Tissue-Engineered Tracheal Reconstruction
Abstract
1. Introduction
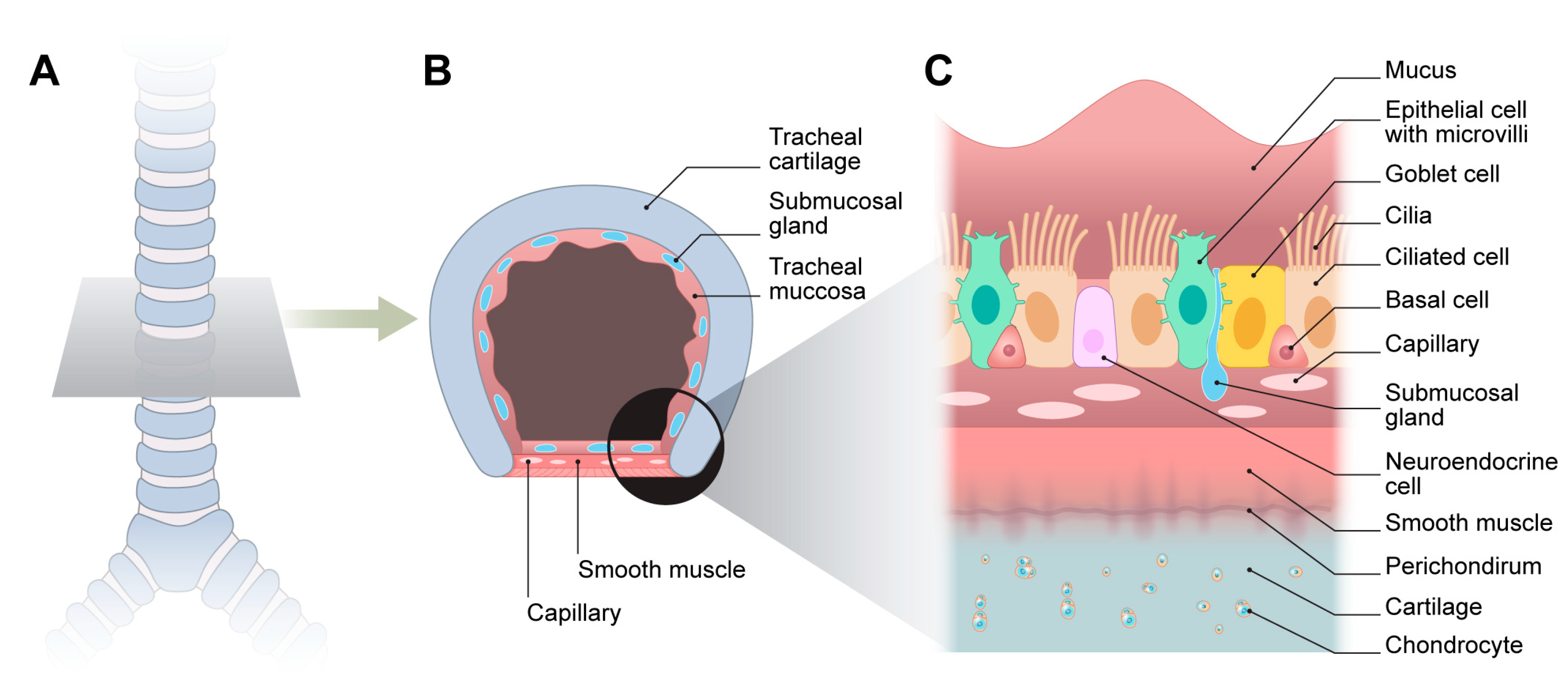
2. Normal Trachea Structure and Physiology
2.1. Mechanical Properties
2.2. Respiratory Epithelium
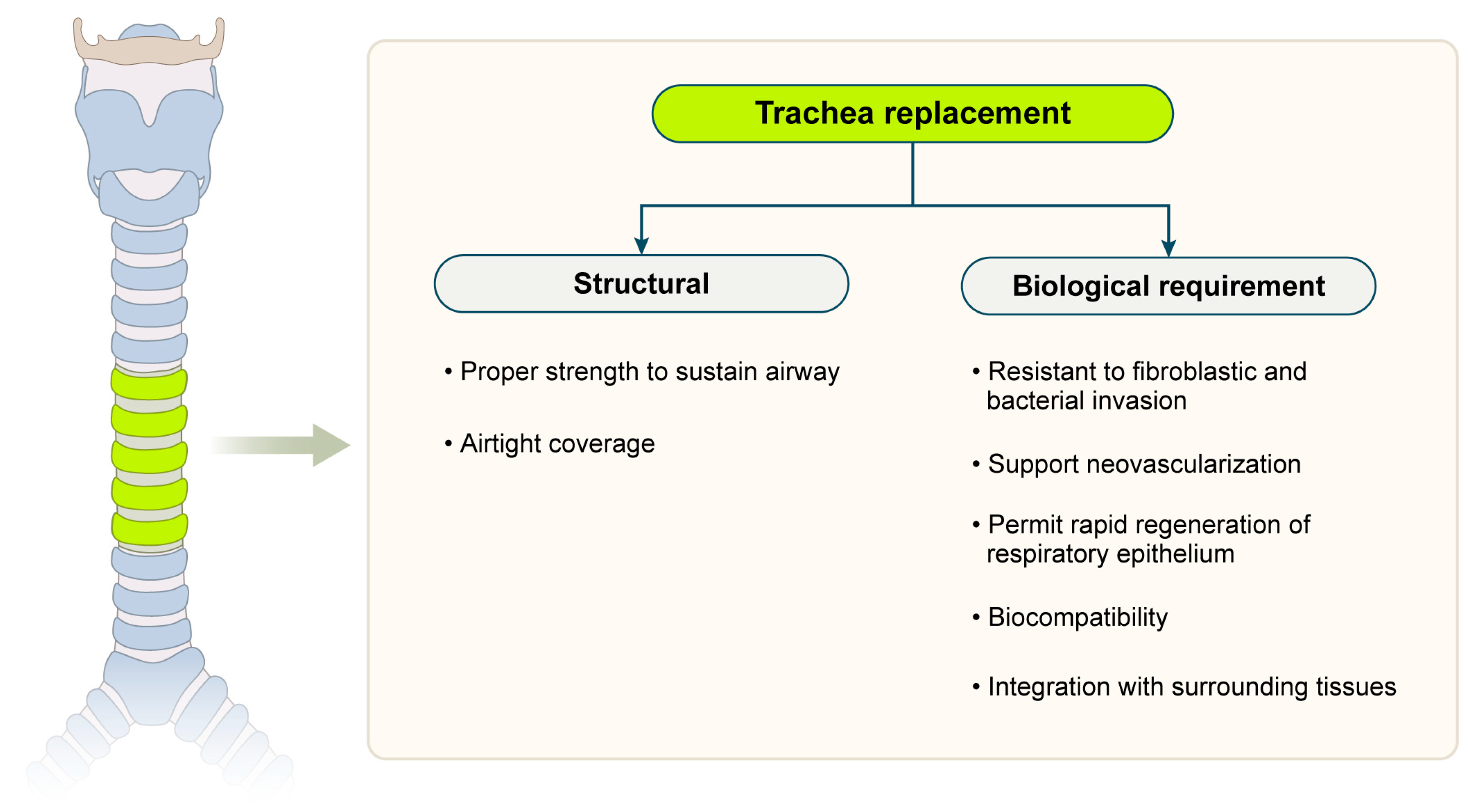
3. Scaffold Strategies
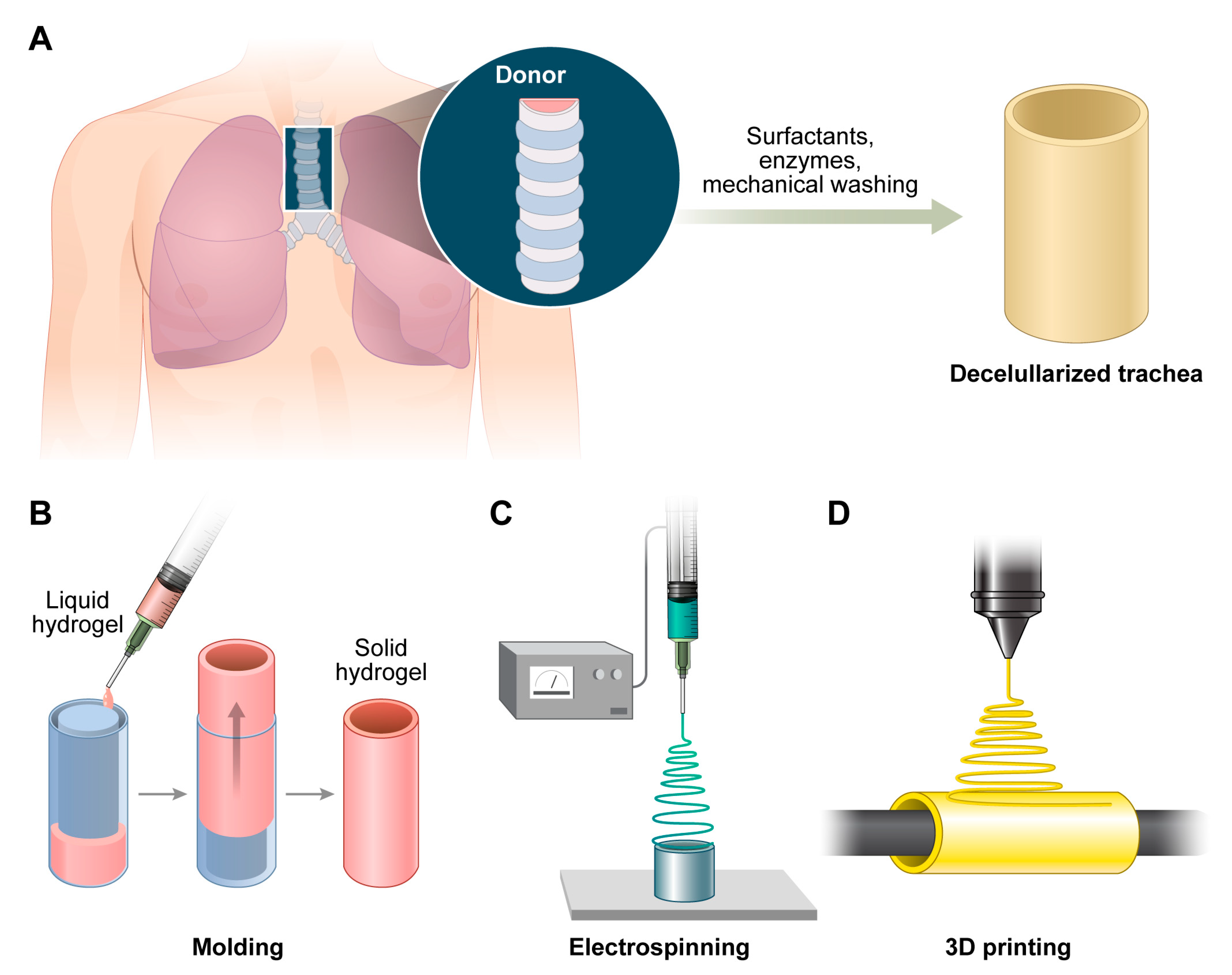
3.1. Decellularized Trachea
3.2. Synthetic Scaffold
3.2.1. Materials
3.2.2. Molding/Electrospinning/3D-Printed Scaffolds
3.2.3. Others
4. Cell Strategy
4.1. Cell Types
4.2. ALI Culture
4.3. Bioreactor Preconditioning
4.3.1. In Vitro Bioreactor
4.3.2. In Vivo Bioreactor
4.4. Cell-Sheet/Spheroid Fusion/Tissue-Strand Techniques
4.4.1. Cell-Sheet Technique
4.4.2. Spheroid Fusion Technique
4.4.3. Tissue-Strand Technique
5. Clinical Application
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Atala, A. Regenerative medicine strategies. J. Pediatr. Surg. 2012, 47, 17–28. [Google Scholar] [CrossRef]
- Martin, I.; Wendt, D.; Heberer, M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004, 22, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H. Bioengineering a human airway using autologous stem cells. Sci. Transl. Med. 2015, 7, 285ra263. [Google Scholar]
- Delaere, P.R.; Van Raemdonck, D. The trachea: The first tissue-engineered organ? J. Thorac. Cardiovasc. Surg. 2014, 147, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Grillo, H.C. Tracheal replacement: A critical review. Ann. Thorac. Surg. 2002, 73, 1995–2004. [Google Scholar] [CrossRef]
- Park, J.H.; Hong, J.M.; Ju, Y.M.; Jung, J.W.; Kang, H.W.; Lee, S.J.; Yoo, J.J.; Kim, S.W.; Kim, S.H.; Cho, D.W. A novel tissue-engineered trachea with a mechanical behavior similar to native trachea. Biomaterials 2015, 62, 106–115. [Google Scholar] [CrossRef]
- Batioglu-Karaaltin, A.; Ovali, E.; Karaaltin, M.V.; Yener, M.; Yilmaz, M.; Eyupoglu, F.; Yilmaz, Y.Z.; Bozkurt, E.R.; Demir, N.; Konuk, E.; et al. Decellularization of Trachea With Combined Techniques for Tissue-Engineered Trachea Transplantation. Clin. Exp. Otorhinolaryngol. 2019, 12, 86–94. [Google Scholar] [CrossRef]
- Haykal, S.; Salna, M.; Zhou, Y.; Marcus, P.; Fatehi, M.; Frost, G.; Machuca, T.; Hofer, S.O.; Waddell, T.K. Double-chamber rotating bioreactor for dynamic perfusion cell seeding of large-segment tracheal allografts: Comparison to conventional static methods. Tissue Eng. Part C Methods 2014, 20, 681–692. [Google Scholar] [CrossRef]
- Kojima, K.; Vacanti, C.A. Tissue engineering in the trachea. Anat. Rec. 2014, 297, 44–50. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, Y.; Luo, F.; Duan, K.; Li, M.; Lv, G. Tissue-engineered tracheal implants: Advancements, challenges, and clinical considerations. Bioeng. Transl. Med. 2024, 9, e10671. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Jiang, Y.; Xu, Y.; Shi, H.; Zhang, S.; Liu, X.; Pan, S.; Ye, G.; Zhang, W.; Zhang, F.; et al. Genipin cross-linked decellularized tracheal tubular matrix for tracheal tissue engineering applications. Sci. Rep. 2016, 6, 24429. [Google Scholar] [CrossRef] [PubMed]
- Khalid, T.; Soriano, L.; Lemoine, M.; Cryan, S.A.; O’Brien, F.J.; O’Leary, C. Development of tissue-engineered tracheal scaffold with refined mechanical properties and vascularisation for tracheal regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1187500. [Google Scholar] [CrossRef]
- Sun, F.; Shan, Y.; Pan, S.; Lu, Y.; Shen, Z.; Zhu, J.; Yuan, L.; Wang, Q.; Chen, W.; Chen, H.; et al. In situ vascularization and epithelialization of segmental bioengineered trachea based on marrow-derived stem/progenitor cells. Mater. Today Bio 2025, 33, 101990. [Google Scholar] [CrossRef]
- Mortola, J.P.; Sant’Ambrogio, G. Mechanics of the trachea and behaviour of its slowly adapting stretch receptors. J. Physiol. 1979, 286, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Furlow, P.W.; Mathisen, D.J. Surgical anatomy of the trachea. Ann. Cardiothorac. Surg. 2018, 7, 255–260. [Google Scholar] [CrossRef]
- Minnich, D.J.; Mathisen, D.J. Anatomy of the trachea, carina, and bronchi. Thorac. Surg. Clin. 2007, 17, 571–585. [Google Scholar] [CrossRef]
- Van Scott, M.R.; Chandler, J.; Olmstead, S.; Brown, J.M.; Mannie, M. Airway Anatomy, Physiology, and Inflammation. In The Toxicant Induction of Irritant Asthma, Rhinitis, and Related Conditions; Springer: New York, NY, USA, 2013; pp. 19–61. [Google Scholar] [CrossRef]
- Walles, T.; Giere, B.; Hofmann, M.; Schanz, J.; Hofmann, F.; Mertsching, H.; Macchiarini, P. Experimental generation of a tissue-engineered functional and vascularized trachea. J. Thorac. Cardiovasc. Surg. 2004, 128, 900–906. [Google Scholar] [CrossRef][Green Version]
- Safshekan, F.; Tafazzoli-Shadpour, M.; Abdouss, M.; Shadmehr, M.B. Mechanical Characterization and Constitutive Modeling of Human Trachea: Age and Gender Dependency. Materials 2016, 9, 456. [Google Scholar] [CrossRef]
- Grillo, H.C. Development of tracheal surgery: A historical review. Part 1: Techniques of tracheal surgery. Ann. Thorac. Surg. 2003, 75, 610–619. [Google Scholar] [CrossRef]
- Belsey, R. Resection and reconstruction of the intrathoracic trachea. Br. J. Surg. 1950, 38, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Neville, W.E.; Bolanowski, P.J.P.; Kotia, G.G. Clinical experience with the silicone tracheal prosthesis. J. Thorac. Cardiovasc. Surg. 1990, 99, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Rock, J.R.; Randell, S.H.; Hogan, B.L.M. Airway basal stem cells: A perspective on their roles in epithelial homeostasis and remodeling. Dis. Model. Mech. 2010, 3, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Hiemstra, P.S.; McCray, P.B., Jr.; Bals, R. The innate immune function of airway epithelial cells in inflammatory lung disease. Eur. Respir. J. 2015, 45, 1150–1162. [Google Scholar] [CrossRef]
- Huang, S.X.L.; Islam, M.N.; O’Neill, J.; Hu, Z.; Yang, Y.G.; Chen, Y.W.; Mumau, M.; Green, M.D.; Vunjak-Novakovic, G.; Bhattacharya, J.; et al. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat. Biotechnol. 2014, 32, 84–91. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Kapat, K.; Gondane, P.; Kumbhakarn, S.; Takle, S.; Sable, R. Challenges and opportunities in developing tracheal substitutes for the recovery of long-segment defects. Macromol. Biosci. 2024, 24, 2400054. [Google Scholar] [CrossRef]
- Park, J.H.; Park, J.Y.; Nam, I.C.; Ahn, M.; Lee, J.Y.; Choi, S.H.; Kim, S.W.; Cho, D.W. A rational tissue engineering strategy based on three-dimensional (3D) printing for extensive circumferential tracheal reconstruction. Biomaterials 2018, 185, 276–283. [Google Scholar] [CrossRef]
- Mammana, M.; Bonis, A.; Verzeletti, V.; Dell’amore, A.; Rea, F. Tracheal Tissue Engineering: Principles and State of the Art. Bioengineering 2024, 11, 198. [Google Scholar] [CrossRef]
- Petersen, T.H.; Calle, E.A.; Zhao, L.; Lee, E.J.; Gui, L.; Raredon, M.B.; Gavrilov, K.; Yi, T.; Zhuang, Z.W.; Breuer, C.; et al. Tissue-engineered lungs for in vivo implantation. Science 2010, 329, 538–541. [Google Scholar] [CrossRef]
- Ott, H.C.; Clippinger, B.; Conrad, C.; Schuetz, C.; Pomerantseva, I.; Ikonomou, L.; Kotton, D.; Vacanti, J.P. Regeneration and orthotopic transplantation of a bioartificial lung. Nat. Med. 2010, 16, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Zopf, D.A.; Hollister, S.; Nelson, M.E.; Ohye, R.G.; Green, G.E. Bioresorbable airway splint created with a three-dimensional printer. N. Engl. J. Med. 2013, 368, 2043–2045. Available online: https://www.nejm.org/doi/10.1056/NEJMc1206319 (accessed on 7 July 2025). [CrossRef]
- Kobayashi, K.; Suzuki, T.; Nomoto, Y.; Tada, Y.; Miyake, M.; Hazama, A.; Wada, I.; Nakamura, T.; Omori, K. A tissue-engineered trachea derived from a framed collagen scaffold, gingival fibroblasts and adipose-derived stem cells. Biomaterials 2010, 31, 4855–4863. [Google Scholar] [CrossRef] [PubMed]
- Crowley, C.; Birchall, M.; Seifalian, A.M. Trachea transplantation: From laboratory to patient. J. Tissue Eng. Regen. Med. 2015, 9, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.M.; Lowdell, M.W.; Birchall, M.A. Stem cell-based organ replacements—Airways and lungs. Tissue Eng. Part. B Rev. 2014, 20, 164–172. [Google Scholar]
- Park, S.H. Biofabrication of multi-layered tracheal tissue constructs using cell-laden bioinks. Biofabrication 2020, 12, 035029. Available online: https://iopscience.iop.org/article/10.1088/1758-5090/ab7e02 (accessed on 7 July 2025).
- Kojima, K.; Bonassar, L.J.; Roy, A.K.; Mizuno, H.; Cortiella, J.; Vacanti, C.A. A composite tissue-engineered trachea using sheep nasal chondrocyte and epithelial cells. FASEB J. 2003, 17, 823–828. [Google Scholar] [CrossRef]
- Taniguchi, D.; Matsumoto, K.; Tsuchiya, T.; Machino, R.; Takeoka, Y.; Elgalad, A.; Gunge, K.; Takagi, K.; Taura, Y.; Hatachi, G.; et al. Scaffold-free trachea regeneration by tissue engineering with bio-3D printing. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 745–752. [Google Scholar] [CrossRef]
- Virk, J.S.; Zhang, H.; Nouraei, R.; Sandhu, G. Prosthetic reconstruction of the trachea: A historical perspective. World J. Clin. Cases 2017, 5, 128–133. [Google Scholar] [CrossRef]
- Serrano-Aroca, A.; Cano-Vicent, A.; Serra, R.S.I.; El-Tanani, M.; Aljabali, A.; Tambuwala, M.M.; Mishra, Y.K. Scaffolds in the microbial resistant era: Fabrication, materials, properties and tissue engineering applications. Mater. Today Bio 2022, 16, 100412. [Google Scholar] [CrossRef]
- Liu, W.; Thomopoulos, S.; Xia, Y. Electrospun nanofibers for regenerative medicine. Adv Healthc Mater 2012, 1, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Galliger, Z.; Vogt, C.D.; Panoskaltsis-Mortari, A. 3D bioprinting for lungs and hollow organs. Transl. Res. 2019, 211, 19–34. [Google Scholar] [CrossRef]
- Griffin, K.H.; Fok, S.W.; Kent Leach, J. Strategies to capitalize on cell spheroid therapeutic potential for tissue repair and disease modeling. NPJ Regen. Med. 2022, 7, 70. [Google Scholar] [CrossRef]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.C.; Reid, R.R. 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Marga, F.; Jakab, K.; Khatiwala, C.; Shepherd, B.; Dorfman, S.; Hubbard, B.; Colbert, S.; Gabor, F. Toward engineering functional organ modules by additive manufacturing. Biofabrication 2012, 4, 022001. [Google Scholar] [CrossRef]
- Itoh, M.; Nakayama, K.; Noguchi, R.; Kamohara, K.; Furukawa, K.; Uchihashi, K.; Toda, S.; Oyama, J.; Node, K.; Morita, S. Scaffold-Free Tubular Tissues Created by a Bio-3D Printer Undergo Remodeling and Endothelialization when Implanted in Rat Aortae. PLoS ONE 2015, 10, e0136681. [Google Scholar] [CrossRef]
- Kanzaki, M.; Yamato, M.; Hatakeyama, H.; Kohno, C.; Yang, J.; Umemoto, T.; Kikuchi, A.; Okano, T.; Onuki, T. Tissue engineered epithelial cell sheets for the creation of a bioartificial trachea. Tissue Eng. 2006, 12, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.J.; De Coppi, P.; Speggiorin, S.; Roebuck, D.; Butler, C.R.; Samuel, E.; Crowley, C.; McLaren, C.; Fierens, A.; Vondrys, D.; et al. Stem-cell-based, tissue engineered tracheal replacement in a child: A 2-year follow-up study. Lancet 2012, 380, 994–1000. [Google Scholar] [CrossRef]
- Go, T.; Jungebluth, P.; Baiguero, S.; Asnaghi, A.; Martorell, J.; Ostertag, H.; Mantero, S.; Birchall, M.; Bader, A.; Macchiarini, P. Both epithelial cells and mesenchymal stem cell-derived chondrocytes contribute to the survival of tissue-engineered airway transplants in pigs. J. Thorac. Cardiovasc. Surg. 2010, 139, 437–443. [Google Scholar] [CrossRef]
- Fulcher, M.L.; Randell, S.H. Human nasal and tracheo-bronchial respiratory epithelial cell culture. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 945, pp. 109–121. [Google Scholar] [CrossRef]
- Pezzulo, A.A.; Starner, T.D.; Scheetz, T.E.; Traver, G.L.; Tilley, A.E.; Harvey, B.G.; Crystal, R.G.; McCray, P.B., Jr.; Zabner, J. The air-liquid interface and use of primary cell cultures are important to recapitulate the transcriptional profile of in vivo airway epithelia. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 300, L25–L31. [Google Scholar] [CrossRef]
- Luengen, A.E.; Kniebs, C.; Buhl, E.M.; Cornelissen, C.G.; Schmitz-Rode, T.; Jockenhoevel, S.; Thiebes, A.L. Choosing the Right Differentiation Medium to Develop Mucociliary Phenotype of Primary Nasal Epithelial Cells In Vitro. Sci. Rep. 2020, 10, 6963. [Google Scholar] [CrossRef] [PubMed]
- Kelly, N.A.; Shontz, K.M.; Bergman, M.; Manning, A.M.; Reynolds, S.D.; Chiang, T. Biobanked tracheal basal cells retain the capacity to differentiate. Laryngoscope Investig. Otolaryngol. 2022, 7, 2119–2125. [Google Scholar] [CrossRef] [PubMed]
- Baldassi, D.; Gabold, B.; Merkel, O.M. Air−liquid interface cultures of the healthy and diseased human respiratory tract: Promises, challenges and future directions. Adv. NanoBiomed Res. 2021, 1, 2000111. [Google Scholar] [CrossRef]
- Dvorak, N.; Liu, Z.; Mouthuy, P.A. Soft bioreactor systems: A necessary step toward engineered MSK soft tissue? Front. Robot. AI 2024, 11, 1287446. [Google Scholar] [CrossRef]
- Weiss, D.J.; Bertoncello, I.; Borok, Z.; Kim, C.; Panoskaltsis-Mortari, A.; Reynolds, S.; Rojas, M.; Stripp, B.; Warburton, D.; Prockop, D.J. Stem cells and cell therapies in lung biology and lung diseases. Proc. Am. Thorac. Soc. 2011, 8, 223–272. [Google Scholar] [CrossRef] [PubMed]
- Chouinard, J.A.; Gagnon, S.; Couture, M.G.; Levesque, A.; Vermette, P. Design and validation of a pulsatile perfusion bioreactor for 3D high cell density cultures. Biotechnol. Bioeng. 2009, 104, 1215–1223. [Google Scholar] [CrossRef]
- Asnaghi, M.A.; Jungebluth, P.; Raimondi, M.T.; Dickinson, S.C.; Rees, L.E.; Go, T.; Cogan, T.A.; Dodson, A.; Parnigotto, P.P.; Hollander, A.P.; et al. A double-chamber rotating bioreactor for the development of tissue-engineered hollow organs: From concept to clinical trial. Biomaterials 2009, 30, 5260–5269. [Google Scholar] [CrossRef]
- Bae, S.W.; Lee, K.W.; Park, J.H.; Lee, J.; Jung, C.R.; Yu, J.; Kim, H.Y.; Kim, D.H. 3D Bioprinted Artificial Trachea with Epithelial Cells and Chondrogenic-Differentiated Bone Marrow-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2018, 19, 1624. [Google Scholar] [CrossRef]
- Yang, M.; Chen, J.; Chen, Y.; Lin, W.; Tang, H.; Fan, Z.; Wang, L.; She, Y.; Jin, F.; Zhang, L.; et al. Scaffold-Free Tracheal Engineering via a Modular Strategy Based on Cartilage and Epithelium Sheets. Adv. Healthc. Mater. 2023, 12, e2302076. [Google Scholar] [CrossRef]
- Ruszymah, B.H.I.; Chua, K.; Latif, M.A.; Hussein, F.N.; Saim, A.B. Formation of in vivo tissue engineered human hyaline cartilage in the shape of a trachea with internal support. Int. J. Pediatr. Otorhinolaryngol. 2005, 69, 1489–1495. [Google Scholar] [CrossRef]
- Hiwatashi, S.; Iwai, R.; Nakayama, Y.; Moriwaki, T.; Okuyama, H. Successful tracheal regeneration using biofabricated autologous analogues without artificial supports. Sci. Rep. 2022, 12, 20279. [Google Scholar] [CrossRef] [PubMed]
- Kushida, A.; Yamato, M.; Konno, C.; Kikuchi, A.; Sakurai, Y.; Okano, T. Decrease in culture temperature releases monolayer endothelial cell sheets together with deposited fibronectin matrix from temperature-responsive culture surfaces. J. Biomed. Mater. Res. 1999, 45, 355–362. [Google Scholar] [CrossRef]
- Patel, N.G.; Zhang, G. Responsive systems for cell sheet detachment. Organogenesis 2013, 9, 93–100. [Google Scholar] [CrossRef]
- Hu, D.; Gao, C.; Li, J.; Tong, P.; Sun, Y. The preparation methods and types of cell sheets engineering. Stem Cell Res. Ther. 2024, 15, 326. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.; Hong, J.; Hwangbo, H.; Kim, G. The utility of biomedical scaffolds laden with spheroids in various tissue engineering applications. Theranostics 2021, 11, 6818–6832. [Google Scholar] [CrossRef]
- Uchida, F.; Matsumoto, K.; Nishimuta, M.; Matsumoto, T.; Oishi, K.; Hara, R.; Machino, R.; Taniguchi, D.; Oyama, S.; Moriyama, M.; et al. Fabrication of a three-dimensional scaffold-free trachea with horseshoe-shaped hyaline cartilage. Eur. J. Cardio-Thorac. Surg. 2024, 66, ezae336. [Google Scholar] [CrossRef]
- Guillaume, O.; Kopinski-Grunwald, O.; Weisgrab, G.; Baumgartner, T.; Arslan, A.; Whitmore, K.; Van Vlierberghe, S.; Ovsianikov, A. Hybrid spheroid microscaffolds as modular tissue units to build macro-tissue assemblies for tissue engineering. Acta Biomater. 2023, 165, 72–85. [Google Scholar] [CrossRef]
- Yu, Y.; Moncal, K.K.; Li, J.; Peng, W.; Rivero, I.; Martin, J.A.; Ozbolat, I.T. Three-dimensional bioprinting using self-assembling scalable scaffold-free “tissue strands” as a new bioink. Sci. Rep. 2016, 6, 28714. [Google Scholar] [CrossRef]
- Machino, R.; Matsumoto, K.; Taniguchi, D.; Tsuchiya, T.; Takeoka, Y.; Taura, Y.; Moriyama, M.; Tetsuo, T.; Oyama, S.; Takagi, K.; et al. Replacement of Rat Tracheas by Layered, Trachea-Like, Scaffold-Free Structures of Human Cells Using a Bio-3D Printing System. Adv. Healthc. Mater. 2019, 8, e1800983. [Google Scholar] [CrossRef]
- Omori, K.; Nakamura, T.; Kanemaru, S.; Asato, R.; Yamashita, M.; Tanaka, S.; Magrufov, A.; Ito, J.; Shimizu, Y. Regenerative medicine of the trachea: The first human case. Ann. Otol. Rhinol. Laryngol. 2005, 114, 429–433. [Google Scholar] [CrossRef]
- Hamilton, N.J.; Kanani, M.; Roebuck, D.J.; Hewitt, R.J.; Cetto, R.; Culme-Seymour, E.J.; Toll, E.; Bates, A.J.; Comerford, A.P.; McLaren, C.A.; et al. Tissue-Engineered Tracheal Replacement in a Child: A 4-Year Follow-Up Study. Am. J. Transplant. 2015, 15, 2750–2757. [Google Scholar] [CrossRef] [PubMed]
- A Phase I Open-label Study to Assess the Safety, Tolerability and Potential Efficacy of a Novel Tracheal Replacement Consisting of a Tissue-engineered Decellularised Tracheal Scaffold With Seeded Autologous Mesenchymal Cells in Subjects With Severe Tracheal Stenosis or Malacia. Available online: https://clinicaltrials.gov/study/NCT02949414 (accessed on 7 July 2025).
- Martinod, E.; Chouahnia, K.; Radu, D.M.; Joudiou, P.; Uzunhan, Y.; Bensidhoum, M.; Santos Portela, A.M.; Guiraudet, P.; Peretti, M.; Destable, M.D.; et al. Feasibility of Bioengineered Tracheal and Bronchial Reconstruction Using Stented Aortic Matrices. JAMA 2018, 319, 2212–2222. [Google Scholar] [CrossRef]
- Development of the Practical Usage Based Technology Using the Patient Customized Bioprinting Trachea for the Regeneration of Respiratory Tract (Trachea). Available online: https://clinicaltrials.gov/study/NCT06051747 (accessed on 7 July 2025).
- Les, A.S.; Ohye, R.G.; Filbrun, A.G.; Ghadimi Mahani, M.; Flanagan, C.L.; Daniels, R.C.; Kidwell, K.M.; Zopf, D.A.; Hollister, S.J.; Green, G.E. 3D-printed, externally-implanted, bioresorbable airway splints for severe tracheobronchomalacia. Laryngoscope 2019, 129, 1763–1771. [Google Scholar] [CrossRef]
- Goh, C.S.; Joethy, J.V.; Tan, B.K.; Wong, M. Large animal models for long-segment tracheal reconstruction: A systematic review. J. Surg. Res. 2018, 231, 140–153. [Google Scholar] [CrossRef]
- Monteiro, A.; Smith, R.L. Bronchial tree Architecture in Mammals of Diverse Body Mass. Int. J. Morphol. 2014, 32, 312–316. [Google Scholar] [CrossRef]
- Dabanoglu, I.; Ocal, M.K.; Kara, M.E. A quantitative study on the trachea of the dog. Anat. Histol. Embryol. 2001, 30, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Drevet, G.; Conti, M.; Deslauriers, J. Surgical anatomy of the tracheobronchial tree. J. Thorac. Dis. 2016, 8, S121–S129. [Google Scholar] [CrossRef]
- Tomkiewicz, R.P.; Albers, G.M.; Ramirez, O.E.; Rubin, B.K.; De Sanctis, G.T.; King, M. Species differences in the physical and transport properties of airway secretions. Can. J. Physiol. Pharmacol. 1995, 73, 165–171. [Google Scholar] [CrossRef]
- Yahaya, B. Understanding cellular mechanisms underlying airway epithelial repair: Selecting the most appropriate animal models. Sci. World J. 2012, 2012, 961684. [Google Scholar] [CrossRef]
- Shai, S.E.; Lai, Y.L.; Hung, Y.W.; Hsieh, C.W.; Su, K.C.; Wang, C.H.; Chao, T.H.; Chiu, Y.T.; Wu, C.C.; Hung, S.C. Long-Term Survival and Regeneration Following Transplantation of 3D-Printed Biodegradable PCL Tracheal Grafts in Large-Scale Porcine Models. Bioengineering 2024, 11, 832. [Google Scholar] [CrossRef]
- Elliott, M.J.; Butler, C.R.; Varanou-Jenkins, A.; Partington, L.; Carvalho, C.; Samuel, E.; Crowley, C.; Lange, P.; Hamilton, N.J.; Hynds, R.E.; et al. Tracheal Replacement Therapy with a Stem Cell-Seeded Graft: Lessons from Compassionate Use Application of a GMP-Compliant Tissue-Engineered Medicine. Stem Cells Transl. Med. 2017, 6, 1458–1464. [Google Scholar] [CrossRef] [PubMed]

| Scaffold Type | Material Example | Biological and Mechanical Properties | Limitations | Clinical/Preclinical Use |
|---|---|---|---|---|
| Natural | Collagen |
|
| Used in early-stage research and animal models [28,34] |
| Fibrin |
|
| Tested for short-segment tracheal repair [28] | |
| Hyaluronic Acid |
|
| Tested in composite scaffolds using animal models [37,38] | |
| Synthetic | PCL (Polycaprolactone) |
|
| Used in pediatric airway stents and clinical trials [29,33,35] |
| PLGA (Poly (lactic-co-glycolic acid)) |
|
| Preclinical animal studies ongoing [28,34,35] | |
| Hybrid | PCL + ECM proteins |
|
| Promising in large- animal models, under study [29,34,35,36,39] |
| Trial | P.I./Institution | Purpose | Approach | Status | Outcomes |
|---|---|---|---|---|---|
| INSPIRE: Phase I Tracheal Replacement Using Seeded Decellularised Scaffold (NCT02949414) [75] | Birchall, M./ University College, London | Evaluate TETG safety in severe stenosis/malacia | Decellularized donor trachea + MSCs + biodegradable stent | Phase I started in 2016, suspended early due to complications | Initial mucosal recovery, high stenosis/granulation halted trial |
| TRITON-01: Airway Replacement with Stented Aortic Matrices (NCT04263129) [76] | Martinod, E./ Avicenne Hospital, Assistance Publique–Hôpitaux de Paris | Assess long-term airway graft integration | Cryo-decell aortic graft + external silicone stent (no recellularization) | Completed (2019–2022, 35 patients) | 0% 30-day mortality; >80% R0 resection; integration with manageable issues |
| Patient-Customized Bioprinting Technology for Practical Regeneration of the Respiratory Tract (Trachea) (NCT06051747) [77] | Bae, J. S./ Catholic University of Korea, Seoul St. Mary’s Hospital | The pilot of patient-specific 3D bioprinted trachea | Three-dimensional printed scaffold with autologous chondrocytes/epithelial cells | Active (pilot Aug 2023); 6-mo follow-up completed | Good graft integration and patency at 6 months |
| Bioresorbable Airway Splint Pivotal Clinical Trial (NCT06406452) [78] | Green, G. E./ C.S. Mott Children’s Hospital, University of Michigan | Evaluate PCL bioresorbable airway splint in infants with severe TBM | Three-dimensional printed PCL external splint, custom-fit | Ongoing (2025–), 8-year follow-up planned in 35 pediatric patients | Compassionate-use cases showed functional restoration and safe biodegradation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeou, S.H.; Shin, Y.S. Tissue-Engineered Tracheal Reconstruction. Biomimetics 2025, 10, 457. https://doi.org/10.3390/biomimetics10070457
Yeou SH, Shin YS. Tissue-Engineered Tracheal Reconstruction. Biomimetics. 2025; 10(7):457. https://doi.org/10.3390/biomimetics10070457
Chicago/Turabian StyleYeou, Se Hyun, and Yoo Seob Shin. 2025. "Tissue-Engineered Tracheal Reconstruction" Biomimetics 10, no. 7: 457. https://doi.org/10.3390/biomimetics10070457
APA StyleYeou, S. H., & Shin, Y. S. (2025). Tissue-Engineered Tracheal Reconstruction. Biomimetics, 10(7), 457. https://doi.org/10.3390/biomimetics10070457






