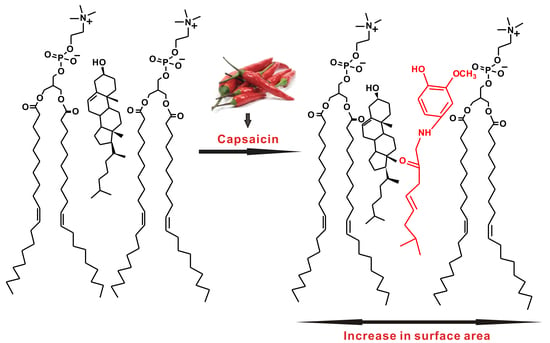
Effects of Capsaicin on Biomimetic Membranes †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cell-Sized Liposomes
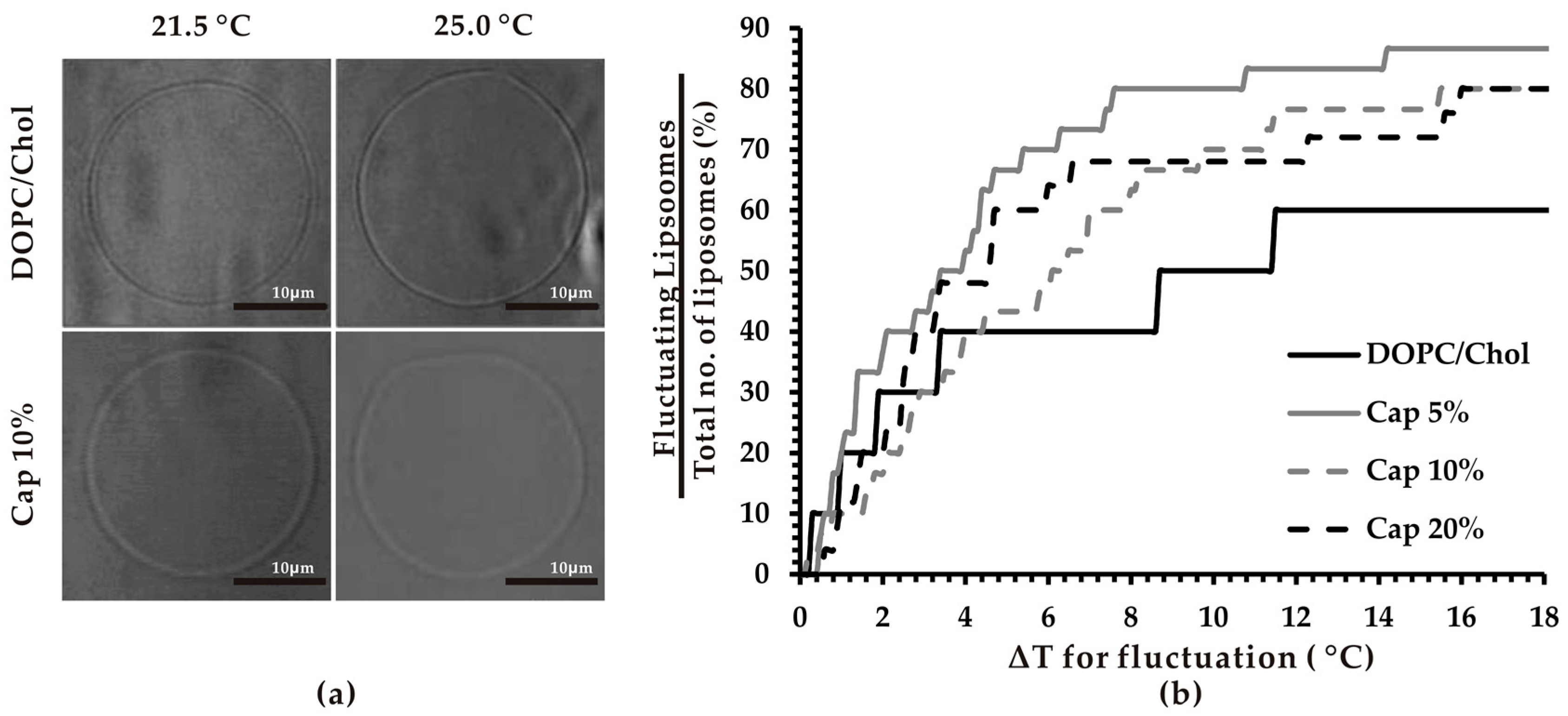
2.3. Thermo-Induced Fluctuation of Lipid Vesicles
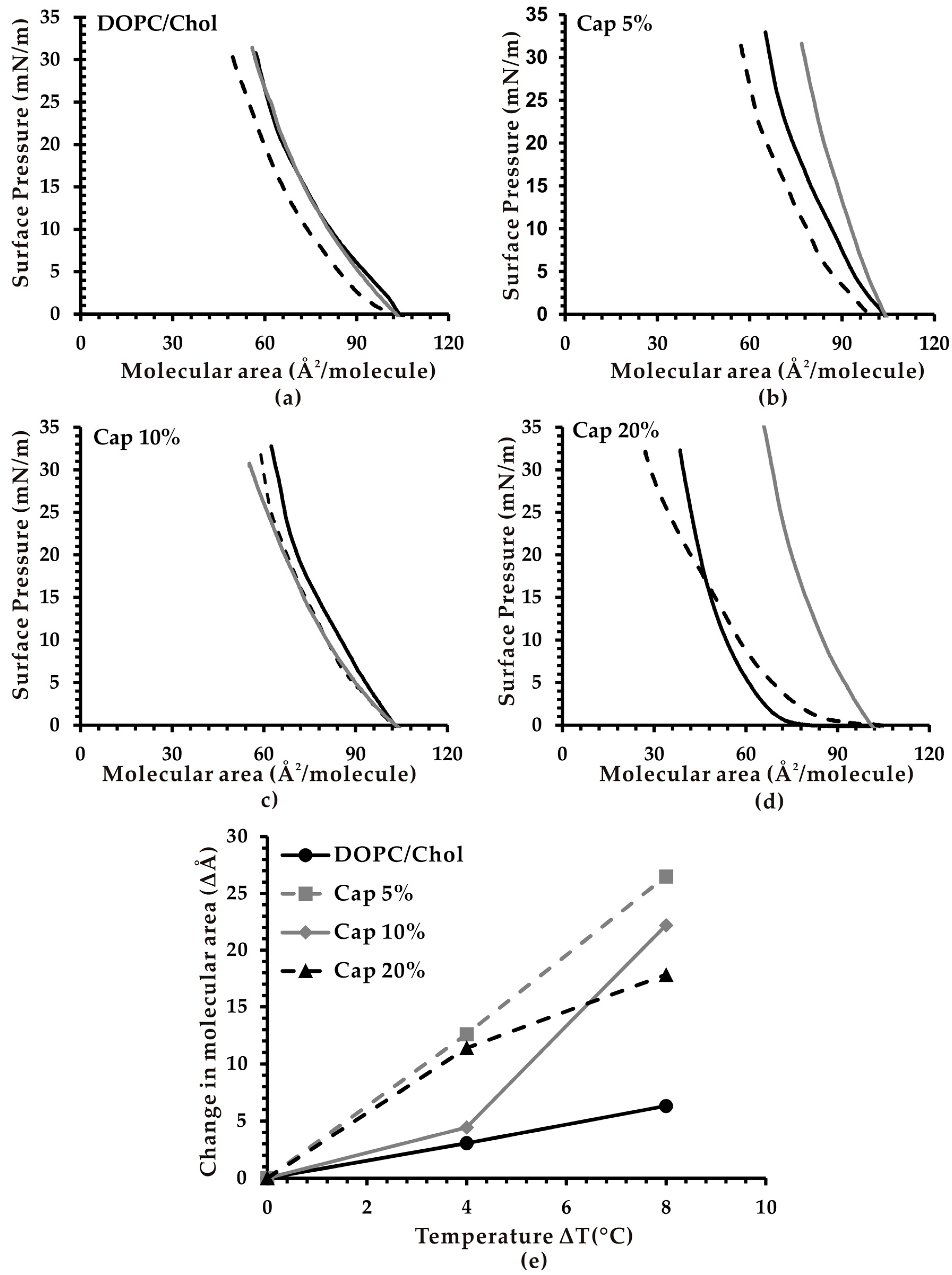
2.4. Effect of Temperature on Molecular Area of Monolayer Lipid Membranes
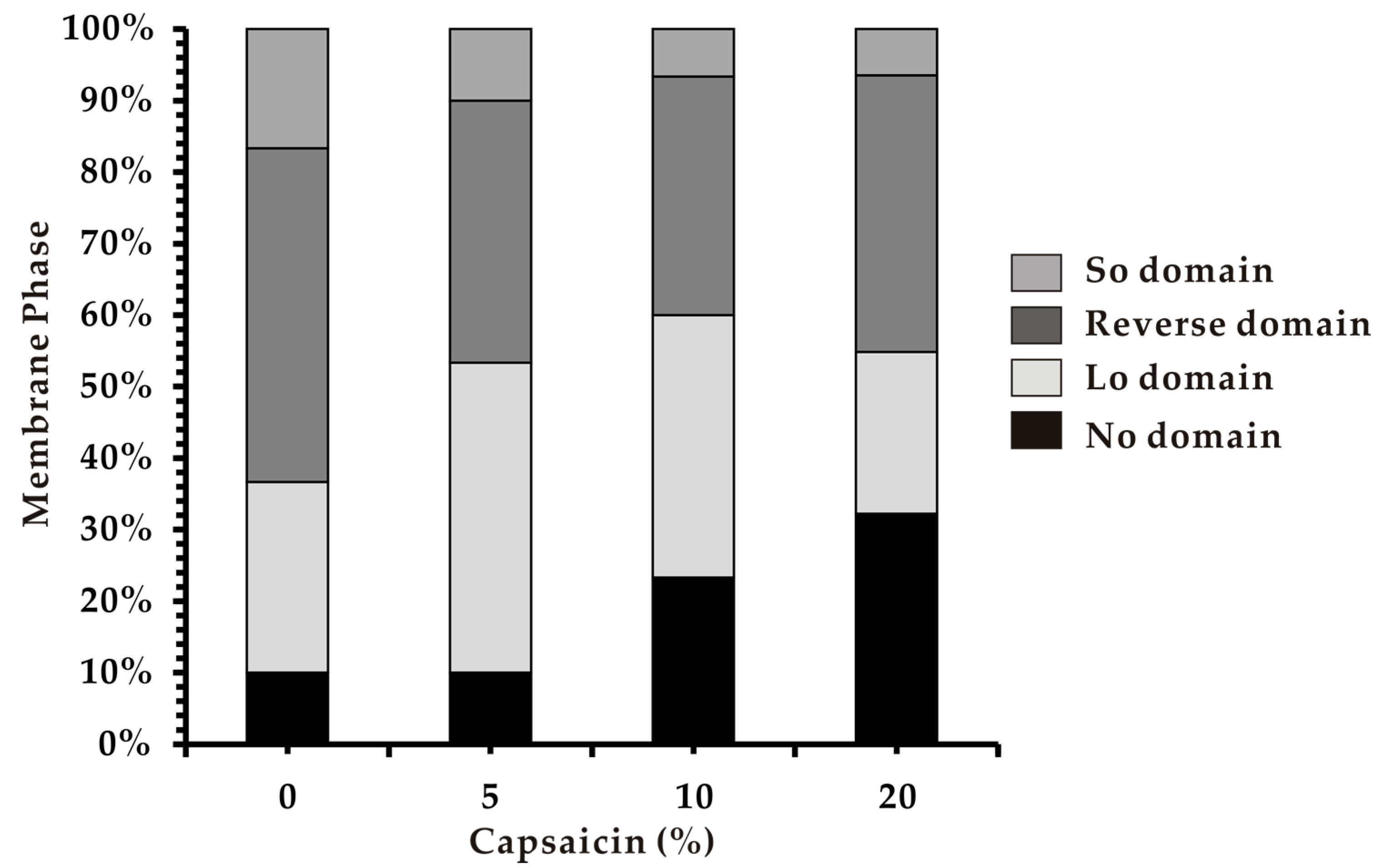
2.5. Multicomponent Phase Separation in Heterogeneous Membrane
2.6. Measurement of Membrane Fluidity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Thermosensitivity of Capsaicin-Containing Vesicles
3.2. Effect of Temperature on the Molecular Area of Monolayer Membranes
3.3. Effect of Capsaicin on Membrane Fluidity
3.4. Phase Separation in Heterogeneous Membranes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-S.; Kawada, T.; Kim, B.-S.; Han, I.S.; Choe, S.Y.; Kurata, T.; Yu, R. Capsaicin exhibits anti-inflammatory property by inhibiting IkB-a degradation in LPS-stimulated peritoneal macrophages. Cell Signal 2003, 15, 299–306. [Google Scholar] [CrossRef]
- Szallasi, A.; Blumberg, P.M. Vanilloid receptors: New insights enhance potential as a therapeutic target. Pain 1996, 68, 195–208. [Google Scholar] [CrossRef]
- Hogaboam, C.M.; Wallace, J.L. Inhibition of platelet aggregation by capsaicin. An effect unrelated to actions on sensory afferent neurons. Eur. J. Pharmacol. 1991, 202, 129–131. [Google Scholar] [CrossRef]
- Cichewicz, R.H.; Thorpe, P.A. The antimicrobial properties of chile peppers (Capsicum species) and their uses in Mayan medicine. J. Ethnopharmacol. 1996, 52, 61–70. [Google Scholar] [CrossRef]
- Zhang, R.; Humphreys, I.; Sahu, R.P.; Shi, Y.; Srivastava, S.K. In vitro and in vivo induction of apoptosis by capsaicin in pancreatic cancer cells is mediated through ROS generation and mitochondrial death pathway. Apoptosis 2008, 13, 1465–1478. [Google Scholar] [CrossRef] [PubMed]
- Kawada, T.; Hagihara, K.-I.; Iwai, K. Effects of capsaicin on lipid metabolism in rats fed a high fat diet. J. Nutr. 1986, 116, 1272–1278. [Google Scholar] [CrossRef]
- Lim, K.; Yoshioka, M.; Kikuzato, S.; Kiyonaga, A.; Tanaka, H.; Shindo, M.; Suzuki, M. Dietary red pepper ingestion increases carbohydrate oxidation at rest and during exercise in runners. Med. Sci. Sports Exerc. 1997, 29, 355–361. [Google Scholar] [CrossRef]
- Yoshioka, M.; St-Pierre, S.; Drapeau, V.; Dionne, I.; Doucet, E.; Suzuki, M.; Tremblay, A. Effects of red pepper on appetite and energy intake. Br. J. Nutr. 1999, 82, 115–123. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1991, 389, 816–824. [Google Scholar] [CrossRef]
- Jancso, G.; Kiraly, E.; Jancsó-Gábor, A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature 1977, 270, 741–743. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P. Capsaicin: Cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol. Rev. 1991, 43, 143–201. [Google Scholar] [PubMed]
- Aranda, F.J.; Villalaín, J.; Gómez-Fernández, J.C. Capsaicin affects the structure and phase organization of phospholipid membranes. Biochim. Biophys. Acta 1995, 1234, 225–234. [Google Scholar] [CrossRef]
- Ai, T.; Bompadre, S.G.; Wang, X.; Hu, S.; Li, M.; Hwang, T.C. Capsaicin potentiates wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride-channel currents. Mol. Pharmacol. 2004, 65, 415–1426. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Yun, H.J.; Choi, J.H.; Han, E.H.; Kim, H.G.; Song, G.Y.; Kwon, K.I.; Jeong, T.C.; Jeong, H.G. Suppression of EGF-induced tumor cell migration and matrix metalloproteinase-9 expression by capsaicin via the inhibition of EGFR-mediated FAK/Akt, PKC/Raf/ERK, p38 MAPK, and AP-1 signaling. Mol. Nutr. Food Res. 2011, 55, 594–605. [Google Scholar] [CrossRef]
- Buck, S.H.; Burks, T.F. The neuropharmacology of capsaicin: Review of some recent observations. Pharmacol. Rev. 1986, 38, 179–226. [Google Scholar]
- Govindarajan, V.S.; Sathyanarayana, M.N. Capsicum—production, technology, chemistry, and quality. Part, V. Impact on physiology, pharmacology, nutrition, and metabolism; structure, pungency, pain, and desensitization sequences. Crit. Rev. Food Sci. Nutr. 1991, 29, 435–474. [Google Scholar] [CrossRef]
- Lundbæk, J.A.; Birn, P.; Tape, S.E.; Toombes, G.E.S.; Sogaard, R.S.R.; Koeppe, R.E., II; Gruner, S.M.; Hansen, A.J.; Andersen, O.S. Capsaicin regulates voltage-dependent sodium channels by altering lipid bilayer elasticity. Mol. Pharmacol. 2005, 68, 680–689. [Google Scholar] [CrossRef]
- Tsuchiya, H. Biphasic membrane effects of capsaicin, an active component in Capsicum species. J. Ethnopharmacol. 2001, 75, 295–299. [Google Scholar] [CrossRef]
- Torrecillas, A.; Schneider, M.; Fernández-Martínez, A.M.; Ausili, A.; de Godos, A.M.; Corbalán-García, S.; Gómez-Fernández, J.C. Capsaicin Fluidifies the Membrane and Localizes Itself near the Lipid–Water Interface. ACS Chem. Neurosci. 2015, 6, 741–1750. [Google Scholar] [CrossRef]
- McMullen, T.P.; Lewis, R.N.; McElhaney, R.N. Cholesterol–phospholipid interactions, the liquid-ordered phase and lipid rafts in model and biological membranes. Curr. Opin. Colloid Interface Sci. 2004, 8, 459–468. [Google Scholar] [CrossRef]
- Veatch, S.L.; Keller, S.L. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys. J. 2003, 85, 3074–3083. [Google Scholar] [CrossRef]
- Morita, M.; Vestergaard, M.C.; Hamada, T.; Takagi, M. Real-time observation of model membrane dynamics induced by Alzheimer’s amyloid beta. Biophys. Chem. 2010, 147, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.T.T.; Yoda, T.; Chahal, B.; Morita, M.; Takagi, M.; Vestergaard, M.C. Structure-dependent interactions of polyphenols with a biomimetic membrane system. Biochim. Biophys. Acta 2014, 1838, 2670–2677. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.C.; Yoda, T.; Hamada, T.; Akazawa Ogawa, Y.; Yoshida, Y.; Takagi, M. The effect of oxycholesterols on thermo-induced membrane dynamics. Biochim. Biophys. Acta 2011, 1808, 2245–2251. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Miura, Y.; Ishii, K.; Araki, S.; Yoshikawa, K.; Vestergaard, M.D.; Takagi, M. Dynamic processes in endocytic transformation of a raft-exhibiting giant liposome. J. Phys. Chem. B 2007, 111, 10853–10857. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Yoshikawa, K. Cell-sized liposomes and droplets: Real-world modeling of living cells. Materials 2012, 5, 2292–2305. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Sato, Y.T.; Yoshikawa, K.; Nagasaki, T. Reversible photoswitching in a cell-sized vesicle. Langmuir 2005, 21, 7626–7628. [Google Scholar] [CrossRef] [PubMed]
- Parasassi, T.; Gratton, E.; Yu, W.M.; Wilson, P.; Levi, M. Two-photon fluorescence microscopy of laurdan generalized polarization domains in model and natural membranes. Biophys. J. 1997, 72, 2413–2429. [Google Scholar] [CrossRef]
- Overgaard, J.; Tomčala, A.; Sørensen, J.G.; Holmstrup, M.; Krogh, P.H.; Simek, P.; Kostál, V. Effects of acclimation temperature on thermal tolerance and membrane phospholipid composition in the fruit fly Drosophila melanogaster. J. Insect Physiol. 2008, 54, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, N.R. The plasma membrane as first responder to heat stress. Plant Cell 2009, 21, 2544. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Hamada, T.; Hatakeyama, M.; Sugimoto, R.; Nagasaki, T.; Takagi, M. Reversible control of exo-and endo-budding transitions in a photosensitive lipid membrane. ChemBioChem 2009, 10, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Swain, J.; Kumar Mishra, A. Location, partitioning behavior, and interaction of capsaicin with lipid bilayer membrane: Study using its intrinsic fluorescence. J. Phys. Chem. B 2015, 119, 12086–12093. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, M.; Walter, P. Molecular Biology of the Cell, 5th ed.; Garland Science: New York, NY, USA, 2008; pp. 620–625. [Google Scholar]
- Cicuta, P.; Keller, S.L.; Veatch, S.L. Diffusion of liquid domains in lipid bilayer membranes. J. Phys. Chem. B 2007, 111, 3328–3331. [Google Scholar] [CrossRef] [PubMed]
- Losada-Pérez, P.; Korshid, M.; Hermans, C.; Robijns, T.; Peeters, M.; Jiménes-Monroy, K.L.; Troung, L.T.N.; Wagner, P. Melittin disruption of raft and non-raft forming biomimetic membranes: A study by quartz crystal microbalance with dissipation monitoring. Colloid Surf. B 2014, 123, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y.; Cevc, G. Structure and Properties of Membranes. In Physical Chemistry of Biological Surfaces; Baszkin, A., Norde, W., Eds.; Marcel Dekker: New York City, NY, USA, 2000; pp. 171–241. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, N.; Phan, H.T.T.; Yoda, T.; Shimokawa, N.; Vestergaard, M.C.; Takagi, M. Effects of Capsaicin on Biomimetic Membranes. Biomimetics 2019, 4, 17. https://doi.org/10.3390/biomimetics4010017
Sharma N, Phan HTT, Yoda T, Shimokawa N, Vestergaard MC, Takagi M. Effects of Capsaicin on Biomimetic Membranes. Biomimetics. 2019; 4(1):17. https://doi.org/10.3390/biomimetics4010017
Chicago/Turabian StyleSharma, Neha, Huong T. T. Phan, Tsuyoshi Yoda, Naofumi Shimokawa, Mun’delanji C. Vestergaard, and Masahiro Takagi. 2019. "Effects of Capsaicin on Biomimetic Membranes" Biomimetics 4, no. 1: 17. https://doi.org/10.3390/biomimetics4010017
APA StyleSharma, N., Phan, H. T. T., Yoda, T., Shimokawa, N., Vestergaard, M. C., & Takagi, M. (2019). Effects of Capsaicin on Biomimetic Membranes. Biomimetics, 4(1), 17. https://doi.org/10.3390/biomimetics4010017