Research Progress and Challenges in 3D Printing of Bioceramics and Bioceramic Matrix Composites
Abstract
1. Introduction
2. Three-Dimensional Printing of Bioceramics
2.1. Direct Ink Writing (DIW)
2.2. Fused Deposition Modeling (FDM)
2.3. Digital Light Processing (DLP)
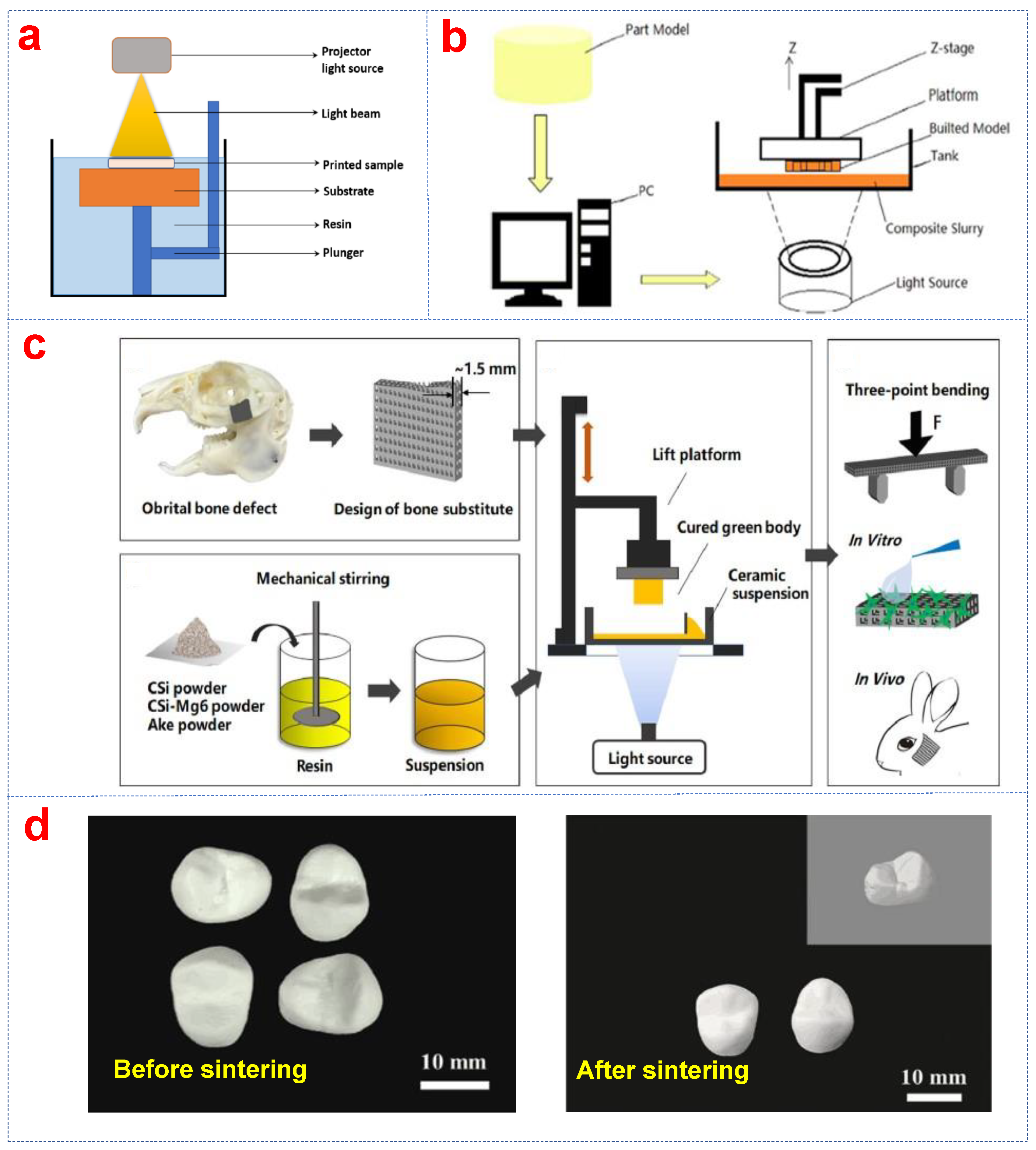

2.4. Stereolithography (SLA)
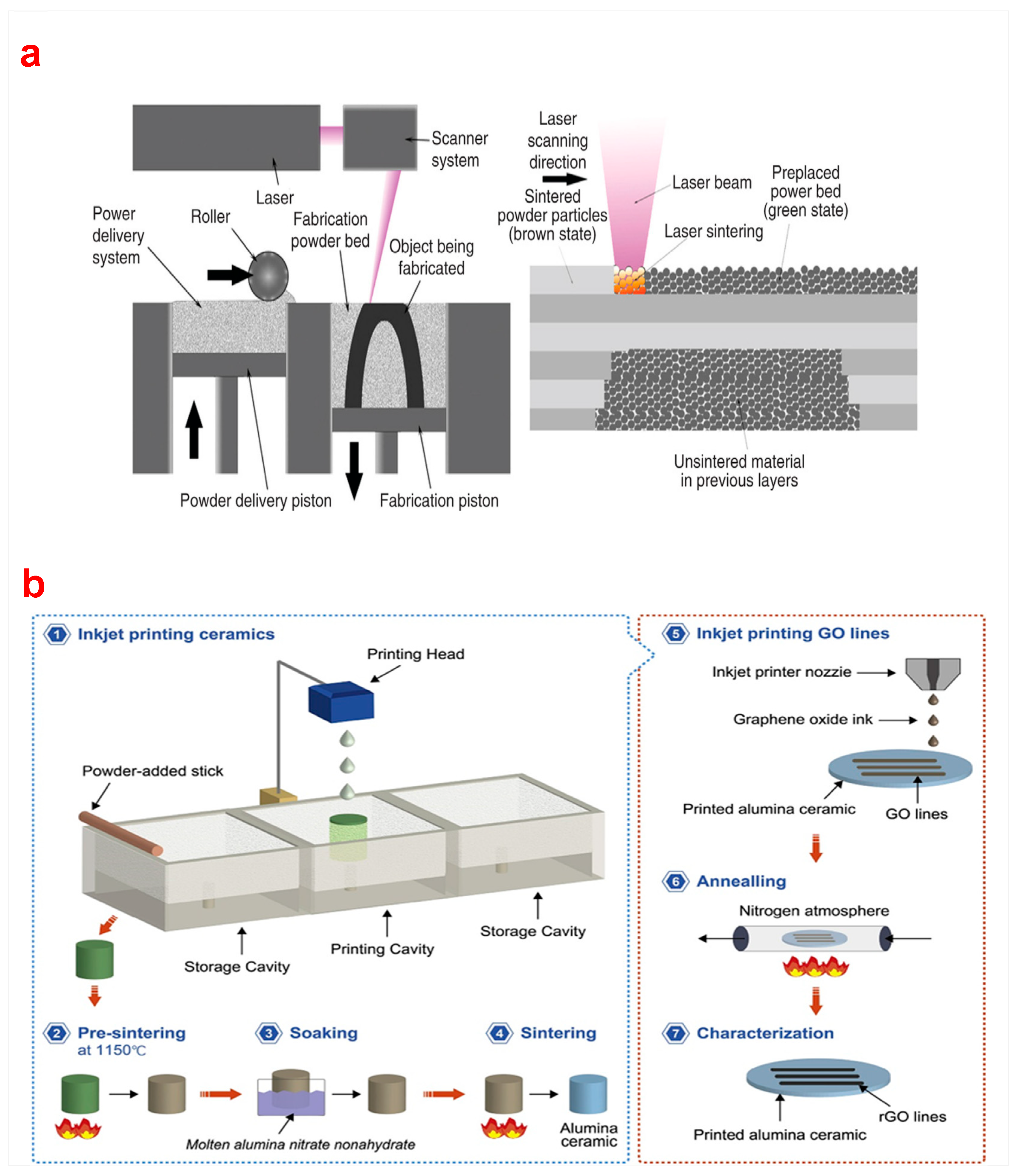
2.5. Selective Laser Sintering (SLS)
2.6. Selective Laser Melting (SLM)
3. Three-Dimensional Printing of Bioceramic Matrix Composites
3.1. Fiber Reinforcements
3.2. Particle Reinforcements
3.3. Whisker Reinforcements
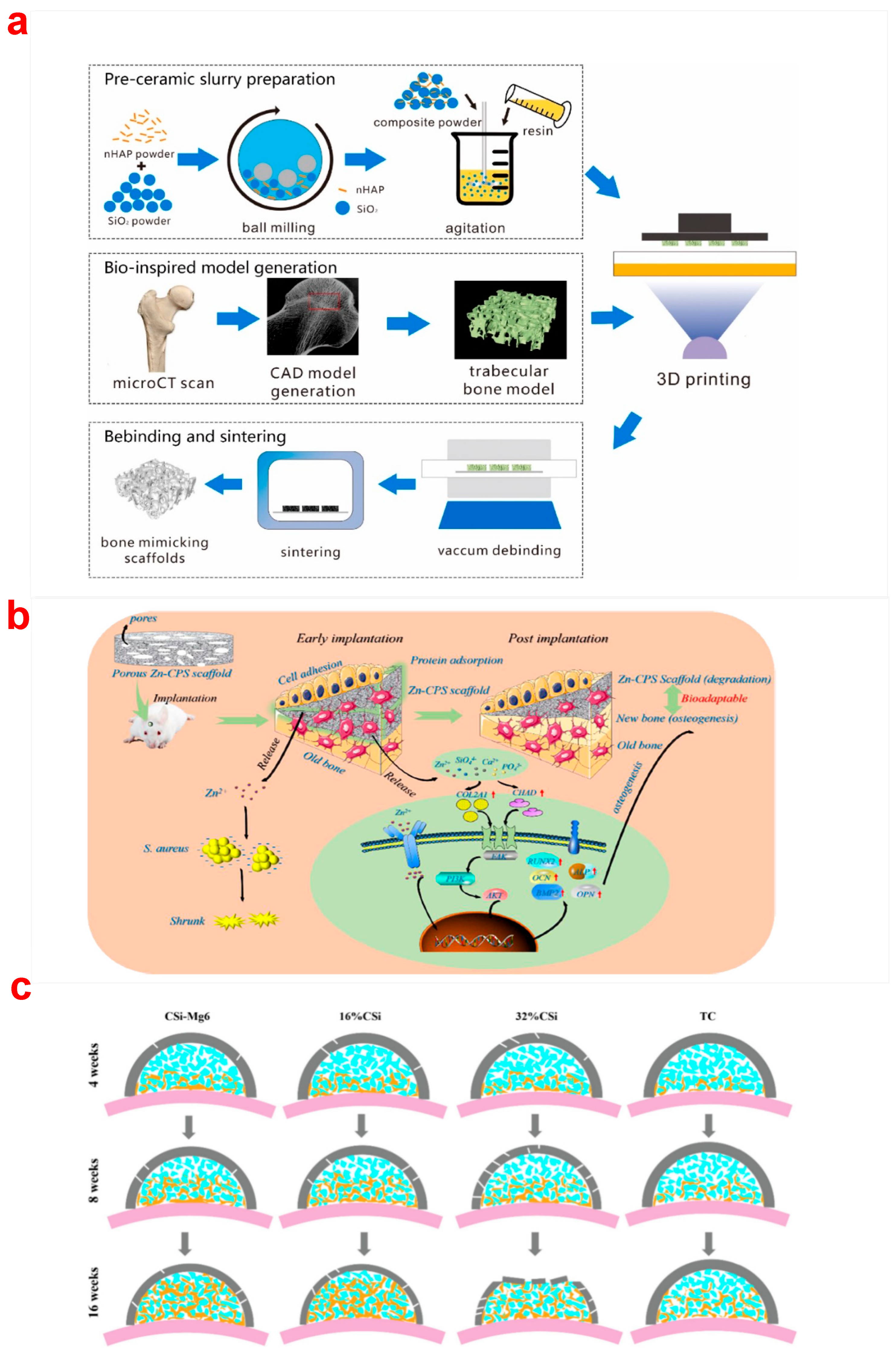
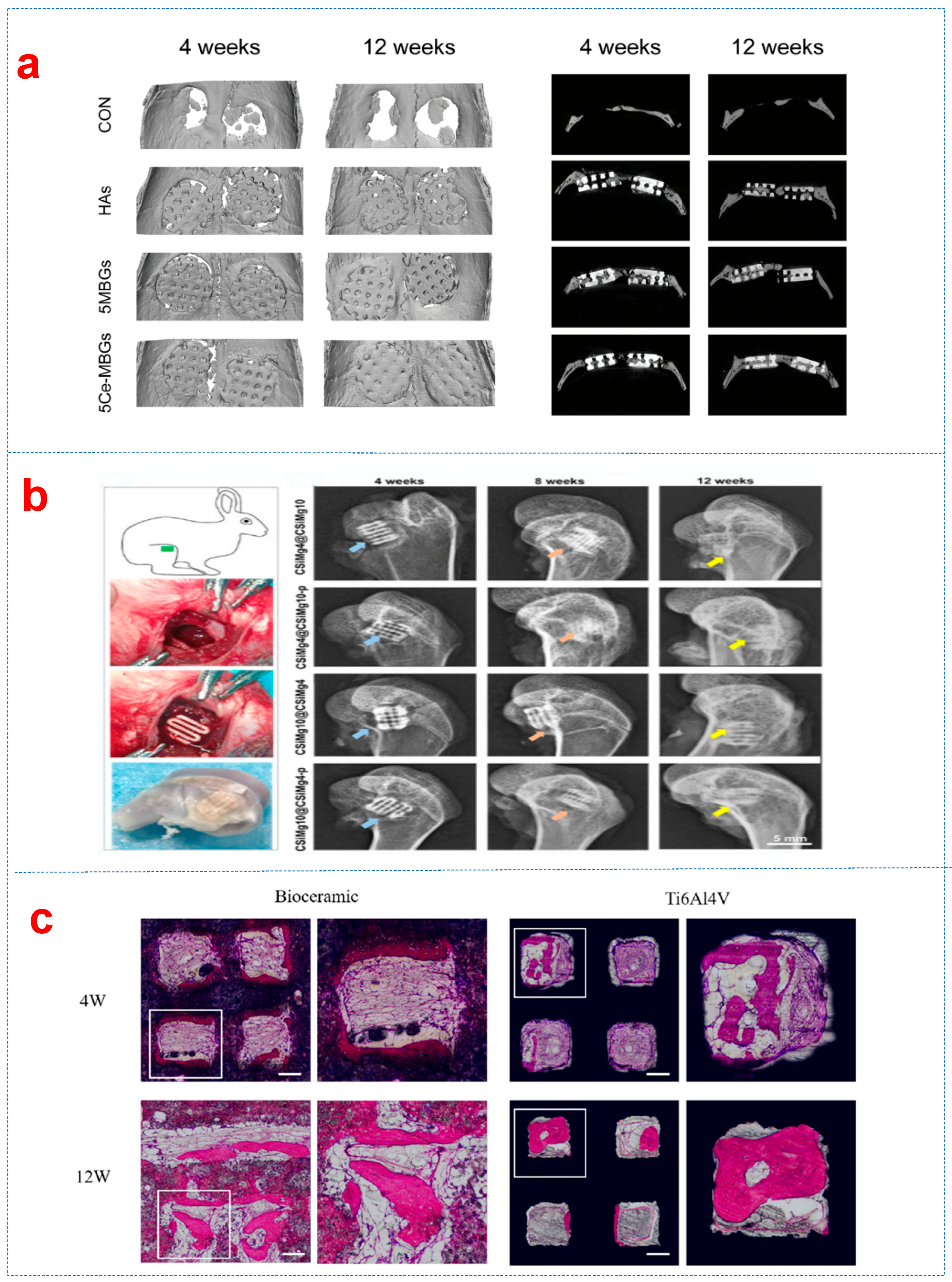
3.4. Ionic Doping Reinforcements
4. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghodbane, S.A.; Murthy, N.S.; Dunn, M.G.; Kohn, J. Achieving molecular orientation in thermally extruded 3D printed objects. Biofabrication. 2019, 11, 045004. [Google Scholar] [CrossRef] [PubMed]
- Shahrubudin, N.; Te Chuan, L.; Ramlan, R. An overview of critical success factors for implementing 3D printing technology in manufacturing firms. J. Appl. Eng. Sci. 2019, 17, 379–385. [Google Scholar] [CrossRef]
- Hull, C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent US4575330A, 8 August 1984. [Google Scholar]
- Wang, X.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D. 3D printing of polymer matrix composites: A review and prospective. Compos. Part B-Eng. 2017, 110, 442–458. [Google Scholar] [CrossRef]
- Leukers, B.; Gülkan, H.; Irsen, S.H.; Milz, S.; Tille, C.; Schieker, M.; Seitz, H. Hydroxyapatite scaffolds for bone tissue engineering made by 3D printing. J. Mater. Sci. Mater. Med. 2005, 16, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, H.; Wu, L.; Ma, L.; Xing, F.; Kong, Q.; Fan, Y.; Zhou, C.; Zhang, X. 3D printing of calcium phosphate bioceramic with tailored biodegradation rate for skull bone tissue reconstruction. Bio-Des. Manuf. 2019, 2, 161–171. [Google Scholar] [CrossRef]
- Rodrigo-Vázquez, C.S.; Kamboj, N.; Aghayan, M.; Sáez, A.; De Aza, A.H.; Rodríguez, M.A.; Hussainova, I. Manufacturing of silicon—Bioactive glass scaffolds by selective laser melting for bone tissue engineering. Ceram. Int. 2020, 46, 26936–26944. [Google Scholar] [CrossRef]
- Song, W.; Sun, W.; Chen, L.; Yuan, Z. In vivo Biocompatibility and Bioactivity of Calcium Silicate-Based Bioceramics in Endodontics. Front. Bioeng. Biotechnol. 2020, 8, 580954. [Google Scholar] [CrossRef]
- Dhinasekaran, D.; Jagannathan, M.; Rajendran, A.R.; Purushothaman, B. Microwave-assisted fabrication of nanostructured borate bioactive glass and its bioactivity. Biomater. Sci. 2024, 12, 4727–4734. [Google Scholar] [CrossRef]
- Deng, C.; Chang, J.; Wu, C. Bioactive scaffolds for osteochondral regeneration. J. Orthop. Translat. 2019, 17, 15–25. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Izquierdo-Barba, I.; Colilla, M. Structure and functionalization of mesoporous bioceramics for bone tissue regeneration and local drug delivery. Philos. Trans. A Math. Phys. Eng. Sci. 2012, 370, 1400–1421. [Google Scholar] [CrossRef]
- Kargozar, S.; Singh, R.K.; Kim, H.-W.; Baino, F. “Hard” ceramics for “Soft” tissue engineering: Paradox or opportunity? Acta Biomater. 2020, 115, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.A.; Monteiro, F.J.; Santos, J.D. Glass-reinforced hydroxyapatite composites: Fracture toughness and hardness dependence on microstructural characteristics. Biomaterials 1999, 20, 2085–2090. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, W.-M.; Fei, Z.-Q.; Chen, J.-L.; Xiong, J.-Y.; Zhang, J.-F.; Duan, L.; Huang, J.; Liu, Z.; Wang, D. The study on biocompatibility of porous nHA/PLGA composite scaffolds for tissue engineering with rabbit chondrocytes in vitro. BioMed Res. Int. 2013, 2013, 412745. [Google Scholar] [CrossRef]
- Gatti, A.; Zaffe, D.; Poli, G. Behaviour of tricalcium phosphate and hydroxyapatite granules in sheep bone defects. Biomaterials 1990, 11, 513–517. [Google Scholar] [CrossRef]
- Böstman, O.; Hirensalo, E.; Vainionpää, S.; Mäkelä, A.; Vihtonen, K.; Törmälä, P.; Rokkanen, P. Ankle fractures treated using biodegradable internal fixation. Clin. Orthop. Relat. Res. 1989, 238, 195–203. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Feng, C.; Chang, J.; Wu, C. 3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta Biomater. 2018, 79, 37–59. [Google Scholar] [CrossRef]
- Song, P.; Hu, C.; Pei, X.; Sun, J.; Sun, H.; Wu, L.; Jiang, Q.; Fan, H.; Yang, B.; Zhou, C. Dual modulation of crystallinity and macro-/microstructures of 3D printed porous titanium implants to enhance stability and osseointegration. J. Mater. Chem. B. 2019, 7, 2865–2877. [Google Scholar] [CrossRef]
- Yang, G.; Sun, Y.; Li, M.; Ou, K.; Fang, J.; Fu, Q. Direct-ink-writing (DIW) 3D printing functional composite materials based on supra-molecular interaction. Compos. Sci. Technol. 2021, 215, 109013. [Google Scholar] [CrossRef]
- Ahlfeld, T.; Köhler, T.; Czichy, C.; Lode, A.; Gelinsky, M. A methylcellulose hydrogel as support for 3D plotting of complex shaped calcium phosphate scaffolds. Gels 2018, 4, 68. [Google Scholar] [CrossRef]
- Tagliaferri, S.; Panagiotopoulos, A.; Mattevi, C. Direct ink writing of energy materials. Mater. Adv. 2021, 2, 540–563. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Luo, H.; Yuan, X.; Wang, X.; Xiong, H.; Zhang, D. Direct ink writing of 3D piezoelectric ceramics with complex unsupported structures. Eur. Ceram. Soc. 2022, 42, 3841–3847. [Google Scholar] [CrossRef]
- Lewis, J.A.; Gratson, G.M. Direct writing in three dimensions. Mater. Today 2004, 7, 32–39. [Google Scholar] [CrossRef]
- Lewis, J.A.; Smay, J.E.; Stuecker, J.; Cesarano, J. Direct ink writing of three-dimensional ceramic structures. J. Am. Ceram. Soc. 2006, 89, 3599–3609. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, L.; Chen, H.; Ventikos, Y.; Narayan, R.J.; Huang, J. Finite element evaluations of the mechanical properties of polycaprolactone/hydroxyapatite scaffolds by direct ink writing: Effects of pore geometry. J. Mech. Behav. Biomed. Mater. 2020, 104, 103665. [Google Scholar] [CrossRef]
- Somers, N.; Jean, F.; Lasgorceix, M.; Preux, N.; Delmotte, C.; Boilet, L.; Petit, F.; Leriche, A. Fabrication of doped β-tricalcium phosphate bioceramics by Direct Ink Writing for bone repair applications. Eur. Ceram. Soc. 2023, 43, 629–638. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, X.; Ditta, A.; Feng, B.; Su, L.; Zhang, Y. Preliminary 3D printing of large inclined-shaped alumina ceramic parts by direct ink writing. J. Adv. Ceram. 2020, 9, 312–319. [Google Scholar] [CrossRef]
- Carloni, D.; Zhang, G.; Wu, Y. Transparent alumina ceramics fabricated by 3D printing and vacuum sintering. Eur. Ceram. Soc. 2021, 41, 781–791. [Google Scholar] [CrossRef]
- Li, Y.-y.; Li, L.-t.; Li, B. Direct write printing of three-dimensional ZrO2 biological scaffolds. Mater. Des. 2015, 72, 16–20. [Google Scholar] [CrossRef]
- Hossain, S.S.; Lu, K. Recent progress of alumina ceramics by direct ink writing: Ink design, printing and post-processing. Ceram. Int. 2023, 49, 10199–10212. [Google Scholar] [CrossRef]
- Bulina, N.V.; Titkov, A.I.; Baev, S.G.; Makarova, S.V.; Khusnutdinov, V.R.; Bessmeltsev, V.P.; Lyakhov, N.Z. Laser sintering of hydroxyapatite for potential fabrication of bioceramic scaffolds. Mater. Today Proc. 2021, 37, 4022–4026. [Google Scholar] [CrossRef]
- Veljovic, D.; Palcevskis, E.; Zalite, I.; Petrovic, R.; Janackovic, D. Two-step microwave sintering—A promising technique for the processing of nanostructured bioceramics. Mater. Lett. 2013, 93, 251–253. [Google Scholar] [CrossRef]
- Fu, L.; Engqvist, H.; Xia, W. Spark plasma sintering of biodegradable Si3N4 bioceramic with Sr, Mg and Si as sintering additives for spinal fusion. Eur. Ceram. Soc. 2018, 38, 2110–2119. [Google Scholar] [CrossRef]
- Wang, C.-J.; Huang, C.-Y. Effect of TiO2 addition on the sintering behavior, hardness and fracture toughness of an ultrafine alumina. Mater. Sci. Eng. A 2008, 492, 306–310. [Google Scholar] [CrossRef]
- Chen, G.; Zu, Y.; Luo, J.; Fu, X.; Zhou, W. Microstructure and superplastic behavior of TiO2-doped Al2O3–ZrO2 (3Y) composite ceramics. Mater. Sci. Eng. A 2012, 554, 6–11. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, P.; Yang, W.; Gao, S.; Li, B.; Li, Q. Direct-writing of 3D periodic TiO2 bio-ceramic scaffolds with a sol-gel ink for in vitro cell growth. Mater. Des. 2018, 144, 304–309. [Google Scholar] [CrossRef]
- Liu, N.; Sun, X.; Chen, Z.; Xu, Z.; Dai, N.; Shi, G.; Cai, S.; Lv, X.; Zheng, C. Direct ink writing of dense alumina ceramics prepared by rapid sintering. Ceram. Int. 2022, 48, 30767–30778. [Google Scholar] [CrossRef]
- Lakhdar, Y.; Tuck, C.; Binner, J.; Terry, A.; Goodridge, R. Additive manufacturing of advanced ceramic materials. Prog. Mater. Sci. 2021, 116, 100736. [Google Scholar] [CrossRef]
- Esslinger, S.; Gadow, R. Additive manufacturing of bioceramic scaffolds by combination of FDM and slip casting. J. Eur. Ceram. Soc. 2020, 40, 3707–3713. [Google Scholar] [CrossRef]
- Kalita, S.J.; Bose, S.; Hosick, H.L.; Bandyopadhyay, A. Development of controlled porosity polymer-ceramic composite scaffolds via fused deposition modeling. Mater. Sci. Eng. C 2003, 23, 611–620. [Google Scholar] [CrossRef]
- Song, P.; Zhou, C.; Fan, H.; Zhang, B.; Pei, X.; Fan, Y.; Jiang, Q.; Bao, R.; Yang, Q.; Dong, Z. Novel 3D porous biocomposite scaffolds fabricated by fused deposition modeling and gas foaming combined technology. Compos. Part B-Eng. 2018, 152, 151–159. [Google Scholar] [CrossRef]
- Arnesano, A.; Padmanabhan, S.K.; Notarangelo, A.; Montagna, F.; Licciulli, A. Fused deposition modeling shaping of glass infiltrated alumina for dental restoration. Ceram. Int. 2020, 46, 2206–2212. [Google Scholar] [CrossRef]
- Singh, D.; Singh, R.; Boparai, K.S. Development and surface improvement of FDM pattern based investment casting of biomedical implants: A state of art review. J. Manuf. Process. 2018, 31, 80–95. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, K.; He, R.; Ding, G.; Xia, M.; Jin, X.; Xie, C. Additive manufacturing of hydroxyapatite bioceramic scaffolds: Dispersion, digital light processing, sintering, mechanical properties, and biocompatibility. J. Adv. Ceram. 2020, 9, 360–373. [Google Scholar] [CrossRef]
- Mahendran, T.; Renold, E.S. Review of Physical, Mechanical, and Biological Characteristics of 3D-Printed Bioceramic Scaffolds for Bone Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2022, 8, 5060–5093. [Google Scholar] [CrossRef]
- He, R.; Liu, W.; Wu, Z.; An, D.; Huang, M.; Wu, H.; Jiang, Q.; Ji, X.; Wu, S.; Xie, Z. Fabrication of complex-shaped zirconia ceramic parts via a DLP-stereolithography-based 3D printing method. Ceram. Int. 2018, 44, 3412–3416. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Li, J.; Liu, C.; Lao, C.; Fu, Y.; Liu, C.; Li, Y.; Wang, P.; He, Y. 3D printing of ceramics: A review. Eur. Ceram. Soc. 2019, 39, 661–687. [Google Scholar] [CrossRef]
- Wang, J.; Dai, X.; Peng, Y.; Liu, M.; Lu, F.; Yang, X.; Gou, Z.; Ye, J. Digital light processing strength-strong ultra-thin bioceramic scaffolds for challengeable orbital bone regeneration and repair in Situ. Appl. Mater. Today. 2021, 22, 100889. [Google Scholar] [CrossRef]
- Huang, J.; Best, S.; Bonfield, W.; Brooks, R.; Rushton, N.; Jayasinghe, S.; Edirisinghe, M. In vitro assessment of the biological response to nano-sized hydroxyapatite. J. Mater. Sci. Mater. Med. 2004, 15, 441–445. [Google Scholar] [CrossRef]
- Salinas, A.J.; Esbrit, P.; Vallet-Regí, M. A tissue engineering approach based on the use of bioceramics for bone repair. Biomater. Sci. 2013, 1, 40–51. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, H.; Shi, T.; Xie, D.; Chen, R.; Han, X.; Shen, L.; Wang, C.; Tian, Z. Additive manufacturing of hydroxyapatite bone scaffolds via digital light processing and in vitro compatibility. Ceram. Int. 2019, 45, 11079–11086. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, J.; Zuo, Y.; Li, K.; Mao, Z.; Jin, X.; Zhang, S.; Gao, H.; Cui, Y. Digital light processing of β-tricalcium phosphate bioceramic scaffolds with controllable porous structures for patient specific craniomaxillofacial bone reconstruction. Mater. Des. 2022, 216, 110558. [Google Scholar] [CrossRef]
- Baino, F.; Magnaterra, G.; Fiume, E.; Schiavi, A.; Tofan, L.P.; Schwentenwein, M.; Verné, E. Digital light processing stereolithography of hydroxyapatite scaffolds with bone-like architecture, permeability, and mechanical properties. J. Am. Ceram. Soc. 2022, 105, 1648–1657. [Google Scholar] [CrossRef]
- Liu, S.; Mo, L.; Bi, G.; Chen, S.; Yan, D.; Yang, J.; Jia, Y.-G.; Ren, L. DLP 3D printing porous β-tricalcium phosphate scaffold by the use of acrylate/ceramic composite slurry. Ceram. Int. 2021, 47, 21108–21116. [Google Scholar] [CrossRef]
- Yin, X.; Li, Q.; Hong, Y.; Yu, X.; Yang, X.; Bao, Z.; Yu, M.; Yang, H.; Gou, Z.; Zhang, B. Customized reconstruction of alveolar cleft by high mechanically stable bioactive ceramic scaffolds fabricated by digital light processing. Mater. Des. 2022, 218, 110659. [Google Scholar] [CrossRef]
- Germaini, M.-M.; Belhabib, S.; Guessasma, S.; Deterre, R.; Corre, P.; Weiss, P. Additive manufacturing of biomaterials for bone tissue engineering–A critical review of the state of the art and new concepts. Prog. Mater. Sci. 2022, 130, 100963. [Google Scholar] [CrossRef]
- Zhang, B.; Xing, F.; Chen, L.; Zhou, C.; Gui, X.; Su, Z.; Fan, S.; Zhou, Z.; Jiang, Q.; Zhao, L. DLP fabrication of customized porous bioceramics with osteoinduction ability for remote isolation bone regeneration. Mat. Sci. Eng. C-Mater. 2023, 145, 213261. [Google Scholar] [CrossRef]
- Liu, W.; Wang, T.; Zhao, X.; Dan, X.; William, W.; Pan, H. Akermanite used as an alkaline biodegradable implants for the treatment of osteoporotic bone defect. Bioact. Mater. 2016, 1, 151–159. [Google Scholar] [CrossRef]
- Liu, Q.; Lian, C.; Shuo, Y.; Chen, L.; Liu, G.; Chang, J.; Cuia, L. A comparative study of proliferation and osteogenic differentiation of adipose-derived stem cells on akermanite and β-TCP ceramics. Biomaterials 2008, 29, 4792–4799. [Google Scholar] [CrossRef]
- Pan, M.-Z.; Hua, S.-B.; Wu, J.-M.; Su, J.-J.; Zhang, X.-Y.; Shi, Y.-S. Composite bioceramic scaffolds with controllable photothermal performance fabricated by digital light processing and vacuum infiltration. Eur. Ceram. Soc. 2023, 43, 2245–2252. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, Y.; Zeng, Y.; Chen, J. DLP 3D printed silica-doped HAp ceramic scaffolds inspired by the trabecular bone structure. Ceram. Int. 2022, 48, 27765–27773. [Google Scholar] [CrossRef]
- Zhang, H.; Jiao, C.; He, Z.; Ge, M.; Tian, Z.; Wang, C.; Wei, Z.; Shen, L.; Liang, H. Fabrication and properties of 3D printed zirconia scaffold coated with calcium silicate/hydroxyapatite. Ceram. Int. 2021, 47, 27032–27041. [Google Scholar] [CrossRef]
- Deng, F.; Bu, Z.; Hu, H.; Huang, X.; Liu, Z.; Ning, C. Bioadaptable bone regeneration of Zn-containing silicocarnotite bioceramics with moderate biodegradation and antibacterial activity. Appl. Mater. Today. 2022, 27, 101433. [Google Scholar] [CrossRef]
- Xu, C.; Wu, F.; Yang, J.; Wang, H.; Jiang, J.; Bao, Z.; Yang, X.; Yang, G.; Gou, Z.; He, F. 3D printed long-term structurally stable bioceramic dome scaffolds with controllable biodegradation favorable for guided bone regeneration. Chem. Eng. J. 2022, 450, 138003. [Google Scholar] [CrossRef]
- Jodati, H.; Evis, Z.; Tezcaner, A.; Alshemary, A.Z.; Motameni, A. 3D porous bioceramic based boron-doped hydroxyapatite/baghdadite composite scaffolds for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2023, 140, 105722. [Google Scholar] [CrossRef]
- Komissarenko, D.; Roland, S.; Seeber, B.S.; Graule, T.; Blugan, G. DLP 3D printing of high strength semi-translucent zirconia ceramics with relatively low-loaded UV-curable formulations. Ceram. Int. 2023, 49, 21008–21016. [Google Scholar] [CrossRef]
- Chen, F.; Zhu, H.; Wu, J.-M.; Chen, S.; Cheng, L.-J.; Shi, Y.-S.; Mo, Y.-C.; Li, C.-H.; Xiao, J. Preparation and biological evaluation of ZrO2 all-ceramic teeth by DLP technology. Ceram. Int. 2020, 46, 11268–11274. [Google Scholar] [CrossRef]
- Zhang, F.; Li, L.-f.; Wang, E.-z. Effect of micro-alumina content on mechanical properties of Al2O3/3Y-TZP composites. Ceram. Int. 2015, 41, 12417–12425. [Google Scholar] [CrossRef]
- Zhao, H.; Xing, H.; Lai, Q.; Zhao, Y.; Chen, Q.; Zou, B. Additive manufacturing of graphene oxide/hydroxyapatite bioceramic scaffolds with reinforced osteoinductivity based on digital light processing technology. Mater. Des. 2022, 223, 111231. [Google Scholar] [CrossRef]
- Hua, S.-B.; Yuan, X.; Wu, J.-M.; Su, J.; Cheng, L.-J.; Zheng, W.; Pan, M.-Z.; Xiao, J.; Shi, Y.-S. Digital light processing porous TPMS structural HA & akermanite bioceramics with optimized performance for cancellous bone repair. Ceram. Int. 2022, 48, 3020–3029. [Google Scholar] [CrossRef]
- Moritz, T.; Maleksaeedi, S. Additive manufacturing of ceramic components. In Additive Manufacturing—Materials, Processes, Quantifications and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 105–161. [Google Scholar]
- Hundley, J.M.; Eckel, Z.C.; Schueller, E.; Cante, K.; Biesboer, S.M.; Yahata, B.D.; Schaedler, T.A. Geometric characterization of additively manufactured polymer derived ceramics. Addit. Manuf. 2017, 18, 95–102. [Google Scholar] [CrossRef]
- Liu, K.; Wu, X.; Liu, J.; Yang, H.; Li, M.; Qiu, T.; Dai, H. Design and manufacture of a customized, large-size and high-strength bioactive HA osteoid composite ceramic by stereolithography. Ceram. Int. 2023, 49, 11630–11640. [Google Scholar] [CrossRef]
- Gauvin, R.; Chen, Y.-C.; Lee, J.W.; Soman, P.; Zorlutuna, P.; Nichol, J.W.; Bae, H.; Chen, S.; Khademhosseini, A. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials 2012, 33, 3824–3834. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wu, Y.-R.; Wu, J.-M.; Zhu, H.; Chen, S.; Hua, S.-B.; He, Z.-X.; Liu, C.-Y.; Xiao, J.; Shi, Y.-S. Preparation and characterization of ZrO2-Al2O3 bioceramics by stereolithography technology for dental restorations. Addit. Manuf. 2021, 44, 102055. [Google Scholar] [CrossRef]
- Wu, H.; Liu, W.; Huang, R.; He, R.; Huang, M.; An, D.; Li, H.; Jiang, Q.; Tian, Z.; Ji, X. Fabrication of high-performance Al2O3-ZrO2 composite by a novel approach that integrates stereolithography-based 3D printing and liquid precursor infiltration. Mater. Chem. Phys. 2018, 209, 31–37. [Google Scholar] [CrossRef]
- Coppola, B.; Lacondemine, T.; Tardivat, C.; Montanaro, L.; Palmero, P. Designing alumina-zirconia composites by DLP-based stereolithography: Microstructural tailoring and mechanical performances. Ceram. Int. 2021, 47, 13457–13468. [Google Scholar] [CrossRef]
- Lu, F.; Wu, R.; Shen, M.; Xie, L.; Liu, M.; Li, Y.; Xu, S.; Wan, L.; Yang, X.; Gao, C. Rational design of bioceramic scaffolds with tuning pore geometry by stereolithography: Microstructure evaluation and mechanical evolution. Eur. Ceram. Soc. 2021, 41, 1672–1682. [Google Scholar] [CrossRef]
- Raghavendra, S.S.; Jadhav, G.R.; Gathani, K.M.; Kotadia, P. Bioceramics in endodontics—A review. J. Istanb. Univ. Fac. Dent. 2017, 51, S128–S137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Q.; He, F.; Zuo, F.; Shi, X. Fabrication of cancellous-bone-mimicking β-tricalcium phosphate bioceramic scaffolds with tunable architecture and mechanical strength by stereolithography 3D printing. Eur. Ceram. Soc. 2022, 42, 6713–6720. [Google Scholar] [CrossRef]
- Liu, R.; Ma, L.; Liu, H.; Xu, B.; Feng, C.; He, R. Effects of pore size on the mechanical and biological properties of stereolithographic 3D printed HAp bioceramic scaffold. Ceram. Int. 2021, 47, 28924–28931. [Google Scholar] [CrossRef]
- Dong, D.; Su, H.; Li, X.; Fan, G.; Zhao, D.; Shen, Z.; Liu, Y.; Guo, Y.; Yang, C.; Liu, L. Microstructures and mechanical properties of biphasic calcium phosphate bioceramics fabricated by SLA 3D printing. J. Manuf. Process. 2022, 81, 433–443. [Google Scholar] [CrossRef]
- Witek, L.; Smay, J.; Silva, N.R.; Guda, T.; Ong, J.L.; Coelho, P.G. Sintering effects on chemical and physical properties of bioactive ceramics. J. Adv. Ceram. 2013, 2, 274–284. [Google Scholar] [CrossRef]
- Le Nihouannen, D.; Duval, L.; Lecomte, A.; Julien, M.; Guicheux, J.; Daculsi, G.; Layrolle, P. Interactions of total bone marrow cells with increasing quantities of macroporous calcium phosphate ceramic granules. J. Mater. Sci. Mater. Med. 2007, 18, 1983–1990. [Google Scholar] [CrossRef]
- Wu, S.-C.; Hsu, H.-C.; Hsu, S.-K.; Wang, W.-H.; Ho, W.-F. Preparation and characterization of four different compositions of calcium phosphate scaffolds for bone tissue engineering. Mater. Charact. 2011, 62, 526–534. [Google Scholar] [CrossRef]
- Melchels, F.P.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef]
- Zhao, L.; Jiang, Z.; Zhang, C.; Guo, W. Regulation of residual stress in stereolithography printing of ZrO2 ceramics. Ceram. Int. 2023, 49, 30801–30810. [Google Scholar] [CrossRef]
- Wang, H.; Shen, F.; Li, Z.; Zhou, B.; Zhao, P.; Wang, W.; Cheng, B.; Yang, J.; Li, B.; Wang, X. Preparation of high-performance ZrO2 bio-ceramics by stereolithography for dental restorations. Ceram. Int. 2023, 49, 28048–28061. [Google Scholar] [CrossRef]
- Molitch-Hou, M. Overview of additive manufacturing process. In Additive Manufacturing—Materials, Processes, Quantifications and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–38. [Google Scholar]
- Sun, S.; Fei, G.; Wang, X.; Xie, M.; Guo, Q.; Fu, D.; Wang, Z.; Wang, H.; Luo, G.; Xia, H. Covalent adaptable networks of polydimethylsiloxane elastomer for selective laser sintering 3D printing. Chem. Eng. J. 2021, 412, 128675. [Google Scholar] [CrossRef]
- Han, J.; Wu, J.; Xiang, X.; Xie, L.; Chen, R.; Li, L.; Ma, K.; Sun, Q.; Yang, R.; Huang, T. Biodegradable BBG/PCL composite scaffolds fabricated by selective laser sintering for directed regeneration of critical-sized bone defects. Mater. Des. 2023, 225, 111543. [Google Scholar] [CrossRef]
- Shuai, C.; Li, P.; Liu, J.; Peng, S. Optimization of TCP/HAP ratio for better properties of calcium phosphate scaffold via selective laser sintering. Mater. Charact. 2013, 77, 23–31. [Google Scholar] [CrossRef]
- Tang, H.-H.; Chiu, M.-L.; Yen, H.-C. Slurry-based selective laser sintering of polymer-coated ceramic powders to fabricate high strength alumina parts. Eur. Ceram. Soc. 2011, 31, 1383–1388. [Google Scholar] [CrossRef]
- Shuai, C.; Nie, Y.; Gao, C.; Feng, P.; Zhuang, J.; Zhou, Y.; Peng, S. The microstructure evolution of nanohydroxapatite powder sintered for bone tissue engineering. J. Exp. Nanosci. 2013, 8, 762–773. [Google Scholar] [CrossRef]
- Liu, H.; Su, H.; Shen, Z.; Wang, E.; Zhao, D.; Guo, M.; Zhang, J.; Liu, L.; Fu, H. Direct formation of Al2O3/GdAlO3/ZrO2 ternary eutectic ceramics by selective laser melting: Microstructure evolutions. Eur. Ceram. Soc. 2018, 38, 5144–5152. [Google Scholar] [CrossRef]
- Huang, Z.; Tang, Y.; Guo, H.; Feng, X.; Zhang, T.; Li, P.; Qian, B.; Xie, Y. 3D printing of ceramics and graphene circuits-on-ceramics by thermal bubble inkjet technology and high temperature sintering. Ceram. Int. 2020, 46, 10096–10104. [Google Scholar] [CrossRef]
- Woo Lee, J.; Cho, D.-W. 3D printing technology over a drug delivery for tissue engineering. Curr. Pharm. Des. 2015, 21, 1606–1617. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, M.; Seitz, H.; Yang, S. A review on 3D micro-additive manufacturing technologies. Int. J. Adv. Manuf. Technol. 2013, 67, 1721–1754. [Google Scholar] [CrossRef]
- Siamak, E.; Azadeh, M.; Pedro, M.; Antonia, P.; Alexandra, L.; José, M.F. Robocasting of 45S5 bioactive glass scaffolds for bone tissue engineering. J. Eur. Ceram. Soc. 2014, 34, 107–118. [Google Scholar] [CrossRef]
- Liu, X.; Miao, Y.; Liang, H.; Diao, J.; Hao, L.; Shi, Z.; Zhao, N.; Wang, Y. 3D-printed bioactive ceramic scaffolds with biomimetic micro/nano-HAp surfaces mediated cell fate and promoted bone augmentation of the bone–implant interface in vivo. Bioact. Mater. 2022, 12, 120–132. [Google Scholar] [CrossRef]
- Shao, H.; Shi, J.; Huang, Z.; Yang, W.; Wang, H. 3D-Printed Bioceramic Scaffolds with High Strength and High Precision. Crystals 2023, 13, 1061. [Google Scholar] [CrossRef]
- Luo, D.; Su, J.; Zou, Y.; Hua, S.; Cheng, L.; Qi, D.; Yuan, X.; Zhu, H.; Liu, C.; Shi, Y.; et al. 3D-printed triply periodic minimal surface bioceramic scaffold for bone defect treatment with tunable structure and mechanical properties. Ceram. Int. 2024, 50, 39020–39031. [Google Scholar] [CrossRef]
- Li, Q.; An, X.; Liang, J.; Liu, Y.; Hu, K.; Lu, Z.; Yue, X.; Li, J.; Zhou, Y.; Sun, X. Balancing flexural strength and porosity in DLP-3D printing Al2O3 cores for hollow turbine blades. J. Mater. Sci. Technol. 2022, 104, 19–32. [Google Scholar] [CrossRef]
- Shan, Y.; Bai, Y.; Yang, S.; Zhou, Q.; Wang, G.; Zhu, B.; Zhou, Y.; Fang, W.; Wen, N.; He, R.; et al. 3D-printed strontium-incorporated β-TCP bioceramic triply periodic minimal surface scaffolds with simultaneous high porosity, enhanced strength, and excellent bioactivity. J. Adv. Ceram. 2023, 12, 1671–1684. [Google Scholar] [CrossRef]
- Hua, S.; Su, J.; Deng, Z.; Wu, J.; Cheng, L.; Yuan, X.; Chen, F.; Zhu, H.; Qi, D.; Xiao, X.; et al. Microstructures and properties of 45S5 bioglass® & BCP bioceramic scaffolds fabricated by digital light processing. Addit. Manuf. 2021, 45, 102074. [Google Scholar] [CrossRef]
- Dmytro, S. Simulation of the selective laser sintering/melting process of bioactive glass 45S5. Simul. Model. Pract. Theory 2024, 136, 103009. [Google Scholar] [CrossRef]
- Nikhil, K.; Miguel, A.R.; Ramin, R.; Konda, G.; Irina, H. Bioceramic scaffolds by additive manufacturing for controlled delivery of the antibiotic vancomycin. Proc. Natl. Acad. Sci. USA 2019, 68, 185–190. [Google Scholar] [CrossRef]
- Kim, C.G.; Han, K.S.; Lee, S.; Kim, M.C.; Kim, S.Y.; Nah, J. Fabrication of Biocompatible Polycaprolactone–Hydroxyapatite Composite Filaments for the FDM 3D Printing of Bone Scaffolds. Appl. Sci. 2021, 11, 6351. [Google Scholar] [CrossRef]
- Carola, E.C.; Francesca, S.; Francesca, G.; Francesco, M.; Alessandro, S.; Alfonso, M. One-step solvent-free process for the fabrication of high loaded PLA/HA composite filament for 3D printing. J. Therm. Anal. 2018, 134, 575–582. [Google Scholar] [CrossRef]
- Bruyas, A.; Lou, F.; Stahl, A.M.; Gardner, M.; Maloney, W.; Goodman, S.; Yang, Y.P. Systematic characterization of 3D-printed PCL/β-TCP scaffolds for biomedical devices and bone tissue engineering: Influence of composition and porosity. J. Mater. Res. 2018, 33, 1948–1959. [Google Scholar] [CrossRef]
- Drummer, D.; Cifuentes-Cuéllar, S.; Rietzel, D. Suitability of PLA/TCP for fused deposition modeling. Rapid Prototyp. J. 2012, 18, 500–507. [Google Scholar] [CrossRef]
- Krishna, C.R.K.; Ming, C.L.; Gregory, E.H.; Mariano, V. Effect of material, process parameters, and simulated body fluids on mechanical properties of 13-93 bioactive glass porous constructs made by selective laser sintering. J. Mech. Behav. Biomed. 2012, 13, 14–24. [Google Scholar] [CrossRef]
- Liu, J.; Gao, C.; Feng, P.; Peng, S.; Shuai, C. Selective laser sintering of β-TCP/nano-58S composite scaffolds with improved mechanical properties. Mater. Des. 2015, 84, 395–401. [Google Scholar] [CrossRef]
- Gao, C.; Yang, B.; Hu, H.; Liu, J.; Shuai, C.; Peng, S. Enhanced sintering ability of biphasic calcium phosphate by polymers used for bone scaffold fabrication. Mater. Sci. Eng. 2013, 33, 53491–53501. [Google Scholar] [CrossRef] [PubMed]
- Passakorn, T.; Ruth, F.; Simon, G.; Robert, L.; Ian, T.; Aldo, R.B.; Jürgen, S. Processing of 45S5 Bioglass® by lithography-based additive manufacturing. Mater. Lett. 2012, 74, 81–84. [Google Scholar] [CrossRef]
- Chugunov, S.; Adams, N.A.; Akhatov, I. Evolution of SLA-Based Al2O3 Microstructure During Additive Manufacturing Process. Materials 2020, 13, 3928. [Google Scholar] [CrossRef]
- Liu, X.; Zou, B.; Xing, H.; Huang, C. The preparation of ZrO2-Al2O3 composite ceramic by SLA-3D printing and sintering processing. Ceram. Int. 2020, 46, 937–944. [Google Scholar] [CrossRef]
- Ahmad, M.; Rodziah, A. HA-SLA: A Hierarchical Autonomic SLA Model for SLA Monitoring in Cloud Computing. J. Softw. Eng. Appl. 2013, 6, 114–117. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, S.; Yin, Y. C-fibre reinforced hydroxyapatite bioceramics. Ceram. Int. 2003, 29, 113–116. [Google Scholar] [CrossRef]
- Mei, H.; Yan, Y.; Feng, L.; Dassios, K.G.; Zhang, H.; Cheng, L. First printing of continuous fibers into ceramics. J. Am. Ceram. Soc. 2019, 102, 3244–3255. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, A.; Zhou, L.; Yang, Z.; Wei, S.; Zhao, Z.; Fan, Q.; Ma, L. 3D printing of bioactive macro/microporous continuous carbon fibre reinforced hydroxyapatite composite scaffolds with synchronously enhanced strength and toughness. Eur. Ceram. Soc. 2022, 42, 4396–4409. [Google Scholar] [CrossRef]
- Inagaki, M.; Kameyama, T. Phase transformation of plasma-sprayed hydroxyapatite coating with preferred crystalline orientation. Biomaterials 2007, 28, 2923–2931. [Google Scholar] [CrossRef]
- Lacroix, J.; Jallot, E.; Lao, J. Gelatin-bioactive glass composites scaffolds with controlled macroporosity. Chem. Eng. J. 2014, 256, 9–13. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Ke, X.; Wang, J.; Yang, G.; Yang, X.; He, D.; Shao, H.; He, Y.; Fu, J. 45S5 Bioglass analogue reinforced akermanite ceramic favorable for additive manufacturing mechanically strong scaffolds. RSC Adv. 2015, 5, 102727–102735. [Google Scholar] [CrossRef]
- Rainer, A.; Giannitelli, S.M.; Abbruzzese, F.; Traversa, E.; Licoccia, S.; Trombetta, M. Fabrication of bioactive glass–ceramic foams mimicking human bone portions for regenerative medicine. Acta Biomater. 2008, 4, 362–369. [Google Scholar] [CrossRef]
- Leach, J.K.; Kaigler, D.; Wang, Z.; Krebsbach, P.H.; Mooney, D.J. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials 2006, 27, 3249–3255. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Tenorio, F.; Giraldo-Estrada, C. Characterization and chemical modification of pullulan produced from a submerged culture of Aureobasidium pullulans ATCC 15233. Polym. Test. 2022, 114, 107686. [Google Scholar] [CrossRef]
- Simorgh, S.; Alasvand, N.; Khodadadi, M.; Ghobadi, F.; Kebria, M.M.; Milan, P.B.; Kargozar, S.; Baino, F.; Mobasheri, A.; Mozafari, M. Additive manufacturing of bioactive glass biomaterials. Methods 2022, 208, 75–91. [Google Scholar] [CrossRef]
- Bose, S.; Bhattacharjee, A.; Banerjee, D.; Boccaccini, A.R.; Bandyopadhyay, A. Influence of random and designed porosities on 3D printed tricalcium phosphate-bioactive glass scaffolds. Addit. Manuf. 2021, 40, 101895. [Google Scholar] [CrossRef] [PubMed]
- Kolan, K.C.R.; Huang, Y.-W.; Semon, J.A.; Leu, M.C. 3D-printed biomimetic bioactive glass scaffolds for bone regeneration in rat calvarial defects. IJB 2020, 6, 274. [Google Scholar] [CrossRef]
- Liu, J.; Hu, H.; Li, P.; Shuai, C.; Peng, S. Fabrication and characterization of porous 45S5 glass scaffolds via direct selective laser sintering. Mater. Manuf. Process. 2013, 28, 610–615. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Shen, Y.; Xiong, Y.; Dong, L.; Zheng, J.; Zhao, S. Fabrication of porous β-TCP/58S bioglass scaffolds via top-down DLP printing with high solid loading ceramic-resin slurry. Mater. Chem. Phys. 2021, 267, 124587. [Google Scholar] [CrossRef]
- Xia, X.; Yang, Y.; Hou, Z.; Cao, Y.; Dai, W.; Li, B.; Dong, T.; SAAD, O.; Ling, S.; Xue, W. Enhanced Osteogenic Potential of Magnesium Oxide-Doped Biphasic Calcium Phosphate Bioceramics Prepared by Stereolithography. Ceram. Int. 2025, 51, 17955–17967. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.; Chen, H.; Zhang, J.; Du, L.; Jiang, F.; Zheng, K. Cerium-Containing Mesoporous Bioactive Glass Nanoparticles Reinforced 3D-Printed Bioceramic Scaffolds toward Enhanced Mechanical, Antioxidant, and Osteogenic Activities for Bone Regeneration. Adv. Healthc. Mater. 2025, 14, 2404346. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, J.; Liu, J.; Wei, Y.; Yang, X.; Lei, L.; Chen, L.; Wu, Y.; Gou, Z. Tuning filament composition and microstructure of 3D-printed bioceramic scaffolds facilitate bone defect regeneration and repair. Regen. Biomater. 2021, 8, rbab007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Darvell, B.W. Synthesis and characterization of hydroxyapatite whiskers by hydrothermal homogeneous precipitation using acetamide. Acta Biomater. 2010, 6, 3216–3222. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Gao, M.; Zhu, D.; Zhang, S.; Pan, Y.; Pan, H.; Liu, Y.; Oliveira, F.J.; Vieira, J.M. SiC whisker reinforced multi-carbides composites prepared from B4C and pyrolyzed rice husks via reactive infiltration. Ceram. Int. 2012, 38, 3519–3527. [Google Scholar] [CrossRef]
- Tanimoto, Y.; Teshima, M.; Nishiyama, N.; Yamaguchi, M.; Hirayama, S.; Shibata, Y.; Miyazaki, T. Tape-cast and sintered β-tricalcium phosphate laminates for biomedical applications: Effect of milled Al2O3 fiber additives on microstructural and mechanical properties. J. Biomed. Mater. Res. B 2012, 100, 2261–2268. [Google Scholar] [CrossRef]
- Ma, C.; Zeng, Q.; Yu, L.; Yu, S.; Song, J.; Ma, Y.; Dong, X. Preparation and characterization of 3D printed hydroxyapatite-whisker-strengthened hydroxyapatite scaffold coated with biphasic calcium phosphate. Chin. J. Mech. Eng. 2023, 2, 100097. [Google Scholar] [CrossRef]
- Feng, P.; Wei, P.; Li, P.; Gao, C.; Shuai, C.; Peng, S. Calcium silicate ceramic scaffolds toughened with hydroxyapatite whiskers for bone tissue engineering. Mater. Charact. 2014, 97, 47–56. [Google Scholar] [CrossRef]
- Wu, C.; Ramaswamy, Y.; Boughton, P.; Zreiqat, H. Improvement of mechanical and biological properties of porous CaSiO3 scaffolds by poly (D, L-lactic acid) modification. Acta Biomater. 2008, 4, 343–353. [Google Scholar] [CrossRef]
- Farhadi, K.; Namini, A.S.; Asl, M.S.; Mohammadzadeh, A.; Kakroudi, M.G. Characterization of hot pressed SiC whisker reinforced TiB2 based composites. Int. J. Refract. Met. Hard Mater. 2016, 61, 84–90. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, J.; Xin, H.; Wang, X.; Zhang, L.; He, F.; Liu, Q.; Zhang, W. Improved dispersion of SiC whisker in nano hydroxyapatite and effect of atmospheres on sintering of the SiC whisker reinforced nano hydroxyapatite composites. Mater. Sci. Eng. C 2018, 91, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Zou, B.; Wang, X.; Hu, Y.; Huang, C.; Xue, K. Fabrication and characterization of SiC whiskers toughened Al2O3 paste for stereolithography 3D printing applications. J. Alloys Compd. 2020, 828, 154347. [Google Scholar] [CrossRef]
- Li, C.; Zhang, W.; Nie, Y.; Du, X.; Huang, C.; Li, L.; Long, J.; Wang, X.; Tong, W.; Qin, L. Time-sequential and multi-functional 3D printed MgO2/PLGA scaffold developed as a novel biodegradable and bioactive bone substitute for challenging postsurgical osteosarcoma treatment. Adv. Mater. 2024, 36, 2308875. [Google Scholar] [CrossRef]
- Wang, S.; Huang, Z.; Liu, L.; Shi, Z.a.; Liu, J.; Li, Z.; Hao, Y. Design and study of in vivo bone formation characteristics of biodegradable bioceramic. Mater. Des. 2021, 212, 110242. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, B.; Lian, F. 3D-printed bioceramic scaffolds for bone defect repair: Bone aging and immune regulation. Front. Bioeng. Biotechnol. 2025, 13, 1557203. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Wang, P.; Guan, J.; Zhang, D. Construction of multileveled and oriented micro/nano channels in Mg doped hydroxyapitite bioceramics and their effect on mimicking mechanical property of cortical bone and biological performance of cancellous bone. Biomater. Adv. 2024, 161, 213871. [Google Scholar] [CrossRef]
- Filion, T.M.; Li, X.; Mason-Savas, A.; Kreider, J.M.; Goldstein, S.A.; Ayers, D.C.; Song, J. Elastomeric osteoconductive synthetic scaffolds with acquired osteoinductivity expedite the repair of critical femoral defects in rats. Tissue Eng. Part A 2011, 17, 503–511. [Google Scholar] [CrossRef]

| 3D Printing Methods | Advantages | Disadvantages | Materials |
|---|---|---|---|
| Direct Ink Writing (DIW) | Suitable for high solids slurries; Flexible structural design; Low cost | Slow printing speed; The rheological properties of the slurry are required | Bioactive glass [100] HAp [101] CSi-Mg [102] β-TCP [27] |
| Digital Light Processing (DLP) | Fast printing speed; High precision; | Add photosensitive resin; Limited materials; Post-processing is required | HA [103] Al2O3 [104] ZrO2 [68] β-TCP [105] Bioactive glass [106] |
| Selective Laser Melting (SLM) | High molding density; Excellent mechanical properties | Expensive equipment; Only suitable for metals or ceramics with high melting points | Bioactive glass [107] CaO3Si [108] |
| Fused Deposition Manufacturing (FDM) | Equipment of low cost; Simple operation; Multi-material printing | Low accuracy; Weak inter-layer adhesion | PCL/HA [109] PLA/HA [110] PCL/β-TCP [111] PLA/β-TCP [112] |
| Selective Laser Sintering (SLS) | No support structure; Suitable for porous and complex geometries; High utilization | The high surface roughness; Post-treatment; High-temperature sintering lead to grain coarsening | Bioactive glass [113] β-TCP [114] HA/β-TCP [115] |
| Stereolithography (SLA) | High resolution; Suitable for fine structures | High cost; Material selection is restricted; Post-processing is required | Bioactive glass [116] Al2O3 [117] ZrO2 [118] HA [119] β-TCP [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Liu, J.; Li, L. Research Progress and Challenges in 3D Printing of Bioceramics and Bioceramic Matrix Composites. Biomimetics 2025, 10, 428. https://doi.org/10.3390/biomimetics10070428
Zhao X, Liu J, Li L. Research Progress and Challenges in 3D Printing of Bioceramics and Bioceramic Matrix Composites. Biomimetics. 2025; 10(7):428. https://doi.org/10.3390/biomimetics10070428
Chicago/Turabian StyleZhao, Xueni, Jizun Liu, and Lingna Li. 2025. "Research Progress and Challenges in 3D Printing of Bioceramics and Bioceramic Matrix Composites" Biomimetics 10, no. 7: 428. https://doi.org/10.3390/biomimetics10070428
APA StyleZhao, X., Liu, J., & Li, L. (2025). Research Progress and Challenges in 3D Printing of Bioceramics and Bioceramic Matrix Composites. Biomimetics, 10(7), 428. https://doi.org/10.3390/biomimetics10070428





