Mechanical Modeling of Healthy and Diseased Calcaneal Fat Pad Surrogates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surrogate Fabrication
2.2. Mechanical Testing
2.3. Material Modeling
2.4. Data Analysis
3. Results and Discussion
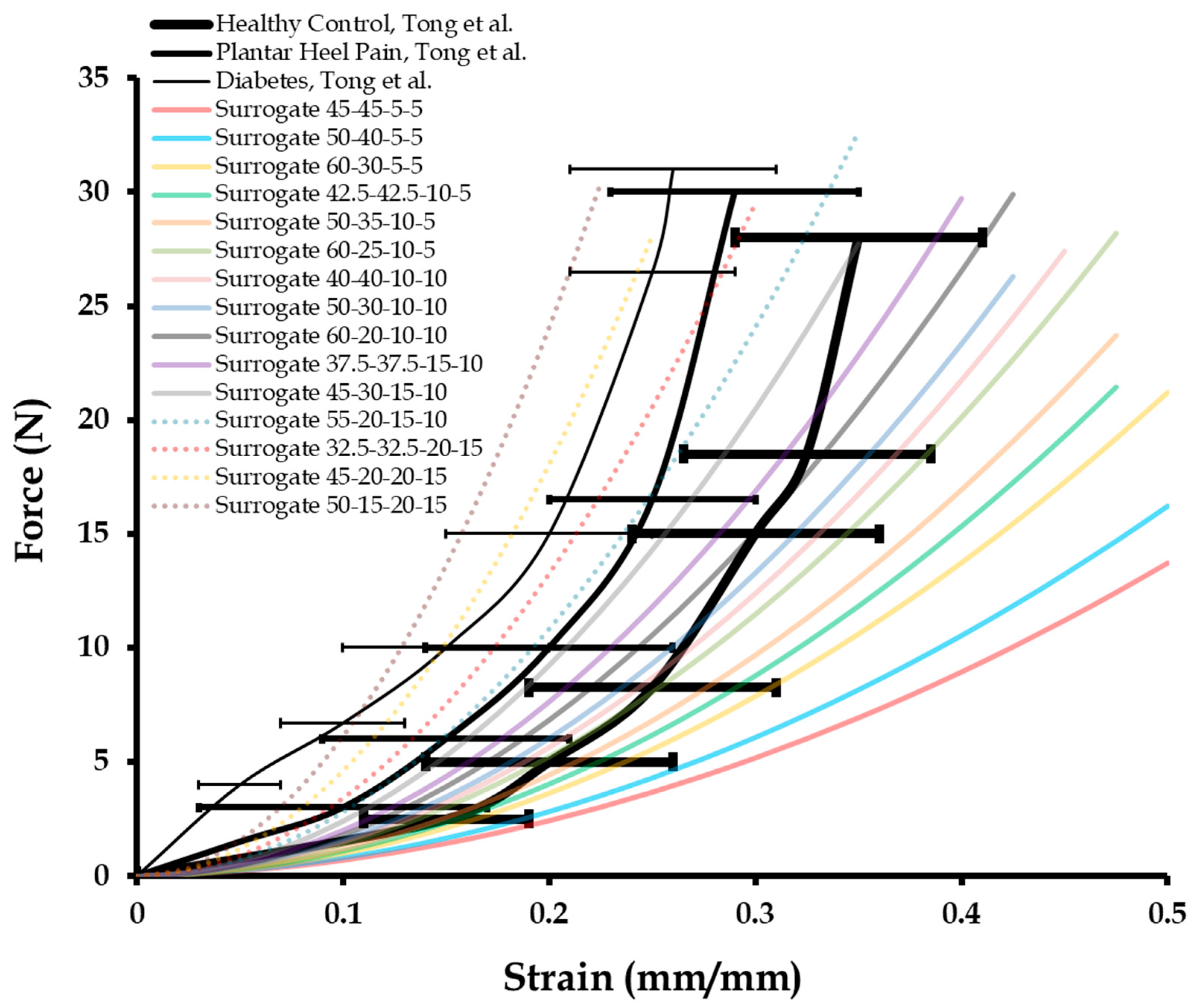
3.1. Dynamic Mechanical Compression Test Results of Calcaneal Heel Pad Surrogates
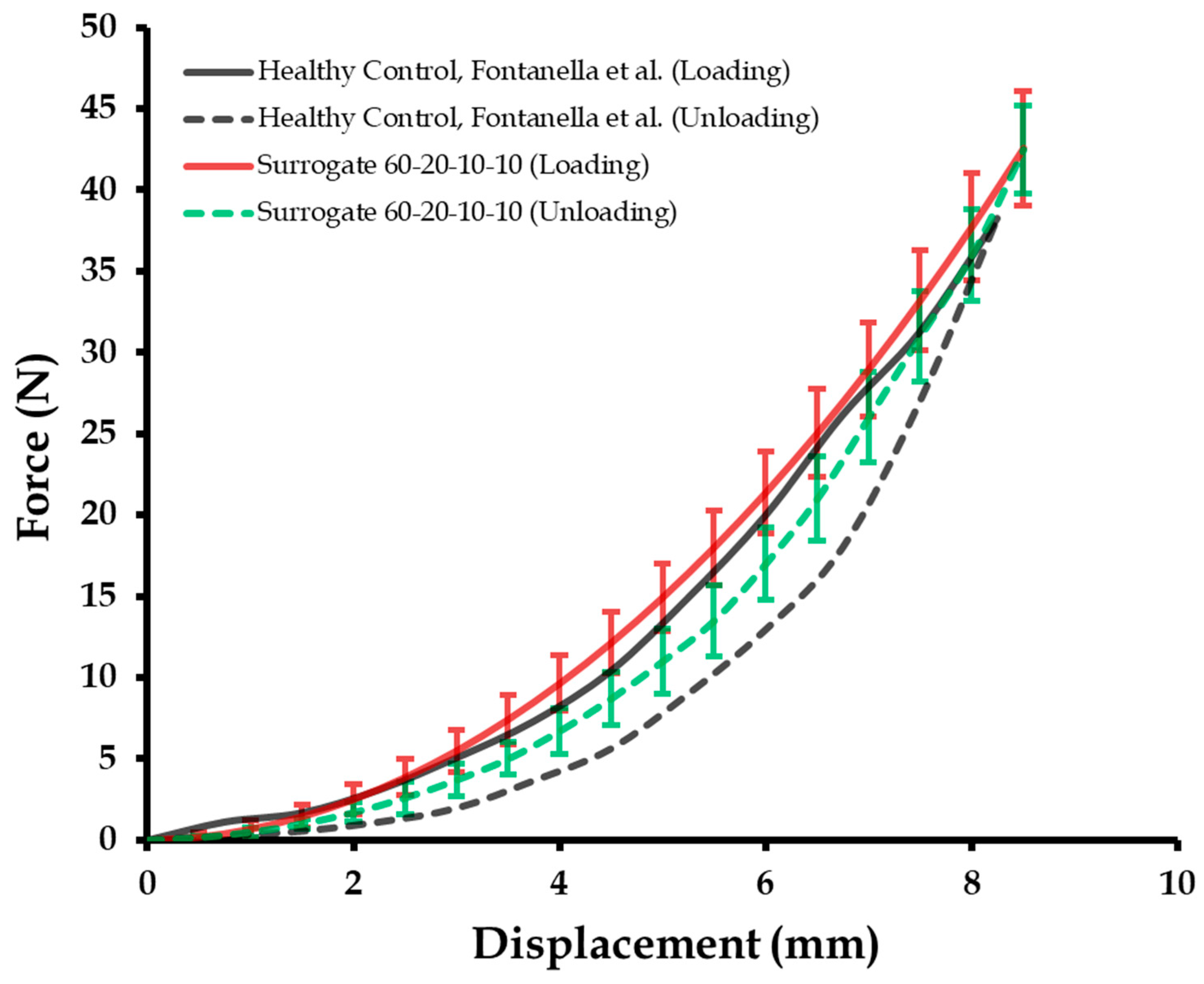
3.2. Cyclic Mechanical Compression Tests and Repeatibility of Calcaneal Heel Pad Surrogates
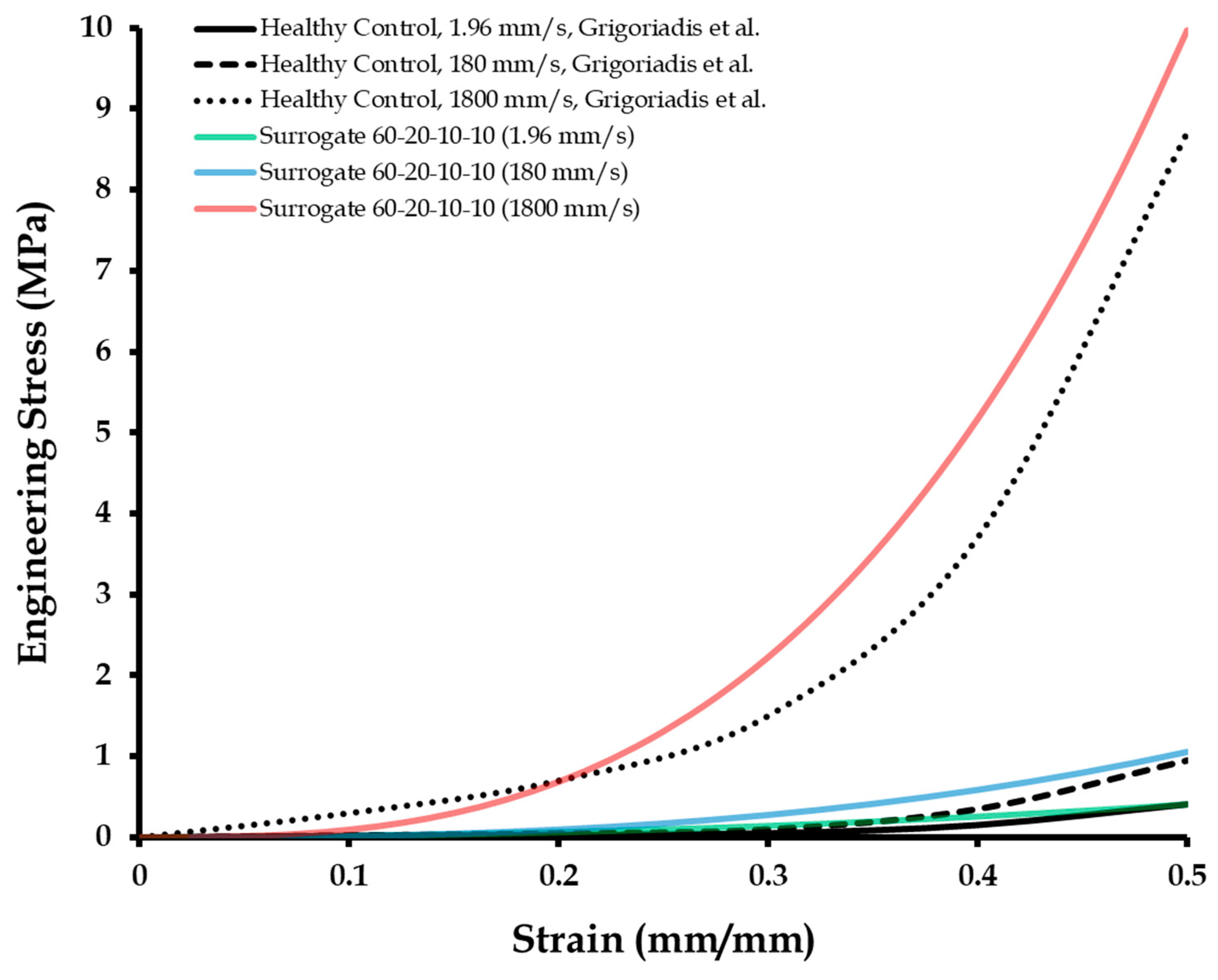
3.3. Compression Tests of Calcaneal Heel Pad Surrogates at High Displacement Rates
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jones, F.W.; Peltier, L.F. Structure and function as seen in the foot. Clin. Orthop. Relat. Res. 2001, 391, 3–6. [Google Scholar] [CrossRef]
- Rome, K. Mechanical properties of the heel pad: Current theory and review of the literature. Foot 1998, 8, 179–185. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Tsai, W.-C.; Wang, C.-L.; Pao, S.-H.; Shau, Y.-W.; Chuan, Y.-S. Microchambers and macrochambers in heel pads: Are they functionally different? J. Appl. Physiol. 2007, 102, 2227–2231. [Google Scholar] [CrossRef] [PubMed]
- Ledoux, W.R.; Blevins, J.J. The compressive material properties of the plantar soft tissue. J. Biomech. 2007, 40, 2975–2981. [Google Scholar] [CrossRef] [PubMed]
- Alcántara, E.; Forner, A.; Ferrús, E.; García, A.-C.; Ramiro, J. Influence of age, gender, and obesity on the mechanical properties of the heel pad under walking impact conditions. J. Appl. Biomech. 2002, 18, 345–356. [Google Scholar] [CrossRef]
- Van Schie, C.; Whalley, A.; Vileikyte, L.; Boulton, A. Efficacy of injected liquid silicone is related to peak plantar foot pressures in the neuropathic diabetic foot. Wounds-A Compend. Clin. Res. Pract. 2002, 14, 26–30. [Google Scholar]
- Gabler, L.F.; Panzer, M.B.; Salzar, R.S. High-Rate Mechanical Properties of Human Heel Pad for Simulation of a Blast Loading Condition. In Proceedings of the IRCOBI Conference, Berlin, Germany, 10–12 September 2014. [Google Scholar]
- Miller-Young, J.E.; Duncan, N.A.; Baroud, G. Material properties of the human calcaneal fat pad in compression: Experiment and theory. J. Biomech. 2002, 35, 1523–1531. [Google Scholar] [CrossRef]
- Natali, A.; Fontanella, C.; Carniel, E. Constitutive formulation and analysis of heel pad tissues mechanics. Med. Eng. Phys. 2010, 32, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Fontanella, C.; Matteoli, S.; Carniel, E.; Wilhjelm, J.E.; Virga, A.; Corvi, A.; Natali, A. Investigation on the load-displacement curves of a human healthy heel pad: In vivo compression data compared to numerical results. Med. Eng. Phys. 2012, 34, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Grigoriadis, G.; Newell, N.; Carpanen, D.; Christou, A.; Bull, A.M.; Masouros, S.D. Material properties of the heel fat pad across strain rates. J. Mech. Behav. Biomed. Mater. 2017, 65, 398–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gefen, A.; Megido-Ravid, M.; Itzchak, Y. In vivo biomechanical behavior of the human heel pad during the stance phase of gait. J. Biomech. 2001, 34, 1661–1665. [Google Scholar] [CrossRef]
- Rome, K.; Webb, P.; Unsworth, A.; Haslock, I. Heel pad stiffness in runners with plantar heel pain. Clin. Biomech. 2001, 16, 901–905. [Google Scholar] [CrossRef]
- Bir, C.; Barbir, A.; Dosquet, F.; Wilhelm, M.; van der Horst, M.; Wolfe, G. Validation of lower limb surrogates as injury assessment tools in floor impacts due to anti-vehicular land mines. Mil. Med. 2008, 173, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Fontanella, C.; Forestiero, A.; Carniel, E.; Natali, A. Analysis of heel pad tissues mechanics at the heel strike in bare and shod conditions. Med. Eng. Phys. 2013, 35, 441–447. [Google Scholar] [CrossRef]
- Tong, J.; Lim, C.; Goh, O. Technique to study the biomechanical properties of the human calcaneal heel pad. Foot 2003, 13, 83–91. [Google Scholar] [CrossRef]
- Chanda, A.; Unnikrishnan, V. Customized Insoles for Diabetic and Pressure Ulcers. Google Patents US20180008000A1, 2018. [Google Scholar]
- Wearing, S.C.; Smeathers, J.E.; Yates, B.; Urry, S.R.; Dubois, P. Bulk compressive properties of the heel fat pad during walking: A pilot investigation in plantar heel pain. Clin. Biomech. 2009, 24, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Baker, W.A.; Untaroiu, C.D.; Crawford, D.M.; Chowdhury, M.R. Mechanical characterization and finite element implementation of the soft materials used in a novel anthropometric test device for simulating underbody blast loading. J. Mech. Behav. Biomed. Mater. 2017, 74, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.M.; Chowdhury, M.R.; Pietsch, H.A. Mechanical Properties of Polymers Used for Anatomical Components in the Warrior Injury Assessment Manikin (WIAMan) Technology Demonstrator; Army Research Lab,: Aberdeen Proving Ground, MD, USA, 2016. [Google Scholar]
- Holst, K.; Liebgott, H.; Wilhjelm, J.E.; Nikolov, S.; Torp-Pedersen, S.T.; Delachartre, P.; Jensen, J.A. Internal strain estimation for quantification of human heel pad elastic modulus: A phantom study. Ultrasonics 2013, 53, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Torp-Pedersen, S.T.; Matteoli, S.; Wilhjelm, J.E.; Amris, K.; Bech, J.I.; Christensen, R.; Danneskiold-Samsøe, B. Diagnostic accuracy of heel pad palpation—A phantom study. J. Forensic Leg. Med. 2008, 15, 437–442. [Google Scholar] [CrossRef]
- Mckay, B.J. Development of Lower Extremity Injury Criteria and Biomechanical Surrogate to Evaluate Military Vehicle Occupant Injury During An Explosive Blast Event. Ph.D. Thesis, Wayne State University, Detroit, MI, USA, 2010. [Google Scholar]
- Baker, W.A.; Chowdhury, M.; Untaroiu, C.D. A finite element model of an anthropomorphic test device lower limb to assess risk of injuries during vertical accelerative loading. J. Biomech. 2018, 81, 104–112. [Google Scholar] [CrossRef]
- Iwamoto, M.; Nakahira, Y.; Kimpara, H. Development and validation of the total human model for safety (THUMS) toward further understanding of occupant injury mechanisms in precrash and during crash. Traffic Inj. Prev. 2015, 16, S36–S48. [Google Scholar] [CrossRef] [PubMed]
- Chanda, A.; Jones, T.G.; Beschorner, K.E. Generalizability of footwear traction performance across flooring and contaminant conditions. IISE Trans. Occup. Ergon. Hum. Factors 2018, 6, 98–108. [Google Scholar] [CrossRef]
- Baker, W.A.; Chowdhury, M.R.; Untaroiu, C.D. Validation of a booted finite element model of the WIAMan ATD lower limb in component and whole-body vertical loading impacts with an assessment of the boot influence model on response. Traffic Inj. Prev. 2018, 19, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Chanda, A.; Unnikrishnan, V. Novel insole design for diabetic foot ulcer management. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.T.-M.; Zhang, M. Parametric design of pressure-relieving foot orthosis using statistics-based finite element method. Med. Eng. Phys. 2008, 30, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Chanda, A.; Unnikrishnan, V. Human Tissue Simulants for Study of Traumatic Brain Injury (TBI). In Proceedings of the American Society for Composites: Thirty-First Technical Conference, Williamsburg, VA, USA, 19–21 September 2016. [Google Scholar]
- Chanda, A. Biofidelic Soft Composites—Experimental and Computational Modeling. Ph.D. Thesis, University of Alabama Libraries, Tuscaloosa, AL, USA, 2017. [Google Scholar]
- Chanda, A.; Callaway, C.; Clifton, C.; Unnikrishnan, V. Biofidelic human brain tissue surrogates. Mech. Adv. Mater. Struct. 2016, 25. [Google Scholar] [CrossRef]
- Chanda, A. Biomechanical modeling of human skin tissue surrogates. Biomimetics 2018, 3, 18. [Google Scholar] [CrossRef]
- Chanda, A.; Graeter, R. Human skin-like composite materials for blast induced injury mitigation. J. Compos. Sci. 2018, 2, 44. [Google Scholar] [CrossRef]
- Chanda, A.; Unnikrishnan, V.; Flynn, Z. Biofidelic human skin simulant. US Patent Appl. US 62/189, 504, 2015. [Google Scholar]
- Chanda, A.; Unnikrishnan, V. A realistic 3D computational model of the closure of skin wound with interrupted sutures. J. Mech. Med. Boil. 2017, 17, 1750025. [Google Scholar] [CrossRef]
- Chanda, A.; Unnikrishnan, V.; Flynn, Z.; Lackey, K. Experimental study on tissue phantoms to understand the effect of injury and suturing on human skin mechanical properties. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2017, 231, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Chanda, A.; Unnikrishnan, V.; Flynn, Z. Biofidelic Skin Simulant. Google Patents US10049601B2, 2018. [Google Scholar]
- Chanda, A.; Curry, K. Patient-specific biofidelic human coronary artery surrogates. J. Mech. Med. Boil. 2018, 18. [Google Scholar] [CrossRef]
- Chanda, A.; Graeter, R.; Unnikrishnan, V. Effect of blasts on subject-specific computational models of skin and bone sections at various locations on the human body. AIMS Mater. Sci. 2015, 2, 425–447. [Google Scholar] [CrossRef]
- Chanda, A. Biofidelic conductive synthetic skin composites. In Proceedings of the American Society for Composites: Thirty-Second Technical Conference, West Lafayette, IN, USA, 23–25 October 2017. [Google Scholar]
- Chanda, A.; Flynn, Z.; Unnikrishnan, V. Biofidelic vaginal tissue surrogate. US. Provisional Patent 62/263, 942, 2015. [Google Scholar]
- Chanda, A.; Flynn, Z.; Unnikrishnan, V. Biomechanical characterization of normal and prolapsed vaginal tissue surrogates. J. Mech. Med. Boil. 2018, 18, 1750100. [Google Scholar] [CrossRef]
- Chanda, A.; Unnikrishnan, V.; Richter, H.E.; Lockhart, M.E. A biofidelic computational model of the female pelvic system to understand effect of bladder fill and progressive vaginal tissue stiffening due to prolapse on anterior vaginal wall. Int. J. Numer. Methods Biomed. Eng. 2016, 32, e02767. [Google Scholar] [CrossRef]
- Chanda, A.; Upchurch, W. Review of recent advances in vaginal mesh tissue interaction. Research & Development in Material Science, 11 October 2018; Preprint. [Google Scholar]
- Chanda, A.; Ruchti, T.; Upchurch, W. Biomechanical modeling of prosthetic mesh and human tissue surrogate interaction. Biomimetics 2018, 3, 27. [Google Scholar] [CrossRef]
- Chanda, A.; Callaway, C. Tissue anisotropy modeling using soft composite materials. Appl. Bionics Biomech. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Chanda, A.; Ghoneim, H. Pumping potential of a two-layer left-ventricle-like flexible-matrix-composite structure. Compos. Struct. 2015, 122, 570–575. [Google Scholar] [CrossRef]
- Ghoneim, H.; Chanda, A. Pumping Potential of a Left-Ventricle-Like Flexiblematrix-Composite Structure. In Proceedings of e-Proceedings of the 19th international conference on composite materials (ICCM19), Montreal, QC, Canada, 28 July–2 August 2013; pp. 7457–7462. [Google Scholar]
- Spears, I.R.; Miller-Young, J.E. The effect of heel-pad thickness and loading protocol on measured heel-pad stiffness and a standardized protocol for inter-subject comparability. Clin. Biomech. 2006, 21, 204–212. [Google Scholar] [CrossRef]
- Erdemir, A.; Viveiros, M.L.; Ulbrecht, J.S.; Cavanagh, P.R. An inverse finite-element model of heel-pad indentation. J. Biomech. 2006, 39, 1279–1286. [Google Scholar] [CrossRef]
- Weijers, R.E.; Kessels, A.G.; Kemerink, G.J. The damping properties of the venous plexus of the heel region of the foot during simulated heelstrike. J. Biomech. 2005, 38, 2423–2430. [Google Scholar] [CrossRef]
- Bennett, M.; Ker, R. The mechanical properties of the human subcalcaneal fat pad in compression. J. Anat. 1990, 171, 131. [Google Scholar] [PubMed]
- Chanda, A.; Ruchti, T.; Unnikrishnan, V. Computational modeling of wound suture: A review. IEEE Rev. Biomed. Eng. 2018, 11, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Chanda, A.; Unnikrishnan, V.; Roy, S.; Richter, H.E. Computational modeling of the female pelvic support structures and organs to understand the mechanism of pelvic organ prolapse: A review. Appl. Mech. Rev. 2015, 67, 040801. [Google Scholar] [CrossRef]
- Chanda, A.; Callaway, C. Computational modeling of blast induced whole-body injury: A review. J. Med. Eng. Technol. 2018, 42, 88–104. [Google Scholar] [CrossRef] [PubMed]
- Chanda, A.; Unnikrishnan, V. Effect of bladder and rectal loads on the vaginal canal and levator ani in varying pelvic floor conditions. Mech. Adv. Mater. Struct. 2017. [Google Scholar] [CrossRef]
- Chanda, A.; Unnikrishnan, V.; Richter, H.E.; Lockhart, M.E. Computational Modeling of Anterior and Posterior Pelvic Organ Prolapse (POP). In Proceedings of the ASME 2016 International Mechanical Engineering Congress and Exposition, Phoenix, AZ, USA; 2016; p. V009T012A085. [Google Scholar]
- Martins, P.; Natal Jorge, R.; Ferreira, A. A comparative study of several material models for prediction of hyperelastic properties: Application to silicone-rubber and soft tissues. Strain 2006, 42, 135–147. [Google Scholar] [CrossRef]
- Chanda, A. Customized human skin simulants. Open Science Framework, 17 October 2018; Preprint. [Google Scholar]
- Chanda, A.; Meyer, I.; Richter, H.E.; Lockhart, M.E.; Moraes, F.R.; Unnikrishnan, V. Vaginal changes due to varying degrees of rectocele prolapse: A computational study. J. Biomech. Eng. 2017, 139, 101001. [Google Scholar] [CrossRef] [PubMed]
- Chanda, A. Anisotropic Soft Composite Based Hyperelastic Model. In Proceedings of the American Society for Composites: Thirty-Third Technical Conference, Seattle, WA, USA, September 2018. [Google Scholar]
- Oehman, C.; Baleani, M.; Viceconti, M. Repeatability of experimental procedures to determine mechanical behaviour of ligaments. Acta Bioeng. Biomech. 2009, 11, 19–23. [Google Scholar]

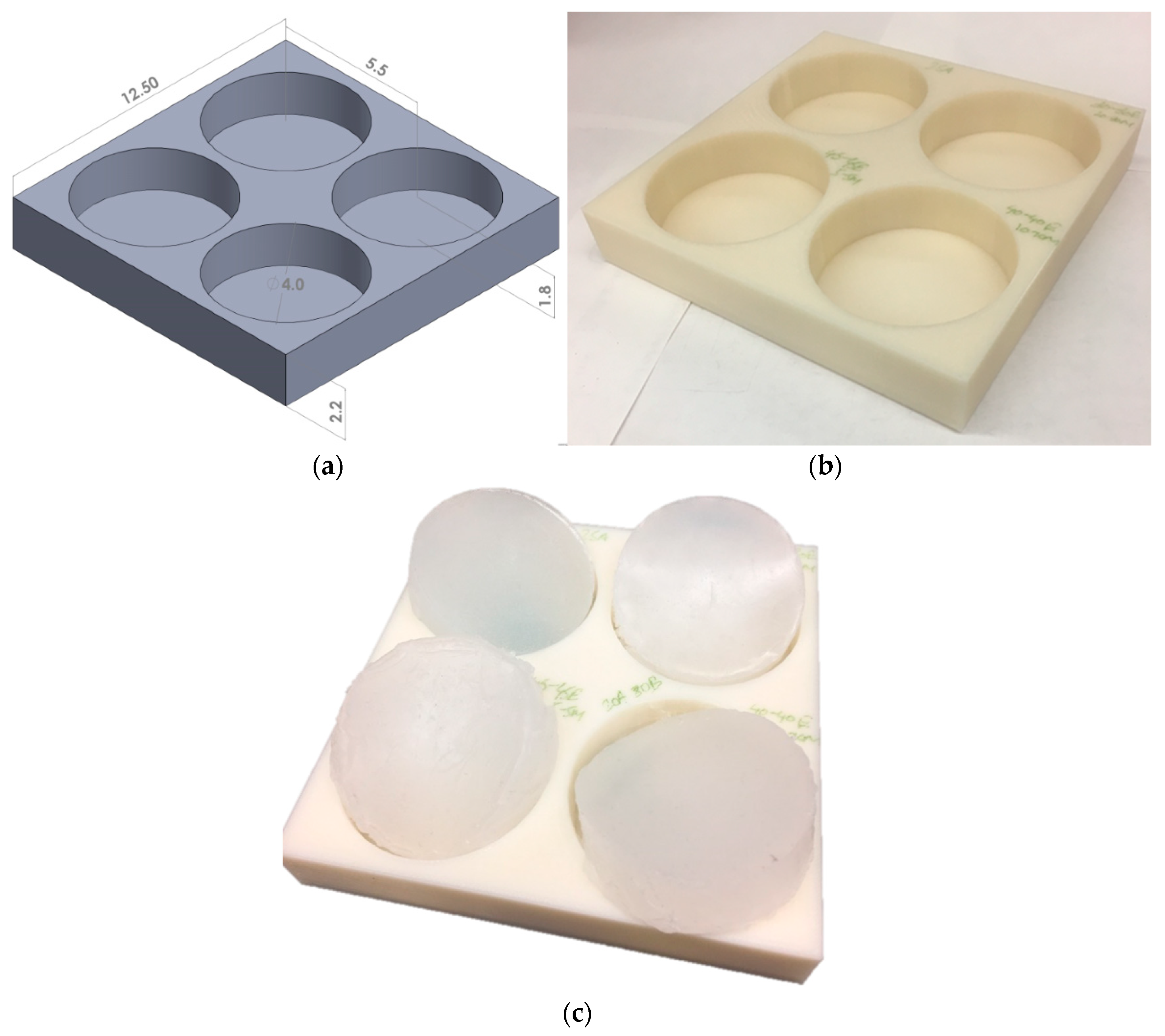
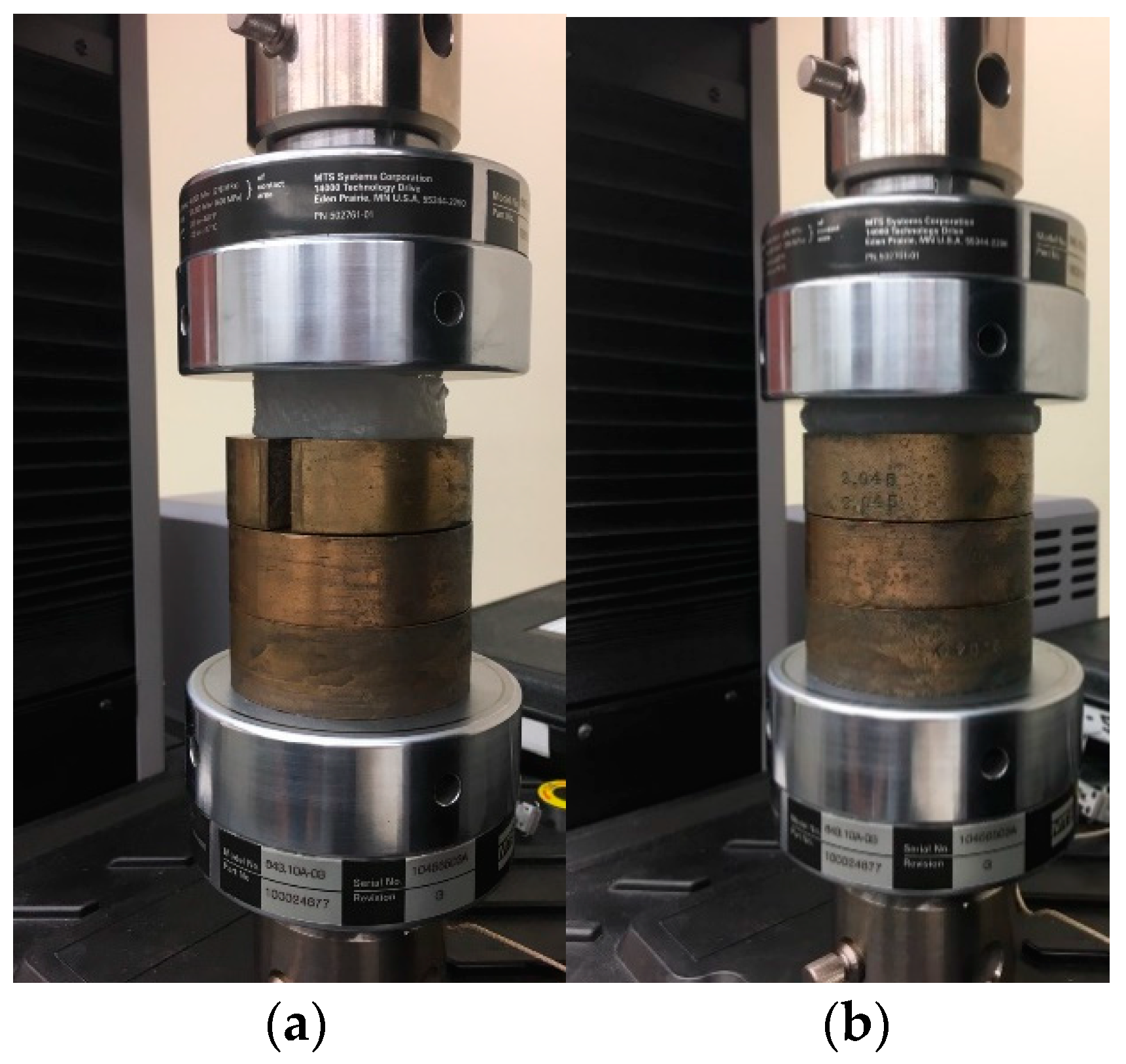
| Test Specimen No. | Shore 00-10 | Shore 30A | ||
|---|---|---|---|---|
| Part A | Part B | Part A | Part B | |
| 1 | 45 | 45 | 5 | 5 |
| 2 | 50 | 40 | 5 | 5 |
| 3 | 60 | 30 | 5 | 5 |
| 4 | 42.5 | 42.5 | 10 | 5 |
| 5 | 50 | 35 | 10 | 5 |
| 6 | 60 | 25 | 10 | 5 |
| 7 | 40 | 40 | 10 | 10 |
| 8 | 50 | 30 | 10 | 10 |
| 9 | 60 | 20 | 10 | 10 |
| 10 | 37.5 | 37.5 | 15 | 10 |
| 11 | 45 | 30 | 15 | 10 |
| 12 | 55 | 20 | 15 | 10 |
| 13 | 32.5 | 32.5 | 20 | 15 |
| 14 | 45 | 20 | 20 | 15 |
| 15 | 50 | 15 | 20 | 15 |
| Surrogate Composition | Healthy Control, Tong et al. | Plantar Heel Pain, Tong et al. | Diabetes, Tong et al. |
|---|---|---|---|
| 45-45-5-5 | 0.32 | 0.17 | 0.02 |
| 50-40-5-5 | 0.36 | 0.20 | 0.05 |
| 60-30-5-5 | 0.39 | 0.24 | 0.10 |
| 42.5-42.5-10-5 | 0.53 | 0.28 | 0.13 |
| 50-35-10-5 | 0.64 | 0.31 | 0.16 |
| 60-25-10-5 | 0.66 | 0.36 | 0.19 |
| 40-40-10-10 | 0.71 | 0.42 | 0.24 |
| 50-30-10-10 | 0.85 | 0.47 | 0.42 |
| 60-20-10-10 a | 0.92 a | 0.53 | 0.49 |
| 37.5-37.5-15-10 | 0.87 | 0.68 | 0.63 |
| 45-30-15-10 | 0.73 | 0.87 | 0.66 |
| 55-20-15-10 a | 0.69 | 0.94 a | 0.71 |
| 32.5-32.5-20-15 | 0.55 | 0.88 | 0.78 |
| 45-20-20-15 a | 0.38 | 0.73 | 0.86 a |
| 50-15-20-15 | 0.22 | 0.66 | 0.80 |
| Surrogate | |||
|---|---|---|---|
| 60-20-10-10 (1.96 mm/s) | 0.0002 | 0.0090 | 0.0080 |
| 60-20-10-10 (180 mm/s) | 0.0050 | 0.0100 | 0.0120 |
| 60-20-10-10 (1800 mm/s) | 0.7200 | 0.3500 | 0.8800 |
| Plantar pain (1.96 mm/s) | 0.0004 | 0.0090 | 0.0100 |
| Diabetes (1.96 mm/min) | 0.0006 | 0.0150 | 0.0100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chanda, A.; McClain, S. Mechanical Modeling of Healthy and Diseased Calcaneal Fat Pad Surrogates. Biomimetics 2019, 4, 1. https://doi.org/10.3390/biomimetics4010001
Chanda A, McClain S. Mechanical Modeling of Healthy and Diseased Calcaneal Fat Pad Surrogates. Biomimetics. 2019; 4(1):1. https://doi.org/10.3390/biomimetics4010001
Chicago/Turabian StyleChanda, Arnab, and Stephen McClain. 2019. "Mechanical Modeling of Healthy and Diseased Calcaneal Fat Pad Surrogates" Biomimetics 4, no. 1: 1. https://doi.org/10.3390/biomimetics4010001
APA StyleChanda, A., & McClain, S. (2019). Mechanical Modeling of Healthy and Diseased Calcaneal Fat Pad Surrogates. Biomimetics, 4(1), 1. https://doi.org/10.3390/biomimetics4010001




