Non-Bactericidal Antifouling Coating Inspired by the “Swinging Effect” of Coral Tentacles in Waves
Abstract
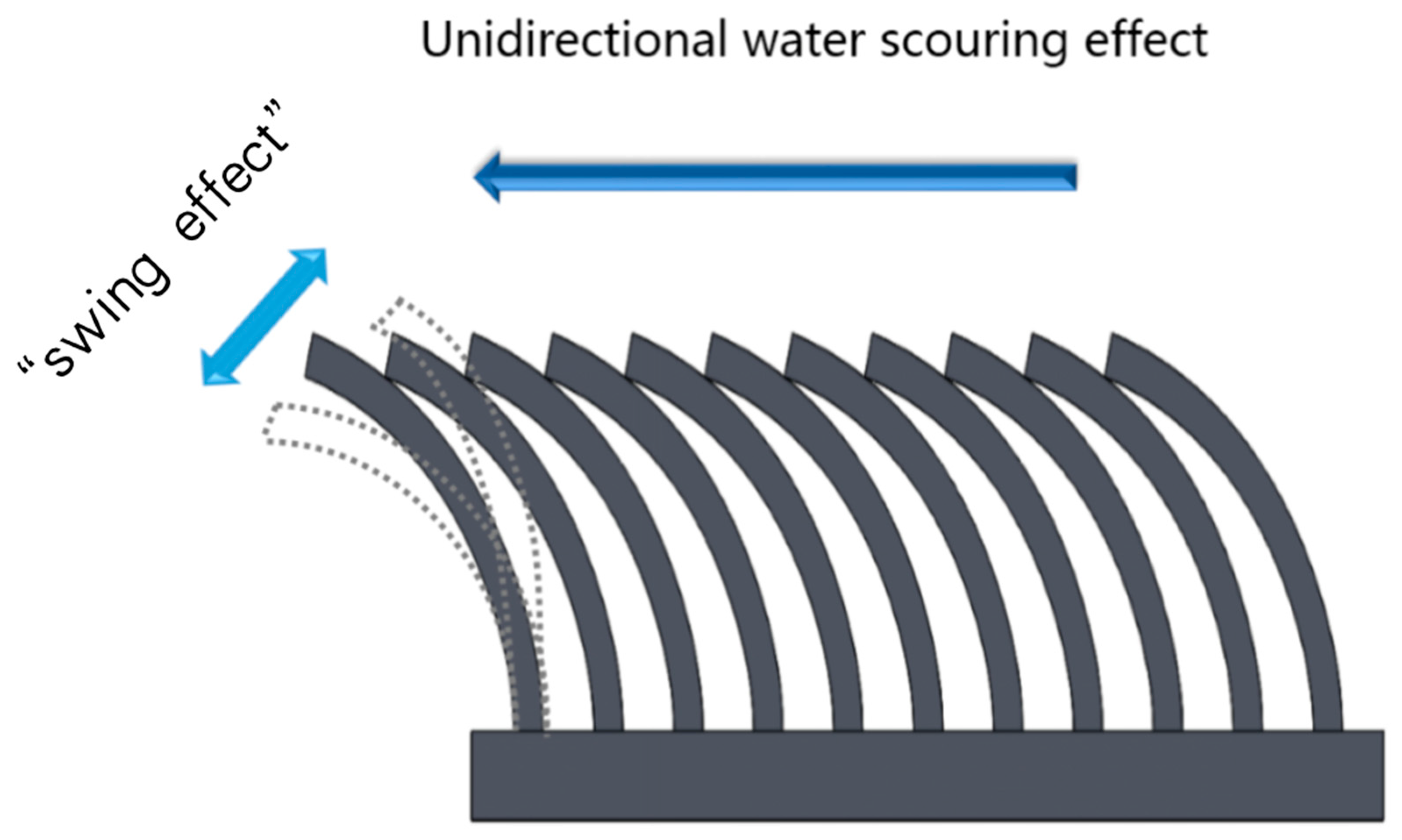
1. Introduction
2. Materials and Methods
2.1. Coral Rearing Environment and Test Equipment
2.1.1. Coral Rearing Environment
2.1.2. Coral Biological Observation Test Equipment
2.2. Simulation of Natural Environment—Coral Tentacles Expansion and Contraction Regularity Test
2.3. Fluid Medium Disturbance Environment—Coral Tentacles Expansion and Contraction Regularity Test
2.4. Coral Tentacles “Swinging Effect” Antifouling Performance Simulation Test
2.5. Design and Preparation of ACMSs with the “Swinging Effect” Based on Coral Tentacles
2.5.1. Materials
2.5.2. Design and Processing of the ACMSs
2.6. Bacteria Culture and the ACMSs Antifouling Performance Test
2.6.1. Bacterial Culture
2.6.2. Antifouling Performance Test
3. Results and Discussion
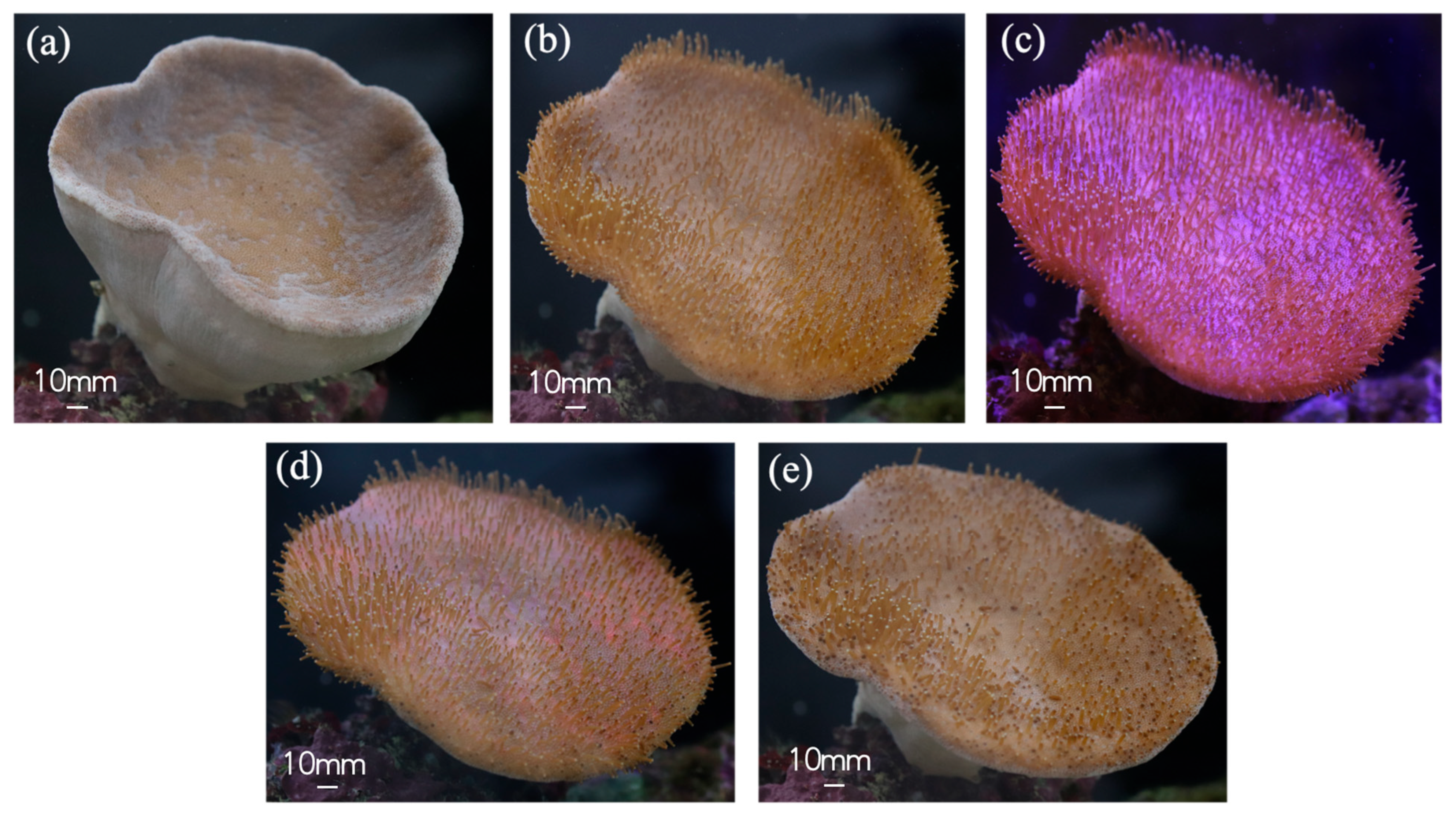
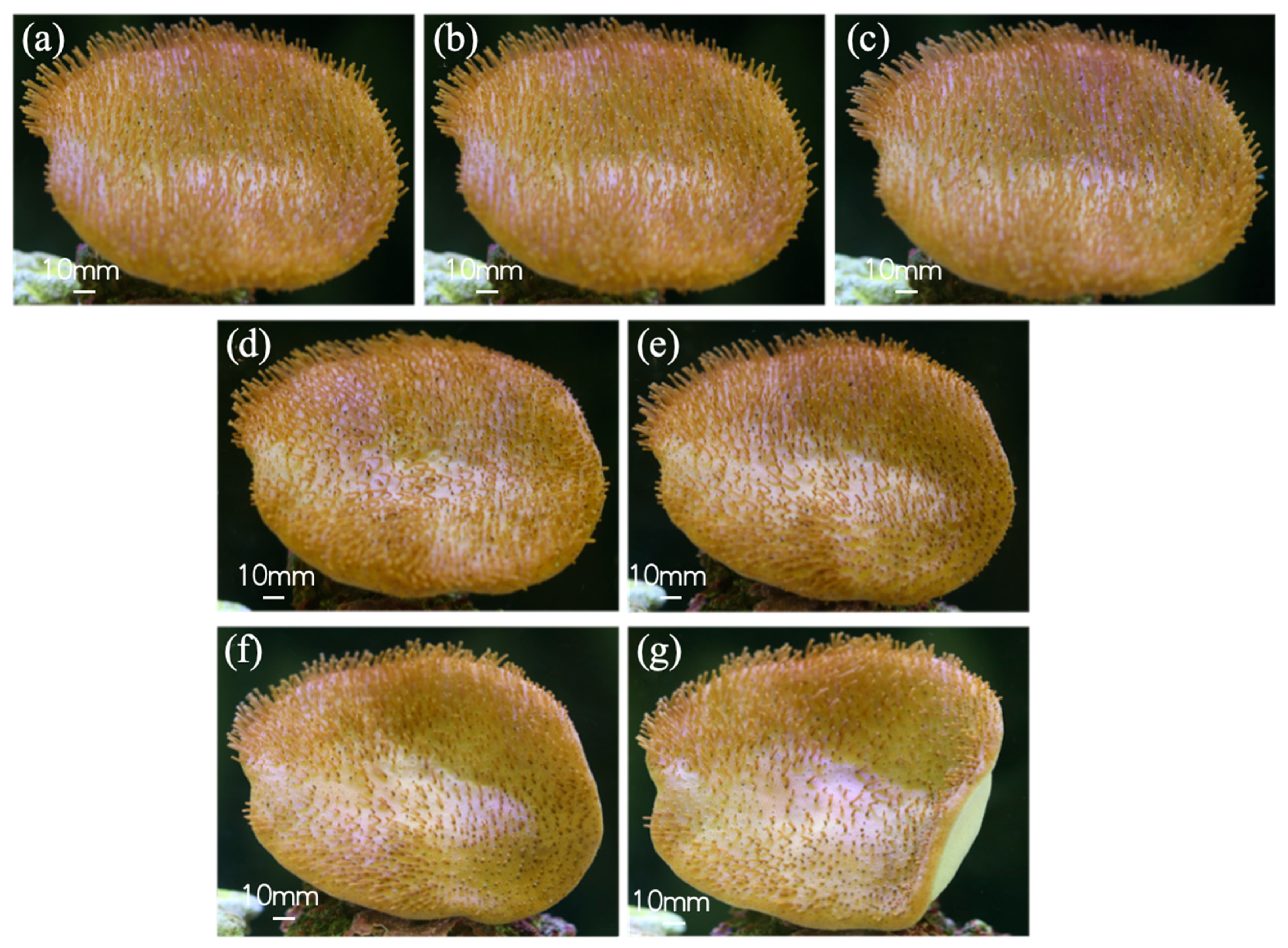
3.1. Natural Environment Simulation—Coral Tentacle Expansion and Contraction Patterns & Biological Sample Information Acquisition
3.2. Fluid Medium Disturbance Environment—Coral Tentacle Response Regularity
3.3. Coral Tentacles “Swinging Effect” Antifouling Performance Test
3.4. Anti-Fouling Performance Test of the ACMSs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, S.Q.; Wu, S.; Xiong, S.L.; Wang, T.S.; Hou, J.B.; Zhao, Y.T.; Li, W.G. Research Progress of Marine Anti-Fouling Coatings. Coatings 2024, 14, 1227. [Google Scholar] [CrossRef]
- Chen, Q.A.; Zhang, Z.P.; Xiong, G.; Wang, L. The effect of different pigment on the adhesion mechanism of marine bacteria and Naviclua tenera on fluorescent antifouling coating. Surf. Interfaces 2025, 60, 106061. [Google Scholar] [CrossRef]
- Marija, K.; Nikola, V.; Neven, H.; Viktor, L. Life-cycle cost assessment of hull protection technologies considering their effect on the environmental friendliness of fishing vessels. Mar. Pollut. Bull. 2024, 209, 117137. [Google Scholar] [CrossRef]
- Liu, G.Z.; Li, K.; Yuan, H.; Zhou, R.; Mao, L.; Zhang, R.F.; Zhang, G.Y. An antifouling epoxy coated metal surface containing silica-immobilized carbonic anhydrase supraparticles for CO2 capture through microalgae. Int. J. Biol. Macromol. 2024, 269, 132075. [Google Scholar] [CrossRef]
- Liang, H.; Shi, X.S.; Li, Y.Z. Technologies in Marine Antifouling and Anti-Corrosion Coatings: A Comprehensive Review. Coatings 2024, 14, 1487. [Google Scholar] [CrossRef]
- Guan, Y.; Chen, R.R.; Zhu, J.H.; Sun, G.H.; Liu, Q.; Liu, J.Y.; Yu, J.; Yang, Z.L.; Wang, J. Dolphins-inspired antifouling self-healing hydrophilic coatings with hierarchical hydrogen bonds for enhanced durability in antifouling performance based on dynamic poly(urethane-urea). Chem. Eng. J. 2025, 515, 163641. [Google Scholar] [CrossRef]
- Fernández-Gutiérrez, J.; Rubal, M.; Sampaio, L.; Moreira, J.; Ramil, F.; Sousa-Pinto, I.; Veiga, P. Structure of Non-Indigenous Fouling Assemblages and Biocontamination Levels in Portuguese Recreational Marinas Under Different Salinity Conditions. Diversity 2025, 17, 245. [Google Scholar] [CrossRef]
- Donelan, S.C.; Miller, A.W.; Ziegler, G.; Ruiz, G. Environmental conditions of vessel routes and arrival ports can alter propagule supply by reproduction in a common biofouling species. Biol. Invasions 2025, 27, 11. [Google Scholar] [CrossRef]
- Barnes, H. The determination of mercury and copper in anti-fouling compositions: Potassium cobalticyanide as complex-forming agent in dithizone technique. Analyst 1946, 71, 578–583. [Google Scholar] [CrossRef]
- Evans, S.M.; Leksono, T.; McKinnell, P.D. Tributyltin pollution: A diminishing problem following legislation limiting the use of TBT-based anti-fouling paints. Mar. Pollut. Bull. 1995, 30, 14–21. [Google Scholar] [CrossRef]
- Satasiya, G.; Kumar, M.A.; Ray, S. Biofouling dynamics and antifouling innovations: Transitioning from traditional biocides to nanotechnological interventions. Environ. Res. 2025, 269, 120943. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, D.; Madkour, S.; Pleskunov, P.; Tafiichuk, R.; Shelemin, A.; Hanuš, J.; Gordeev, I.; Sysolyatina, E.; Lavrikova, A.; Ermolaeva, S.; et al. Cu nanoparticles constrain segmental dynamics of cross-linked polyethers: A trade-off between non-fouling and antibacterial properties. Soft Matter 2019, 15, 2884–2896. [Google Scholar] [CrossRef] [PubMed]
- Weiss, C.M. The comparative tolerances of some fouling organisms to copper and mercury. Biol. Bull. 1947, 93, 56–63. [Google Scholar] [CrossRef]
- Sanchez-Salcedo, S.; Vallet-Regí, M.; Shahin, S.A.; Glackin, C.A.; Zink, J.I. Mesoporous core-shell silica nanoparticles with anti-fouling properties for ovarian cancer therapy. Chem. Eng. J. 2018, 340, 114–124. [Google Scholar] [CrossRef]
- Nurioglu, A.G.; Esteves, A.C.C.; De, G. Non-toxic, non-biocide-release antifouling coatings based on molecular structure design for marine applications. J. Mater. Chem. B 2015, 3, 6547–6570. [Google Scholar] [CrossRef]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology-Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Gu, G.J.; Yang, X.B.; Li, Y.X.; Guo, J.; Huang, J.H.; Nxumalo, E.N.; Mamba, B.B.; Shao, L. Advanced zwitterionic polymeric membranes for diverse applications beyond antifouling. Sep. Purif. Technol. 2025, 356, 129848. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, W.; Mo, M.; Zhou, C.; Liu, G.; Zeng, Z.; Wu, X.; Xue, Q. Architecture of modified silica resin coatings with various micro/nano patterns for fouling resistance: Microstructure and antifouling performance. RSC Adv. 2015, 5, 97862–97873. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, F.; Zhang, Z.; Liu, Y.; Li, Z.; Wei, X.; Wang, T.; Fan, C.; Jiang, Z. Electrostatic interaction enhanced surface segregation towards heterogeneous antifouling membrane for oil/water separation. Chem. Eng. Sci. 2025, 306, 121283. [Google Scholar] [CrossRef]
- Peng, Y.L.; Lin, C.G.; Wang, L. The preliminary study on antifouling mechanism of shark skin. Adv. Mater. Res. 2009, 79–82, 977–980. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, W.; Chen, Z.; Liu, Z.; Cao, H.; Zhou, C.; Cui, P. Influence of biomimetic boundary structure on the antifouling performances of siloxane modified resin coatings. Colloid Surf. A 2017, 528, 57–64. [Google Scholar] [CrossRef]
- Selim, M.S.; El-Safty, S.A.; Shenashen, M.A.; Higazy, S.A.; Elmarakbi, A. Progress in biomimetic leverages for marine antifouling using nanocomposite coatings. J. Mater. Chem. B 2020, 8, 3701–3732. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.; Ma, L.; Che, D.; Zhang, D.; Sudarshan, T.S. Investigation on large-area fabrication of vivid shark skin with superior surface functions. Appl. Surf. Sci. 2014, 316, 124–131. [Google Scholar] [CrossRef]
- Tian, L.; Yin, Y.; Bing, W.; Jin, E. Antifouling Technology Trends in Marine Environmental Protection. J. Bionic Eng. 2021, 18, 239–263. [Google Scholar] [CrossRef]
- Jin, H.C.; Tian, L.M.; Bing, W.; Zhao, J.; Ren, L.Q. Bioinspired marine antifouling coatings: Status, prospects, and future. Prog. Mater. Sci. 2022, 124, 100889. [Google Scholar] [CrossRef]
- Tian, L.M.; Yin, Y.; Jin, H.C.; Bing, W.; Jin, E.; Jin, H.C.; Ren, L.Q. Novel marine antifouling coatings inspired by corals. Mater. Today Chem. 2020, 17, 100294. [Google Scholar] [CrossRef]
- Lesser, M.P.; Mazel, C.H.; Gorbunov, M.Y.; Falkowski, P.G. Discovery of Symbiotic Nitrogen-Fixing Cyanobacteria in Corals. Science 2004, 305, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.C.; Bing, W.; Jin, E.; Tian, L.M.; Jiang, Y. Bioinspired PDMS—Phosphor—Silicone Rubber Sandwich-Structure Coatings for Combating Biofouling. Adv. Mater. Interfaces 2020, 7, 1901577. [Google Scholar] [CrossRef]
- Tian, L.; Wang, Y.; Li, Z.; Mei, H.; Shang, Y. The thermal conductivity-dependant drag reduction mechanism of water droplets controlled by graphene/silicone rubber composites. Exp. Therm. Fluid Sci. 2017, 85, 363–368. [Google Scholar] [CrossRef]
- Bing, W.; Tian, L.M.; Wang, Y.; Jin, H.C.; Ren, L.Q.; Dong, S. Bio-Inspired Non-Bactericidal Coating Used for Antibiofouling. Adv. Mater. Technol. 2018, 4, 1800480. [Google Scholar] [CrossRef]
- Tian, L.M.; Jin, E.; Sun, H.; Shang, Y.G.; Bing, W. Novel Anti-fouling Strategies of Live and Dead Soft Corals (Sarcophyton trocheliophorum): Combined Physical and Chemical Mechanisms. J. Bionic Eng. 2020, 17, 677–685. [Google Scholar] [CrossRef]


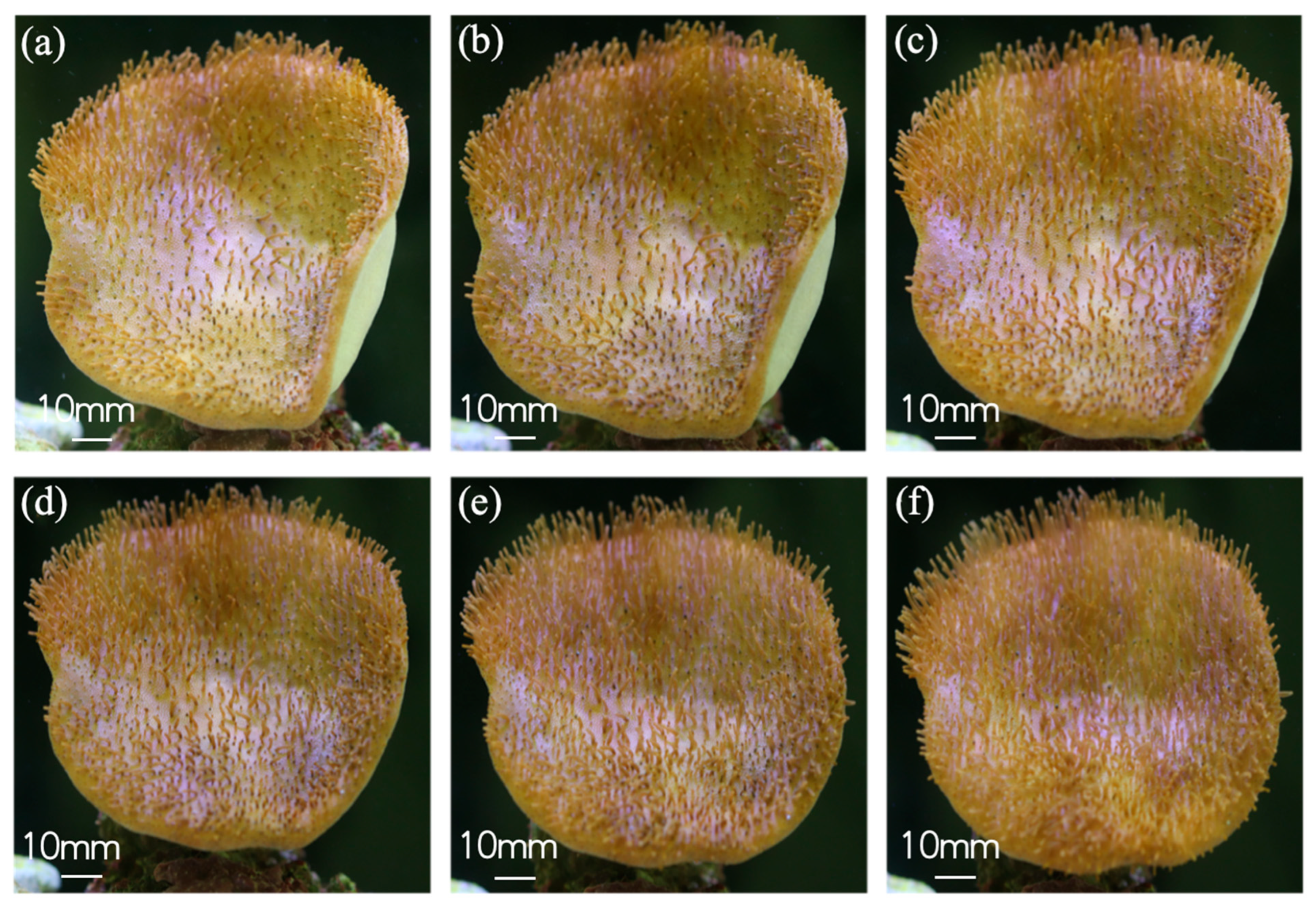
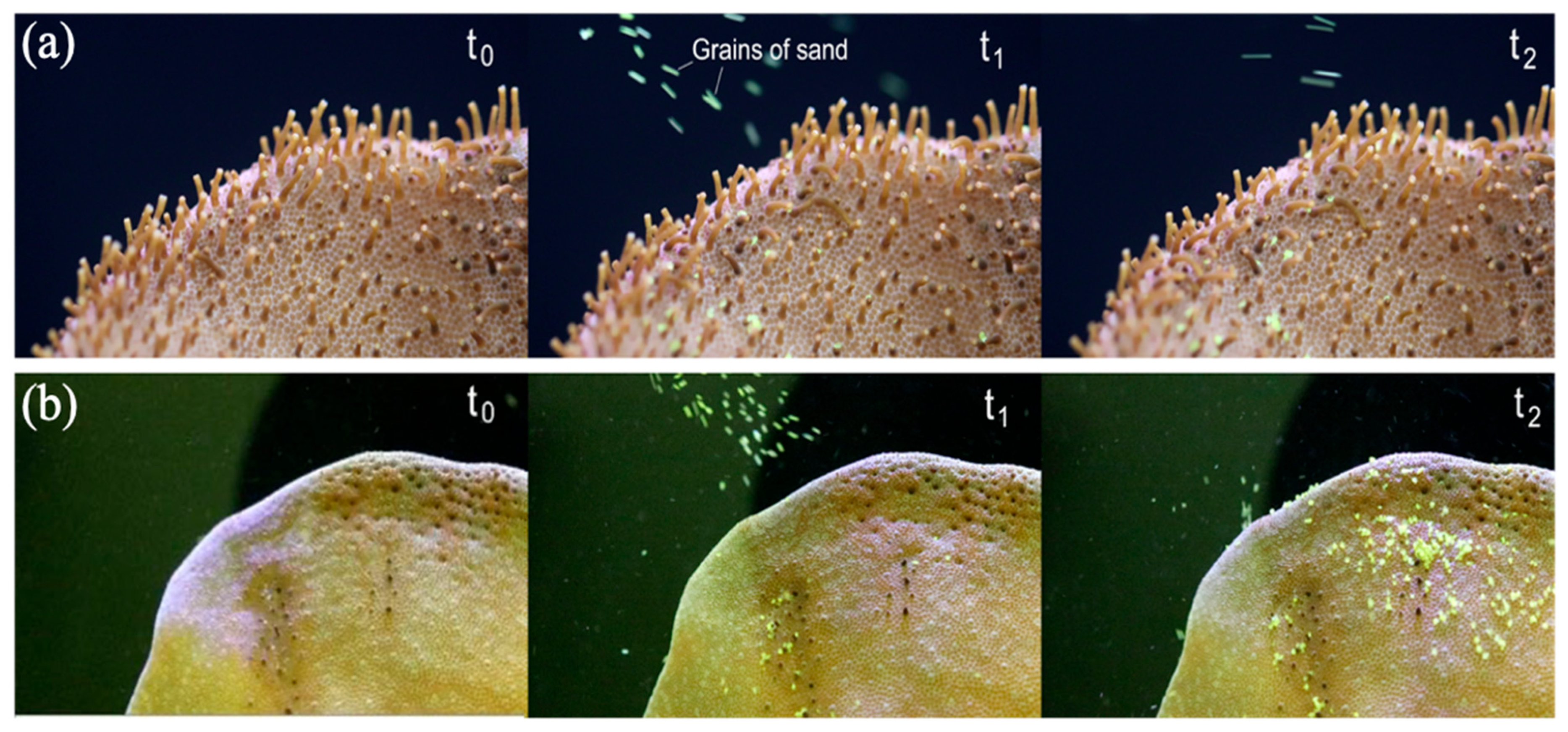
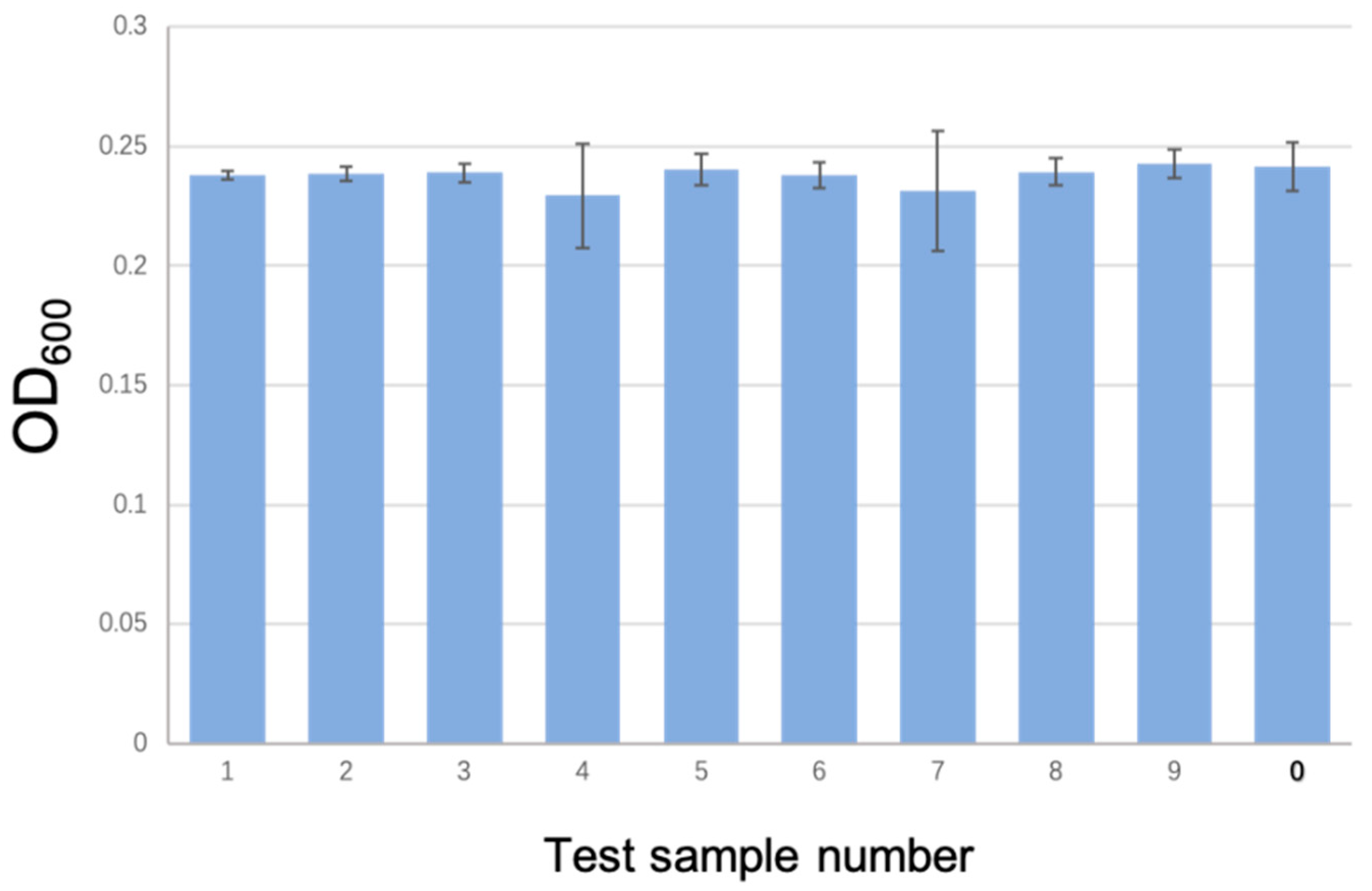

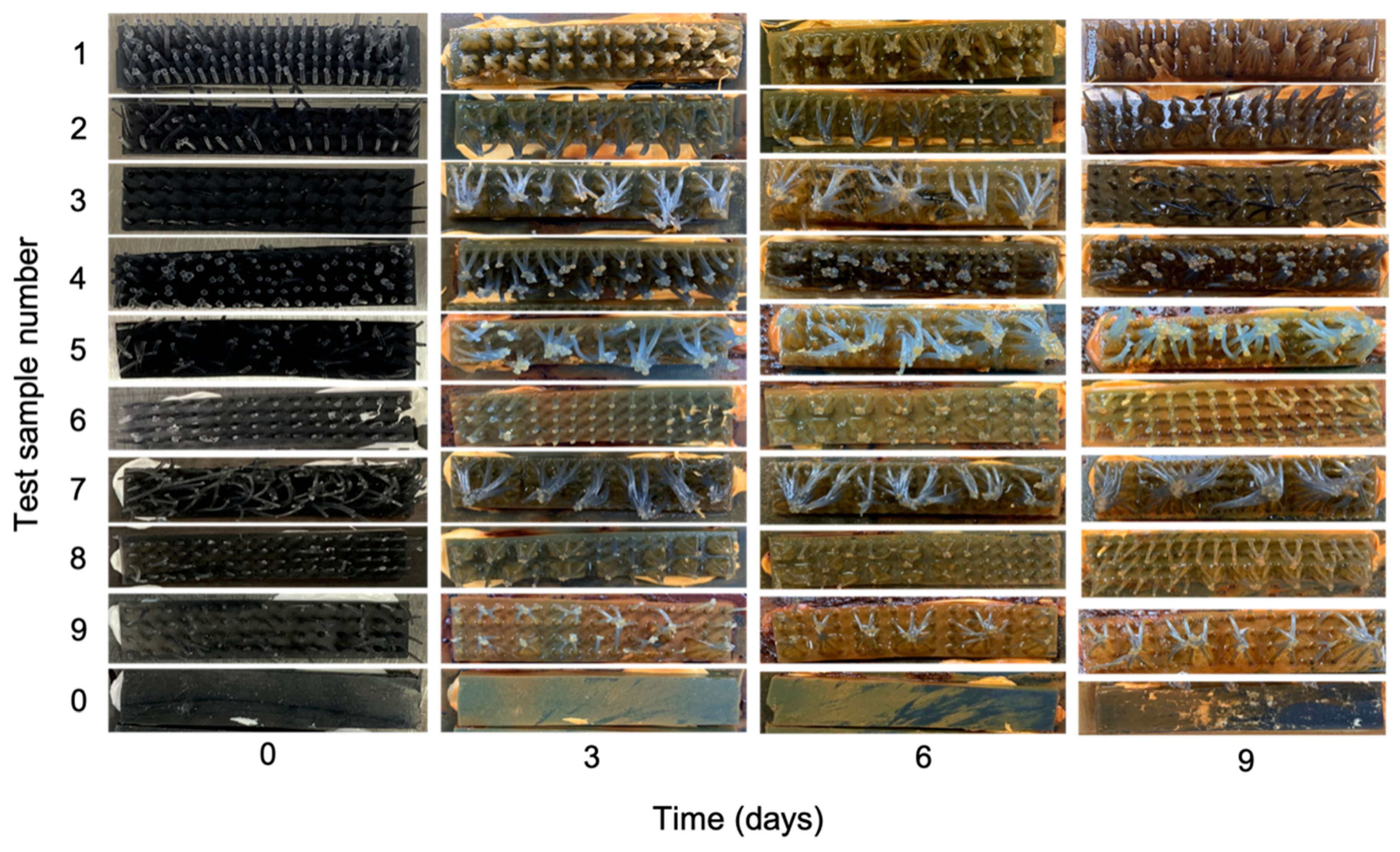
| Parameter | Tentacle Distance | Tentacle Length | |
|---|---|---|---|
| Number | |||
| 1 | 2 | 7 | |
| 2 | 2.5 | 10 | |
| 3 | 3 | 13 | |
| 4 | 2 | 10 | |
| 5 | 2.5 | 13 | |
| 6 | 3 | 7 | |
| 7 | 2 | 13 | |
| 8 | 2.5 | 7 | |
| 9 | 3 | 10 | |
| 0 | 0 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Wang, J.; Zheng, X. Non-Bactericidal Antifouling Coating Inspired by the “Swinging Effect” of Coral Tentacles in Waves. Biomimetics 2025, 10, 606. https://doi.org/10.3390/biomimetics10090606
Yin Y, Wang J, Zheng X. Non-Bactericidal Antifouling Coating Inspired by the “Swinging Effect” of Coral Tentacles in Waves. Biomimetics. 2025; 10(9):606. https://doi.org/10.3390/biomimetics10090606
Chicago/Turabian StyleYin, Yue, Jianfu Wang, and Xu Zheng. 2025. "Non-Bactericidal Antifouling Coating Inspired by the “Swinging Effect” of Coral Tentacles in Waves" Biomimetics 10, no. 9: 606. https://doi.org/10.3390/biomimetics10090606
APA StyleYin, Y., Wang, J., & Zheng, X. (2025). Non-Bactericidal Antifouling Coating Inspired by the “Swinging Effect” of Coral Tentacles in Waves. Biomimetics, 10(9), 606. https://doi.org/10.3390/biomimetics10090606