Healing through Histidine: Bioinspired Pathways to Self-Healing Polymers via Imidazole–Metal Coordination
Abstract
1. Introduction: The Role of Histidine–Metal Coordination in Self-Healing Behaviors of Biopolymeric Materials
1.1. Brief Introduction to Self-Healing Materials
1.2. Biological Role Models for Self-Healing Materials
1.3. Metal Coordination as a Dynamic Cross-Linking Strategy in Natural Materials
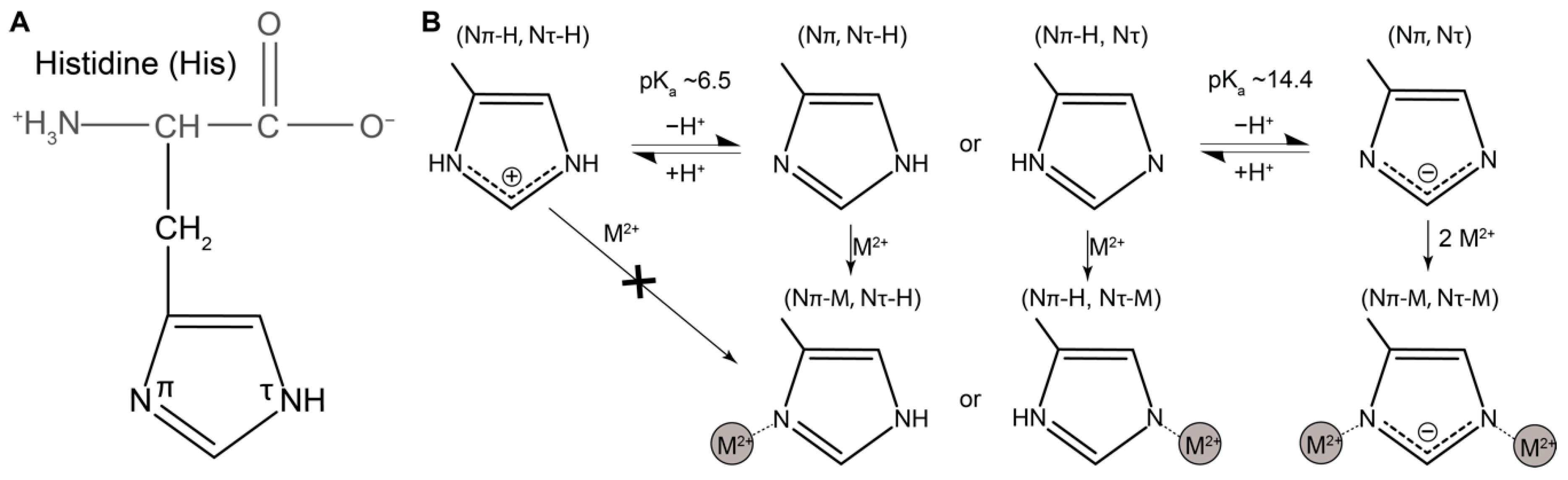
1.4. Histidine as a Versatile Ligand for Tuning Mechanical Performance of Biopolymers
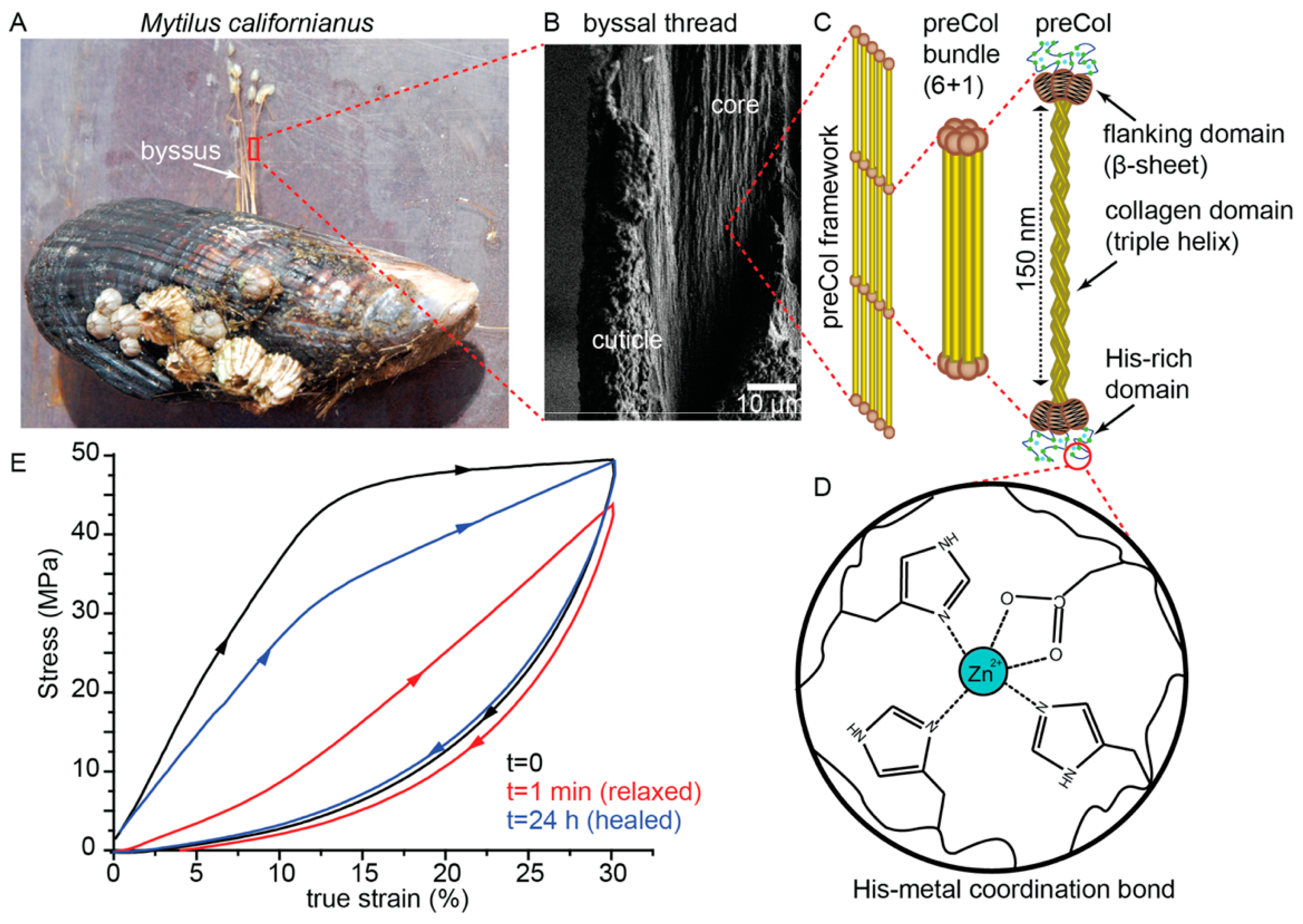
2. Mussel Byssus as Inspiration for Self-Healing Polymeric Materials
2.1. Mussel Byssus Background
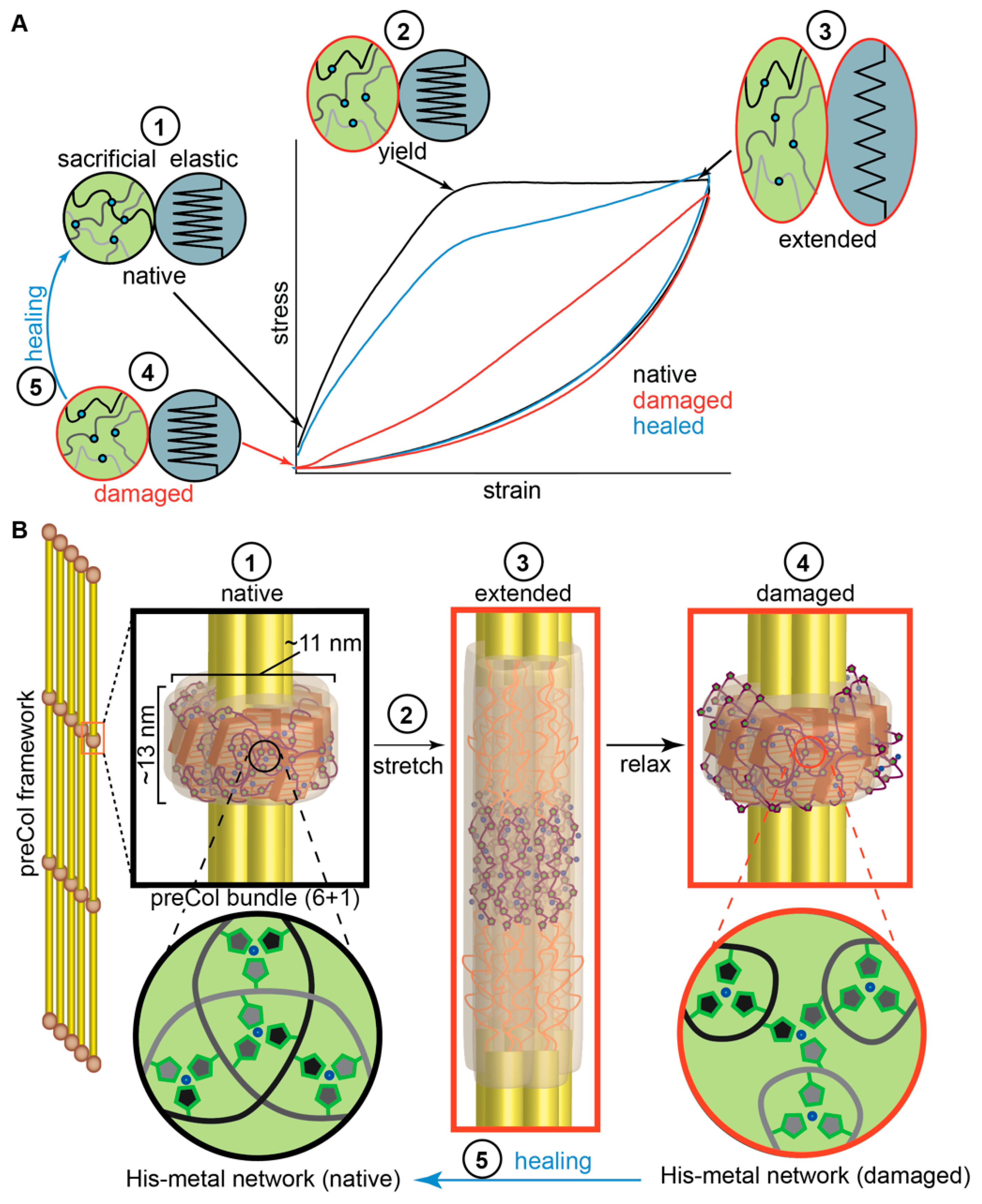
2.2. Influence of Hierarchical Structure on Byssus Self-Healing
3. Bioinspired Metallopolymers
3.1. General Aspects of Metal–Ligand-Based Polymers
3.2. His-Functionalized Polymers: Using Imidazole plus Metal Ions to Achieve Dynamic and Self-Healing Ability
3.3. Using Histidine–Metal Complexes for Advanced Healing Systems
3.4. Future Developments of Synthetic Bioinspired Metallopolymers
4. Conclusions
Funding
Conflicts of Interest
References
- Blaiszik, B.J.; Kramer, S.L.B.; Olugebefola, S.C.; Moore, J.S.; Sottos, N.R.; White, S.R. Self-healing polymers and composites. Annu. Rev. Mater. Res. 2010, 40, 179–211. [Google Scholar] [CrossRef]
- Hager, M.D.; Greil, P.; Leyens, C.; van der Zwaag, S.; Schubert, U.S. Self-healing materials. Adv. Mater. 2010, 22, 5424–5430. [Google Scholar] [CrossRef] [PubMed]
- Diesendruck, C.E.; Sottos, N.R.; Moore, J.S.; White, S.R. Biomimetic self-healing. Angew. Chem. Int. Ed. 2015. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, A.; Bertinetti, L.; Fratzl, P.; Harrington, M.J. Cooperative behavior of a sacrificial bond network and elastic framework in providing self-healing capacity in mussel byssal threads. J. Struct. Biol. 2016, 196, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Cordier, P.; Tournilhac, F.; Soulie-Ziakovic, C.; Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature 2008, 451, 977–980. [Google Scholar] [CrossRef] [PubMed]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Fratzl, P.; Weinkamer, R. Nature’s hierarchical materials. Prog. Mater. Sci. 2007, 52, 1263–1334. [Google Scholar] [CrossRef]
- Van der Zwaag, S.; Brinkman, E. Self-Healing Materials: Pioneering Work in The Netherlands; IOS Press: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Trask, R.S.; Williams, H.R.; Bond, I.P. Self-healing polymer composites: Mimicking nature to enhance performance. Bioinspir. Biomim. 2007, 2, P1–P9. [Google Scholar] [CrossRef] [PubMed]
- Degtyar, E.; Harrington, M.J.; Politi, Y.; Fratzl, P. The mechanical role of metal ions in biogenic protein-based materials. Angew. Chem. Int. Ed. 2014, 53, 12026–12044. [Google Scholar] [CrossRef] [PubMed]
- Fratzl, P.; Weinkamer, R. Hierarchical structure and repair of bone: Deformation, remodelling, healing. In Self Healing Materials: An Alternative Approach to 20 Centuries of Materials Science; van der Zwaag, S., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 323–335. [Google Scholar]
- Speck, T.; Mülhaupt, R.; Speck, O. Self-healing in plants as bio-inspiration for self-repairing polymers. In Self-Healing Polymers: From Principles to Applications, 1st ed.; Wiley: Hoboken, NJ, USA, 2013; pp. 61–89. [Google Scholar]
- Ashton, N.N.; Stewart, R.J. Self-recovering caddisfly silk: Energy dissipating, Ca2+-dependent, double dynamic network fibers. Soft Matter 2015, 11, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Harrington, M.; Scott Wasko, S.; Masic, A.; Dieter Fischer, F.; Gupta, H.; Fratzl, P. Pseudoelastic behaviour of a natural material is achieved via reversible changes in protein backbone conformation. J. R. Soc. Interface 2012, 9, 2911–2922. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, L.K.; Bryant, H.E.; Weinstein, J.A. Transition metal complexes as photosensitisers in one- and two-photon photodynamic therapy. Coord. Chem. Rev. 2019, 379, 2–29. [Google Scholar] [CrossRef]
- Ragni, R.; Punzi, A.; Babudri, F.; Farinola, G.M. Organic and organometallic fluorinated materials for electronics and optoelectronics: A survey on recent research. Eur. J. Org. Chem. 2018, 2018, 3500–3519. [Google Scholar] [CrossRef]
- Mills, I.N.; Porras, J.A.; Bernhard, S. Judicious design of cationic, cyclometalated Ir(III) complexes for photochemical energy conversion and optoelectronics. Acc. Chem. Res. 2018, 51, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Dudev, T.; Lim, C. Metal binding affinity and selectivity in metalloproteins: Insights from computational studies. Annu. Rev. Biophys. 2008, 37, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Harding, M.M.; Nowicki, M.W.; Walkinshaw, M.D. Metals in protein structures: A review of their principal features. Crystallogr. Rev. 2010, 16, 247–302. [Google Scholar] [CrossRef]
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-molecule mechanics of mussel adhesion. Proc. Nat. Acad. Sci. USA 2006, 103, 12999–13003. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, L.; Ludwig, M.; Gaub, H.E.; Tampe, R. A Metal-chelating microscopy tip as a new toolbox for single-molecule experiments by atomic force microscopy. Biophys. J. 2000, 78, 3275–3285. [Google Scholar] [CrossRef]
- Grindy, S.C.; Learsch, R.; Mozhdehi, D.; Cheng, J.; Barrett, D.G.; Guan, Z.; Messersmith, P.B.; Holten-Andersen, N. Control of hierarchical polymer mechanics with bioinspired metal-coordination dynamics. Nat. Mater. 2015, 14, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Krogsgaard, M.; Nue, V.; Birkedal, H. Mussel-inspired materials: Self-healing through coordination chemistry. Chem. Eur. J. 2016, 22, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Creighton, T.E. Proteins: Structures and Molecular Properties, 2nd ed.; W. H. Freeman: New York, NY, USA, 1992. [Google Scholar]
- Takeuchi, H. Raman structural markers of tryptophan and histidine side chains in proteins. Biopolymers 2003, 72, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Chakrabati, P. Geometry of interaction of metal ions with histidine residues in protein structures. Prot. Eng. 1990, 4, 57–63. [Google Scholar] [CrossRef]
- Trapaidze, A.; D’Antuono, M.; Fratzl, P.; Harrington, M.J. Exploring mussel byssus fabrication with peptide-polymer hybrids: Role of pH and metal coordination in self-assembly and mechanics of histidine-rich domains. Eur. Polym. J. 2018, 109, 229–236. [Google Scholar] [CrossRef]
- Venkataraman, D.; Du, Y.; Wilson, S.R.; Hirsch, K.A.; Zhang, P.; Moore, J.S. A coordination geometry table of the d-block elements and their ions. J. Chem. Educ. 1997, 74, 915. [Google Scholar] [CrossRef]
- Broomell, C.C.; Zok, F.W.; Waite, J.H. Role of transition metals in sclerotization of biological tissue. Acta Biomater. 2008, 4, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Lichtenegger, H.C.; Schoberl, T.; Ruokolainen, J.T.; Cross, J.O.; Heald, S.M.; Birkedal, H.; Waite, J.H.; Stucky, G.D. Zinc and mechanical prowess in the jaws of Nereis, a marine worm. Proc. Nat. Acad. Sci. USA 2003, 100, 9144–9149. [Google Scholar] [CrossRef] [PubMed]
- Schofield, R.M.S.; Nesson, M.H.; Richardson, K.A.; Wyeth, P. Zinc is incorporated into cuticular “tools” after ecdysis: The time course of the zinc distribution in “tools” and whole bodies of an ant and a scorpion. J. Insect Physiol. 2003, 49, 31–44. [Google Scholar] [CrossRef]
- Politi, Y.; Priewasser, M.; Pippel, E.; Zaslansky, P.; Hartmann, J.; Siegel, S.; Li, C.H.; Barth, F.G.; Fratzl, P. A Spider’s fang: How to design an injection needle using chitin-based composite material. Adv. Funct. Mater. 2012, 22, 2519–2528. [Google Scholar] [CrossRef]
- Broomell, C.C.; Khan, R.K.; Moses, D.N.; Miserez, A.; Pontin, M.G.; Stucky, G.D.; Zok, F.W.; Waite, J.H. Mineral minimization in nature’s alternative teeth. J. R. Soc. Interfaces 2007, 4, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Broomell, C.C.; Chase, S.F.; Laue, T.; Waite, J.H. Cutting edge structural protein from the jaws of Nereis virens. Biomacromolecules 2008, 9, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Harrington, M.J.; Waite, J.H. Short-order tendons: Liquid crystal mesophases, metal-complexation and protein gradients in the externalized collagens of mussel byssal threads. In Fibrous Proteins; Taylor & Francis Group: Abingdon, UK, 2008. [Google Scholar]
- Priemel, T.; Degtyar, E.; Dean, M.N.; Harrington, M.J. Rapid self-assembly of complex biomolecular architectures during mussel byssus biofabrication. Nat. Commun. 2017, 8, 14539. [Google Scholar] [CrossRef] [PubMed]
- Carrington, E. The ecomechanics of mussel attachment: From molecules to ecosystems. Integr. Comp. Biol. 2002, 42, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Harrington, M.J. Iron-clad fibers: A metal-based biological strategy for hard flexible coatings. Science 2010, 328, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Holten-Andersen, N.; Fantner, G.E.; Hohlbauch, S.; Waite, J.H.; Zok, F.W. Protective coatings on extensible biofibres. Nat. Mater. 2007, 6, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.; Gosline, J. Mechanical design of mussel byssus: Material yield enhances attachment strength. J. Exp. Biol. 1996, 199 Pt 4, 1005–1017. [Google Scholar]
- Carrington, E.; Gosline, J. Mechanical design of mussel byssus: Load cycle and strain rate dependence. Am. Malacol. Bull. 2004, 18, 135–142. [Google Scholar]
- Lachance, A.A.; Myrand, B.; Tremblay, R.; Koutitonsky, V.; Carrington, E. Biotic and abiotic factors influencing attachment strength of blue mussels Mytilus edulis in suspended culture. Aquat. Biol. 2008, 2, 119–129. [Google Scholar] [CrossRef]
- Carrington, E.; Moeser, G.M.; Dimond, J.; Mello, J.J.; Boller, M.L. Seasonal disturbance to mussel beds: Field test of a mechanistic model predicting wave dislodgment. Limnol. Oceanogr. 2009, 54, 978–986. [Google Scholar] [CrossRef]
- Bouhlel, Z.; Genard, B.; Ibrahim, N.; Carrington, E.; Babarro, J.M.F.; Lok, A.; Flores, A.A.V.; Pellerin, C.; Tremblay, R.; Marcotte, I. Interspecies comparison of the mechanical properties and biochemical composition of byssal threads. J. Exp. Biol. 2017, 220, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Brazee, S.L.; Carrington, E. Interspecific comparison of the mechanical properties of mussel byssus. Biol. Bull. 2006, 211, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Gosline, J.; Lillie, M.; Carrington, E.; Guerette, P.; Ortlepp, C.; Savage, K. Elastic proteins: Biological roles and mechanical properties. Philos. Trans. R. Soc. Biol. Sci. 2002, 357, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Pearce, T.; LaBarbera, M. A comparative study of the mechanical properties of mytilid byssal threads. J. Exp. Biol. 2009, 212, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, E.; Waite, J.H. Yield and post-yield behavior of mussel byssal thread: A self-healing biomolecular material. Biomacromolecules 2001, 2, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Harrington, M.J.; Gupta, H.S.; Fratzl, P.; Waite, J.H. Collagen insulated from tensile damage by domains that unfold reversibly: In situ X-ray investigation of mechanical yield and damage repair in the mussel byssus. J. Struct. Biol. 2009, 167, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Rudall, K.M. The distribution of collagen and chitin. Symp. Soc. Exp. Biol. 1955, 9, 49–71. [Google Scholar]
- Mercer, E.H. Observations on the molecular structure of byssus fibres. Mar. Freshw. Res. 1952, 3, 199–204. [Google Scholar] [CrossRef]
- Qin, X.; Waite, J.H. Exotic collagen gradients in the byssus of the mussel Mytilus edulis. J. Exp. Biol. 1995, 198, 633–644. [Google Scholar] [PubMed]
- Coyne, K.J.; Qin, X.-X.; Waite, J.H. Extensible collagen in mussel byssus: A natural block copolymer. Science 1997, 277, 1830–1832. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.X.; Coyne, K.J.; Waite, J.H. Tough tendons—Mussel byssus has collagen with silk-like domains. J. Biol. Chem. 1997, 272, 32623–32627. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.X.; Waite, J.H. A potential mediator of collagenous block copolymer gradients in mussel byssal threads. Proc. Natl Acad. Sci. USA 1998, 95, 10517–10522. [Google Scholar] [CrossRef] [PubMed]
- Waite, J.H.; Lichtenegger, H.C.; Stucky, G.D.; Hansma, P. Exploring molecular and mechanical gradients in structural bioscaffolds. Biochemistry 2004, 43, 7653–7662. [Google Scholar] [CrossRef] [PubMed]
- Waite, J.H.; Qin, X.X.; Coyne, K.J. The peculiar collagens of mussel byssus. Matrix Biol. 1998, 17, 93–106. [Google Scholar] [CrossRef]
- Schmitt, C.N.Z.; Politi, Y.; Reinecke, A.; Harrington, M.J. Role of sacrificial protein–metal bond exchange in mussel byssal thread self-healing. Biomacromolecules 2015, 16, 2852–2861. [Google Scholar] [CrossRef] [PubMed]
- Harrington, M.J.; Waite, J.H. Holdfast heroics: Comparing the molecular and mechanical properties of Mytilus californianus byssal threads. J. Exp. Biol. 2007, 210, 4307–4318. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Reinecke, A.; Wojcik, F.; Pussak, D.; Hartmann, L.; Harrington, M.J. Metal-mediated molecular self-healing in histidine-rich mussel peptides. Biomacromolecules 2014, 15, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.A.; Byette, F.; Seguin-Heine, M.O.; LeBlanc, A.; Sleno, L.; Tremblay, R.; Pellerin, C.; Marcotte, I. Solid-state NMR structure determination of whole anchoring threads from the blue mussel Mytilus edulis. Biomacromolecules 2013, 14, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Hagenau, A.; Papadopoulos, P.; Kremer, F.; Scheibel, T. Mussel collagen molecules with silk-like domains as load-bearing elements in distal byssal threads. J. Struct. Biol. 2011, 175, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Krauss, S.; Metzger, T.H.; Fratzl, P.; Harrington, M.J. Self-repair of a biological fiber guided by an ordered elastic framework. Biomacromolecules 2013, 14, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Hassenkam, T.; Gutsmann, T.; Hansma, P.; Sagert, J.; Waite, J.H. Giant bent-core mesogens in the thread forming process of marine mussels. Biomacromolecules 2004, 5, 1351–1354. [Google Scholar] [CrossRef] [PubMed]
- Tirrell, D.A. Hierarchical Structures in Biology as a Guide for New Materials Technology; National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- Reinecke, A.; Harrington, M.J. The role of metal ions in the mussel byssus. Physiol. Mollusca 2016, 1, 113–152. [Google Scholar]
- Whittell, G.R.; Hager, M.D.; Schubert, U.S.; Manners, I. Functional soft materials from metallopolymers and metallosupramolecular polymers. Nat. Mater. 2011, 10, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Eloi, J.-C.; Chabanne, L.; Whittell, G.R.; Manners, I. Metallopolymers with emerging applications. Mater. Today 2008, 11, 28–36. [Google Scholar] [CrossRef]
- Keefe, M.H.; Benkstein, K.D.; Hupp, J.T. Luminescent sensor molecules based on coordinated metals: A review of recent developments. Coord. Chem. Rev. 2000, 205, 201–228. [Google Scholar] [CrossRef]
- Sandmann, B.; Bode, S.; Hager, M.D.; Schubert, U.S. Metallopolymers as an emerging class of self-healing materials. Adv. Polym. Sci. 2013, 262, 239–257. [Google Scholar]
- Kurth, D.G.; Higuchi, M. Transition metal ions: Weak links for strong polymers. Soft Matter 2006, 2, 915–927. [Google Scholar] [CrossRef]
- Stanley, J.M.; Holliday, B.J. Luminescent lanthanide-containing metallopolymers. Coord. Chem. Rev. 2012, 256, 1520–1530. [Google Scholar] [CrossRef]
- Voorhaar, L.; Hoogenboom, R. Supramolecular polymer networks: Hydrogels and bulk materials. Chem. Soc. Rev. 2016, 45, 4013–4031. [Google Scholar] [CrossRef] [PubMed]
- Koga, Y.; Matsubara, K. Phosphorescent organic light-emitting diodes using an iridium complex polymer as the solution-processible host material. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 4358–4365. [Google Scholar] [CrossRef]
- Irina, S.; Oleksandra, B.; Olena, T.; Yaroslav, F.; Sergiy, S.; Nataliya, R. Monomer and metallopolymer compounds of Tb(III) as precursors for OLEDs. Appl. Nanosci. 2018. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, J.; Ren, L.; Tang, C. Metal-containing and related polymers for biomedical applications. Chem. Soc. Rev. 2016, 45, 5232–5263. [Google Scholar] [CrossRef] [PubMed]
- Callari, M.; Aldrich-Wright, J.R.; de Souza, P.L.; Stenzel, M.H. Polymers with platinum drugs and other macromolecular metal complexes for cancer treatment. Prog. Polym. Sci. 2014, 39, 1614–1643. [Google Scholar] [CrossRef]
- Janib, S.M.; Moses, A.S.; MacKay, J.A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Kumpfer, J.R.; Rowan, S.J. Thermo-, photo-, and chemo-responsive shape-memory properties from photo-cross-linked metallo-supramolecular polymers. J. Am. Chem. Soc. 2011, 133, 12866–12874. [Google Scholar] [CrossRef] [PubMed]
- Enke, M.; Döhler, D.; Bode, S.; Binder, W.H.; Hager, M.D.; Schubert, U.S. Intrinsic self-healing polymers based on supramolecular interactions: State of the art and future directions. Adv. Polym. Sci. 2016, 273, 59–112. [Google Scholar]
- Bode, S.; Zedler, L.; Schacher, F.H.; Dietzek, B.; Schmitt, M.; Popp, J.; Hager, M.D.; Schubert, U.S. Self-healing polymer coatings based on crosslinked metallosupramolecular copolymers. Adv. Mater. 2013, 25, 1634–1638. [Google Scholar] [CrossRef] [PubMed]
- Burnworth, M.; Tang, L.; Kumpfer, J.R.; Duncan, A.J.; Beyer, F.L.; Fiore, G.L.; Rowan, S.J.; Weder, C. Optically healable supramolecular polymers. Nature 2011, 472, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Bode, S.; Enke, M.; Bose, R.K.; Schacher, F.H.; Garcia, S.J.; van der Zwaag, S.; Hager, M.D.; Schubert, U.S. Correlation between scratch healing and rheological behavior for terpyridine complex based metallopolymers. J. Mater. Chem. A 2015, 3, 22145–22153. [Google Scholar] [CrossRef]
- Bode, S.; Bose, R.K.; Matthes, S.; Ehrhardt, M.; Seifert, A.; Schacher, F.H.; Paulus, R.M.; Stumpf, S.; Sandmann, B.; Vitz, J.; et al. Self-healing metallopolymers based on cadmium bis(terpyridine) complex containing polymer networks. Polym. Chem. 2013, 4, 4966–4973. [Google Scholar] [CrossRef]
- Hillewaere, X.K.D.; Du Prez, F.E. Fifteen chemistries for autonomous external self-healing polymers and composites. Prog. Polym. Sci. 2015, 49–50, 121–153. [Google Scholar] [CrossRef]
- Diba, M.; Spaans, S.; Ning, K.; Ippel, B.D.; Yang, F.; Loomans, B.; Dankers, P.Y.W.; Leeuwenburgh, S.C.G. Self-healing biomaterials: From molecular concepts to clinical applications. Adv. Mater. Interfaces 2018, 5, 1800118. [Google Scholar] [CrossRef]
- Webber, M.J.; Appel, E.A.; Meijer, E.W.; Langer, R. Supramolecular biomaterials. Nat. Mater. 2015, 15, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.N.; Xiong, X.M.; Wang, J.; Rong, M.Z.; Zhang, M.Q. A seawater triggered dynamic coordinate bond and its application for underwater self-healing and reclaiming of lipophilic polymer. Chem. Sci. 2016, 7, 2736–2742. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.P.; Messersmith, P.B.; Israelachvili, J.N.; Waite, J.H. Mussel-inspired adhesives and coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Lu, X.; Liu, K.; Wang, K.; Fang, L.; Weng, L.-T.; Zhang, H.; Tang, Y.; Ren, F.; Zhao, C.; et al. Mussel-inspired adhesive and tough hydrogel based on nanoclay confined dopamine polymerization. ACS Nano 2017, 11, 2561–2574. [Google Scholar] [CrossRef] [PubMed]
- Krogsgaard, M.; Behrens, M.A.; Pedersen, J.S.; Birkedal, H. Self-healing mussel-inspired multi-pH-responsive hydrogels. Biomacromolecules 2013, 14, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Faure, E.; Falentin-Daudré, C.; Jérôme, C.; Lyskawa, J.; Fournier, D.; Woisel, P.; Detrembleur, C. Catechols as versatile platforms in polymer chemistry. Prog. Polym. Sci. 2013, 38, 236–270. [Google Scholar] [CrossRef]
- Holten-Andersen, N.; Harrington, M.J.; Birkedal, H.; Lee, B.P.; Messersmith, P.B.; Lee, K.Y.C.; Waite, J.H. pH-induced metal-ligand cross-links inspired by mussel yield self-healing polymer networks with near-covalent elastic moduli. Proc. Nat. Acad. Sci. USA 2011, 108, 2651–2655. [Google Scholar] [CrossRef] [PubMed]
- Holten-Andersen, N.; Jaishankar, A.; Harrington, M.J.; Fullenkamp, D.E.; DiMarco, G.; He, L.; McKinley, G.H.; Messersmith, P.B.; Lee, K.Y.C. Metal-coordination: Using one of nature’s tricks to control soft material mechanics. J. Mater. Chem. B 2014, 2, 2467–2472. [Google Scholar] [CrossRef] [PubMed]
- Krogsgaard, M.; Hansen, M.R.; Birkedal, H. Metals & polymers in the mix: Fine-tuning the mechanical properties & color of self-healing mussel-inspired hydrogels. J. Mater. Chem. B 2014, 2, 8292–8297. [Google Scholar]
- Menyo, M.S.; Hawker, C.J.; Waite, J.H. Versatile tuning of supramolecular hydrogels through metal complexation of oxidation-resistant catechol-inspired ligands. Soft Matter 2013, 9, 10314–10323. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z. Mechanics of metal-catecholate complexes: The roles of coordination state and metal types. Sci. Rep. 2013, 3, 2914. [Google Scholar] [CrossRef] [PubMed]
- Vatankhah-Varnoosfaderani, M.; Hashmi, S.; GhavamiNejad, A.; Stadler, F.J. Rapid self-healing and triple stimuli responsiveness of a supramolecular polymer gel based on boron–catechol interactions in a novel water-soluble mussel-inspired copolymer. Polym. Chem. 2014, 5, 512–523. [Google Scholar] [CrossRef]
- Sundberg, R.J.; Martin, R.B. Interactions of histidine and other imidazole derivatives with transition metal ions in chemical and biological systems. Chem. Rev. 1974, 74, 471–517. [Google Scholar] [CrossRef]
- Enke, M.; Jehle, F.; Bode, S.; Vitz, J.; Harrington, M.J.; Hager, M.D.; Schubert, U.S. Histidine–zinc interactions investigated by isothermal titration calorimetry (ITC) and their application in self-healing polymers. Macromol. Chem. Phys. 2017, 218, 1600458. [Google Scholar] [CrossRef]
- Enke, M.; Bode, S.; Vitz, J.; Schacher, F.H.; Harrington, M.J.; Hager, M.D.; Schubert, U.S. Self-healing response in supramolecular polymers based on reversible zinc–histidine interactions. Polymer 2015, 69, 274–282. [Google Scholar] [CrossRef]
- Pham, T.T.H.; van der Gucht, J.; Mieke Kleijn, J.; Cohen Stuart, M.A. Reversible polypeptide hydrogels from asymmetric telechelics with temperature-dependent and Ni2+-dependent connectors. Soft Matter 2016, 12, 4979–4984. [Google Scholar] [CrossRef] [PubMed]
- Mozhdehi, D.; Neal, J.A.; Grindy, S.C.; Cordeau, Y.; Ayala, S.; Holten-Andersen, N.; Guan, Z. Tuning dynamic mechanical response in metallopolymer networks through simultaneous control of structural and temporal properties of the networks. Macromolecules 2016, 49, 6310–6321. [Google Scholar] [CrossRef]
- Wegner, S.V.; Schenk, F.C.; Witzel, S.; Bialas, F.; Spatz, J.P. Cobalt cross-linked redox-responsive PEG hydrogels: From viscoelastic liquids to elastic solids. Macromolecules 2016, 49, 4229–4235. [Google Scholar] [CrossRef]
- Tang, Q.; Zhao, D.; Zhou, Q.; Yang, H.; Peng, K.; Zhang, X. Polyhistidine-based metal coordination hydrogels with physiologically relevant pH responsiveness and enhanced stability through a novel synthesis. Macromol. Rapid Commun. 2018, 39, 1800109. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, P.; Pageni, P.; Tang, C. Recent advances in metal-containing polymer hydrogels. Macromol. Rapid Commun. 2017, 38, 1700109. [Google Scholar] [CrossRef] [PubMed]
- Ahner, J.; Pretzel, D.; Enke, M.; Geitner, R.; Zechel, S.; Popp, J.; Schubert, U.S.; Hager, M.D. Conjugated oligomers as fluorescence marker for the determination of the self-healing efficiency in mussel-inspired polymers. Chem. Mater. 2018, 30, 2791–2799. [Google Scholar] [CrossRef]
- Tang, S.; Olsen, B.D. Relaxation Processes in supramolecular metallogels based on histidine–nickel coordination bonds. Macromolecules 2016, 49, 9163–9175. [Google Scholar] [CrossRef]
- Fullenkamp, D.E.; He, L.; Barrett, D.G.; Burghardt, W.R.; Messersmith, P.B. Mussel-inspired histidine-based transient network metal coordination hydrogels. Macromolecules 2013, 46, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Grindy, S.C.; Holten-Andersen, N. Bio-inspired metal-coordinate hydrogels with programmable viscoelastic material functions controlled by longwave UV light. Soft Matter 2017, 13, 4057–4065. [Google Scholar] [CrossRef] [PubMed]
- Grindy, S.C.; Lenz, M.; Holten-Andersen, N. Engineering elasticity and relaxation time in metal-coordinate cross-linked hydrogels. Macromolecules 2016, 49, 8306–8312. [Google Scholar] [CrossRef]
- Tang, Q.; Zhao, D.; Yang, H.; Wang, L.; Zhang, X. A pH-responsive self-healing hydrogel based on multivalent coordination of Ni2+ with polyhistidine-terminated PEG and IDA-modified oligochitosan. J. Mater. Chem. B 2018, 7, 30–42. [Google Scholar] [CrossRef]
- Mozhdehi, D.; Ayala, S.; Cromwell, O.R.; Guan, Z. Self-healing multiphase polymers via dynamic metal–ligand interactions. J. Am. Chem. Soc. 2014, 136, 16128–16131. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Wang, Y.; Zhu, J.; Yu, J.; Hu, Z. Disulfide bonds and metal-ligand co-crosslinked network with improved mechanical and self-healing properties. Mater. Today Commun. 2017, 13, 282–289. [Google Scholar] [CrossRef]
- Xu, J.; Ye, S.; Fu, J. Novel sea cucumber-inspired material based on stiff, strong yet tough elastomer with unique self-healing and recyclable functionalities. J. Mater. Chem. A 2018, 6, 24291–24297. [Google Scholar] [CrossRef]
- Liu, X.; Su, G.; Guo, Q.; Lu, C.; Zhou, T.; Zhou, C.; Zhang, X. Hierarchically structured self-healing sensors with tunable positive/negative piezoresistivity. Adv. Funct. Mater. 2018, 28, 1706658. [Google Scholar] [CrossRef]
- Zhao, H.; Waite, J.H. Proteins in Load-Bearing Junctions: The histidine-rich metal-binding protein of mussel byssus. Biochemistry 2006, 45, 14223–14231. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, A.; Brezesinski, G.; Harrington, M.J. pH-responsive self-organization of metal-binding protein motifs from biomolecular junctions in mussel byssus. Adv. Mater. Interfaces 2016, 4. [Google Scholar] [CrossRef]
- Enke, M.; Bose, R.K.; Zechel, S.; Vitz, J.; Deubler, R.; Garcia, S.J.; van der Zwaag, S.; Schacher, F.H.; Hager, M.D.; Schubert, U.S. A translation of the structure of mussel byssal threads into synthetic materials by the utilization of histidine-rich block copolymers. Polym. Chem. 2018, 9, 3543–3551. [Google Scholar] [CrossRef]
- Balkenende, D.W.R.; Coulibaly, S.; Balog, S.; Simon, Y.C.; Fiore, G.L.; Weder, C. Mechanochemistry with metallosupramolecular polymers. J. Am. Chem. Soc. 2014, 136, 10493–10498. [Google Scholar] [CrossRef] [PubMed]
- Enke, M.; Köps, L.; Zechel, S.; Brendel, J.C.; Vitz, J.; Hager, M.D.; Schubert, U.S. Influence of aspartate moieties on the self-healing behavior of histidine-rich supramolecular polymers. Macromol. Rapid Commun. 2018, 39, 1700742. [Google Scholar] [CrossRef] [PubMed]

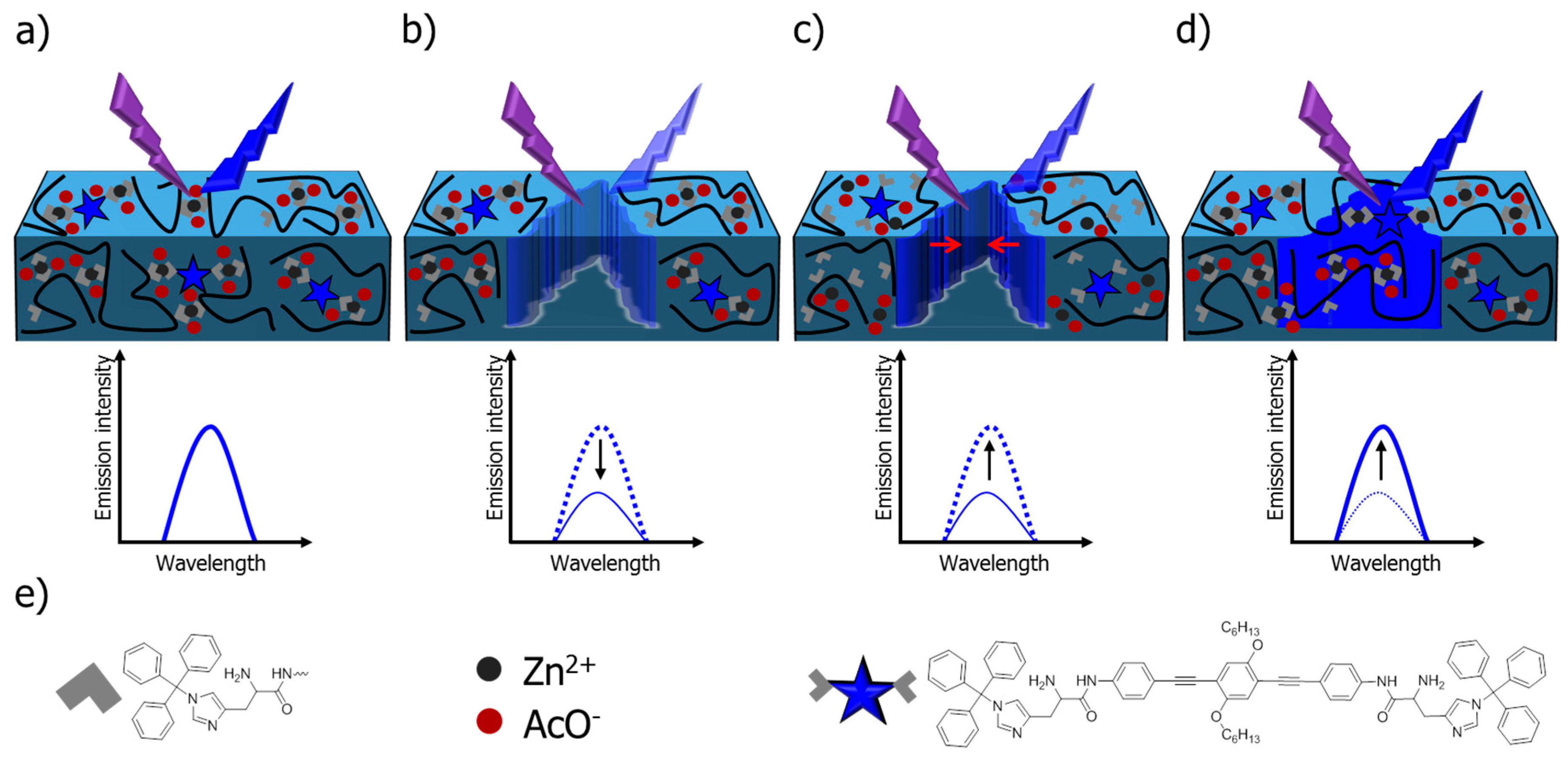
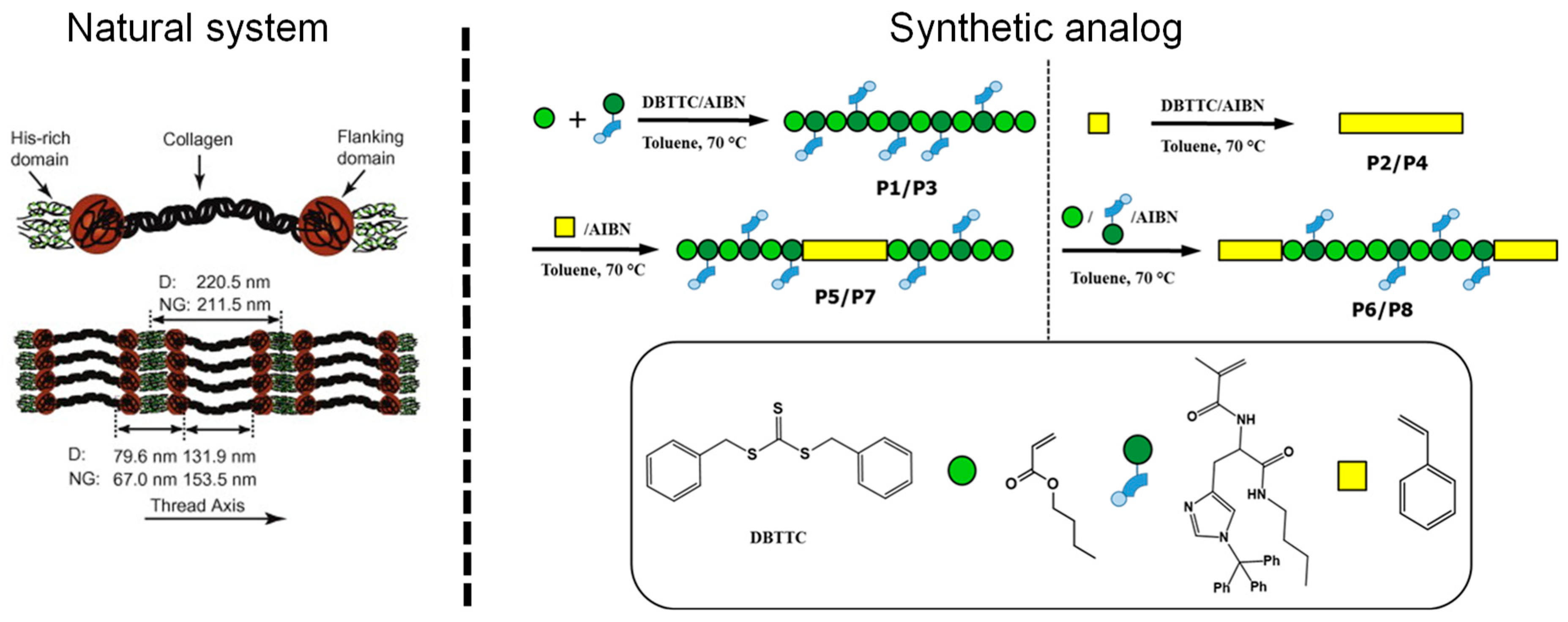
| System | Proposed Mechanism | References | |
|---|---|---|---|
| Cellular | Wound healing |
| [7] |
| Bone healing |
| [12] | |
| Latex-bearing plants |
| [13] | |
| Acellular | Mussel byssus | Reversible sacrificial bonds and hidden length based on protein–metal coordination | [4] |
| Caddisfly silk | Reversible inter- and intramolecular interactions between phosphoserine and Ca2+/Mg2+ ions | [14] | |
| Whelk egg capsules | Hidden length based on reversible unfolding and refolding of protein backbone (α-helix ⟷ β-sheet) | [15] |
| Authors | Ligand | Metal ion | Polymer | Bulk/Gel | Reference |
|---|---|---|---|---|---|
| Enke and colleagues | Histidine | Zinc(II) | Poly(methacrylate) | Bulk/Film | [101,102] |
| Ahner et al. | Histidine | Zinc(II) | Poly(methacrylate) | Bulk/Film | [108] |
| Tang et al. | Histidine | Nickel(II) | Polyacrylamide | Hydrogel | [109] |
| Fullenkamp et al. | Histidine | Zinc (II), copper(II), nickel(II), cobalt(II) | Poly(ethylene glycol) | Hydrogel | [110] |
| Grindy and colleagues | Histidine | Copper(II), nickel(II), cobalt(II) | Poly(ethylene glycol) | Hydrogel | [23,111,112] |
| Harrington and colleagues | Histidine | Zinc (II) and nickel (II) | Poly(ethylene glycol)/peptides | Hydrogel | [28,61] |
| Wegner et al. | Histidine | Cobalt(II/III) | Poly(ethylene glycol) | Hydrogel | [105] |
| Tang and colleagues | Histidine | Nickel(II) | Poly(ethylene glycol) and chitosan | Hydrogel | [106,113] |
| Pham et al. | Histidine | Nickel(II) | Peptide | Hydrogel | [103] |
| Mozhdehi and colleagues | Imidazole | Zinc(II), copper(II), cobalt(II) | Polystyrene-graft-poly(acrylate) | Bulk | [104,114] |
| Liu et al. | Imidazole | Zinc(II) | Poly(propylene glycol) | Bulk/Film | [115] |
| Xu et al. | Imidazole | Zinc(II) | Poly(acrylate) | Bulk/Film | [116] |
| Liu et al. | Imidazole | Zinc(II) | Cellulose | Bulk/Composite | [117] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zechel, S.; Hager, M.D.; Priemel, T.; Harrington, M.J. Healing through Histidine: Bioinspired Pathways to Self-Healing Polymers via Imidazole–Metal Coordination. Biomimetics 2019, 4, 20. https://doi.org/10.3390/biomimetics4010020
Zechel S, Hager MD, Priemel T, Harrington MJ. Healing through Histidine: Bioinspired Pathways to Self-Healing Polymers via Imidazole–Metal Coordination. Biomimetics. 2019; 4(1):20. https://doi.org/10.3390/biomimetics4010020
Chicago/Turabian StyleZechel, Stefan, Martin D. Hager, Tobias Priemel, and Matthew J. Harrington. 2019. "Healing through Histidine: Bioinspired Pathways to Self-Healing Polymers via Imidazole–Metal Coordination" Biomimetics 4, no. 1: 20. https://doi.org/10.3390/biomimetics4010020
APA StyleZechel, S., Hager, M. D., Priemel, T., & Harrington, M. J. (2019). Healing through Histidine: Bioinspired Pathways to Self-Healing Polymers via Imidazole–Metal Coordination. Biomimetics, 4(1), 20. https://doi.org/10.3390/biomimetics4010020







