Polyaniline/Ti3C2 MXene Composites with Artificial 3D Biomimetic Surface Structure of Natural Macaw Feather Applied for Anticorrosion Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instruments and Methods
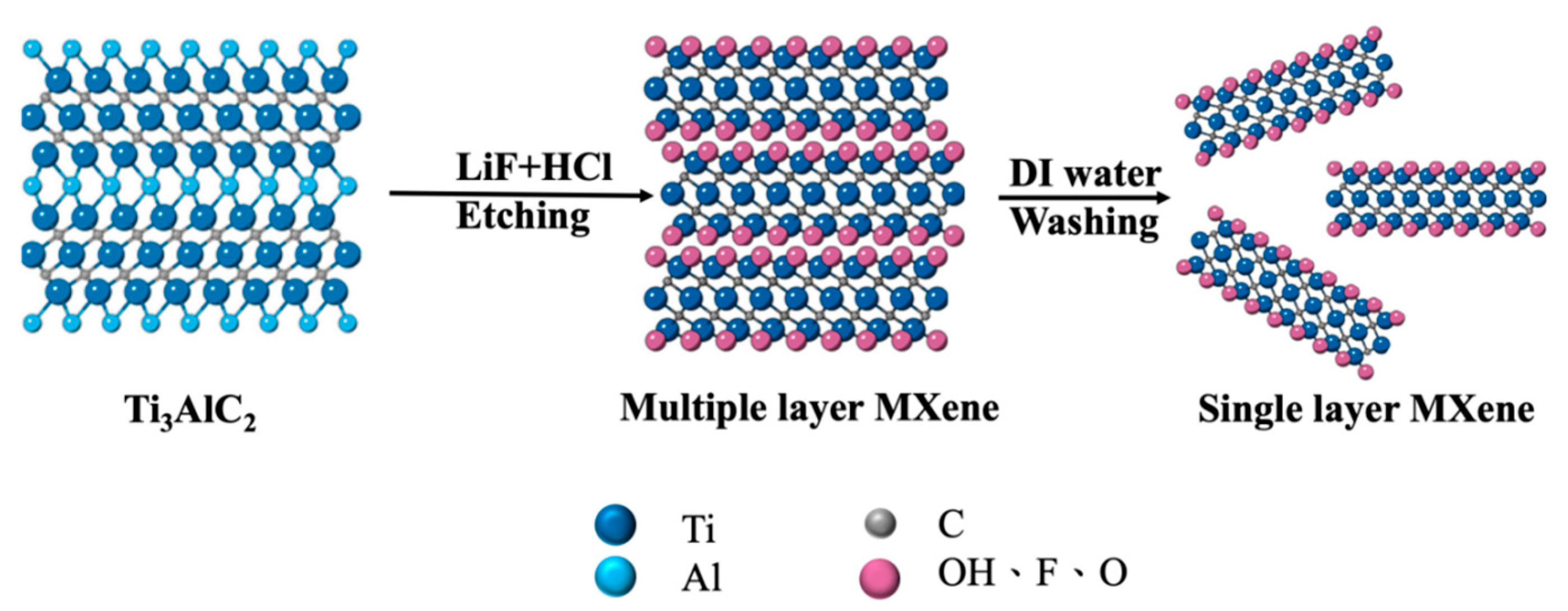
2.3. Preparation of Ti3C2 MXene Nanosheets
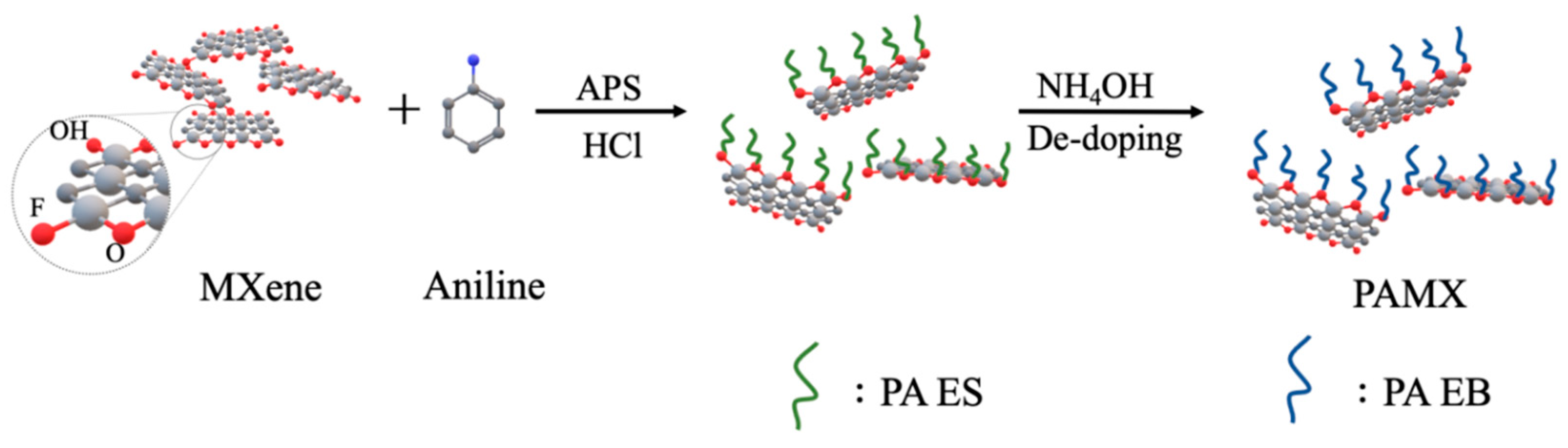
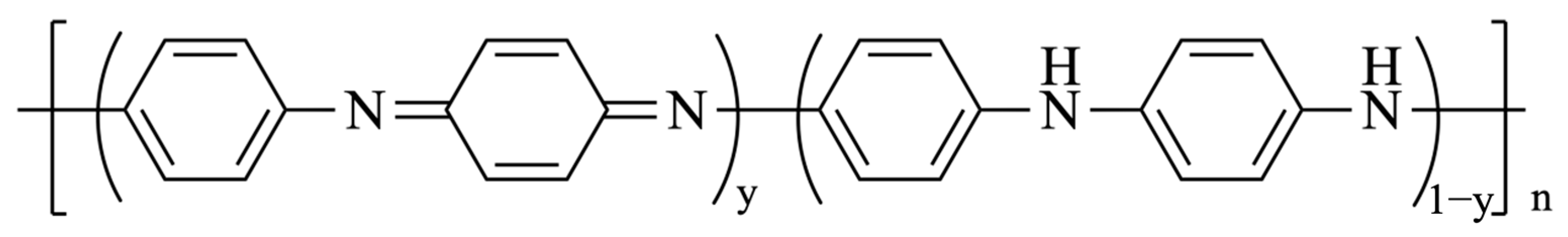
2.4. Synthesis of Polyaniline (PANI)
2.5. Preparation of PA-MX-5
2.6. Preparation of Free-Standing Membranes of PANI and Its Composites and Corresponding Barrier Property Measurements
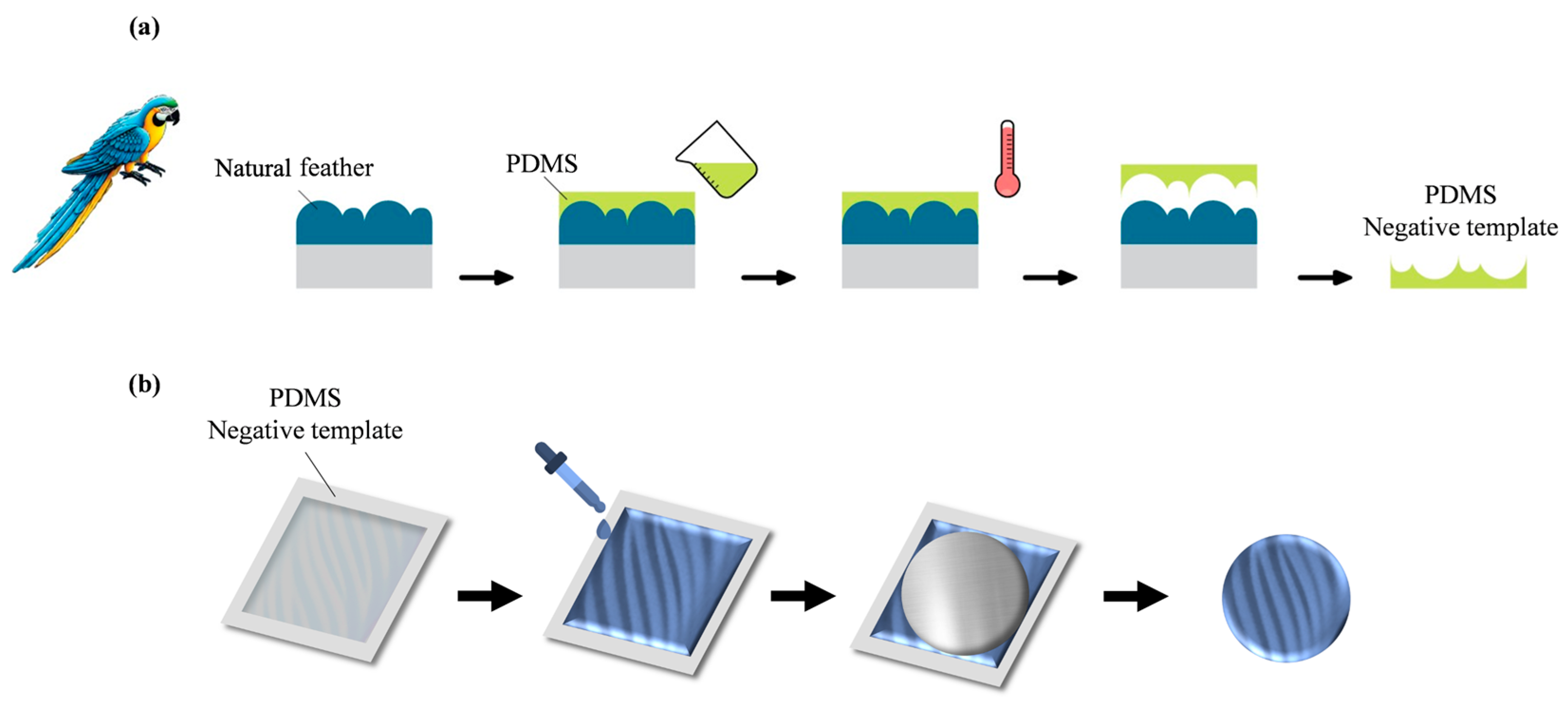
2.7. Preparation of PDMS Negative Template [26,27,28,29,30,31,32,33,34]
2.8. Preparation of Coatings Without/with Biomimetic Structure of Macaw Feather
2.8.1. Fabrication of PANI and PA-MX-5 Film
2.8.2. PANI and PA-MX with 3D Biomimetic Surface Structure of Macaw Feather
2.9. Electrochemical Corrosion Measurements
3. Results and Discussion
3.1. Identification of MXene
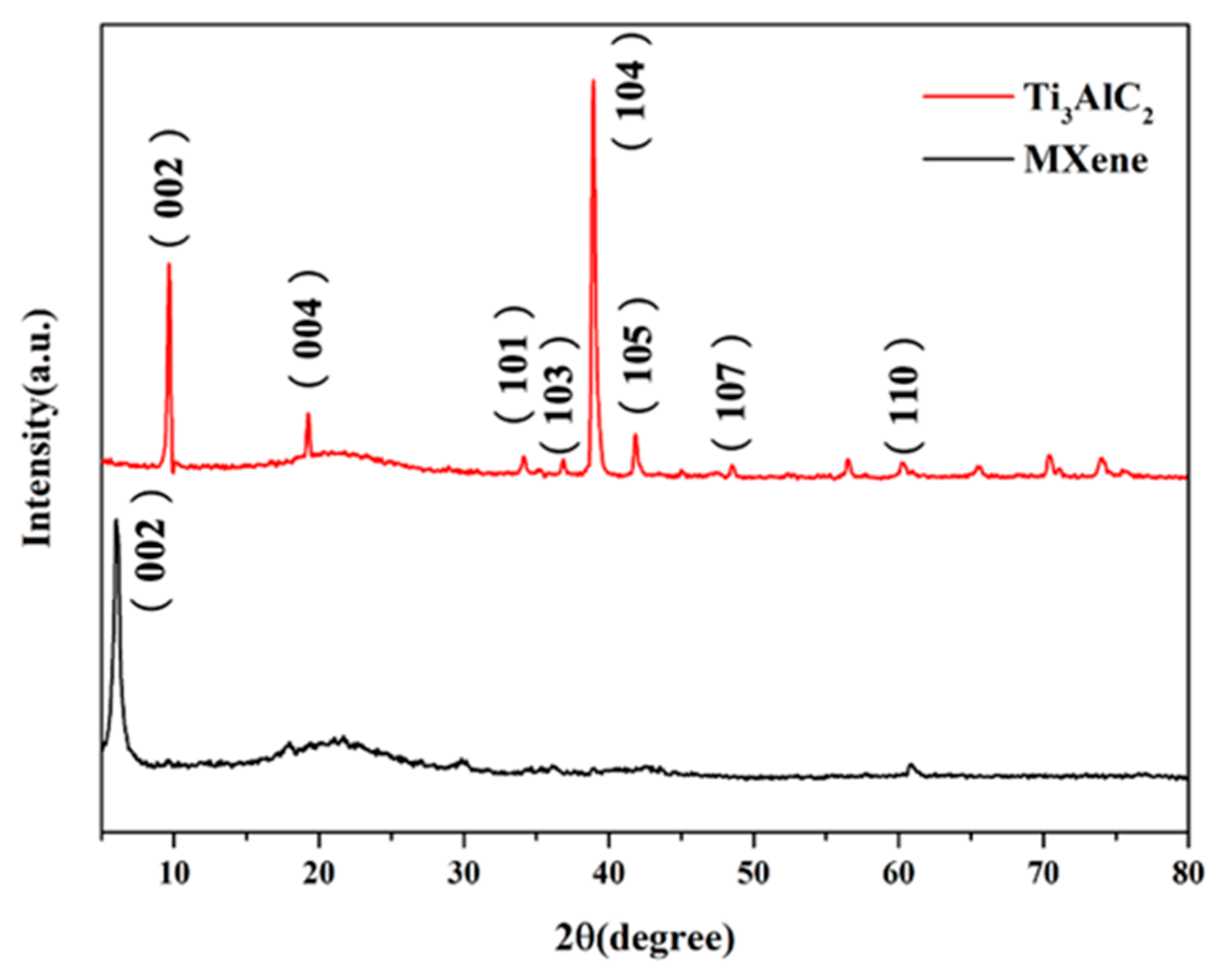
3.1.1. XRD Analysis [14,15,16,17,18,19,20]
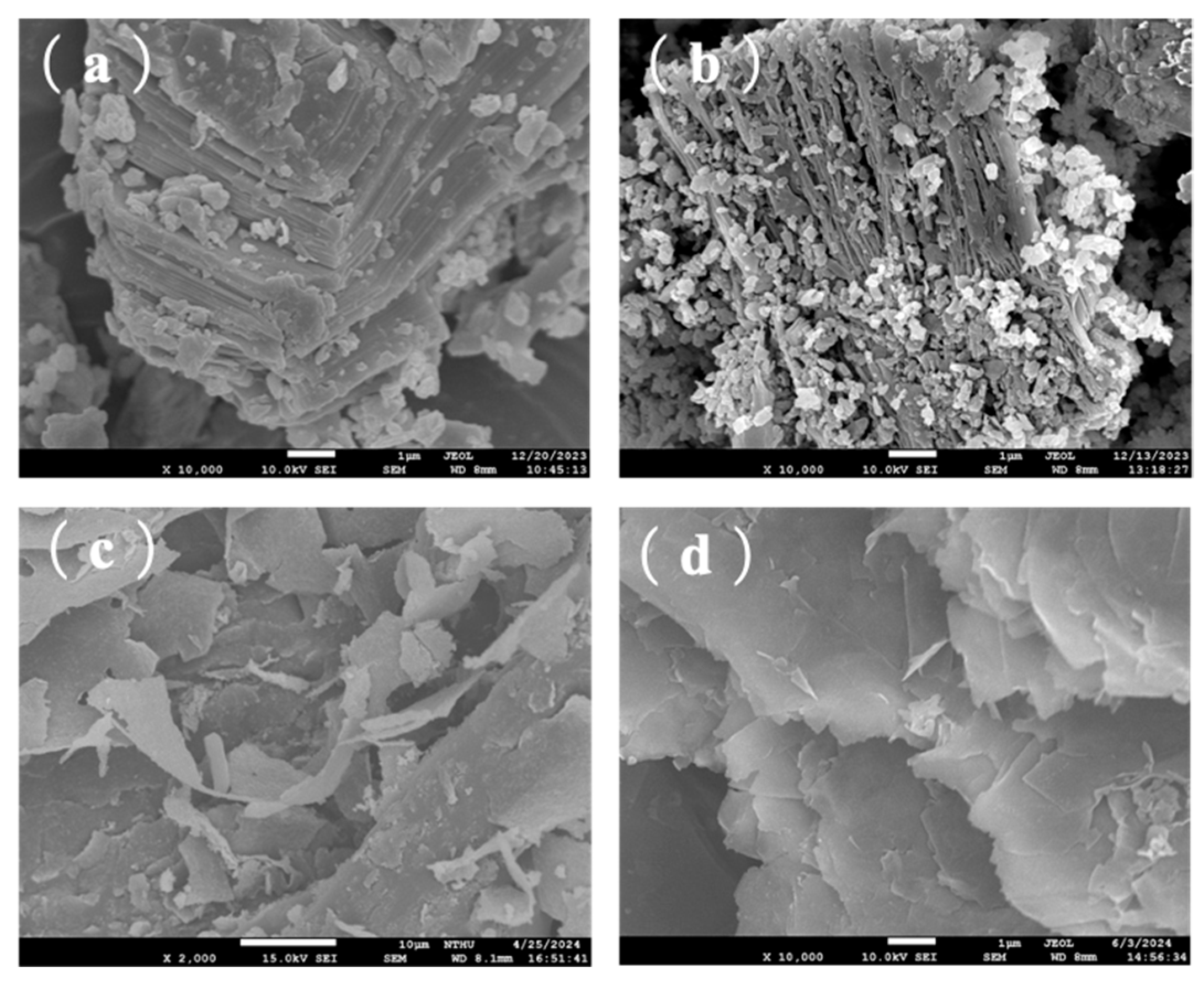
3.1.2. SEM Analysis [14,15,16,17,18,19,20]
3.2. Identification of PANI and PA-MX-5
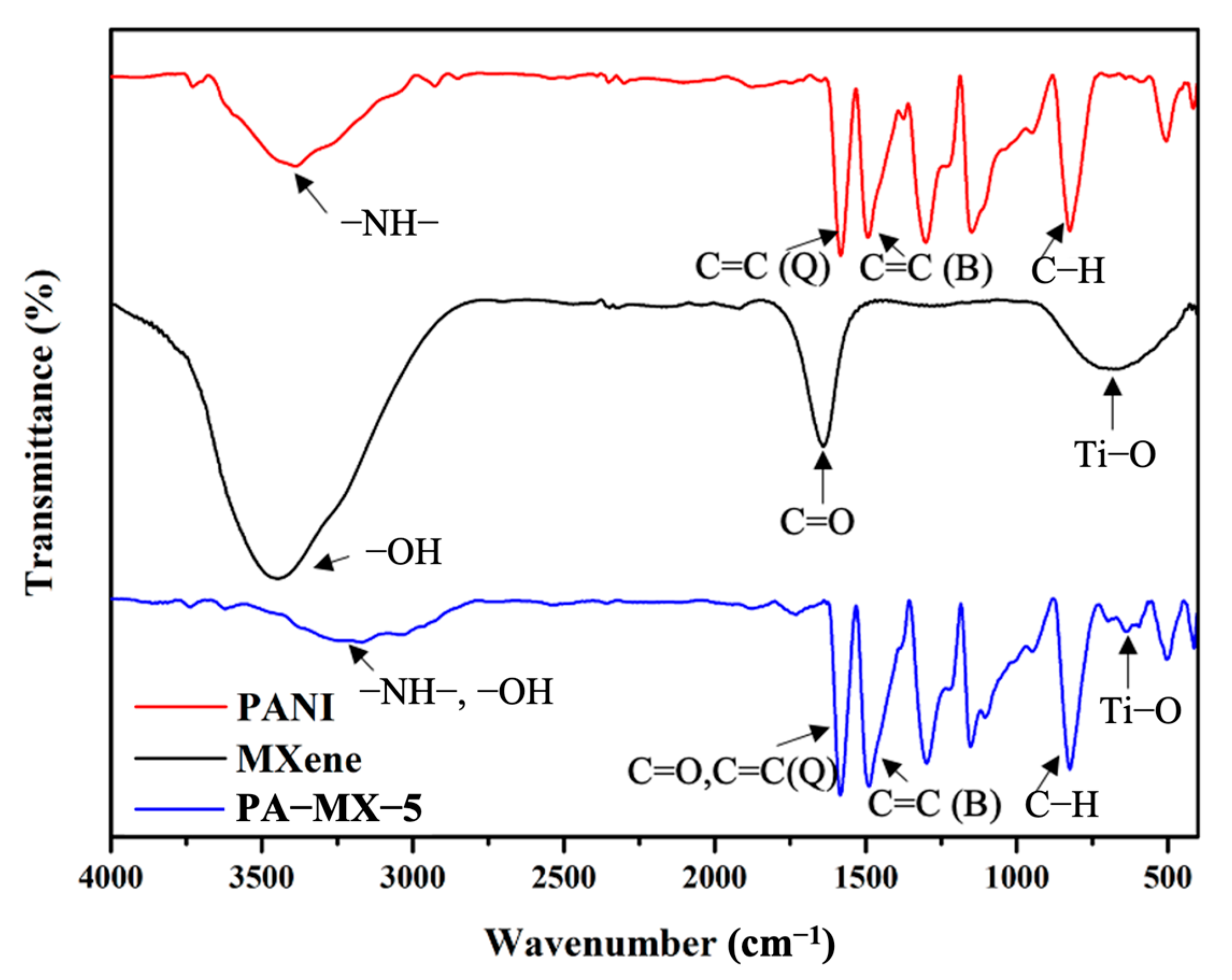
3.2.1. FTIR Analysis of PANI, Ti3C2 MXene, and PA-MX-5
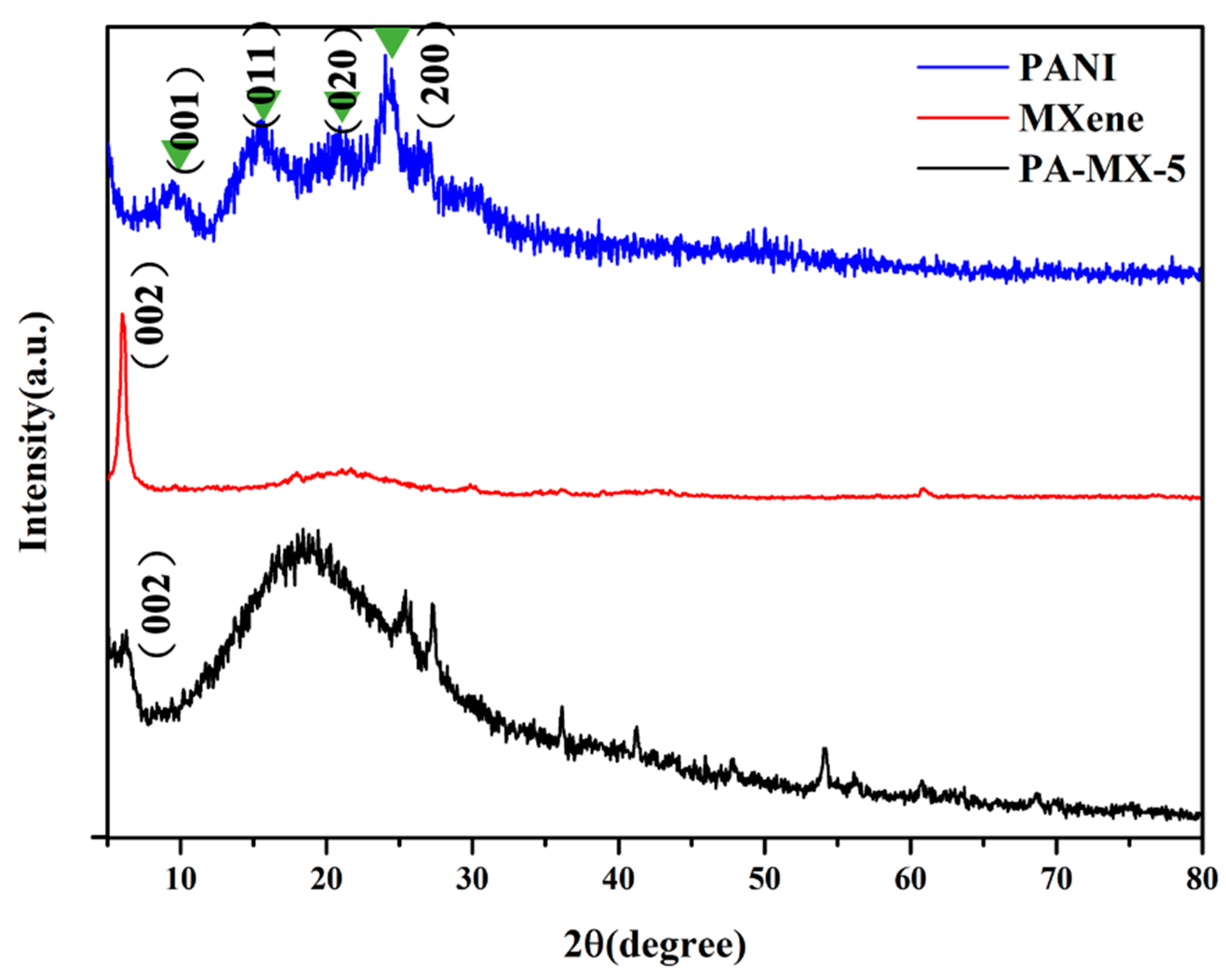
3.2.2. XRD Analysis of PANI, Ti3C2 MXene, and PA-MX-5
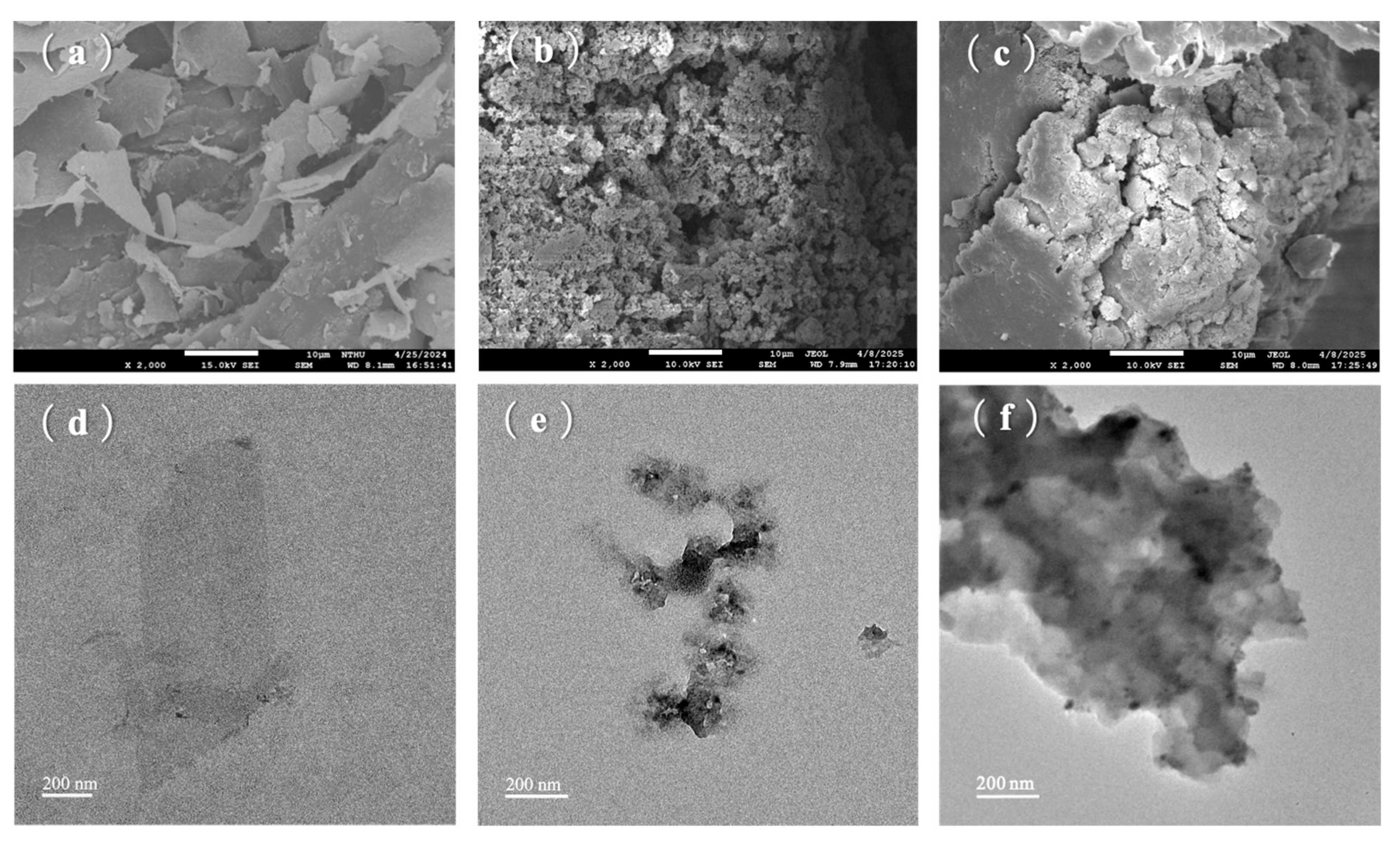
3.2.3. SEM/TEM Observation of PANI, Ti3C2 MXene, and PA-MX-5
3.2.4. Redox Capability Characterization
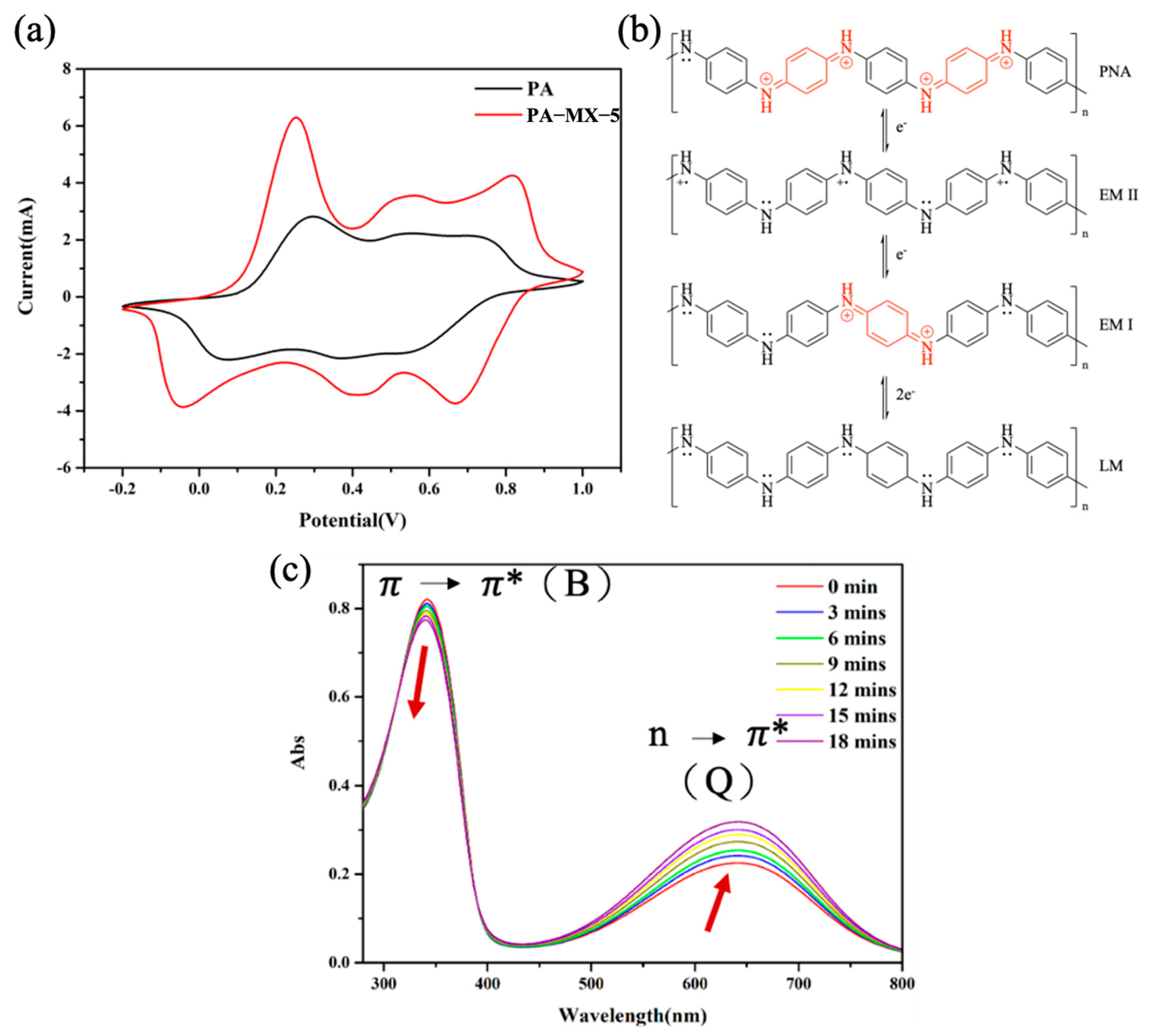
Electrochemical Cyclic Voltammetry (CV) of PANI and PA-MX-5
UV-Visible Absorption Spectroscopy of PA-MX-5
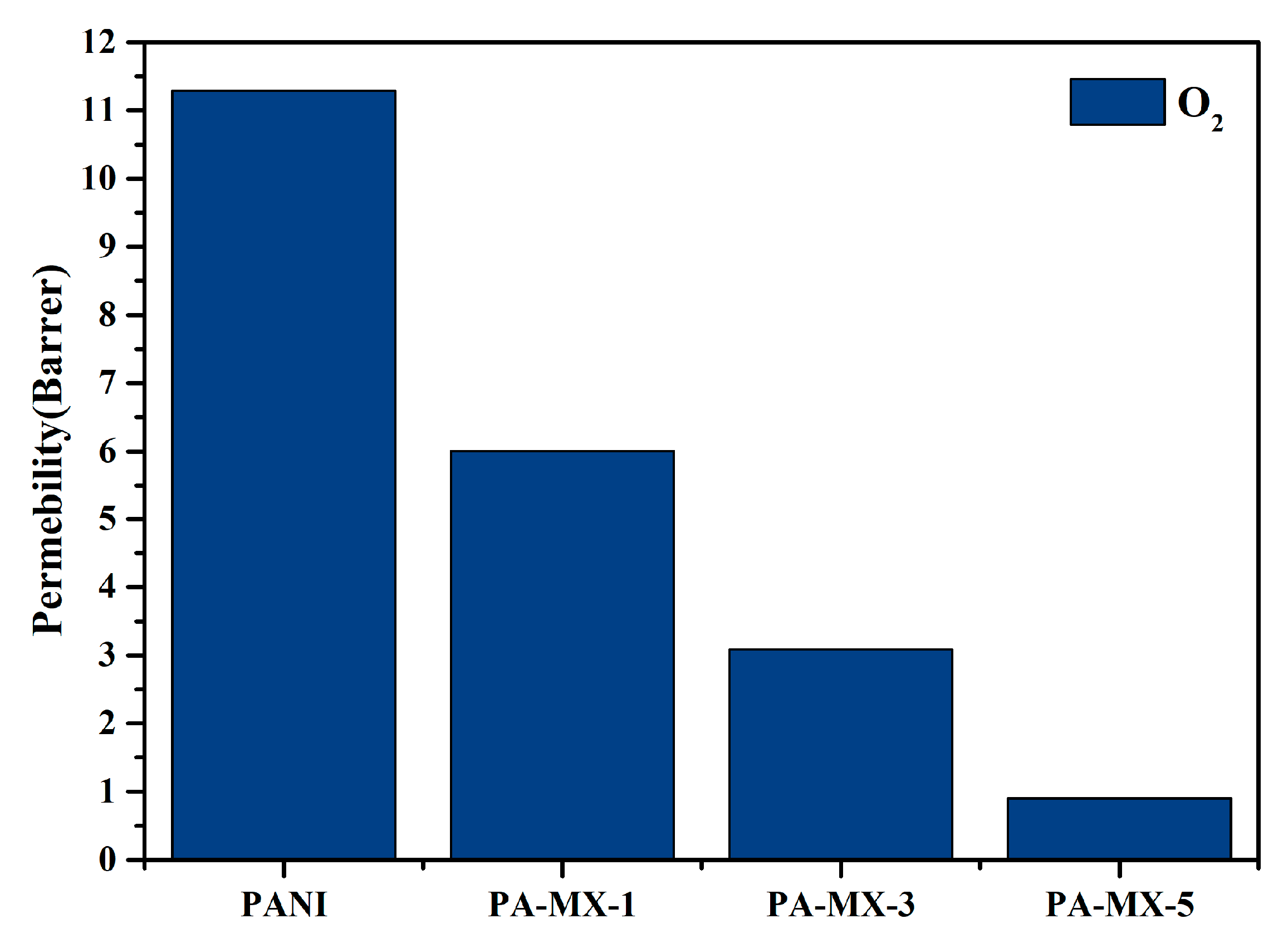
3.2.5. Oxygen Gas Permeability Analysis of PANI and Ti3C2 MXene-Based PANI Composite Free-Standing Membranes
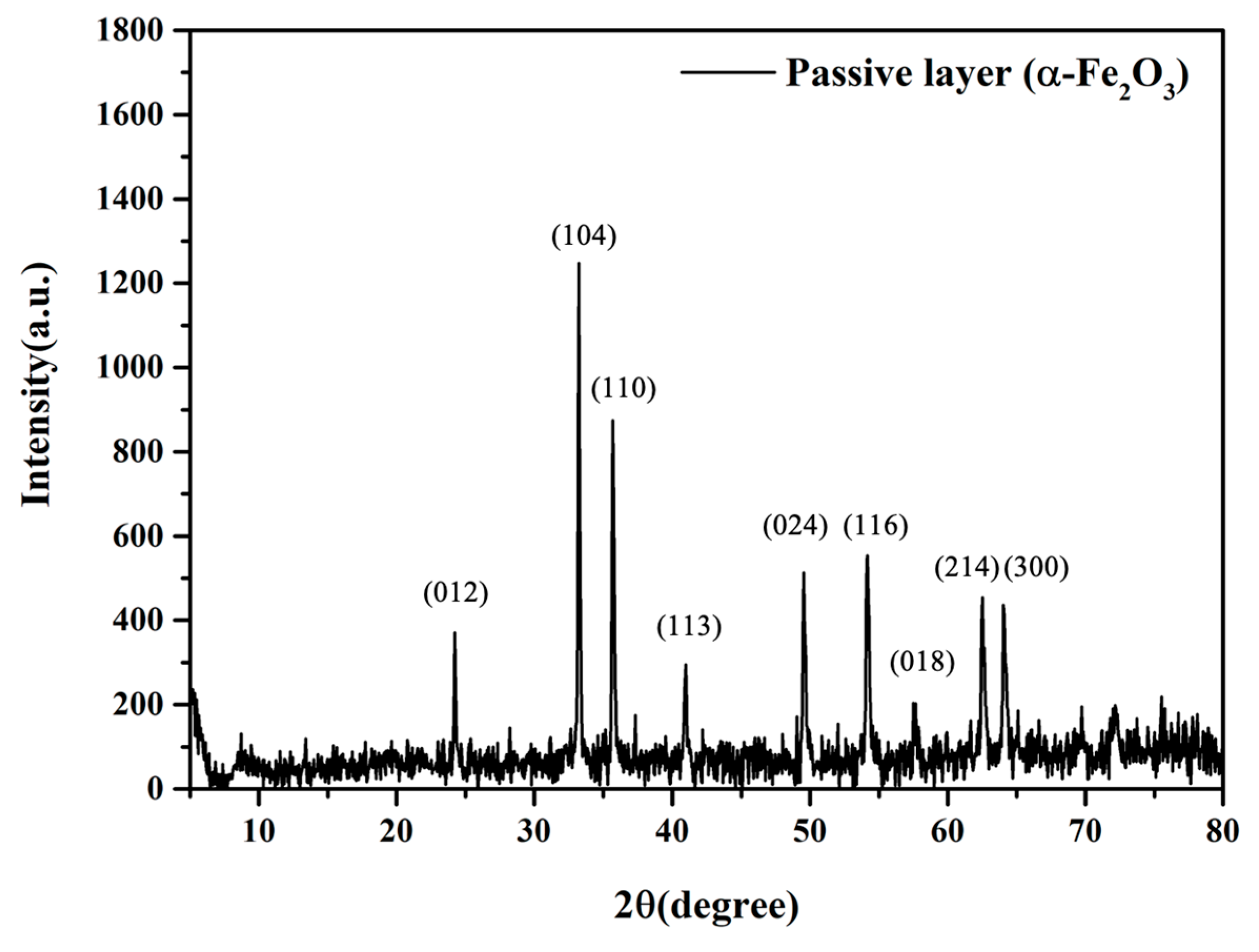
3.2.6. XRD Characterization of Densely Passive Metal Oxide Layer Induced by the Electro-Catalyzed Capability of PA-MX-5
3.3. Structural Characterization of Bio-PANI and Bio-PA-MX-5
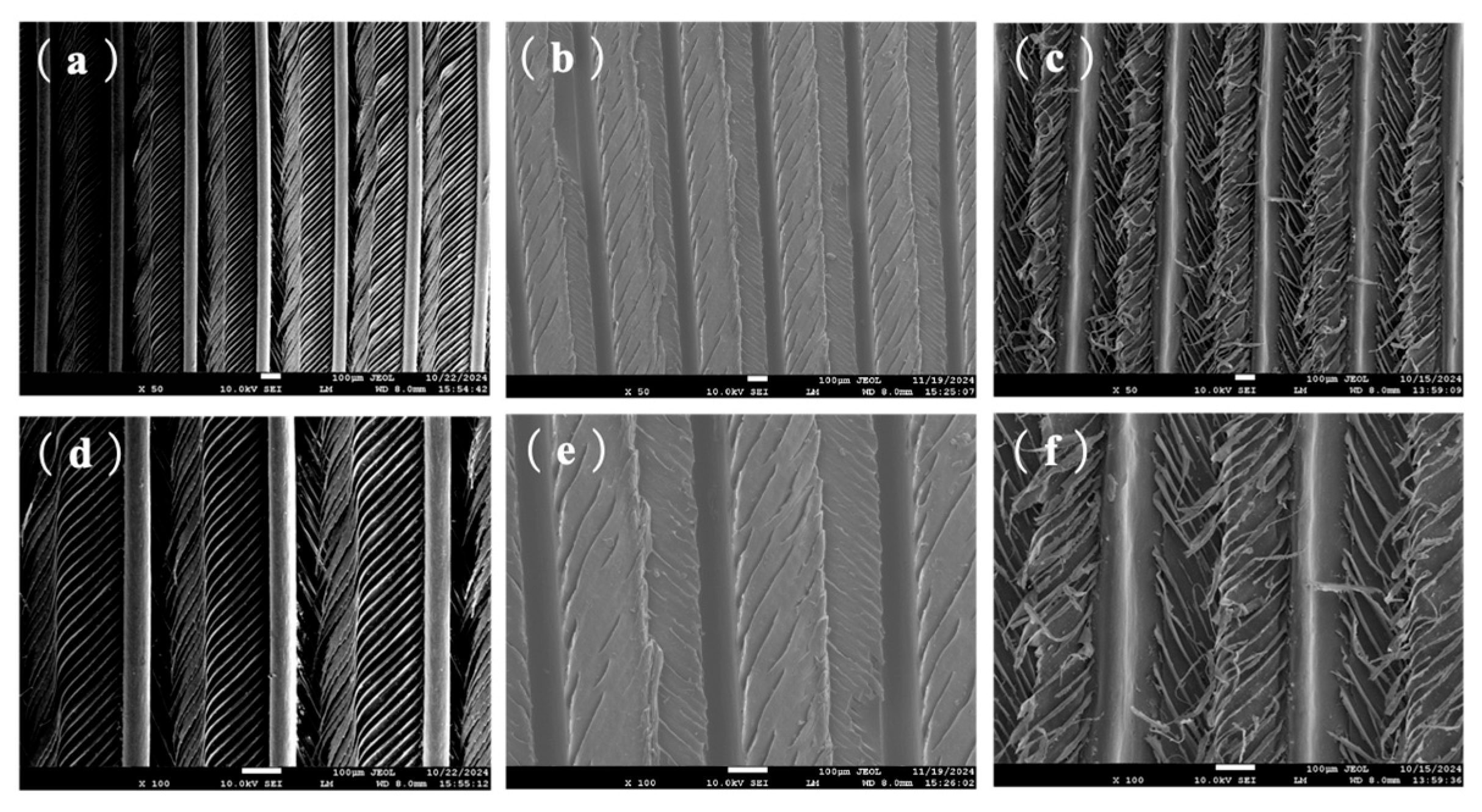
3.3.1. Surface Morphology Observed by Scanning Electron Microscopy (SEM)
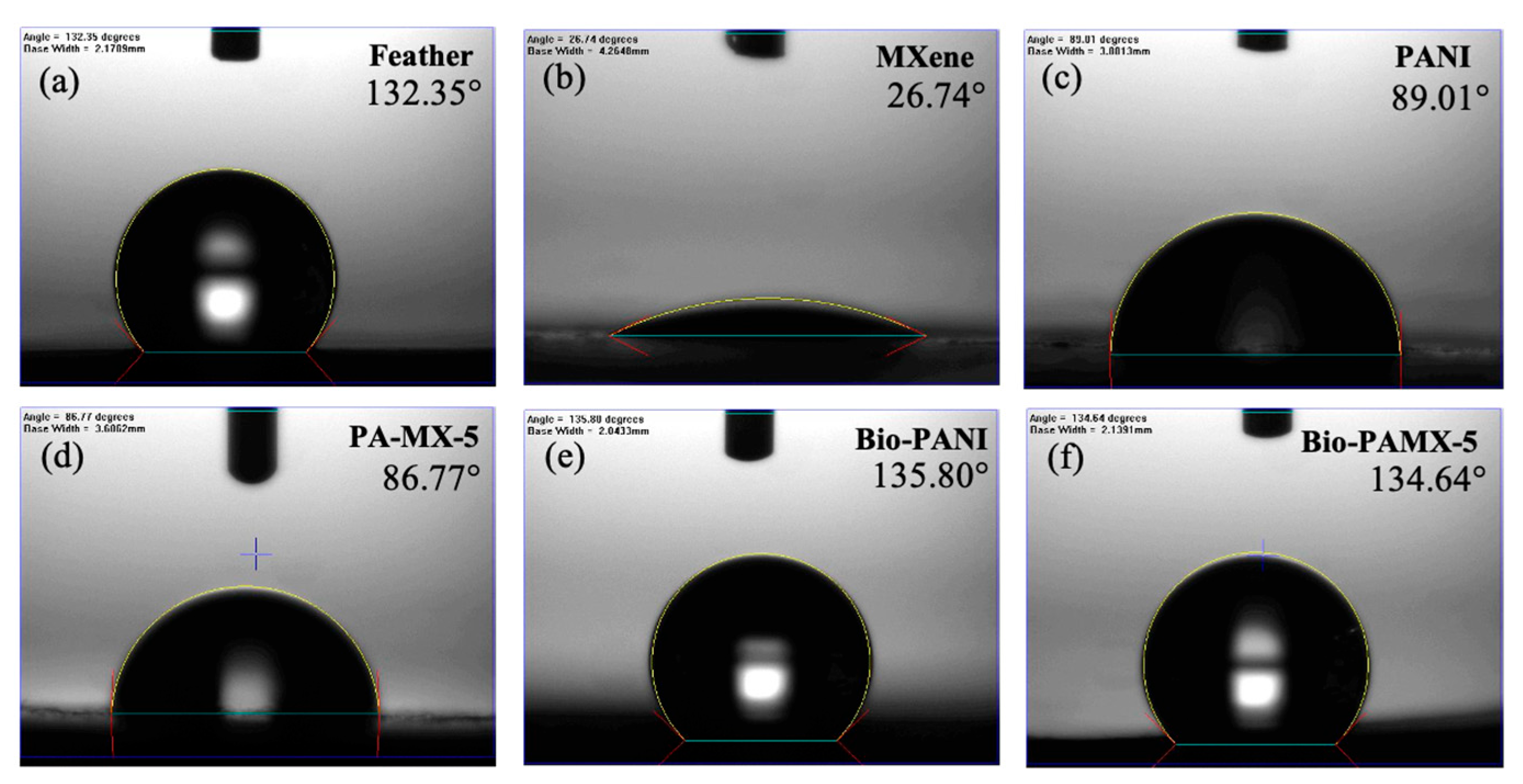
3.3.2. Water Contact Angle (CA)
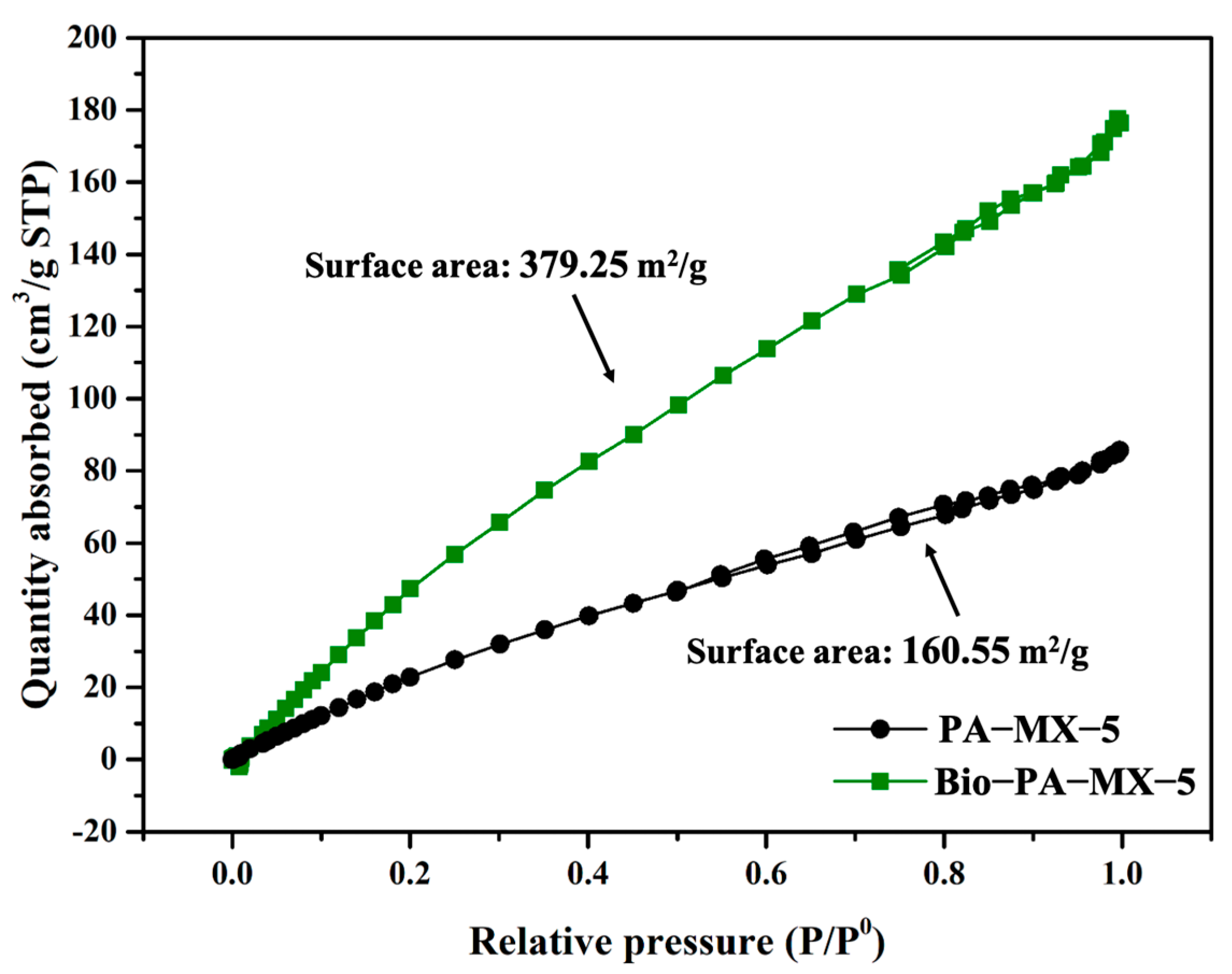
3.3.3. BET Study
3.4. Corrosion Protection Determined by Electro-Chemical Measurements
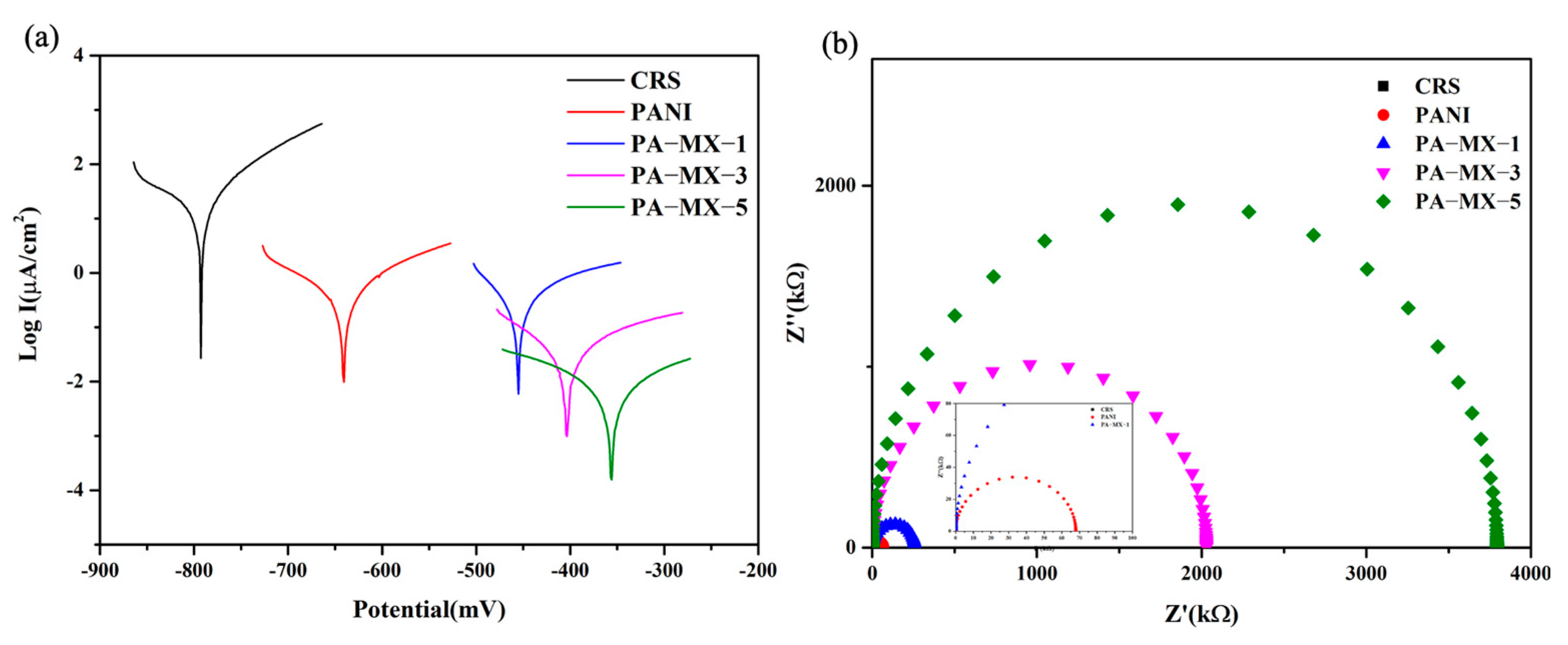
3.4.1. MXene-Based PANI Composites with Distinctive Loading of Ti3C2 Nanosheets
Tafel Test
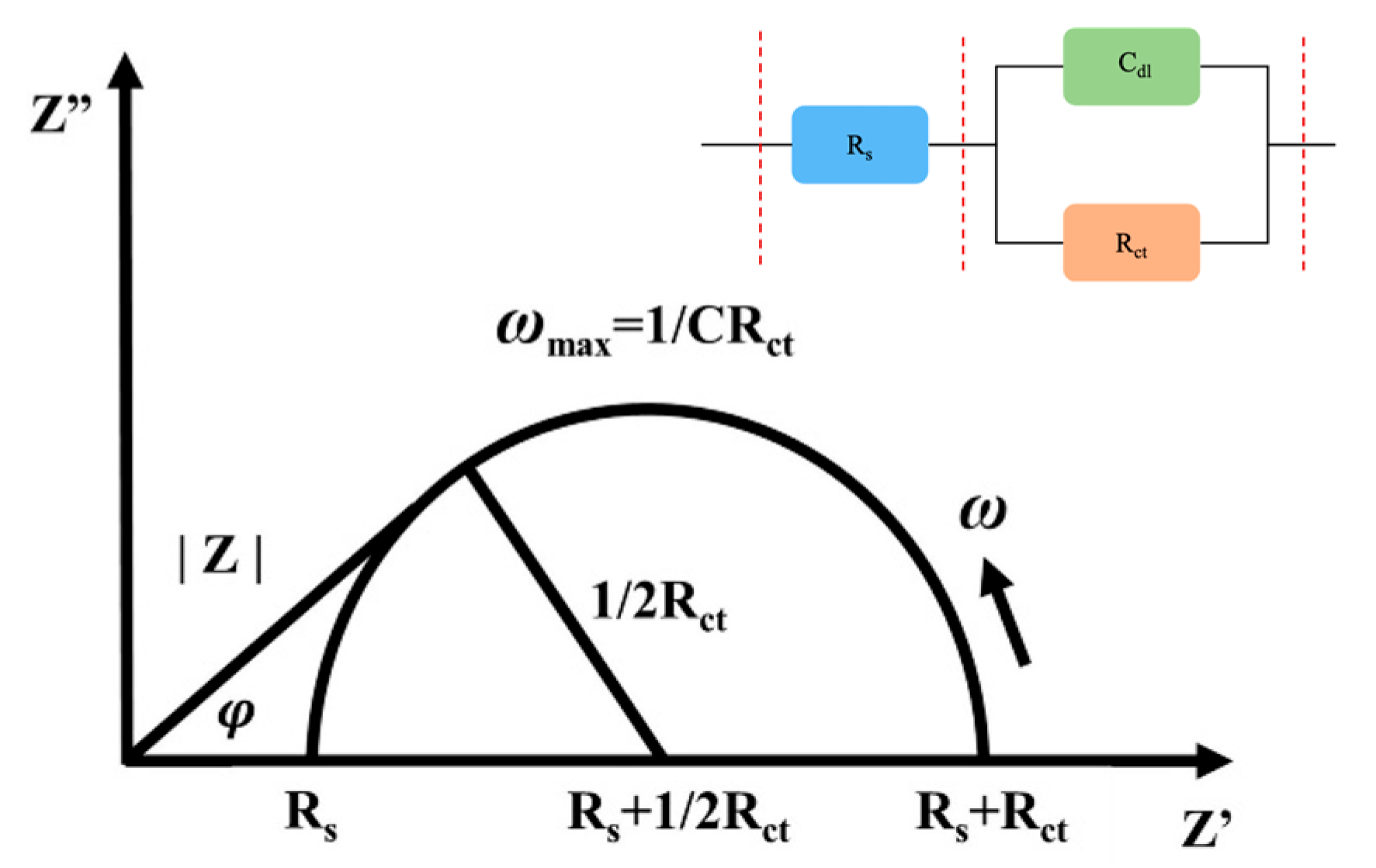
Nyquist Plots Determined by Impedance Spectroscopy (EIS)
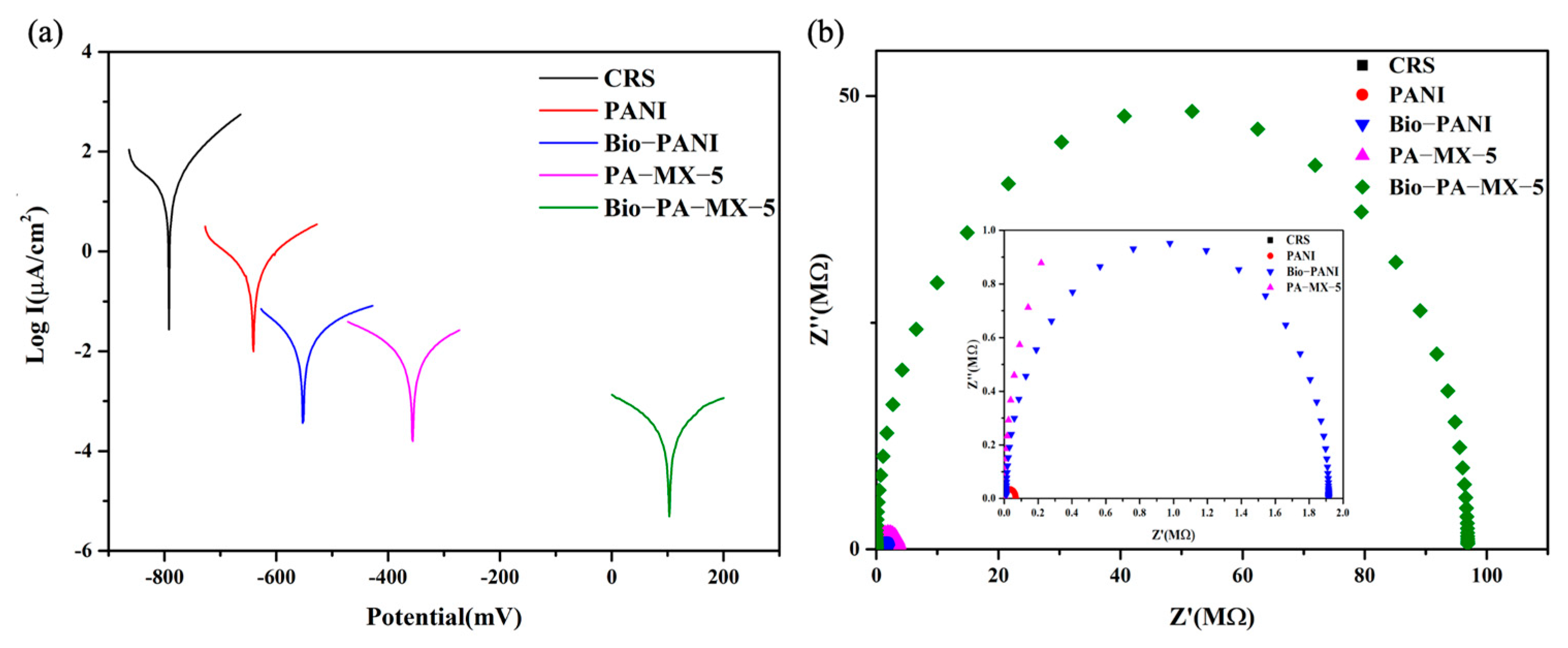
3.4.2. Corrosion Protection of Coatings with Biomimetic Surface Structure Prepared from the Nano-Casting Technique
Tafel Test
Nyquist Plots Determined by Impedance Spectroscopy (EIS)
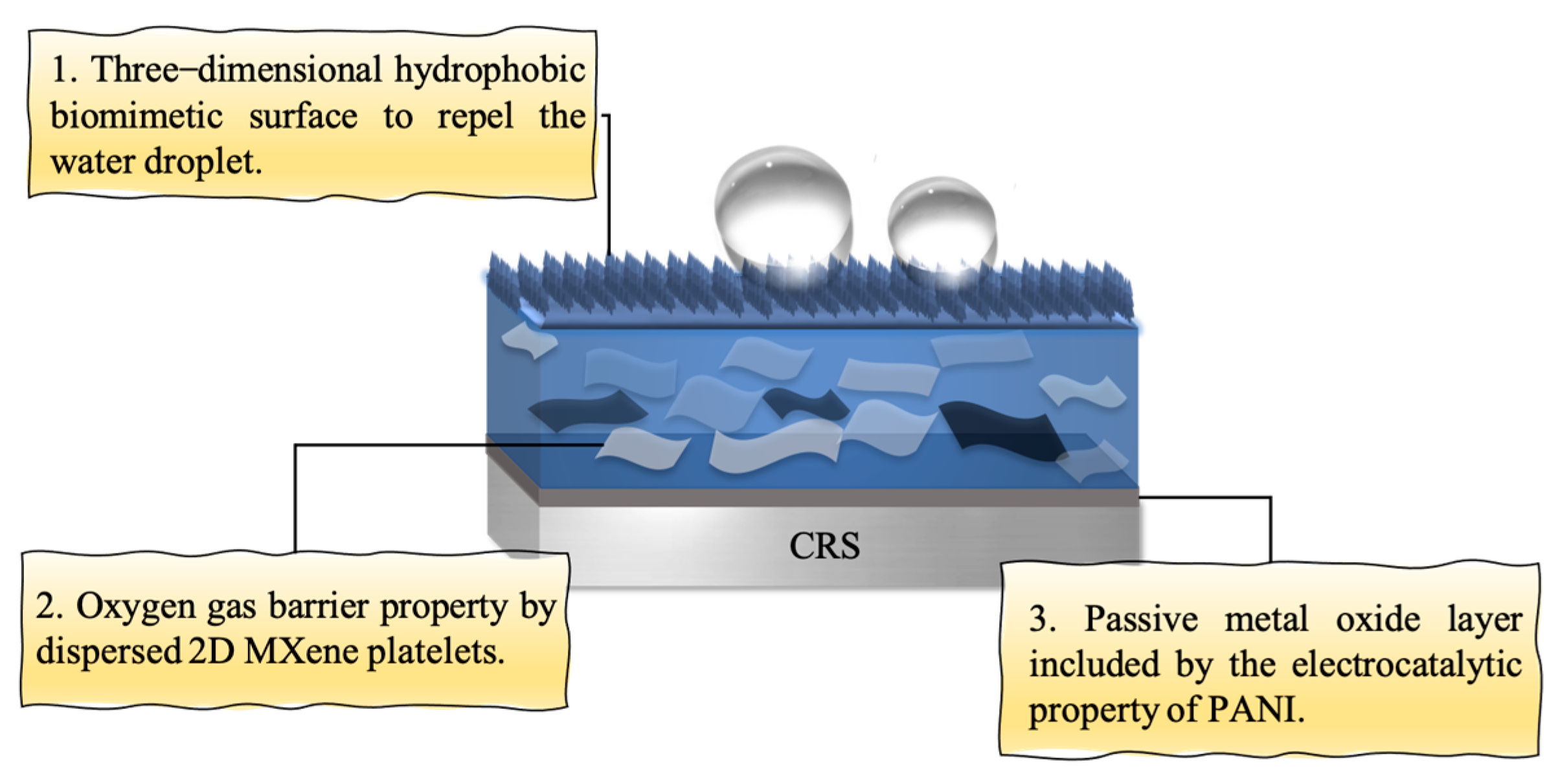
3.5. Mechanism for Enhancement of Anticorrosion of CRS Electrode Coated with Ti3C2 MXene-Based PANI Composite with Artificial Biomimetic Surface Structure
- (i)
- Electrocatalytic passive-film formation by PANI.
- (ii)
- Gas-permeability barrier from 2D MXene nanosheets.
- (iii)
- Hydrophobicity via biomimetic surface structuring.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Zhang, D.; Liu, Z.; Li, Z.; Du, C.; Dong, C. Materials science: Share corrosion data. Nature 2015, 527, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, X.G.; Du, C.W.; Cheng, Y.F. Effect of cathodic protection om corrosion of pipeline steel under disbanded coating. Corros. Sci. 2009, 51, 2242–2245. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, Q.; Li, Y.; Hou, B. Facile fluorine-free one step fabrication of superhydrophobic aluminum surface towards self-cleaning and marine anticorrosion. Chem. Eng. J. 2018, 352, 625–633. [Google Scholar] [CrossRef]
- Zhang, B.; Hu, X.; Zhu, Q.; Wang, X.; Zhao, X.; Sun, C.; Li, Y.; Hou, B. Controllable dianthus caryophyllus-like superhydrophilic/superhydrophobic hierarchical structure based on self-congregated nanowires for corrosion inhibition and biofouling mitigation. Chem. Eng. J. 2017, 312, 317–327. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Niroumandrad, S.; Almadi, A.; Mahdavian, M.; Moghadam, M.H.M. Enhancement of barrier and corrosion protection performance of an epoxy coating through wet transfer of amino functionalized graphene oxide. Corros. Sci. 2016, 103, 283–304. [Google Scholar] [CrossRef]
- Qiu, S.; Chen, C.; Cui, M.; Li, W.; Zhao, H.; Wang, L. Corrosion protection performance of waterborne epoxy coatings containing self-doped polyaniline nanofiber. Appl. Surf. Sci. 2017, 407, 213–222. [Google Scholar] [CrossRef]
- Hu, H.; Gan, M.; Ma, L.; Li, Z.; Li, Y.; Ge, C.; Tu, Y.; Yu, L.; Huang, H.; Yang, F. Synthesis and anticorrosion property of poly(2,3-dimethylaniline)/organic-attapulgite nanofibers via self-assembling and graft polymerization. Compos. Sci. Technol. 2014, 104, 9–17. [Google Scholar] [CrossRef]
- Li, J.; Feng, Q.; Cui, J.; Yuan, Q.; Qiu, H.; Gao, S.; Yang, J. Self-assembled graphene oxide microcapsules in pickering emulsions for self-healing waterborne polyurethane coatings. Compos. Sci. Technol. 2017, 151, 282–290. [Google Scholar] [CrossRef]
- Huang, Y.C.; Lo, T.Y.; Chao, C.G.; Whang, W.T. Anticorrosion characteristics of polyimide/h-boron nitride composite films with different polymer configurations. Surf. Coat. Technol. 2014, 260, 113–117. [Google Scholar] [CrossRef]
- Yeh, J.M.; Liou, S.J.; Lai, C.Y.; Wu, P.C.; Ysai, T.Y. Enhancement of corrosion protection effect in polyaniline via the formation of polyaniline-clay nanocomposite materials. Chem. Mater. 2001, 13, 1131–1136. [Google Scholar] [CrossRef]
- Hantanasirisakul, K.; Zhao, M.Q.; Urbankowski, P.; Halim, J.; Anasori, B.; Kota, S.; Ren, C.E.; Barsoum, M.W.; Gogotsi, Y. Fabrication of Ti3C2Tx MXene transparent thin films with tunable optoelectronic properties. Adv. Funct. Mat. 2016, 2, 1600050. [Google Scholar] [CrossRef]
- Zhang, C.; Kim, S.J.; Ghidiu, M.; Zhao, M.Q.; Barsoum, M.W.; Nicolosi, V.; Gogotsi, Y. Layered orthorhombic Nb2O5@Nb4C3Tx and TiO2@Ti3C2Tx hierarchical composites for high performance Li-ion batteries. Adv. Funct. Mat. 2016, 26, 4143–4151. [Google Scholar] [CrossRef]
- Li, X.; Qian, Y.; Liu, T.; Cao, F.; Zang, Z.; Sun, X.; Sun, S.; Niu, Q.; Wu, J. Enhanced lithium and electron diffusion of LiFePO4 cathode with two dimensional Ti3C2 MXene nanosheets. J. Mater. Sci. 2018, 53, 11078–11090. [Google Scholar] [CrossRef]
- Yan, H.; Li, W.; Li, H.; Fan, X.; Zhu, M. Ti3C2 MXene nanosheets towards high-performance corrosion inhibitors for epoxy coating. Prog. Org. Coat. 2019, 135, 156–167. [Google Scholar] [CrossRef]
- Cai, M.; Yan, H.; Li, Y.; Li, W.; Li, H.; Fan, X.; Zhu, M. Ti3C2Tx/PANI composites with tunable conductivity towards anticorrosion application. Chem. Eng. J. 2021, 410, 128310. [Google Scholar] [CrossRef]
- Li, C.C.; Xu, J.; Xue, G.M.; Yu, H.K.; Wang, X.T.; Lu, J.Y.; Cui, G.Z.; Gu, G.X. Synthesis of Ti3C2 MXene@PANI compoites for excellent anticorrosion performance of waterborne epoxy coating. Prog. Org. Coat. 2022, 165, 106673. [Google Scholar] [CrossRef]
- Kaewsaneha, C.; Thanankul, K.; Opaprakasit, P.; Tangboriboonrat, P.; Sreearunothai, P. Hybrid MXene (Ti3C2Tx)/polyaniline nanosheets as additives for enhancing anticorrosion properties of Zn-epoxy coating. Prog. Org. Coat. 2022, 173, 107173. [Google Scholar] [CrossRef]
- Ahadiparsa, M.; Dehghani, A.; Ramezanzadeh, B. Sulfonated polyaniline-grafted two-demensional Ti3C2-MXene (SPANI-MXene) nanoplatform for designing an advanced smart self-healable coating system. ACS Appl. Mater. Inter. 2023, 15, 24756–24768. [Google Scholar] [CrossRef] [PubMed]
- Goswarmi, R.N.; Saini, R.; Mehta, S.; Verma, G.; Ray, A.; Khatri, O.P. Ti3C2Tx MXene-polyaniline nanocomposites for redesigning epoxy coatings to improve anticorrosive performance. ACS Appl. Mater. Inter. 2024, 2, 1396–1410. [Google Scholar] [CrossRef]
- Zhu, Q.S.; Lei, X.Y.; Zha, X.Q.; Elsharkawy, E.R.; Ren, C.Y.; Chang, H.Y.; Bahy, S.M.E.; Ren, J.N.; Wang, R.J.; Bahy, Z.M.E. Polyaniline nanoparticles intercalated Ti3C2 MXene reinforced waterborne epoxy nanocomposites for electromagnetic wave absorption and anticorrosion coating applications. Compos. Part A 2025, 188, 108557. [Google Scholar] [CrossRef]
- Liao, R.; Ma, K.; Tang, S.; Liu, C.; Yue, H.; Liang, B. Biomimetic mineralization to fabricate superhydrophilic and underwater superoleophobic filter mesh for oil–water separations. Ind. Eng. Chem. Res. 2020, 59, 6226–6235. [Google Scholar] [CrossRef]
- Li, M.; Xu, Q.; Wu, X.; Li, W.; Lan, W.; Heng, L.; Street, J.; Xia, Z. Tough reversible adhesion properties of a dry self-cleaning biomimetic surface. ACS App. Mater. Interfaces 2018, 10, 26787–26794. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.W.; Wang, Z.; Feng, X.M.; Li, B.; Mu, Z.Z.; Zhang, J.Q.; Niu, S.C.; Ren, L.Q. Antireflective surface inspired from biology: A review. Biosurf. Biotribol. 2016, 2, 137–150. [Google Scholar] [CrossRef]
- Liu, D.; Ma, Z.; Zhang, W.; Huang, B.; Zhao, H.; Ren, L. Superior antiwear biomimetic artificial joint based on high-entropy alloy coating on porous Ti6Al4V. Tribol. Int. 2021, 158, 106937. [Google Scholar] [CrossRef]
- Bar-Cohen, Y. Biomimetics: Biologically Inspired Technologies; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Weng, C.J.; Chang, C.H.; Peng, C.W.; Chen, S.W.; Yeh, J.M.; Wei, Y. Advanced anticorrosive coatings prepared from the mimicked Xanthosoma Sagittifolium-leaf like electroactive epoxy with synergistic effect of super-hydrophobicity and redox catalytic capability. Chem. Mater. 2011, 23, 2075–2083. [Google Scholar] [CrossRef]
- Yang, T.I.; Peng, C.W.; Lin, Y.L.; Weng, C.J.; Edgington, G.; Mylonakis, A.; Huang, T.C.; Hsu, C.H.; Yeh, J.M.; Wei, Y. Synergistic effect of electroactivity and hydrophobicity on the anticorrosion property of room-temperature-cured epoxy coatings with multi-scale structures mimicking the surface of Xanthosoma sagittifolium leaf. J. Mater. Chem. 2012, 22, 15845–15852. [Google Scholar] [CrossRef]
- Peng, C.W.; Chang, K.C.; Weng, C.J.; Chang, C.H.; Hsu, C.H.; Li, P.L.; Hsu, C.L.; Yeh, J.M. UV-Curable nanocasting technique to prepare advanced anticorrosive coatings with bio-mimicked leaf-like non-fluorinated super-hydrophobic polymeric surfaces. Polym. Chem. 2013, 4, 926–932. [Google Scholar] [CrossRef]
- Peng, C.W.; Chang, K.C.; Weng, C.J.; Lai, M.C.; Hsu, C.H.; Hsu, S.C.; Hsu, Y.Y.; Wei, Y.; Yeh, J.M. Nano-casting technique to prepare polyaniline surface with biomimetic super-hydrophobic structures for anticorrosion application. Electrochim. Acta 2013, 95, 192–199. [Google Scholar] [CrossRef]
- Ahmed, M.M.M.; Ji, Y.T.; Hung, Y.H.; Reyes, L.M.C.; Yeh, J.M. UV-curable electro-active polyurethane acrylate coatings with super-hydrophobic surface structure of biomimetic peacock feather for anticorrosion application. Prog. Org. Coat. 2022, 165, 106679. [Google Scholar] [CrossRef]
- Chang, C.H.; Hsu, M.H.; Weng, C.J.; Peng, C.W.; Hung, W.I.; Chang, K.C.; Chuang, T.L.; Yen, Y.C.; Yeh, J.M. 3D-dioprinting approach to fabricate superhydrophobic epoxy/organophilic clay as advanced anticorrosive coatings with a synergistic effect of superhydrophobicity and gas barrier property. J. Mater. Chem. A 2013, 1, 13869–13877. [Google Scholar] [CrossRef]
- Hwang, J.J.; Li, M.X.; Balitaan, J.N.I.; Luo, K.H.; Yang, Y.Y.; Lin, S.R.; Yeh, J.M. Bioinspired superhydrophobic coatings: ZnO-based epoxy composites with the biomimetic structure of parrot feathers for anti-corrosion/anti-biofilm applications. Prog. Org. Coat. 2024, 194, 108610. [Google Scholar] [CrossRef]
- Hwang, J.J.; Chen, P.Y.; Luo, K.H.; Wang, Y.C.; Lai, T.Y.; Balitaan, J.N.I.; Lin, S.R.; Yeh, J.M. Leaf on a film: Mesoporous silica-based epoxy composites with superhydrophobic biomimetic surface structure as anti-corrosion and antibiofilm coatings. Polymers 2024, 16, 1673. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.J.; Hu, F.H.; Li, M.X.; Luo, K.H.; Liu, Y.H.; Lin, S.R.; Yeh, J.M. Superhydrophobic surface of biomass carbon-based PANI composite coatings with the biomimetic structure og goose feather for anticorrosion/antibiofilm applications. Surf. Coat. Technol. 2024, 482, 130700. [Google Scholar] [CrossRef]
- Yeh, J.M.; Liou, S.J.; Lin, C.Y.; Cheng, C.Y.; Chang, Y.W.; Lee, K.R. Anticorrosively enhanced PMMA-clay nanocomposite materials with quaternary alkylphosphonium salt as an intercalating agent. Chem. Mater. 2002, 14, 154. [Google Scholar] [CrossRef]
- Lan, Y.X.; Cho, Y.C.; Liu, W.R.; Wong, W.T.; Sun, C.F.; Yeh, J.M. Small-load rGO as partial replacement for the large amount of zinc dust in epoxy zinc-rich composites applied in heavy-duty anticorrosion coatings. Prog. Org. Coat. 2023, 175, 107332. [Google Scholar] [CrossRef]
- Qiu, M.; Zhang, Y.; Wen, B.Y. Facile synthesis of polyaniline nanostructures with effective electromagnetic interference shielding performance. J. Mater. Sci. Mater. Electron. 2018, 29, 10437–10444. [Google Scholar] [CrossRef]
- Albuquerque, J.E.D.; Mattoso, L.H.C.; Faria, R.M.; Masters, J.G.; MacDiarmid, A.G. Study of the interconversion of polyaniline oxidation states by optical absorption spectroscopy. Synth. Met. 2004, 146, 1–10. [Google Scholar] [CrossRef]
- Wessling, B. Passivation of metals by coating with polyaniline: Corrosion potential shift and morphological changes. Adv. Mater. 1994, 9, 226–228. [Google Scholar] [CrossRef]
| Sample Code | Composition Ratio | Electrochemical Corrosion Measurement | PEF (%) | Z′ (kΩ) | Oxygen Permeability (Barrer) | Thickness (µm) | |||
|---|---|---|---|---|---|---|---|---|---|
| Aniline | MXene | Ecorr (mV) | Rp (kΩcm2) | Icorr (µA/cm2) | |||||
| CRS | - | - | −791.9 | 8.71 × 10−1 | 5.74 × 10 | - | 0.0259 | - | - |
| PANI | 100 | 0 | −588.3 | 4.63 × 101 | 1.09 | 98.10 | 67 | 11.2910 | 82 ± 2 |
| PA-MX-1 | 100 | 1 | −455.2 | 5.53 × 101 | 4.73 × 10−1 | 99.17 | 258 | 6.0034 | 80 ± 2 |
| PA-MX-3 | 100 | 3 | −402.4 | 5.86 × 102 | 6.52 × 10−2 | 99.88 | 2029 | 3.0795 | 81 ± 2 |
| PA-MX-5 | 100 | 5 | −356.0 | 3.19 × 103 | 3.52 × 10−2 | 99.93 | 3794 | 0.8978 | 80 ± 1 |
| Bio-PANI | 100 | 0 | −552.0 | 1.36 × 102 | 4.76 × 10−2 | 99.91 | 1915 | - | 85 ± 5 |
| Bio-PA-MX-5 | 100 | 5 | 103.6 | 8.65 × 104 | 7.22 × 10−4 | 99.998 | 96875 | - | 85 ± 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chien, C.-C.; Liu, Y.-H.; Luo, K.-H.; Liu, T.-Y.; Kao, Y.-T.; Yang, S.-H.; Yeh, J.-M. Polyaniline/Ti3C2 MXene Composites with Artificial 3D Biomimetic Surface Structure of Natural Macaw Feather Applied for Anticorrosion Coatings. Biomimetics 2025, 10, 465. https://doi.org/10.3390/biomimetics10070465
Chien C-C, Liu Y-H, Luo K-H, Liu T-Y, Kao Y-T, Yang S-H, Yeh J-M. Polyaniline/Ti3C2 MXene Composites with Artificial 3D Biomimetic Surface Structure of Natural Macaw Feather Applied for Anticorrosion Coatings. Biomimetics. 2025; 10(7):465. https://doi.org/10.3390/biomimetics10070465
Chicago/Turabian StyleChien, Chen-Cheng, Yu-Hsuan Liu, Kun-Hao Luo, Ting-Yun Liu, Yi-Ting Kao, Shih-Harn Yang, and Jui-Ming Yeh. 2025. "Polyaniline/Ti3C2 MXene Composites with Artificial 3D Biomimetic Surface Structure of Natural Macaw Feather Applied for Anticorrosion Coatings" Biomimetics 10, no. 7: 465. https://doi.org/10.3390/biomimetics10070465
APA StyleChien, C.-C., Liu, Y.-H., Luo, K.-H., Liu, T.-Y., Kao, Y.-T., Yang, S.-H., & Yeh, J.-M. (2025). Polyaniline/Ti3C2 MXene Composites with Artificial 3D Biomimetic Surface Structure of Natural Macaw Feather Applied for Anticorrosion Coatings. Biomimetics, 10(7), 465. https://doi.org/10.3390/biomimetics10070465








