Influence of Scaffold Structure and Biomimetic Properties on Adipose Stem Cell Homing in Personalized Reconstructive Medicine
Abstract
1. Introduction
2. Phenotype and Functional Properties of Human Adipose Stem Cells
3. ASC Homing
4. Strategies to Enhance ASC Homing for Improved Regenerative Outcomes
4.1. Chemotactic Signaling
4.2. Immunomodulation for ASC Recruitment
4.3. Preconditioning Strategies
4.4. Cell-Based Strategies for Enhanced Homing
4.5. Surface Modification and Genetic Engineering
4.6. Biomaterial-Assisted Homing
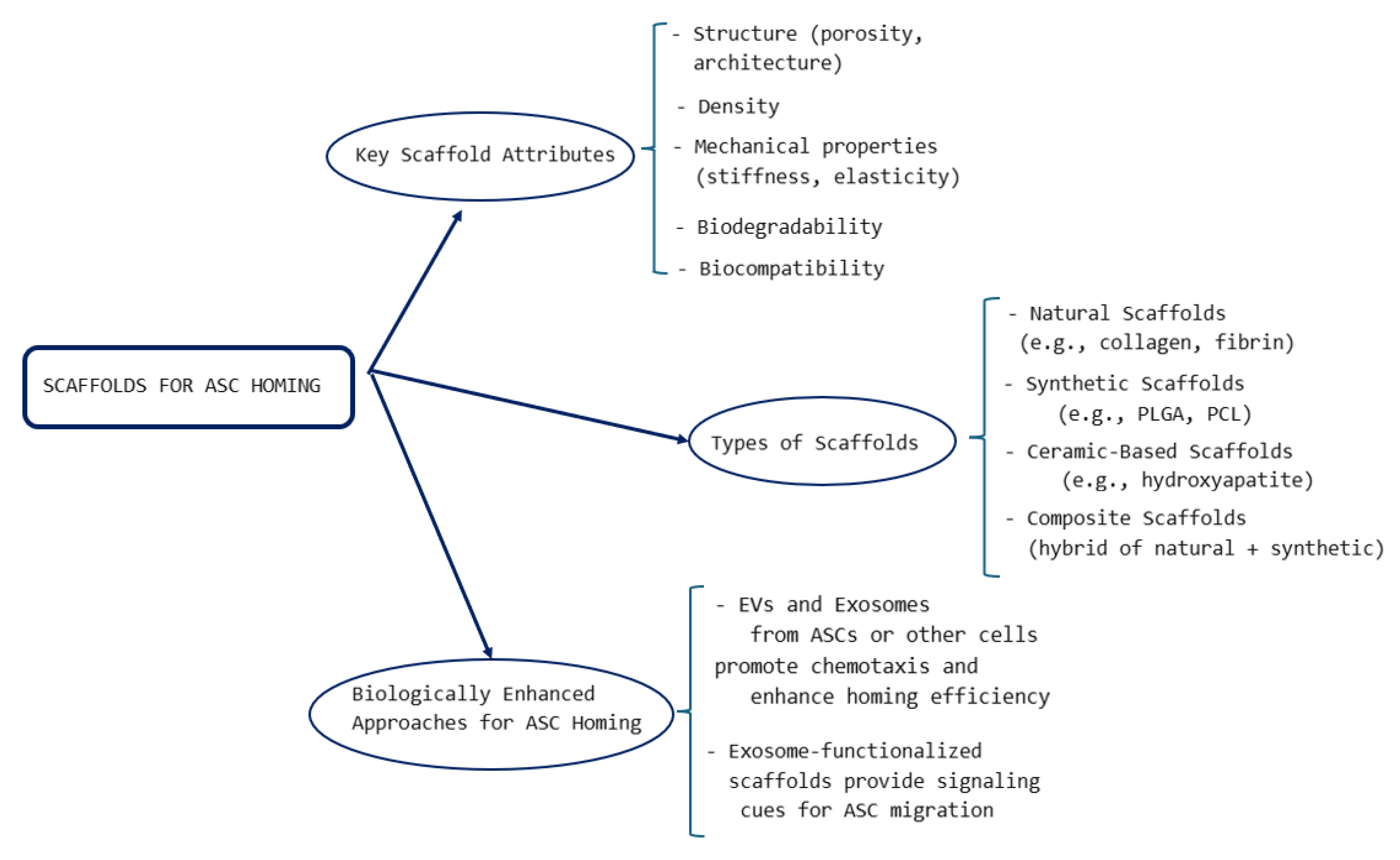
4.6.1. Key Attributes of Scaffolds Used for Tissue Regeneration
4.6.2. Natural Scaffolds for Adipose Stem Cell-Based Tissue Engineering
4.6.3. Synthetic Scaffolds for Tissue Regeneration
| Synthetic Scaffolds | Material | Properties | Applications | References |
|---|---|---|---|---|
| Polylactic acid (PLA) | Biodegradable polymer | Slow degradation, low osteoinductive capacity | Bone, cartilage regeneration | [162,163,164] |
| Polyglycolic acid (PGA) | Biodegradable polymer | Rapid degradation | Sutures, soft tissue repair | [165] |
| Poly (lactic-co-glycolic acid) (PLGA) | Copolymer | Tunable degradation, drug-delivery applications | Drug delivery, scaffolding | [165,167] |
| Polycaprolactone (PCL) | Biodegradable polymer | Long degradation time, high mechanical strength | Bone, nerve regeneration | [166,167,168] |
| Polyethylene glycol (PEG) | Non-biodegradable polymer | Biocompatible, hydrophilic | Drug delivery, cell encapsulation | [169,170] |
| Polymethylmethacrylate (PMMA) | Non-biodegradable polymer | High stability, used in bone cements | Orthopedics, dental applications | [171] |
| Polyurethane (PU) | Synthetic polymer | Flexible, high mechanical strength | Cardiovascular, orthopedic applications | [172] |
4.6.4. Ceramic-Based Scaffolds
4.6.5. Composite Scaffolds
4.7. Extracellular Vesicles (EVs) and Exosome-Based Approaches
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANG-2 | angiopoietin-2 |
| ASC | adipose stem cell |
| bFGF | basic fibroblast growth factor |
| BC | bacterial cellulose |
| BCP | biphasic calcium phosphate |
| BDNF | brain-derived neurotrophic factor |
| BMP | bone morphogenetic protein |
| 2D | bi-dimensional |
| CaP | calcium phosphate |
| CCR | C-C motif chemokine receptor |
| CXCL | C-X-C motif chemokine ligand |
| CXCR | C-X-C motif chemokine receptor |
| CNT | carbon nanotube |
| dECM | decellularized extracellular matrix |
| Erk1/2 | extracellular signal-regulated kinase 1/2 |
| ECM | extracellular matrix |
| EV | extracellular vesicle |
| FDA | Food and Drug Administration |
| FBS | fetal bovine serum |
| FGF | fibroblast growth factor |
| GDNF | glial cell-derived neurotrophic factor |
| GNP | graphene nanoplatelet |
| GPI | glycosylphosphatidylinositol |
| HA | hyaluronic acid |
| HIF | hypoxia inducible factor |
| HLA | human leukocyte antigen |
| HPV | human papilloma virus |
| HGF | hepatocyte growth factor |
| ICAM | intercellular adhesion molecule |
| IGF | insulin-like growth factor |
| JNK | jun N-terminus kinase |
| LPA | lysophosphatidic acid |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MAT | micronized adipose tissue |
| MHC | major histocompatibility complex |
| MMP | matrix metalloproteinase |
| mRNA | messenger ribonucleic acid |
| miRNA | microribonucleic acid |
| MSC | mesenchymal stem cell |
| MCP-1 | monocyte chemoattractant protein-1 |
| NGF | nerve growth factor |
| Oct | octamer transcription factor |
| PCL | polycaprolactone |
| PDGFR | platelet-derived growth factor receptor |
| PEG | polyethylene glycol |
| PGA | polyglycolic acid |
| PI3K | phosphatidylinositol-3-kinase |
| PLA | polylactic acid |
| PLGA | poly(lactic-co-glycolic acid) |
| PMMA | polymethylmethacrylate |
| PODXL | podocalyxin |
| PRP | platelet-rich plasma |
| PU | polyurethane |
| ROS | reactive oxygen species |
| RNA | ribonucleic acid |
| SVF | stromal vascular fraction |
| TERT | telomerase reverse transcriptase |
| 3D | three-dimensional |
| tPRP | thrombin-activated platelet-rich plasma |
| tRNA | transfer ribonucleic acid |
| VCAM | vascular cell adhesion molecule 1 |
| VEGF | vascular endothelial growth factor |
| VLA-4 | very late antigen-4 |
References
- Kozaniti, F.K.; Deligianni, D.D.; Georgiou, M.D.; Portan, D.V. The Role of Substrate Topography and Stiffness on MSC Cells Functions: Key Material Properties for Biomimetic Bone Tissue Engineering. Biomimetics 2021, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Sideridis, E.; Theocaris, P.S.; Papanicolaou, G.C. The elastic modulus of particulate composites using the concept of a mesophase. Rheol. Acta 1986, 25, 350–358. [Google Scholar] [CrossRef]
- Anifantis, N.K.; Kakavas, P.A.; Papanicolaou, G.C. Thermal stress concentration due to imperfect adhesion in fiber-reinforced composites. Compos. Sci. Technol. 1997, 57, 687–696. [Google Scholar] [CrossRef]
- Portan, D.; Papaefthymiou, K.; Pirvu, C.; Papanicolaou, G.; Demetrescu, I. Manufacturing and characterization of TiO(2) nanotubes on pure titanium surfaces for advanced biomedical applications. UPB Bul. Stiintific Ser. B Chem. Mater. Sci. 2011, 73, 181–196. [Google Scholar]
- Portan, D.V.; Papanicolaou, G.C.; Jiga, G.; Caposi, M. A novel experimental method for obtaining multi-layered TiO2 nanotubes through electrochemical anodizing. J. Appl. Electrochem. 2012, 42, 1013–1024. [Google Scholar] [CrossRef]
- Papanicolaou, G.C.; Charitidis, C.A.; Portan, D.V.; Perivoliotis, D.K.; Koklioti, M.A. Investigation of nanomechanical properties of multilayered hybrid nanocomposites. Meccanica 2014, 49, 2645–2655. [Google Scholar] [CrossRef]
- Portan, D.V.; Koliadima, A.; Kapolos, J.; Azamfirei, L. Biomimetic Design and Assessment via Microenvironmental Testing: From Food Packaging Biomaterials to Implantable Medical Devices. Biomimetics 2025, 10, 370. [Google Scholar] [CrossRef]
- Feier, A.M.; Portan, D.; Manu, D.R.; Kostopoulos, V.; Kotrotsos, A.; Strnad, G.; Dobreanu, M.; Salcudean, A.; Bataga, T. Primary MSCs for Personalized Medicine: Ethical Challenges, Isolation and Biocompatibility Evaluation of 3D Electrospun and Printed Scaffolds. Biomedicines 2022, 10, 1563. [Google Scholar] [CrossRef]
- Mei, R.; Wan, Z.; Yang, C.; Shen, X.; Wang, R.; Zhang, H.; Yang, R.; Li, J.; Song, Y.; Su, H. Advances and clinical challenges of mesenchymal stem cell therapy. Front. Immunol. 2024, 15, 1421854. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1421854/full (accessed on 29 March 2025). [CrossRef]
- Wang, M.; Wang, J.; Xu, X.; Li, E.; Xu, P. Engineering gene-activated bioprinted scaffolds for enhancing articular cartilage repair. Mater. Today Bio 2024, 29, 101351. [Google Scholar] [CrossRef]
- Da Silva, K.; Kumar, P.; Choonara, Y.E. The paradigm of stem cell secretome in tissue repair and regeneration: Present and future perspectives. Wound Repair Regen. 2025, 33, e13251. Available online: https://onlinelibrary.wiley.com/doi/10.1111/wrr.13251 (accessed on 29 March 2025). [CrossRef] [PubMed]
- Yan, S.; Campos De Souza, S.; Xie, Z.; Bao, Y. Research progress in clinical trials of stem cell therapy for stroke and neurodegenerative diseases. Ibrain 2023, 9, 214–230. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Mi, B.B.; Lin, Z.; Hu, Y.Q.; Yu, L.; Zha, K.K.; Panayi, A.C.; Yu, T.; Chen, L.; Liu, Z.-P.; et al. The role of the immune microenvironment in bone, cartilage, and soft tissue regeneration: From mechanism to therapeutic opportunity. Mil. Med. Res. 2022, 9, 65. [Google Scholar] [CrossRef]
- Trigo, C.M.; Rodrigues, J.S.; Camões, S.P.; Solá, S.; Miranda, J.P. Mesenchymal stem cell secretome for regenerative medicine: Where do we stand? J. Adv. Res. 2025, 70, 103–124. [Google Scholar] [CrossRef] [PubMed]
- Kozaniti, F.K.; Manara, A.E.; Kostopoulos, V.; Mallis, P.; Michalopoulos, E.; Polyzos, D.; Deligianni, D.D.; Portan, D.V. Computational and Experimental Investigation of the Combined Effect of Various 3D Scaffolds and Bioreactor Stimulation on Human Cells’ Feedback. Appl. Biosci. 2023, 2, 249–277. [Google Scholar] [CrossRef]
- Skrypnyk, M. Current progress and limitations of research regarding the therapeutic use of adipose-derived stem cells: Literature review. J. Umm Al-Qura Univ. Appl. Sci. 2024, 11, 63–75. Available online: https://link.springer.com/10.1007/s43994-024-00147-9 (accessed on 12 February 2025). [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Al-Ghadban, S.; Artiles, M.; Bunnell, B.A. Adipose Stem Cells in Regenerative Medicine: Looking Forward. Front. Bioeng. Biotechnol. 2022, 9, 837464. Available online: https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2021.837464 (accessed on 18 February 2025). [CrossRef]
- Al-Ghadban, S.; Bunnell, B.A. Adipose Tissue-Derived Stem Cells: Immunomodulatory Effects and Therapeutic Potential. Physiology 2020, 35, 125–133. [Google Scholar] [CrossRef]
- Ullah, M.; Liu, D.D.; Thakor, A.S. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience 2019, 15, 421–438. [Google Scholar] [CrossRef]
- Sajjad, U.; Ahmed, M.; Iqbal, M.Z.; Riaz, M.; Mustafa, M.; Biedermann, T.; Klar, A.S. Exploring mesenchymal stem cells homing mechanisms and improvement strategies. Stem Cells Transl. Med. 2024, 13, 1161–1177. [Google Scholar] [CrossRef] [PubMed]
- Portan, D.V.; Angelopoulou, A.; Koliadima, A.; Kontaxis, L.C.; Michanetzis, G.; Kouzoudis, D.; Michalopoulos, E.; Papanicolaou, G.C. Biodegradation, mechanical and biocompatibility evaluation of compact and porous polylactic acid after long term dynamic fluid immersion. J. Chem. Technol. Biotechnol. 2025; in process. [Google Scholar] [CrossRef]
- Dobreanu, M.; Manu, D.; Portan, D. Modern methods and techniques for testing artificial substrates biocompatibility. In Proceedings of the ICSAAM 2023 Book of Abstracts, Zakynthos, Greece, 10–14 September 2023; p. 12. [Google Scholar]
- Si, Z.; Wang, X.; Sun, C.; Kang, Y.; Xu, J.; Wang, X.; Hui, Y. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed. Pharmacother. 2019, 114, 108765. [Google Scholar] [CrossRef] [PubMed]
- Aronowitz, J.A.; Lockhart, R.A.; Hakakian, C.S. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. SpringerPlus 2015, 4, 713. [Google Scholar] [CrossRef]
- Cremona, M.; Gallazzi, M.; Rusconi, G.; Mariotta, L.; Gola, M.; Soldati, G. State of the Art in the Standardization of Stromal Vascular Fraction Processing. Biomolecules 2025, 15, 199. [Google Scholar] [CrossRef]
- Available online: https://www.isscr.org/basic-research-standards (accessed on 25 February 2025).
- Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/regulatory-considerations-human-cells-tissues-and-cellular-and-tissue-based-products-minimal (accessed on 25 February 2025).
- Li, J.; Huang, H.; Xu, X. Biological characteristics and karyotiping of a new isolation method for human adipose mesenchymal stem cells in vitro. Tissue Cell 2017, 49, 376–382. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar]
- Typiak, M.; Żurawa-Janicka, D. Not an immune cell, but they may act like one—Cells with immune properties outside the immune system. Immunol. Cell Biol. 2024, 102, 487–499. [Google Scholar] [CrossRef]
- Han, S.M.; Han, S.H.; Coh, Y.R.; Jang, G.; Chan Ra, J.; Kang, S.K.; Lee, H.W.; Youn, H.Y. Enhanced proliferation and differentiation of Oct4- and Sox2-overexpressing human adipose tissue mesenchymal stem cells. Exp. Mol. Med. 2014, 46, e101. [Google Scholar] [CrossRef]
- Ghiasi, M.; Kalhor, N.; Tabatabaei Qomi, R.; Sheykhhasan, M. The effects of synthetic and natural scaffolds on viability and proliferation of adipose-derived stem cells. Front. Life Sci. 2016, 9, 32–43. [Google Scholar] [CrossRef]
- Hatzmann, F.M.; Ejaz, A.; Wiegers, G.J.; Mandl, M.; Brucker, C.; Lechner, S.; Rauchenwald, T.; Zwierzina, M.; Baumgarten, S.; Wagner, S.; et al. Quiescence, Stemness and Adipogenic Differentiation Capacity in Human DLK1−/CD34+/CD24+ Adipose Stem/Progenitor Cells. Cells 2021, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Pierantozzi, E.; Gava, B.; Manini, I.; Roviello, F.; Marotta, G.; Chiavarelli, M.; Sorrentino, V. Pluripotency Regulators in Human Mesenchymal Stem Cells: Expression of NANOG But Not of OCT-4 and SOX-2. Stem Cells Dev. 2010, 20, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Zeineddine, D.; Hammoud, A.A.; Mortada, M.; Boeuf, H. The Oct4 protein: More than a magic stemness marker. Am. J. Stem Cells 2014, 3, 74–82. [Google Scholar]
- Tsubaki, T.; Chijimatsu, R.; Takeda, T.; Abe, M.; Ochiya, T.; Tsuji, S.; Inoue, K.; Matsuzaki, T.; Iwanaga, Y.; Omata, Y.; et al. Aging and cell expansion enhance microRNA diversity in small extracellular vesicles produced from human adipose-derived stem cells. Cytotechnology 2025, 77, 15. [Google Scholar] [CrossRef]
- Suga, H.; Matsumoto, D.; Eto, H.; Inoue, K.; Aoi, N.; Kato, H.; Araki, J.; Yoshimura, K. Functional Implications of CD34 Expression in Human Adipose–Derived Stem/Progenitor Cells. Stem Cells Dev. 2009, 18, 1201–1210. [Google Scholar] [CrossRef]
- Davies, O.G.; Cooper, P.R.; Shelton, R.M.; Smith, A.J.; Scheven, B.A. Isolation of adipose and bone marrow mesenchymal stem cells using CD29 and CD90 modifies their capacity for osteogenic and adipogenic differentiation. J. Tissue Eng. 2015, 6, 2041731415592356. [Google Scholar] [CrossRef]
- Mildmay-White, A.; Khan, W. Cell Surface Markers on Adipose-Derived Stem Cells: A Systematic Review. Curr. Stem Cell Res. Ther. 2017, 12, 484–492. [Google Scholar] [CrossRef]
- Stojanović, S.; Najman, S.; Korać, A. Stem Cells Derived from Lipoma and Adipose Tissue—Similar Mesenchymal Phenotype but Different Differentiation Capacity Governed by Distinct Molecular Signature. Cells 2018, 7, 260. [Google Scholar] [CrossRef]
- Zhou, W.; Lin, J.; Zhao, K.; Jin, K.; He, Q.; Hu, Y.; Feng, G.; Cai, Y.; Xia, C.; Liu, H.; et al. Single-Cell Profiles and Clinically Useful Properties of Human Mesenchymal Stem Cells of Adipose and Bone Marrow Origin. Am. J. Sports Med. 2019, 47, 1722–1733. [Google Scholar] [CrossRef]
- Bunnell, B.A. Adipose Tissue-Derived Mesenchymal Stem Cells. Cells 2021, 10, 3433. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, X.; Min, J.; Kong, W.; Hu, X.; Zhang, J.; Chen, L. Advancements in culture technology of adipose-derived stromal/stem cells: Implications for diabetes and its complications. Front. Endocrinol. 2024, 15, 1343255. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Xiong, H.; Chen, J.; Yang, X.; Liu, Y.; Guo, J.; Jiang, T.; Xu, Z.; Yuan, M.; Liu, Y.; et al. The whole profiling and competing endogenous RNA network analyses of noncoding RNAs in adipose-derived stem cells from diabetic, old, and young patients. Stem. Cell Res. Ther. 2021, 12, 313. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.; David, G.; Jacobs, D.; Kuczwara, P.; Woessner, A.E.; Kim, J.W.; Quinn, K.P.; Song, Y. Neuro-regenerative behavior of adipose-derived stem cells in aligned collagen I hydrogels. Mater. Today Bio 2023, 22, 100762. [Google Scholar] [CrossRef]
- Almalki, S.G.; Agrawal, D.K. Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation 2016, 92, 41–51. [Google Scholar] [CrossRef]
- Tomita, K.; Madura, T.; Sakai, Y.; Yano, K.; Terenghi, G.; Hosokawa, K. Glial differentiation of human adipose-derived stem cells: Implications for cell-based transplantation therapy. Neuroscience 2013, 236, 55–65. [Google Scholar] [CrossRef]
- Luo, L.; Hu, D.H.; Yin, J.Q.; Xu, R.X. Molecular Mechanisms of Transdifferentiation of Adipose-Derived Stem Cells into Neural Cells: Current Status and Perspectives. Stem Cells Int. 2018, 2018, 5630802. [Google Scholar] [CrossRef]
- Mahoney, A.L.G.; Nassif, N.T.; O’Brien, B.A.; Simpson, A.M. Pancreatic Transdifferentiation Using β-Cell Transcription Factors for Type 1 Diabetes Treatment. Cells 2022, 11, 2145. [Google Scholar] [CrossRef]
- Choi, Y.S.; Dusting, G.J.; Stubbs, S.; Arunothayaraj, S.; Han, X.L.; Collas, P.; Morrison, W.A.; Dilley, R.J. Differentiation of human adipose-derived stem cells into beating cardiomyocytes. J. Cell. Mol. Med. 2010, 14, 878–889. [Google Scholar] [CrossRef]
- Walewska, A.; Janucik, A.; Tynecka, M.; Moniuszko, M.; Eljaszewicz, A. Mesenchymal stem cells under epigenetic control—The role of epigenetic machinery in fate decision and functional properties. Cell Death Dis. 2023, 14, 720. [Google Scholar] [CrossRef]
- Ozkul, Y.; Galderisi, U. The Impact of Epigenetics on Mesenchymal Stem Cell Biology. J. Cell. Physiol. 2016, 231, 2393–2401. [Google Scholar] [CrossRef] [PubMed]
- Kant, R.J.; Coulombe, K.L.K. Integrated approaches to spatiotemporally directing angiogenesis in host and engineered tissues. Acta Biomater. 2018, 69, 42–62. [Google Scholar] [CrossRef]
- Ghaedi, M.; Tuleuova, N.; Zern, M.A.; Wu, J.; Revzin, A. Bottom-up signaling from HGF-containing surfaces promotes hepatic differentiation of mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2011, 407, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Tirza, G.; Solodeev, I.; Sela, M.; Greenberg, I.; Pasmanik-Chor, M.; Gur, E.; Shani, N. Reduced culture temperature attenuates oxidative stress and inflammatory response facilitating expansion and differentiation of adipose-derived stem cells. Stem Cell Res. Ther. 2020, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Devitt, S.M.; Carter, C.M.; Dierov, R.; Weiss, S.; Gersch, R.P.; Percec, I. Successful Isolation of Viable Adipose-Derived Stem Cells from Human Adipose Tissue Subject to Long-Term Cryopreservation: Positive Implications for Adult Stem Cell-Based Therapeutics in Patients of Advanced Age. Stem Cells Int. 2015, 2015, 146421. [Google Scholar] [CrossRef]
- Kang, S.K.; Putnam, L.; Dufour, J.; Ylostalo, J.; Jung, J.S.; Bunnell, B.A. Expression of Telomerase Extends the Lifespan and Enhances Osteogenic Differentiation of Adipose Tissue–Derived Stromal Cells. Stem Cells 2004, 22, 1356–1372. [Google Scholar] [CrossRef]
- Tátrai, P.; Szepesi, Á.; Matula, Z.; Szigeti, A.; Buchan, G.; Mádi, A.; Uher, F.; Német, K. Combined introduction of Bmi-1 and hTERT immortalizes human adipose tissue-derived stromal cells with low risk of transformation. Biochem. Biophys. Res. Commun. 2012, 422, 28–35. [Google Scholar] [CrossRef]
- Balducci, L.; Blasi, A.; Saldarelli, M.; Soleti, A.; Pessina, A.; Bonomi, A.; Coccè, V.; Dossena, M.; Tosetti, V.; Ceserani, V.; et al. Immortalization of human adipose-derived stromal cells: Production of cell lines with high growth rate, mesenchymal marker expression and capability to secrete high levels of angiogenic factors. Stem Cell Res. Ther. 2014, 5, 63. [Google Scholar] [CrossRef]
- Locke, M.; Feisst, V.; Meidinger, S. From bench to bedside: Use of human adipose-derived stem cells. Stem Cells Cloning Adv. Appl. 2015, 8, 149–162. [Google Scholar] [CrossRef]
- Bailey, A.M.; Lawrence, M.B.; Shang, H.; Katz, A.J.; Peirce, S.M. Agent-Based Model of Therapeutic Adipose-Derived Stromal Cell Trafficking during Ischemia Predicts Ability To Roll on P-Selectin. PLoS Comput. Biol. 2009, 5, e1000294. [Google Scholar] [CrossRef]
- Suila, H.; Hirvonen, T.; Kotovuori, A.; Ritamo, I.; Kerkelä, E.; Anderson, H.; Natunen, S.; Tuimala, J.; Laitinen, S.; Nystedt, J.; et al. Human Umbilical Cord Blood-Derived Mesenchymal Stromal Cells Display a Novel Interaction between P-Selectin and Galectin-1. Scand. J. Immunol. 2014, 80, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ning, H.; Banie, L.; Wang, G.; Lin, G.; Lue, T.F.; Lin, C.-S. Adipose tissue-derived stem cells secrete CXCL5 cytokine with chemoattractant and angiogenic properties. Biochem. Biophys. Res. Commun. 2010, 402, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Jeon, E.S.; Lee, J.S.; Cho, M.; Suh, D.; Chang, C.L.; Kim, J.H. Lysophosphatidic acid in malignant ascites stimulates migration of human mesenchymal stem cells. J. Cell. Biochem. 2008, 104, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Han, B.; Cai, S.; Lei, Y.; Sun, T.; Sheng, Z. Migration of bone marrow-derived mesenchymal stem cells induced by tumor necrosis factor-α and its possible role in wound healing. Wound Repair Regen. 2009, 17, 185–191. [Google Scholar] [CrossRef]
- Yuan, L.; Sakamoto, N.; Song, G.; Sato, M. Low-Level Shear Stress Induces Human Mesenchymal Stem Cell Migration Through the SDF-1/CXCR4 Axis Via MAPK Signaling Pathways. Stem Cells Dev. 2013, 22, 2384–2393. [Google Scholar] [CrossRef]
- Ryu, C.H.; Park, S.A.; Kim, S.M.; Lim, J.Y.; Jeong, C.H.; Jun, J.A.; Oh, J.H.; Park, S.H.; Oh, W.-I.; Jeun, S.-S. Migration of human umbilical cord blood mesenchymal stem cells mediated by stromal cell-derived factor-1/CXCR4 axis via Akt, ERK, and p38 signal transduction pathways. Biochem. Biophys. Res. Commun. 2010, 398, 105–110. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, H.M.; Shang, D.S.; Fang, W.G.; He, Z.Y.; Chen, Y.H. The Involvement of CXCL11 in Bone Marrow-Derived Mesenchymal Stem Cell Migration Through Human Brain Microvascular Endothelial Cells. Neurochem. Res. 2014, 39, 700–706. [Google Scholar] [CrossRef]
- Zha, Y.; He, J.; Mei, Y.; Yin, T.; Mao, L. Zinc-finger transcription factor Snail accelerates survival, migration and expression of matrix metalloproteinase-2 in human bone mesenchymal stem cells. Cell Biol. Int. 2007, 31, 1089–1096. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.G.; Song, S.Y.; Kim, J.K.; Sung, J.H. Reactive oxygen species-responsive miR-210 regulates proliferation and migration of adipose-derived stem cells via PTPN2. Cell Death Dis. 2013, 4, e588. [Google Scholar] [CrossRef]
- Li, J.; Deng, T.; Zhu, S.; Xie, P.; Wang, W.; Zhou, H.; Xu, C. The SDF-1/CXCR4 axis is involved in adipose-derived stem cell migration. Neurourol. Urodyn. 2024, 43, 2279–2289. [Google Scholar] [CrossRef]
- Gan, F.; Liu, L.; Zhou, Q.; Huang, W.; Huang, X.; Zhao, X. Effects of adipose-derived stromal cells and endothelial progenitor cells on adipose transplant survival and angiogenesis. PLoS ONE 2022, 17, e0261498. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Han, J.; Hwang, S.J.; Sung, J.H. An update on niche composition, signaling and functional regulation of the adipose-derived stem cells. Expert Opin. Biol. Ther. 2014, 14, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Ko, G.R.; Lee, J.S. Engineering of Immune Microenvironment for Enhanced Tissue Remodeling. Tissue Eng. Regen. Med. 2022, 19, 221–236. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, M.; Lu, G.; Huang, B.; Wang, D.; Shao, Y.; Lu, M. Hypoxic Preconditioning Enhances Cellular Viability and Pro-angiogenic Paracrine Activity: The Roles of VEGF-A and SDF-1a in Rat Adipose Stem Cells. Front. Cell Dev. Biol. 2020, 8, 580131. [Google Scholar] [CrossRef]
- McIlhenny, S.E.; Hager, E.S.; Grabo, D.J.; DiMatteo, C.; Shapiro, I.M.; Tulenko, T.N.; DiMuzio, P.J. Linear Shear Conditioning Improves Vascular Graft Retention of Adipose-Derived Stem Cells by Upregulation of the α5 β1 Integrin. Tissue Eng. Part A 2010, 16, 245–255. [Google Scholar] [CrossRef]
- Casson, J.; O’Kane, S.; Smith, C.A.; Dalby, M.; Berry, C. Interleukin 6 Plays a Role in the Migration of Magnetically Levitated Mesenchymal Stem Cells Spheroids. Appl. Sci. 2018, 8, 412. [Google Scholar] [CrossRef]
- Li, W.; Liu, Q.; Shi, J.; Xu, X.; Xu, J. The role of TNF-α in the fate regulation and functional reprogramming of mesenchymal stem cells in an inflammatory microenvironment. Front. Immunol. 2023, 14, 1074863. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Z.; Jin, L.; Zhao, L.; Meng, L.; Kong, F.; He, C.; Kong, F.; Zheng, L.; Liang, F. LPS-pretreatment adipose-derived mesenchymal stromal cells promote wound healing in diabetic rats by improving angiogenesis. Injury 2022, 53, 3920–3929. [Google Scholar] [CrossRef]
- Liang, X.; Huang, X.; Zhou, Y.; Jin, R.; Li, Q. Mechanical Stretching Promotes Skin Tissue Regeneration via Enhancing Mesenchymal Stem Cell Homing and Transdifferentiation. Stem Cells Transl. Med. 2016, 5, 960–969. [Google Scholar] [CrossRef]
- Iwasa, S.N.; Babona-Pilipos, R.; Morshead, C.M. Environmental Factors That Influence Stem Cell Migration: An “Electric Field”. Stem Cells Int. 2017, 2017, 4276927. [Google Scholar] [CrossRef]
- Hammam, I.A.; Winters, R.; Hong, Z. Advancements in the application of biomaterials in neural tissue engineering: A review. Biomed. Eng. Adv. 2024, 8, 100132. [Google Scholar] [CrossRef]
- Steiner, D.; Mutschall, H.; Winkler, S.; Horch, R.E.; Arkudas, A. The Adipose-Derived Stem Cell and Endothelial Cell Coculture System—Role of Growth Factors? Cells 2021, 10, 2074. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, R.H.; Højgaard, L.D.; Reese-Petersen, A.L.; Hoeeg, C.; Mathiasen, A.B.; Haack-Sørensen, M.; Follin, B.; Genovese, F.; Kastrup, J.; Juhl, M.; et al. Adipose-derived stromal cells increase the formation of collagens through paracrine and juxtacrine mechanisms in a fibroblast co-culture model utilizing macromolecular crowding. Stem Cell Res. Ther. 2022, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.-T.; Li, H.-M.; Yin, Q.-S.; Liu, D.-L.; Nan, H.; Zhao, P.-R.; Liang, S.-W. Human Breast Adipose-Derived Stem Cells Transfected with the Stromal Cell-Derived Factor-1 Receptor CXCR4 Exhibit Enhanced Viability in Human Autologous Free Fat Grafts. Cell. Physiol. Biochem. 2014, 34, 2091–2104. [Google Scholar] [CrossRef]
- Resch, A.; Wolf, S.; Mann, A.; Weiss, T.; Stetco, A.L.; Radtke, C. Co-Culturing Human Adipose Derived Stem Cells and Schwann Cells on Spider Silk—A New Approach as Prerequisite for Enhanced Nerve Regeneration. Int. J. Mol. Sci. 2018, 20, 71. [Google Scholar] [CrossRef]
- Yang, F.; Cohen, R.N.; Brey, E.M. Optimization of Co-Culture Conditions for a Human Vascularized Adipose Tissue Model. Bioengineering 2020, 7, 114. [Google Scholar] [CrossRef]
- Dash, S.; Shakeel, A.; Mohanty, S. Impact of Nanotechnology on the Realm of Stem Cells and Regenerative Medicine. ChemNanoMat 2022, 8, e202200177. [Google Scholar] [CrossRef]
- Park, H.W.; Lee, C.E.; Kim, S.; Jeong, W.J.; Kim, K. Ex Vivo Peptide Decoration Strategies on Stem Cell Surfaces for Augmenting Endothelium Interaction. Tissue Eng. Part B Rev. 2024, 30, 327–339. [Google Scholar] [CrossRef]
- Bobis-Wozowicz, S.; Miekus, K.; Wybieralska, E.; Jarocha, D.; Zawisz, A.; Madeja, Z.; Majka, M. Genetically modified adipose tissue−derived mesenchymal stem cells overexpressing CXCR4 display increased motility, invasiveness, and homing to bone marrow of NOD/SCID mice. Exp. Hematol. 2011, 39, 686–696.e4. [Google Scholar] [CrossRef]
- Dai, R.; Wang, Z.; Samanipour, R.; Koo Kin Kim, K. Adipose-Derived Stem Cells for Tissue Engineering and Regenerative Medicine Applications. Stem Cells Int. 2016, 2016, 6737345. [Google Scholar] [CrossRef]
- Boccardo, S.; Gaudiello, E.; Melly, L.; Cerino, G.; Ricci, D.; Martin, I.; Eckstein, F.; Banfi, A.; Marsano, A. Engineered mesenchymal cell-based patches as controlled VEGF delivery systems to induce extrinsic angiogenesis. Acta Biomater. 2016, 42, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Luo, Y. Three-dimensional scaffolds. In Principles of Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 343–360. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780128184226000204 (accessed on 27 February 2025).
- Mukasheva, F.; Adilova, L.; Dyussenbinov, A.; Yernaimanova, B.; Abilev, M.; Akilbekova, D. Optimizing scaffold pore size for tissue engineering: Insights across various tissue types. Front. Bioeng. Biotechnol. 2024, 12, 1444986. [Google Scholar] [CrossRef]
- Nellinger, S.; Kluger, P.J. How Mechanical and Physicochemical Material Characteristics Influence Adipose-Derived Stem Cell Fate. Int. J. Mol. Sci. 2023, 24, 3551. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.S.; Edwards, J.; Jeon, H. Electrospun Fibrous Scaffolds for Tissue Engineering: Viewpoints on Architecture and Fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Golebiowska, A.A.; Intravaia, J.T.; Sathe, V.M.; Kumbar, S.G.; Nukavarapu, S.P. Decellularized extracellular matrix biomaterials for regenerative therapies: Advances, challenges and clinical prospects. Bioact. Mater. 2024, 32, 98–123. [Google Scholar] [CrossRef]
- Krishani, M.; Shin, W.Y.; Suhaimi, H.; Sambudi, N.S. Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review. Gels 2023, 9, 100. [Google Scholar] [CrossRef]
- Suamte, L.; Tirkey, A.; Barman, J.; Jayasekhar Babu, P. Various manufacturing methods and ideal properties of scaffolds for tissue engineering applications. Smart Mater. Manuf. 2023, 1, 100011. [Google Scholar] [CrossRef]
- Zonderland, J.; Moroni, L. Steering cell behavior through mechanobiology in 3D: A regenerative medicine perspective. Biomaterials 2021, 268, 120572. [Google Scholar] [CrossRef]
- Montanheiro, T.L.D.A.; Schatkoski, V.M.; De Menezes, B.R.C.; Pereira, R.M.; Ribas, R.G.; De Freitas, A.D.S.M.; Lemes, A.P.; Fernandes, M.H.F.V.; Thim, G.P. Recent progress on polymer scaffolds production: Methods, main results, advantages and disadvantages. Express Polym. Lett. 2022, 16, 197–219. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Yan, J.; Zhang, K.; Lin, F.; Xiang, L.; Deng, L.; Guan, Z.; Cui, W.; Zhang, H. Pharmaceutical electrospinning and 3D printing scaffold design for bone regeneration. Adv. Drug Deliv. Rev. 2021, 174, 504–534. [Google Scholar] [CrossRef] [PubMed]
- Fereshteh, Z. Freeze-drying technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds; Elsevier: Amsterdam, The Netherlands, 2018; pp. 151–174. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780081009796000070 (accessed on 22 February 2025).
- Sudhakar, K.; Ji, S.M.; Kummara, M.R.; Han, S.S. Recent Progress on Hyaluronan-Based Products for Wound Healing Applications. Pharmaceutics 2022, 14, 2235. [Google Scholar] [CrossRef] [PubMed]
- Man, E.; Lamprou, D.; Easdon, C.; McLellan, I.; Yiu, H.H.P.; Hoskins, C. Exploration of Dual Ionic Cross-Linked Alginate Hydrogels Via Cations of Varying Valences towards Wound Healing. Polymers 2022, 14, 5192. [Google Scholar] [CrossRef]
- Wu, K.; Yan, Z.; Wu, Z.; Li, J.; Zhong, W.; Ding, L.; Zhong, T.; Jiang, T. Recent Advances in the Preparation, Antibacterial Mechanisms, and Applications of Chitosan. J. Funct. Biomater. 2024, 15, 318. [Google Scholar] [CrossRef]
- Geng, Y.; Xue, H.; Zhang, Z.; Panayi, A.C.; Knoedler, S.; Zhou, W.; Mi, B.; Liu, G. Recent advances in carboxymethyl chitosan-based materials for biomedical applications. Carbohydr. Polym. 2023, 305, 120555. [Google Scholar] [CrossRef]
- Horue, M.; Silva, J.M.; Berti, I.R.; Brandão, L.R.; Barud, H.D.S.; Castro, G.R. Bacterial Cellulose-Based Materials as Dressings for Wound Healing. Pharmaceutics 2023, 15, 424. [Google Scholar] [CrossRef]
- Wosicka-Frąckowiak, H.; Poniedziałek, K.; Woźny, S.; Kuprianowicz, M.; Nyga, M.; Jadach, B.; Milanowski, B. Collagen and Its Derivatives Serving Biomedical Purposes: A Review. Polymers 2024, 16, 2668. [Google Scholar] [CrossRef]
- Chung, E.; Rytlewski, J.A.; Merchant, A.G.; Dhada, K.S.; Lewis, E.W.; Suggs, L.J. Fibrin-based 3D matrices induce angiogenic behavior of adipose-derived stem cells. Acta Biomater. 2015, 17, 78–88. [Google Scholar] [CrossRef]
- Hinsenkamp, A.; Fülöp, Á.; Hricisák, L.; Pál, É.; Kun, K.; Majer, A.; Varga, V.; Lacza, Z.; Hornyák, I. Application of Injectable, Crosslinked, Fibrin-Containing Hyaluronic Acid Scaffolds for In Vivo Remodeling. J. Funct. Biomater. 2022, 13, 119. [Google Scholar] [CrossRef]
- Bindi, B.; Perioli, A.; Melo, P.; Mattu, C.; Ferreira, A.M. Bioinspired Collagen/Hyaluronic Acid/Fibrin-Based Hydrogels for Soft Tissue Engineering: Design, Synthesis, and In Vitro Characterization. J. Funct. Biomater. 2023, 14, 495. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhuang, Y.; Cai, M.; Wang, X.; Lin, K. Decellularized extracellular matrix: A promising strategy for skin repair and regeneration. Eng. Regen. 2023, 4, 357–374. [Google Scholar] [CrossRef]
- Izadyari Aghmiuni, A.; Heidari Keshel, S.; Sefat, F.; AkbarzadehKhiyavi, A. Fabrication of 3D hybrid scaffold by combination technique of electrospinning-like and freeze-drying to create mechanotransduction signals and mimic extracellular matrix function of skin. Mater. Sci. Eng. C 2021, 120, 111752. [Google Scholar] [CrossRef]
- Hennink, W.E.; Van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose–Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, D.A.; Wu, D.; He, F.; Wang, H.; Huang, L.; Shi, D.; Liu, Q.; Ni, N.; Pakvasa, M.; et al. Applications of Biocompatible Scaffold Materials in Stem Cell-Based Cartilage Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 603444. [Google Scholar] [CrossRef]
- Lang, Z.; Chen, T.; Zhu, S.; Wu, X.; Wu, Y.; Miao, X.; Wang, Q.; Zhao, L.; Zhu, Z.; Xu, R.X. Construction of vascular grafts based on tissue-engineered scaffolds. Mater. Today Bio 2024, 29, 101336. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Jiang, X.; Dai, B.; Zhang, L.; Zhu, Y. Decellularized extracellular matrix materials for treatment of ischemic cardiomyopathy. Bioact. Mater. 2024, 33, 460–482. [Google Scholar] [CrossRef]
- Li, Q.; Li, M.; Li, M.; Zhang, Z.; Ma, H.; Zhao, L.; Zhang, M.; Wang, G. Adipose-derived mesenchymal stem cell seeded Atelocollagen scaffolds for cardiac tissue engineering. J. Mater. Sci. Mater. Med. 2020, 31, 83. [Google Scholar] [CrossRef]
- Rickel, A.P.; Deng, X.; Engebretson, D.; Hong, Z. Electrospun nanofiber scaffold for vascular tissue engineering. Mater. Sci. Eng. C 2021, 129, 112373. [Google Scholar] [CrossRef]
- Kim, J.J.; Cho, D.W. Advanced strategies in 3D bioprinting for vascular tissue engineering and disease modelling using smart bioinks. Virtual Phys. Prototyp. 2024, 19, e2395470. [Google Scholar] [CrossRef]
- Xu, R.; Dou, B.; Yu, S.; Wang, Z.; Zhang, Y.; Leng, L.; Ouyang, L.; Sun, W. Enabling 3D printability and vascular morphogenesis with double network dynamic hydrogels. Mater Today. 2025, 84, 10–27. [Google Scholar] [CrossRef]
- Carvalho, C.R.; Oliveira, J.M.; Reis, R.L. Modern Trends for Peripheral Nerve Repair and Regeneration: Beyond the Hollow Nerve Guidance Conduit. Front Bioeng. Biotechnol. 2019, 7, 337. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, T.; Li, Y. 3D Printing and Bioprinting Nerve Conduits for Neural Tissue Engineering. Polymers 2020, 12, 1637. [Google Scholar] [CrossRef]
- Tarricone, G.; Carmagnola, I.; Chiono, V. Tissue-Engineered Models of the Human Brain: State-of-the-Art Analysis and Challenges. J. Funct. Biomater. 2022, 13, 146. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Z.; Chen, X.; Li, R.; Li, D.; Feng, S. A Silk Fibroin/Collagen Nerve Scaffold Seeded with a Co-Culture of Schwann Cells and Adipose-Derived Stem Cells for Sciatic Nerve Regeneration. PLoS ONE 2016, 11, e0147184. [Google Scholar] [CrossRef]
- Dai, W.; Yang, Y.; Yang, Y.; Liu, W. Material advancement in tissue-engineered nerve conduit. Nanotechnol. Rev. 2021, 10, 488–503. [Google Scholar] [CrossRef]
- Desiderio, V.; De Francesco, F.; Schiraldi, C.; De Rosa, A.; La Gatta, A.; Paino, F.; d’Aquino, R.; Ferraro, G.A.; Tirino, V.; Papaccio, G. Human Ng2+ adipose stem cells loaded in vivo on a new crosslinked hyaluronic acid-lys scaffold fabricate a skeletal muscle tissue. J. Cell. Physiol. 2013, 228, 1762–1773. [Google Scholar] [CrossRef]
- Xie, J.; Bian, H.; Qi, S.; Xu, Y.; Tang, J.; Li, T.; Liu, X. Effects of basic fibroblast growth factor on the expression of extracellular matrix and matrix metalloproteinase-1 in wound healing. Clin. Exp. Dermatol. 2008, 33, 176–182. [Google Scholar] [CrossRef]
- Saberianpour, S.; Heidarzadeh, M.; Geranmayeh, M.H.; Hosseinkhani, H.; Rahbarghazi, R.; Nouri, M. Tissue engineering strategies for the induction of angiogenesis using biomaterials. J. Biol. Eng. 2018, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Bian, D.; Wu, Y.; Song, G. Basic fibroblast growth factor combined with extracellular matrix-inspired mimetic systems for effective skin regeneration and wound healing. Mater. Today Commun. 2023, 35, 105876. [Google Scholar] [CrossRef]
- Fadera, S.; Cheng, N.C.; Young, T.H.; Lee, I.C. In vitro study of SDF-1α-loaded injectable and thermally responsive hydrogels for adipose stem cell therapy by SDF-1/CXCR4 axis. J. Mater. Chem. B 2020, 8, 10360–10372. [Google Scholar] [CrossRef]
- Beederman, M.; Lamplot, J.D.; Nan, G.; Wang, J.; Liu, X.; Yin, L.; Li, R.; Shui, W.; Zhang, H.; Kim, S.H.; et al. BMP signaling in mesenchymal stem cell differentiation and bone formation. J. Biomed. Sci. Eng. 2013, 06, 32–52. [Google Scholar] [CrossRef]
- Wen, C.; Xu, L.; Xu, X.; Wang, D.; Liang, Y.; Duan, L. Insulin-like growth factor-1 in articular cartilage repair for osteoarthritis treatment. Arthritis Res. Ther. 2021, 23, 277. [Google Scholar] [CrossRef]
- Whitty, C.; Pernstich, C.; Marris, C.; McCaskie, A.; Jones, M.; Henson, F. Sustained delivery of the bone morphogenetic proteins BMP-2 and BMP-7 for cartilage repair and regeneration in osteoarthritis. Osteoarthr. Cartil. Open. 2022, 4, 100240. [Google Scholar] [CrossRef]
- Wang, T.; Yang, F. A comparative study of chondroitin sulfate and heparan sulfate for directing three-dimensional chondrogenesis of mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 284. [Google Scholar] [CrossRef]
- Li, X.; Ding, J.; Zhuang, X.; Chang, F.; Wang, J.; Chen, X. Chitosan-Based Scaffolds for Cartilage Regeneration. In Chitin and Chitosan for Regenerative Medicine; Springer Series on Polymer and Composite Materials; Dutta, P.K., Ed.; Springer: New Delhi, India, 2016; pp. 61–82. Available online: https://link.springer.com/10.1007/978-81-322-2511-9_3 (accessed on 22 February 2025).
- Zhao, T.; Li, X.; Li, H.; Deng, H.; Li, J.; Yang, Z.; He, S.; Jiang, S.; Sui, X.; Guo, Q.; et al. Advancing drug delivery to articular cartilage: From single to multiple strategies. Acta Pharm. Sin. B 2023, 13, 4127–4148. [Google Scholar] [CrossRef]
- Yu, J.; Wang, A.; Tang, Z.; Henry, J.; Li-Ping Lee, B.; Zhu, Y.; Yuan, F.; Huang, F.; Li, S. The effect of stromal cell-derived factor-1α/heparin coating of biodegradable vascular grafts on the recruitment of both endothelial and smooth muscle progenitor cells for accelerated regeneration. Biomaterials 2012, 33, 8062–8074. [Google Scholar] [CrossRef]
- Wei, Z.; Volkova, E.; Blatchley, M.R.; Gerecht, S. Hydrogel vehicles for sequential delivery of protein drugs to promote vascular regeneration. Adv. Drug Deliv. Rev. 2019, 149–150, 95–106. [Google Scholar] [CrossRef]
- Chen, S.; An, J.; Weng, L.; Li, Y.; Xu, H.; Wang, Y.; Ding, D.; Kong, D.; Wang, S. Construction and biofunctional evaluation of electrospun vascular graft loaded with selenocystamine for in situ catalytic generation of nitric oxide. Mater. Sci. Eng. C 2014, 45, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Fakhr, M.J.; Kavakebian, F.; Ababzadeh, S.; Rezapour, A. Challenges and Advances in Peripheral Nerve Tissue Engineering Critical Factors Affecting Nerve Regeneration. J. Tissue Eng. Regen. Med. 2024, 2024, 8868411. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, E.J.; Moon, L.D.F.; Popat, R.J.; King, V.R.; Bennett, G.S.; Patel, P.N.; Fawcett, J.W.; McMahon, S.B. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 2002, 416, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, M.A.; El-Fawal, H.A.N.; Allam, N.K. Biocompatible PCL-nanofibers scaffold with immobilized fibronectin and laminin for neuronal tissue regeneration. Mater. Sci. Eng. C. 2021, 119, 111550. [Google Scholar] [CrossRef]
- Xu, P.; Wu, Y.; Zhou, L.; Yang, Z.; Zhang, X.; Hu, X.; Yang, J.; Wang, M.; Wang, B.; Luo, G.; et al. Platelet-rich plasma accelerates skin wound healing by promoting re-epithelialization. Burns Trauma 2020, 8, tkaa028. [Google Scholar] [CrossRef]
- Ghiasi, M.; Farzaneh, S.; Bigdelo, M. Assessment of human cartilage regeneration in a patient with knee osteoarthritis using autologous adipose-tissue-derived stem cells and Platelet-rich plasma: A case study. J. Surg. Trauma 2020, 8, 73–78. [Google Scholar]
- Chen, X.B.; Fazel Anvari-Yazdi, A.; Duan, X.; Zimmerling, A.; Gharraei, R.; Sharma, N.K.; Sweilem, S.; Ning, L. Biomaterials/bioinks and extrusion bioprinting. Bioact. Mater. 2023, 28, 511–536. [Google Scholar] [CrossRef]
- Xiang, J.Y.; Kang, L.; Li, Z.M.; Tseng, S.L.; Wang, L.Q.; Li, T.H.; Li, Z.-J.; Huang, J.-Z.; Yu, N.-Z.; Long, X. Biological scaffold as potential platforms for stem cells: Current development and applications in wound healing. World J. Stem Cells 2024, 16, 334–352. [Google Scholar] [CrossRef]
- Flynn, L.; Prestwich, G.D.; Semple, J.L.; Woodhouse, K.A. Adipose tissue engineering with naturally derived scaffolds and adipose-derived stem cells. Biomaterials 2007, 28, 3834–3842. [Google Scholar] [CrossRef]
- Kang, H.; Peng, J.; Lu, S.; Liu, S.; Zhang, L.; Huang, J.; Sui, X.; Zhao, B.; Wang, A.; Xu, W.; et al. In vivo cartilage repair using adipose-derived stem cell-loaded decellularized cartilage ECM scaffolds: In vivo cartilage repair using ADSC-loaded decellularized cartilage ECM scaffolds. J. Tissue Eng. Regen. Med. 2014, 8, 442–453. [Google Scholar] [CrossRef]
- He, Y.; Lu, F. Development of Synthetic and Natural Materials for Tissue Engineering Applications Using Adipose Stem Cells. Stem Cells Int. 2016, 2016, 5786257. [Google Scholar] [CrossRef] [PubMed]
- Barbulescu, G.I.; Bojin, F.M.; Ordodi, V.L.; Goje, I.D.; Barbulescu, A.S.; Paunescu, V. Decellularized Extracellular Matrix Scaffolds for Cardiovascular Tissue Engineering: Current Techniques and Challenges. Int. J. Mol. Sci. 2022, 23, 13040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chen, Y.; Hao, M.; Xia, Y. Putting Hybrid Nanomaterials to Work for Biomedical Applications. Angew. Chem. Int. Ed. 2024, 63, e202319567. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Holzwarth, J.M.; Ma, P.X. Functionalized Synthetic Biodegradable Polymer Scaffolds for Tissue Engineering. Macromol. Biosci. 2012, 12, 911–919. [Google Scholar] [CrossRef]
- Echeverria Molina, M.I.; Malollari, K.G.; Komvopoulos, K. Design Challenges in Polymeric Scaffolds for Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 617141. [Google Scholar] [CrossRef]
- Yang, Z.; Yin, G.; Sun, S.; Xu, P. Medical applications and prospects of polylactic acid materials. iScience 2024, 27, 111512. [Google Scholar] [CrossRef]
- Kontaxis, L.C.; Zachos, D.; Georgali-Fickel, A.; Portan, D.V.; Zaoutsos, S.P.; Papanicolaou, G.C. 3D-Printed PLA Mechanical and Viscoelastic Behavior Dependence on the Nozzle Temperature and Printing Orientation. Polymers 2025, 17, 913. [Google Scholar] [CrossRef]
- Rahman, G.; Frazier, T.P.; Gimble, J.M.; Mohiuddin, O.A. The Emerging Use of ASC/Scaffold Composites for the Regeneration of Osteochondral Defects. Front. Bioeng. Biotechnol. 2022, 10, 893992. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, X.; Fu, Y.; Jin, Y.; Weng, Y.; Bian, X.; Chen, X. Degradation Behaviors of Polylactic Acid, Polyglycolic Acid, and Their Copolymer Films in Simulated Marine Environments. Polymers 2024, 16, 1765. [Google Scholar] [CrossRef]
- Barbarisi, M.; Marino, G.; Armenia, E.; Vincenzo, Q.; Rosso, F.; Porcelli, M.; Barbarisi, A. Use of polycaprolactone (PCL) as scaffolds for the regeneration of nerve tissue. J. Biomed. Mater. Res. A 2015, 103, 1755–1760. [Google Scholar] [CrossRef]
- Liang, H.Y.; Lee, W.K.; Hsu, J.T.; Shih, J.Y.; Ma, T.L.; Vo, T.T.T.; Lee, C.-W.; Cheng, M.-T.; Lee, I.-T. Polycaprolactone in Bone Tissue Engineering: A Comprehensive Review of Innovations in Scaffold Fabrication and Surface Modifications. J. Funct. Biomater. 2024, 15, 243. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.; Narayana Kalkura, S.; Balme, S.; Bohatier, C.P.; Miele, P.; Bechelany, M. Nanofibrous Scaffolds for Tissue Engineering Application. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A.S.H., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 665–691. Available online: http://link.springer.com/10.1007/978-3-319-53655-2_30 (accessed on 27 February 2025).
- Wang, Z.; Ye, Q.; Yu, S.; Akhavan, B. Poly Ethylene Glycol (PEG)-Based Hydrogels for Drug Delivery in Cancer Therapy: A Comprehensive Review. Adv. Healthc. Mater. 2023, 12, 2300105. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.G.; Revzin, A.; Pishko, M.V. Poly(ethylene glycol) Hydrogel Microstructures Encapsulating Living Cells. Langmuir 2002, 18, 2459–2462. [Google Scholar] [CrossRef]
- Ramanathan, S.; Lin, Y.C.; Thirumurugan, S.; Hu, C.C.; Duann, Y.F.; Chung, R.J. Poly(methyl methacrylate) in Orthopedics: Strategies, Challenges, and Prospects in Bone Tissue Engineering. Polymers 2024, 16, 367. [Google Scholar] [CrossRef]
- Miri, Z.; Farè, S.; Ma, Q.; Haugen, H.J. Updates on polyurethane and its multifunctional applications in biomedical engineering. Prog. Biomed. Eng. 2023, 5, 042001. [Google Scholar] [CrossRef]
- Ribeiro, M.; Simões, M.; Vitorino, C.; Mascarenhas-Melo, F. Hydrogels in Cutaneous Wound Healing: Insights into Characterization, Properties, Formulation and Therapeutic Potential. Gels 2024, 10, 188. [Google Scholar] [CrossRef]
- Richbourg, N.R.; Peppas, N.A.; Sikavitsas, V.I. Tuning the biomimetic behavior of scaffolds for regenerative medicine through surface modifications. J. Tissue Eng. Regen. Med. 2019, 13, 1275–1293. [Google Scholar] [CrossRef]
- Shineh, G.; Patel, K.; Mobaraki, M.; Tayebi, L. Functional Approaches in Promoting Vascularization and Angiogenesis in Bone Critical-Sized Defects via Delivery of Cells, Growth Factors, Drugs, and Particles. J. Funct. Biomater. 2023, 14, 99. [Google Scholar] [CrossRef]
- Mansour, A.; Romani, M.; Acharya, A.B.; Rahman, B.; Verron, E.; Badran, Z. Drug Delivery Systems in Regenerative Medicine: An Updated Review. Pharmaceutics 2023, 15, 695. [Google Scholar] [CrossRef]
- Budharaju, H.; Suresh, S.; Sekar, M.P.; De Vega, B.; Sethuraman, S.; Sundaramurthi, D.; Kalaskar, D.M. Ceramic materials for 3D printing of biomimetic bone scaffolds—Current state-of-the-art & future perspectives. Mater. Des. 2023, 231, 112064. [Google Scholar]
- Portan, D.V.; Deligianni, D.D.; Papanicolaou, G.C.; Kostopoulos, V.; Psarras, G.C.; Tyllianakis, M. Combined Optimized Effect of a Highly Self-Organized Nanosubstrate and an Electric Field on Osteoblast Bone Cells Activity. BioMed Res. Int. 2019, 2019, 7574635. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Li, P.; Liu, J.; Peng, S. Optimization of TCP/HAP ratio for better properties of calcium phosphate scaffold via selective laser sintering. Mater. Charact. 2013, 77, 23–31. [Google Scholar] [CrossRef]
- Fernandes, H.R.; Gaddam, A.; Rebelo, A.; Brazete, D.; Stan, G.E.; Ferreira, J.M.F. Bioactive Glasses and Glass-Ceramics for Healthcare Applications in Bone Regeneration and Tissue Engineering. Materials 2018, 11, 2530. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Fu, S.; Zhu, Y. 3D printing of ceramic-based scaffolds for bone tissue engineering: An overview. J. Mater. Chem. B 2018, 6, 4397–4412. [Google Scholar] [CrossRef]
- Cinici, B.; Yaba, S.; Kurt, M.; Yalcin, H.C.; Duta, L.; Gunduz, O. Fabrication Strategies for Bioceramic Scaffolds in Bone Tissue Engineering with Generative Design Applications. Biomimetics 2024, 9, 409. [Google Scholar] [CrossRef]
- Tanvir, M.A.H.; Khaleque, M.A.; Kim, G.H.; Yoo, W.Y.; Kim, Y.Y. The Role of Bioceramics for Bone Regeneration: History, Mechanisms, and Future Perspectives. Biomimetics 2024, 9, 230. [Google Scholar] [CrossRef]
- De Carvalho, A.B.G.; Rahimnejad, M.; Oliveira, R.L.M.S.; Sikder, P.; Saavedra, G.S.F.A.; Bhaduri, S.B.; Gawlitta, D.; Malda, J.; Kaigler, D.; Trichês, E.S.; et al. Personalized bioceramic grafts for craniomaxillofacial bone regeneration. Int. J. Oral Sci. 2024, 16, 62. [Google Scholar] [CrossRef]
- Arcos, D.; Vallet-Regí, M. Bioceramics for drug delivery. Acta Mater. 2013, 61, 890–911. [Google Scholar] [CrossRef]
- Huang, B.; Caetano, G.; Vyas, C.; Blaker, J.; Diver, C.; Bártolo, P. Polymer-Ceramic Composite Scaffolds: The Effect of Hydroxyapatite and β-tri-Calcium Phosphate. Materials 2018, 11, 129. [Google Scholar] [CrossRef]
- Farjaminejad, S.; Farjaminejad, R.; Hasani, M.; Garcia-Godoy, F.; Abdouss, M.; Marya, A.; Harsoputranto, A.; Jamilian, A. Advances and Challenges in Polymer-Based Scaffolds for Bone Tissue Engineering: A Path Towards Personalized Regenerative Medicine. Polymers 2024, 16, 3303. [Google Scholar] [CrossRef]
- Sousa, A.C.; Alvites, R.; Lopes, B.; Sousa, P.; Moreira, A.; Coelho, A.; Rêma, A.; Biscaia, S.; Cordeiro, R.; Faria, F.; et al. Hybrid scaffolds for bone tissue engineering: Integration of composites and bioactive hydrogels loaded with hDPSCs. Biomater. Adv. 2025, 166, 214042. [Google Scholar] [CrossRef] [PubMed]
- Becerra, J.; Rodriguez, M.; Leal, D.; Noris-Suarez, K.; Gonzalez, G. Chitosan-collagen-hydroxyapatite membranes for tissue engineering. J. Mater. Sci. Mater. Med. 2022, 33, 18. [Google Scholar] [CrossRef] [PubMed]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. Nanomed. 2017, 12, 4937–4961. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; Giuffrida, R.; Forte, S.; Fabbi, C.; Figallo, E.; Salvatorelli, L.; Memeo, L.; Parenti, R.; Gulisano, M.; Gulino, R. Human adipose-derived mesenchymal stem cells seeded into a collagen-hydroxyapatite scaffold promote bone augmentation after implantation in the mouse. Sci. Rep. 2017, 7, 7110. [Google Scholar] [CrossRef]
- Si, Y.; Yu, J.; Tang, X.; Ge, J.; Ding, B. Ultralight nanofibre-assembled cellular aerogels with superelasticity and multifunctionality. Nat. Commun. 2014, 5, 5802. [Google Scholar] [CrossRef]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.M.; Mahapatra, C.; Kim, H.-W.; Knowles, J.C. Sol–gel based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Liebig, B.E.; Kisiday, J.D.; Bahney, C.S.; Ehrhart, N.P.; Goodrich, L.R. The platelet-rich plasma and mesenchymal stem cell milieu: A review of therapeutic effects on bone healing. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2020, 38, 2539–2550. [Google Scholar] [CrossRef]
- Oktaş, B.; Çırpar, M.; Şanlı, E.; Canbeyli, İ.D.; Bozdoğan, Ö. The effect of the platelet-rich plasma on osteogenic potential of the periosteum in an animal bone defect model. Jt. Dis. Relat. Surg. 2021, 32, 668–675. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Kawazoe, N.; Chen, G. Preparation of dexamethasone-loaded calcium phosphate nanoparticles for the osteogenic differentiation of human mesenchymal stem cells. J. Mater. Chem. B 2017, 5, 6801–6810. [Google Scholar] [CrossRef]
- Costa, P.F.; Puga, A.M.; Díaz-Gomez, L.; Concheiro, A.; Busch, D.H.; Alvarez-Lorenzo, C. Additive manufacturing of scaffolds with dexamethasone controlled release for enhanced bone regeneration. Int. J. Pharm. 2015, 496, 541–550. [Google Scholar] [CrossRef]
- Gao, S.; Calcagni, M.; Welti, M.; Hemmi, S.; Hild, N.; Stark, W.J.; Bürgisser, G.M.; Wanner, G.A.; Cinelli, P.; Buschmann, J. Proliferation of ASC-derived endothelial cells in a 3D electrospun mesh: Impact of bone-biomimetic nanocomposite and co-culture with ASC-derived osteoblasts. Injury 2014, 45, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [PubMed]
- Nayak, G.S.; Carradò, A.; Masson, P.; Pourroy, G.; Mouillard, F.; Migonney, V.; Falentin-Daudre, C.; Pereira, C.; Palkowski, H. Trends in Metal-Based Composite Biomaterials for Hard Tissue Applications. JOM 2022, 74, 102–125. [Google Scholar] [CrossRef]
- Bommala, V.K.; Krishna, M.G.; Rao, C.T. Magnesium matrix composites for biomedical applications: A review. J. Magnes. Alloys 2019, 7, 72–79. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, J.; Wang, Y.; Chen, C.; Gu, H.; Chai, Y.; Wang, Y. Silver nanoparticles-decorated and mesoporous silica coated single-walled carbon nanotubes with an enhanced antibacterial activity for killing drug-resistant bacteria. Nano Res. 2020, 13, 389–400. [Google Scholar] [CrossRef]
- MacDonald, R.A.; Voge, C.M.; Kariolis, M.; Stegemann, J.P. Carbon nanotubes increase the electrical conductivity of fibroblast-seeded collagen hydrogels. Acta Biomater. 2008, 4, 1583–1592. [Google Scholar] [CrossRef]
- Yang, G.; Waheed, S.; Wang, C.; Shekh, M.; Li, Z.; Wu, J. Exosomes and Their Bioengineering Strategies in the Cutaneous Wound Healing and Related Complications: Current Knowledge and Future Perspectives. Int. J. Biol. Sci. 2023, 19, 1430–1454. [Google Scholar] [CrossRef]
- Moghassemi, S.; Dadashzadeh, A.; Sousa, M.J.; Vlieghe, H.; Yang, J.; León-Félix, C.M.; Amorim, C.A. Extracellular vesicles in nanomedicine and regenerative medicine: A review over the last decade. Bioact. Mater. 2024, 36, 126–156. [Google Scholar] [CrossRef]
- Fan, M.H.; Pi, J.K.; Zou, C.Y.; Jiang, Y.L.; Li, Q.J.; Zhang, X.Z.; Xing, F.; Nie, R.; Han, C.; Xie, H.Q. Hydrogel-exosome system in tissue engineering: A promising therapeutic strategy. Bioact. Mater. 2024, 38, 1–30. [Google Scholar] [CrossRef]
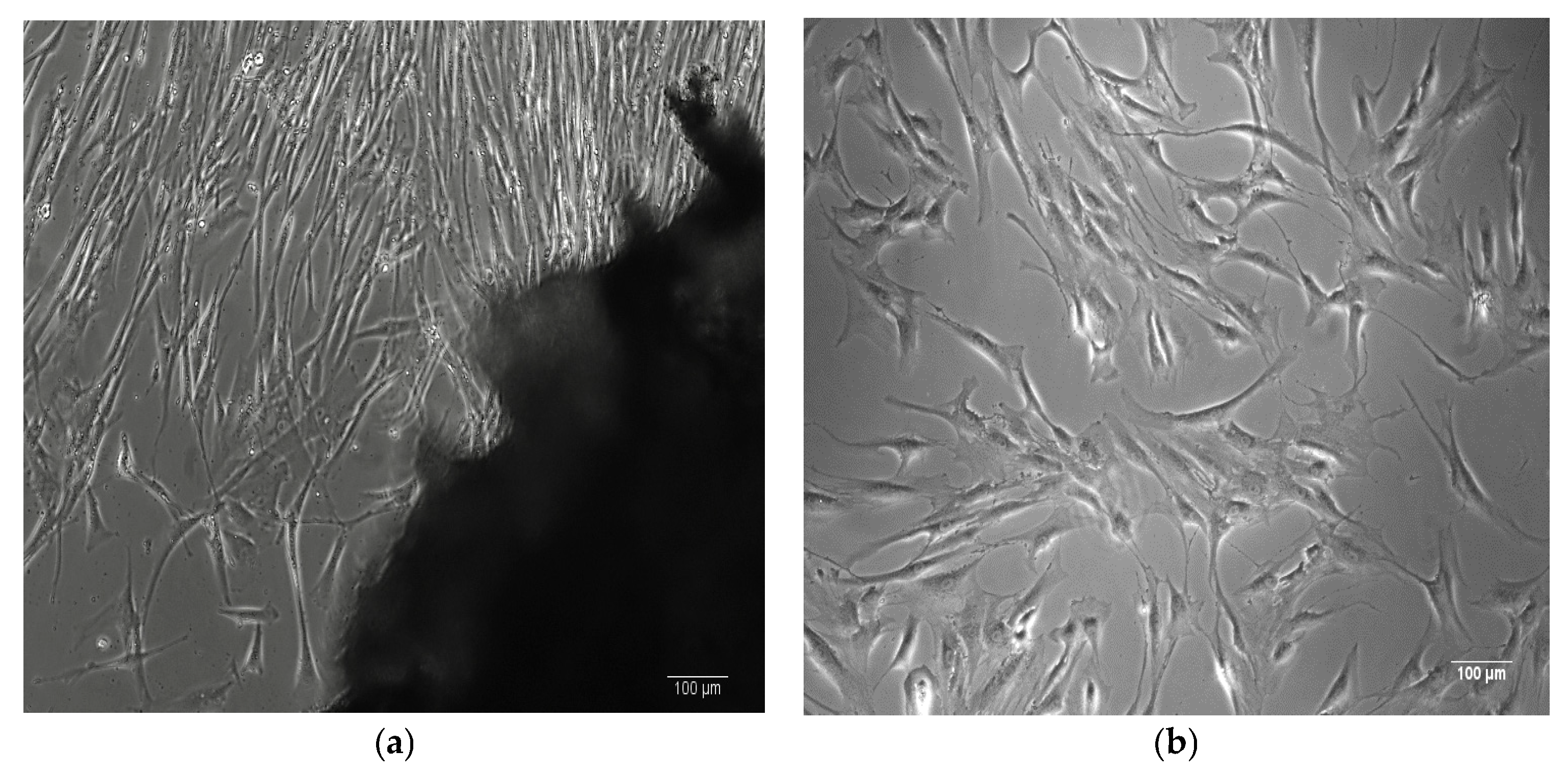
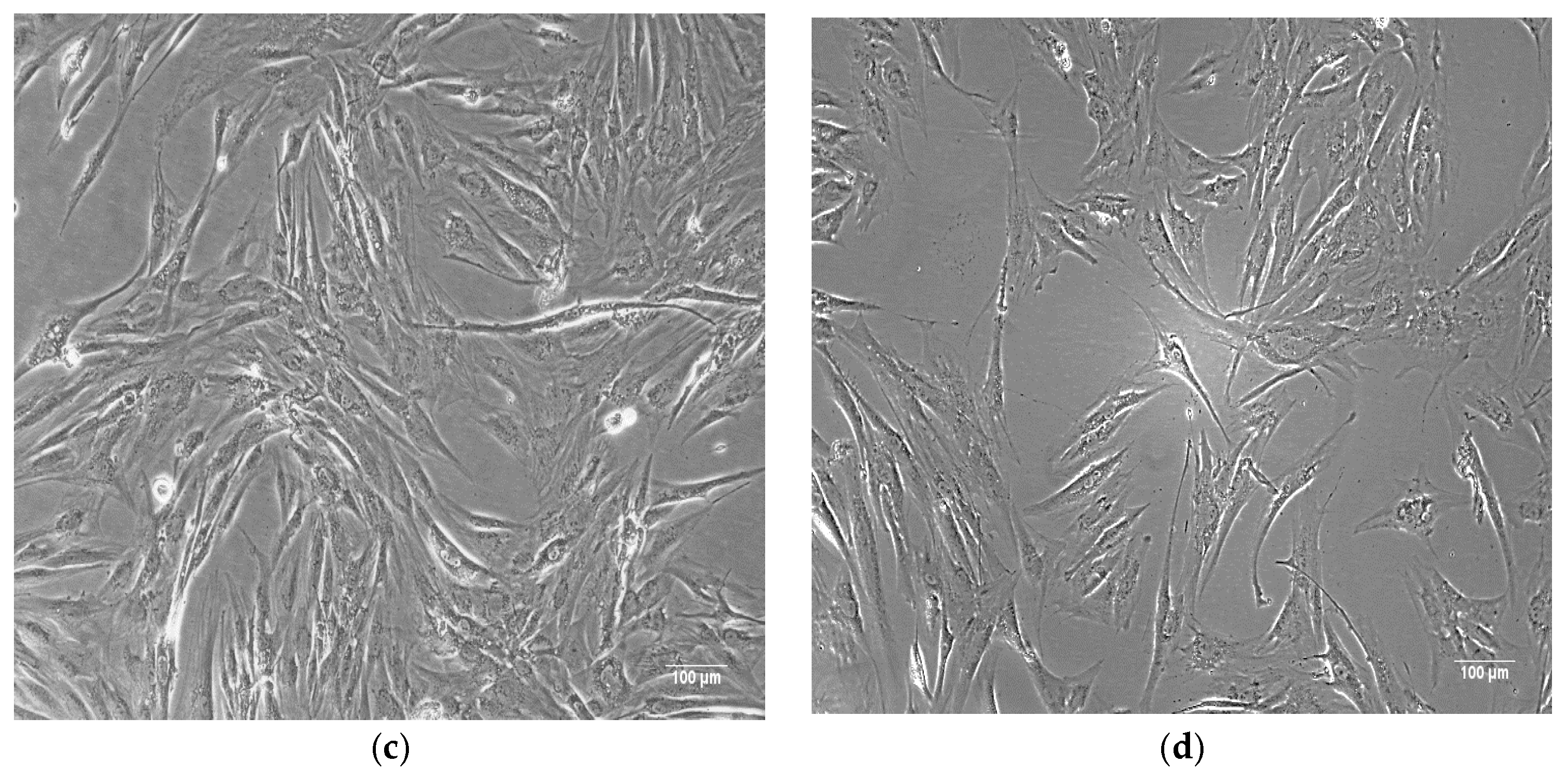
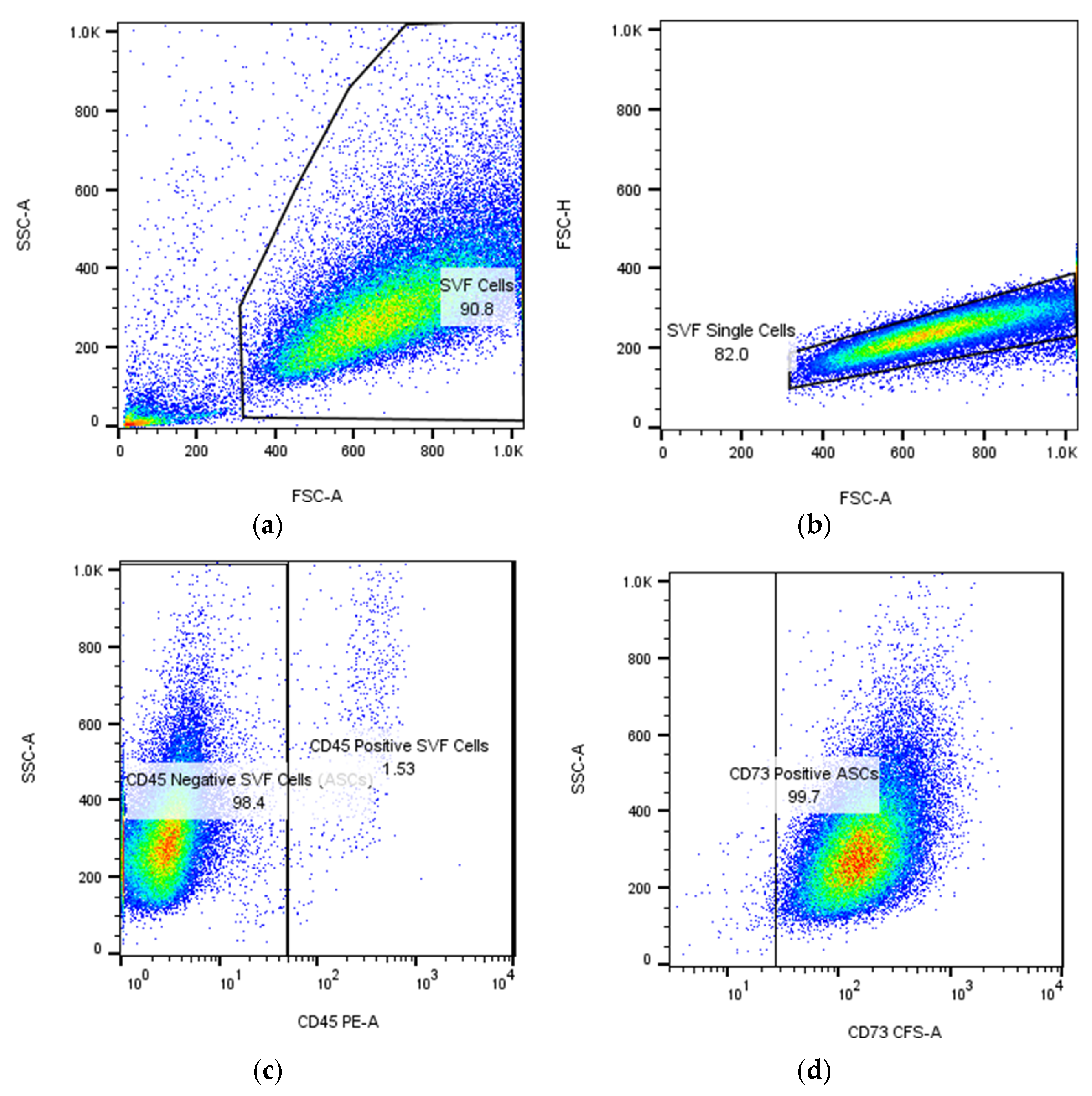
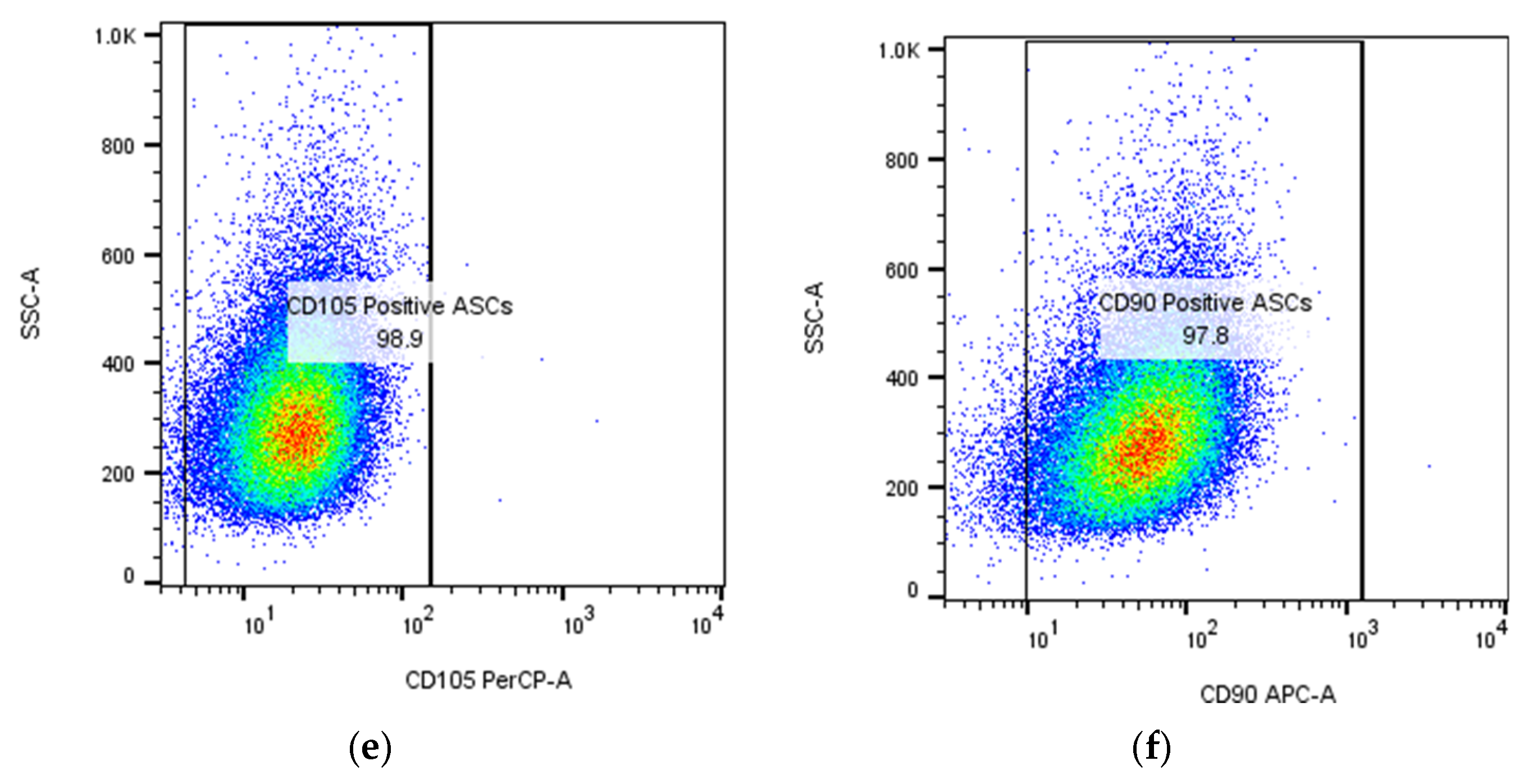

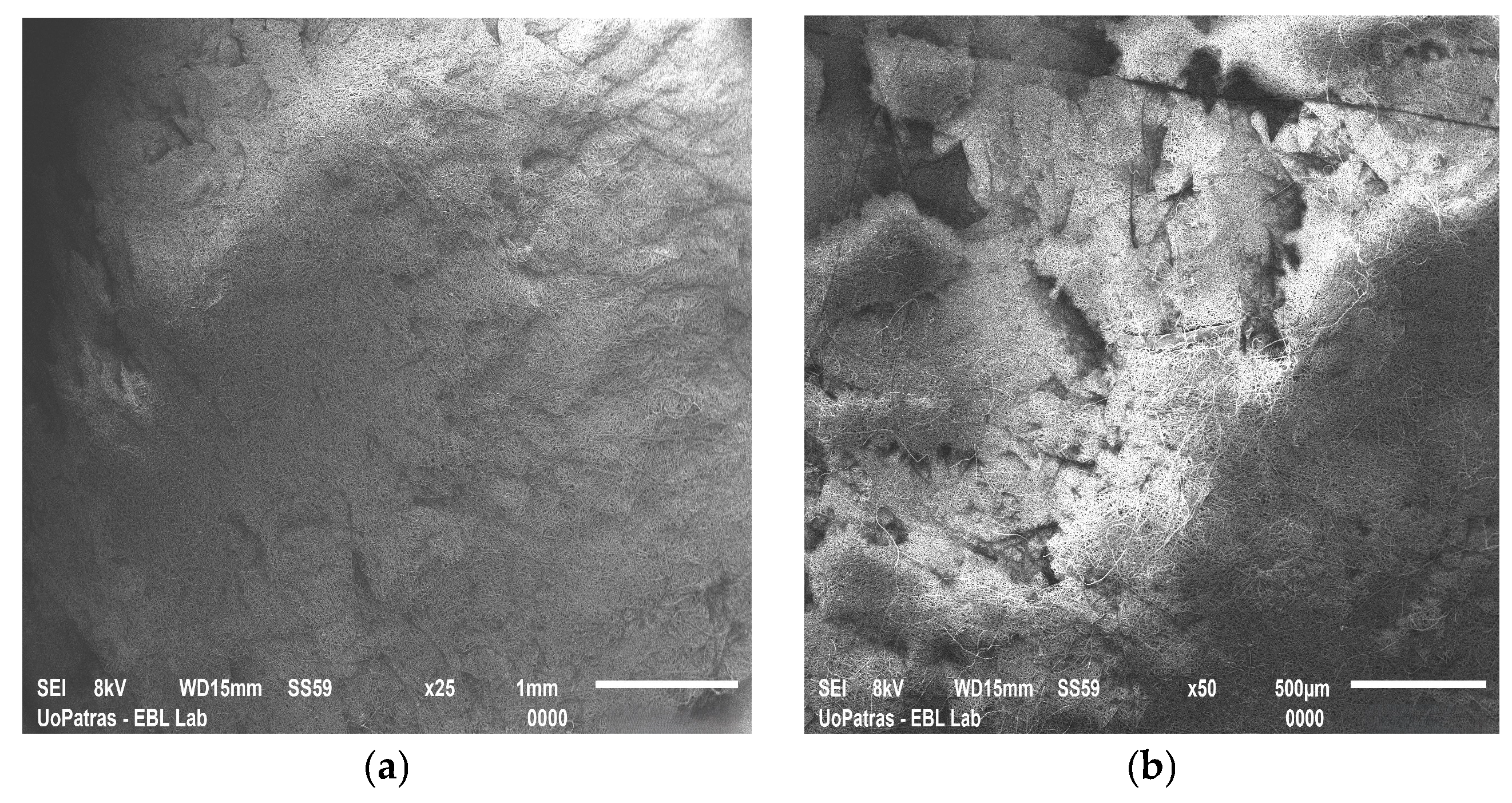
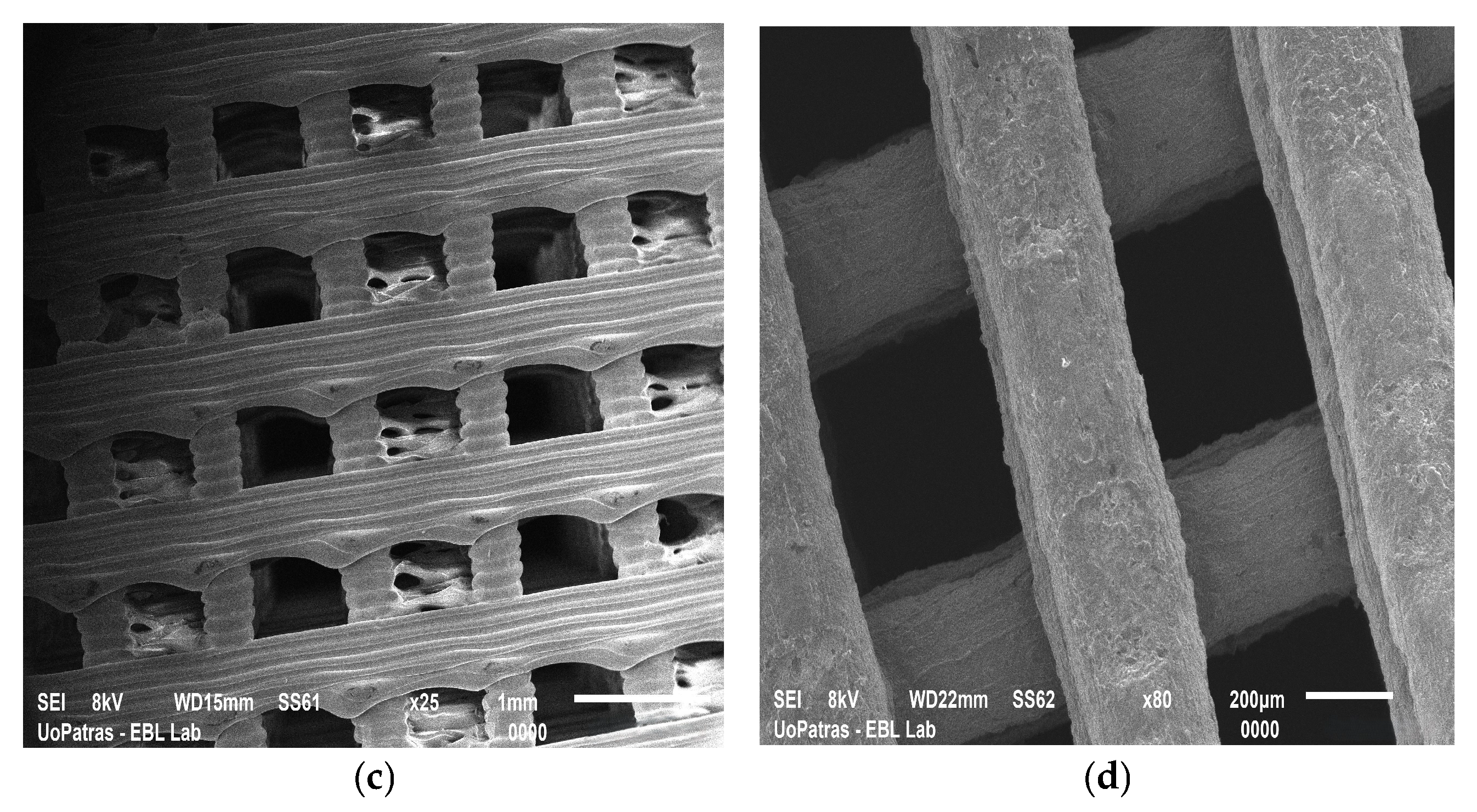
| Natural Scaffolds | Material | Properties | Applications | References |
|---|---|---|---|---|
| Hyaluronic acid (HA) | Glycosaminoglycan | Supports fibroblast proliferation, keratinocyte migration; low mechanical strength | Wound healing, soft tissue regeneration | [108] |
| Alginate | Polysaccharide (algae/bacteria) | Forms gels via ion exchange; good biocompatibility | Wound healing, cardiovascular applications | [109] |
| Chitosan | Cationic polysaccharide | Antibacterial, hemostatic, biodegradable; low mechanical strength | Tissue regeneration, wound healing | [110] |
| Carboxymethyl chitosan | Modified chitosan | Enhanced strength, biocompatibility, antibacterial activity | Wound healing, tissue regeneration | [111] |
| Bacterial cellulose (BC) | Microbial-derived polysaccharide | High porosity, excellent biocompatibility | Wound care, bone/cartilage regeneration | [112] |
| Collagen | ECM protein | Supports wound healing; low load-bearing capacity | Soft-tissue engineering, nerve regeneration | [113] |
| Fibrin | Protein-based hydrogel | Supports vascularization, cell viability | Cardiovascular, Soft-tissue engineering | [114] |
| Hydrogels | Collagen derivative | Supports cell adhesion; drug/growth factor carrier | Soft-tissue engineering | [102,119,120,121,122] |
| Platelet-rich plasma (PRP) | Blood-derived | Promotes angiogenesis, cell adhesion | Soft tissue repair, wound healing | [151,152] |
| Decellularized ECM (dECM) | Tissue-derived ECM | Maintains native ECM composition, biocompatible | Adipose, cartilage, liver, cardiovascular tissue engineering | [101,153,154,155,156,157,158,159] |
| Hybrid/Composite Scaffolds | Material | Properties | Applications | References |
|---|---|---|---|---|
| Collagen–HA | Protein–polysaccharide composite | Supports ASC migration, ECM deposition | Soft-tissue engineering | [189,191] |
| Fibrin–HA | Protein–polysaccharide composite | High biocompatibility, vascularization | Soft tissue, cardiovascular applications | [116] |
| Gelatin–dECM | Protein–tissue composite | Supports cell adhesion, tunable degradation | Soft-tissue engineering | [117] |
| Polymer–ceramic composites | PLGA, PCL, hydroxyapatite, tricalcium phosphate | Bioactive, osteoconductive | Bone regeneration, orthopedic implants | [186,187,188] |
| Ceramic–metal composites | Hydroxyapatite–titanium, magnesium | High mechanical strength, osteoinductive | Bone repair, orthopedic applications | [201] |
| Nanocomposite scaffolds | Electrospun PCL, PLGA, hydroxyapatite | ECM-mimicking, improves osteogenic differentiation | Bone, cartilage regeneration | [159,196,197,202,203] |
| Conductive polymers | Polypyrrole, graphene | Enhances ASC migration via electrical stimulation | Nerve, cardiac repair | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manu, D.R.; Portan, D.V.; Vuţă, M.; Dobreanu, M. Influence of Scaffold Structure and Biomimetic Properties on Adipose Stem Cell Homing in Personalized Reconstructive Medicine. Biomimetics 2025, 10, 438. https://doi.org/10.3390/biomimetics10070438
Manu DR, Portan DV, Vuţă M, Dobreanu M. Influence of Scaffold Structure and Biomimetic Properties on Adipose Stem Cell Homing in Personalized Reconstructive Medicine. Biomimetics. 2025; 10(7):438. https://doi.org/10.3390/biomimetics10070438
Chicago/Turabian StyleManu, Doina Ramona, Diana V. Portan, Monica Vuţă, and Minodora Dobreanu. 2025. "Influence of Scaffold Structure and Biomimetic Properties on Adipose Stem Cell Homing in Personalized Reconstructive Medicine" Biomimetics 10, no. 7: 438. https://doi.org/10.3390/biomimetics10070438
APA StyleManu, D. R., Portan, D. V., Vuţă, M., & Dobreanu, M. (2025). Influence of Scaffold Structure and Biomimetic Properties on Adipose Stem Cell Homing in Personalized Reconstructive Medicine. Biomimetics, 10(7), 438. https://doi.org/10.3390/biomimetics10070438






