Cell Culture Platforms with Controllable Stiffness for Chick Embryonic Cardiomyocytes
Abstract
1. Introduction
2. Materials and Methods
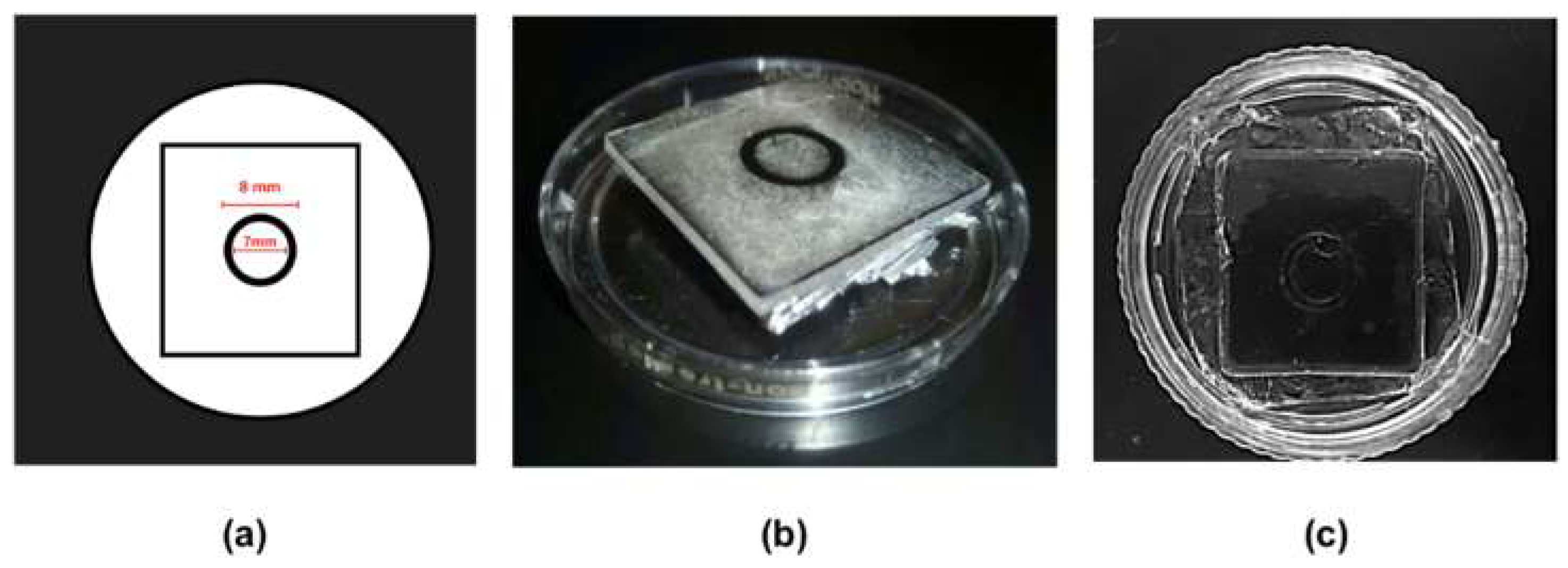
2.1. Manufacture of PDMS Microscaffolds
2.2. Cell Culture
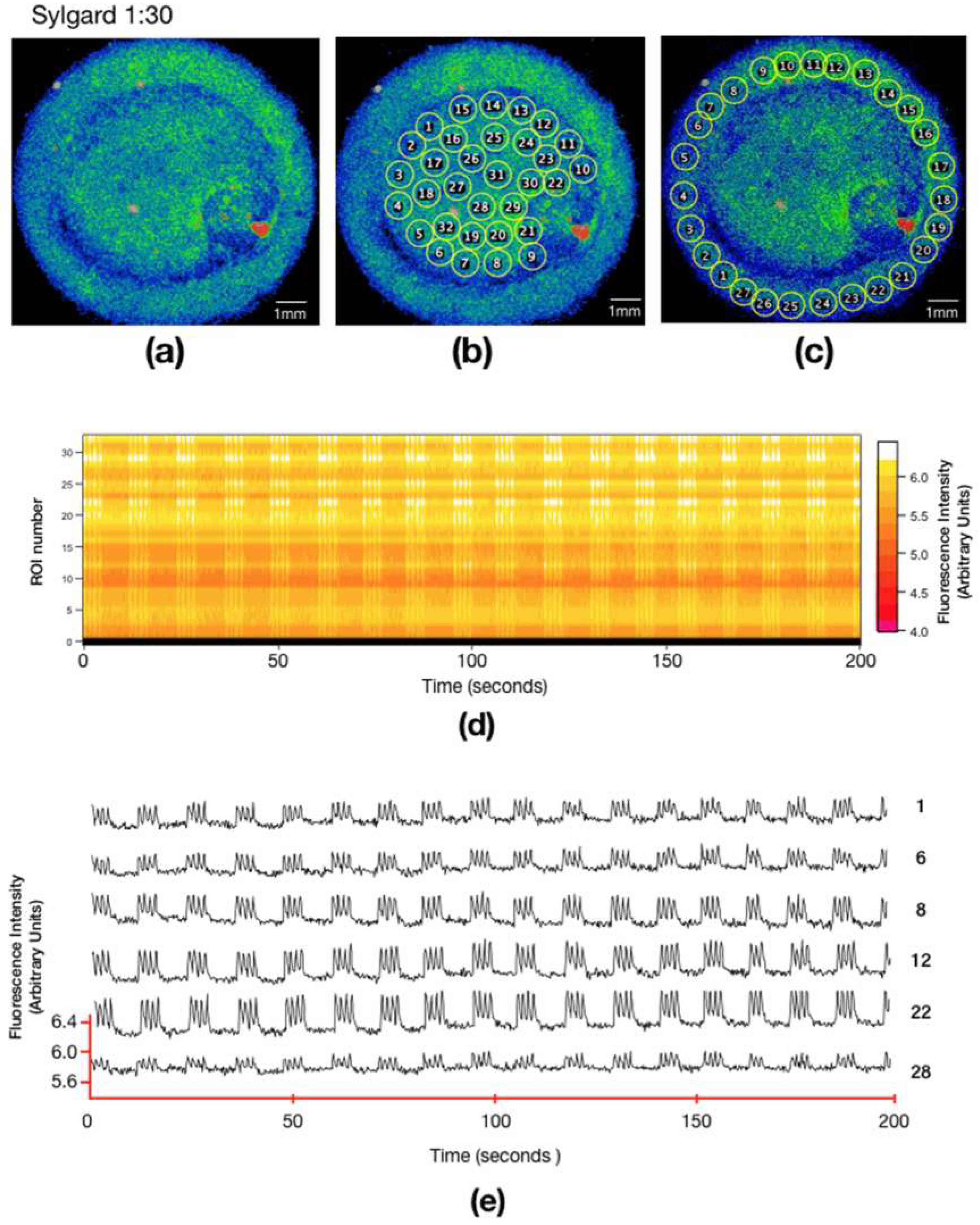
2.3. Intracellular Calcium Imaging
2.4. Cell Viability
2.5. Data Analysis
3. Results
3.1. Biocompatibility, Adhesion, and Monolayer Formation.
3.2. Calcium Signaling in Different Substrates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mitcheson, J.S.; Hancox, J.C.; Levi, A.J. Cultured adult cardiac myocytes: Future applications, culture methods, morphological and electrophysiological properties. Cardiovasc. Res. 1998, 39, 280–300. [Google Scholar] [CrossRef]
- González, H.; Nagai, Y.; Bub, G.; Glass, L.; Shrier, A. Reentrant waves in a ring of embryonic chick ventricular cells imaged with a Ca2+ sensitive dye. Biosystems 2003, 71, 71–80. [Google Scholar] [CrossRef]
- Snopko, R.M.; Aromolaran, A.S.; Karko, K.L.; Ramos-Franco, J.; Blatter, L.A.; Mejía-Alvarez, R. Cell culture modifies Ca2+ signaling during excitation-contraction coupling in neonate cardiac myocytes. Cell Calc. 2007, 41, 13–25. [Google Scholar] [CrossRef]
- Nuss, H.B.; Marban, E. Electrophysiological properties of neonatal mouse cardiac myocytes in primary culture. J. Physiol. 1994, 479, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Krogh-Madsen, T.; Schaffer, P.; Skriver, A.D.; Taylor, L.K.; Pelzmann, B.; Koidl, B.; Guevara, M.R. An ionic model for rhythmic activity in small clusters of embryonic chick ventricular cells. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H398–H413. [Google Scholar] [CrossRef]
- Engler, A.J.; Carag-Krieger, C.; Johnson, C.P.; Raab, M.; Tang, H.-Y.; Speicher, D.W.; Sanger, J.W.; Sanger, J.M.; Discher, D.E. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: Scar-like rigidity inhibits beating. J. Cell Sci. 2008, 121, 3794–3802. [Google Scholar] [CrossRef]
- Leipzig, N.D.; Shoichet, M.S. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials 2009, 30, 6867–6878. [Google Scholar] [CrossRef]
- Boothe, S.D.; Myers, J.D.; Pok, S.; Sun, J.; Xi, Y.; Nieto, R.M.; Cheng, J.; Jacot, J.G. The effect of substrate stiffness on cardiomyocyte action potentials. Cell Biochem. Biophys. 2016, 74, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Berk, B.C.; Fujiwara, K.; Lehoux, S. ECM remodeling in hypertensive heart disease. J. Clin. Investig. 2007, 117, 568–575. [Google Scholar] [CrossRef]
- Van Deel, E.D.; Najafi, A.; Fontoura, D.; Valent, E.; Goebel, M.; Kardux, K.; Falcão-Pires, I.; van der Velden, J.; Pandey, P.; Hawkes, W.; et al. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J. Mol. Cell. Cardiol. 2017, 44, 326–336. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Bhana, B.; Iyer, R.K.; Chen, W.L.K.; Zhao, R.; Sider, K.L.; Likhitpanichkul, M.; Simmons, C.A.; Radisic, M. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol. Bioeng. 2010, 105, 1148–1160. [Google Scholar] [CrossRef] [PubMed]
- Jacot, J.G.; McCulloch, A.D.; Omens, J.H. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys. J. 2008, 95, 3479–3487. [Google Scholar] [CrossRef]
- Rodriguez, A.G.; Han, S.J.; Regnier, M.; Sniadecki, N.J. Substrate stiffness increases twitch power of neonatal cardiomyocytes in correlation with changes in myofibril structure and intracellular calcium. Biophys. J. 2011, 101, 2455–2464. [Google Scholar] [CrossRef]
- Young, J.L.; Engler, A.J. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials 2011, 32, 1002–1009. [Google Scholar] [CrossRef]
- Van Spreeuwel, A.A.; Bax, N.N.; Bouten, C.C. The relevance of extracellular matrix structure and composition in engineering the diseased cardiac microenvironment. OA Tissue Eng. 2014, 18, 2. [Google Scholar]
- Ni, M.; Tong, W.H.; Choudhury, D.; Rahim, N.A.A.; Iliescu, C.; Yu, H. Cell culture on MEMS platforms: A review. Int. J. Mol. Sci. 2009, 10, 5411–5541. [Google Scholar] [CrossRef]
- Efimenko, K.; Wallace, W.E.; Genzer, J. Surface modification of Sylgard-184 poly (dimethyl siloxane) networks by ultraviolet and ultraviolet/ozone treatment. J. Colloid Interface Sci. 2002, 254, 306–315. [Google Scholar] [CrossRef]
- Palchesko, R.N.; Zhang, L.; Sun, Y.; Feinberg, A.W. Development of polydimethylsiloxane substrates with tunable elastic modulus to study cell mechanobiology in muscle and nerve. PLoS ONE 2012, 7, e51499. [Google Scholar] [CrossRef]
- Asano, Y.; Shimoda, H.; Okano, D.; Matsusaki, M.; Akashi, M. Transplantation of three-dimensional artificial human vascular tissues fabricated using an extracellular matrix nanofilm-based cell-accumulation technique. J. Tissue Eng. Regen. Med. 2017, 11, 1303–1307. [Google Scholar] [CrossRef]
- Karamichos, D.; Skinner, J.; Brown, R.; Mudera, V. Matrix stiffness and serum concentration effects matrix remodelling and ECM regulatory genes of human bone marrow stem cells. J. Tissue Eng. Regen. Med. 2008, 2, 97–105. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Datta, A.; Berg, J.M.; Gangopadhyay, S. Studies on surface wettability of poly (dimethyl) siloxane (pdms) and glass under oxygen-plasma. J. Microelectromech. Syst. 2005, 14, 590–597. [Google Scholar] [CrossRef]
- Grimes, A.; Breslauer, D.N.; Long, M.; Pegan, J.; Lee, L.P.; Khine, M. Shrinky-dink microfluidics: Rapid generation of deep and rounded patterns. Lab Chip 2007, 8, 170–172. [Google Scholar] [CrossRef]
- Bub, G.; Glass, L.; Publicover, N.G.; Shrier, A.; Hrier, A.L.S. Bursting calcium rotors in cultured cardiac myocyte monolayers. Proc. Natl. Acad. Sci. USA 1998, 95, 10283–10287. [Google Scholar] [CrossRef] [PubMed]
- Durán-Pastén, M.L.; Fiordelisio-Coll, T.; Hernández-Cruz, A. Castration-induced modifications of GnRH-elicited [Ca2+](i) signaling patterns in male mouse pituitary gonadotrophs in situ: Studies in the acute pituitary slice preparation. Biol. Reprod. 2013, 88, 38. [Google Scholar] [CrossRef]
- Piper, H.M.; Probst, I.; Schwartz, P.; Hütter, F.J.; Spieckermann, P.G. Culturing of calcium stable adult cardiac myocytes. J. Mol. Cell. Cardiol. 1982, 14, 397–412. [Google Scholar] [CrossRef]
- Fearnley, C.J.; Llewelyn Roderick, H.; Bootman, M.D. Calcium signaling in cardiac myocytes. Cold Spring Harb. Perspect. Biol. 2011, 3, a004242. [Google Scholar] [CrossRef]
- Sedmera, D.; Pexieder, T.; Rychterova, V.; Hu, N.; Clark, E.B. Remodeling of chick embryonic ventricular myoarchitecture under experimentally changed loading conditions. Anat. Rec. 1999, 254, 238–252. [Google Scholar] [CrossRef]
- Koide, M.; Akins, R.E.; Harayama, H.; Yasui, K.; Yokota, M.; Tuan, R.S. Atrial natriuretic peptide accelerates proliferation of chick embryonic cardiomyocytes in vitro. Differentiation 1996, 61, 1–11. [Google Scholar] [CrossRef]
- Jones, S.P.; Kennedy, S.W. Chicken embryo cardiomyocyte cultures—A new approach for studying effects of halogenated aromatic hydrocarbons in the avian heart. Toxicol. Sci. 2009, 109, 66–74. [Google Scholar] [CrossRef]
- Ebihara, L.; Shigeto, N.; Lieberman, M.; Johnson, E.A. The initial inward current in spherical clusters of chick embryonic heart cells. J. Gen. Physiol. 1980, 75, 437–456. [Google Scholar] [CrossRef]
- You, M.J.; Langfield, P.; Campanari, L.; Dobbs, M.; Shrier, A.; Glass, L. Demonstration of cardiac rotor and source mapping techniques in embryonic chick monolayers. Chaos 2017, 27, 093938. [Google Scholar] [CrossRef] [PubMed]
- Slotvitsky, M.; Tsvelaya, V.; Frolova, S.; Dementyeva, E.; Agladze, K. Arrhythmogenicity test based on a human induced pluripotent stem cell (iPSC)-derived cardiomyocyte layer. Toxicol. Sci. 2018, 168, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Freire, V.; Lee, A.S.; Hu, S.; Abilez, O.J.; Liang, P.; Lan, F.; Huber, B.C.; Ong, S.G.; Hong, W.X.; Huang, M.; et al. Effect of human donor cell source on differentiation and function of cardiac induced pluripotent stem cells. J. Am. Coll. Cardiol. 2014, 64, 436–448. [Google Scholar] [CrossRef]
- Obergrussberger, A.; Haarmann, C.; Stölzle-Feix, S.; Becker, N.; Ohtsuki, A.; Brüggemann, A.; George, M.; Fertig, N. Automated patch clamp recordings of human stem cell-derived cardiomyocytes. In Stem Cell-Derived Models in Toxicology; Humana Press: New York, NY, USA, 2016. [Google Scholar]
- Koltai, M.Z.; Balogh, I.; Wagner, M.; Pogátsa, G. Diabetic myocardial alterations in ultrastructure and function. Exp. Pathol. 1984, 25, 215–221. [Google Scholar] [CrossRef]
- Mazhari, R.; Omens, J.H.; Covell, J.W.; McCulloch, A.D. Structural basis of regional dysfunction in acutely ischemic myocardium. Cardiovasc. Res. 2000, 47, 284–293. [Google Scholar] [CrossRef]
- Herrmann, K.L.; McCulloch, A.D.; Omens, J.H. Glycated collagen cross-linking alters cardiac mechanics in volume-overload hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1277–H1284. [Google Scholar] [CrossRef]
- Omens, J.H.; Usyk, T.P.; Li, Z.; McCulloch, A.D. Muscle LIM protein deficiency leads to alterations in passive ventricular mechanics. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H680–H687. [Google Scholar] [CrossRef][Green Version]
- Weis, S.M.; Emery, J.L.; Becker, K.D.; McBride, D.J.; Omens, J.H.; McCulloch, A.D. Myocardial mechanics and collagen structure in the osteogenesis imperfecta murine (oim). Circ. Res. 2000, 87, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.F.; Engler, A.J.; Woo, Y.J.; Pirolli, T.J.; Bish, L.T.; Jayasankar, V.; Morine, K.J.; Gardner, T.J.; Discher, D.E.; Sweeney, H.L. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2196–H2203. [Google Scholar] [CrossRef] [PubMed]
- Boudreau-Béland, J.; Duverger, J.E.; Petitjean, E.; Maguy, A.; Ledoux, J.; Comtois, P. Spatiotemporal stability of neonatal rat cardiomyocyte monolayers spontaneous activity is dependent on the culture substrate. PLoS ONE 2015, 10, e0127977. [Google Scholar] [CrossRef] [PubMed]
- Ospina-Orejarena, A.; Vera-Graziano, R.; Castillo-Ortega, M.M.; Hinestroza, J.P.; Rodriguez-Gonzalez, M.; Palomares-Aguilera, L.; Morales-Moctezuma, M.; Maciel-Cerda, A. Grafting collagen on poly (lactic acid) by a simple route to produce electrospun scaffolds, and their cell adhesion evaluation. Tissue Eng. Regen. Med. 2016, 13, 375–387. [Google Scholar] [CrossRef] [PubMed]



| Mixture | Ratio | Young’s modulus (kPa) (mean ± SD) |
|---|---|---|
| 527 only | 0 | 15.16 ± 4.45 |
| 184 only | 1 | 1100 ± 77.45 |
| 30:1 | 0.03 | 25.76 ± 6.21 |
| 10:1 | 0.1 | 54.87 ± 3.62 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durán-Pastén, M.L.; Cortes, D.; Valencia-Amaya, A.E.; King, S.; González-Gómez, G.H.; Hautefeuille, M. Cell Culture Platforms with Controllable Stiffness for Chick Embryonic Cardiomyocytes. Biomimetics 2019, 4, 33. https://doi.org/10.3390/biomimetics4020033
Durán-Pastén ML, Cortes D, Valencia-Amaya AE, King S, González-Gómez GH, Hautefeuille M. Cell Culture Platforms with Controllable Stiffness for Chick Embryonic Cardiomyocytes. Biomimetics. 2019; 4(2):33. https://doi.org/10.3390/biomimetics4020033
Chicago/Turabian StyleDurán-Pastén, María Luisa, Daniela Cortes, Alan E. Valencia-Amaya, Santiago King, Gertrudis Hortensia González-Gómez, and Mathieu Hautefeuille. 2019. "Cell Culture Platforms with Controllable Stiffness for Chick Embryonic Cardiomyocytes" Biomimetics 4, no. 2: 33. https://doi.org/10.3390/biomimetics4020033
APA StyleDurán-Pastén, M. L., Cortes, D., Valencia-Amaya, A. E., King, S., González-Gómez, G. H., & Hautefeuille, M. (2019). Cell Culture Platforms with Controllable Stiffness for Chick Embryonic Cardiomyocytes. Biomimetics, 4(2), 33. https://doi.org/10.3390/biomimetics4020033





