured nanoparticles in human alveolar epithelial and macrophage cell lines. Part Fibre Toxicol. 2009, 6, 1–12. [Google Scholar] [CrossRef]
- Sahu, D.; Kannan, G.M.; Tailang, M.; Vijayaraghavan, R. In Vitro Cytotoxicity of Nanoparticles: A Comparison between Particle Size and Cell Type. J. Nanosci. 2016, 2016, 4023852. [Google Scholar] [CrossRef]
- Chhabra, H.; Deshpande, R.; Kanitkar, M.; Jaiswal, A.; Kale, V.P.; Bellare, J.R. A nano zinc oxide doped electrospun scaffold improves wound healing in a rodent model. RSC Adv. 2016, 6, 1428–1439. [Google Scholar] [CrossRef]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef]
- Hanini, A.; Schmitt, A.; Kacem, K.; Chau, F.; Ammar, S.; Gavard, J. Evaluation of iron oxide nanoparticle biocompatibility. Int. J. Nanomed. 2011, 6, 787–794. [Google Scholar]
- Roy, R.; Kumar, D.; Sharma, A.; Gupta, P.; Chaudhari, B.P.; Tripathi, A.; Das, M.; Dwivedi, P.D. ZnO nanoparticles induced adjuvant effect via toll-like receptors and Src signalling in Balb/c mice. Toxicol. Lett. 2014, 230, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H.; Grainger, D. Nanoparticle Uptake: The Phagocyte Problem HHS Public Access. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, X.; Zhang, G.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S.-Y. Interaction of mesoporous silica nanoparticles with human red blood cell membranes: Size and surface effects. ACS Nano 2011, 5, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Rizk, M.Z.; Ali, S.A.; Hamed, M.A.; El-Rigal, N.S.; Alya, H.F.; Sala, H.H. Toxicity of titanium dioxide nanoparticles: Effect of dose and time on biochemical disturbance, oxidative stress and genotoxicity in mice. Biomed. Pharmacother. 2017, 90, 466–472. [Google Scholar] [CrossRef]
- Morán-Martínez, J.; Beltrán del Río-Parra, R.; Betancourt-Martínez, N.D.; García-Garza, R.; Jiménez-Villarreal, J.; Niño-Castañeda, M.S.; Nava-Rivera, L.E.; Facio Umaña, J.A.; Carranza-Rosales, P.; Pérez-Vertti, R.D.A. Evaluation of the Coating with TiO2 Nanoparticles as an Option for the Improvement of the Characteristics of NiTi Archwires: Histopathological, Cytotoxic and Genotoxic Evidence. J. Nanomater. 2018, 2018, 2585918. [Google Scholar] [CrossRef]
- Moghaddam, B.; Moniri, M.; Azizi, S.; Rahim, R.A.; Ariff, A.B.; Navaderi, M.; Mohamad, R. Eco-friendly formulated zinc oxide nanoparticles: Induction of cell cycle arrest and apoptosis in the MCF-7 cancer cell line. Genes 2017, 8, 281. [Google Scholar] [CrossRef]
- García-Hevia, L.; Valiente, R.; Martín-Rodríguez, R.; Renero-Lecuna, C.; González, J.; Rodríguez-Fernández, L.; Aguado, F.; Villegas, J.C.; Fanarraga, M.L. Nano-ZnO lead to tubulin macro tube assembly and actin-bundling triggering, cytoskeletal catastrophe and cell necrosis. Nanoscale 2016, 8, 10963–10973. [Google Scholar] [CrossRef]
- Mao, Z.; Xu, B.; Ji, X.; Zhou, K.; Zhang, X.; Chen, M.; Han, X.; Tang, Q.; Wang, X.; Xia, Y. Titanium dioxide nanoparticles alter cellular morphology via disturbing the microtubule dynamics. Nanoscale 2015, 7, 8466–8475. [Google Scholar] [CrossRef]
- Rojas, J.M.; Sanz-Ortega, L.; Mulens-Arias, V.; Gutiérrez, L.; Pérez-Yagüe, S.; Barber, D.F. Superparamagnetic iron oxide nanoparticle uptake alters M2 macrophage phenotype, iron metabolism, migration and invasion. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1127–1138. [Google Scholar] [CrossRef]
- Somsanith, N.; Kim, Y.-K.; Jang, Y.-S.; Lee, Y.-H.; Yi, H.-K.; Jang, J.-H.; Kim, K.-A.; Bae, T.-S.; Lee, M.-H. Enhancing of Osseointegration with Propolis-Loaded TiO2 Nanotubes in Rat Mandible for Dental Implants. Materials 2018, 11, 61. [Google Scholar] [CrossRef]
- Bogdan, J.; Pławińska-Czarnak, J.; Zarzyńska, J. Nanoparticles of Titanium and Zinc Oxides as Novel Agents in Tumor Treatment: A Review. Nanoscale Res. Lett. 2017, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sheng-Nan, S.; Chao, W.; Zan-Zan, Z.; Yang-Long, H.; Venkatraman, S.S.; Zhi-Chuan, X. Magnetic iron oxide nanoparticles: Synthesis and surface coating techniques for biomedical applications. Chin. Phys. B 2014, 23, 037503. [Google Scholar]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Arias, L.S.; Pessa, J.P.; Vieira, A.P.M.; Toito de Lima, T.M.; Delbem ID, A.C.B.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef]
- Peng, L.; He, M.; Chen, B.; Wu, Q.; Zhang, Z.; Pang, D.; Zhu, Y.; Hu, B. Cellular uptake, elimination and toxicity of CdSe/ZnS quantum dots in HepG2 cells. Biomaterials 2013, 34, 9545–9558. [Google Scholar] [CrossRef]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M.A. Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug delivery. Int. J. Mol. Sci. 2015, 16, 8070–8101. [Google Scholar] [CrossRef]
- Indira, T.K.; Lakshmi, P.K. Magnetic Nanoparticles—A Review. Int. J. Pharmaceut. Sci. Nanotechnol. 2010, 3, 1035–1042. [Google Scholar]
- Giustini, A.J.; Petryk, A.A.; Cassim, S.M.; Tate, J.A.; Baker, I.; Hoopes, P.J. Magnetic Nanoparticle Hyperthermia in Cancer Treatment. Nano Life 2010. [Google Scholar] [CrossRef]
- Hilger, I.; Hiergeist, R.; Hergt, R.; Winnefeld, K.; Schubert, H.; Kaiser, W.A. Thermal ablation of tumors using magnetic nanoparticles: An in vivo feasibility study. Invest. Radiol. 2002, 37, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Hurbankova, M.; Volkovova, K.; Hraskova, D.; Wimmerova, S.; Moricova, S. Respiratory toxicity of Fe3O4 nanoparticles: An experimental study. Rev. Environ. Health. 2017, 32, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.-F.; Jia, J.-F.; Guo, X.-K.; Zhao, Y.-P.; Chen, D.-S.; Guo, Y.-Y.; Cheng, T.; Zhang, X.-L. Biocompatibility of chitosan-coated iron oxide nanoparticles with osteoblast cells. Int. J. Nanomed. 2012, 7, 5593–5602. [Google Scholar]
- Kalidasan, V.; Liu, X.L.; Herng, T.S.; Yang, Y.; Ding, J. Bovine Serum Albumin-Conjugated Ferrimagnetic Iron Oxide Nanoparticles to Enhance the Biocompatibility and Magnetic Hyperthermia Performance. Nano-Micro Lett. 2016, 8, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Vismara, E.; Bongio, C.; Coletti, A.; Edelman, R.; Serafini, A.; Mauri, M.; Simonutti, R.; Bertini, S.; Urso, E.; Assaraf, Y.G.; et al. Albumin and Hyaluronic Acid-Coated Superparamagnetic Iron Oxide Nanoparticles Loaded with Paclitaxel for Biomedical Applications. Molecules 2017, 22, 1030. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Xia, X.; Wu, M.; Cui, C.; Zhang, Y.; Liu, L.; Wu, B.; Wang, C.; Zhang, L.; Zhou, X.; et al. Folic acid-conjugated iron oxide porous nanorods loaded with doxorubicin for targeted drug delivery. Colloids Surf. B 2014, 120, 142–151. [Google Scholar] [CrossRef]
- Arokiyaraj, S.; Saravanan, M.; Prakash, N.K.U.; Arasu, M.V.; Vijayakumar, B.; Vincent, S. Enhanced antibacterial activity of iron oxide magnetic nanoparticles treated with Argemone mexicana L. leaf extract: An in vitro study. Mater. Res. Bull. 2013, 48, 3323–3327. [Google Scholar] [CrossRef]
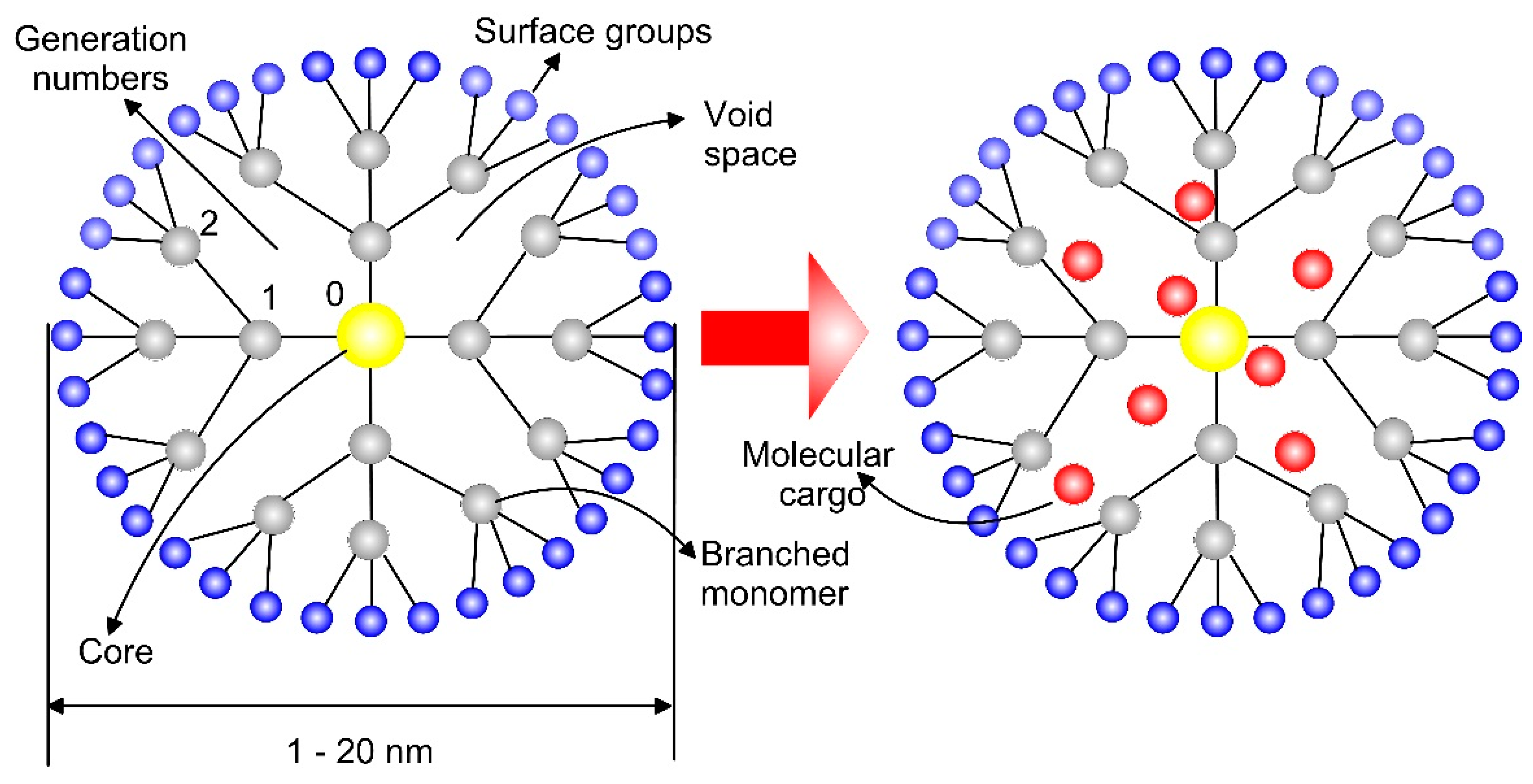
- Chauhan, A.S. Dendrimers for Drug Delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef]
- Naha, P.C.; Mukherjee, S.P.; Byrne, H.J. Toxicology of Engineered Nanoparticles: Focus on Poly(amidoamine) Dendrimers. Int. J. Environ. Res. Public Health 2018, 15, 338. [Google Scholar] [CrossRef]
- Mendes, L.P.; Pan, J.; Torchilin, V.P. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef]
- Agrawal, A.; Min, D.-H.; Singh, N.; Zhu, H.; Birjiniuk, A.; von Maltzahn, G.; Harris, T.J.; Xing, D.; Woolfenden, S.D.; Sharp, P.A.; et al. Functional Delivery of siRNA in Mice Using Dendriworms. ACS Nano 2009, 3, 2495–2504. [Google Scholar] [CrossRef]
- Prieto, M.J.; del Rio Zabala, N.E.; Marotta, C.H.; Gutierrez, H.C.; Arévalo, R.A.; Chiaramoni, N.S.; del Valle Alonso, S. Optimization and In Vivo Toxicity Evaluation of G4.5 Pamam Dendrimer-Risperidoneing Complexes. PLoS ONE 2014, 9, e90393. [Google Scholar] [CrossRef]
- Khodadust, R.; Unsoy, G.; Yalcın, S.; Gunduz, G.; Gunduz, U. PAMAM dendrimer-coated iron oxide nanoparticles: Synthesis and characterization of different generations. J. Nanoparticle Res. 2013, 15, 1488. [Google Scholar] [CrossRef]
- Salimi, M.; Sarkar, S.; Fathi, S.; Alizadeh, A.M.; Saber, R.; Moradi, F.; Delavari, H. Biodistribution, pharmacokinetics and toxicity of dendrimer-coated iron oxide nanoparticles in BALB/c mice. Int. J. Nanomed. 2018, 13, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Bisht, G.; Rayamajhi, S. ZnO nanoparticles: A promising anticancer agent. Nanobiomedicine 2016, 3, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, H. The synergistic effect and mechanism of doxorubicin-ZnO nanocomplexes as a multimodal agent integrating diverse anticancer therapeutics. Int. J. Nanomed. 2013, 8, 1835–1841. [Google Scholar]
- Puvvada, N.; Rajput, S.; Prashanth Kumar, B.; Sarkar, S.; Konar, S.; Brunt, K.R.; Rao, R.R.; Mazumdar, A.; Das, S.K.; Basu, R.; et al. Novel ZnO hollow-nanocarriers containing paclitaxel targeting folate-receptors in a malignant pH-microenvironment for effective monitoring and promoting breast tumor regression. Sci. Rep. 2015, 5, 1–15. [Google Scholar] [CrossRef]
- Dhivya, R.; Ranjani, J.; Rajendhran, J.; Mayandi, J.; Annaraj, J. Enhancing the anti-gastric cancer activity of curcumin with biocompatible and pH-sensitive PMMA-AA/ZnO nanoparticles. Mater. Sci. Eng. C 2018, 82, 182–189. [Google Scholar] [CrossRef]
- Malizia, R.; Scorsone, A.; D’Angelo, P.; Lo Pinto, C.; Pitrolo, L.; Giordano, C. Zinc deficiency and cell-mediated and humoral autoimmunity of insulin-dependent diabetes in thalassemic subjects. J. Pediatr. Endocrinol. Metab. 1998, 11, 981–984. [Google Scholar]
- Hussein, J.; El-Banna, M.; Razik, T.A.; El-Naggar, M.E. Biocompatible zinc oxide nanocrystals stabilized via hydroxyethyl cellulose for mitigation of diabetic complications. Int. J. Biol. Macromol. 2018, 107, 748–754. [Google Scholar] [CrossRef]
- Haugen, H.J.; Monjo, M.; Rubert, M.; Verket, A.; Lyngstadaas, S.P.; Ellingsen, J.E.; Rønold, H.J.; Wohlfahrt, J.C. Porous ceramic titanium dioxide scaffolds promote bone formation in rabbit peri-implant cortical defect model. Acta Biomater. 2012, 9, 5390–5399. [Google Scholar] [CrossRef]
- Martin, P.; Leibovich, S.J. Inflammatory cells during wound repair: The good, the bad and the ugly. Trends Cell Biol. 2005, 15, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cao, S.; Tie, X.; Qiu, B.; Wu, A.; Zheng, Z. Induction of cytotoxicity by photoexcitation of TiO2 can prolong survival in glioma-bearing mice. Mol. Biol. Rep. 2011, 38, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Zeni, P.F.; Santos, D.P.D.; Canevarolo, R.R.; Yunes, J.A.; Padilha, F.F.; de Albuquerque, J.R.L.C.; Egues, S.M.; Hernández-Macedo, M.L. Photocatalytic and Cytotoxic Effects of Nitrogen-Doped TiO2 Nanoparticles on Melanoma Cells. J. Nanosci. Nanotechnol. 2018, 18, 3722–3728. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Li, M.; Cui, J.; Xing, Z.; Li, Z.; Wu, X.; Song, E.; Gong, M.; Zhou, W. 808 nm light triggered black TiO2 nanoparticles for killing of bladder cancer cells. Mater. Sci. Eng. C 2017, 81, 252–260. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, C.; Luo, F.; Li, P.; Xiao, X. P25 nanoparticles decorated on titania nanotubes arrays as effective drug delivery system for ibuprofen. Appl. Surf. Sci. 2015, 324, 621–626. [Google Scholar] [CrossRef]
- Kumeria, T.; Mon, H.; Aw, M.S.; Gulati, K.; Santos, A.; Griesser, H.J.; Losic, D. Advanced biopolymer-coated drug-releasing titania nanotubes (TNTs) implants with simultaneously enhanced osteoblast adhesion and antibacterial properties. Colloids Surf. B 2015, 130, 255–263. [Google Scholar] [CrossRef]
- Masoudi, M.; Mashreghi, M.; Goharshadi, E.; Meshkini, A. Multifunctional fluorescent titania nanoparticles: Green preparation and applications as antibacterial and cancer theranostic agents. Artif. Cells Nanomed. Biotechnol. 2018, 46, 248–259. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhang, C. Synthesis of Diamond-Shaped Mesoporous Titania Nanobricks aspH-Responsive Drug Delivery Vehicles for Cancer Therapy. Chem. Select. 2019, 4, 8225–8228. [Google Scholar]
- Hussain, S.; Vanoirbeek, J.A.; Hoet, P.H. Interactions of nanomaterials with the immune system. Wiley Interdiscip Rev. Nanomed. Nanobiotechnol. 2011, 4, 169–183. [Google Scholar] [CrossRef]
- Elsabahy, M.; Wooley, K.L. Cytokines as biomarkers of nanoparticle immunotoxicity. Chem. Soc. Rev. 2013, 42, 5552–5576. [Google Scholar] [CrossRef]
- Singh, L.; Kruger, H.G.; Maguire, G.E.M.; Govender, T.; Parboosing, R. The role of nanotechnology in the treatment of viral infections. Adv. Infect. Dis. 2017, 4, 105–131. [Google Scholar] [CrossRef] [PubMed]
- Yadavalli, T.; Shukla, D. Role of metal and metal oxide nanoparticles as diagnostic and therapeutic tools for highly prevalent viral infections. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Gorantla, S.; Dou, H.; Boska, M.; Destache, C.J.; Nelson, J.; Poluektova, L.; Rabinow, B.E.; Gendelman, H.E.; Mosley, R.L. Quantitative magnetic resonance and SPECT imaging for macrophage tissue migration and nanoformulated drug delivery. J. Leukoc. Biol. 2006, 80, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Fiandra, L.; Colombo, M.; Mazzucchelli, S.; Truffi, M.; Santini, B.; Allevi, R.; Nebuloni, M.; Capetti, A.; Rizzardini, G.; Prosperi, D.; et al. Nanoformulation of antiretroviral drugs enhance their penetration across the blood-brain barrier in mice. Nanomedicine 2015, 11, 1387–1397. [Google Scholar] [CrossRef]
- Neto, L.M.M.; Kipnis, A.; Junqueira-Kipnis, A.P. Role of Metallic Nanoparticles in Vaccinology: Implications for Infectious Disease Vaccine Development. Front. Immunol. 2017, 8, 1–10. [Google Scholar]
- Laskar, A.; Eilertsen, J.; Li, W.; Yuan, X.M. SPION primes THP1 derived M2 macrophages towards M1-like macrophages. Biochem. Biophys. Res. Commun. 2013, 441, 737–742. [Google Scholar] [CrossRef]
- Shevtsov, M.A.; Nikolaev, B.P.; Yakovleva, L.Y.; Parr, M.A.; Marchenko, Y.Y.; Eliseev, I.; Yudenko, A.; Dobrodumov, A.V.; Zlobina, O.; Zhakhov, A.; et al. 70-kDa heat shock protein-coated magnetic nanocarriers as a nanovaccine for induction of anti-tumor immune response in experimental glioma. J. Control Release A 2015, 200, 329–340. [Google Scholar] [CrossRef]
- Neto, L.M.M.; Zufelato, N.; de Sousa-Júnior, A.A.; Trentini, M.M.; Castro da Costa, A.; Bakuzis, A.F.; Kipnis, A.; Junqueira-Kipnis, A.P. Specific T cell induction using iron oxide-based nanoparticles as subunit vaccine adjuvant. Hum. Vaccin. Immunother. 2018, 14, 2786–2801. [Google Scholar] [CrossRef]
- Mieloch, A.A.; Kręcisz, M.; Rybka, J.D.; Strugała, A.; Krupiński, M.; Urbanowicz, A.; Kozak, M.; Skalski, B.; Figlerowicz, M.; Giersig, M. The influence of ligand charge and length on the assembly of Brome mosaic virus-derived virus-like particles with a magnetic core. AIP Adv. 2018, 8, 035005. [Google Scholar] [CrossRef]
- Li, L.-L.; Yu, P.; Wang, X.; Yu, S.-S.; Mathieu, J.; Yu, H.-Q.; Alvarez, P.J.J. Enhanced biofilm penetration for microbial control by polyvalent phages conjugated with magnetic colloidal nanoparticle clusters (CNCs). Environ. Sci. Nano 2017, 4, 1817–1826. [Google Scholar] [CrossRef]
- Ghosh, D.; Lee, Y.; Thomas, S.; Kohli, A.G.; Yun, D.S.; Belcher, A.M.; Kelly, K.A. M13-templated magnetic nanoparticles for targeted in vivo imaging of prostate cancer. Nat. Nanotechnol. 2012, 7, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Schütz, C.; Varela, J.; Perica, K.; Haupt, C.; Oelke, M.; Schneck, J.P. Antigen-specific T cell Redirectors: A nanoparticle based approach for redirecting T cells. Oncotarget 2016, 7, 68503–68512. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.C.; Zhao, X.; Tan, E.C.; Khamis, N.; Assodani, A.; Xiong, S.; Ruedl, C.; Ng KW, L.J.S. Evaluation of the cytotoxic and inflammatory potential of differentially shaped zinc oxide nanoparticles. Arch. Toxicol. 2011, 85, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Simon-Vazquez, R.; Lozano-Fernandez, T.; Davila-Grana, A.; Gonzalez-Fernandez, A. Metal oxide nanoparticles interact with immune cells and activate different cellular responses. Int. J. Nanomed. 2016, 11, 4657–4668. [Google Scholar] [CrossRef]
- Agelidis, A.; Koujah, L.; Suryawanshi, R.; Yadavalli, T.; Mishra, Y.K.; Adelung, R.; Shukla, D. An Intra-Vaginal Zinc Oxide Tetrapod Nanoparticles (ZOTEN) and Genital Herpesvirus Cocktail Can Provide a Novel Platform for Live Virus Vaccine. Front. Immunol. 2019, 10, 500. [Google Scholar] [CrossRef]
- Wang, C.; Hu, X.; Gao, Y.; Ji, Y. ZnO Nanoparticles Treatment Induces Apoptosis by Increasing Intracellular ROS Levels in LTEP-a-2 Cells. BioMed. Res. Int. 2015, 2015, 423287. [Google Scholar] [CrossRef]
- Thatoi, P.; Kerry, R.G.; Gouda, S.; Das, G.; Pramanik, K.; Thatoi, H.; Patra, J.K. Photo-mediated green synthesis of silver and zinc oxide nanoparticles using aqueous extracts of two mangrove plant species, Heritiera fomes and Sonneratia apetala and investigation of their biomedical applications. J. Photochem. Photobiol. B 2016, 163, 311–318. [Google Scholar] [CrossRef]
- Sharma, P.; Shin, J.B.; Park, B.C.; Lee, J.W.; Byun, S.W.; Jang, N.Y.; Kim, Y.J.; Kim, Y.; Kim, Y.K.; Cho, N.H. Application of radially grown ZnO nanowires on poly-l-lactide microfibers complexed with a tumor antigen for cancer immunotherapy. Nanoscale 2019, 11, 4591–4600. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, H.R.; Dong, L.; Xu, M.R.; Zhang, L.; Ding, W.P.; Zhang, J.Q.; Lin, J.; Zhang, Y.J.; Qiu, B.S.; et al. Enhancing tumor chemotherapy and overcoming drug resistance through autophagy-mediated intracellular dissolution of zinc oxide nanoparticles. Nanoscale 2019, 11, 11789–11807. [Google Scholar] [CrossRef]
- Charitidis, C.A.; Georgiou, P.; Koklioti, M.A.; Trompeta, A.-F.; Markakis, V. Manufacturing nanomaterials: From research to industry. Manuf. Rev. 2014, 1, 1–11. [Google Scholar] [CrossRef]
- Wierzbinski, K.R.; Szymanski, T.; Rozwadowska, N.; Rybka, J.D.; Zimna, A.; Zalewski, T.; Nowicka-Bauer, K.; Malcher, A.; Nowaczyk, M.; Krupinski, M.; et al. Potential use of superparamagnetic iron oxide nanoparticles for in vitro and in vivo bioimaging of human myoblasts. Sci. Rep. 2018, 8, 3682. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, K.; Ihsan, N.; Irie, K.; Mishima, K.; Okuyama, T. Bioimaging application of highly luminescent silica-coated ZnO-nanoparticle quantum dots with biotin. J. Colloid Interface Sci. 2013, 399, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Barua, S.; Sharma, G.; Dey, S.K.; Rege, K. Inorganic nanoparticles for cancer imaging and therapy. J. Control Release 2011, 155, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Estelrich, J.; Sánchez-Martín, M.J.; Busquets, M.A. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar]
- Yoo, D.; Lee, J.H.; Shin, T.H.; Cheon, J. Theranostic magnetic nanoparticles. Acc. Chem Res. 2011, 44, 863–874. [Google Scholar] [CrossRef]
- Jang, J.T.; Nah, H.; Lee, J.H.; Moon, S.H.; Kim, M.G.; Cheon, J. Critical enhancements of MRI contrast and hyperthermic effects by dopant-controlled magnetic nanoparticles. Angew. Chem. Int. Ed. Engl. 2009, 48, 1234–1238. [Google Scholar] [CrossRef]
- Soenen, S.J.; Himmelreich, U.; Nuytten, N.; Pisanic 2nd, T.R.; Ferrari, A.; De Cuyper, M. Intracellular nanoparticle coating stability determines nanoparticle diagnostics efficacy and cell functionality. Small 2010, 6, 2136–2145. [Google Scholar] [CrossRef]
- Javidi, M.; Zarei, M.; Naghavi, N.; Mortazavi, M.; Nejat, A.H. Zinc oxide nanoparticles as a sealer in endodontics and its sealing ability. Contemp. Clin. Dent. 2014, 5, 20–24. [Google Scholar]
- Zebarjad, S.M. Synthesis and characterization of nanoparticles and nanocomposite of ZnO and MgO by the sonochemical method and their application for zinc polycarboxylate dental cement preparation. Int. Nano Lett. 2011, 1, 43–51. [Google Scholar]
- Cierech, M.; Osica, I.; Kolenda, A.; Wojnarowicz, J.; Szmigiel, D.; Łojkowski, W.; Kurzydłowski, K.; Ariga, K.; Mierzwinska-Nastalska, K. Mechanical and Physicochemical Properties of Newly Formed ZnO-PMMA Nanocomposites for Denture Bases. Nanomaterials 2018, 8, 305. [Google Scholar] [CrossRef]
- Huang, L.; Jing, S.; Zhuo, O.; Meng, X.; Wang, X. Surface Hydrophilicity and Antifungal Properties of TiO2 Films Coated on a Co-Cr Substrate. BioMed. Res. Int. 2017, 2017, 2054723. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wen, J.; Li, B.; Li, W.; Qiao, W.; Shen, J.; Jin, W.; Jiang, X.; Yeung, K.W.K.; Chu, P.K. Valence State Manipulation of Cerium Oxide Nanoparticles on a Titanium Surface for Modulating Cell Fate and Bone Formation. Adv. Sci. 2018, 5, 1700678. [Google Scholar] [CrossRef] [PubMed]
- Karakoti, A.S.; Tsigkou, O.; Yue, S.; Lee, P.D.; Stevens, M.M.; Jones, J.R.; Seal, S. Rare earth oxides as nano-additives in 3-D nanocomposite scaffolds for bone regeneration. J. Mater. Chem. 2010, 20, 8912–9819. [Google Scholar] [CrossRef]
- Khlusov, I.; Litvinova, L.; Shupletsova, V.; Khaziakhmatova, O.; Melashchenko, E.; Yurova, K.; Leitsin, V.; Khlusova, M.; Pichugin, V.; Sharkeev, Y. Rough Titanium Oxide Coating Prepared by Micro-Arc Oxidation Causes Down-Regulation of hTERT Expression, Molecular Presentation and Cytokine Secretion in Tumor Jurkat T Cells. Materials 2018, 11, 360. [Google Scholar] [CrossRef] [PubMed]
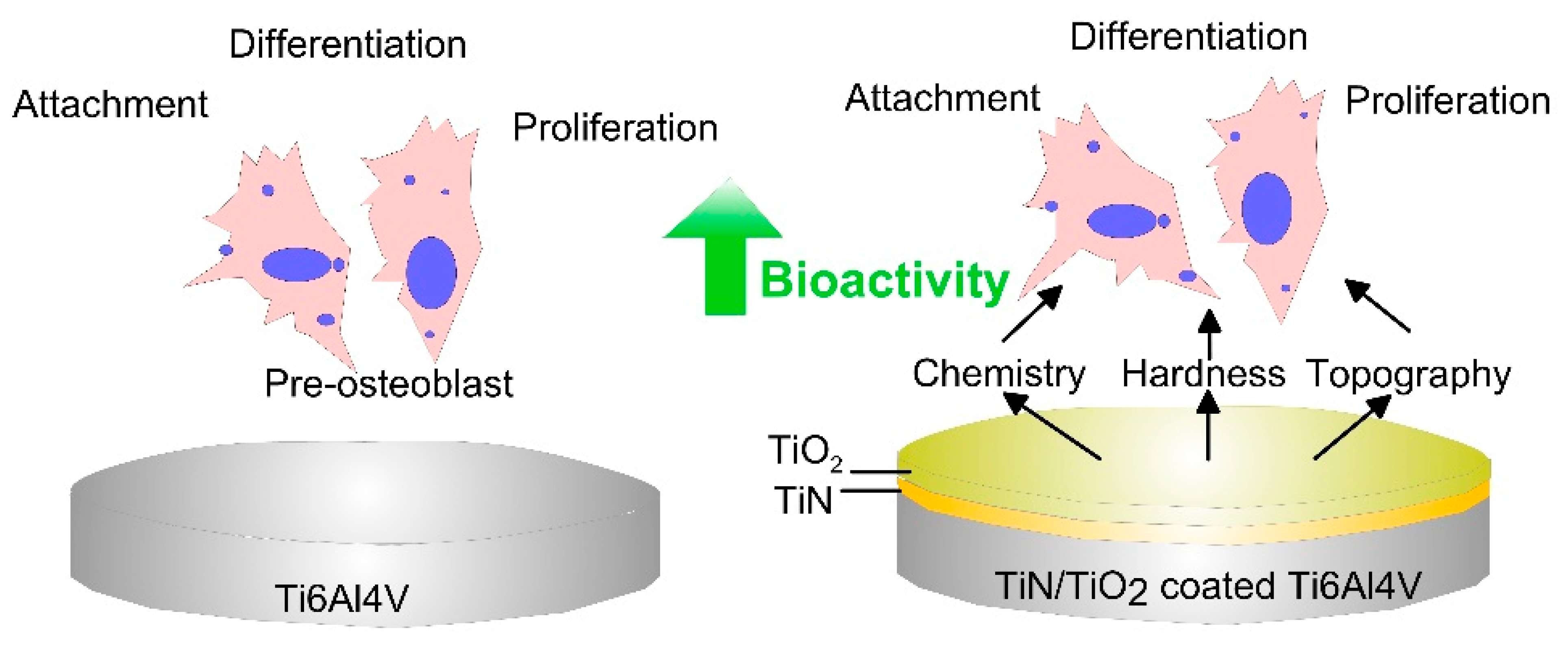
- Nikolova, M.P.; Yankov, E.; Petrov, P.; Valkov, S.; Ormanova, M.; Zaharieva, V.; Tonev, D.; Andreeva, A. Electron beam surface modification of Ti5Al4V alloy for biomedical applications. In Proceedings of the METAL 2017 International Conference on Metallurgy and Materials, Brno, Czech Republic, 24–26 May 2017; pp. 1555–1559. [Google Scholar]
- Petrov, P.; Dechev, D.; Ivanov, N.; Hikov, T.; Valkov, S.; Nikolova, M.; Yankov, E.; Parshorov, S.; Bezdushnyi, R.; Andreeva, A. Study of the influence of electron beam treatment of Ti5Al4V substrate on the mechanical properties and surface topography of multilayer TiN/TiO2 coatings. Vacuum 2018, 154, 264–271. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Nikolova, V.; Ivanova, V.L.; Valkov, S.; Petrov, P.; Apostolova, M.D. Mechanical Properties and In Vitro Biocompatibility Evaluation of TiN/TiO2 Coated Ti6Al4V Alloy. Mater. Today Proc. 2020, in press. [Google Scholar] [CrossRef]
- Wang, N.; Li, H.; Lü, W.; Li, J.; Wang, J.; Zhang, Z.; Liu, Y. Effects of TiO2 nanotubes with different diameters on gene expression and osseointegration of implants in minipigs. Biomaterials 2011, 32, 6900–6911. [Google Scholar] [CrossRef]
- Labouta, H.I.; Schneider, M. Interaction of inorganic nanoparticles with the skin barrier: Current status and critical review. Nanomedicine 2013, 9, 39–54. [Google Scholar] [CrossRef]
- Shokri, N.; Javar, H.A. Comparison of calcium phosphate and zinc oxide nanoparticles as dermal penetration enhancers for albumin. Indian J. Pharm. Sci. 2015, 77, 694–704. [Google Scholar] [CrossRef]
- Niethammer, P.; Grabher, C.; Look, A.T.; Mitchison, T.J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 2009, 459, 996–999. [Google Scholar] [CrossRef]
- Augustine, R.; Malik, H.N.; Singhal, D.K.; Mukherjee, A.; Malakar, D.; Kalarikkal, N.; Thomas, S. Electrospun polycaprolactone/ZnO nanocomposite membranes as biomaterials with antibacterial and cell adhesion properties. J. Polym. Res. 2014, 21, 347. [Google Scholar] [CrossRef]
- Balen, R.; de Costa, W.V.; de Lara Andrade, J.; Piai, J.F.; Muniz, E.C.; Companhoni, M.V.; Nakamura, T.U.; Lima, S.M.; da Cunha Andrade, L.H.; Bittencourt, P.R.S.; et al. Structural, thermal, optical properties and cytotoxicity of PMMA/ZnO fibres and films: Potential application in tissue engineering. Appl. Surf. Sci. 2016, 385, 257–267. [Google Scholar] [CrossRef]
- Lu, Z.; Gao, J.; He, Q.; Wu, J.; Liang, D.; Yang, H.; Chen, R. Enhanced antibacterial and wound healing activities of microporous chitosan-Ag/ZnO composite dressing. Carbohydr. Polym. 2017, 156, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Babitha, S.; Korrapati, P.S. Biodegradable zein-polydopamine polymeric scaffold impregnated with TiO2 nanoparticles for skin tissue engineering. Biomed. Mater. 2017, 12, 055008. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Ullah, H.; Ul-Islam, M.; Khan, R.; Khan, S.; Ahmad, F.; Khan, T.; Wahid, F. Bacterial cellulose–TiO2 nanocomposites promote healing and tissue regeneration in burn mice model. RSC Adv. 2017, 7, 47662. [Google Scholar] [CrossRef]
- AlKahtani, R.N. The implications and applications of nanotechnology in dentistry: A review. Saudi Dent. J. 2018, 30, 107–116. [Google Scholar] [CrossRef] [PubMed]
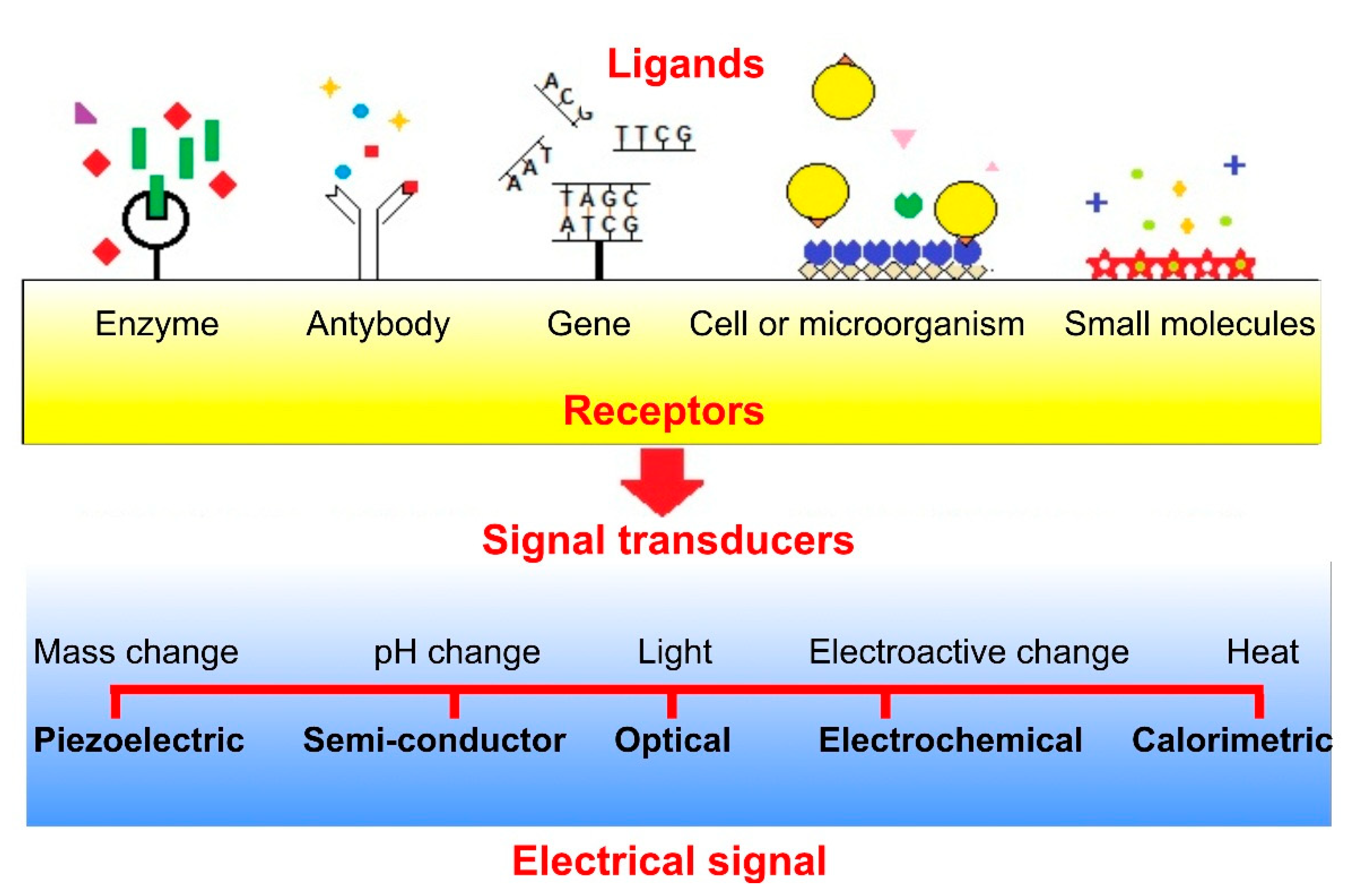
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xia, J.; Wang, L.; Qian, J.; Li, H.; Wang, G.; Sun, K.; He, M. α-Fe2O3 Cubes with High Visible-Light-Activated Photoelectrochemical Activity towards Glucose: Hydrothermal Synthesis Assisted by a Hydrophobic Ionic Liquid. Chem. Eur. J. 2014, 20, 2244–2253. [Google Scholar] [CrossRef]
- Solanki, P.R.; Kaushik, A.; Agrawal, V.V.; Malhotra, B.D. Nanostructured metal oxide-based biosensors. NPG Asia Mater. 2011, 3, 17–24. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, K.; Wei, Y.; Ma, Y.; Liu, L.; Huang, X. Laccase Biosensor Based on Phytic Acid Modification of Nanostructured SiO2 Surface for Sensitive Detection of Dopamine. Langmuir 2014, 30, 11131–11137. [Google Scholar] [CrossRef]
- Cheng, J.; Di, J.; Hong, J.; Yao, K.; Sun, Y.; Zhuang, J.; Xu, Q.; Zheng, H.; Bi, S. The promotion effect of titania nanoparticles on the direct electrochemistry of lactate dehydrogenase sol-gel modified gold electrode. Talanta 2008, 76, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Wang, N.; Lidong, L.; Lin, G. Synthesis and characterization of waxberry-like microstructures ZnO for biosensors. Sens. Actuators B Chem. 2008, 129, 268–273. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ahammad, A.J.S.; Jin, J.-H.; Ahn, S.J.; Lee, J.-J. A comprehensive review of glucose biosensors based on nanostructured metal-oxides. Sensors 2010, 10, 4855–4886. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, H.; Ni, Y.; Zhang, Q.; Chen, J. Porous nanosheet-based ZnO microspheres for the construction of direct electrochemical biosensors. Biosens. Bioelectron. 2008, 24, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Abu-Salah, K.M.; Alrokyan, S.A.; Khan, M.N.; Ansari, A.A. Nanomaterials as Analytical Tools for Genosensors. Sensors 2010, 10, 963–993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, T.; Zhuang, X.; Guo, Z.; Jiao, K. An ionic liquid supported CeO2 nanoshuttles-carbon nanotubes composite as a platform for impedance DNA hybridization sensing. Biosens. Bioelectron. 2009, 24, 2417–2422. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, T.; Li, X.; Wang, D.; Jiao, K. Conductive architecture of Fe2O3 microspheres/self-doped polyaniline nanofibers on carbon ionic liquid electrode for impedance sensing of DNA hybridization. Biosens. Bioelectron. 2009, 25, 428–434. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, J.G.; Maji, S.; Malhotra, B.D. Nanostructured zirconia decorated reduced graphene oxide-based efficient biosensing platform for non-invasive oral cancer detection. Biosens. Bioelectron. 2016, 78, 497–504. [Google Scholar] [CrossRef]
- Kaushik, A.; Solanki, P.R.; Ansari, A.A.; Ahmad, S.; Malhotra, B.D. A nanostructured cerium oxide film-based immunosensor for mycotoxin detection. Nanotechnology 2009, 20, 055105. [Google Scholar] [CrossRef]
- Chang, C.C.; Chiu, N.F.; Lin, D.S.; Chu-Su, Y.; Liang, Y.H.; Lin, C.W. High-sensitivity detection of carbohydrate antigen 15-3 using a gold/zinc oxide thin film surface plasmon resonance-based biosensor. Anal. Chem. 2010, 82, 1207–1212. [Google Scholar] [CrossRef]
- Wang, R.; Ruan, C.; Kanayeva, D.; Lassiter, K.; Li, Y. TiO2 nanowire bundle microelectrode based impedance immunosensor for rapid and sensitive detection of Listeria monocytogenes. Nano Lett. 2008, 8, 2625–2631. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Ju, H. Applications of Nanomaterials in Sensors and Diagnostics, Signal Amplification Using Nanomaterials for Biosensing; Tuantranont, A., Ed.; Springer Series on Chemical Sensors and Biosensors; Springer: Berlin/Heildelberg, Germany, 2013; pp. 17–42. [Google Scholar]
- Arlett, J.; Myers, E.; Roukes, M. Comparative advantages of mechanical biosensors. Nat. Nanotechnol. 2011, 6, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Achkar, J.M.; Fries, B.C. Candida infections of the genitourinary tract. Clin. Microbiol. Rev. 2010, 23, 253–273. [Google Scholar] [CrossRef] [PubMed]
- Knetsch, M.L.; Koole, L.H. New strategies in the development of antimicrobial coatings: The example of increasing usage of silver and silver nanoparticles. Polymers 2011, 3, 340–366. [Google Scholar] [CrossRef]
- Yamamoto, O.; Sawai, J.; Sasamoto, T. Change in antibacterial characteristics with a doping amount of ZnO in MgO-ZnO solid solution. Int. J. Inorg. Mater. 2000, 2, 451–454. [Google Scholar] [CrossRef]
- Sawai, J.; Kojima, H.; Igarashi, H.; Hashimoto, A.; Shoji, S.; Sawaki, T.; Hakoda, A.; Kawada, E.; Kokugan, T.; Shimizu, M. Antibacterial characteristics of magnesium oxide powder. World J. Microbiol. Biotechnol. 2000, 16, 187–194. [Google Scholar] [CrossRef]
- Ijaz, F.; Shahid, S.; Khan, S.A.; Ahmad, W.; Zaman, S. Green synthesis of copper oxide nanoparticles using Abutilon indicum leaf extract: Antimicrobial, antioxidant and photocatalytic dye degradation activities. Trop. J. Pharm. Res. 2017, 16, 743–753. [Google Scholar] [CrossRef]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative Antimicrobial Approach: Nano-Antimicrobial Materials. Evid. Based Complementary Altern. Med. 2015, 2015, 246012. [Google Scholar] [CrossRef]
- Devi, L.G.; Nagaraj, B. Disinfection of Escherichia coli gram-negative bacteria using surface modified TiO2: Optimization of Ag metallization and depiction of the charge transfer mechanism. J. Photoch. Photobio. A 2014, 90, 1089–1098. [Google Scholar]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Yuan, X.; Wang, X.; Shao, P. Zinc accumulation and synthesis of ZnO nanoparticles using Physalis alkekengi L. Environ. Pollut. 2011, 159, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- Ledwith, D.M.; Whelan, A.M.; Kelly, J.M. A rapid, straightforward method for controlling the morphology of stable silver nanoparticles. J. Mater. Chem. 2007, 17, 2459–2464. [Google Scholar] [CrossRef]
- Sorbiun, M.; Mehr, E.S.; Ramazani, A.; Malekzadeh, A.M. Biosynthesis of metallic nanoparticles using plant extracts and evaluation of their antibacterial properties. Nanochem. Res. 2018, 3, 1–16. [Google Scholar]
- Agarwal, H.; Kumar, S.V.; Rajeshkumar, S. A review of the green synthesis of zinc oxide nanoparticles—An eco-friendly approach. Resour. Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Hemalatha, S.; Makeswari, M. Green Synthesis, Characterization and Antibacterial Studies of CuO Nanoparticles from Eichhornia crassipes. Rasayan J. Chem. 2017, 10, 838–843. [Google Scholar]
- Rezaie, A.B.; Montazer, M.; Rad, M.M. A cleaner route for nano colouration of wool fabric via green assembling of cupric oxide nanoparticles along with antibacterial and UV protection properties. J. Clean Prod. 2017, 166, 221–231. [Google Scholar] [CrossRef]
- Sharma, D.; Thakur, N.; Vashistt, J.; Bisht, G.S. Antibacterial Evaluation of Cuprous Oxide Nanoparticles Synthesized Using Leaf Extract of Callistemon viminalis. Indian J. Pharmaceut. Edu. Res. 2018, 52, 449–455. [Google Scholar] [CrossRef]
- Devi, H.S.; Boda, M.A.; Shah, M.A.; Parveen, S.; Wani, A.H. Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity. Green Process. Synth. 2019, 8, 38–45. [Google Scholar] [CrossRef]
- Kanagasubbulakshmi, S.; Kadirvelu, K. Green Synthesis of Iron Oxide Nanoparticles using Lagenaria Siceraria and Evaluation of its Antimicrobial Activity. Def. Life Sci. J. 2017, 2, 422–427. [Google Scholar] [CrossRef]
- Saranya, S.; Vijayarani, K.; Pavithra, S. Green Synthesis of Iron Nanoparticles using an aqueous Extract of Musa ornata Flower Sheath against Pathogenic Bacteria. Indian J. Pharmaceut. Sci. 2017, 79, 688–694. [Google Scholar] [CrossRef]
- Santhoshkumar, J.; Kumar, S.V.; Rajeshkumar, S. Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resour. Effic. Technol. 2017, 3, 459–465. [Google Scholar] [CrossRef]
- Ramesh, M.; Anbuvannan, M.; Viruthagiri, G. Green synthesis of ZnO nanoparticles using Solanum nigrum leaf extract and their antibacterial activity. Spectrochim. Acta A. 2015, 136, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhabi, N.A.; Arasu, M.V. Environmentally-Friendly Green Approach for the Production of Zinc Oxide Nanoparticles and Their Anti-Fungal, Ovicidal and Larvicidal Properties. Nanomaterials 2018, 8, 500. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Mahadevan, S.; Arulmozhi, P.; Sriram, S.; Praseetha, P.K. Green synthesis of zinc oxide nanoparticles using Atalantia monophylla leaf extracts: Characterization and antimicrobial analysis. Mater. Sci. Semicon Proc. 2018, 82, 39–45. [Google Scholar] [CrossRef]
- Irshad, S.; Salamat, A.; Anjum, A.A.; Sana, S.; Saleem, R.S.Z.; Naheed, A.; Iqbal, A. Green tea leaves mediated ZnO nanoparticles and its antimicrobial activity. Cogent Chem. 2018, 4, 1469207. [Google Scholar] [CrossRef]
- Hajiashrafi, S.; Motakef-Kazemi, N. Green synthesis of zinc oxide nanoparticles using parsley extract. Nanomed. Res. J. 2018, 3, 44–50. [Google Scholar]
- Sharmila, G.; Muthukumaran, C.; Sandiya, K.; Pradeep, R.S.; Kumar, N.M.; Suriyanarayanan, N.; Thirumarimurugan, M. Biosynthesis, characterization and antibacterial activity of zinc oxide nanoparticles derived from Bauhinia tomentosa leaf extract. J. Nanostructure Chem. 2018, 8, 293–299. [Google Scholar] [CrossRef]
- Khatami, M.; Alijani, H.Q.; Heli, H.; Sharifi, I. Rectangular shaped zinc oxide nanoparticles: Green synthesis by Stevia and its biomedical efficiency. Ceram. Int. 2018, 44, 15596–15602. [Google Scholar] [CrossRef]
- Khan, S.A.; Noreen, F.; Kanwal, S.; Iqbal, A.; Hussain, G. Green synthesis of ZnO and Cu-doped ZnO nanoparticles from leaf extracts of Abutilon indicum, Clerodendrum infortunatum, Clerodendrum inerme and investigation of their biological and photocatalytic activities. Mater. Sci. Eng. C 2017, 82, 46–59. [Google Scholar] [CrossRef]
- Kannan, S.K.; Sundararajan, M. Green synthesis of ruthenium oxide nanoparticles: Characterization and its antibacterial activity. Adv. Powder Technol. 2015, 26, 1505–1511. [Google Scholar] [CrossRef]
- Arumugam, A.; Karthikeyan, C.; Hameed, A.S.H.; Gopinath, K.; Gowri, S.; Karthika, V. Synthesis of cerium oxide nanoparticles using Gloriosa superba L. leaf extract and their structural, optical and antibacterial properties. Mater. Sci. Eng. C 2015, 49, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Radhika, D.; Nikolova, M.P.; Sadasivuni, K.K.; Mahdizadeh, H.; Verma, U. Structural studies of bio-mediated NiO nanoparticles for photocatalytic and antibacterial activities. Inorg Chem. Commun. 2020, 113, 107755. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Abdul Rahuman, A.; Vishnu Kirthi, A.; Marimuthu, S.; Santhoshkumar, T.; Bagavan, A.; Gaurav, K.; Karthik, L.; Rao, K.V.B. Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim. Acta A 2012, 90, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, M.; Gopinath, V.; Chaurasia, M.K.; Syed, A.; Ameen, F.; Purushothaman, N. Green synthesis of anisotropic zinc oxide nanoparticles with antibacterial and cytofriendly properties. Microb. Pathog. 2018, 115, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, G.; Abdulrahman, A.; Roopan, S.M.; Khanna, V.G.; Elango, G.; Kamaraj, C.; Zahir, A.A.V.; Velayutham, K. Fungus-mediated biosynthesis and characterization of TiO2 nanoparticles and their activity against pathogenic bacteria. Spectroch. Acta A 2012, 91, 23–29. [Google Scholar] [CrossRef]
- Ibrahem, E.J.; Thalij, K.M.; Saleh, M.K.; Badawy, A.S. Biosynthesis of Zinc Oxide Nanoparticles and Assay of Antibacterial Activity. Am. J. Biochem. Biotechnol. 2017, 13, 63–69. [Google Scholar] [CrossRef]
- Kalpana, V.N.; Kataru, B.A.S.; Sravani, N.; Vigneshwari, T.; Panneerselvam, A.; Rajeswari, V.D. Biosynthesis of zinc oxide nanoparticles using culture filtrates of Aspergillus niger: Antimicrobial textiles and dye degradation studies. OpenNano 2018, 3, 48–55. [Google Scholar] [CrossRef]
- Abboud, Y.; Saffaj, T.; Chagraoui, A.; El Bouari, A.; Brouzi, K.; Tanane, O.; Ihssane, B. Biosynthesis, characterization and antimicrobial activity of copper oxide nanoparticles (CONPs) produced using brown alga extract (Bifurcaria bifurcata). Appl. Nanosci. 2014, 4, 571–576. [Google Scholar] [CrossRef]
- Saran, S.; Sharma, G.; Kumar, M.; Ali, M.I. Biosynthesis of Copper Oxide Nanoparticles using Cyanobacteria Spirulina Platensys and its Antibacterial activity. Int. J. Pharmaceut. Sci. Res. 2017, 8, 3887–3892. [Google Scholar]
- Alsaggaf, M.S. Applicable Control of Antimicrobial Resistant Skin Pathogens using Algal-Synthesized Zinc Oxide Nanoparticles. Biosci. Biotechnol. Res. Asia 2018, 15, 111–117. [Google Scholar] [CrossRef]
- Geetha, A.; Sakthivel, R.; Mallika, J.; Kannusamy, R.; Rajendran, R. Green Synthesis of antibacterial Zinc oxide Nanoparticles using biopolymer Azadirachtaindica gum. Orient. J. Chem. 2016, 32, 955–963. [Google Scholar] [CrossRef]
- Chikkanna, M.M.; Neelagund, S.; Hiremath, M.B. Biological Synthesis, Characterization and Antibacterial Activity of Novel Copper Oxide Nanoparticles (CuONPs). J. Bionanosci. 2018, 12, 1–8. [Google Scholar] [CrossRef]
- Joost, U.; Juganson, K.; Visnapuu, M.; Mortimer, M.; Kahru, A.; Nõmmiste, E.; Joost, U.; Kisand, V.; Ivask, A. Photocatalytic antibacterial activity of nano-TiO2 (anatase)-based thin films: Effects on Escherichia coli cells and fatty acids. J. Photochem. Photobiol. B 2015, 142, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Chung, H.; Choi, W.; Yoon, J. Linear correlation between inactivation of E. coli and OH radical concentration in TiO2 photocatalytic disinfection. Water Res. 2004, 38, 1069–1077. [Google Scholar] [CrossRef]
- Kiwi, J.; Nadtochenko, V. New evidence for TiO2 photocatalysis during bilayer lipid peroxidation. J. Phys. Chem. B 2004, 108, 17675–17684. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G. Potent antibacterial activities of Ag/TiO2 nanocomposite powders synthesized by a one-pot sol-gel method. Environ. Sci. Technol. 2009, 43, 2905–2910. [Google Scholar] [CrossRef]
- Desai, V.S.; Kowshik, M. Antimicrobial Activity of Titanium Dioxide Nanoparticles Synthesized by Sol-Gel Technique. Res. J. Microbiol. 2009, 4, 97–103. [Google Scholar]
- Kubacka, A.; Diez, M.S.; Rojo, D.; Bargiela, R.; Ciordia, S.; Zapico, I.; Albar, J.P.; Barbas, C.; Martins dos Santos, V.A.P.; Fernández-García, M.; et al. Understanding the antimicrobial mechanism of TiO2-based nanocomposite films in a pathogenic bacterium. Sci. Rep. 2014, 4, 4134. [Google Scholar] [CrossRef]
- Ashkarran, A.A.; Aghigh, S.M.; Farahani, N.J. Visible light photo-and bioactivity of Ag/TiO2 nanocomposite with various silver contents. Curr. Appl. Phys. 2011, 11, 1048–1055. [Google Scholar] [CrossRef]
- Nakano, R.; Hara, M.; Ishiguro, H.; Yao, Y.; Ochiai, T.; Nakata, K.; Murakami, T.; Kajioka, J.; Sunada, K.; Hashimoto, K.; et al. Broad-spectrum microbicidal activity of photocatalysis by TiO2. Catalysts 2013, 3, 310–323. [Google Scholar] [CrossRef]
- Senarathna, U.L.N.H.; Fernando, S.S.N.; Gunasekara, T.D.C.P.; Weerasekera, M.M.; Hewageegana, H.G.S.P.; Arachchi, N.D.H.; Siriwardena, H.D.; Jayaweera, P.M. Enhanced antibacterial activity of TiO2 nanoparticle surface modified with Garcinia zeylanica extract. Chem. Central J. 2017, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Jiang, J.; Gu, W.; Sun, C.; Wu, D.; Yang, T.; Yang, G. Photocatalytic inactivation efficiency of anatase nano-TiO2 sol on the H9N2 avian influenza virus. Photochem. Photobiol. 2010, 86, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Levina, A.S.; Repkova, M.N.; Mazurkova, N.A.; Makarevich, E.V.; Ismagilov, Z.R.; Zarytova, V.F. Knockdown of different influenza a virus subtypes in cell culture by a single antisense oligodeoxyribonucleotide. Int. J. Antimicrob. Agents 2015, 46, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.S.; Nisha, M.M.; Joice, M.; Shilpa, P.N. Antimicrobial activity of zinc oxide (ZnO) nanoparticle against Klebsiella pneumonia. Pharmaceut. Biol. 2014, 52, 1388–1397. [Google Scholar] [CrossRef]
- Kasraei, S.; Sami, L.; Hendi, S.; Ali Khani, M.-Y.; Rezaei-Soufi, L.; Khamverdi, Z. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutants and Lactobacillus. Restor. Dent. Endod. 2014, 39, 109–114. [Google Scholar] [CrossRef]
- Palanikumar, L.; Ramasamy, S.N.; Balachandran, C. Size-dependent antimicrobial response of zinc oxide nanoparticles. IET Nanobiotechnol. 2014, 8, 111–117. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, B.; Jiang, H.; Wang, C.; Wang, H.; Wang, X. A strategy for ZnO nanorod mediated multi-mode cancer treatment. Biomaterials 2011, 32, 1906–1914. [Google Scholar] [CrossRef]
- Hirota, K.; Sugimoto, M.; Kato, M.; Tsukagoshi, K.; Tanigawa, T.; Sugimoto, H. Preparation of zinc oxide ceramics with a sustainable antibacterial activity under dark conditions. Ceram. Int. 2010, 36, 497–506. [Google Scholar] [CrossRef]
- Ramani, M.; Ponnusamy, S.; Muthamizhchelvan, C.; Marsili, E. Amino acid-mediated synthesis of zinc oxide nanostructures and evaluation of their facet-dependent antimicrobial activity. Colloids Surf. B 2014, 117, 233–239. [Google Scholar] [CrossRef]
- Mostafa, A.A. Antibacterial Activity of Zinc Oxide Nanoparticles against Toxigenic Bacillus cereus and Staphylococcus aureus Isolated from Some Egyptian Food. Int. J. Microbiol. Res. 2015, 6, 145–154. [Google Scholar]
- Leung, Y.H.; Chan, C.M.N.; Ng, A.M.C.; Chan, H.T.; Chiang, M.W.L.; Djurišić, A.B.; Ng, Y.H.; Jim, W.Y.; Guo, M.Y.; Leung, F.C.C.; et al. Antibacterial activity of ZnO nanoparticles with a modified surface under ambient illumination. Nanotechnology 2012, 23, 475703. [Google Scholar] [CrossRef] [PubMed]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal oxide nanoparticles as bactericidal agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Kadiyala, U.; Turali-Emre, E.S.; Bahng, J.H.; Kotov, N.A.; VanEpps, J.S. Unexpected insights into the antibacterial activity of zinc oxide nanoparticles against methicillin-resistant Staphylococcus aureus (MRSA). Nanoscale 2018, 10, 4927–4939. [Google Scholar] [CrossRef] [PubMed]
- Shaalan, M.I.; El-Mahdy, M.M.; Theiner, S.; El-Matbouli, M.; Saleh, M. In vitro assessment of the antimicrobial activity of silver and zinc oxide nanoparticles against fish pathogens. Acta Vet. Scand. 2017, 59, 49. [Google Scholar] [CrossRef] [PubMed]
- Antoine, T.E.; Mishra, Y.K.; Trigilio, J.; Tiwari, V.; Adelung, R.; Shukla, D.P. Prophylactic, Therapeutic and neutralizing effects of zinc oxide tetrapod structures against herpes simplex virus type-2 infection. Antiviral Res. 2012, 96, 363–375. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Adelung, R.; Röhl, C.; Shukla, D.; Spors, F.; Tiwari, V. Virostatic potential of micro-nano filopodia-like ZnO structures against herpes simplex virus-1. Antiviral. Res. 2011, 92, 305–312. [Google Scholar] [CrossRef]
- Wang, Z.; Von Dem Bussche, A.; Kabadi, P.K.; Kane, A.B.; Hurt, R.H. Biological and environmental transformations of copper-based nanomaterials. ACS Nano 2013, 7, 8715–8727. [Google Scholar] [CrossRef]
- Jo, H.J.; Choi, J.W.; Lee, S.H.; Hong, S.W. Acute toxicity of Ag and CuO nanoparticle suspensions against Daphnia magna: The importance of their dissolved fraction varying with preparation methods. J. Hazard. Mater. 2012, 227, 301–308. [Google Scholar] [CrossRef]
- Agarwala, M.; Choudhury, B.; Yadav, R.N.S. Comparative study of antibiofilm activity of copper oxide and iron oxide nanoparticles against multidrug-resistant biofilm forming uropathogens. Indian J. Microbiol. 2014, 54, 365–368. [Google Scholar] [CrossRef]
- Hsueh, Y.-H.; Tsai, P.-H.; Lin, K.-S. pH-Dependent Antimicrobial Properties of Copper Oxide Nanoparticles in Staphylococcus aureus. Int. J. Mol. Sci. 2017, 18, 793. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, L.M.; Albalghiti, E.M.; Fishman, Z.S.; Perreault, F.; Corredor, C.; Posner, J.D.; Elimelech, M.; Pfefferle, L.D.; Zimmerman, J.B. Shape-dependent surface reactivity and antimicrobial activity of nano-cupric oxide. Environ. Sci. Technol. 2016, 50, 3975–3984. [Google Scholar] [CrossRef] [PubMed]
- Meghana, S.; Kabra, P.; Chakraborty, S.; Padmavathy, N. Understanding the pathway of antibacterial activity of copper oxide nanoparticles. RSC Adv. 2015, 5, 12293–12299. [Google Scholar] [CrossRef]
- Khan, S.T.; Ahamed, M.; Al-Khedhairy, A.; Musarrat, J. Biocidal effect of copper and zinc oxide nanoparticles on the human oral microbiome and biofilm formation. Mater. Lett. 2013, 97, 67–70. [Google Scholar] [CrossRef]
- Semisch, A.; Ohle, J.; Witt, B.; Hartwig, A. Cytotoxicity and genotoxicity of nano-and microparticulate copper oxide: The role of solubility and intracellular bioavailability. Part Fibre Toxicol. 2014, 11, 1–16. [Google Scholar] [CrossRef]
- Jin, S.-E.; Hwang, W.; Lee, H.J.; Jin, H.-E. Dual UV irradiation-based metal oxide nanoparticles for enhanced antimicrobial activity in Escherichia coli and M13 bacteriophage. Int. J. Nanomed. 2017, 12, 8057–8070. [Google Scholar] [CrossRef]
- Guo, J.; Gao, S.-H.; Lu, J.; Bond, P.L.; Verstraete, W.; Yuan, Z. Copper Oxide Nanoparticles Induce Lysogenic Bacteriophage and Metal-Resistance Genes in Pseudomonas aeruginosa PAO1. ACS Appl. Mater. Interfaces 2017, 9, 22298–22307. [Google Scholar] [CrossRef]
- Nies, D.H. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 1999, 51, 730–750. [Google Scholar] [CrossRef]
- Jung, W.; Koo, H.; Kim, K.; Shin, S.; Kim, S.; Park, Y. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef]
- Walder, B.; Pittet, D.; Tramer, M. Prevention of bloodstream infections with central venous catheters treated with anti-infective agents depends on catheter type and insertion time: Evidence from a meta-analysis. Infect. Cont Hosp. Ep. 2002, 23, 748–756. [Google Scholar] [CrossRef]
- Fernandez, C.; Thomas, A.; Shailaja Raj, M. Green Synthesis of Silver Oxide Nanoparticle and Its Antimicrobial Activity against Organisms Causing Dental Plaques. Int. J. Pharm. Bio. Sci. 2016, 7, 14–19. [Google Scholar] [CrossRef]
- Ashokraja, C.; Sakar, M.; Balakumar, S. A perspective on the hemolytic activity of chemical and green-synthesized silver and silver oxide nanoparticles. Mater. Res. Express 2017, 4, 105406. [Google Scholar] [CrossRef]
- Suresh, S.; Karthikeyan, S.; Saravanan, P.; Jayamoorthy, K.; Dhanalekshmi, K.I. Comparison of antibacterial and antifungal activity of 5-amino-2-mercapto benzimidazole and functionalized Ag3O4 nanoparticles. Karbala Int. J. Modern Sci. 2016, 2, 129–137. [Google Scholar] [CrossRef]
- Martinez-Boubeta, C.; Balcells, L.; Cristofol, R.; Sanfeliu, C.; Rodriguez, E.; Weissleder, R.; Piedrafita, S.L.; Simeonidis, K.; Angelakeris, M.; Sandiumenge, F.; et al. Self-assembled multifunctional Fe/MgO nanospheres for magnetic resonance imaging and hyperthermia. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wu, D.; Zhu, X.; Wang, W.; Tan, F.; Chen, J.; Qiao, X.; Qiu, X. Sol-gel preparation of Ag-doped MgO nanoparticles with high efficiency for bacterial inactivation. Ceram Int. 2017, 43, 1066–1072. [Google Scholar] [CrossRef]
- Sierra-Fernandez, A.; De La Rosa-Garcia, S.C.; Gomez-Villalba, L.S.; Gomez-Cornelio, S.; Rabanal, M.E.; Fort, R.; Quintana, P. Synthesis, photocatalytic and antifungal properties of MgO, ZnO and Zn/Mg oxide nanoparticles for the protection of calcareous stone heritage. ACS Appl. Mater. Interfaces 2017, 9, 24873–24886. [Google Scholar] [CrossRef]
- Sawai, J.; Igarashi, H.; Hashimoto, A.; Kokugan, T.; Shimizu, M. Effect of particle size and the heating temperature of ceramic powders on antibacterial activity of their slurries. J. Chem. Eng. Jpn. 1996, 29, 251–256. [Google Scholar] [CrossRef]
- Huang, L.; Li, D.Q.; Lin, Y.J.; Wei, M.; Evans, D.G.; Duan, X. Controllable preparation of nano-MgO and investigation of its bactericidal properties. J. Inorg. Biochem. 2005, 99, 986–993. [Google Scholar] [CrossRef]
- Yao, C.; Wang, W.; Wang, P.; Zhao, M.; Li, X.; Zhang, F. Near-infrared upconversion mesoporous cerium oxide hollow biophotocatalyst for concurrent pH-/H2O2-Responsive O2-Evolving synergetic cancer therapy. Adv. Mater. 2018, 30, 1704833. [Google Scholar] [CrossRef]
- Cai, L.; Chen, J.; Liu, Z.; Wang, H.; Yang, H.; Ding, W. Magnesium Oxide Nanoparticles: Effective Agricultural Antibacterial Agent against Ralstonia solanacearum. Front. Microbiol. 2018, 9, 790. [Google Scholar] [CrossRef]
- Jin, T.; He, Y. Antibacterial activities of magnesium oxide (MgO) nanoparticles against foodborne pathogens. J. Nanoparticle Res. 2011, 13, 6877–6885. [Google Scholar] [CrossRef]
- Ge, S.; Wang, G.; Shen, Y.; Zhang, Q.; Jia, D.; Wang, H.; Dong, Q.; Yin, T. Cytotoxic effects of MgO nanoparticles on human umbilical vein endothelial cells in vitro. IET Nanobiotechnol. 2011, 5, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Sawai, J.; Shiga, S.; Kojima, H. Kinetic analysis of the death of bacteria in CaO powder slurry. Int. Biodeterior. Biodegrad. 2001, 47, 23–26. [Google Scholar] [CrossRef]
- Sawai, J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by the conductimetric assay. J. Microbiol. Methods 2003, 54, 177–182. [Google Scholar] [CrossRef]
- Makhluf, S.; Dror, R.; Nitzan, Y.; Abramovich, Y.; Jelinek, R.; Gedanken, A. Microwave-assisted synthesis of nanocrystalline MgO and its use as a bacteriocide. Adv. Funct. Mater. 2005, 15, 1708–1715. [Google Scholar] [CrossRef]
- Sawai, J.; Yoshikawa, T. Quantitative evaluation of the antifungal activity of metallic oxide powders (MgO, CaO and ZnO) by an indirect conductimetric assay. J. Appl. Microbiol. 2004, 96, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Latgé, J.P. The cell wall: A carbohydrate armour for the fungal cell. Mol. Microbiol. 2007, 66, 279–290. [Google Scholar] [CrossRef]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Cameotra, S.S.; Saquib, Q.; Musarrat, J. Interaction of Al2O3 nanoparticles with Escherichia coli and their cell envelope biomolecules. J. Appl. Microbiol. 2013, 116, 772–783. [Google Scholar] [CrossRef]
- Balasubramanyam, A.; Sailaja, N.; Mahboob, M.; Rahman, M.F.; Hussain, S.M.; Grover, P. In vitro mutagenicity assessment of aluminium oxide nanomaterials using the Salmonella/microsome assay. Toxicol. In Vitro 2010, 24, 1871–1876. [Google Scholar] [CrossRef]
- Jiang, W.; Mashayekhi, H.; Xing, B. Bacterial toxicity comparison between nano- and micro-scale oxide particles. Environ. Pollut. 2009, 157, 1619–1625. [Google Scholar] [CrossRef]
- Bala, T.; Armstrong, G.; Laffir, F.; Thornton, R. Titania-silver and alumina-silver composite nanoparticles: A novel, versatile synthesis, reaction mechanism and potential antimicrobial application. J. Colloid Interface Sci. 2011, 356, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.; Chen, X.; Xiang, Q.; Li, Q.; Xing, X. Recyclable magnetite-silver heterodimer nanocomposites with durable antibacterial performance. Bioact Mater. 2018, 3, 80–86. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef] [PubMed]
- Ismail, R.A.; Sulaiman, G.M.; Abdulrahman, S.A.; Marzoog, T.R. Antibacterial activity of magnetic iron oxide nanoparticles synthesized by laser ablation in liquid. Mater Sci. Eng. C 2015, 53, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Groiss, S.; Selvaraj, R.; Varadavenkatesan, T.; Vinayagam, R. Structural characterization, the antibacterial and catalytic effect of iron oxide nanoparticles synthesised using the leaf extract of Cynometra ramiflora. J. Mol. Struct. 2017, 1128, 572–578. [Google Scholar] [CrossRef]
- Kannan, K.; Radhika, D.; Nikolova, M.P.; Nivea, R.; Sharma, G.; Sadasivunia, K.K.; Namitha, R. Structural and Functional Properties of Rare Earth Based (NiO-CGO) Nanocomposite Produced by Effective Multiple Doping Approach via Co-precipitation. Mater Technol. Adv Perform Mater. 2020, in press. [Google Scholar] [CrossRef]
- Suresh, S.; Karthikeyan, S.; Saravanan, P.; Jayamoorthy, K. Comparison of antibacterial and antifungal activities of 5-amino-2-mercaptobenzimidazole and functionalized NiO nanoparticles. Karbala Int. J. Modern Sci. 2016, 2, 188–195. [Google Scholar] [CrossRef]
- Ezhilarasi, A.A.; Vijaya, J.J.; Kaviyarasu, K.; Kennedy, L.J.; Ramalingam, R.J.; Al-Lohedan, H.A. Green synthesis of NiO nanoparticles using Aegle marmelos leaf extract for the evaluation of in-vitro cytotoxicity, antibacterial and photocatalytic properties. J. Photochem. Photobiol. B 2018, 180, 39–50. [Google Scholar] [CrossRef]
- Helan, V.; Prince, J.J.; Al-Dhabi, N.A.; Arasu, M.V.; Ayeshamariam, A.; Madhumitha, G.; Roopan, S.M.; Jayachandran, M. Neem leaves mediated preparation of NiO nanoparticles and its magnetization, coercivity and antibacterial analysis. Results Phys. 2016, 6, 712–718. [Google Scholar] [CrossRef]
- Farias, I.A.P.; Lima dos Santos, C.C.; Sampaio, F.C. Antimicrobial Activity of Cerium Oxide Nanoparticles on Opportunistic Microorganisms: A Systematic Review. BioMed. Res. Int. 2018, 2018, 1923606. [Google Scholar] [CrossRef]
- Thill, A.; Zeyons, O.; Spalla, O.; Chauvat, F.; Rose, J.; Auffan, M.; Flank, A.M. Cytotoxicity of CeO2 nanoparticles for Escherichia coli: Physico-chemical insight of the toxicity mechanism. Environ. Sci. Technol. 2006, 40, 6151–6156. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, D.A.; Suresh, A.K.; Holton, G.A.; McKeown, C.K.; Wang, W.; Gu, B.; Mortensen, N.P.; Allison, D.P.; Joy, D.C.; Allison, M.R.; et al. Effects of engineered cerium oxide nanoparticles on bacterial growth and viability. Appl. Environ. Microbiol. 2010, 76, 7981–7989. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, T.N.; Ramakrishnappa, T.; Nagaraju, G.; Rajanaika, H. Synthesis and Characterization of CeO2 Nanoparticles viaSolution Combustion Method for Photocatalytic and Antibacterial Activity Studies. Chem. Open 2014, 4, 146–154. [Google Scholar]
- Bakkiyaraj, R.; Balakrishnan, M.; Bharath, G.; Ponpandian, N. Facile synthesis, structural characterization, photocatalytic and antimicrobial activities of Zr doped CeO2 nanoparticles. J. Alloys Compd. 2017, 724, 555–564. [Google Scholar] [CrossRef]
- Geraets, L.; Oomen, A.G.; Schroeter, J.D.; Coleman, V.A.; Cassee, F.R. Tissue Distribution of Inhaled Micro- and Nano-sized Cerium Oxide Particles in Rats: Results From a 28-Day Exposure Study. Toxicol. Sci. 2012, 127, 463–473. [Google Scholar] [CrossRef]
- Heinemann, C.; Heinemann, S.; Bernhardt, A.; Worch, H.; Hanke, T. Novel textile chitosan scaffolds promote spreading, proliferation and differentiation of osteoblasts. Biomacromolecules 2008, 9, 2913–2920. [Google Scholar] [CrossRef]
- Jarockyte, G.; Daugelaite, E.; Stasys, M.; Statkute, U.; Poderys, V.; Tseng, T.; Hsu, S.; Karabanovas, V.; Rotomskis, R. Accumulation and toxicity of superparamagnetic iron oxide nanoparticles in cells and experimental animals. Int. J. Mol. Sci. 2016, 17, 1193. [Google Scholar] [CrossRef]
- Marín-Barba, M.; Gavilán, H.; Gutiérrez, L.; Lozano-Velasco, E.; Rodríguez-Ramiro, I.; Wheeler, G.N.; Morris, C.J.; Morales, M.P.; Ruiz, A. Unravelling the mechanisms that determine the uptake and metabolism of magnetic single and multicore nanoparticles in a Xenopus laevis model. Nanoscale 2018, 10, 690–704. [Google Scholar] [CrossRef]
- Lamme, T.; Sturve, J. Assessment of titanium dioxide nanoparticle toxicity in the rainbow trout (Onchorynchus mykiss) liver and gill cell lines RTL-W1 and RTgill-W1 under particular consideration of nanoparticle stability and interference with fluorometric assays. NanoImpact 2018, 11, 1–19. [Google Scholar] [CrossRef]
- Rehman, F.U.; Zhao, C.; Jiang, H.; Selke, M.; Wang, X. Protective effect of TiO2 nanowhiskers on Tetra Sulphonatophenyl Porphyrin (TSPP) complexes induced oxidative stress during photodynamic therapy. Photodiagnosis Photodyn. Ther. 2016, 13, 267–275. [Google Scholar] [CrossRef]
- Sharma, P.; Jang, N.Y.; Lee, J.W.; Park, B.C.; Kim, Y.K.; Cho, N.H. Application of ZnO-Based Nanocomposites for Vaccines and Cancer Immunotherapy. Pharmaceutics 2019, 11, 493. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Ho, C.-C.; Yang, C.S.; Chang, W.-H.; Tsai, M.-H.; Tsai, H.-T.; Lin, P. Involvement of MyD88 in zinc oxide nanoparticle-induced lung inflammation. Exp. Toxicol. Pathol. 2013, 65, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Aula, S.; Lakkireddy, S.; Kapley, A.; Adimadhyam, V.N.; Sharma, R.K.; Uppin, S.G.; Jamil, K. Route of administration induced in vivo effects and toxicity responses of Zinc Oxide nanorods at molecular and genetic levels. Int. J. Nano Dimens. 2018, 9, 158–169. [Google Scholar]
- Choi, J.; Kim, H.; Kim, P.; Jo, E.; Kim, H.M.; Lee, M.Y.; Jin, S.M.; Park, K. Toxicity of zinc oxide nanoparticles in rats treated by two different routes: Single intravenous injection and single oral administration. J. Toxicol. Environ. Health A 2015, 78, 226–243. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Tian, Y.; Zhang, T.; Ren, G.; Yang, Z. Nano-zinc oxide damages spatial cognition capability via over-enhanced long-term potentiation in the hippocampus of Wistar rats. Int. J. Nanomed. 2011, 6, 1453–1461. [Google Scholar]
- Roca, A.G.; Gutiérrez, L.; Gavilán, H.; Brollo, M.E.F.; Veintemillas-Verdaguer, S.; del P Morales, M. Design strategies for shape-controlled magnetic iron oxide nanoparticles. Adv. Drug Deliv Rev. 2019, 138, 68–104. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.S.; Duffin, R.; Howie, S.E.; Scotton, C.J.; Wallace, W.A.; Macnee, W.; Bradley, M.; Megson, I.L.; Donaldson, K. Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn2+ dissolution inside lysosomes. Part. Fibre Toxicol. 2011, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Saptarshi, S.R.; Duschl, A.; Lopata, A.L. Biological reactivity of zinc oxide nanoparticles with mammalian test systems: An overview. Nanomedicine 2015, 10, 2075–2092. [Google Scholar] [CrossRef] [PubMed]
- Lartigue, L.; Alloyeau, D.; Kolosnjaj-Tabi, J.; Javed, Y.; Guardia, P.; Riedinger, A.; Péchoux, C.; Pellegrino, T.; Wilhelm, C.; Gazeau, F. Biodegradation of iron oxide nanocubes: High-resolution in situ monitoring. ACS Nano 2013, 7, 3939–3952. [Google Scholar] [CrossRef]
- Lewerenz, J.; Ates, G.; Methner, A.; Conrad, M.; Maher, P. Oxytosis/Ferroptosis—(Re-) Emerging roles for oxidative stress-dependent non-apoptotic cell death in diseases of the central nervous system. Front. Neurosci. 2018, 12, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Monian, P.; Quadri, N.; Ramasamy, R.; Jiang, X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol. Cell 2015, 59, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Steinhäuser, K.G.; Sayre, P.G.; Nowack, B. Reliability of methods and data for regulatory assessment of nanomaterial risks. NanoImpact 2018, 10, 68–69. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef] [PubMed]
| MO | Precursor | Biosynthesis | NP Size [nm] | Tested Organism | Biological Activity/ Effect/Outcome | Ref. |
|---|---|---|---|---|---|---|
| Biosynthesis of MONPs from plants | ||||||
| CuO | CuSO4 | Leaf extract of Eichhornia crassipes | 20–22 | S. pneumonia—S. aureus, K. pneumonia |
| [171] |
| CuO | Cu(O2CCH3)2 | Stems of Seidlitzia rosmarinus ashes | 8–40 | S. aureus, E. coli |
| [172] |
| CuO | Cu(NO3)2. 3H2O | Aqueous leaf extract of Abutilon indicum | 16.8 | G− (E. coli) and G+ (B. subtilis, S. aureus and Klebsiella) bacteria |
| [162] |
| Cu2O | CuSO4.5H2O | Aqueous leaf extract of Callistemon viminalis | 423 | E. coli, Acinetobacter baumannii |
| [173] |
| α-Fe2O3 γ-Fe2O3 | Fe(NO3)3.9H2O | Leaf extract of Platanus orientalis | 38 | Aspergillus niger, Mucor piriformis |
| [174] |
| Fe3O4 | FeCl3 | Seeds, leaves and fruits of Lagenaria siceraria | 30–100 | E.coli, S. aureus |
| [175] |
| Fe3O4 | FeSO4 | Flower sheath extract of Musa ornate | 43.69 | S. aureus, Streptococcus agalactiae, E.coli, Salmonella enterica |
| [176] |
| ZnO | Zn(O2CCH3)2 | Plant extract of Passiflora caerulea | 30–50 | Klebsiella sp., E.coli, Enterococcus sp., Streptococcus sp. |
| [177] |
| ZnO | Zn(NO3)2 | Aqueous leaf extract of Solanum nigrum | 20–30 | S. aureus, Salmonella paratyphi, Vibrio cholerae, E. coli |
| [178] |
| ZnO | Zn(O2CCH3)2 | Leaf powder aqueous extract of Scadoxus multiflorus | 31 ± 2 | Aedes aegypti (larvae and eggs); Aspergillus niger; Aspergillus flavus |
| [179] |
| ZnO | Zn(O2CCH3)2. (H2O)2 | Leaf extract Atalantia monophylla | 20–45 | Bacterial (B. subtilis, B. cereus, S. aureus, P. aeruginosa, Klebsiella pneumonia) and fungal species (C. albicans, A. niger) |
| [180] |
| ZnO | Zn(O2CCH3)2. (H2O)2 | Green tea leaves (Camellia sinensis) | 30–40 | G+ (S. aureus) and G− (E. coli) bacteria; fungal species (A. niger) |
| [181] |
| ZnO | Zn(O2CCH3)2. (H2O)2 | Aqueous extract of parsley (Petroselinum crispum) | 50 nm (at RT) 40 nm (at 90 °C) | E. coli |
| [182] |
| ZnO | ZnSO4 | Leaf extract of Bauhinia tomentosa | 22–94 | G− (P. aeruginosa, E. coli) and G+ (B. subtilis, S. aureus) |
| [183] |
| ZnO | Zn(O2CCH3)2 | Leaf extract from Stevia | 10–90 | Parasitic strain: Leishmaniasis major Bacteria: S. aureus and Escherichia coli |
| [184] |
| ZnO and Cu- doped ZnO | Zn(NO3)2.6H2O and Cu(NO3)2 |
| ZnO—16.7; Cu-doped ZnO method 1–17.5; Cu-doped ZnO method 2–20.7 | Bacteria: S. aureus, B. subtilis, Klebsiella, E. coli; fungal strains A. niger, A. flavus, Trichoderma harzianum; An anticancer activity using human breast carcinoma cells |
| [185] |
| RuO2 | RuCl3.xH2O | Plant extract of Acalypha indica | 6–25 | E. coli, P. aeruginosa, Serratia marcescens S. aureus |
| [186] |
| CeO2 | CeCl3 | Leaf extract of Gloriosa superba L. | 5 | E. coli, S. aureus, S. dysenteriae, P. aeruginosa, P. vulgaris, K. pneumonia, S. pneumoniae |
| [187] |
| NiO | NiO(CH3COO)2.4H2O | Citrus fruit juice of Limoinia acidissima Chrism | 20 | G+ (S. aureus) and G− (P. aeruginosa, E. coli, K. pneumonia) bacteria |
| [188] |
| Biosynthesis of MONPs from bacteria, fungi, algae and natural compounds | ||||||
| ZnO | ZnO powder | Culture of bacteria Aeromonas hydrophila | 42–64 | Aeromonas hydrophila, E. coli, S. aureus, P. aeruginosa, Enterococcus faecalis, Streptococcus pyogenes; Aspergillus flavus, A. niger, C. albicans |
| [189] |
| ZnO | Zn(NO3)2 | Culture of Bacillus megaterium | 45–150 | Helicobacter pylori |
| [190] |
| TiO2 | TiO(OH)2 | Mycelium of Aspergillus flavus | 62–74 | S. aureus, E. coli, P. aeruginosa, Klebsiella pneumoniae, B. subtilis |
| [191] |
| ZnO | ZnCl2 | A fungal isolate of Aspergillus niger | 41–75 | S. aureus, E. coli |
| [192] |
| ZnO | Zn(NO3)2 | Culture medium of Aspergillus niger | 84–91 | S. aureus, E. coli |
| [193] |
| CuO and Cu2O | CuSO4 | Algae extract of brown algae Bufurcaria bufurcata | 5–45 | Enterobacter aerogenes, S. aureus |
| [194] |
| CuO | Cu(O2CCH3)2. H2O | Algae extract of cyanobacteria Spirulina Platensys | 30–40 | G−: E. coli, Proteus vulgaris, Klebsiella pneumonia G+: S. aureus; S. epidermidis, Bacillus cereus |
| [195] |
| ZnO | Zn(NO3)2.6H2O | Algal extract of marine microalgae Sargassum multicum | 4–23 | S. aureus—sensitive and resistant; C. albicans—sensitive and resistant; |
| [196] |
| ZnO | ZnNO3.6H2O | Al-gum extrudates of Azadirachta indica | 30–60 | E. coli, S. aureus |
| [197] |
| CuO | CuSO4 | Goat (GFM) and sheep (SFM) fecal matter | 29.2 ± 15.9 for GFM; 32.3 ± 32.2 for SFM | Salmonella typhi, B. subtilis |
| [198] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolova, M.P.; Chavali, M.S. Metal Oxide Nanoparticles as Biomedical Materials. Biomimetics 2020, 5, 27. https://doi.org/10.3390/biomimetics5020027
Nikolova MP, Chavali MS. Metal Oxide Nanoparticles as Biomedical Materials. Biomimetics. 2020; 5(2):27. https://doi.org/10.3390/biomimetics5020027
Chicago/Turabian StyleNikolova, Maria P., and Murthy S. Chavali. 2020. "Metal Oxide Nanoparticles as Biomedical Materials" Biomimetics 5, no. 2: 27. https://doi.org/10.3390/biomimetics5020027
APA StyleNikolova, M. P., & Chavali, M. S. (2020). Metal Oxide Nanoparticles as Biomedical Materials. Biomimetics, 5(2), 27. https://doi.org/10.3390/biomimetics5020027