Towards Health Status Determination and Local Weather Forecasts from Vitis vinifera Electrome
Abstract
1. Introduction
2. Materials and Methods
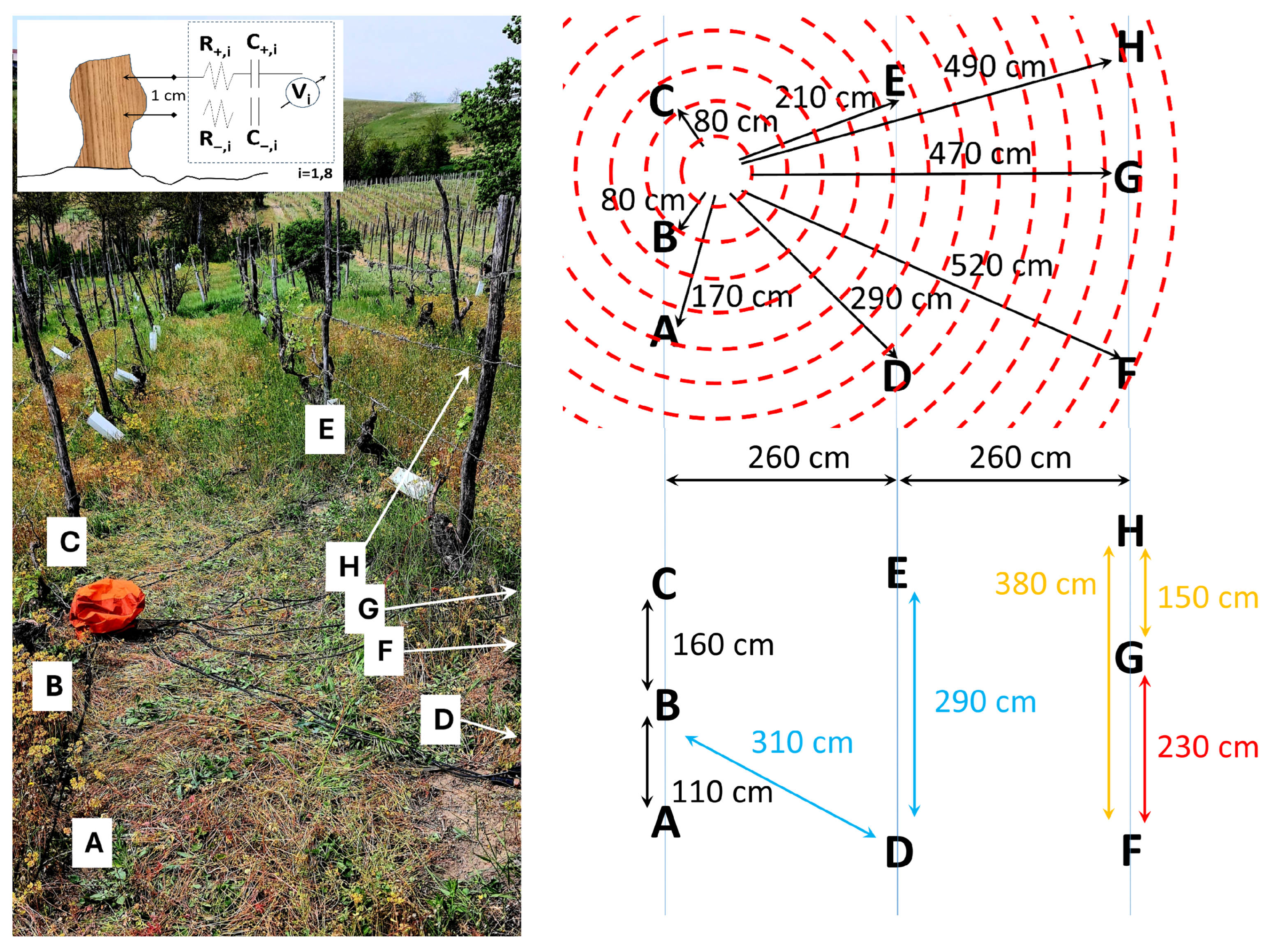
2.1. Experimental Setup and Crop Features
2.2. Electrome Correlation Analyses and Spectral Features
2.3. Attempting to Resolve the Health Status by Means of Signal Features
- A healthy plant is characterized by the unique features of having a small range of values, a negative median value, and a highly negative skewness; the small range could be due to tight homeostatic control of water status and ion fluxes that keeps excursions modest. The negative median baseline is slightly “below zero” vs. reference and could be due to tension-dominated xylem potential, mildly hyperpolarized tissues, or electrode offset); the highly negative skewness could be explained by the fact that many small fluctuations with occasional sharper downward events occur (nighttime recharge, brief stomatal closures, cavitation micro-events [20]), producing a long negative tail, while positive bursts are rare.
- A plant which has undergone recovery is characterized by the unique features of having a small range, a negative median value, a slightly negative skewness, and a flat FFT. The small range and negative median indicate that homeostasis is mostly restored, the baseline is still on the negative side, the skewness is only slightly negative (the large negative tail has subsided), there are fewer acute stress dips, and there are reduced spectral peaks (flatter spectrum) indicating damped oscillations post-stress [21].
- A plant which has undergone recovery and brings fruits is characterized by the unique features of having a positive median value; the fruit load shifts source–sink relations, sustained phloem loading, and turgor dominate the measured signal (e.g., more depolarized tissues/greater pressure), pushing the baseline above zero; sugar transport and anabolic activity bias the distribution to the positive side, even if variability stays modest [22].
- A plant which features ongoing Flavescence dorée is characterized by the unique features of having near-zero skewness and Kurtosis, a very broad range, and a peculiar three-stage FFT. The broad range might be due to phytoplasma-driven phloem dysfunction (callose deposition), hormonal imbalance, and carbohydrate backlog, which causes alternating episodes of hydraulic/electrical instability; large excursions in both directions; near-zero skewness and Kurtosis; variability is symmetric and mesokurtic, erratic but not dominated by single-sided bursts or heavy tails; and a “Three-stage” FFT, where distinct bands emerge from (i) slow phenological/metabolic drift, (ii) diurnal transpiration–stomatal cycles decoupled by pathology, and (iii) fast stress/compensatory oscillations from disrupted sieve-tube transport and ion flux feedbacks [23].
- A dead stump is characterized by the unique features of having an extended range and a negative median value. The extended range can be explained by the fact that, without living regulation, the sensor tracks have unbuffered environmental swings (temperature, humidity, soil moisture, EM noise), so spread explodes; the negative median is due to persistent desiccation/ionic drift or an electrode half-cell offset, which keeps the baseline on the negative side in the absence of an active metabolism [10].
- A control vine log is characterized by the unique features of having a negative skewness, a zero Kurtosis, a small range, and a negative median value. The small range and mesokurtic tails are typical of stable, near-Gaussian fluctuations around a steady setpoint; the negative skewness and negative median are like regulated baselines, as in healthy vines.
2.4. Software Setup
| Model: "sequential" | ||
 | ||
| Layer (type) | Output Shape | Param # |
 | ||
| bidirectional (Bidirectional) | (None, 48, 32) | 3296 |
| multi_head_attention (MultiHeadAttention) | (None, 48, 32) | 4224 |
| dense (Dense) | (None, 48, 32) | 1056 |
| dropout (Dropout) | (None, 48, 32) | 0 |
| dense_1 (Dense) | (None, 48, 1) | 33 |
 | ||
| Total params: 8609 (33.63 KB) | ||
| Trainable params: 8609 (33.63 KB) | ||
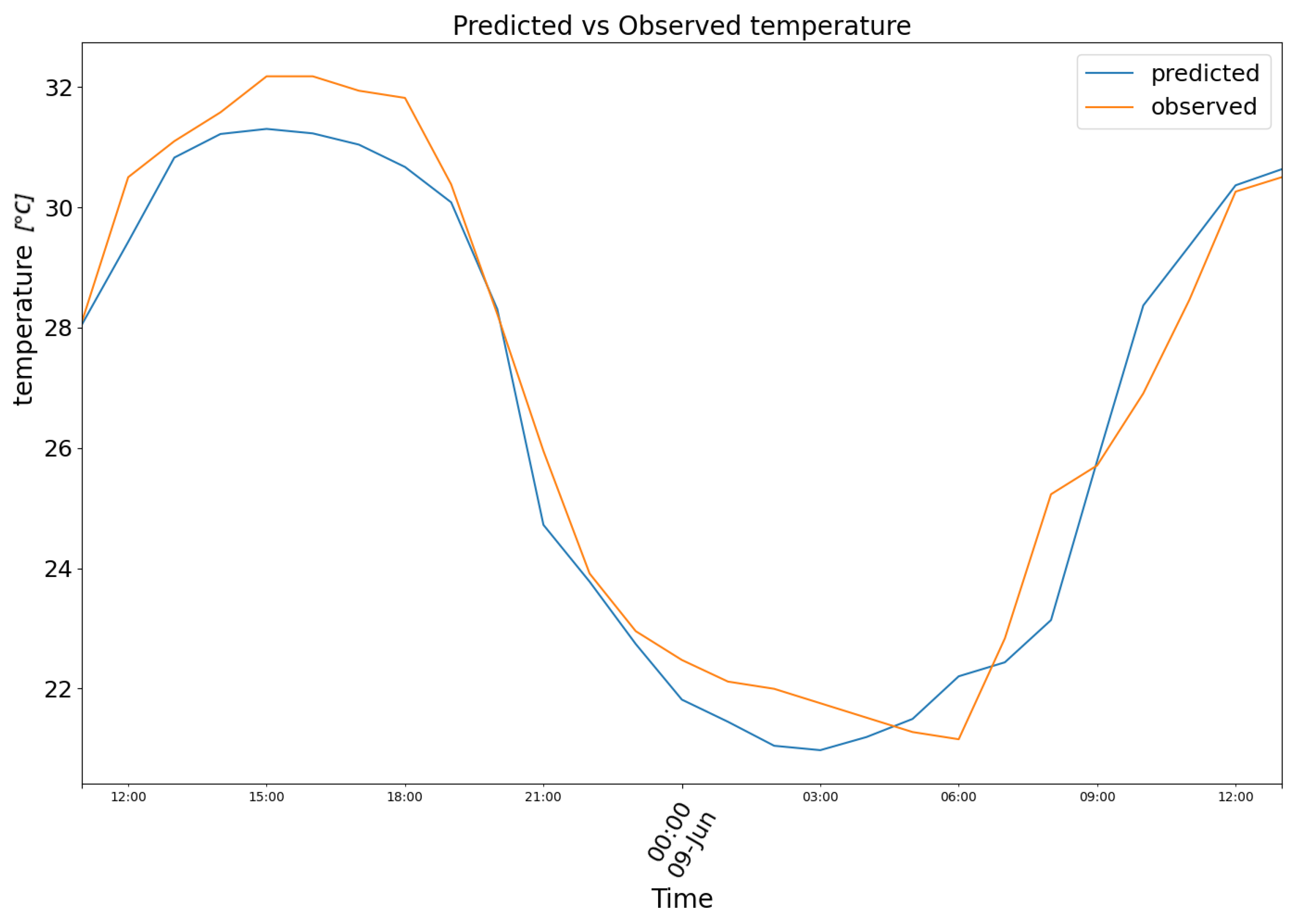
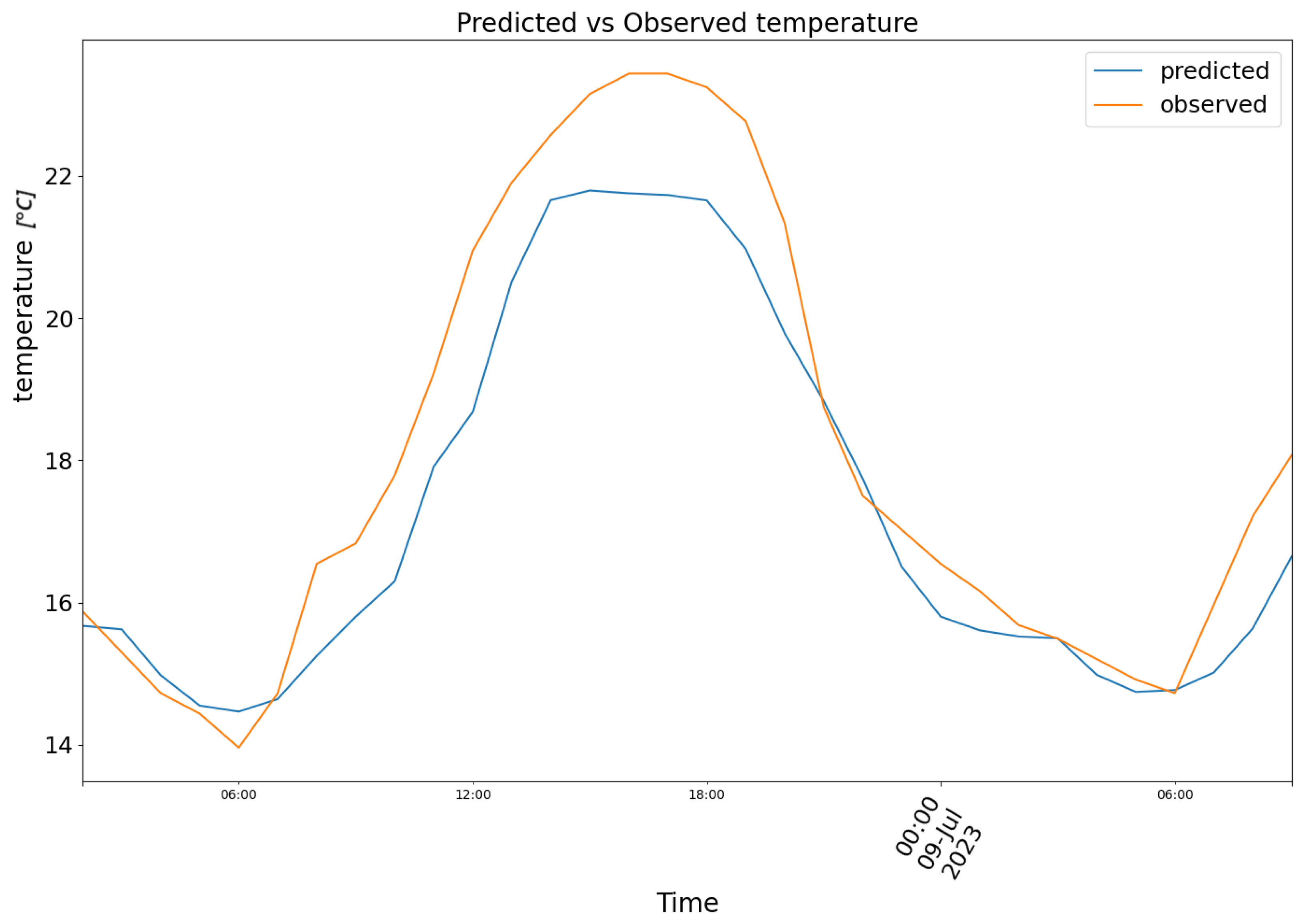
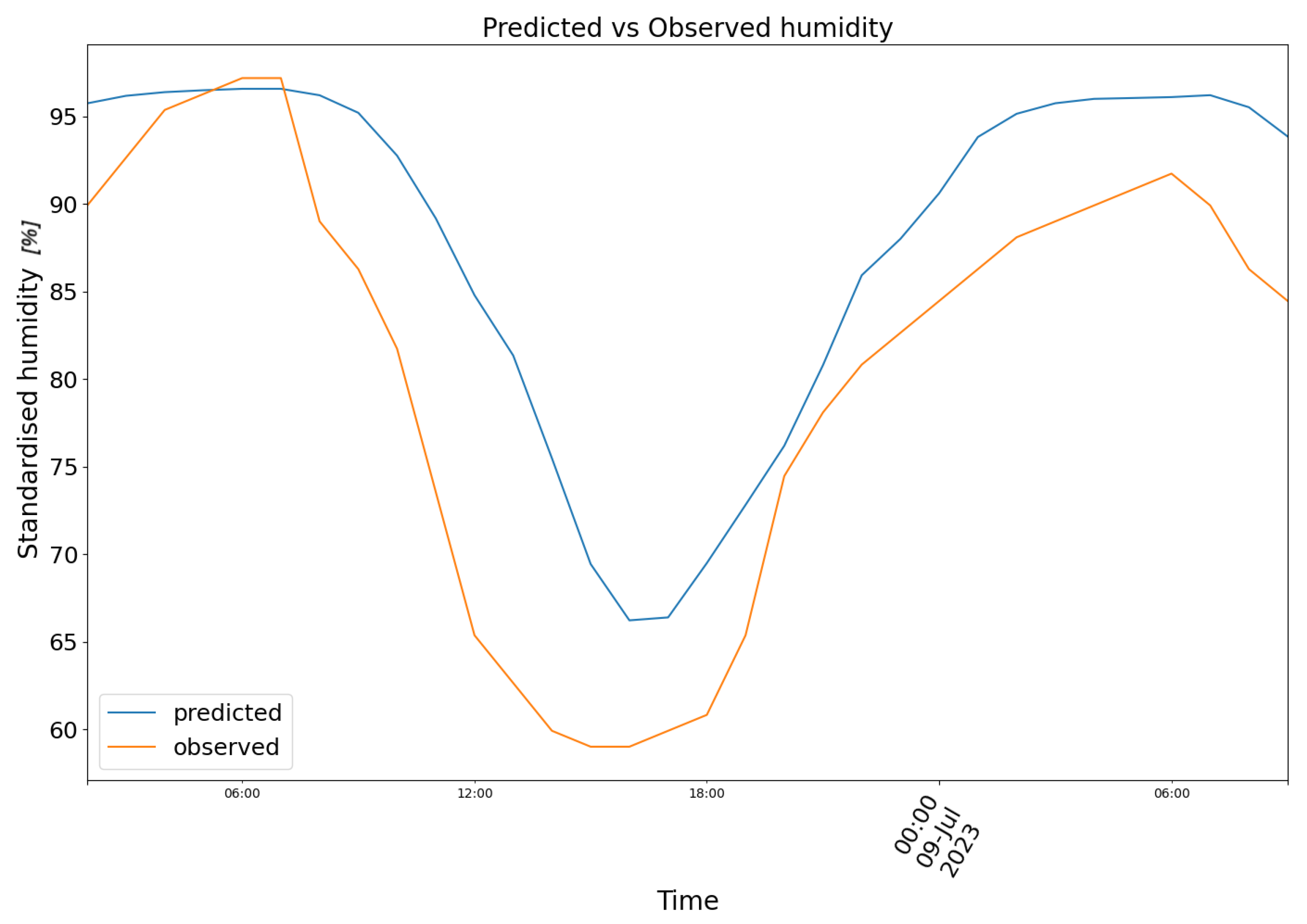
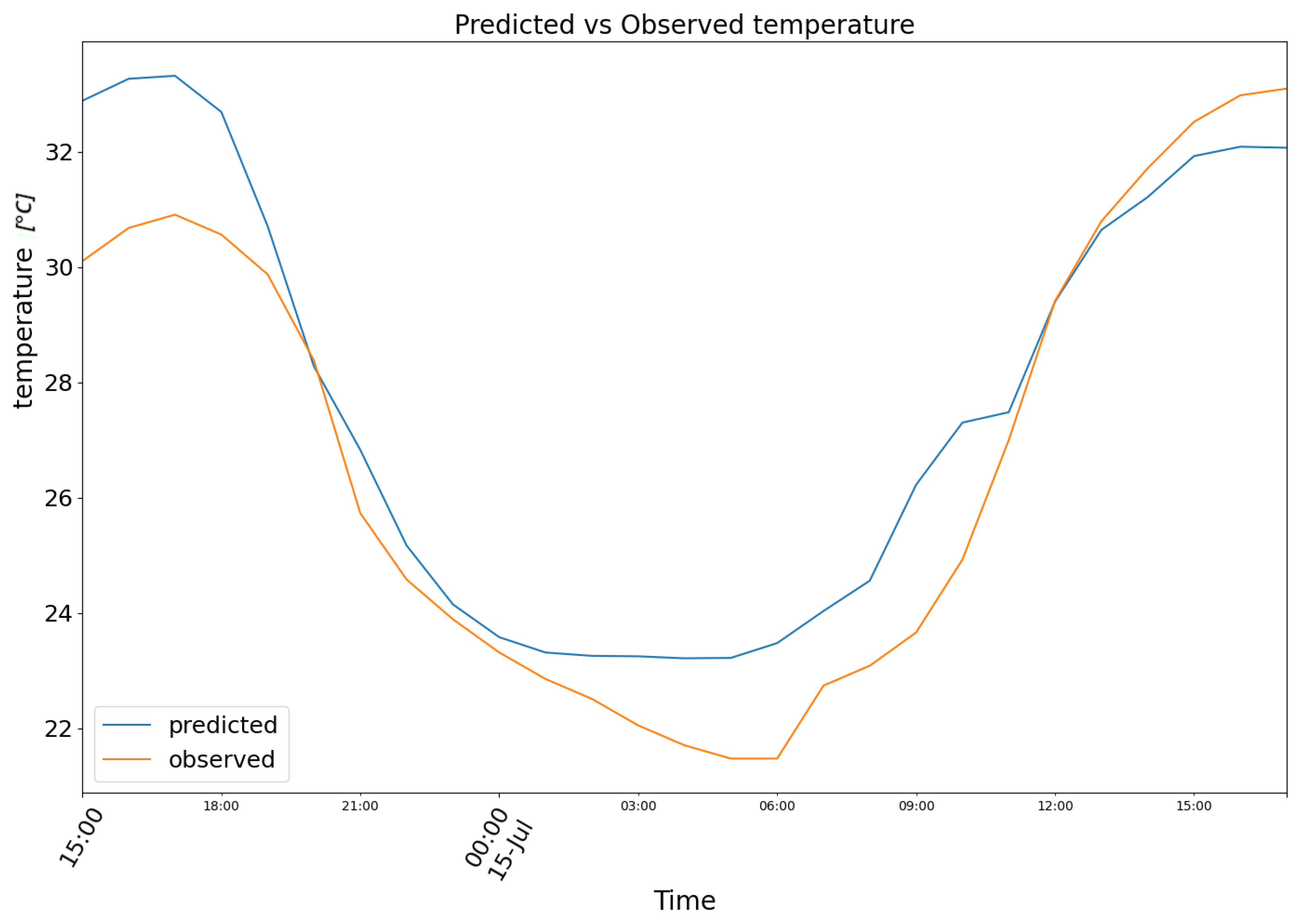
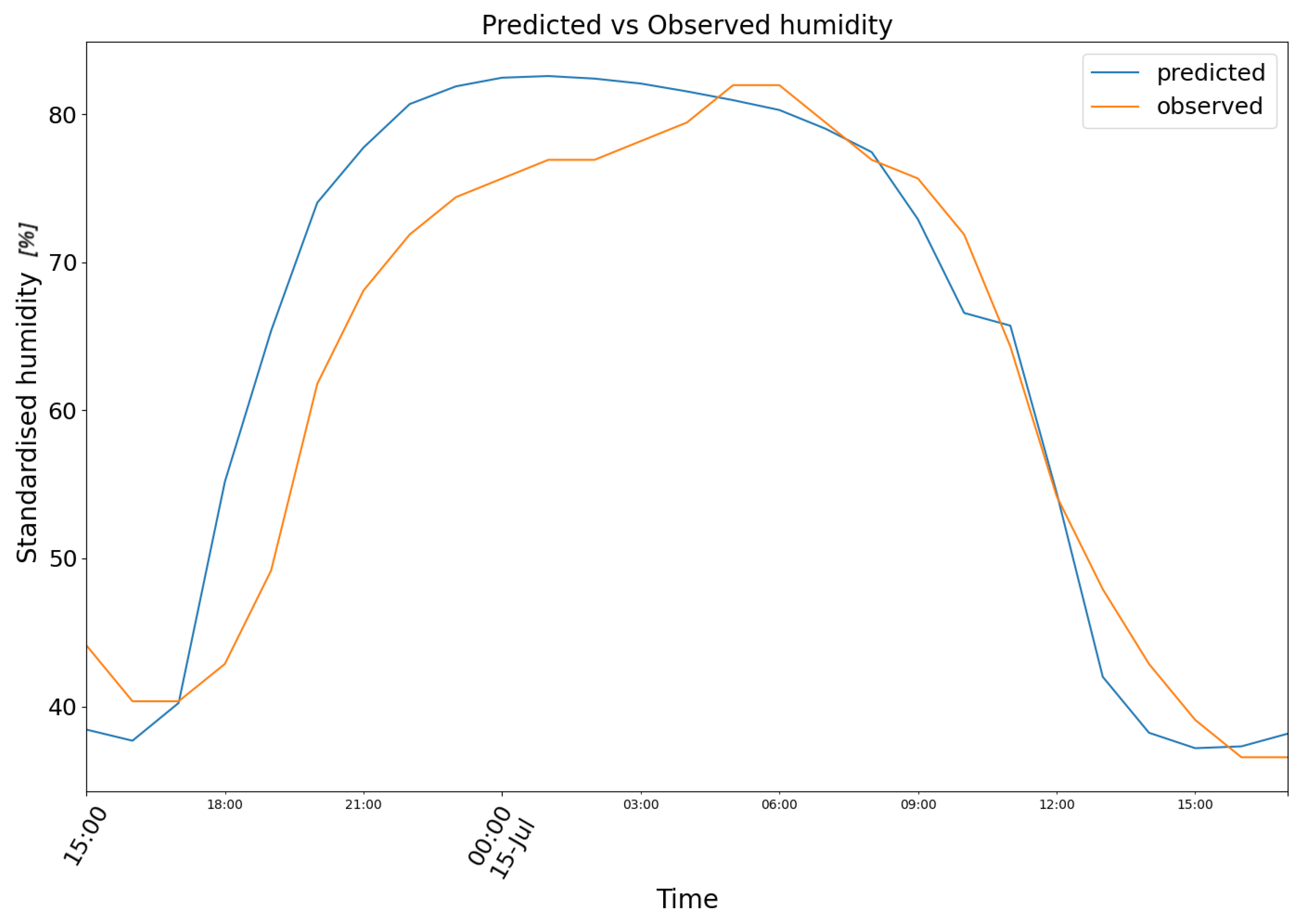
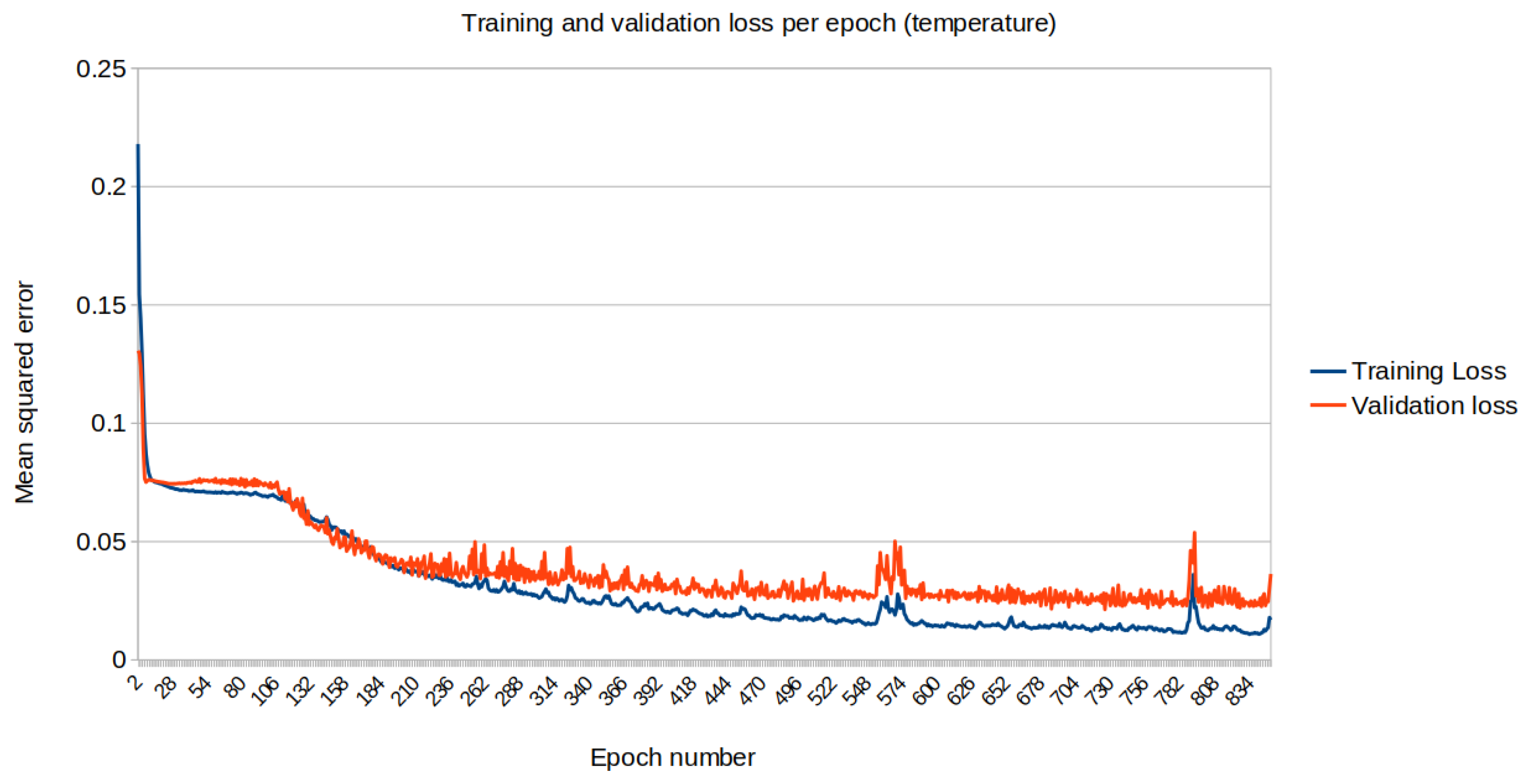
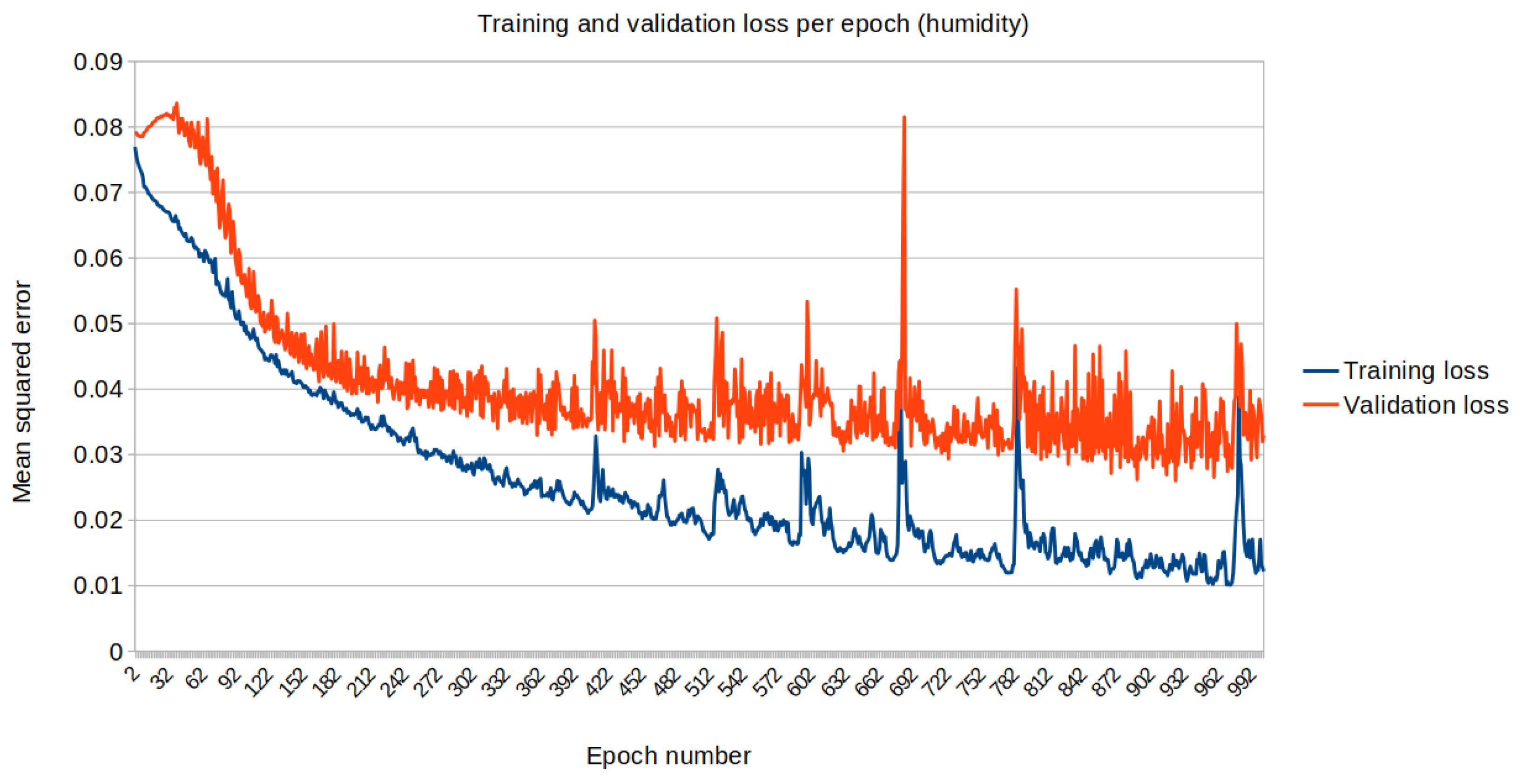
3. Results
4. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaudhary, S.; Bajaj, S.B.; Mongia, S. Analysis of a Novel Integrated Machine Learning Model for Yield and Weather Prediction: Punjab Versus Maharashtra. In Proceedings of International Conference on Recent Innovations in Computing. ICRIC 2022; Lecture Notes in Electrical Engineering; Singh, Y., Verma, C., Zoltán, I., Chhabra, J.K., Singh, P.K., Eds.; Springer: Singapore, 2023; pp. 83–93. [Google Scholar]
- Covert, M.W.; Gillies, T.E.; Takamasa, K.; Agmon, E. A forecast for large-scale, predictive biology: Lessons from meteorology. Cell Syst. 2021, 12, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Chiolerio, A.; Gagliano, M.; Pilia, S.; Pilia, P.; Vitiello, G.; Dehshibi, M.; Adamatzky, A. Bioelectrical synchronization of Picea abies during a solar eclipse. R. Soc. Open Sci. 2025, 12, 241786. [Google Scholar] [CrossRef] [PubMed]
- Randriamandimbisoa, M.V.; Nany Razafindralambo, N.A.M.; Fakra, D.; Ravoajanahary, D.A.; Gatina, J.C.; Jaffrezic-Renault, N. Electrical response of plants to environmental stimuli: A short review and perspectives for meteorological applications. Sens. Int. 2020, 1, 100053. [Google Scholar] [CrossRef]
- de Toledo, G.R.A.; Parise, A.G.; Simmi, F.Z.; Costa, A.V.L.; Senko, L.G.S.; Debono, M.-W.; Souza, G.M. Plant electrome: The electrical dimension of plant life. Theor. Exp. Plant Physiol. 2019, 31, 21–46. [Google Scholar] [CrossRef]
- Tran, D.; Dutoit, F.; Najdenovska, E.; Wallbridge, N.; Plummer, C.; Mazza, M.; Raileanu, L.E.; Camps, C. Electrophysiological assessment of plant status outside a Faraday cage using supervised machine learning. Sci. Rep. 2019, 9, 17073. [Google Scholar] [CrossRef]
- Madariaga, D.; Arro, D.; Irarrázaval, C.; Soto, A.; Guerra, F.; Romero, A.; Ovalle, F.; Fedrigolli, E.; DesRosiers, T.; Serbe-Kamp, E.; et al. A library of electrophysiological responses in plants—A model of transversal education and open science. Plant Signal. Behav. 2024, 19, 2310977. [Google Scholar] [CrossRef]
- Saraiva, G.F.R.; Ferreira, A.S.; Souza, G.M. Osmotic stress decreases complexity underlying the electrophysiological dynamic in soybean. Plant Biol. 2017, 19, 702–708. [Google Scholar] [CrossRef]
- Najdenovska, E.; Dutoit, F.; Tran, D.; Rochat, A.; Vu, B.; Mazza, M.; Camps, C.; Plummer, C.; Wallbridge, N.; Raileanu, L.E. Identifying General Stress in Commercial Tomatoes Based on Machine Learning Applied to Plant Electrophysiology. Appl. Sci. 2021, 11, 5640. [Google Scholar] [CrossRef]
- Kozlova, E.; Yudina, L.; Sukhova, E.; Sukhov, V. Analysis of Electrome as a Tool for Plant Monitoring: Progress and Perspectives. Plants 2025, 14, 1500. [Google Scholar] [CrossRef] [PubMed]
- Xia, A.; Wu, Y.; Zhai, K.; Xiang, D.; Li, L.; Qin, Z.; Twagirayezu, G. Plant Electrophysiological Parameters Represent Leaf Intracellular Water–Nutrient Metabolism and Immunoregulations in Brassica rapa During Plasmodiophora Infection. Plants 2025, 14, 2337. [Google Scholar] [CrossRef]
- Armada-Moreira, A.; Dar, A.M.; Zhao, Z.; Cea, C.; Gelinas, J.; Berggren, M.; Costa, A.; Khodagholy, D.; Stavrinidou, E. Plant electrophysiology with conformable organic electronics: Deciphering the propagation of Venus flytrap action potentials. Sci. Adv. 2023, 9, eadh4443. [Google Scholar] [CrossRef] [PubMed]
- Imam, T.; Hasri, A.; Hidetaka, N.; Rofiq, A.R.; Ades, T. Deep learning algorithm for room temperature detection using bioelectric potential of plant data. Biomed. Signal Process. Control 2025, 101, 107214. [Google Scholar]
- Cattani, A.; de Riedmatten, L.; Roulet, J.; Smit-Sadki, T.; Alfonso, E.; Kurenda, A.; Graeff, M.; Remolif, E.; Rienth, M. Water status assessment in grapevines using plant electrophysiology. OENO One 2024, 58, 8209. [Google Scholar] [CrossRef]
- Pereira, D.R.; Papa, J.P.; Saraiva, G.F.R.; Souza, G.M. Automatic classification of plant electrophysiological responses to environmental stimuli using machine learning and interval arithmetic. Comput. Electron. Agric. 2018, 145, 35–42. [Google Scholar] [CrossRef]
- Rizzoli, A.; Jelmini, L.; Pezzatti, G.B.; Jermini, M.; Schumpp, O.; Debonneville, C.; Marcolin, E.; Krebs, P.; Conedera, M. Impact of the “Flavescence Dorée” Phytoplasma on Xylem Growth and Anatomical Characteristics in Trunks of ‘Chardonnay’ Grapevines (Vitis vinifera). Biology 2022, 11, 978. [Google Scholar] [CrossRef] [PubMed]
- de Toledo, G.R.A.; Reissig, G.N.; Senko, L.G.S.; Pereira, D.R.; da Silva, A.F.; Souza, G.M. Common bean under different water availability reveals classifiable stimuli-specific signatures in plant electrome. Plant Signal. Behav. 2024, 19, 2333144. [Google Scholar] [CrossRef]
- Szechyńska-Hebda, M.; Lewandowska, M.; Witoń, D.; Fichman, Y.; Mittler, R.; Karpiński, S.M. Aboveground plant-to-plant electrical signaling mediates network acquired acclimation. Plant Cell 2022, 34, 3047–3065. [Google Scholar] [CrossRef]
- Dixon, T.A.; Williams, T.C.; Pretorius, I.S. Sensing the future of bio-informational engineering. Nat. Commun. 2021, 12, 388. [Google Scholar] [CrossRef]
- Zufferey, V.; Cochard, H.; Ameglio, T.; Spring, J.-L.; Viret, O. Diurnal cycles of embolism formation and repair in petioles of grapevine (Vitis vinifera cv. Chasselas). J. Exp. Bot. 2011, 62, 3885–3894. [Google Scholar] [CrossRef]
- Pagliarani, C.; Gambino, G.; Ferrandino, A.; Chitarra, W.; Vrhovsek, U.; Cantu, D.; Palmano, S.; Marzachì, C.; Schubert, A. Molecular memory of Flavescence dorée phytoplasma in recovering grapevines. Hortic. Resour. 2020, 7, 126. [Google Scholar] [CrossRef]
- Li, Y.-M.; Forney, C.; Bondada, B.; Leng, F.; Xie, Z.-S. The Molecular Regulation of Carbon Sink Strength in Grapevine (Vitis vinifera L.). Front. Plant Sci. 2021, 11, 606918. [Google Scholar] [CrossRef]
- Oliveira, M.J.R.A.; Castro, S.; Paltrinieri, S.; Bertaccini, A.; Sottomayor, M.; Santos, C.S.; Vasconcelos, M.W.; Carvalho, S.M.P. “Flavescence dorée” impacts growth, productivity and ultrastructure of Vitis vinifera plants in Portuguese “Vinhos Verdes” region. Sci. Hortic. 2020, 261, 108742. [Google Scholar] [CrossRef]
- Hochreiter, S.; Schmidhuber, J. Long short-term memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef] [PubMed]
- Gers, F.A.; Schmidhuber, J. Recurrent nets that time and count. In Proceedings of the IEEE-INNS-ENNS International Joint Conference on Neural Networks. IJCNN 2000. Neural Computing: New Challenges and Perspectives for the New Millenniu, Como, Italy, 24–27 July 2000; Volume 3, p. 2. [Google Scholar]
- Openweather. Available online: https://openweather.co.uk/accuracy-and-quality (accessed on 21 September 2025).
- Aguila, S.F.; Fuentes Barrios, A.; Lorenzo, S.L. Evaluation of the Nowcasting and very Short-Range Prediction System of the National Meteorological Service of Cuba. Environ. Sci. Proc. 2021, 8, 36. [Google Scholar]

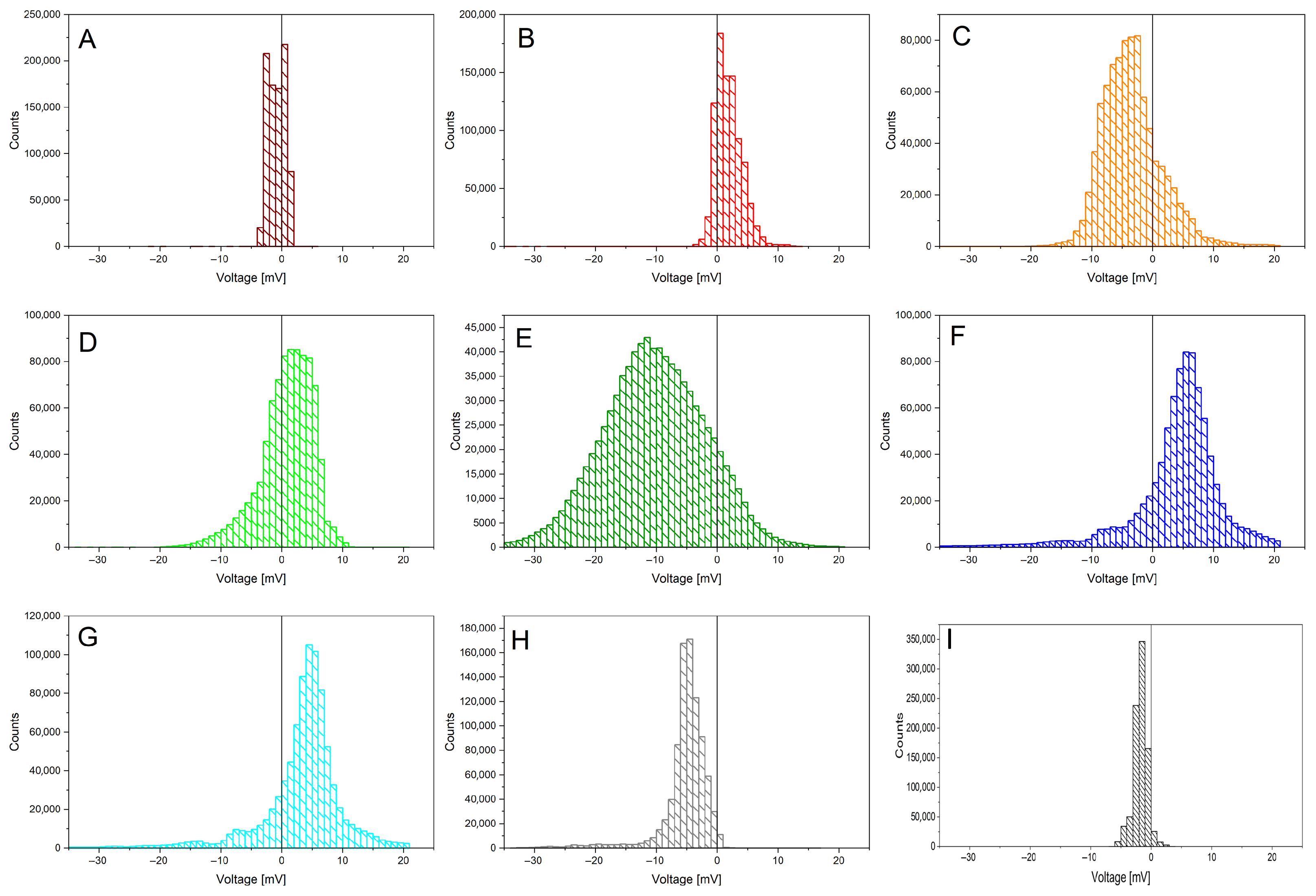
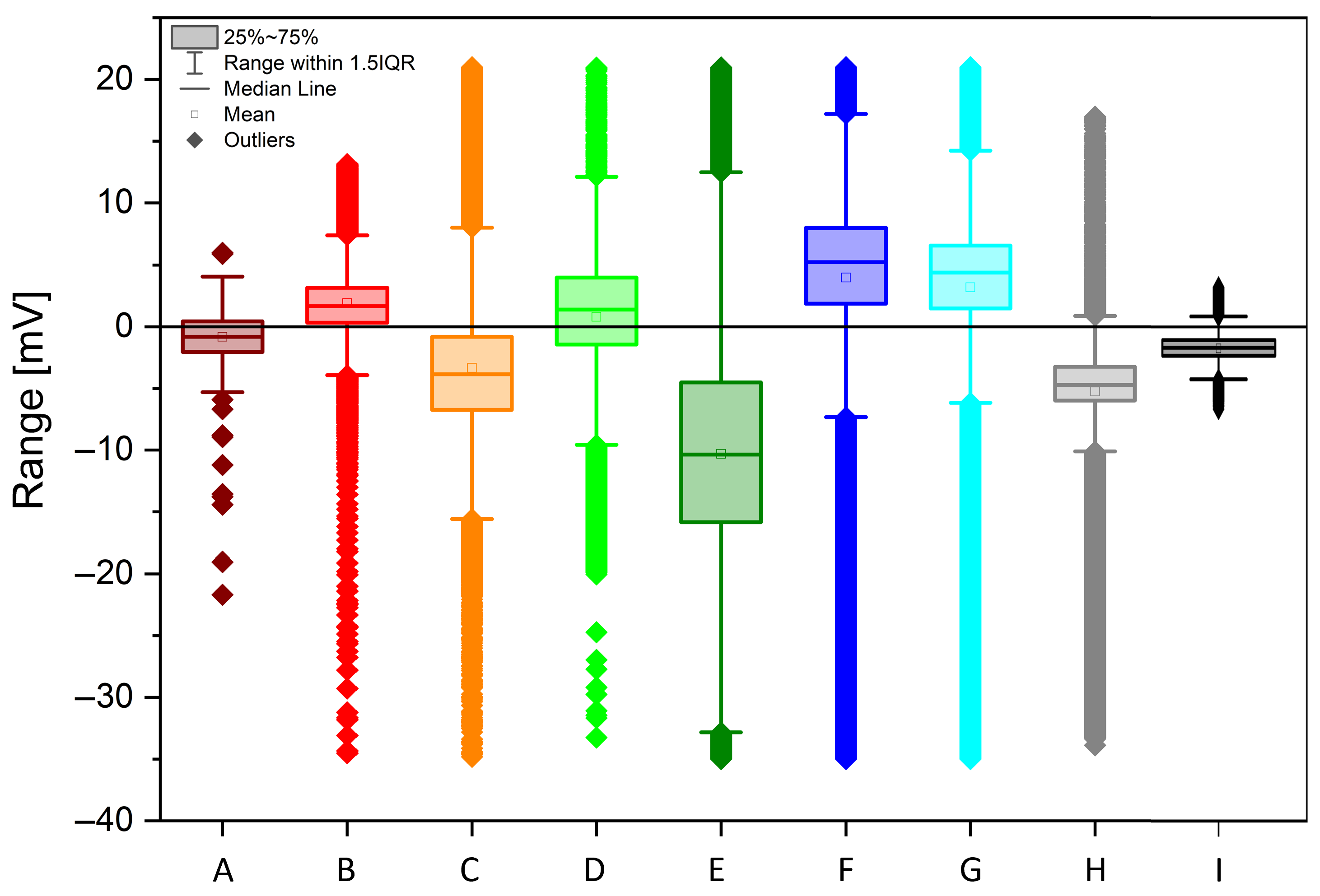
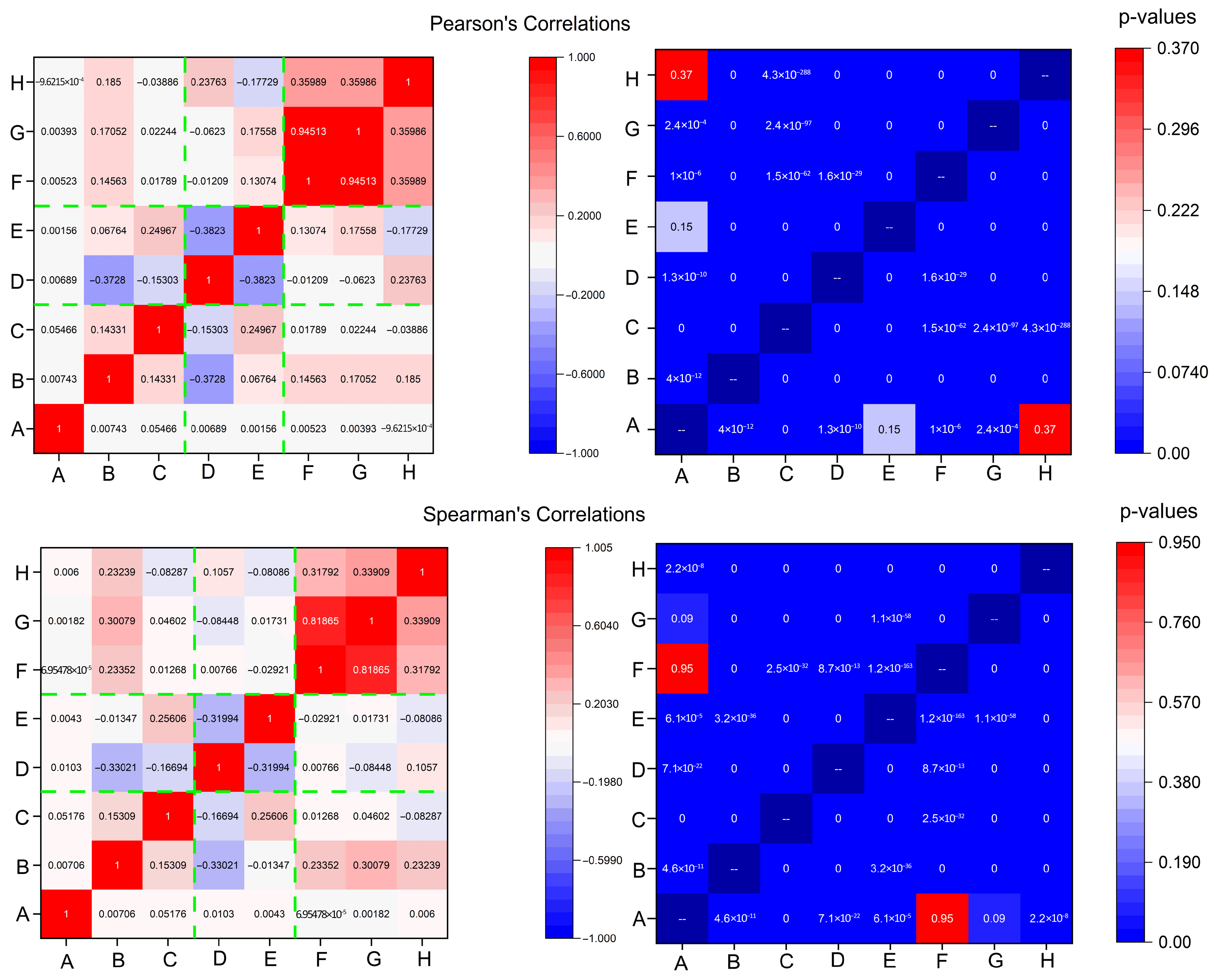
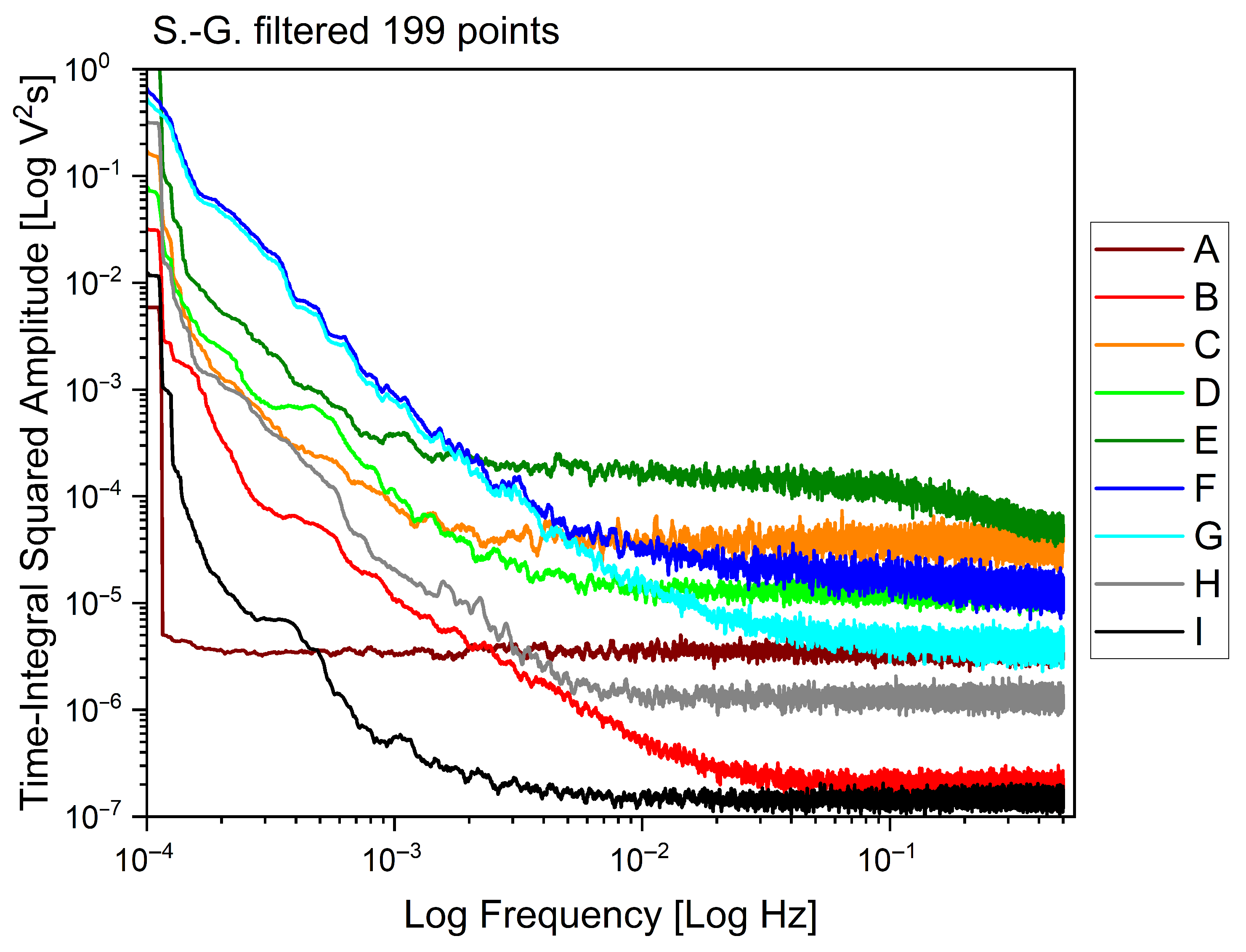

| ID | Health Status | Skewness | Kurtosis | Range | Med | Avg/Med | FFT |
|---|---|---|---|---|---|---|---|
| A | recovery | < | > | − | − | = | flat |
| B | recovery + fruits | > | > | − | + | > | lin + flat |
| C | stump | > | > | + | − | > | lin + flat |
| D | recovery + fruits | < | > | + | + | < | lin + flat |
| E | flavescent | o | o | +++ | − | = | lin + flat + lin |
| F | stump | << | > | + | + | < | lin + flat |
| G | recovery + fruits | << | > | + | + | < | lin + flat |
| H | healthy | << | > | − | − | < | lin + flat |
| I | control | < | o | − | − | = | lin + flat |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiolerio, A.; Taranto, F.; Brandino, G.P. Towards Health Status Determination and Local Weather Forecasts from Vitis vinifera Electrome. Biomimetics 2025, 10, 636. https://doi.org/10.3390/biomimetics10090636
Chiolerio A, Taranto F, Brandino GP. Towards Health Status Determination and Local Weather Forecasts from Vitis vinifera Electrome. Biomimetics. 2025; 10(9):636. https://doi.org/10.3390/biomimetics10090636
Chicago/Turabian StyleChiolerio, Alessandro, Federico Taranto, and Giuseppe Piero Brandino. 2025. "Towards Health Status Determination and Local Weather Forecasts from Vitis vinifera Electrome" Biomimetics 10, no. 9: 636. https://doi.org/10.3390/biomimetics10090636
APA StyleChiolerio, A., Taranto, F., & Brandino, G. P. (2025). Towards Health Status Determination and Local Weather Forecasts from Vitis vinifera Electrome. Biomimetics, 10(9), 636. https://doi.org/10.3390/biomimetics10090636







