Abstract
Human adipose stem cells (ASCs) are multipotent cells expressing mesenchymal stem cell (MSC) markers that are capable of multilineage differentiation and secretion of bioactive factors. Their “homing” to injured tissues is mediated by chemokines, cytokines, adhesion molecules, and signaling pathways. Enhancing ASC homing is critical for improving regenerative therapies. Strategies include boosting chemotactic signaling, modulating immune responses to create a supportive environment, preconditioning ASCs with hypoxia or mechanical stimuli, co-culturing with supportive cells, applying surface modifications or genetic engineering, and using biomaterials to promote ASC recruitment, retention, and integration at injury sites. Scaffolds provide structural support and a biomimetic environment for ASC-based tissue regeneration. Natural scaffolds promote adhesion and differentiation but have mechanical limitations, while synthetic scaffolds offer tunable properties and controlled degradation. Functionalization with bioactive molecules improves the regenerative outcomes of different tissue types. Ceramic-based scaffolds, due to their strength and bioactivity, are ideal for bone healing. Composite scaffolds, combining polymers, ceramics, or metals, further optimize mechanical and biological properties, supporting personalized regenerative therapies. This review integrates concepts from cell biology, biomaterials science, and regenerative medicine to offer a comprehensive understanding of ASC homing and its impact on tissue engineering and clinical applications.
1. Introduction
Regenerative medicine is an interdisciplinary field that focuses on repairing, replacing, or regenerating aged, disease-affected, or damaged tissues and organs by leveraging the body’s natural healing mechanisms. It combines principles from stem cell biology, biomaterials, gene therapy, and tissue engineering to develop therapies that restore normal function in diseased or injured tissues.
Studies on biomaterials proved that cellular responses, including cell morphology, motility, proliferation, protein abundance, adhesion, and gene regulation, can be modulated by applying an efficient biomimetic design to biomaterials. Meanwhile, the potential range of stem cell applications in tissue engineering continues to grow in convergence with appropriate implants capable of creating tightly defined artificial microenvironments for each targeted organ [1]. Advancements in biomaterial manufacturing have enabled a shift from composites produced through complex concepts [2,3] to nano-processed materials, such as orthopedic metals [4,5], to multi-phase functional materials that incorporate metals, carbon-based elements, plastics [6], and sometimes 3D-printed components [7]. Moreover, autologous cell therapy using patients’ own cells may be delivered via 3D materials to deliver precise and ideal treatment through a personalized medicine approach [8].
Stem cell therapy uses MSCs within different tissues (bone marrow, adipose, placental, limb bud, amniotic fluid, dental, skin) or induced pluripotent stem cells to regenerate tissues through multiple mechanisms. One involves the direct replacement of damaged cells and tissues, while another relies on paracrine signaling, which modulates the microenvironment [9,10,11]. This includes activating the native immune response, exerting anti-inflammatory effects, preventing fibrosis, alleviating pain through cytokine secretion, regulating cell death, and providing immunomodulatory benefits [12,13,14]. Meanwhile, tissue engineering is developing bioengineered scaffolds [15] that support cell growth and tissue regeneration for applications such as skin grafts, cartilage or bone repair, and organ regeneration [16].
Adipose-derived stem cells (ASCs) have emerged as a promising tool in regenerative medicine due to their accessibility, multipotency, and immunomodulatory properties [17,18,19]. A critical factor in their therapeutic success is efficient homing, the process by which ASCs migrate and engraft at target tissues. Understanding the biological mechanisms governing ASC homing, as well as the role of scaffold materials in directing this process, is essential for optimizing regenerative strategies [20,21].
This review explores the key aspects of ASC homing, beginning with the biological mechanisms that drive their migration, including chemotaxis, receptor–ligand interactions, and major signaling pathways in contact with innovative biomaterials. Scaffolds reproduce the anatomy and physiology of tissue. An ideal scaffold should possess some attributes such as (1) porous structure, (2) biocompatibility, (3) bioactivity, and (4) excellent mechanical properties [22]. The validation of its biomimetic efficiency for fast biointegration involves primary cell cultures [23]. In this context, we examine the influence of scaffold chemical composition, comparing natural, synthetic, and hybrid biomaterials and their impact on ASC adhesion and migration.
We then discuss strategies for enhancing homing efficiency, such as chemotactic signaling to promote ASC migration to injury sites; immunomodulation that enhances ASC retention; and preconditioning strategies such as hypoxia, mechanical stimulation, and inflammatory cytokine exposure to further improve ASC adhesion and survival. Co-culturing with specific tissue cells enhances integration, while genetic engineering enhances migratory capacity and tissue targeting. Smart scaffolds with tunable properties provide an optimal microenvironment for ASC recruitment, viability, and differentiation. Functionalization with bioactive molecules improves targeted homing and adhesion, while advanced biomaterials, including 3D-printed, injectable, or electrospun scaffolds, support ASC retention and guided differentiation for tissue regeneration. By integrating insights from cell biology, biomaterials science, and regenerative medicine, this review aims to provide a comprehensive understanding of ASC homing and its implications for tissue engineering and clinical applications. Finally, we provide current challenges and future directions in scaffold-based ASC therapies.
2. Phenotype and Functional Properties of Human Adipose Stem Cells
ASCs are a rich, ubiquitous, and easily accessible source of multipotent stem cells [24]. Based on isolation methods from adipose tissue, ASCs, stromal vascular fraction (SVF), and micronized adipose tissue (MAT) can be obtained. The term ASCs specifically refers to mesenchymal stem cells (MSCs) extracted from adipose tissue and expanded in culture. SVF is obtained through a process involving centrifugation and enzymatic digestion with collagenase, which releases a heterogeneous cell population from the collagen matrix, such as ASCs, pericytes, smooth muscle cells, endothelial cells, immune cells, and progenitor cells. In contrast, MAT is produced through the mechanical separation of adipose tissue without collagenase, allowing cells to be released from the lipoaspirate with minimal manipulation. Unlike SVF, MAT retains the extracellular matrix (ECM) and ASCs, fibroblasts, and pericytes embedded within the ECM. Mechanical methods yield lower nucleated cells retained in niche, and lower frequencies of progenitor cells, than the enzymatic approaches. ASCs are localized in perivascular niches, and mechanical isolation methods release fewer cells from these spaces due to reduced ECM disruption compared with enzymatic methods [25,26].
Mechanical methods were initially developed to isolate “minimally manipulated” cells to potentially bypass strict FDA regulations, whereas enzymatic methods produce cells considered “more than minimally manipulated” and are regulated as drugs; however, recent FDA guidance aims to classify all SVF isolation methods, both enzymatic and mechanical, as yielding “more than minimally manipulated” cells, subjecting them to stricter oversight [27,28].
The stem cells isolated from adipose tissue exhibit a characteristic spindle-shaped, bipolar morphology typical of mesenchymal stem cells (MSCs) [29], as shown in Figure 1.
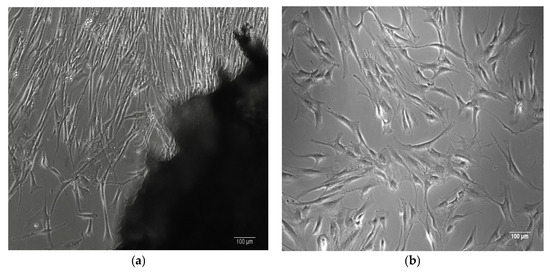
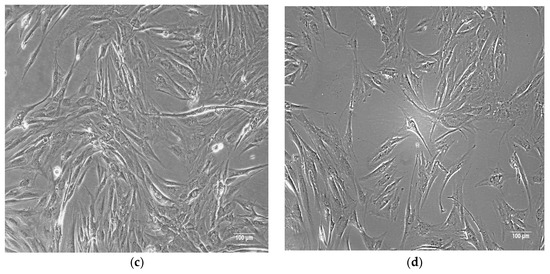
Figure 1.
(a) Human bone-derived MSCs migrating from a bone explant. (b) Human bone-derived MSCs proliferating at passage 1. (c,d) Human ASCs proliferating at passage 1. Images captured at 100× magnification using phase-contrast microscopy.
Primary cells were isolated from human tissues by the authors at the Department of Immunology, Center for Advanced Medical and Pharmaceutical Research, Târgu Mureș.
ASCs express typical MSC markers, including receptor molecules (CD90 and CD105) and the GPI-anchored enzyme CD73 (CD105, CD73, and CD90 are expressed on virtually all mesenchymal stem cells, regardless of source), as illustrated in Figure 2.
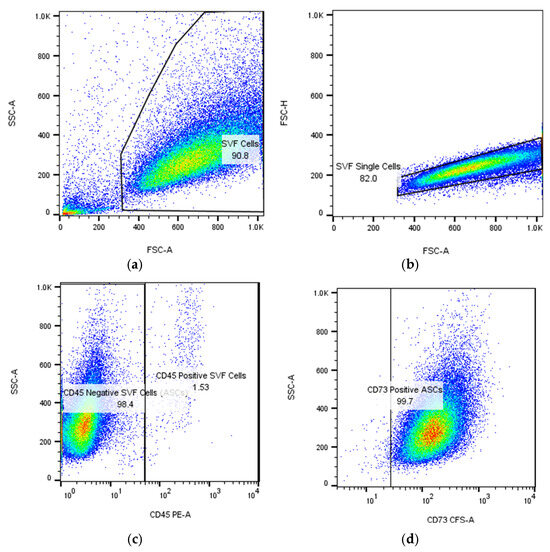
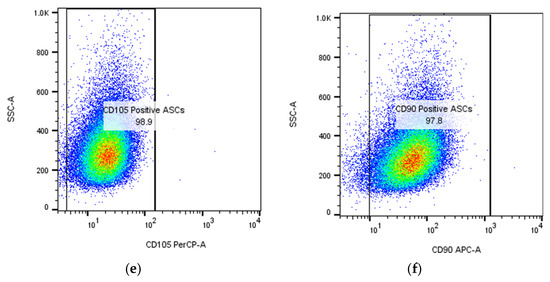
Figure 2.
Flow cytometric identification in BD FACS Aria III of adipose-derived stem cells (ASCs) expanded in culture after passage 1 (P1): (a,b) gating strategy to isolate single cells; the numbers represent the percentage of cells and singlets, respectively; (c) detection of ASCs as CD45-negative cells (more than 98%); (d–f) expression of mesenchymal stem cell markers CD73, CD90, and CD105 on ASCs. The cells were isolated, cultured, submitted to passage 1, re-cultured until confluence, and then analyzed in flow cytometer at the Department of Immunology, Center for Advanced Medical and Pharmaceutical Research, Târgu Mureș.
ASCs also express adhesion molecules characteristic of different tissues (CD29 in placental MSCs; CD44 in adipose, skin, and dental MSCs; CD146 in endometrial MSCs; and CD166 in skin MSCs). Additionally, markers such as CD13, CD10, CD49e, and CD59 are usually positive [30].
In low-passage cultures, ASCs often express CD34, a marker commonly used to identify hematopoietic or endothelial progenitor cells, although its expression is influenced by passage number and culture conditions. Typically, ASCs show minimal expression of hematopoietic markers (CD11b, CD14, CD19, and CD45) and endothelial markers (CD31 and HLA-DR) [31].
ASCs are considered relatively immune-privileged because they lack HLA-DR (MHC class II) and costimulatory molecules (e.g., CD80, CD86, CD40, and CD40L) required for T- and B-cell activation. Moreover, they express low levels of MHC class I [19,32].
In early passages, ASCs express low levels of stemness-related transcriptional factors such as Oct4, Nanog, and Sox2, which are essential for pluripotency, self-renewal, proliferation, and survival of MSCs [33,34]. These pluripotency markers were barely detectable in a study conducted by Hatzmann et al. [35]. In another study, Pierantozzi et al. showed that Nanog, but not OCT-4 or SOX-2, is expressed in cultured MSCs, appearing only after in vitro culture and restricted to proliferating cells. Its expression declines in late passages for adipose and heart-derived MSCs. Despite this, Nanog levels do not correlate with proliferation, differentiation, or stemness, suggesting its activation reflects adaptation to in vitro conditions rather than a regulatory role [36].
The maintenance and regulation of pluripotency involves a complex interplay between intrinsic factors like Oct4, Sox2, and Nanog, and extrinsic signals such as the LIF/STAT3 pathway, acting in a context-dependent manner. Oct4 exerts dose-dependent effects on stem cell fate, promoting pluripotency at intermediate levels and driving differentiation at low or high levels through partner switching with Sox2 or Sox17. Its activity and stability are finely regulated by post-translational modifications such as phosphorylation, ubiquitination, and sumoylation, which influence its DNA binding, protein interactions, and functional roles in pluripotency and differentiation [37].
ASCs from both younger and older donors exhibit similar antigen profiles [38]. Single-cell RNA sequencing analysis has revealed low transcriptomic heterogeneity among ASCs [31,39,40,41,42,43]. However, no exclusive marker has yet been identified to distinguish ASCs; notably, ASCs do not express markers specific to bone marrow-derived MSCs, such as adhesion marker CD106 or the sialoglycoprotein podocalyxin (PODXL) [44].
ASCs from obese donors exhibit reduced expression of stemness markers compared with those from lean individuals, while promoting a pro-inflammatory phenotype. White adipose tissue exists as subcutaneous and visceral fat, with ASCs from both sharing viability and markers but differing in motility, secretion, and gene expression. Subcutaneous ASCs have greater adipogenic and osteogenic potential, while visceral ASCs proliferate slower and secrete more inflammatory cytokines. ASCs in superficial layers show angiogenic capacity, whereas deeper ASCs resemble visceral ASCs [45].
ASCs produce a wide range of soluble mediators, including cytokines, chemokines (both pro- and anti-inflammatory), adipokines, antioxidative molecules, pro-angiogenic and anti-apoptotic factors, and growth factors such as Vascular Endothelial Growth Factor (VEGF), Hepatocyte Growth Factor (HGF), Fibroblast Growth Factor (FGF), and Insulin-like Growth Factor (IGF-1). ASCs also secrete brain-derived neurotrophic factor (BDNF) and various interleukins (e.g., IL-1Ra, IL-6, IL-7, IL-8, and IL-11). Furthermore, extracellular vesicles (EVs) derived from ASCs transfer functional biomolecules (proteins; nucleic acids such as mRNA, microRNAs, tRNAs, and other non-coding RNAs; and lipids) that influence cell proliferation, migration, apoptosis, immune modulation, angiogenesis, metabolism, nerve regeneration, and tumorigenesis [19,46].
In addition to these phenotypic characteristics, ASCs possess multilineage differentiation potential, an essential property for their use in cell therapy and tissue engineering. They can differentiate into cells of ectodermal, endodermal, and mesodermal lineages. Notably, ASCs can give rise to adipogenic, osteogenic, chondrogenic, myogenic, angiogenic, cardiomyogenic, tenogenic, and periodontogenic cell types, reflecting their mesodermal origin. Their neuro-regenerative potential has also been evaluated [47]. In vitro differentiation is typically induced by culture in media supplemented with lineage-specific factors [24,48].
Under specific induction conditions, ASCs can transdifferentiate beyond mesodermal lineages (e.g., adipocytes, osteocytes) into ectodermal lineages (neurons, glial cells) [49,50], endodermal lineages (hepatocyte-like cells, pancreatic β-like cells) [51], or cardiomyocyte-like cells [52].
This plasticity can be driven by epigenetic remodeling, via chromatin modification or DNA methylation, by lineage-transcription factor overexpression or microRNA regulation [53,54].
ASCs transdifferentiation also occurs under niche signals such as specific growth factor/cytokine signaling (e.g., FGF, EGF, HGF) [55,56].
However, several limitations must be considered regarding this process. Stem cell transdifferentiation may be incomplete; although the resulting cells often express markers of the target lineage, they may not fully replicate their function. This requires stable reprogramming of the cell’s genome and epigenetic modifications. Functional integration into host tissue and long-term survival remain significant challenges.
The culture and expansion of ASCs, as well as freezing and long-term storage at ultra-low temperatures, are preparative steps that may influence their phenotype and function. Human ASCs retain their stemness in serum-free conditions, though FBS deprivation for 48 h reduces metabolism and proliferation without inducing apoptosis or necrosis. Alternative FBS substitutes such as human platelet lysates show superior support for hASC proliferation while maintaining their undifferentiated state. Similarly, thrombin-activated platelet-rich plasma (tPRP) and pooled human serum sustain ASC properties, though tPRP may reduce reactive oxygen species (ROS) accumulation and genetic instability, which can be mitigated by lowering the culture temperature [57].
Although cryopreserved lipoaspirate-derived cells show reduced viability and lower colony-forming unit percentages compared with fresh cells, the remaining viable cells retain adhesive and proliferative properties, which can offset the negative effects with continued growth. Prolonged cryopreservation at −70 °C further reduces both the number of viable cells and their viability [58].
ASCs rapidly undergo replicative senescence and lose stem cell properties after 21 days in 2D culture. Senescent cells exhibit decreased proliferation, morphological changes, and increased ROS, raising concerns about ASC stability and safety for application. Immortalization techniques, such as ectopic telomerase reverse transcriptase (TERT) expression, can prevent senescence and maintain proliferative and differentiation potential. While TERT overexpression has successfully immortalized ASCs in some studies, issues like chromosomal aberrations and loss of differentiation ability remain [59]. Co-transducing hTERT with other genes, like Bmi-1 or HPV-E6/E7, has also been explored, but results vary. Gene editing can immortalize cells, but karyotype variation must be considered [60,61].
3. ASC Homing
In stem cell science, homing refers to the ability of stem cells to locate their specific niches. This process involves preparing the niche, enhancing cellular migratory responsiveness, and increasing sensitivity to niche-derived signals. Stem cell homing mechanisms can be categorized as either non-systemic or systemic.
Non-systemic homing involves the recruitment of local or transplanted stem cells that navigate along a chemokine gradient released from an injured tissue site. Direct application of ASCs has proven effective for tissue regeneration in various conditions, including intracardiac, intra-articular, intramuscular, intraosseous, and intrathecal. MSC retention can be enhanced through targeted delivery methods such as intracerebral administration for neurological disorders, intratracheal delivery for lung disease, or intramyocardial injection for cardiac conditions [62].
Systemic homing occurs following intravascular transplantation. In this process, stem cells exhibit a migration pattern similar to that of leukocytes, enabling them to home to sites of injury or inflammation. This mechanism is mediated primarily by interactions among chemokines, cytokines, and adhesion molecules. For instance, in stromal cells derived from human adipose tissue, galectin-1 and CD24 have been identified as potential ligands for P-selectins [63,64]. ASCs express chemokine receptors such as CXC chemokine receptor (CXCR) type 4 and type 7, which respond to stromal cell-derived factor-1 (SDF-1/CXCL12). The CXCL5/CXCR2 axis induces ASC migration [65]. Additionally, monocyte chemoattractant protein-1 (MCP-1) enhances homing by binding to CC chemokine receptor type 2 (CCR2). The signaling molecules Talin and Kindlin interact with very late antigen-4 (VLA-4) upon SDF-1 activation, promoting migration. Moreover, the vascular cell adhesion molecule 1 (VCAM-1) expressed on endothelial cells binds to activated VLA-4 integrin. ASCs traverse endothelial cell junctions in response to inflammatory signals—a process known as transendothelial migration—and secrete matrix metalloproteinases (MMPs) to degrade extracellular matrix components, facilitating their infiltration into damaged tissues. Lysophosphatidic acid (LPA), a small bioactive phospholipid expressed in ASCs, stimulates ASC migration via the G protein-coupled receptor [66]. Mitogen-activated protein kinases (MAPKs), including Jun N-terminus kinase (JNK), p38, and extracellular signal-regulated kinase 1/2 (Erk1/2) are involved in stem cell migration. Erk1/2 induces mobility via the SDF-1/CXCR4 [67,68,69] and CXCL11/CXCR3 axis [70]. ASC migration and proliferation are enhanced via activation of the phosphatidylinositol-3 kinase (PI3K)/Akt signaling pathway [71]. ASC migration significantly increased through platelet-derived growth factor receptor-β (PDGFR)-β signaling, leading to oxygen species generation and microRNA-210 up-regulation [72]. Inflammatory chemokines also contribute to MSC migration [21].
A thorough understanding of the molecular events underlying ASC homing is essential for developing strategies to optimize this process for therapeutic applications.
4. Strategies to Enhance ASC Homing for Improved Regenerative Outcomes
Enhancing ASC homing to target tissues is crucial for improving regenerative outcomes. Several strategies have been developed to optimize ASC recruitment, retention, and functional integration at injury or defect sites. The main mechanisms may be seen in the diagram in Figure 3, followed by detailed descriptions below.
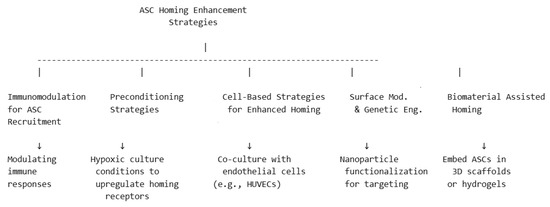
Figure 3.
Schematic representation of strategies for ASC enhanced homing.
4.1. Chemotactic Signaling
The SDF-1α/CXCR4 axis guides ASC migration to injury sites where SDF-1α is upregulated [73]. VEGF and angiopoietin-1 can enhance ASC recruitment by promoting vascularization, which in turn improves ASC delivery to damaged tissues [74]. PDGF and FGF stimulate ASC proliferation and migration via the activation of PI3K/Akt and MAPK pathways [75].
4.2. Immunomodulation for ASC Recruitment
Modulating immune responses with IL-4, IL-10, or TGF-β promotes an anti-inflammatory environment that, in turn, enhances ASC retention and differentiation. Reducing complement-mediated ASC clearance using complement inhibitors (e.g., C3a or C5a blockers) enhances ASC survival and engraftment. Promoting regulatory T cell (Treg) expansion reduces local inflammation, improving ASC homing and integration [13,76].
4.3. Preconditioning Strategies
Culturing ASCs under hypoxic conditions upregulates SDF-1 α and VEGF, enhancing migration and survival after transplantation [77].
Pre-exposing ASCs to fluid shear stress enhances their ability to adhere to vascular endothelial cells [78].
Exposure to inflammatory cytokines like TNF-α and IL-6 increases ASC expression of adhesion molecules (ICAM-1, VCAM-1), improving retention at injury sites [79,80]. Pre-treating ASCs with lipopolysaccharides (LPSs) has been shown to enhance wound healing and angiogenesis, while also increasing the secretion of growth factors involved in tissue regeneration and immune modulation [81].
Mechanical stretching or electrical stimulation can enhance ASC responsiveness to chemotactic cues, increasing homing efficiency [82,83].
Attaching magnetic nanoparticles to ASCs allows for targeted delivery using external magnetic fields, increasing homing efficiency [84].
4.4. Cell-Based Strategies for Enhanced Homing
Co-culturing ASCs with endothelial cells [85] or fibroblasts [86] can mimic the native microenvironment, preconditioning ASCs for better migration and integration upon transplantation [87].
For nerve regeneration, ASCs co-seeded with Schwann cells improve homing to injured peripheral nerves and enhance axonal growth [88].
Forming ASC spheroids with supporting cells (e.g., pericytes, fibroblasts) enhances their survival and homing efficiency [89].
4.5. Surface Modification and Genetic Engineering
Functionalizing ASCs with nanoparticles loaded with SDF-1α, peptides, or growth factors improves targeted homing, adhesion, proliferation, and stem cell differentiation [90]. Modifying ASC membranes with homing peptides increases their recruitment to injured sites [91].
Engineering ASCs to overexpress CXCR4 or VEGF enhances their migratory capacity and adhesion to endothelial cells [87,92,93,94].
4.6. Biomaterial-Assisted Homing
Current studies suggest that incorporating ASCs within 3D scaffolds may offer a promising alternative for wound healing, orthopedic tissue repair, cardiovascular grafts, and post-surgical tissue reconstruction. The ideal scaffolds create a biomimetic environment that supports ASCs’ viability, proliferation, and differentiation. Furthermore, scaffolds designed to encapsulate ASCs can stimulate their differentiation into specific cell types. Advanced fabrication techniques allow tailoring scaffolds’ features and functionality. Between them, electrospinning and 3D printing are the most popular; scaffolds manufactured by the authors may be seen in Figure 4.
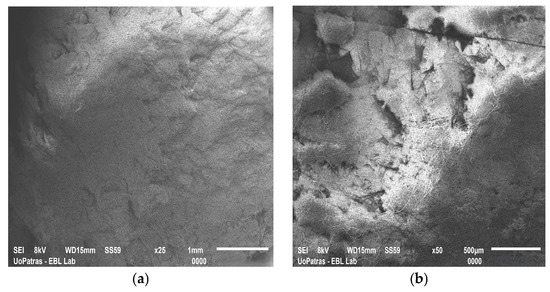
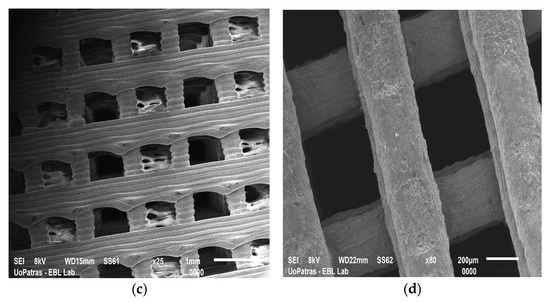
Figure 4.
SEM images of (a) graphene nanoplatelet-reinforced polyetherimide ketone scaffold fabricated by electrospinning; (b) double reinforcement: graphene nanoplatelet- and hydroxyapatite-reinforced polyetherimide ketone scaffold fabricated by electrospinning; (c) polylactic acid scaffold fabricated by 3D printing; and (d) carbon nanotube-reinforced polylactic acid scaffold fabricated by 3D printing. The images have been provided by the Department of Mechanical Engineering and Aeronautics from the University of Patras.
In Figure 5, we may see a schematic representation of the most important aspects related to ASC homing via scaffolds. The decisive factors of successful homing are correlated with the scaffold features like structure, mechanical properties, biocompatibility, and others. There are several classes of scaffolds (natural, synthetic thermoplastic, ceramic-based scaffolds), while the procedures may be considerably enhanced by the addition of biological components. The more detailed descriptions are given in the sections below.

Figure 5.
Schematic representation of the important aspects related to ASC homing via scaffolds.
4.6.1. Key Attributes of Scaffolds Used for Tissue Regeneration
A scaffold is a temporary or permanent template that provides structural support and an optimal environment for seeding stem cells during tissue regeneration. Scaffolds should be designed to mimic the regulatory functions of the extracellular matrix (ECM) in tissue formation with adjustable chemical, electrical, and physical properties [95].
Scaffolds can be porous, offering high surface area and enhanced cell migration. The architecture of porous scaffolds—including pore size and interconnectivity—must ensure proper cell attachment, cell-to-cell interactions, and migration. Porosity also facilitates tissue penetration, vascularization, and nutrient/oxygen diffusion, which are essential for cell survival [96,97]. Furthermore, pore size and geometry influence differentiation. Larger pores favor osteogenic differentiation, while smaller pores promote adipogenic differentiation [98].
Fibrous scaffolds composed of biocompatible polymers arranged in various scales, dimensions, and spatial configurations mimic the structural complexity of the ECM [99].
Another type of scaffold is hydrogels, which are highly hydrated networks formed by crosslinked polymer chains used for soft-tissue applications [100].
Derived from native tissues, decellularized ECM (dECM) preserves the architecture and mechanics of the ECM, is non-immunogenic, and offers advantages such as promoting tissue regeneration via chemotactic stimuli and the presence of native growth factors [101].
A scaffold must be biocompatible to promote cell adhesion, proliferation, migration, and ECM restoration. Scaffold bioactivity refers to its ability to enhance tissue regeneration via cell attachment ligands, growth factors that induce differentiation, and topographical cues for proper spatial organization. The biodegradation rate should be commensurate with tissue regeneration, and degradation products must be non-toxic and either incorporated into other biochemical pathways or eliminated without harming adjacent tissues [102].
Moreover, scaffolds should possess mechanical properties similar to the target tissue to support cell growth and mimic the native ECM. The elasticity and degradation rate should be tailored to match the target tissue, ensuring gradual replacement by the native ECM. Key mechanical parameters include the elastic modulus (also called Young’s modulus) for resistance to deformation under stress; tensile strength, providing maximum stress before failure under tension; fracture toughness for resistance to crack propagation; fatigue resistance, the ability to withstand cyclic loading over time; and elongation at break, providing stretchability before failure [103,104].
Additionally, scaffold stiffness influences lineage commitment—soft substrates favor adipogenesis and chondrogenesis, while stiffer substrates promote osteogenesis [105].
Common fabrication techniques include salt leaching, solvent casting, gas foaming, thermally induced phase separation, freeze-drying, electrospinning, thermally induced self-agglomeration, and three-dimensional (3D) printing [105]. These methods yield scaffolds with the mechanical and bioactive properties required for specific target tissues. Combining scaffold types can further enhance these properties and modulate degradation rates via microstructural adjustments. Incorporating bioactive factors into hydrogels, nanofiber scaffolds, microparticles, or bioprinted scaffolds ensures sustained, localized release that optimizes stem cell function and patient-specific tissue regeneration [102]. In particular, 3D bioprinting and electrospinning offer personalized scaffold architectures with effective bioactive factor delivery [106], while freeze-drying and hydrogel crosslinking remain valuable for tuning pore size and morphology [107].
4.6.2. Natural Scaffolds for Adipose Stem Cell-Based Tissue Engineering
Natural scaffolds—typically derived from polysaccharides or proteins—mimic the extracellular matrix (ECM), providing a supportive microenvironment that promotes cell adhesion, proliferation, and differentiation. Natural scaffolds provide both physical and biochemical platforms that facilitate ASC attachment and growth, which are critical for tissue regeneration.
Hyaluronic acid (HA), a skin/connective tissue-derived glycosaminoglycan, aids wound healing by supporting fibroblast proliferation and keratinocyte migration; however, its low mechanical strength limits its use in high-strength applications [108]. Alginates, derived from algae and bacteria and forming gels via ion exchange when in contact with body exudates, are also used in wound healing [109].
Chitosan, a cationic polysaccharide, is valued for its antibacterial, hemostatic, and biodegradable properties, although its low mechanical strength restricts its high-load applications [110]. Carboxymethyl-chitosan, with improved strength, biocompatibility, and antibacterial activity, supports tissue regeneration and wound healing [111].
Bacterial cellulose (BC) is ideal for tissue engineering due to its purity, high porosity, and excellent biocompatibility; it aids wound care by maintaining a moist environment, absorbing exudates, and preventing infection. BC-based nanocomposites and hydrogels show promise for bone and cartilage regeneration [112].
Collagen, a major ECM protein, supports wound healing but has low load-bearing capacity, whereas gelatin (a collagen derivative) supports cell adhesion and can serve as a carrier for growth factors or drugs [113]. Constructs comprising human platelet-poor plasma, alginate, and fibrin gel exhibited enhanced adipogenic differentiation and VEGF expression. Although collagen sponges offered favorable mechanical properties and supported cell viability, they resulted in poor adipogenic differentiation. Conversely, fibrin gel was identified as the optimal compromise, providing superior mechanical properties, high cell viability, and stimulation of adipogenic and angiogenic factor secretion [114]. The porous structures (e.g., those made from collagen, HA, or BC), ensure adequate nutrient and oxygen supply, ASC migration, and ECM deposition. For soft tissue regeneration common naturally-derived scaffolds include collagen-HA, fibrin-HA [115,116], and gelatin-dECM [117]. These can be fabricated via electrospinning (producing nanofibrous ECM-mimicking structures) or freeze-drying (yielding highly porous constructs for cell infiltration) [118].
Hydrogels, formed through UV- or enzyme-mediated crosslinking, offer injectable or 3D matrices with tunable degradation [119]. For cartilage repair, 3D bioprinting enables precise hydrogel structuring to create patient-specific scaffolds [120], while freeze-drying combined with crosslinking improves the hydrogel’s mechanical properties and water retention for osteochondral defect repair [121]. Additionally, microfluidic gelation facilitates microsphere or microgel obtaining for cell encapsulation [122].
The primary objectives in cardiovascular tissue engineering are vascularization, endothelialization, and functional remodeling. Cardiovascular tissue engineering requires scaffold materials such as fibrin, collagen, and alginate-HA-dECM composites to support vascularization and remodeling [123,124]. ASCs can grow and proliferate well on Atelocollagen (a biological collagen from cattle), and induced by 5-azacytidine, the cells can differentiate into myocardial cells [125]. Techniques like electrospinning with cell seeding produce fibrous scaffolds that mimic blood vessel walls [126], while 3D printing of hydrogels enables the formation of vascularized networks that support endothelial cell growth and stem cell differentiation [127,128].
For nerve regeneration, scaffolds composed of collagen–laminin, chitosan–alginate, and fibrin–HA–dECM facilitate axonal growth and functional recovery. Electrospinning can create aligned nanofibers that serve as nerve guidance conduits [84,129], while 3D bioprinting with cell-laden hydrogels enables precise nerve conduit designs that enhance neural differentiation [130]. Freeze-drying of collagen-hydrogel models and scaffolds with laminin coating increase scaffold bioactivity for spinal cord and brain tissue engineering [131]. Nerve tissue engineering focuses on neurogenic differentiation, axonal outgrowth, and functional recovery. A co-culture system of Schwann cells and ASCs seeded into a silk fibroin/collagen scaffold was able to construct a tissue-engineered nerve conduit [132,133].
ASCs possess an intrinsic myogenic potential and can differentiate into skeletal myoblasts, as indicated by the expression of markers such as MyoD, Myogenin, and α-skeletal actinin. In one study, a cross-linked HA scaffold loaded with ASCs positive for NG2, a pericytic surface marker, differentiated into skeletal muscle tissue [134].
The naturally derived scaffolds can be functionalized with growth factors, cytokines, or other bioactive molecules to enhance ASC differentiation into specific cell types (e.g., adipocytes, osteocytes, chondrocytes) based on therapeutic needs. These scaffolds can be enriched with bioactive factors such as VEGF, angiopoietin-2 (Ang-2), elastin, basic fibroblast growth factor (bFGF), or transforming growth factor-β1 (TGF-β1), to stimulate blood vessel formation, fibroblast proliferation, ECM production, and adipogenesis, while HA fragments and PDGF further enhance hydration, cell migration, and tissue repair [135,136,137]. Hydrogels infused with SDF-1α or HA promote localized ASC retention and survival post-injection [138]. ECM-mimicking scaffolds incorporating growth factors improve ASC adhesion and differentiation at the target site [139]. In the particular case of optimal cartilage regeneration, scaffolds should promote chondrogenic differentiation, ECM accumulation, and mechanical strength. The incorporation of bioactive factors such as bone morphogenetic protein-2 (BMP-2), BMP-7, and IGF-1 enhances chondrocyte proliferation and ECM formation and supports cell survival [140,141], while chondroitin sulfate and heparan sulfate improve chondrocyte adhesion [142]. Advanced microparticle-loaded hydrogels providing sustained release of TGF-β1, SDF-1, BMP-2, and IGF-1, as well as chitosan–collagen 3D scaffolds infused with growth factors, have been developed for long-term cartilage repair [143,144]. In cardiovascular disease, key bioactive factors for vascular repair include VEGF (to promote vessel formation), angiopoietin-1 (to stabilize new vessels) [134], and SDF-1α (to recruit endothelial and smooth muscle progenitors [145,146]. Nitric oxide-releasing compounds improve endothelial function and reduce thrombosis, while bFGF and fibronectin enhance cardiomyocyte proliferation. Scaffolds such as injectable HA–fibrin hydrogels loaded with VEGF and SDF-1α [136] and electrospun vascular grafts coated with nitric oxide-releasing polymers further optimize regeneration [147].
In neurological disorders, bioactive factors such as nerve growth factor (NGF) promote stem cell differentiation into neural-like cells, while brain-derived neurotrophic factor (BDNF) and glial cell-derived neurotrophic factor (GDNF) enhance neurite outgrowth and Schwann cell function [148]. Additionally, chondroitinase ABC degrades inhibitory ECM molecules to facilitate nerve regeneration [145], and laminin plus fibronectin improve cell adhesion [149]. Aligned electrospun nanofibers coated with NGF and BDNF serve as effective nerve conduits, and hydrogel-based drug delivery systems provide the controlled release of neurotrophic factors [150].
Controlled release of homing factors from biodegradable scaffolds enhances ASC recruitment and integration [144]. Being biodegradable, they allow gradual replacement by new tissue, reducing the risk of long-term inflammation or rejection, which is particularly advantageous in clinical applications.
Platelet-rich plasma (PRP), derived from the patient’s blood, enhances tissue healing and regeneration by promoting cell adhesion, angiogenesis, and tissue repair [151]. Ghiasi et al. demonstrated that ASC viability and proliferation were significantly higher in active platelet-rich plasma compared with fibrinogen glue and alginate, likely due to the abundant release of growth factors and cytokines [152].
dECM derived from native tissues preserves the structural and biochemical properties needed to support tissue regeneration while minimizing immune rejection [153,154]. In a study by Flynn et al. [155], decellularized human placenta facilitated ASC attachment and spreading, while adipogenic differentiation was enhanced when cells were encapsulated in non-cell-adhesive, crosslinked hyaluronan scaffold gels. In contrast, lower cell viability was observed when ASCs were encapsulated versus when they adhered to the scaffold matrix; moreover, non-adhesive scaffolds promoted adipogenic differentiation relative to cell-adhesive matrices. In 2022, Ren et al. [46] published findings showing that seeding ASCs in decellularized adipose tissue ECM facilitated wound healing by enhancing proliferation, angiogenesis, epithelization, and anti-inflammatory effects. The decellularized adipose tissue ECM preserved its 3D structure and key components—including collagen, sulfated glycosaminoglycans, and VEGF—while lacking MHC class I. Cartilage dECM-derived scaffolds with a 3D interconnected porous structure, when seeded with ASCs, have shown cartilage tissue formation [156]. Acellular cartilage matrices, which have excellent physicochemical properties and biocompatibility, are ideal for cartilage tissue engineering [157]. dECM processing combined with bioreactor culturing preserves native ECM signals for use in heart patches and vascularized constructs [158]. Liver dECM preserves the macroscopic 3D architecture, native composition, and ultrastructure and has proven valuable for promoting ASCs’ differentiation into hepatocytes in both the presence and absence of growth factors [159].
These insights underscore the importance of scaffold selection and functionalization in targeted tissue engineering applications. Although naturally derived scaffolds offer benefits such as biocompatibility, biodegradability, bioactivity, and reduced immune response, they also present some limitations (e.g., low mechanical strength, rapid degradation, limited tunability). The main types and characteristics of naturally derived scaffolds are summarized in Table 1. The challenges can be overcome by combining natural scaffolds with bioengineering techniques to enhance their structural and functional properties for more effective ASC-based regenerative medicine.

Table 1.
Summarizing types of natural scaffolds used in studies, illustrating advancements in biomaterial-assisted strategies for enhancing ASC homing and differentiation.
4.6.3. Synthetic Scaffolds for Tissue Regeneration
Natural polymers offer excellent biocompatibility and cell adhesion, while synthetic polymers allow better control over porosity and provide controlled degradation, tunable physical, chemical, and mechanical properties, and scalability for mass production. Their tissue integration depends on optimizing degradation rates, mechanical properties, and bioactivity [160,161].
Synthetic scaffolds can be categorized into biodegradable and non-biodegradable types. The main synthetic scaffolds with their characteristics are summarized in Table 2.
Biodegradable synthetic scaffolds gradually degrade, allowing natural tissue to replace them. Common materials include polylactic acid (PLA), which degrades slowly and is used in bone and cartilage regeneration [162,163]. However, resorbable polymers like PLA have limited osteoinductive capacity [164]. Polyglycolic acid (PGA) degrades more rapidly and is used in sutures and soft tissue repair; poly (lactic-co-glycolic acid) (PLGA) is a copolymer with adjustable degradation properties used in drug delivery and scaffolding [165]; polycaprolactone (PCL) has a longer degradation time and high mechanical strength, and is suitable for bone and nerve regeneration [166,167]. Nanofibrous scaffolds such as electrospun PCL or PLGA mimic the ECM, improving cell attachment and differentiation [168].

Table 2.
Summarizing synthetic scaffolds used in studies illustrating advancements in strategies for enhancing ASC homing and differentiation.
Table 2.
Summarizing synthetic scaffolds used in studies illustrating advancements in strategies for enhancing ASC homing and differentiation.
| Synthetic Scaffolds | Material | Properties | Applications | References |
|---|---|---|---|---|
| Polylactic acid (PLA) | Biodegradable polymer | Slow degradation, low osteoinductive capacity | Bone, cartilage regeneration | [162,163,164] |
| Polyglycolic acid (PGA) | Biodegradable polymer | Rapid degradation | Sutures, soft tissue repair | [165] |
| Poly (lactic-co-glycolic acid) (PLGA) | Copolymer | Tunable degradation, drug-delivery applications | Drug delivery, scaffolding | [165,167] |
| Polycaprolactone (PCL) | Biodegradable polymer | Long degradation time, high mechanical strength | Bone, nerve regeneration | [166,167,168] |
| Polyethylene glycol (PEG) | Non-biodegradable polymer | Biocompatible, hydrophilic | Drug delivery, cell encapsulation | [169,170] |
| Polymethylmethacrylate (PMMA) | Non-biodegradable polymer | High stability, used in bone cements | Orthopedics, dental applications | [171] |
| Polyurethane (PU) | Synthetic polymer | Flexible, high mechanical strength | Cardiovascular, orthopedic applications | [172] |
Non-biodegradable synthetic scaffolds are used when long-term structural support is required. These materials exhibit strong mechanical properties but may require permanent implantation or later removal. Examples include polyethylene glycol (PEG), which forms hydrogels commonly used for drug delivery [169] and cell encapsulation [170]. PEG is widely used in synthetic scaffolds due to its biocompatibility, hydrophilicity, and low immunogenicity, supporting chondrocyte viability and ECM deposition. PCL, an FDA-approved polymer, offers tunable mechanical strength and can be fabricated as electrospun nanofibers or porous scaffolds. Polymethylmethacrylate (PMMA) offers stability in bone cements and dental applications [171]. Polyurethane (PU), valued for its flexibility and strength, is frequently used in cardiovascular and orthopedic applications, drug delivery, and wound healing [172]. In addition, PEG, PU, and PCL hydrogels are beneficial for skin and wound healing [173].
Because synthetic scaffolds inherently lack bioactivity, surface modifications with bioactive molecules (e.g., ECM proteins, RGD peptides, or growth factors) are often necessary to enhance cell adhesion, proliferation, and differentiation [174]. Incorporating VEGF, TGF-β, FGF, or BMPs further promotes stem cells’ differentiation and tissue regeneration [175]. Moreover, integrating controlled drug delivery [176] or gene delivery systems [10] within synthetic scaffolds can improve stem cell-based regenerative outcomes.
Integrating conductive polymers (e.g., polypyrrole, graphene) into scaffolds enhances ASC migration via electrical stimulation, which is beneficial for nerve and cardiac repair [129].
4.6.4. Ceramic-Based Scaffolds
Ceramic-based scaffolds possess a 3D structure that facilitates cell attachment, proliferation, and differentiation, making them ideal for bone regeneration. In addition to biocompatibility, hydrophilicity, and bioactivity, these scaffolds must have porosity that supports nutrient diffusion and cell migration as well as mechanical strength comparable to native bone. While higher porosity may compromise the mechanical performance, lower porosity can hinder nutrient/oxygen diffusion and integration with the host bone. Ceramic materials offer mechanical strength similar to native bone and exhibit degradability that matches the rate of bone formation [177].
Bioactive ceramics used in scaffold fabrication include calcium phosphate-based materials such as hydroxyapatite, tricalcium phosphate (TCP), and biphasic calcium phosphate (BCP), a combination of hydroxyapatite and TCP. Electrical properties are important to ensure a biomimetic environment for the cell culture [178]. In this sense, hydroxyapatite offers excellent osteoconductivity, but it degrades slowly, whereas TCP is more favorable for bone remodeling [179]. TCP and hydroxyapatite promote osteoinduction but are brittle and poorly absorbed [164].
Bioactive glasses composed of silica-based materials form a bone-like apatite layer upon interacting with body fluids, releasing ions that enhance bioactivity. Low-alkali or nearly alkali-free bioactive glasses are particularly suitable for bone regeneration and tissue engineering [180].
Zirconia, silicon nitride, and alumina are bioinert ceramic-based scaffolds that provide high mechanical strength and antibacterial properties but exhibit lower bioactivity compared with calcium-based ceramics [181].
The fabrication of ceramic scaffolds can be optimized to achieve ideal architecture, porosity, and mechanical properties with the incorporation of bioactive ions or molecules to create patient-specific implants [182].
Ceramic-based scaffolds can serve as orthopedic and dental implants that promote bone healing and regeneration [183] and are used in craniofacial reconstruction to repair bone defects [184]. Ceramic scaffolds can also function as drug delivery systems, releasing growth factors or antibiotics in a controlled manner to enhance healing. Limitations in mechanical strength and bioactivity can be mitigated by incorporating nanoparticles or growth factors [185].
4.6.5. Composite Scaffolds
Composite scaffolds provide tailored properties for personalized medicine and regenerative therapies by overcoming the limitations of single-component scaffolds. They enhance mechanical strength, bioactivity, and degradation control.
The most common composite scaffolds are polymer–ceramic composites, which combine the versatility and degradability of polymers with the bioactivity and mechanical strength of ceramics. The ceramic component enhances osteoconductivity and supplies the mineral fraction essential for bone regeneration. Such composites are widely used in orthopedic applications, bone grafts, and dental implants because they closely mimic the composition and structure of native bone [186,187,188]. Bone regeneration often relies on scaffold combinations such as collagen–hydroxyapatite–chitosan [189] or bone dECM with fibrin [190] to mimic the natural bone environment. In a study performed by Calabrese et al. [191], a composite material comprising mineralized type I collagen and magnesium-enriched hydroxyapatite, designed to mimic bone, was used to study osteogenesis and vasculogenesis during bone tissue regeneration. Collagen stimulates MSC differentiation into osteoblasts, and the incorporation of hydroxyapatite enhances cell proliferation, differentiation, and bone matrix formation. The collagen/Mg-HA scaffold recruited host cells in vivo that migrated into the scaffold, underwent osteogenic differentiation, and promoted vasculogenesis. Moreover, seeding human ASCs onto these scaffolds prior to implantation significantly improved bone regeneration. Techniques such as electrospinning combined with freeze-drying produce porous nanofibers that promote osteogenic differentiation [192], while sol-gel calcium phosphate-based materials enhance mineralization and accelerate stem cell differentiation [193]. Moreover, 3D bioprinting enables the creation of customized scaffolds for large bone defects that integrate bioactive molecules and growth factors [106].
Key objectives in bone regeneration include osteogenic differentiation, mineralization, and vascularization. Factors such as BMP-2 BMP-7, and BMP-9 stimulate osteoblast differentiation and bone matrix formation [139], while PRP growth factors enhance MSC osteogenic potential [194,195]. Controlled dexamethasone dosing further promotes osteogenesis, and calcium phosphate nanoparticles provide bioactive mineralization cues [196]. Improved scaffold integration may be achieved using bioceramic-coated scaffolds (e.g., with hydroxyapatite or tricalcium phosphate) or electrospun collagen scaffolds designed for sustained dexamethasone release [197]. In a study by Gao et al. [198], ASC-derived endothelial cells, alone or in co-culture with ASC-derived osteoblasts, were seeded onto a PLGA-based electrospun nanocomposite scaffold containing amorphous calcium phosphate (CaP) nanoparticles. The addition of CaP nanoparticles slowed cell proliferation but enhanced the differentiation of osteoblasts compared with pure PLGA scaffolds. Co-cultures of endothelial cells and osteoblasts promoted mutual differentiation. The CaP nanoparticles also improved the scaffold’s mechanical stability, bioactivity, and osteoconductivity, making it ideal for seeding with pre-differentiated cells.
Another category is polymer–polymer composites, which combine two or more polymers to optimize mechanical strength, degradation rate, and bioactivity. Natural blends (e.g., collagen with chitosan), enhance cellular interactions while remaining biodegradable, making them ideal for soft-tissue engineering. Synthetic combinations (e.g., PLGA with PCL) offer improved structural integrity and prolonged degradation for load-bearing applications. These composites are frequently used in cartilage repair, nerve regeneration, and wound healing. In addition, various bioactive materials have been employed to create injectable hydrogel scaffolds for cartilage regeneration [187,199].
Ceramic–metal composites combine the bioactivity of ceramics with the high mechanical strength of metals and are useful for load-bearing applications such as orthopedic and dental implants.
Metals like titanium (Ti), magnesium (Mg), and stainless steel enhance scaffold strength, addressing ceramic fragility. Metallic biomaterials may be bioinert (e.g., Ti and stainless-steel implants) or prone to corrosion (e.g., Mg- or Zn-based implants). Incorporating bioactive ceramics and polymers can improve surface bioactivity and corrosion resistance [200]. Magnesium-based composites are attractive for their biodegradability and bone-forming ability, whereas titanium-based composites offer excellent biocompatibility and corrosion resistance. These materials are common in spinal fusion devices, joint replacements, and maxillofacial reconstructions [201].
Hybrid composites incorporating nanomaterials have also gained attention due to enhanced mechanical and biological properties. Embedding nanoparticles such as carbon nanotubes (CNTs), silver nanoparticles, or silica nanoparticles improves scaffold strength, imparts antibacterial properties, and enables the delivery of bioactive molecules. For instance, silver nanoparticles reduce infection risks [202], while carbon nanotube-reinforced scaffolds provide electrical conductivity beneficial for neural tissue engineering and bone healing [203]. Such nanocomposites are promising smart scaffolds with controlled drug release and tissue-specific regeneration [159]. Ceramic and composite scaffold types, with their main characteristics, are summarized in Table 3.

Table 3.
Summary of hybrid and composite scaffolds used in the study of ASC homing and differentiation.
4.7. Extracellular Vesicles (EVs) and Exosome-Based Approaches
Pre-treating ASCs with EVs or exosomes from injured tissues enhances their homing ability by activating migration-related pathways [204].
Loading ASCs with exosomes rich in SDF-1α, miR-21, HIF-1α, or other factors improves their ability to localize to ischemic and inflamed tissues [205]. Incorporating exosomes into hydrogels or scaffolds creates a sustained-release system that recruits ASCs to the injury site [206].
5. Conclusions
ASCs hold great promise in regenerative medicine due to their accessibility, multipotency, and immunomodulatory properties. Their ability to differentiate into multiple lineages and secrete bioactive factors makes them valuable for tissue repair. However, challenges such as donor variability, culture-induced changes, and regulatory constraints must be addressed. Optimizing ASC homing, including by using scaffolds designed to mimic the regulatory functions of the natural extracellular matrix and engraftment through better delivery strategies and molecular modulation, will enhance their therapeutic potential. Overcoming these hurdles is key to translating ASCs into effective, standardized clinical treatments.
Author Contributions
Conceptualization, M.D. and D.R.M.; methodology, D.R.M. and D.V.P.; software, D.R.M. and M.V.; validation D.R.M. and D.V.P.; formal analysis, D.R.M. and M.V.; investigation, D.R.M., D.V.P. and M.V.; resources, D.R.M. and D.V.P.; data curation, D.R.M., D.V.P. and M.V.; writing—original draft preparation, D.R.M., D.V.P. and M.D.; writing—review and editing, D.R.M., D.V.P. and M.D.; visualization, D.V.P. and M.V.; supervision, M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was conducted as part of the doctoral research of V.M. at the Doctoral School of Medicine and Pharmacy, “George Emil Palade” University of Medicine, Pharmacy, Science and Technology of Târgu Mures, Romania, contributing to the development of the author’s doctoral thesis with guidance and collaboration from D.M., M.D.R. and D.V.P.
Acknowledgments
The authors express their acknowledgement to Vassilis Kostopoulos, Department of Mechanical Engineering and Aeronautics, University of Patras, Greece, who provided the scaffolds.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ANG-2 | angiopoietin-2 |
| ASC | adipose stem cell |
| bFGF | basic fibroblast growth factor |
| BC | bacterial cellulose |
| BCP | biphasic calcium phosphate |
| BDNF | brain-derived neurotrophic factor |
| BMP | bone morphogenetic protein |
| 2D | bi-dimensional |
| CaP | calcium phosphate |
| CCR | C-C motif chemokine receptor |
| CXCL | C-X-C motif chemokine ligand |
| CXCR | C-X-C motif chemokine receptor |
| CNT | carbon nanotube |
| dECM | decellularized extracellular matrix |
| Erk1/2 | extracellular signal-regulated kinase 1/2 |
| ECM | extracellular matrix |
| EV | extracellular vesicle |
| FDA | Food and Drug Administration |
| FBS | fetal bovine serum |
| FGF | fibroblast growth factor |
| GDNF | glial cell-derived neurotrophic factor |
| GNP | graphene nanoplatelet |
| GPI | glycosylphosphatidylinositol |
| HA | hyaluronic acid |
| HIF | hypoxia inducible factor |
| HLA | human leukocyte antigen |
| HPV | human papilloma virus |
| HGF | hepatocyte growth factor |
| ICAM | intercellular adhesion molecule |
| IGF | insulin-like growth factor |
| JNK | jun N-terminus kinase |
| LPA | lysophosphatidic acid |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MAT | micronized adipose tissue |
| MHC | major histocompatibility complex |
| MMP | matrix metalloproteinase |
| mRNA | messenger ribonucleic acid |
| miRNA | microribonucleic acid |
| MSC | mesenchymal stem cell |
| MCP-1 | monocyte chemoattractant protein-1 |
| NGF | nerve growth factor |
| Oct | octamer transcription factor |
| PCL | polycaprolactone |
| PDGFR | platelet-derived growth factor receptor |
| PEG | polyethylene glycol |
| PGA | polyglycolic acid |
| PI3K | phosphatidylinositol-3-kinase |
| PLA | polylactic acid |
| PLGA | poly(lactic-co-glycolic acid) |
| PMMA | polymethylmethacrylate |
| PODXL | podocalyxin |
| PRP | platelet-rich plasma |
| PU | polyurethane |
| ROS | reactive oxygen species |
| RNA | ribonucleic acid |
| SVF | stromal vascular fraction |
| TERT | telomerase reverse transcriptase |
| 3D | three-dimensional |
| tPRP | thrombin-activated platelet-rich plasma |
| tRNA | transfer ribonucleic acid |
| VCAM | vascular cell adhesion molecule 1 |
| VEGF | vascular endothelial growth factor |
| VLA-4 | very late antigen-4 |
References
- Kozaniti, F.K.; Deligianni, D.D.; Georgiou, M.D.; Portan, D.V. The Role of Substrate Topography and Stiffness on MSC Cells Functions: Key Material Properties for Biomimetic Bone Tissue Engineering. Biomimetics 2021, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Sideridis, E.; Theocaris, P.S.; Papanicolaou, G.C. The elastic modulus of particulate composites using the concept of a mesophase. Rheol. Acta 1986, 25, 350–358. [Google Scholar] [CrossRef]
- Anifantis, N.K.; Kakavas, P.A.; Papanicolaou, G.C. Thermal stress concentration due to imperfect adhesion in fiber-reinforced composites. Compos. Sci. Technol. 1997, 57, 687–696. [Google Scholar] [CrossRef]
- Portan, D.; Papaefthymiou, K.; Pirvu, C.; Papanicolaou, G.; Demetrescu, I. Manufacturing and characterization of TiO(2) nanotubes on pure titanium surfaces for advanced biomedical applications. UPB Bul. Stiintific Ser. B Chem. Mater. Sci. 2011, 73, 181–196. [Google Scholar]
- Portan, D.V.; Papanicolaou, G.C.; Jiga, G.; Caposi, M. A novel experimental method for obtaining multi-layered TiO2 nanotubes through electrochemical anodizing. J. Appl. Electrochem. 2012, 42, 1013–1024. [Google Scholar] [CrossRef]
- Papanicolaou, G.C.; Charitidis, C.A.; Portan, D.V.; Perivoliotis, D.K.; Koklioti, M.A. Investigation of nanomechanical properties of multilayered hybrid nanocomposites. Meccanica 2014, 49, 2645–2655. [Google Scholar] [CrossRef]
- Portan, D.V.; Koliadima, A.; Kapolos, J.; Azamfirei, L. Biomimetic Design and Assessment via Microenvironmental Testing: From Food Packaging Biomaterials to Implantable Medical Devices. Biomimetics 2025, 10, 370. [Google Scholar] [CrossRef]
- Feier, A.M.; Portan, D.; Manu, D.R.; Kostopoulos, V.; Kotrotsos, A.; Strnad, G.; Dobreanu, M.; Salcudean, A.; Bataga, T. Primary MSCs for Personalized Medicine: Ethical Challenges, Isolation and Biocompatibility Evaluation of 3D Electrospun and Printed Scaffolds. Biomedicines 2022, 10, 1563. [Google Scholar] [CrossRef]
- Mei, R.; Wan, Z.; Yang, C.; Shen, X.; Wang, R.; Zhang, H.; Yang, R.; Li, J.; Song, Y.; Su, H. Advances and clinical challenges of mesenchymal stem cell therapy. Front. Immunol. 2024, 15, 1421854. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1421854/full (accessed on 29 March 2025). [CrossRef]
- Wang, M.; Wang, J.; Xu, X.; Li, E.; Xu, P. Engineering gene-activated bioprinted scaffolds for enhancing articular cartilage repair. Mater. Today Bio 2024, 29, 101351. [Google Scholar] [CrossRef]
- Da Silva, K.; Kumar, P.; Choonara, Y.E. The paradigm of stem cell secretome in tissue repair and regeneration: Present and future perspectives. Wound Repair Regen. 2025, 33, e13251. Available online: https://onlinelibrary.wiley.com/doi/10.1111/wrr.13251 (accessed on 29 March 2025). [CrossRef] [PubMed]
- Yan, S.; Campos De Souza, S.; Xie, Z.; Bao, Y. Research progress in clinical trials of stem cell therapy for stroke and neurodegenerative diseases. Ibrain 2023, 9, 214–230. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Mi, B.B.; Lin, Z.; Hu, Y.Q.; Yu, L.; Zha, K.K.; Panayi, A.C.; Yu, T.; Chen, L.; Liu, Z.-P.; et al. The role of the immune microenvironment in bone, cartilage, and soft tissue regeneration: From mechanism to therapeutic opportunity. Mil. Med. Res. 2022, 9, 65. [Google Scholar] [CrossRef]
- Trigo, C.M.; Rodrigues, J.S.; Camões, S.P.; Solá, S.; Miranda, J.P. Mesenchymal stem cell secretome for regenerative medicine: Where do we stand? J. Adv. Res. 2025, 70, 103–124. [Google Scholar] [CrossRef] [PubMed]
- Kozaniti, F.K.; Manara, A.E.; Kostopoulos, V.; Mallis, P.; Michalopoulos, E.; Polyzos, D.; Deligianni, D.D.; Portan, D.V. Computational and Experimental Investigation of the Combined Effect of Various 3D Scaffolds and Bioreactor Stimulation on Human Cells’ Feedback. Appl. Biosci. 2023, 2, 249–277. [Google Scholar] [CrossRef]
- Skrypnyk, M. Current progress and limitations of research regarding the therapeutic use of adipose-derived stem cells: Literature review. J. Umm Al-Qura Univ. Appl. Sci. 2024, 11, 63–75. Available online: https://link.springer.com/10.1007/s43994-024-00147-9 (accessed on 12 February 2025). [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Al-Ghadban, S.; Artiles, M.; Bunnell, B.A. Adipose Stem Cells in Regenerative Medicine: Looking Forward. Front. Bioeng. Biotechnol. 2022, 9, 837464. Available online: https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2021.837464 (accessed on 18 February 2025). [CrossRef]
- Al-Ghadban, S.; Bunnell, B.A. Adipose Tissue-Derived Stem Cells: Immunomodulatory Effects and Therapeutic Potential. Physiology 2020, 35, 125–133. [Google Scholar] [CrossRef]
- Ullah, M.; Liu, D.D.; Thakor, A.S. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience 2019, 15, 421–438. [Google Scholar] [CrossRef]
- Sajjad, U.; Ahmed, M.; Iqbal, M.Z.; Riaz, M.; Mustafa, M.; Biedermann, T.; Klar, A.S. Exploring mesenchymal stem cells homing mechanisms and improvement strategies. Stem Cells Transl. Med. 2024, 13, 1161–1177. [Google Scholar] [CrossRef] [PubMed]
- Portan, D.V.; Angelopoulou, A.; Koliadima, A.; Kontaxis, L.C.; Michanetzis, G.; Kouzoudis, D.; Michalopoulos, E.; Papanicolaou, G.C. Biodegradation, mechanical and biocompatibility evaluation of compact and porous polylactic acid after long term dynamic fluid immersion. J. Chem. Technol. Biotechnol. 2025; in process. [Google Scholar] [CrossRef]
- Dobreanu, M.; Manu, D.; Portan, D. Modern methods and techniques for testing artificial substrates biocompatibility. In Proceedings of the ICSAAM 2023 Book of Abstracts, Zakynthos, Greece, 10–14 September 2023; p. 12. [Google Scholar]
- Si, Z.; Wang, X.; Sun, C.; Kang, Y.; Xu, J.; Wang, X.; Hui, Y. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed. Pharmacother. 2019, 114, 108765. [Google Scholar] [CrossRef] [PubMed]
- Aronowitz, J.A.; Lockhart, R.A.; Hakakian, C.S. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. SpringerPlus 2015, 4, 713. [Google Scholar] [CrossRef]
- Cremona, M.; Gallazzi, M.; Rusconi, G.; Mariotta, L.; Gola, M.; Soldati, G. State of the Art in the Standardization of Stromal Vascular Fraction Processing. Biomolecules 2025, 15, 199. [Google Scholar] [CrossRef]
- Available online: https://www.isscr.org/basic-research-standards (accessed on 25 February 2025).
- Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/regulatory-considerations-human-cells-tissues-and-cellular-and-tissue-based-products-minimal (accessed on 25 February 2025).
- Li, J.; Huang, H.; Xu, X. Biological characteristics and karyotiping of a new isolation method for human adipose mesenchymal stem cells in vitro. Tissue Cell 2017, 49, 376–382. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar]
- Typiak, M.; Żurawa-Janicka, D. Not an immune cell, but they may act like one—Cells with immune properties outside the immune system. Immunol. Cell Biol. 2024, 102, 487–499. [Google Scholar] [CrossRef]
- Han, S.M.; Han, S.H.; Coh, Y.R.; Jang, G.; Chan Ra, J.; Kang, S.K.; Lee, H.W.; Youn, H.Y. Enhanced proliferation and differentiation of Oct4- and Sox2-overexpressing human adipose tissue mesenchymal stem cells. Exp. Mol. Med. 2014, 46, e101. [Google Scholar] [CrossRef]
- Ghiasi, M.; Kalhor, N.; Tabatabaei Qomi, R.; Sheykhhasan, M. The effects of synthetic and natural scaffolds on viability and proliferation of adipose-derived stem cells. Front. Life Sci. 2016, 9, 32–43. [Google Scholar] [CrossRef]
- Hatzmann, F.M.; Ejaz, A.; Wiegers, G.J.; Mandl, M.; Brucker, C.; Lechner, S.; Rauchenwald, T.; Zwierzina, M.; Baumgarten, S.; Wagner, S.; et al. Quiescence, Stemness and Adipogenic Differentiation Capacity in Human DLK1−/CD34+/CD24+ Adipose Stem/Progenitor Cells. Cells 2021, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Pierantozzi, E.; Gava, B.; Manini, I.; Roviello, F.; Marotta, G.; Chiavarelli, M.; Sorrentino, V. Pluripotency Regulators in Human Mesenchymal Stem Cells: Expression of NANOG But Not of OCT-4 and SOX-2. Stem Cells Dev. 2010, 20, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Zeineddine, D.; Hammoud, A.A.; Mortada, M.; Boeuf, H. The Oct4 protein: More than a magic stemness marker. Am. J. Stem Cells 2014, 3, 74–82. [Google Scholar]
- Tsubaki, T.; Chijimatsu, R.; Takeda, T.; Abe, M.; Ochiya, T.; Tsuji, S.; Inoue, K.; Matsuzaki, T.; Iwanaga, Y.; Omata, Y.; et al. Aging and cell expansion enhance microRNA diversity in small extracellular vesicles produced from human adipose-derived stem cells. Cytotechnology 2025, 77, 15. [Google Scholar] [CrossRef]
- Suga, H.; Matsumoto, D.; Eto, H.; Inoue, K.; Aoi, N.; Kato, H.; Araki, J.; Yoshimura, K. Functional Implications of CD34 Expression in Human Adipose–Derived Stem/Progenitor Cells. Stem Cells Dev. 2009, 18, 1201–1210. [Google Scholar] [CrossRef]
- Davies, O.G.; Cooper, P.R.; Shelton, R.M.; Smith, A.J.; Scheven, B.A. Isolation of adipose and bone marrow mesenchymal stem cells using CD29 and CD90 modifies their capacity for osteogenic and adipogenic differentiation. J. Tissue Eng. 2015, 6, 2041731415592356. [Google Scholar] [CrossRef]
- Mildmay-White, A.; Khan, W. Cell Surface Markers on Adipose-Derived Stem Cells: A Systematic Review. Curr. Stem Cell Res. Ther. 2017, 12, 484–492. [Google Scholar] [CrossRef]
- Stojanović, S.; Najman, S.; Korać, A. Stem Cells Derived from Lipoma and Adipose Tissue—Similar Mesenchymal Phenotype but Different Differentiation Capacity Governed by Distinct Molecular Signature. Cells 2018, 7, 260. [Google Scholar] [CrossRef]
- Zhou, W.; Lin, J.; Zhao, K.; Jin, K.; He, Q.; Hu, Y.; Feng, G.; Cai, Y.; Xia, C.; Liu, H.; et al. Single-Cell Profiles and Clinically Useful Properties of Human Mesenchymal Stem Cells of Adipose and Bone Marrow Origin. Am. J. Sports Med. 2019, 47, 1722–1733. [Google Scholar] [CrossRef]
- Bunnell, B.A. Adipose Tissue-Derived Mesenchymal Stem Cells. Cells 2021, 10, 3433. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, X.; Min, J.; Kong, W.; Hu, X.; Zhang, J.; Chen, L. Advancements in culture technology of adipose-derived stromal/stem cells: Implications for diabetes and its complications. Front. Endocrinol. 2024, 15, 1343255. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Xiong, H.; Chen, J.; Yang, X.; Liu, Y.; Guo, J.; Jiang, T.; Xu, Z.; Yuan, M.; Liu, Y.; et al. The whole profiling and competing endogenous RNA network analyses of noncoding RNAs in adipose-derived stem cells from diabetic, old, and young patients. Stem. Cell Res. Ther. 2021, 12, 313. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.; David, G.; Jacobs, D.; Kuczwara, P.; Woessner, A.E.; Kim, J.W.; Quinn, K.P.; Song, Y. Neuro-regenerative behavior of adipose-derived stem cells in aligned collagen I hydrogels. Mater. Today Bio 2023, 22, 100762. [Google Scholar] [CrossRef]
- Almalki, S.G.; Agrawal, D.K. Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation 2016, 92, 41–51. [Google Scholar] [CrossRef]
- Tomita, K.; Madura, T.; Sakai, Y.; Yano, K.; Terenghi, G.; Hosokawa, K. Glial differentiation of human adipose-derived stem cells: Implications for cell-based transplantation therapy. Neuroscience 2013, 236, 55–65. [Google Scholar] [CrossRef]
- Luo, L.; Hu, D.H.; Yin, J.Q.; Xu, R.X. Molecular Mechanisms of Transdifferentiation of Adipose-Derived Stem Cells into Neural Cells: Current Status and Perspectives. Stem Cells Int. 2018, 2018, 5630802. [Google Scholar] [CrossRef]
- Mahoney, A.L.G.; Nassif, N.T.; O’Brien, B.A.; Simpson, A.M. Pancreatic Transdifferentiation Using β-Cell Transcription Factors for Type 1 Diabetes Treatment. Cells 2022, 11, 2145. [Google Scholar] [CrossRef]
- Choi, Y.S.; Dusting, G.J.; Stubbs, S.; Arunothayaraj, S.; Han, X.L.; Collas, P.; Morrison, W.A.; Dilley, R.J. Differentiation of human adipose-derived stem cells into beating cardiomyocytes. J. Cell. Mol. Med. 2010, 14, 878–889. [Google Scholar] [CrossRef]
- Walewska, A.; Janucik, A.; Tynecka, M.; Moniuszko, M.; Eljaszewicz, A. Mesenchymal stem cells under epigenetic control—The role of epigenetic machinery in fate decision and functional properties. Cell Death Dis. 2023, 14, 720. [Google Scholar] [CrossRef]
- Ozkul, Y.; Galderisi, U. The Impact of Epigenetics on Mesenchymal Stem Cell Biology. J. Cell. Physiol. 2016, 231, 2393–2401. [Google Scholar] [CrossRef] [PubMed]
- Kant, R.J.; Coulombe, K.L.K. Integrated approaches to spatiotemporally directing angiogenesis in host and engineered tissues. Acta Biomater. 2018, 69, 42–62. [Google Scholar] [CrossRef]
- Ghaedi, M.; Tuleuova, N.; Zern, M.A.; Wu, J.; Revzin, A. Bottom-up signaling from HGF-containing surfaces promotes hepatic differentiation of mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2011, 407, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Tirza, G.; Solodeev, I.; Sela, M.; Greenberg, I.; Pasmanik-Chor, M.; Gur, E.; Shani, N. Reduced culture temperature attenuates oxidative stress and inflammatory response facilitating expansion and differentiation of adipose-derived stem cells. Stem Cell Res. Ther. 2020, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Devitt, S.M.; Carter, C.M.; Dierov, R.; Weiss, S.; Gersch, R.P.; Percec, I. Successful Isolation of Viable Adipose-Derived Stem Cells from Human Adipose Tissue Subject to Long-Term Cryopreservation: Positive Implications for Adult Stem Cell-Based Therapeutics in Patients of Advanced Age. Stem Cells Int. 2015, 2015, 146421. [Google Scholar] [CrossRef]
- Kang, S.K.; Putnam, L.; Dufour, J.; Ylostalo, J.; Jung, J.S.; Bunnell, B.A. Expression of Telomerase Extends the Lifespan and Enhances Osteogenic Differentiation of Adipose Tissue–Derived Stromal Cells. Stem Cells 2004, 22, 1356–1372. [Google Scholar] [CrossRef]
- Tátrai, P.; Szepesi, Á.; Matula, Z.; Szigeti, A.; Buchan, G.; Mádi, A.; Uher, F.; Német, K. Combined introduction of Bmi-1 and hTERT immortalizes human adipose tissue-derived stromal cells with low risk of transformation. Biochem. Biophys. Res. Commun. 2012, 422, 28–35. [Google Scholar] [CrossRef]
- Balducci, L.; Blasi, A.; Saldarelli, M.; Soleti, A.; Pessina, A.; Bonomi, A.; Coccè, V.; Dossena, M.; Tosetti, V.; Ceserani, V.; et al. Immortalization of human adipose-derived stromal cells: Production of cell lines with high growth rate, mesenchymal marker expression and capability to secrete high levels of angiogenic factors. Stem Cell Res. Ther. 2014, 5, 63. [Google Scholar] [CrossRef]
- Locke, M.; Feisst, V.; Meidinger, S. From bench to bedside: Use of human adipose-derived stem cells. Stem Cells Cloning Adv. Appl. 2015, 8, 149–162. [Google Scholar] [CrossRef]
- Bailey, A.M.; Lawrence, M.B.; Shang, H.; Katz, A.J.; Peirce, S.M. Agent-Based Model of Therapeutic Adipose-Derived Stromal Cell Trafficking during Ischemia Predicts Ability To Roll on P-Selectin. PLoS Comput. Biol. 2009, 5, e1000294. [Google Scholar] [CrossRef]
- Suila, H.; Hirvonen, T.; Kotovuori, A.; Ritamo, I.; Kerkelä, E.; Anderson, H.; Natunen, S.; Tuimala, J.; Laitinen, S.; Nystedt, J.; et al. Human Umbilical Cord Blood-Derived Mesenchymal Stromal Cells Display a Novel Interaction between P-Selectin and Galectin-1. Scand. J. Immunol. 2014, 80, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ning, H.; Banie, L.; Wang, G.; Lin, G.; Lue, T.F.; Lin, C.-S. Adipose tissue-derived stem cells secrete CXCL5 cytokine with chemoattractant and angiogenic properties. Biochem. Biophys. Res. Commun. 2010, 402, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Jeon, E.S.; Lee, J.S.; Cho, M.; Suh, D.; Chang, C.L.; Kim, J.H. Lysophosphatidic acid in malignant ascites stimulates migration of human mesenchymal stem cells. J. Cell. Biochem. 2008, 104, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Han, B.; Cai, S.; Lei, Y.; Sun, T.; Sheng, Z. Migration of bone marrow-derived mesenchymal stem cells induced by tumor necrosis factor-α and its possible role in wound healing. Wound Repair Regen. 2009, 17, 185–191. [Google Scholar] [CrossRef]
- Yuan, L.; Sakamoto, N.; Song, G.; Sato, M. Low-Level Shear Stress Induces Human Mesenchymal Stem Cell Migration Through the SDF-1/CXCR4 Axis Via MAPK Signaling Pathways. Stem Cells Dev. 2013, 22, 2384–2393. [Google Scholar] [CrossRef]
- Ryu, C.H.; Park, S.A.; Kim, S.M.; Lim, J.Y.; Jeong, C.H.; Jun, J.A.; Oh, J.H.; Park, S.H.; Oh, W.-I.; Jeun, S.-S. Migration of human umbilical cord blood mesenchymal stem cells mediated by stromal cell-derived factor-1/CXCR4 axis via Akt, ERK, and p38 signal transduction pathways. Biochem. Biophys. Res. Commun. 2010, 398, 105–110. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, H.M.; Shang, D.S.; Fang, W.G.; He, Z.Y.; Chen, Y.H. The Involvement of CXCL11 in Bone Marrow-Derived Mesenchymal Stem Cell Migration Through Human Brain Microvascular Endothelial Cells. Neurochem. Res. 2014, 39, 700–706. [Google Scholar] [CrossRef]
- Zha, Y.; He, J.; Mei, Y.; Yin, T.; Mao, L. Zinc-finger transcription factor Snail accelerates survival, migration and expression of matrix metalloproteinase-2 in human bone mesenchymal stem cells. Cell Biol. Int. 2007, 31, 1089–1096. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.G.; Song, S.Y.; Kim, J.K.; Sung, J.H. Reactive oxygen species-responsive miR-210 regulates proliferation and migration of adipose-derived stem cells via PTPN2. Cell Death Dis. 2013, 4, e588. [Google Scholar] [CrossRef]
- Li, J.; Deng, T.; Zhu, S.; Xie, P.; Wang, W.; Zhou, H.; Xu, C. The SDF-1/CXCR4 axis is involved in adipose-derived stem cell migration. Neurourol. Urodyn. 2024, 43, 2279–2289. [Google Scholar] [CrossRef]
- Gan, F.; Liu, L.; Zhou, Q.; Huang, W.; Huang, X.; Zhao, X. Effects of adipose-derived stromal cells and endothelial progenitor cells on adipose transplant survival and angiogenesis. PLoS ONE 2022, 17, e0261498. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Han, J.; Hwang, S.J.; Sung, J.H. An update on niche composition, signaling and functional regulation of the adipose-derived stem cells. Expert Opin. Biol. Ther. 2014, 14, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Ko, G.R.; Lee, J.S. Engineering of Immune Microenvironment for Enhanced Tissue Remodeling. Tissue Eng. Regen. Med. 2022, 19, 221–236. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, M.; Lu, G.; Huang, B.; Wang, D.; Shao, Y.; Lu, M. Hypoxic Preconditioning Enhances Cellular Viability and Pro-angiogenic Paracrine Activity: The Roles of VEGF-A and SDF-1a in Rat Adipose Stem Cells. Front. Cell Dev. Biol. 2020, 8, 580131. [Google Scholar] [CrossRef]
- McIlhenny, S.E.; Hager, E.S.; Grabo, D.J.; DiMatteo, C.; Shapiro, I.M.; Tulenko, T.N.; DiMuzio, P.J. Linear Shear Conditioning Improves Vascular Graft Retention of Adipose-Derived Stem Cells by Upregulation of the α5 β1 Integrin. Tissue Eng. Part A 2010, 16, 245–255. [Google Scholar] [CrossRef]
- Casson, J.; O’Kane, S.; Smith, C.A.; Dalby, M.; Berry, C. Interleukin 6 Plays a Role in the Migration of Magnetically Levitated Mesenchymal Stem Cells Spheroids. Appl. Sci. 2018, 8, 412. [Google Scholar] [CrossRef]
- Li, W.; Liu, Q.; Shi, J.; Xu, X.; Xu, J. The role of TNF-α in the fate regulation and functional reprogramming of mesenchymal stem cells in an inflammatory microenvironment. Front. Immunol. 2023, 14, 1074863. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Z.; Jin, L.; Zhao, L.; Meng, L.; Kong, F.; He, C.; Kong, F.; Zheng, L.; Liang, F. LPS-pretreatment adipose-derived mesenchymal stromal cells promote wound healing in diabetic rats by improving angiogenesis. Injury 2022, 53, 3920–3929. [Google Scholar] [CrossRef]
- Liang, X.; Huang, X.; Zhou, Y.; Jin, R.; Li, Q. Mechanical Stretching Promotes Skin Tissue Regeneration via Enhancing Mesenchymal Stem Cell Homing and Transdifferentiation. Stem Cells Transl. Med. 2016, 5, 960–969. [Google Scholar] [CrossRef]
- Iwasa, S.N.; Babona-Pilipos, R.; Morshead, C.M. Environmental Factors That Influence Stem Cell Migration: An “Electric Field”. Stem Cells Int. 2017, 2017, 4276927. [Google Scholar] [CrossRef]
- Hammam, I.A.; Winters, R.; Hong, Z. Advancements in the application of biomaterials in neural tissue engineering: A review. Biomed. Eng. Adv. 2024, 8, 100132. [Google Scholar] [CrossRef]
- Steiner, D.; Mutschall, H.; Winkler, S.; Horch, R.E.; Arkudas, A. The Adipose-Derived Stem Cell and Endothelial Cell Coculture System—Role of Growth Factors? Cells 2021, 10, 2074. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, R.H.; Højgaard, L.D.; Reese-Petersen, A.L.; Hoeeg, C.; Mathiasen, A.B.; Haack-Sørensen, M.; Follin, B.; Genovese, F.; Kastrup, J.; Juhl, M.; et al. Adipose-derived stromal cells increase the formation of collagens through paracrine and juxtacrine mechanisms in a fibroblast co-culture model utilizing macromolecular crowding. Stem Cell Res. Ther. 2022, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.-T.; Li, H.-M.; Yin, Q.-S.; Liu, D.-L.; Nan, H.; Zhao, P.-R.; Liang, S.-W. Human Breast Adipose-Derived Stem Cells Transfected with the Stromal Cell-Derived Factor-1 Receptor CXCR4 Exhibit Enhanced Viability in Human Autologous Free Fat Grafts. Cell. Physiol. Biochem. 2014, 34, 2091–2104. [Google Scholar] [CrossRef]
- Resch, A.; Wolf, S.; Mann, A.; Weiss, T.; Stetco, A.L.; Radtke, C. Co-Culturing Human Adipose Derived Stem Cells and Schwann Cells on Spider Silk—A New Approach as Prerequisite for Enhanced Nerve Regeneration. Int. J. Mol. Sci. 2018, 20, 71. [Google Scholar] [CrossRef]
- Yang, F.; Cohen, R.N.; Brey, E.M. Optimization of Co-Culture Conditions for a Human Vascularized Adipose Tissue Model. Bioengineering 2020, 7, 114. [Google Scholar] [CrossRef]
- Dash, S.; Shakeel, A.; Mohanty, S. Impact of Nanotechnology on the Realm of Stem Cells and Regenerative Medicine. ChemNanoMat 2022, 8, e202200177. [Google Scholar] [CrossRef]
- Park, H.W.; Lee, C.E.; Kim, S.; Jeong, W.J.; Kim, K. Ex Vivo Peptide Decoration Strategies on Stem Cell Surfaces for Augmenting Endothelium Interaction. Tissue Eng. Part B Rev. 2024, 30, 327–339. [Google Scholar] [CrossRef]
- Bobis-Wozowicz, S.; Miekus, K.; Wybieralska, E.; Jarocha, D.; Zawisz, A.; Madeja, Z.; Majka, M. Genetically modified adipose tissue−derived mesenchymal stem cells overexpressing CXCR4 display increased motility, invasiveness, and homing to bone marrow of NOD/SCID mice. Exp. Hematol. 2011, 39, 686–696.e4. [Google Scholar] [CrossRef]
- Dai, R.; Wang, Z.; Samanipour, R.; Koo Kin Kim, K. Adipose-Derived Stem Cells for Tissue Engineering and Regenerative Medicine Applications. Stem Cells Int. 2016, 2016, 6737345. [Google Scholar] [CrossRef]
- Boccardo, S.; Gaudiello, E.; Melly, L.; Cerino, G.; Ricci, D.; Martin, I.; Eckstein, F.; Banfi, A.; Marsano, A. Engineered mesenchymal cell-based patches as controlled VEGF delivery systems to induce extrinsic angiogenesis. Acta Biomater. 2016, 42, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Luo, Y. Three-dimensional scaffolds. In Principles of Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 343–360. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780128184226000204 (accessed on 27 February 2025).
- Mukasheva, F.; Adilova, L.; Dyussenbinov, A.; Yernaimanova, B.; Abilev, M.; Akilbekova, D. Optimizing scaffold pore size for tissue engineering: Insights across various tissue types. Front. Bioeng. Biotechnol. 2024, 12, 1444986. [Google Scholar] [CrossRef]
- Nellinger, S.; Kluger, P.J. How Mechanical and Physicochemical Material Characteristics Influence Adipose-Derived Stem Cell Fate. Int. J. Mol. Sci. 2023, 24, 3551. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.S.; Edwards, J.; Jeon, H. Electrospun Fibrous Scaffolds for Tissue Engineering: Viewpoints on Architecture and Fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Golebiowska, A.A.; Intravaia, J.T.; Sathe, V.M.; Kumbar, S.G.; Nukavarapu, S.P. Decellularized extracellular matrix biomaterials for regenerative therapies: Advances, challenges and clinical prospects. Bioact. Mater. 2024, 32, 98–123. [Google Scholar] [CrossRef]
- Krishani, M.; Shin, W.Y.; Suhaimi, H.; Sambudi, N.S. Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review. Gels 2023, 9, 100. [Google Scholar] [CrossRef]
- Suamte, L.; Tirkey, A.; Barman, J.; Jayasekhar Babu, P. Various manufacturing methods and ideal properties of scaffolds for tissue engineering applications. Smart Mater. Manuf. 2023, 1, 100011. [Google Scholar] [CrossRef]
- Zonderland, J.; Moroni, L. Steering cell behavior through mechanobiology in 3D: A regenerative medicine perspective. Biomaterials 2021, 268, 120572. [Google Scholar] [CrossRef]
- Montanheiro, T.L.D.A.; Schatkoski, V.M.; De Menezes, B.R.C.; Pereira, R.M.; Ribas, R.G.; De Freitas, A.D.S.M.; Lemes, A.P.; Fernandes, M.H.F.V.; Thim, G.P. Recent progress on polymer scaffolds production: Methods, main results, advantages and disadvantages. Express Polym. Lett. 2022, 16, 197–219. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Yan, J.; Zhang, K.; Lin, F.; Xiang, L.; Deng, L.; Guan, Z.; Cui, W.; Zhang, H. Pharmaceutical electrospinning and 3D printing scaffold design for bone regeneration. Adv. Drug Deliv. Rev. 2021, 174, 504–534. [Google Scholar] [CrossRef] [PubMed]
- Fereshteh, Z. Freeze-drying technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds; Elsevier: Amsterdam, The Netherlands, 2018; pp. 151–174. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780081009796000070 (accessed on 22 February 2025).
- Sudhakar, K.; Ji, S.M.; Kummara, M.R.; Han, S.S. Recent Progress on Hyaluronan-Based Products for Wound Healing Applications. Pharmaceutics 2022, 14, 2235. [Google Scholar] [CrossRef] [PubMed]
- Man, E.; Lamprou, D.; Easdon, C.; McLellan, I.; Yiu, H.H.P.; Hoskins, C. Exploration of Dual Ionic Cross-Linked Alginate Hydrogels Via Cations of Varying Valences towards Wound Healing. Polymers 2022, 14, 5192. [Google Scholar] [CrossRef]
- Wu, K.; Yan, Z.; Wu, Z.; Li, J.; Zhong, W.; Ding, L.; Zhong, T.; Jiang, T. Recent Advances in the Preparation, Antibacterial Mechanisms, and Applications of Chitosan. J. Funct. Biomater. 2024, 15, 318. [Google Scholar] [CrossRef]
- Geng, Y.; Xue, H.; Zhang, Z.; Panayi, A.C.; Knoedler, S.; Zhou, W.; Mi, B.; Liu, G. Recent advances in carboxymethyl chitosan-based materials for biomedical applications. Carbohydr. Polym. 2023, 305, 120555. [Google Scholar] [CrossRef]
- Horue, M.; Silva, J.M.; Berti, I.R.; Brandão, L.R.; Barud, H.D.S.; Castro, G.R. Bacterial Cellulose-Based Materials as Dressings for Wound Healing. Pharmaceutics 2023, 15, 424. [Google Scholar] [CrossRef]
- Wosicka-Frąckowiak, H.; Poniedziałek, K.; Woźny, S.; Kuprianowicz, M.; Nyga, M.; Jadach, B.; Milanowski, B. Collagen and Its Derivatives Serving Biomedical Purposes: A Review. Polymers 2024, 16, 2668. [Google Scholar] [CrossRef]
- Chung, E.; Rytlewski, J.A.; Merchant, A.G.; Dhada, K.S.; Lewis, E.W.; Suggs, L.J. Fibrin-based 3D matrices induce angiogenic behavior of adipose-derived stem cells. Acta Biomater. 2015, 17, 78–88. [Google Scholar] [CrossRef]
- Hinsenkamp, A.; Fülöp, Á.; Hricisák, L.; Pál, É.; Kun, K.; Majer, A.; Varga, V.; Lacza, Z.; Hornyák, I. Application of Injectable, Crosslinked, Fibrin-Containing Hyaluronic Acid Scaffolds for In Vivo Remodeling. J. Funct. Biomater. 2022, 13, 119. [Google Scholar] [CrossRef]
- Bindi, B.; Perioli, A.; Melo, P.; Mattu, C.; Ferreira, A.M. Bioinspired Collagen/Hyaluronic Acid/Fibrin-Based Hydrogels for Soft Tissue Engineering: Design, Synthesis, and In Vitro Characterization. J. Funct. Biomater. 2023, 14, 495. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhuang, Y.; Cai, M.; Wang, X.; Lin, K. Decellularized extracellular matrix: A promising strategy for skin repair and regeneration. Eng. Regen. 2023, 4, 357–374. [Google Scholar] [CrossRef]
- Izadyari Aghmiuni, A.; Heidari Keshel, S.; Sefat, F.; AkbarzadehKhiyavi, A. Fabrication of 3D hybrid scaffold by combination technique of electrospinning-like and freeze-drying to create mechanotransduction signals and mimic extracellular matrix function of skin. Mater. Sci. Eng. C 2021, 120, 111752. [Google Scholar] [CrossRef]
- Hennink, W.E.; Van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose–Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, D.A.; Wu, D.; He, F.; Wang, H.; Huang, L.; Shi, D.; Liu, Q.; Ni, N.; Pakvasa, M.; et al. Applications of Biocompatible Scaffold Materials in Stem Cell-Based Cartilage Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 603444. [Google Scholar] [CrossRef]
- Lang, Z.; Chen, T.; Zhu, S.; Wu, X.; Wu, Y.; Miao, X.; Wang, Q.; Zhao, L.; Zhu, Z.; Xu, R.X. Construction of vascular grafts based on tissue-engineered scaffolds. Mater. Today Bio 2024, 29, 101336. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Jiang, X.; Dai, B.; Zhang, L.; Zhu, Y. Decellularized extracellular matrix materials for treatment of ischemic cardiomyopathy. Bioact. Mater. 2024, 33, 460–482. [Google Scholar] [CrossRef]
- Li, Q.; Li, M.; Li, M.; Zhang, Z.; Ma, H.; Zhao, L.; Zhang, M.; Wang, G. Adipose-derived mesenchymal stem cell seeded Atelocollagen scaffolds for cardiac tissue engineering. J. Mater. Sci. Mater. Med. 2020, 31, 83. [Google Scholar] [CrossRef]
- Rickel, A.P.; Deng, X.; Engebretson, D.; Hong, Z. Electrospun nanofiber scaffold for vascular tissue engineering. Mater. Sci. Eng. C 2021, 129, 112373. [Google Scholar] [CrossRef]
- Kim, J.J.; Cho, D.W. Advanced strategies in 3D bioprinting for vascular tissue engineering and disease modelling using smart bioinks. Virtual Phys. Prototyp. 2024, 19, e2395470. [Google Scholar] [CrossRef]
- Xu, R.; Dou, B.; Yu, S.; Wang, Z.; Zhang, Y.; Leng, L.; Ouyang, L.; Sun, W. Enabling 3D printability and vascular morphogenesis with double network dynamic hydrogels. Mater Today. 2025, 84, 10–27. [Google Scholar] [CrossRef]
- Carvalho, C.R.; Oliveira, J.M.; Reis, R.L. Modern Trends for Peripheral Nerve Repair and Regeneration: Beyond the Hollow Nerve Guidance Conduit. Front Bioeng. Biotechnol. 2019, 7, 337. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, T.; Li, Y. 3D Printing and Bioprinting Nerve Conduits for Neural Tissue Engineering. Polymers 2020, 12, 1637. [Google Scholar] [CrossRef]
- Tarricone, G.; Carmagnola, I.; Chiono, V. Tissue-Engineered Models of the Human Brain: State-of-the-Art Analysis and Challenges. J. Funct. Biomater. 2022, 13, 146. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Z.; Chen, X.; Li, R.; Li, D.; Feng, S. A Silk Fibroin/Collagen Nerve Scaffold Seeded with a Co-Culture of Schwann Cells and Adipose-Derived Stem Cells for Sciatic Nerve Regeneration. PLoS ONE 2016, 11, e0147184. [Google Scholar] [CrossRef]
- Dai, W.; Yang, Y.; Yang, Y.; Liu, W. Material advancement in tissue-engineered nerve conduit. Nanotechnol. Rev. 2021, 10, 488–503. [Google Scholar] [CrossRef]
- Desiderio, V.; De Francesco, F.; Schiraldi, C.; De Rosa, A.; La Gatta, A.; Paino, F.; d’Aquino, R.; Ferraro, G.A.; Tirino, V.; Papaccio, G. Human Ng2+ adipose stem cells loaded in vivo on a new crosslinked hyaluronic acid-lys scaffold fabricate a skeletal muscle tissue. J. Cell. Physiol. 2013, 228, 1762–1773. [Google Scholar] [CrossRef]
- Xie, J.; Bian, H.; Qi, S.; Xu, Y.; Tang, J.; Li, T.; Liu, X. Effects of basic fibroblast growth factor on the expression of extracellular matrix and matrix metalloproteinase-1 in wound healing. Clin. Exp. Dermatol. 2008, 33, 176–182. [Google Scholar] [CrossRef]
- Saberianpour, S.; Heidarzadeh, M.; Geranmayeh, M.H.; Hosseinkhani, H.; Rahbarghazi, R.; Nouri, M. Tissue engineering strategies for the induction of angiogenesis using biomaterials. J. Biol. Eng. 2018, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Bian, D.; Wu, Y.; Song, G. Basic fibroblast growth factor combined with extracellular matrix-inspired mimetic systems for effective skin regeneration and wound healing. Mater. Today Commun. 2023, 35, 105876. [Google Scholar] [CrossRef]
- Fadera, S.; Cheng, N.C.; Young, T.H.; Lee, I.C. In vitro study of SDF-1α-loaded injectable and thermally responsive hydrogels for adipose stem cell therapy by SDF-1/CXCR4 axis. J. Mater. Chem. B 2020, 8, 10360–10372. [Google Scholar] [CrossRef]
- Beederman, M.; Lamplot, J.D.; Nan, G.; Wang, J.; Liu, X.; Yin, L.; Li, R.; Shui, W.; Zhang, H.; Kim, S.H.; et al. BMP signaling in mesenchymal stem cell differentiation and bone formation. J. Biomed. Sci. Eng. 2013, 06, 32–52. [Google Scholar] [CrossRef]
- Wen, C.; Xu, L.; Xu, X.; Wang, D.; Liang, Y.; Duan, L. Insulin-like growth factor-1 in articular cartilage repair for osteoarthritis treatment. Arthritis Res. Ther. 2021, 23, 277. [Google Scholar] [CrossRef]
- Whitty, C.; Pernstich, C.; Marris, C.; McCaskie, A.; Jones, M.; Henson, F. Sustained delivery of the bone morphogenetic proteins BMP-2 and BMP-7 for cartilage repair and regeneration in osteoarthritis. Osteoarthr. Cartil. Open. 2022, 4, 100240. [Google Scholar] [CrossRef]
- Wang, T.; Yang, F. A comparative study of chondroitin sulfate and heparan sulfate for directing three-dimensional chondrogenesis of mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 284. [Google Scholar] [CrossRef]
- Li, X.; Ding, J.; Zhuang, X.; Chang, F.; Wang, J.; Chen, X. Chitosan-Based Scaffolds for Cartilage Regeneration. In Chitin and Chitosan for Regenerative Medicine; Springer Series on Polymer and Composite Materials; Dutta, P.K., Ed.; Springer: New Delhi, India, 2016; pp. 61–82. Available online: https://link.springer.com/10.1007/978-81-322-2511-9_3 (accessed on 22 February 2025).
- Zhao, T.; Li, X.; Li, H.; Deng, H.; Li, J.; Yang, Z.; He, S.; Jiang, S.; Sui, X.; Guo, Q.; et al. Advancing drug delivery to articular cartilage: From single to multiple strategies. Acta Pharm. Sin. B 2023, 13, 4127–4148. [Google Scholar] [CrossRef]
- Yu, J.; Wang, A.; Tang, Z.; Henry, J.; Li-Ping Lee, B.; Zhu, Y.; Yuan, F.; Huang, F.; Li, S. The effect of stromal cell-derived factor-1α/heparin coating of biodegradable vascular grafts on the recruitment of both endothelial and smooth muscle progenitor cells for accelerated regeneration. Biomaterials 2012, 33, 8062–8074. [Google Scholar] [CrossRef]
- Wei, Z.; Volkova, E.; Blatchley, M.R.; Gerecht, S. Hydrogel vehicles for sequential delivery of protein drugs to promote vascular regeneration. Adv. Drug Deliv. Rev. 2019, 149–150, 95–106. [Google Scholar] [CrossRef]
- Chen, S.; An, J.; Weng, L.; Li, Y.; Xu, H.; Wang, Y.; Ding, D.; Kong, D.; Wang, S. Construction and biofunctional evaluation of electrospun vascular graft loaded with selenocystamine for in situ catalytic generation of nitric oxide. Mater. Sci. Eng. C 2014, 45, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Fakhr, M.J.; Kavakebian, F.; Ababzadeh, S.; Rezapour, A. Challenges and Advances in Peripheral Nerve Tissue Engineering Critical Factors Affecting Nerve Regeneration. J. Tissue Eng. Regen. Med. 2024, 2024, 8868411. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, E.J.; Moon, L.D.F.; Popat, R.J.; King, V.R.; Bennett, G.S.; Patel, P.N.; Fawcett, J.W.; McMahon, S.B. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 2002, 416, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, M.A.; El-Fawal, H.A.N.; Allam, N.K. Biocompatible PCL-nanofibers scaffold with immobilized fibronectin and laminin for neuronal tissue regeneration. Mater. Sci. Eng. C. 2021, 119, 111550. [Google Scholar] [CrossRef]
- Xu, P.; Wu, Y.; Zhou, L.; Yang, Z.; Zhang, X.; Hu, X.; Yang, J.; Wang, M.; Wang, B.; Luo, G.; et al. Platelet-rich plasma accelerates skin wound healing by promoting re-epithelialization. Burns Trauma 2020, 8, tkaa028. [Google Scholar] [CrossRef]
- Ghiasi, M.; Farzaneh, S.; Bigdelo, M. Assessment of human cartilage regeneration in a patient with knee osteoarthritis using autologous adipose-tissue-derived stem cells and Platelet-rich plasma: A case study. J. Surg. Trauma 2020, 8, 73–78. [Google Scholar]
- Chen, X.B.; Fazel Anvari-Yazdi, A.; Duan, X.; Zimmerling, A.; Gharraei, R.; Sharma, N.K.; Sweilem, S.; Ning, L. Biomaterials/bioinks and extrusion bioprinting. Bioact. Mater. 2023, 28, 511–536. [Google Scholar] [CrossRef]
- Xiang, J.Y.; Kang, L.; Li, Z.M.; Tseng, S.L.; Wang, L.Q.; Li, T.H.; Li, Z.-J.; Huang, J.-Z.; Yu, N.-Z.; Long, X. Biological scaffold as potential platforms for stem cells: Current development and applications in wound healing. World J. Stem Cells 2024, 16, 334–352. [Google Scholar] [CrossRef]
- Flynn, L.; Prestwich, G.D.; Semple, J.L.; Woodhouse, K.A. Adipose tissue engineering with naturally derived scaffolds and adipose-derived stem cells. Biomaterials 2007, 28, 3834–3842. [Google Scholar] [CrossRef]
- Kang, H.; Peng, J.; Lu, S.; Liu, S.; Zhang, L.; Huang, J.; Sui, X.; Zhao, B.; Wang, A.; Xu, W.; et al. In vivo cartilage repair using adipose-derived stem cell-loaded decellularized cartilage ECM scaffolds: In vivo cartilage repair using ADSC-loaded decellularized cartilage ECM scaffolds. J. Tissue Eng. Regen. Med. 2014, 8, 442–453. [Google Scholar] [CrossRef]
- He, Y.; Lu, F. Development of Synthetic and Natural Materials for Tissue Engineering Applications Using Adipose Stem Cells. Stem Cells Int. 2016, 2016, 5786257. [Google Scholar] [CrossRef] [PubMed]
- Barbulescu, G.I.; Bojin, F.M.; Ordodi, V.L.; Goje, I.D.; Barbulescu, A.S.; Paunescu, V. Decellularized Extracellular Matrix Scaffolds for Cardiovascular Tissue Engineering: Current Techniques and Challenges. Int. J. Mol. Sci. 2022, 23, 13040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chen, Y.; Hao, M.; Xia, Y. Putting Hybrid Nanomaterials to Work for Biomedical Applications. Angew. Chem. Int. Ed. 2024, 63, e202319567. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Holzwarth, J.M.; Ma, P.X. Functionalized Synthetic Biodegradable Polymer Scaffolds for Tissue Engineering. Macromol. Biosci. 2012, 12, 911–919. [Google Scholar] [CrossRef]
- Echeverria Molina, M.I.; Malollari, K.G.; Komvopoulos, K. Design Challenges in Polymeric Scaffolds for Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 617141. [Google Scholar] [CrossRef]
- Yang, Z.; Yin, G.; Sun, S.; Xu, P. Medical applications and prospects of polylactic acid materials. iScience 2024, 27, 111512. [Google Scholar] [CrossRef]
- Kontaxis, L.C.; Zachos, D.; Georgali-Fickel, A.; Portan, D.V.; Zaoutsos, S.P.; Papanicolaou, G.C. 3D-Printed PLA Mechanical and Viscoelastic Behavior Dependence on the Nozzle Temperature and Printing Orientation. Polymers 2025, 17, 913. [Google Scholar] [CrossRef]
- Rahman, G.; Frazier, T.P.; Gimble, J.M.; Mohiuddin, O.A. The Emerging Use of ASC/Scaffold Composites for the Regeneration of Osteochondral Defects. Front. Bioeng. Biotechnol. 2022, 10, 893992. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, X.; Fu, Y.; Jin, Y.; Weng, Y.; Bian, X.; Chen, X. Degradation Behaviors of Polylactic Acid, Polyglycolic Acid, and Their Copolymer Films in Simulated Marine Environments. Polymers 2024, 16, 1765. [Google Scholar] [CrossRef]
- Barbarisi, M.; Marino, G.; Armenia, E.; Vincenzo, Q.; Rosso, F.; Porcelli, M.; Barbarisi, A. Use of polycaprolactone (PCL) as scaffolds for the regeneration of nerve tissue. J. Biomed. Mater. Res. A 2015, 103, 1755–1760. [Google Scholar] [CrossRef]
- Liang, H.Y.; Lee, W.K.; Hsu, J.T.; Shih, J.Y.; Ma, T.L.; Vo, T.T.T.; Lee, C.-W.; Cheng, M.-T.; Lee, I.-T. Polycaprolactone in Bone Tissue Engineering: A Comprehensive Review of Innovations in Scaffold Fabrication and Surface Modifications. J. Funct. Biomater. 2024, 15, 243. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.; Narayana Kalkura, S.; Balme, S.; Bohatier, C.P.; Miele, P.; Bechelany, M. Nanofibrous Scaffolds for Tissue Engineering Application. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A.S.H., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 665–691. Available online: http://link.springer.com/10.1007/978-3-319-53655-2_30 (accessed on 27 February 2025).
- Wang, Z.; Ye, Q.; Yu, S.; Akhavan, B. Poly Ethylene Glycol (PEG)-Based Hydrogels for Drug Delivery in Cancer Therapy: A Comprehensive Review. Adv. Healthc. Mater. 2023, 12, 2300105. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.G.; Revzin, A.; Pishko, M.V. Poly(ethylene glycol) Hydrogel Microstructures Encapsulating Living Cells. Langmuir 2002, 18, 2459–2462. [Google Scholar] [CrossRef]
- Ramanathan, S.; Lin, Y.C.; Thirumurugan, S.; Hu, C.C.; Duann, Y.F.; Chung, R.J. Poly(methyl methacrylate) in Orthopedics: Strategies, Challenges, and Prospects in Bone Tissue Engineering. Polymers 2024, 16, 367. [Google Scholar] [CrossRef]
- Miri, Z.; Farè, S.; Ma, Q.; Haugen, H.J. Updates on polyurethane and its multifunctional applications in biomedical engineering. Prog. Biomed. Eng. 2023, 5, 042001. [Google Scholar] [CrossRef]
- Ribeiro, M.; Simões, M.; Vitorino, C.; Mascarenhas-Melo, F. Hydrogels in Cutaneous Wound Healing: Insights into Characterization, Properties, Formulation and Therapeutic Potential. Gels 2024, 10, 188. [Google Scholar] [CrossRef]
- Richbourg, N.R.; Peppas, N.A.; Sikavitsas, V.I. Tuning the biomimetic behavior of scaffolds for regenerative medicine through surface modifications. J. Tissue Eng. Regen. Med. 2019, 13, 1275–1293. [Google Scholar] [CrossRef]
- Shineh, G.; Patel, K.; Mobaraki, M.; Tayebi, L. Functional Approaches in Promoting Vascularization and Angiogenesis in Bone Critical-Sized Defects via Delivery of Cells, Growth Factors, Drugs, and Particles. J. Funct. Biomater. 2023, 14, 99. [Google Scholar] [CrossRef]
- Mansour, A.; Romani, M.; Acharya, A.B.; Rahman, B.; Verron, E.; Badran, Z. Drug Delivery Systems in Regenerative Medicine: An Updated Review. Pharmaceutics 2023, 15, 695. [Google Scholar] [CrossRef]
- Budharaju, H.; Suresh, S.; Sekar, M.P.; De Vega, B.; Sethuraman, S.; Sundaramurthi, D.; Kalaskar, D.M. Ceramic materials for 3D printing of biomimetic bone scaffolds—Current state-of-the-art & future perspectives. Mater. Des. 2023, 231, 112064. [Google Scholar]
- Portan, D.V.; Deligianni, D.D.; Papanicolaou, G.C.; Kostopoulos, V.; Psarras, G.C.; Tyllianakis, M. Combined Optimized Effect of a Highly Self-Organized Nanosubstrate and an Electric Field on Osteoblast Bone Cells Activity. BioMed Res. Int. 2019, 2019, 7574635. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Li, P.; Liu, J.; Peng, S. Optimization of TCP/HAP ratio for better properties of calcium phosphate scaffold via selective laser sintering. Mater. Charact. 2013, 77, 23–31. [Google Scholar] [CrossRef]
- Fernandes, H.R.; Gaddam, A.; Rebelo, A.; Brazete, D.; Stan, G.E.; Ferreira, J.M.F. Bioactive Glasses and Glass-Ceramics for Healthcare Applications in Bone Regeneration and Tissue Engineering. Materials 2018, 11, 2530. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Fu, S.; Zhu, Y. 3D printing of ceramic-based scaffolds for bone tissue engineering: An overview. J. Mater. Chem. B 2018, 6, 4397–4412. [Google Scholar] [CrossRef]
- Cinici, B.; Yaba, S.; Kurt, M.; Yalcin, H.C.; Duta, L.; Gunduz, O. Fabrication Strategies for Bioceramic Scaffolds in Bone Tissue Engineering with Generative Design Applications. Biomimetics 2024, 9, 409. [Google Scholar] [CrossRef]
- Tanvir, M.A.H.; Khaleque, M.A.; Kim, G.H.; Yoo, W.Y.; Kim, Y.Y. The Role of Bioceramics for Bone Regeneration: History, Mechanisms, and Future Perspectives. Biomimetics 2024, 9, 230. [Google Scholar] [CrossRef]
- De Carvalho, A.B.G.; Rahimnejad, M.; Oliveira, R.L.M.S.; Sikder, P.; Saavedra, G.S.F.A.; Bhaduri, S.B.; Gawlitta, D.; Malda, J.; Kaigler, D.; Trichês, E.S.; et al. Personalized bioceramic grafts for craniomaxillofacial bone regeneration. Int. J. Oral Sci. 2024, 16, 62. [Google Scholar] [CrossRef]
- Arcos, D.; Vallet-Regí, M. Bioceramics for drug delivery. Acta Mater. 2013, 61, 890–911. [Google Scholar] [CrossRef]
- Huang, B.; Caetano, G.; Vyas, C.; Blaker, J.; Diver, C.; Bártolo, P. Polymer-Ceramic Composite Scaffolds: The Effect of Hydroxyapatite and β-tri-Calcium Phosphate. Materials 2018, 11, 129. [Google Scholar] [CrossRef]
- Farjaminejad, S.; Farjaminejad, R.; Hasani, M.; Garcia-Godoy, F.; Abdouss, M.; Marya, A.; Harsoputranto, A.; Jamilian, A. Advances and Challenges in Polymer-Based Scaffolds for Bone Tissue Engineering: A Path Towards Personalized Regenerative Medicine. Polymers 2024, 16, 3303. [Google Scholar] [CrossRef]
- Sousa, A.C.; Alvites, R.; Lopes, B.; Sousa, P.; Moreira, A.; Coelho, A.; Rêma, A.; Biscaia, S.; Cordeiro, R.; Faria, F.; et al. Hybrid scaffolds for bone tissue engineering: Integration of composites and bioactive hydrogels loaded with hDPSCs. Biomater. Adv. 2025, 166, 214042. [Google Scholar] [CrossRef] [PubMed]
- Becerra, J.; Rodriguez, M.; Leal, D.; Noris-Suarez, K.; Gonzalez, G. Chitosan-collagen-hydroxyapatite membranes for tissue engineering. J. Mater. Sci. Mater. Med. 2022, 33, 18. [Google Scholar] [CrossRef] [PubMed]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. Nanomed. 2017, 12, 4937–4961. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; Giuffrida, R.; Forte, S.; Fabbi, C.; Figallo, E.; Salvatorelli, L.; Memeo, L.; Parenti, R.; Gulisano, M.; Gulino, R. Human adipose-derived mesenchymal stem cells seeded into a collagen-hydroxyapatite scaffold promote bone augmentation after implantation in the mouse. Sci. Rep. 2017, 7, 7110. [Google Scholar] [CrossRef]
- Si, Y.; Yu, J.; Tang, X.; Ge, J.; Ding, B. Ultralight nanofibre-assembled cellular aerogels with superelasticity and multifunctionality. Nat. Commun. 2014, 5, 5802. [Google Scholar] [CrossRef]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.M.; Mahapatra, C.; Kim, H.-W.; Knowles, J.C. Sol–gel based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Liebig, B.E.; Kisiday, J.D.; Bahney, C.S.; Ehrhart, N.P.; Goodrich, L.R. The platelet-rich plasma and mesenchymal stem cell milieu: A review of therapeutic effects on bone healing. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2020, 38, 2539–2550. [Google Scholar] [CrossRef]
- Oktaş, B.; Çırpar, M.; Şanlı, E.; Canbeyli, İ.D.; Bozdoğan, Ö. The effect of the platelet-rich plasma on osteogenic potential of the periosteum in an animal bone defect model. Jt. Dis. Relat. Surg. 2021, 32, 668–675. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Kawazoe, N.; Chen, G. Preparation of dexamethasone-loaded calcium phosphate nanoparticles for the osteogenic differentiation of human mesenchymal stem cells. J. Mater. Chem. B 2017, 5, 6801–6810. [Google Scholar] [CrossRef]
- Costa, P.F.; Puga, A.M.; Díaz-Gomez, L.; Concheiro, A.; Busch, D.H.; Alvarez-Lorenzo, C. Additive manufacturing of scaffolds with dexamethasone controlled release for enhanced bone regeneration. Int. J. Pharm. 2015, 496, 541–550. [Google Scholar] [CrossRef]
- Gao, S.; Calcagni, M.; Welti, M.; Hemmi, S.; Hild, N.; Stark, W.J.; Bürgisser, G.M.; Wanner, G.A.; Cinelli, P.; Buschmann, J. Proliferation of ASC-derived endothelial cells in a 3D electrospun mesh: Impact of bone-biomimetic nanocomposite and co-culture with ASC-derived osteoblasts. Injury 2014, 45, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [PubMed]
- Nayak, G.S.; Carradò, A.; Masson, P.; Pourroy, G.; Mouillard, F.; Migonney, V.; Falentin-Daudre, C.; Pereira, C.; Palkowski, H. Trends in Metal-Based Composite Biomaterials for Hard Tissue Applications. JOM 2022, 74, 102–125. [Google Scholar] [CrossRef]
- Bommala, V.K.; Krishna, M.G.; Rao, C.T. Magnesium matrix composites for biomedical applications: A review. J. Magnes. Alloys 2019, 7, 72–79. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, J.; Wang, Y.; Chen, C.; Gu, H.; Chai, Y.; Wang, Y. Silver nanoparticles-decorated and mesoporous silica coated single-walled carbon nanotubes with an enhanced antibacterial activity for killing drug-resistant bacteria. Nano Res. 2020, 13, 389–400. [Google Scholar] [CrossRef]
- MacDonald, R.A.; Voge, C.M.; Kariolis, M.; Stegemann, J.P. Carbon nanotubes increase the electrical conductivity of fibroblast-seeded collagen hydrogels. Acta Biomater. 2008, 4, 1583–1592. [Google Scholar] [CrossRef]
- Yang, G.; Waheed, S.; Wang, C.; Shekh, M.; Li, Z.; Wu, J. Exosomes and Their Bioengineering Strategies in the Cutaneous Wound Healing and Related Complications: Current Knowledge and Future Perspectives. Int. J. Biol. Sci. 2023, 19, 1430–1454. [Google Scholar] [CrossRef]
- Moghassemi, S.; Dadashzadeh, A.; Sousa, M.J.; Vlieghe, H.; Yang, J.; León-Félix, C.M.; Amorim, C.A. Extracellular vesicles in nanomedicine and regenerative medicine: A review over the last decade. Bioact. Mater. 2024, 36, 126–156. [Google Scholar] [CrossRef]
- Fan, M.H.; Pi, J.K.; Zou, C.Y.; Jiang, Y.L.; Li, Q.J.; Zhang, X.Z.; Xing, F.; Nie, R.; Han, C.; Xie, H.Q. Hydrogel-exosome system in tissue engineering: A promising therapeutic strategy. Bioact. Mater. 2024, 38, 1–30. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).