Three-Dimensionally Printed Scaffolds and Drug Delivery Systems in Treatment of Osteoporosis
Abstract
1. Introduction
2. Osteoporosis Medication
3. Biomaterials in Osteoporosis Management
4. DDS for Osteoporosis Treatment
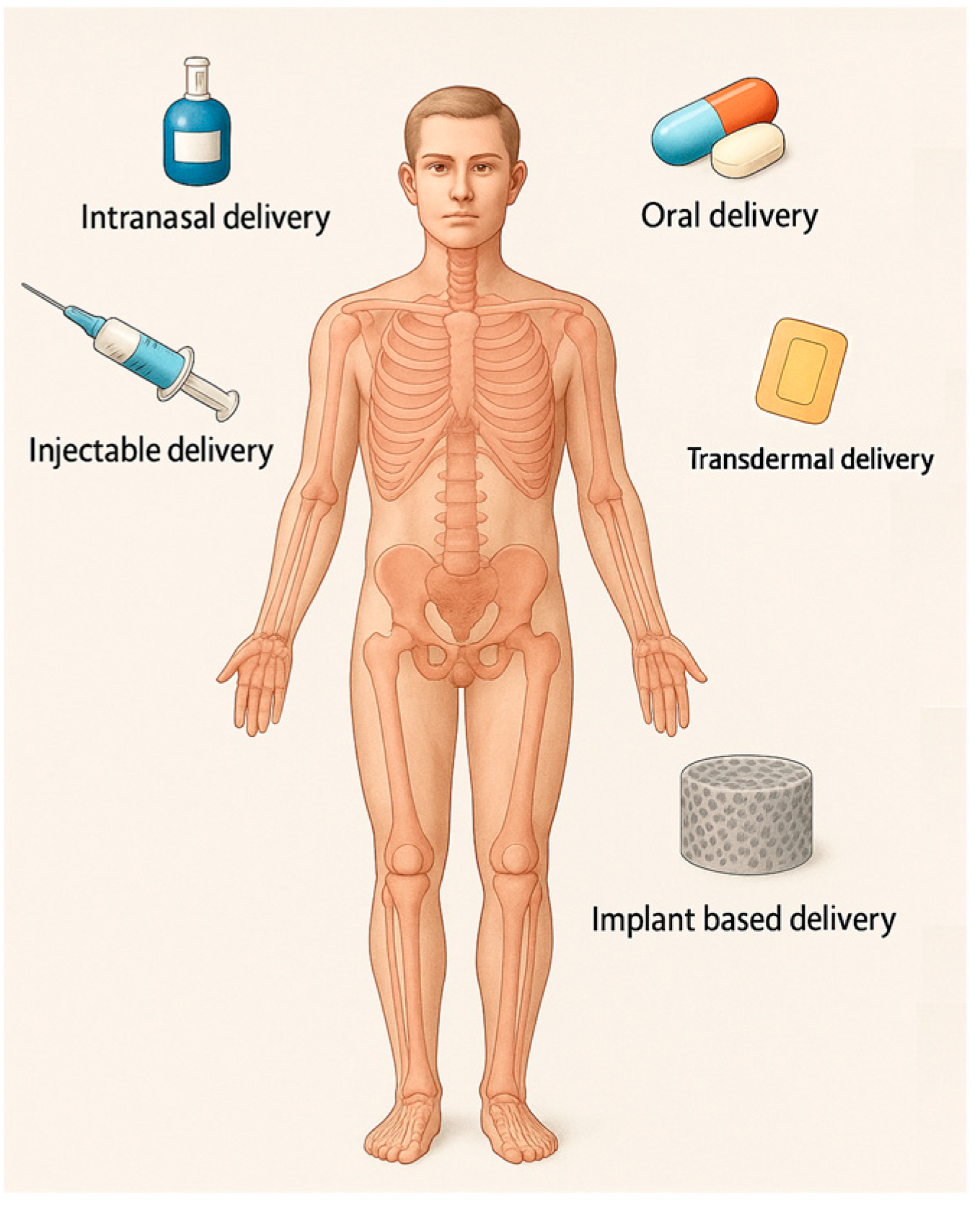
4.1. Route of Administration
- Oral administration DDSs. Oral administration is broadly considered the safest, most convenient, and economically advantageous route for drug delivery. It is commonly used but has limitations such as low bioavailability and adverse effects due to systemic distribution. Although oral drug delivery is highly desirable from a patient care perspective, it faces substantial physiological challenges that compromise drug stability, absorption, and overall bioavailability. The acidic gastric environment promotes acid-catalyzed degradation, resulting in the loss of drug efficacy. In addition, enzymatic degradation, primarily through proteolytic enzymes, leads to the breakdown of macromolecular therapeutics and other labile compounds prior to their absorption. The gastrointestinal tract further presents physical and biochemical barriers, including the mucus layer and the epithelial lining. The mucus entraps larger molecules, impeding their diffusion, while the tight junctions of the epithelial cells limit paracellular transport, thereby restricting the systemic uptake of poorly permeable drugs. Moreover, orally administered drugs are subject to first-pass hepatic metabolism, wherein a considerable fraction is metabolized by liver enzymes before reaching systemic circulation, significantly reducing therapeutic availability [80].
- Parenteral Administration DDSs. Injections can improve bioavailability but still pose challenges like patient compliance and side effects. When recombinant salmon calcitonin is utilized in the form of injections, it is associated with adverse effects such as nausea and facial rash, attributable to the high plasma concentration peaks following administration [13]. DDSs that avoid first-pass metabolism by using a parenteral route to deliver a drug directly into the bloodstream enable comparatively less material to achieve the same therapeutic effect in a well-controlled manner [25].
- Transdermal patch and microneedle DDSs. These systems, such as those developed for risedronate, are used as an adverse-effects-reducing method, with the potential to improve patient compliance [81]. Transdermal microneedle array patches deliver drugs to the epidermis or upper dermis, avoiding the cutaneous pain receptors and allowing for painless drug delivery [25]. The avoidance of drug degradation within the gastrointestinal tract, coupled with the circumvention of hepatic first-pass metabolism, enables controlled drug delivery, enhances tolerability, and broadens the potential scope of therapeutic applications. For a drug to successfully reach systemic circulation via transdermal delivery, it must traverse the aqueous environment of the viable epidermis. Molecules exhibiting a Log P (octanol–water partition coefficient) value between 2 and 3, indicative of intermediate lipophilicity, are considered optimal candidates. Additional requirements include a molecular weight below 500 Da, sufficient solubility, low ionization, high pharmacological potency, and good skin tolerability [13].
- Intranasal administration DDSs. For drugs like raloxifene, intranasal delivery using chitosan nanoparticles can enhance bioavailability and provide a promising alternative to oral administration [82]. Challenges come from the fact that the nasal mucosa contains a diverse array of xenobiotic-metabolizing enzymes, including cytochrome P450-dependent enzymes, as well as those involved in phase I and phase II metabolic pathways. Additionally, the nasal mucociliary clearance system constitutes a critical component of the nasal cavity’s innate defense mechanisms, playing a key role in protecting against inhaled pathogens and foreign substances [83]. Bisphosphates and hormones have been used to treat osteoporosis, with treatment approaches like oral and intranasal delivery [48]. Recombinant salmon calcitonin administered via nasal spray can lead to complications, including epistaxis, rhinitis, and ulceration of the nasal mucosa [13]. This route is used more often to deliver anti-seizure medications, migraine medications, cholinesterase inhibitors, and antidepressants [25].
- Local DDSs offer a promising strategy for osteoporosis treatment by targeting therapeutic agents directly to bone tissue. Approaches such as bone-targeting nanoparticles and hydrogel implants enhance drug localization, reduce systemic side effects, and improve efficacy. While encouraging results have been observed in preclinical studies, these technologies remain largely experimental. As drug-loaded bone scaffolds function as localized DDSs, they are capable of targeting bone tissue locally and specifically, thereby facilitating the further treatment of the injured area and promoting the healing process. Bone scaffolds can function as prolonged-release platforms for therapeutic agents. This can be achieved either by direct drug incorporation into the scaffold or by embedding drug-loaded nanoparticles, which address issues such as toxicity and poor solubility inherent to many drugs. Additionally, adjuvant therapies can enable precise cell targeting through strategies such as stimulus-responsive nanocarriers or the surface functionalization of carriers with targeting ligands [59]. Some authors mention that DDSs in preclinical development for OP include HA-containing scaffolds [25]. This is further discussed in the following section, titled 3D scaffold-based systems.
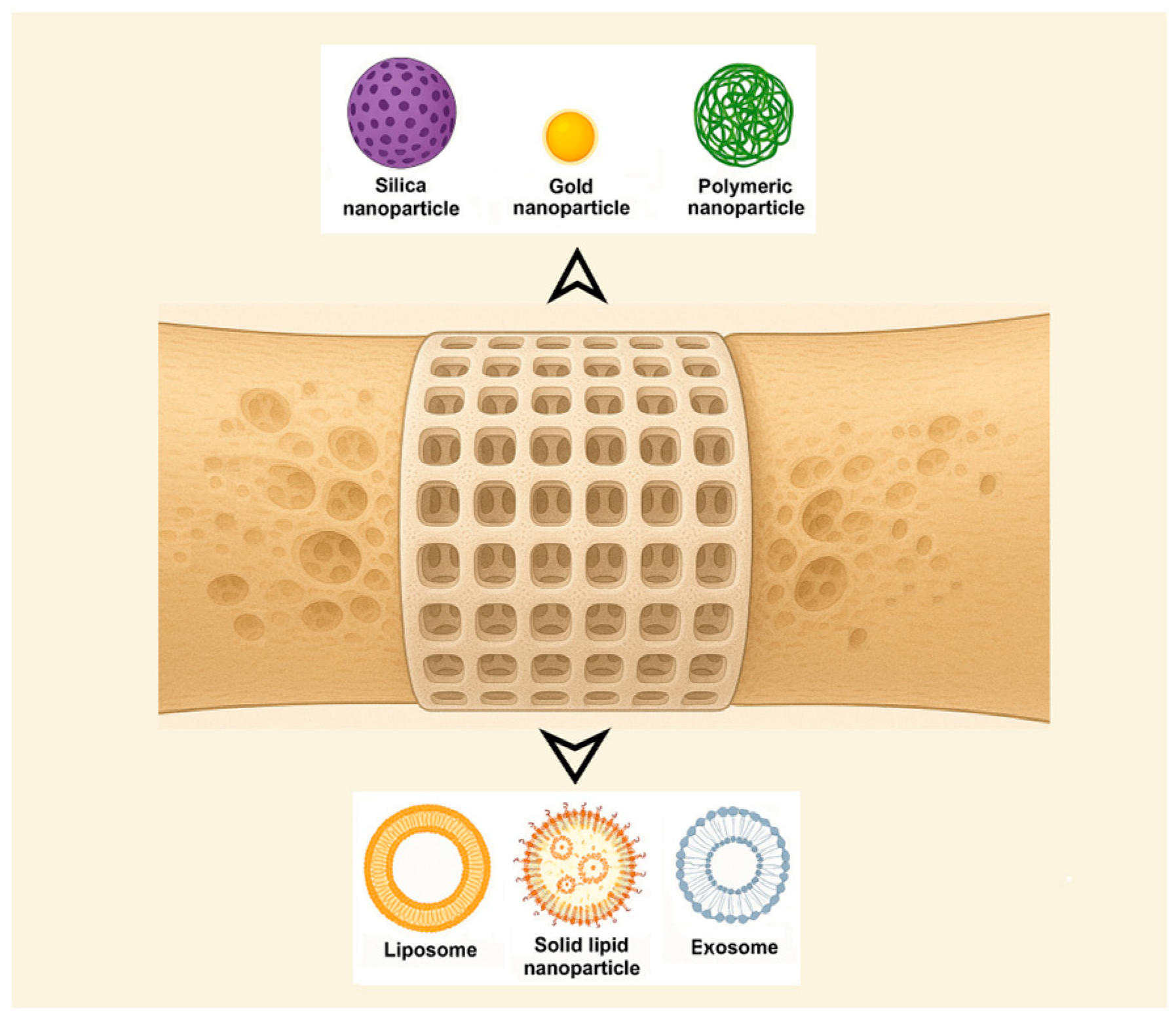
4.2. Compositional and Structural Types
- Nanoparticle-based systems. A promising approach for DDSs involves leveraging innovative nanoparticles (NPs) while using conventional medications. NPs (1–100 nm size range) have been extensively researched for their unique physical and chemical characteristics, making them valuable in biomedical applications such as disease diagnosis and treatment, biological imaging, and biosensor development [24]. Also, nanotechnology has been applied in orthopedics for bone tissue engineering, implant and prosthesis surface modification, targeted drug delivery, and advanced diagnostics [26]. Among their useful proprieties are their small size, large surface area-to-volume ratio, and tunable surface characteristics [48]. The pharmacokinetics of nanoparticle-based DDSs are primarily influenced by their physicochemical characteristics, which are determined by factors such as material composition, size, shape, surface charge, and modifications. NPs can enhance the stability and solubility of encapsulated compounds, facilitate membrane penetration, and extend circulation time, ultimately improving both safety and therapeutic effectiveness [79] while reducing formulation costs [76]. NPs offer several advantages, including a high drug-loading capacity relative to their size, enhanced solubility, improved drug stability, reduced side effects, and increased efficiency in facilitating drug transport and internalization within specific organelles [48]. The diverse range of requirements can be met by designing NPs tailored to specific patient groups, disease types, or a combination of both [79]. Numerous foundational studies have explored material selection and size optimization to enhance DDS effectiveness. Encapsulating conventional drugs within nanoparticles has been shown to enhance targeting precision while minimizing adverse effects in the treatment of various diseases [24]. The following classification of NPs used for DDSs was made by some authors: inorganic nanomaterials (silica NPs, HA NPs, metallic NPs, magnetic NPs, titanium nanotubes, nanospheres of mesoporous bioactive glass, etc.), polymeric NPs (gelatin NPs, chitosan NPs, nanogels, polyurethane nanomicelles), and lipid-based NPs [46,48]. Inorganic NPs, encompassing metals, metal/non-metal oxides, semiconductor NPs, etc., exhibit unique properties, such as surface effects, quantum size effects, and macroscopic quantum tunneling effects, while organic NPs, such as polymeric micelles, vesicles, liposomes, and dendritic polymers, hold a central role in improving the stability of drugs and genes. Nanoparticles composed of calcium phosphate have demonstrated the ability to activate cellular functions, enhance mineralization processes, and stimulate bone tissue growth [48]. Bioceramic NPs are the best alternative for reparative and restorative bone treatment due to their biomimetic composition, bioactivity, and good assimilation into the natural bone structure [26]. Organic nanocarriers enhance solubility, refine pharmacokinetic profiles, promote accumulation at targeted sites, and contribute to the reduction in systemic toxicity and adverse effects, thereby optimizing therapeutic efficacy [76]. It is worth mentioning also that microparticles, typically ranging from 1 to 1000 μm in size, are also studied. Microparticles are particularly effective for the delivery of larger therapeutic payloads and facilitate localized, controlled drug release at the target site [80]. NPs face limitations in regard to their potential antigenicity and immunogenicity, interacting with the mononuclear phagocyte system or the complement system, which leads to their rapid clearance [76].
- Liposome-based systems. Liposomes have become one of the most prominent nanocarriers in the field of targeted drug delivery, owing to their low immunogenicity, high degree of versatility, and well-established therapeutic efficacy. A liposome is a closed, spherical, lipid bilayer artificial membrane, similar to a cell membrane, that forms an internal cavity capable of carrying various substances [85]. However, the physiological characteristics associated with each specific disease vary considerably, necessitating the tailored formulation of each liposomal system to align with the particular pathological conditions [86]. Used for bone-targeted delivery, liposomes can encapsulate drugs and deliver them specifically to bone tissue, although clinical translation remains challenging [87]. An example of a bone-targeting liposome is the system containing an oligopeptide of eight aspartate residues (Asp8), which had previously been shown to specifically target the bone, encapsulating icaritin. In vivo, researchers found that the Asp8-icaritin-liposome enhanced bone formation in ovariectomized mice compared to an icaritin-liposome control lacking the Asp8 moiety [88]. Other authors mention developing an α-cyperone-containing liposome-based nano-drug delivery system that specifically targets the bone resorption interface [89]. Liposomes can be tailored to be pH- or temperature-sensitive. Concerns exist regarding the long-term stability of liposomal formulations. Due to their high surface-area-to-volume ratio, liposomes possess elevated surface free energy, which can result in physical instabilities such as particle aggregation or fusion. These phenomena may lead to an increased particle size and unintended drug leakage. In addition, chemical instabilities, such as the degradation of ligands conjugated to the liposomal surface, can adversely affect the precision and efficacy of the targeting mechanism [86].
- Solid lipid nanoparticle-based systems. Solid lipid nanoparticles (SLNs) are potentially biocompatible and efficient nanocomposite systems. SLNs were developed as an alternative to liposomes. They are colloidal dispersions in an aqueous medium, characterized by a matrix composed of solid, biodegradable lipids [90]. These systems offer several significant advantages, including sustained drug release, enhanced bioavailability, improved drug encapsulation efficiency, and broad applicability across various therapeutic areas [91,92], and facilitate the use of poorly water-soluble active pharmaceutical ingredients [93]. When they are combined with thermosensitive injectable sol–gel systems, SLNs turn into an effective and efficient drug delivery system [94]. SLNs can incorporate both hydrophilic and lipophilic compounds within their matrix [91]. Biocompatible lipids such as Compritol®888 ATO, Precirol® ATO5, cetyl alcohol, cetylpalmitate, glyceryl monostearate, trimyristin/Dynasan®114, tristearin/Dynasan®118, stearic acid, and Imwitor®900 are used in the formulation of SLNs and appear to be well tolerated physiologically when administered in vivo [49]. Nanostructured lipid carriers (NLCs), representing the next generation of SLN-based systems, are composed of solid and liquid lipids at the nanoscale and have been developed to address certain limitations associated with SLNs, including their limited drug-loading capacity and the tendency for drug expulsion during storage [49,90]. Future developments of SLN anti-OP solutions will focus on the development of reproducible and scalable formulations of a lipid carrier for poorly soluble drug substances.
- Hydrogel-based systems. Hydrogels are 3D networks composed of hydrophilic polymers that, through the chemical or physical cross-linking of polymer chains, can retain substantial amounts of water while preserving structural integrity. This architecture enables the encapsulation of therapeutic agents, including nucleic-acid-based drugs, thereby forming a sustained-release reservoir. The release kinetics can be modulated by adjusting the degree of cross-linking within the hydrogel matrix [80]. Injectable hydrogels represent a highly promising approach for localized DDSs in the management of bone-related conditions such as osteoporosis, osteonecrosis, osteoarthritis, osteomyelitis, and osteosarcoma, offering a viable approach for mimicking the topography and properties of the extracellular matrix [95]. The development of advanced smart hydrogels and nanogels that respond dynamically to external stimuli, such as changes in pH or temperature, offers a promising strategy for improving controlled drug release. These materials can undergo reversible structural transformations, thereby enhancing delivery precision and therapeutic efficacy. Furthermore, integrating hydrogels with nanogels may combine their respective advantages. For example, the use of biopolymers can enhance structural stability, mechanical strength, and drug-loading capacity. Incorporating nanoparticles into hydrogel matrices may further improve both mechanical performance and the efficiency of drug encapsulation [96]. Key challenges include ensuring mechanical stability and controlled degradation, achieving precise drug release and optimal bioavailability, and managing immune responses and ensuring biocompatibility, as well as addressing issues related to scalability and reproducibility in production [95]. Polymers can act as carriers that increase drug efficacy and reduce adverse effects by allowing for localized and prolonged drug release. They improve patient compliance by reducing the frequency of drug administration [23]. The ability to release biomolecules at a desired rate will play critical roles in the future development of bone scaffolds [22].
- Moiety-based systems. These systems use water-soluble polymers to direct drugs specifically to some tissues, reducing side effects and improving therapeutic efficacy. Surface functionalization for targeted drug delivery represents a promising approach for reducing systemic toxicity and off-target effects commonly associated with nonspecific drug distribution. By attaching targeting moieties to the surface of drug carriers, the specificity of delivery to diseased tissues can be significantly enhanced. In particular, ligand-mediated strategies, employing antibodies, peptides, or small molecules that selectively bind to overexpressed receptors on target cells can substantially improve the accuracy and therapeutic efficiency of the delivery system [96]. Other authors mention as well that, by coating the inorganic NP with additional surface ligands (i.e., proteins, peptides, carbohydrates, etc.), higher reactivity and enhanced functionality can be achieved [54].
- Stimuli-responsive systems. Researchers are exploring strategies to regulate the spatial and temporal release of therapeutic agents from controlled and targeted DDSs, such as on-demand release with stimuli-responsive functional groups after advancing to the targeted tissue [77]. This allows therapeutic agents to be precisely delivered to the intended site at the optimal dosage. Several types of stimuli-responsive smart materials have been developed for drug delivery. This strategy ensures a customized spatiotemporal release, reducing side effects while maximizing therapeutic impact. Stimuli-responsive biomaterials have the ability to react to external or internal triggers, including mechanical forces, electrical or magnetic fields, temperature fluctuations, pH variations, and enzymatic activity, to induce a specific functional response or behavior [20,21]. Some of the accounted stimuli are specific to the pathological microenvironments, such as elevated reactive oxygen species and mild acidity in tumors, pH reduction and bacterial enzyme activity in infections, and localized electronegative potentials at bone defect sites, which can serve as effective biochemical triggers for activating bone disease therapies and promoting regeneration [20]. An example is the DDS based on poly(lactic-co-glycolic acid) NPs co-loaded with 17β estradiol (E2) and iron oxide (Fe3O4), modified with alendronate to achieve bone targeting and realize a magnetically remote-controllable drug release [27].
- Macromolecular therapeutic-based systems. These systems use water-soluble polymers to direct drugs specifically to bone [10]. Polymers enable selective targeting, extended circulation, enhanced delivery, and controlled cargo release via mechanisms such as physical adsorption, chemical conjugation, and internal loading. Biodegradable, biocompatible, and physicochemically stable polymers are ideal carriers, and biomimetic, bio-inspired systems based on these materials hold significant promises for overcoming current drug delivery challenges [80]. Natural endogenous materials (NEMs), including cells, cell derivatives, polysaccharides, proteins, peptides, and nucleic-acid-based vectors, offer inherent biocompatibility, biodegradability, and natural targeting capabilities, minimizing adverse in vivo reactions and enhancing drug delivery efficacy. Various NEM-based drug delivery systems have been developed to address challenges associated with macromolecules, such as their large size, complex structure, low permeability, and environmental instability [97]. Among polymer-based DDSs, natural polysaccharides are highly favored due to their abundance in nature and renewability [98]. There are even stimuli-responsive polymer-based DDSs that enable selective and targeted therapeutic release at specific tissue sites. Some authors mention examples of pH-, redox-, thermo-, hypoxia-, and enzyme-responsive DDSs [80].
- Dual-targeting systems. These systems deliver both anabolic and antiresorptive agents simultaneously to different zones of bone, potentially improving therapeutic outcomes without severe adverse effects. Anabolic drugs are favored as they promote the formation of new bone, whereas antiresorptive drugs terminate the further deterioration of bone. The non-specific delivery of anabolic agents results in adverse effects. Several clinical trials have been reported for the combinational therapy of anabolic agents and antiresorptive agents for osteoporosis. However, none of them have proven their cumulative effects in the treatment of disease. Some authors emphasize a dual-targeting drug delivery approach comprising bone anabolic and antiresorptive agents that would simultaneously deliver the therapeutic agents. This approach is believed to intensify the explicit interaction between the therapeutic agent and bone surfaces separately without developing severe adverse effects and to improve osteoporotic therapy effectively compared to non-targeted drug delivery [99].
- Vesicles derived from macrophages-based systems, as well as other biomimetic carriers. Exosomes, microvesicles, and vesicles originating from reconstructed membranes may retain their chemotactic migration capability and excellent biocompatibility. Exosomes are emerging as novel nanoscale biopharmaceutical DDSs due to their ability to mediate signal exchange through ligands, nucleic acids, or protein factors embedded in their membranes or encapsulated within them [60]. Due to these distinctive characteristics, these systems emerge as promising contenders for innovative drug delivery systems in precision nanomedicine [24]. Biomimetic nanocarriers are developed by incorporating entire cells or cellular components sourced from autologous biological materials. These systems offer excellent biocompatibility and benefit from relatively straightforward fabrication methods, avoiding the need for extensive chemical modification. Derived from natural cellular structures, these bioinspired nanocarriers preserve intrinsic cellular functions. When tailored for specific therapeutic applications, they demonstrate high target specificity, thereby enabling safer and more effective strategies for disease diagnosis and treatment [76]. To encapsulate NPs for drug delivery within a biomimetic outer shell, some authors have mentioned using membranes from white blood cells, red blood cells, platelets, cancer cells, dendritic cells, keratinocytes, VLA-4, or outer membrane vesicles derived from Gram-negative bacteria, as well as hybrid cell platelet–macrophage membranes, hybrid cell erythrocyte–macrophage membranes, and exosomes, which are naturally occurring secreted membrane vesicles found in various biological fluids [76]. Exosomes, like endogenous substances, exhibit excellent biocompatibility; however, their application is limited by complex purification processes and a low yield. Enhancing exosome production remains a critical focus for future research [26]. Extracellular vesicles, consisting of a lipid bilayer with transmembrane proteins and encapsulated cytosolic contents such as mRNAs and miRNAs, function as mediators of intercellular communication both locally and systemically [100]. Bacterial extracellular vesicles (BEVs) are lipid bilayer nanostructures (20–400 nm) secreted via bacterial autolysis and membrane budding, encapsulating bioactive cargo such as endotoxins, peptidoglycans, periplasmic proteins, and nucleic acids [52]. A notable example involves BEVs engineered to overexpress CXCR4 and incorporate ClyA on their surface, enabling the delivery of SOST siRNA as a promising strategy for refractory osteoporosis therapy [47]. However, the intricate architecture of biological systems presents a significant challenge for the large-scale or industrial production of bioinspired DDSs [52].Table 3. Recent DDSs developed for anti-OP treatment or with significant anti-OP potential, categorized based on their structure.Table 3. Recent DDSs developed for anti-OP treatment or with significant anti-OP potential, categorized based on their structure.
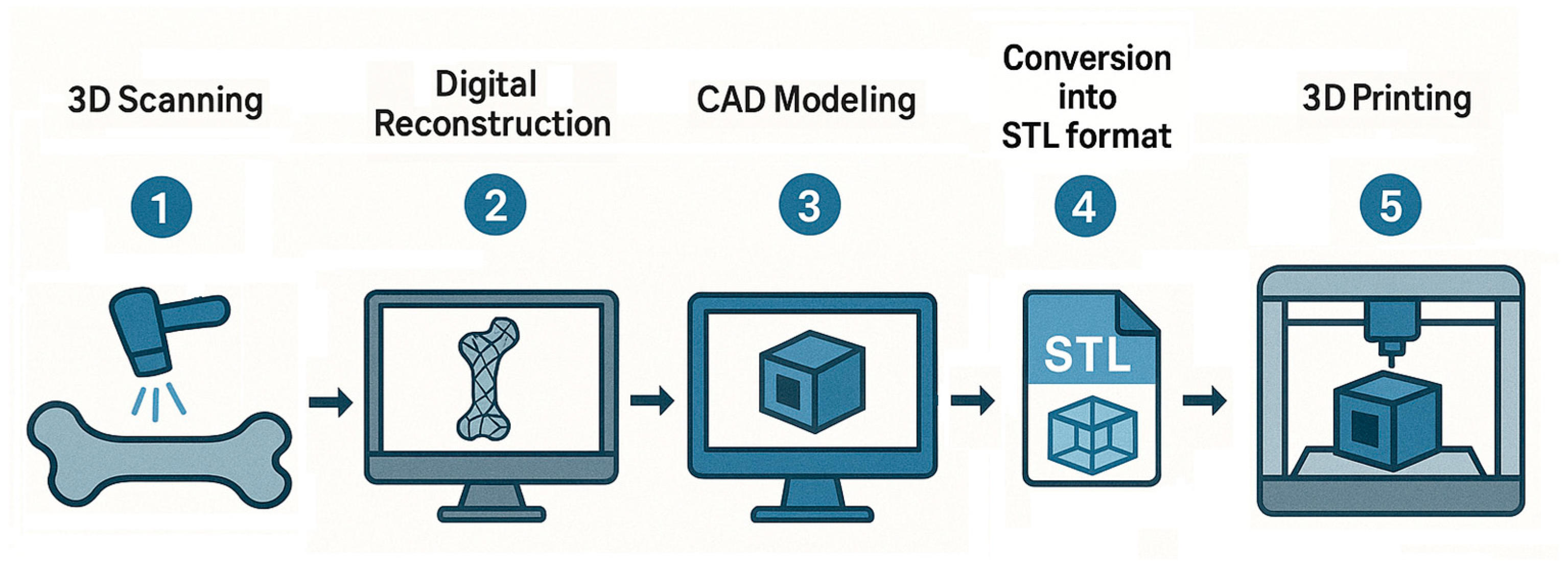
Category Basic Material Type of Carrier Type of Drug References Inorganic NPs Calcium phosphate NPs HA co-doped with iron oxide Small non-coding MiR (microRNA)-21/124 [101] Zinc-rich amorphous calcium phosphate - [102] Tetracycline modified and monostearin coated amorphous calcium carbonate platform Simvastatin [103] Silica NPs SBA-15/12-tungstophosphoric acid (TPA)-functionalized SBA-15 Alendronate sodium [104] Zinc-based zeolite imidazolium skeleton (ZIF-8) NPs doped with cerium ions and coated with poly(sodium 4-styrenesulfonate) Alendronate sodium [105] Thiolated mesoporous silica - [106] Mesoporous silica and Fe3O4 composite Baicalein (5,6,7-trihydroxyflavone) [107] Gold NPs Selenium-gold multi-shell nanocomposites Icariin [108] Carbon NPs Carbon nanohorns—calcium phosphates Ibandronate [109] Bioactive glass Bioactive glass doped with magnesium–strontium [110] Polymer NPs PLGA NPs PLGA loaded with secretome C–X–C chemokine receptor type 4 (CXCR4) [111] PLGA NPs PLGA modified with alendronate 17β estradiol (E2) and Fe3O4 [27] Bisphosphonate-based coordination polymers (BPCPs) Benzene 1,4-bis(bisphosphonic acid) (BBPA)—CA, Zn, Mg 5-fluorouracil [112] Microparticles/Microspheres Calcium phosphates Carbonated apatite microspheres Antibodies against sclerostin [113] Liposomes Lipid NPs DLin-MC3-DMA, Zoledronic acid-DSPC, cholesterol, and DMG-PEG-2000 m7G methylated Runx2 mRNA [114] Solid lipid NPs Compritol® 888 ATO Raloxifene [115] Extracellular vesicles Exosomes Exosomes secreted by mesenchymal stem cells (BT-Exo-siShn3) siRNA of the Shn3 gene [116] Hybrid exosomes Fused CXCR4+ exosomes with liposomes Antagomir-188 (inhibiting miR-188 oligonucleotid) [117] Microvesicles Small osteoblast vesicles [100] Hydrogels Collagen Dense collagen hydrogels Sclerostin Antibody [118] Silica hydrogels Sodium silicate pentahydrate and silicic-acid-based hydrogels bis-phosphonates [119] Collagen/chitosan/hyaluronic-acid-based hydrogel Mesoporous silica particles decorated with hydroxyapatite immobilized in collagen/chitosan/hyaluronic-acid-based hydrogel. Alendronate [120] Micelles Polymers mPEG_PLGA Alendronate and astragaloside [121] AL-P(LLA-CL)-PEG-P(LLA-CL)-MY micelles Myricetin [122] Amphiphilic polymer composed of methoxypolyethylene glycol amine-glutathione-palmitic acid (mPEG-GSHn-PA) Dexamethasone [123] Capsaicin nano vesicles Phosphorylated capsaicin (Cap-p) nano vesicles Curcumin [124] Three-Dimensionally Printed Scaffolds Hydrogel Three-dimensionally printed triple-crosslinked hydrogel scaffold based on methylpropenylated gelatin (GelMA) and methylpropenylated alginate (AlgMA) Glycopyrrolate (GA) and epigallocatechin gallate (EGCG) [125] Electrospunned nanofibrous membranes PLGA PLGA-PLL (Poly (lactic-glycolic acid-L-lysine)) Parathyroid hormone relative peptides [126] - Three-dimensional scaffold-based systems. Drug delivery through scaffolds represents an innovative alternative to traditional pharmaceutical formulations, enabling the controlled spatiotemporal release of therapeutic agents [58]. There are expectations of developing 3D scaffolds that serve as grafts while simultaneously delivering multiple drugs for anesthesia, anti-inflammatory effects, and other therapeutic purposes [65]. Various strategies have been employed to integrate drug delivery functionalities into bone scaffolds, including drug incorporation within the interparticle porosity of bioceramics, loading into polymer matrices, and the surface coating of porous or mesoporous nanoparticles to achieve high drug loading and sustained release [59]. In the case of osteoporotic fractures, convenient local drug delivery approaches are implant coatings, injectable bone cements, and gels [6]. Scaffolds are not only a substitute for the extracellular matrix (ECM) but can also serve as the delivery vehicle for cells and the carrier for growth factors. Scaffolds affect seeded cells, including cell attachment, migration, and proliferation, thus affecting the efficacy of regenerative medicine [8,67]. In addition to the ability of smart scaffolds to interact with cells and induce the desired cell functions for tissue regeneration, scaffolds can also be used to deliver drugs [8]. Smart scaffolds have been designed with the incorporation of bioactive molecules and nanoparticles and the use of tailored modifications of the physical and chemical properties of the scaffolds. Enhancing bone grafts with additional capabilities, such as drug delivery, opens the door to the development of multifunctional implant materials with improved therapeutic potential [66] (Figure 3).
- Low drug-loading capacity: For example, PLGA nanoparticles, while widely studied, suffer from a low drug-loading capacity, limiting their clinical translation [127]. Efforts to improve drug loading are crucial.
- Targeting specificity and delivery efficiency: For example, nanocrystals have been found to be superior to other nanoparticle systems as carriers for hydrophobic drug delivery due to their high drug-loading capacity, minimal excipient requirements, chemical stability, and low toxicity. Their nanoscale enhances the bioavailability of poorly water-soluble drugs, making them favorable for industrial application [128]. Improving targeting methods and delivery efficiency remains a challenge.
- Controlled drug release: Developing systems that offer precise control over drug release to optimize therapeutic outcomes through an autonomous response to lesion-specific pathological cues or external physical stimuli, thus influencing cell behavior and promoting bone repair [20].
- Biocompatibility and biodegradability: Ensuring that nanomaterials are biocompatible and biodegradable to avoid long-term toxicity. While nanomaterials have shown promising therapeutic effects, caution is needed due to the heterogeneity between animal models and human diseases, as well as variability across disease stages and patient populations [129].
- Clinical translation: Translating preclinical research to clinical trials requires overcoming hurdles related to biocompatibility, toxicity, and regulatory approval. Regulatory challenges in developing drug delivery need to demonstrate safety and efficacy amid complex drug–carrier–host interactions. Comprehensive preclinical studies and the development of representative animal models are essential. Achieving controlled, sustained drug release while minimizing systemic side effects remains a critical hurdle [130].
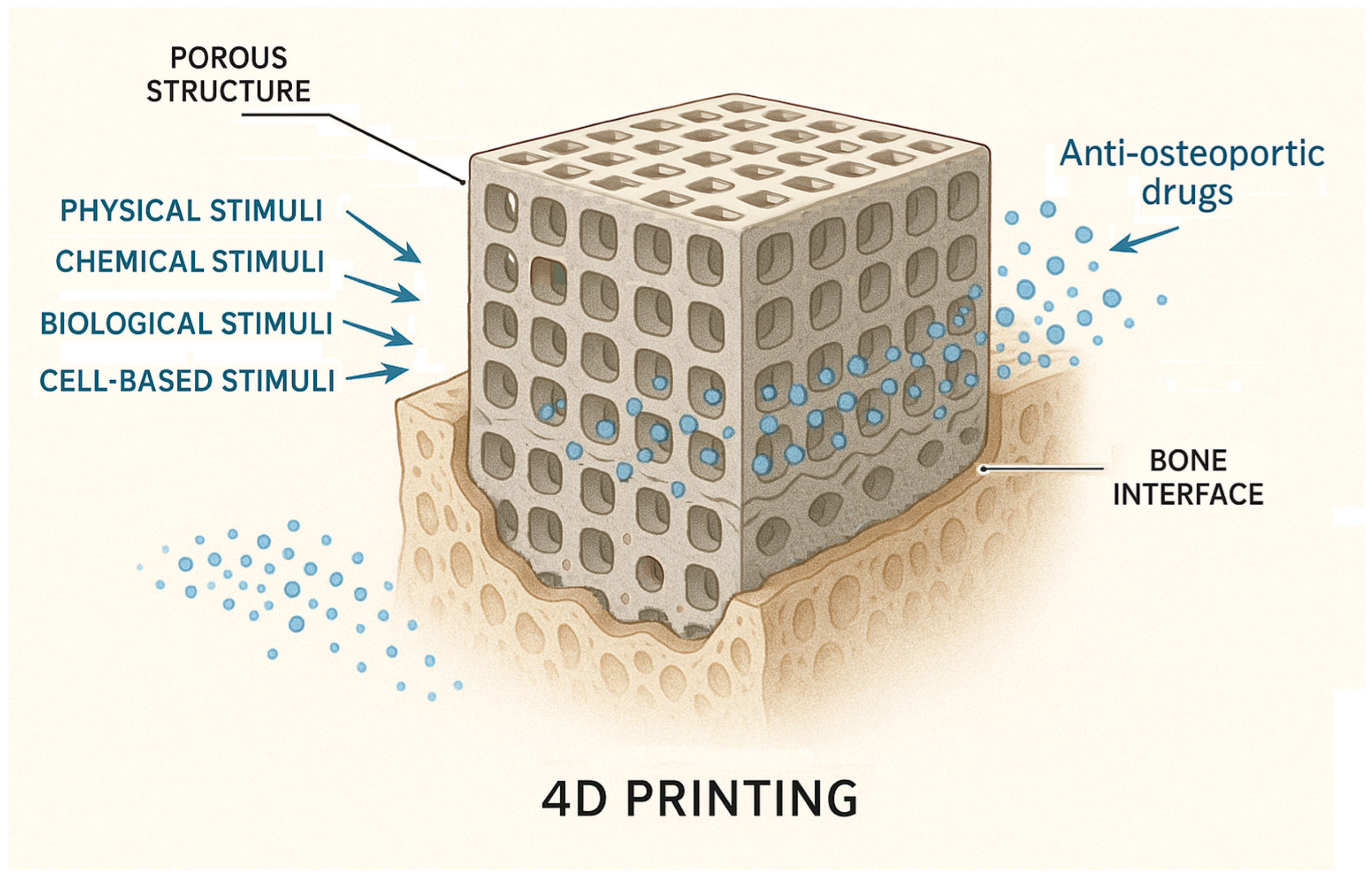
4.3. Smart Materials and 4D Printing
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tarantino, U.; Iolascon, G.; Cianferotti, L.; Masi, L.; Marcucci, G.; Giusti, F.; Marini, F.; Parri, S.; Feola, M.; Rao, C.; et al. Clinical Guidelines for the Prevention and Treatment of Osteoporosis: Summary Statements and Recommendations from the Italian Society for Orthopaedics and Traumatology. J. Orthop. Traumatol. 2017, 18 (Suppl. S1), 3–36. [Google Scholar] [CrossRef]
- Wildemann, B.; Ignatius, A.; Leung, F.; Taitsman, L.A.; Smith, R.M.; Pesántez, R.; Stoddart, M.J.; Richards, R.G.; Jupiter, J.B. Non-Union Bone Fractures. Nat. Rev. Dis. Primers 2021, 7, 57. [Google Scholar] [CrossRef]
- Trikha, V.; Kumar, A. Osteoporotic Distal Femur Fractures: An Overview. Indian J. Orthop. 2025, 59, 311–325. [Google Scholar] [CrossRef]
- Zhou, S.; Tao, Z.; Zhu, Y.; Tao, L. Mapping Theme Trends and Recognizing Hot Spots in Postmenopausal Osteoporosis Research: A Bibliometric Analysis. PeerJ 2019, 7, e8145. [Google Scholar] [CrossRef]
- Wang, H.; Luo, Y.; Wang, H.; Li, F.; Yu, F.; Ye, L. Mechanistic Advances in Osteoporosis and Anti-Osteoporosis Therapies. MedComm 2023, 4, e244. [Google Scholar] [CrossRef] [PubMed]
- Codrea, C.I.; Croitoru, A.-M.; Baciu, C.C.; Melinescu, A.; Ficai, D.; Fruth, V.; Ficai, A. Advances in Osteoporotic Bone Tissue Engineering. J. Clin. Med. 2021, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Chandran, M.; Mitchell, P.J.; Amphansap, T.; Bhadada, S.K.; Chadha, M.; Chan, D.-C.; Chung, Y.-S.; Ebeling, P.; Gilchrist, N.; Habib Khan, A.; et al. Development of the Asia Pacific Consortium on Osteoporosis (APCO) Framework: Clinical Standards of Care for the Screening, Diagnosis, and Management of Osteoporosis in the Asia-Pacific Region. Osteoporos. Int. 2021, 32, 1249–1275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, S.; Zhou, C.; Cheng, L.; Gao, X.; Xie, X.; Sun, J.; Wang, H.; Weir, M.D.; Reynolds, M.A.; et al. Advanced Smart Biomaterials and Constructs for Hard Tissue Engineering and Regeneration. Bone Res. 2018, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Özpolat Bulut, Ö.; Alyanak, B.; Dede, B.T.; Temel, M.H.; Yıldızgören, M.T.; Bağcıer, F. Bridging Academia and Public Interest: The Impact of Altmetrics on Osteoporosis Treatment Research. Arch. Osteoporos. 2025, 20, 13. [Google Scholar] [CrossRef]
- Wang, D.; Miller, S.C.; Kopečková, P.; Kopeček, J. Bone-Targeting Macromolecular Therapeutics. Adv. Drug Deliv. Rev. 2005, 57, 1049–1076. [Google Scholar] [CrossRef]
- Petersen, T.G.; Rubin, K.H.; Javaid, M.K.; Hermann, A.P.; Åkesson, K.E.; Abrahamsen, B. Long-Term Adherence to Anti-Osteoporosis Medication and Determinants of Adherence in the Population-Based Screening Trial ROSE. Osteoporos. Int. 2025, 36, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Gerges, M.; Raynor, W.Y.; Park, P.S.U.; Nguyen, E.; Chan, D.H.; Gholamrezanezhad, A. State of the Art Imaging of Osteoporosis. Semin. Nucl. Med. 2024, 54, 415–426. [Google Scholar] [CrossRef]
- Villanueva-Martínez, A.; Merino, V.; Ganem-Rondero, A. Transdermal Formulations and Strategies for the Treatment of Osteoporosis. J. Drug Deliv. Sci. Technol. 2022, 69, 103111. [Google Scholar] [CrossRef]
- Ashrafi, M.; Ghalichi, F.; Mirzakouchaki, B.; Doblare, M. On the Effect of Antiresorptive Drugs on the Bone Remodeling of the Mandible after Dental Implantation: A Mathematical Model. Sci. Rep. 2021, 11, 2792. [Google Scholar] [CrossRef]
- Liu, T.; Huang, J.; Xu, D.; Li, Y. Identifying a Possible New Target for Diagnosis and Treatment of Postmenopausal Osteoporosis through Bioinformatics and Clinical Sample Analysis. Ann. Transl. Med. 2021, 9, 1154. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, X.; Gu, Y.; Song, D.; Ding, M.; Liao, L.; Wang, J.; Ni, J.; He, G. The Effect of Bisphosphonates on Fracture Healing Time and Changes in Bone Mass Density: A Meta-Analysis. Front. Endocrinol. 2021, 12, 1031. [Google Scholar] [CrossRef] [PubMed]
- Rinonapoli, G.; Ruggiero, C.; Meccariello, L.; Bisaccia, M.; Ceccarini, P.; Caraffa, A. Osteoporosis in Men: A Review of an Underestimated Bone Condition. Int. J. Mol. Sci. 2021, 22, 2105. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Hung, M.-C.; Chang, S.-F.; Tsuang, F.-Y.; Chang, J.Z.-C.; Sun, J.-S. Efficacy and Safety of Postmenopausal Osteoporosis Treatments: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2021, 10, 3043. [Google Scholar] [CrossRef]
- Zhou, Z.; Feng, W.; Moghadas, B.K.; Baneshi, N.; Noshadi, B.; Baghaei, S.; Dehkordi, D.A. Review of Recent Advances in Bone Scaffold Fabrication Methods for Tissue Engineering for Treating Bone Diseases and Sport Injuries. Tissue Cell 2024, 88, 102390. [Google Scholar] [CrossRef]
- Wei, H.; Cui, J.; Lin, K.; Xie, J.; Wang, X. Recent Advances in Smart Stimuli-Responsive Biomaterials for Bone Therapeutics and Regeneration. Bone Res. 2022, 10, 17. [Google Scholar] [CrossRef]
- Montoya, C.; Du, Y.; Gianforcaro, A.L.; Orrego, S.; Yang, M.; Lelkes, P.I. On the Road to Smart Biomaterials for Bone Research: Definitions, Concepts, Advances, and Outlook. Bone Res. 2021, 9, 12. [Google Scholar] [CrossRef]
- Gong, T.; Xie, J.; Liao, J.; Zhang, T.; Lin, S.; Lin, Y. Nanomaterials and Bone Regeneration. Bone Res. 2015, 3, 15029. [Google Scholar] [CrossRef] [PubMed]
- Nazrun Shuid, A.; Izzah Ibrahim, N.; Cairul Iqbal Mohd Amin, M.; Naina Mohamed, I. Drug Delivery Systems for Prevention and Treatment of Osteoporotic Fracture. Curr. Drug Targets 2013, 14, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-J.; Cai, J.-X.; Li, Y.-J.; Wu, J.-Y.; Xiang, D. Recent Progress of Macrophage Vesicle-Based Drug Delivery Systems. Drug Deliv. Transl. Res. 2022, 12, 2287–2302. [Google Scholar] [CrossRef] [PubMed]
- Baryakova, T.H.; Pogostin, B.H.; Langer, R.; McHugh, K.J. Overcoming Barriers to Patient Adherence: The Case for Developing Innovative Drug Delivery Systems. Nat. Rev. Drug Discov. 2023, 22, 387–409. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Zhou, C.; Jin, S.; Fu, L.; Zhang, H.; Huang, X.; Long, H.; Ming, W.; Zhao, J. An Update on the Advances in the Field of Nanostructured Drug Delivery Systems for a Variety of Orthopedic Applications. Drug Deliv. 2023, 30, 2241667. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Y.; Shi, C.; Wu, T.; Cui, Y.; Wang, S.; Liu, P.; Feng, X.; He, Y.; Fu, D. Remote-Controllable Bone-Targeted Delivery of Estradiol for the Treatment of Ovariectomy-Induced Osteoporosis in Rats. J. Nanobiotechnol. 2021, 19, 248. [Google Scholar] [CrossRef]
- Kobayashi, T.; Morimoto, T.; Ito, K.; Mawatari, M.; Shimazaki, T. Denosumab vs. Bisphosphonates in Primary Osteoporosis: A Meta-Analysis of Comparative Safety in Randomized Controlled Trials. Osteoporos. Int. 2024, 35, 1377–1393. [Google Scholar] [CrossRef]
- Migliorini, F.; Maffulli, N.; Colarossi, G.; Eschweiler, J.; Tingart, M.; Betsch, M. Effect of Drugs on Bone Mineral Density in Postmenopausal Osteoporosis: A Bayesian Network Meta-Analysis. J. Orthop. Surg. Res. 2021, 16, 533. [Google Scholar] [CrossRef]
- Mäkinen, V.-N.; Sølling, A.S.; McClung, M.; Langdahl, B.L. Romosozumab for the Treatment of Osteoporosis—A Systematic Review. J. Endocrinol. Investig. 2025, 48, 547–572. [Google Scholar] [CrossRef]
- Liu, L.; Wu, S.; Wei, L.; Xia, Z.; Ji, J.; Huang, D. Romosozumab Adverse Event Profile: A Pharmacovigilance Analysis Based on the FDA Adverse Event Reporting System (FAERS) from 2019 to 2023. Aging Clin. Exp. Res. 2025, 37, 23. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; James, V.; Prajapati, B. An Update on Selective Estrogen Receptor Modulator: Repurposing and Formulations. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 6311–6333. [Google Scholar] [CrossRef]
- Macedo, L.G.; Mulinari-Santos, G.; Siqueira, N.B.; Pitol-Palin, L.; Silva, A.C.; Frigério, P.B.; Botacin, P.R.; Lisboa-Filho, P.N.; Okamoto, R. Enhancing Bone Repair: Impact of Raloxifene-Functionalized Cerabone® on Rat Calvarial Defects. J. Funct. Biomater. 2025, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Mirkazemi, M.; Nadimi, B.; Soltani, P.; Eslami, G.; Parvisi, Y.; Garousi, M.; Izanloo, A. Clinical Evaluation of Therapeutic Efficacy of Teriparatide in Osteoporotic Patients with Vertebral Degeneration. J. Orthop. 2025, 66, 104–109. [Google Scholar] [CrossRef]
- Codrea, C.I.; Lincu, D.; Ene, V.L.; Nicoară, A.I.; Stan, M.S.; Ficai, D.; Ficai, A. Three-Dimensional-Printed Composite Scaffolds Containing Poly-ε-Caprolactone and Strontium-Doped Hydroxyapatite for Osteoporotic Bone Restoration. Polymers 2024, 16, 1511. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Xu, K.; Zhao, X.; Han, Y. Development of a Strontium-Containing Hydroxyapatite Bone Cement. Biomaterials 2005, 26, 4073–4083. [Google Scholar] [CrossRef]
- Verberckmoes, S.C.; Behets, G.J.; Oste, L.; Bervoets, A.R.; Lamberts, L.V.; Drakopoulos, M.; Somogyi, A.; Cool, P.; Dorriné, W.; De Broe, M.E.; et al. Effects of Strontium on the Physicochemical Characteristics of Hydroxyapatite. Calcif. Tissue Int. 2004, 75, 405–415. [Google Scholar] [CrossRef]
- Frasnelli, M.; Cristofaro, F.; Sglavo, V.M.; Dirè, S.; Callone, E.; Ceccato, R.; Bruni, G.; Cornaglia, A.I.; Visai, L. Synthesis and Characterization of Strontium-Substituted Hydroxyapatite Nanoparticles for Bone Regeneration. Mater. Sci. Eng. C 2017, 71, 653–662. [Google Scholar] [CrossRef]
- Codrea, C.I.; Lincu, D.; Atkinson, I.; Culita, D.C.; Croitoru, A.-M.; Dolete, G.; Trusca, R.; Vasile, B.S.; Stan, M.S.; Ficai, D.; et al. Comparison between Two Different Synthesis Methods of Strontium-Doped Hydroxyapatite Designed for Osteoporotic Bone Restoration. Materials 2024, 17, 1472. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, I.B.; Chaves Filho, G.; Silva, E.G.B.; Nogueira, L.F.B.; de Mendonça, T.S.; Furtado, T.C.S.; Ferreira GasparNeto, P.C.; Dias, L.G.; Fukada, S.Y.; Ciancaglini, P.; et al. Synthesis and Characterization of a Strontium–Quercetin Complex and Its In Vitro and In Vivo Potential for Application in Bone Regeneration. ACS Omega 2025, 10, 4836–4846. [Google Scholar] [CrossRef]
- Huang, Y.-R.; Ding, S.-J. Exploring Processing–Structure–Property Relationships of Chemically Precipitated Strontium Silicate Particles for Medical Applications. J. Mater. Chem. B 2025, 13, 3990–4005. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Wen, J.; Ye, W.-H.; Li, Z.; Huang, X.; Chen, S.; Ma, J.-C.; Wu, Y.; Chen, R.; Cui, Z.-K. A Facile and Smart Strategy to Enhance Bone Regeneration with Efficient Vitamin D3 Delivery through Sterosome Technology. J. Control. Release 2024, 370, 140–151. [Google Scholar] [CrossRef]
- Lei, S.; Zhang, X.; Song, L.; Wen, J.; Zhang, Z.; Tian, J.; Yang, R.; Xu, S.; Qiu, S.; MacIsaac, R.J.; et al. Expert Consensus on Vitamin D in Osteoporosis. Ann. Jt. 2025, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Majumdar, U.; Bose, S. Vitamin D3 Release from MgO Doped 3D Printed TCP Scaffolds for Bone Regeneration. ACS Biomater. Sci. Eng. 2024, 10, 1676–1685. [Google Scholar] [CrossRef]
- Bose, S.; Chaudhari, V.S.; Kushram, P. 3D Printed Scaffolds with Quercetin and Vitamin D3 Nanocarriers: In Vitro Cellular Evaluation. J. Biomed. Mater. Res. Part A 2024, 112, 2110–2123. [Google Scholar] [CrossRef]
- Zeghoud, S.; Ben Amor, I.; Alnazza Alhamad, A.; Darwish, L.; Hemmami, H. Osteoporosis Therapy Using Nanoparticles: A Review. Ann. Med. Surg. 2024, 86, 284–291. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Wang, S.; Cui, J.; Weng, W.; Liu, X.; Tang, H.; Hu, Y.; Li, X.; Zhang, K.; et al. Bone-Targeted Bioengineered Bacterial Extracellular Vesicles Delivering siRNA to Ameliorate Osteoporosis. Compos. Part B Eng. 2023, 255, 110610. [Google Scholar] [CrossRef]
- Dayanandan, A.P.; Cho, W.J.; Kang, H.; Bello, A.B.; Kim, B.J.; Arai, Y.; Lee, S.-H. Emerging Nano-Scale Delivery Systems for the Treatment of Osteoporosis. Biomater. Res. 2023, 27, 68. [Google Scholar] [CrossRef]
- Ezzati Nazhad Dolatabadi, J.; Omidi, Y. Solid Lipid-Based Nanocarriers as Efficient Targeted Drug and Gene Delivery Systems. TrAC Trends Anal. Chem. 2016, 77, 100–108. [Google Scholar] [CrossRef]
- Anish, R.J.; Nair, A. Osteoporosis Management-Current and Future Perspectives—A Systemic Review. J. Orthop. 2024, 53, 101–113. [Google Scholar] [CrossRef]
- Chen, Y.; Jia, L.; Han, T.; Zhao, Z.; Yang, J.; Xiao, J.; Yang, H.-J.; Yang, K. Osteoporosis Treatment: Current Drugs and Future Developments. Front. Pharmacol. 2024, 15, 1456796. [Google Scholar]
- Ming, L.; Wu, H.; Fan, Q.; Dong, Z.; Huang, J.; Xiao, Z.; Xiao, N.; Huang, H.; Liu, H.; Li, Z. Bio-Inspired Drug Delivery Systems: A New Attempt from Bioinspiration to Biomedical Applications. Int. J. Pharm. 2024, 658, 124221. [Google Scholar] [CrossRef] [PubMed]
- Ding, A.; Tang, F.; Alsberg, E. 4D Printing: A Comprehensive Review of Technologies, Materials, Stimuli, Design, and Emerging Applications. Chem. Rev. 2025, 125, 3663–3771. [Google Scholar] [CrossRef]
- Burdușel, A.-C.; Gherasim, O.; Andronescu, E.; Grumezescu, A.M.; Ficai, A. Inorganic Nanoparticles in Bone Healing Applications. Pharmaceutics 2022, 14, 770. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Singh, I.; Khademhosseini, A. Engineered Biomaterials for in Situ Tissue Regeneration. Nat. Rev. Mater. 2020, 5, 686–705. [Google Scholar] [CrossRef]
- Prakash, A.; Malviya, R.; Sridhar, S.B.; Shareef, J. 4D Printing in Dynamic and Adaptive Bone Implants: Progress in Bone Tissue Engineering. Bioprinting 2024, 44, e00373. [Google Scholar] [CrossRef]
- Zhao, R.; Shang, T.; Yuan, B.; Zhu, X.; Zhang, X.; Yang, X. Osteoporotic Bone Recovery by a Bamboo-Structured Bioceramic with Controlled Release of Hydroxyapatite Nanoparticles. Bioact. Mater. 2022, 17, 379–393. [Google Scholar] [CrossRef]
- Poorirani, S.; Taheri, S.L.; Mostafavi, S.A. Scaffolds: A Biomaterial Engineering in Targeted Drug Delivery for Osteoporosis. Osteoporos. Int. 2023, 34, 255–267. [Google Scholar] [CrossRef]
- Liang, W.; Long, H.; Zhang, H.; Bai, J.; Jiang, B.; Wang, J.; Fu, L.; Ming, W.; Zhao, J.; Zeng, B. Bone Scaffolds-Based Localized Drugs Delivery for Osteosarcoma: Current Status and Future Perspective. Drug Deliv. 2024, 31, 2391001. [Google Scholar] [CrossRef] [PubMed]
- He, X.-Y.; Yu, H.-M.; Lin, S.; Li, Y.-Z. Advances in the Application of Mesenchymal Stem Cells, Exosomes, Biomimetic Materials, and 3D Printing in Osteoporosis Treatment. Cell. Mol. Biol. Lett. 2021, 26, 47. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Yan, J.; Zhang, K.; Lin, F.; Xiang, L.; Deng, L.; Guan, Z.; Cui, W.; Zhang, H. Pharmaceutical Electrospinning and 3D Printing Scaffold Design for Bone Regeneration. Adv. Drug Deliv. Rev. 2021, 174, 504–534. [Google Scholar] [CrossRef]
- Poh, P.S.; Lingner, T.; Kalkhof, S.; Märdian, S.; Baumbach, J.; Dondl, P.; Duda, G.N.; Checa, S. Enabling Technologies towards Personalization of Scaffolds for Large Bone Defect Regeneration. Curr. Opin. Biotechnol. 2022, 74, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.H.; Friederichs, R.J.; Best, S.M. 11—Synthetic Hydroxyapatite for Tissue Engineering Applications. In Hydroxyapatite (Hap) for Biomedical Applications; Mucalo, M., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 235–267. [Google Scholar] [CrossRef]
- Carmona, F.J.; Dal Sasso, G.; Bertolotti, F.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M.; Pedersen, J.S.; Masciocchi, N.; Guagliardi, A. The Role of Nanoparticle Structure and Morphology in the Dissolution Kinetics and Nutrient Release of Nitrate-Doped Calcium Phosphate Nanofertilizers. Sci. Rep. 2020, 10, 12396. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Chang, J. 1—Structure and Properties of Hydroxyapatite for Biomedical Applications. In Hydroxyapatite (Hap) for Biomedical Applications; Mucalo, M., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 3–19. [Google Scholar] [CrossRef]
- Loca, D.; Locs, J.; Dubnika, A.; Zalite, V.; Berzina-Cimdina, L. 9—Porous Hydroxyapatite for Drug Delivery. In Hydroxyapatite (Hap) for Biomedical Applications; Mucalo, M., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 189–209. [Google Scholar] [CrossRef]
- Bordone, M.; Bettencourt, A. Management of Bone Diseases: Looking at Scaffold-Based Strategies for Drug Delivery. Drug Deliv. Transl. Res. 2023, 13, 79–104. [Google Scholar] [CrossRef]
- Wang, C.; Liu, A.; Zhao, Z.; Ying, T.; Deng, S.; Jian, Z.; Zhang, X.; Yi, C.; Li, D. Application and Progress of 3D Printed Biomaterials in Osteoporosis. Front. Bioeng. Biotechnol. 2025, 13, 1541746. [Google Scholar]
- Ejnisman, L.; Gobbato, B.; de França Camargo, A.F.; Zancul, E. Three-Dimensional Printing in Orthopedics: From the Basics to Surgical Applications. Curr. Rev. Musculoskelet. Med. 2021, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, O.; Patel, R.; Thahir, A.; Sy, J.; Jou, E. The Use of Three-Dimensional Printing in Orthopaedics: A Systematic Review and Meta-Analysis. Arch. Bone Jt. Surg. 2024, 12, 441–456. [Google Scholar] [CrossRef]
- Palmquist, A.; Jolic, M.; Hryha, E.; Shah, F.A. Complex Geometry and Integrated Macro-Porosity: Clinical Applications of Electron Beam Melting to Fabricate Bespoke Bone-Anchored Implants. Acta Biomater. 2023, 156, 125–145. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.; Wang, W.; Liu, J. Current Developments in 3D Printing Technology for Orthopedic Trauma: A Review. Medicine 2025, 104, e41946. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.; Zhao, J.; Hussain, M.; Wang, M. Additive Manufacturing in Orthopedics: A Review. ACS Biomater. Sci. Eng. 2022, 8, 1367–1380. [Google Scholar] [CrossRef]
- Pingale, P.; Dawre, S.; Dhapte-Pawar, V.; Dhas, N.; Rajput, A. Advances in 4D Printing: From Stimulation to Simulation. Drug Deliv. Transl. Res. 2023, 13, 164–188. [Google Scholar] [CrossRef]
- Wu, W.; Sabharwal, S.; Bunker, M.; Sabharwal, S. 3D Printing Technology in Pediatric Orthopedics: A Primer for the Clinician. Curr. Rev. Musculoskelet. Med. 2023, 16, 398–409. [Google Scholar] [CrossRef]
- Kang, W.; Xu, Z.; Lu, H.; Liu, S.; Li, J.; Ding, C.; Lu, Y. Advances in Biomimetic Nanomaterial Delivery Systems: Harnessing Nature’s Inspiration for Targeted Drug Delivery. J. Mater. Chem. B 2024, 12, 7001–7019. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Nagpal, M.; Aggarwal, G. Nanotechnology for Targeted Drug Delivery to Treat Osteoporosis. Curr. Drug Targets 2023, 24, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in Drug Delivery Systems, Challenges and Future Directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Ding, S.; Alexander, E.; Liang, H.; Kulchar, R.J.; Singh, R.; Herzog, R.W.; Daniell, H.; Leong, K.W. Synthetic and Biogenic Materials for Oral Delivery of Biologics: From Bench to Bedside. Chem. Rev. 2025, 125, 4009–4068. [Google Scholar] [CrossRef]
- Pasqualone, M.; Andreetta, H.A.; Cortizo, M.S. Risedronate Transdermal Delivery System Based on a Fumaric Copolymer for Therapy of Osteoporosis. Mater. Sci. Eng. C 2017, 76, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Saini, D.; Fazil, M.; Ali, M.M.; Baboota, S.; Ameeduzzafar; Ali, J. Formulation, Development and Optimization of Raloxifene-Loaded Chitosan Nanoparticles for Treatment of Osteoporosis. Drug Deliv. 2015, 22, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.-A.; Merkel, O.; Popp, A. Intranasal Drug Delivery: Opportunities and Toxicologic Challenges during Drug Development. Drug Deliv. Transl. Res. 2022, 12, 735–757. [Google Scholar] [CrossRef]
- Garg, S.; Kumar, M.; Maurya, R.; Alam, M.I.; Karwal, A.; Yadav, A.K.; Yadav, V.K.; Shukla, A.K. Specialized Drug Delivery Systems: An Overview. In Novel Carrier Systems for Targeted and Controlled Drug Delivery; Yadav, A.K., Jain, K., Eds.; Springer Nature: Singapore, 2024; pp. 391–458. [Google Scholar] [CrossRef]
- He, K.; Tang, M. Safety of Novel Liposomal Drugs for Cancer Treatment: Advances and Prospects. Chem.-Biol. Interact. 2018, 295, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Gatto, M.S.; Johnson, M.P.; Najahi-Missaoui, W. Targeted Liposomal Drug Delivery: Overview of the Current Applications and Challenges. Life 2024, 14, 672. [Google Scholar] [CrossRef]
- Nirwan, N.; Nikita; Sultana, Y.; Vohora, D. Liposomes as Multifaceted Delivery System in the Treatment of Osteoporosis. Expert. Opin. Drug Deliv. 2021, 18, 761–775. [Google Scholar] [CrossRef]
- Huang, L.; Wang, X.; Cao, H.; Li, L.; Chow, D.H.-K.; Tian, L.; Wu, H.; Zhang, J.; Wang, N.; Zheng, L.; et al. A Bone-Targeting Delivery System Carrying Osteogenic Phytomolecule Icaritin Prevents Osteoporosis in Mice. Biomaterials 2018, 182, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; An, X.; Gong, W.; Wu, W.; Liu, B.; Shao, X.; Xian, Y.; Peng, R.; Guo, B.; Jiang, Q. Liposomal α-Cyperone Targeting Bone Resorption Surfaces Suppresses Osteoclast Differentiation and Osteoporosis Progression via the PI3K/Akt Axis. Nano Res. 2024, 17, 2949–2959. [Google Scholar] [CrossRef]
- Munir, M.; Zaman, M.; Waqar, M.A.; Khan, M.A.; Alvi, M.N. Solid Lipid Nanoparticles: A Versatile Approach for Controlled Release and Targeted Drug Delivery. J. Liposome Res. 2024, 34, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Alwani, S.; Wasan, E.K.; Badea, I. Solid Lipid Nanoparticles for Pulmonary Delivery of Biopharmaceuticals: A Review of Opportunities, Challenges, and Delivery Applications. Mol. Pharm. 2024, 21, 3084–3102. [Google Scholar] [CrossRef]
- Attama, A.A.; Umeyor, C.E. The Use of Solid Lipid Nanoparticles for Sustained Drug Release. Ther. Deliv. 2015, 6, 669–684. [Google Scholar] [CrossRef]
- Wolska, E.; Sznitowska, M. Modeling the Analysis Process of a Lipid-Based, Multi-Compartment Drug Delivery System. Processes 2025, 13, 460. [Google Scholar] [CrossRef]
- Das, T.; Venkatesh, M.P.; Pramod Kumar, T.M.; Koland, M. SLN Based Alendronate in Situ Gel as an Implantable Drug Delivery System—A Full Factorial Design Approach. J. Drug Deliv. Sci. Technol. 2020, 55, 101415. [Google Scholar] [CrossRef]
- Salama, A.M.; Hardy, J.G.; Yessuf, A.M.; Chen, J.; Ni, M.; Huang, C.; Zhang, Q.; Liu, Y. Injectable Hydrogel Technologies for Bone Disease Treatment. ACS Appl. Bio Mater. 2025, 8, 2691–2715. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Pujol, E.J.; Martínez, G.; Casado-Jurado, D.; Vázquez, J.; León-Barberena, J.; Rodríguez-Lucena, D.; Torres, Y.; Alcudia, A.; Begines, B. Hydrogels and Nanogels: Pioneering the Future of Advanced Drug Delivery Systems. Pharmaceutics 2025, 17, 215. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Du, K.; Wang, M.; Zhu, Z.; Lei, L.; Shi, Y. Natural Endogenous Material-Based Vehicles for Delivery of Macromolecular Drugs. Chin. J. Nat. Med. 2024, 22, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Agrawal, P.; Singh, S.K.; Chhonker, Y.S.; Sun, J.; Murry, D.J. Polymer-Based Drug Delivery Systems for Cancer Therapeutics. Polymers 2024, 16, 843. [Google Scholar] [CrossRef] [PubMed]
- Salave, S.; Rana, D.; Benival, D. Dual Targeting Anti-Osteoporotic Therapy Through Potential Nanotherapeutic Approaches. Pharm. Nanotechnol. 2022, 10, 384–392. [Google Scholar] [CrossRef]
- Uenaka, M.; Yamashita, E.; Kikuta, J.; Morimoto, A.; Ao, T.; Mizuno, H.; Furuya, M.; Hasegawa, T.; Tsukazaki, H.; Sudo, T.; et al. Osteoblast-Derived Vesicles Induce a Switch from Bone-Formation to Bone-Resorption in Vivo. Nat. Commun. 2022, 13, 1066. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Śmieszek, A.; Kornicka-Garbowska, K.; Pielok, A.; Janeczek, M.; Lipińska, A.; Nikodem, A.; Filipiak, J.; Sobierajska, P.; Nedelec, J.M.; et al. Novel Nanohydroxyapatite (nHAp)-Based Scaffold Doped with Iron Oxide Nanoparticles (IO), Functionalized with Small Non-Coding RNA (miR-21/124) Modulates Expression of Runt-Related Transcriptional Factor 2 and Osteopontin, Promoting Regeneration of Osteoporotic Bone in Bilateral Cranial Defects in a Senescence-Accelerated Mouse Model (SAM/P6). PART 2. Int. J. Nanomed. 2021, 16, 6049–6065. [Google Scholar] [CrossRef]
- Liu, J.; Bian, H.; Zhang, Y.; Long, Y.; Li, C.; Zhang, R.; Mao, Z.; Wu, H.; Li, B.; Zhi, C.; et al. Formation of Stable Zinc-Rich Amorphous Calcium Phosphate. Cryst. Growth Des. 2024, 24, 9492–9501. [Google Scholar] [CrossRef]
- Tao, S.; Yu, F.; Song, Y.; Zhou, W.; Lv, J.; Zhao, R.; Wang, C.; Hu, F.; Yuan, H. Water/pH Dual Responsive in Situ Calcium Supplement Collaborates Simvastatin for Osteoblast Promotion Mediated Osteoporosis Therapy via Oral Medication. J. Control. Release 2021, 329, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Mehta, S. Functionalized SBA-15: Engineering, Detailed Study on Release and Kinetics of Alendronate as Well as Its Anti-Tumour Properties towards Osteosarcoma. RSC Pharm. 2024, 1, 797–805. [Google Scholar] [CrossRef]
- Yao, K.; Zhang, Q.; Weng, L.; Li, S.; Zheng, X.; Hu, L.; Luo, Y.; Huang, X.; Gong, Z.; Wang, Z.; et al. Cerium-Doped, Alendronate-Loaded, Metal–Organic Framework Nanodrug for Delayed Osteoporosis Progress. ACS Appl. Nano Mater. 2024, 7, 28504–28518. [Google Scholar] [CrossRef]
- Rasool, N.; Negi, D.; Singh, Y. Thiol-Functionalized, Antioxidant, and Osteogenic Mesoporous Silica Nanoparticles for Osteoporosis. ACS Biomater. Sci. Eng. 2023, 9, 3535–3545. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Lin, Z.; Xiong, Y.; Xue, H.; Song, W.; Yu, T.; Chen, L.; Hu, Y.; Panayi, A.C.; Sun, Y.; et al. Dual-Targeted Nanoplatform Regulating the Bone Immune Microenvironment Enhances Fracture Healing. ACS Appl. Mater. Interfaces 2021, 13, 56944–56960. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, X.; Li, Y.; Song, Q.; Long, N.; Fu, X.; Wang, Y.; He, Y.; Yan, H.; Li, C.; et al. Icariin-Loaded Selenium-Gold Multi-Shell Nanocomposites with NIR-II Response Release to Relieve Post-Damaged Bone Microenvironment for Osteoporosis Synergy Therapy. Chem. Eng. J. 2024, 499, 156421. [Google Scholar] [CrossRef]
- Nakamura, M.; Ueda, K.; Yamamoto, Y.; Aoki, K.; Zhang, M.; Saito, N.; Yudasaka, M. Ibandronate-Loaded Carbon Nanohorns Fabricated Using Calcium Phosphates as Mediators and Their Effects on Macrophages and Osteoclasts. ACS Appl. Mater. Interfaces 2021, 13, 3701–3712. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Wang, J.; Liao, L.; Li, X.; Zhang, Z.; Yang, X.; Yu, X.; Fan, B.; Li, B.; et al. Binary Doping of Strontium–Magnesium to Bioactive Glasses to Enhance Antibacterial and Osteogenic Effects. ACS Omega 2025, 10, 215–229. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, W.; Zhu, D.; Li, Z.; Wang, Z.; Li, J.; Mei, X.; Xu, W.; Cheng, K.; Zhong, B. Nanoparticles Functionalized with Stem Cell Secretome and CXCR4-Overexpressing Endothelial Membrane for Targeted Osteoporosis Therapy. J. Nanobiotechnol. 2022, 20, 35. [Google Scholar] [CrossRef]
- Carmona-Sarabia, L.; Quiñones Vélez, G.; Escalera-Joy, A.M.; Mojica-Vázquez, D.; Esteves-Vega, S.; Peterson-Peguero, E.A.; López-Mejías, V. Design of Extended Bisphosphonate-Based Coordination Polymers as Bone-Targeted Drug Delivery Systems for Breast Cancer-Induced Osteolytic Metastasis and Other Bone Therapies. Inorg. Chem. 2023, 62, 9440–9453. [Google Scholar] [CrossRef]
- Hayashi, K.; Zhang, C.; Taleb Alashkar, A.N.; Ishikawa, K. Carbonate Apatite Honeycomb Scaffold-Based Drug Delivery System for Repairing Osteoporotic Bone Defects. ACS Appl. Mater. Interfaces 2024, 16, 45956–45968. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Wu, Y.; Li, G.; Ji, N.; Han, R.; Tang, H.; Liu, X.; Liu, H.; Wang, C.; et al. Delivery of m7G Methylated Runx2 mRNA by Bone-Targeted Lipid Nanoparticle Promotes Osteoblastic Bone Formation in Senile Osteoporosis. Nano Today 2024, 54, 102074. [Google Scholar] [CrossRef]
- Guo, Z.; Qi, P.; Pei, D.; Zhang, X. Raloxifene-Loaded Solid Lipid Nanoparticles Decorated Gel with Enhanced Treatment Potential of Osteoporosis. J. Drug Deliv. Sci. Technol. 2022, 75, 103733. [Google Scholar] [CrossRef]
- Cui, Y.; Guo, Y.; Kong, L.; Shi, J.; Liu, P.; Li, R.; Geng, Y.; Gao, W.; Zhang, Z.; Fu, D. A Bone-Targeted Engineered Exosome Platform Delivering siRNA to Treat Osteoporosis. Bioact. Mater. 2022, 10, 207–221. [Google Scholar] [CrossRef]
- Hu, Y.; Li, X.; Zhang, Q.; Gu, Z.; Luo, Y.; Guo, J.; Wang, X.; Jing, Y.; Chen, X.; Su, J. Exosome-Guided Bone Targeted Delivery of Antagomir-188 as an Anabolic Therapy for Bone Loss. Bioact. Mater. 2021, 6, 2905–2913. [Google Scholar] [CrossRef]
- Sicard, L.; Maillard, S.; Mbita Akoa, D.; Torrens, C.; Collignon, A.-M.; Coradin, T.; Chaussain, C. Sclerostin Antibody-Loaded Dense Collagen Hydrogels Promote Critical-Size Bone Defect Repair. ACS Biomater. Sci. Eng. 2024, 10, 6451–6464. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, K.E.; Turhanen, P.; Brückner, S.I.; Brunner, E.; Demadis, K.D. Smart, Programmable and Responsive Injectable Hydrogels for Controlled Release of Cargo Osteoporosis Drugs. Sci. Rep. 2017, 7, 4743. [Google Scholar] [CrossRef]
- Klara, J.; Hinz, A.; Bzowska, M.; Horak, W.; Lewandowska-Łańcucka, J. In Vitro/Ex Vivo Evaluation of Multifunctional Collagen/Chitosan/Hyaluronic Acid Hydrogel-Based Alendronate Delivery Systems. Int. J. Biol. Macromol. 2024, 262, 130142. [Google Scholar] [CrossRef]
- Xi, Y.; Wang, W.; Ma, L.; Xu, N.; Shi, C.; Xu, G.; He, H.; Pan, W. Alendronate Modified mPEG-PLGA Nano-Micelle Drug Delivery System Loaded with Astragaloside Has Anti-Osteoporotic Effect in Rats. Drug Deliv. 2022, 29, 2386–2402. [Google Scholar] [CrossRef]
- Xi, Y.; Wang, W.; Xu, N.; Shi, C.; Xu, G.; Sun, J.; He, H.; Jiang, T. Myricetin Loaded Nano-Micelles Delivery System Reduces Bone Loss Induced by Ovariectomy in Rats Through Inhibition of Osteoclast Formation. J. Pharm. Sci. 2022, 111, 2341–2352. [Google Scholar] [CrossRef]
- Lima, A.C.; Reis, R.L.; Ferreira, H.; Neves, N.M. Glutathione Reductase-Sensitive Polymeric Micelles for Controlled Drug Delivery on Arthritic Diseases. ACS Biomater. Sci. Eng. 2021, 7, 3229–3241. [Google Scholar] [CrossRef]
- Liu, D.; Zou, Z.; Hu, X.; Huang, S.; Huang, T.; Gu, J.; Zhu, W.; Liu, M.; Huang, S.; Zhang, X. Self-Assembly of Phosphorylated Capsaicin and Curcumin for Treatment of Triple-Negative Breast Cancer via Combination of Chemotherapy and Photodynamic Therapy. Colloids Surf. A Physicochem. Eng. Asp. 2025, 720, 137108. [Google Scholar] [CrossRef]
- Yang, Y.; Xiang, Y.; Xu, P.; Zhang, W.; Wang, Y.; Feng, L.; She, R. Immuno-Osteoinductive 3D Printed Hydrogel Scaffolds with Triple Crosslinking and GA/EGCG Release for Bone Healing. Colloids Surf. B Biointerfaces 2025, 252, 114651. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, J.; Chen, L.; Li, Z.; Feng, Q.; Chen, F.; Hao, Z.; Chen, T.; Shi, G.; Zhang, Q.; et al. Electrospunned Nanofiber Membranes of PTH-Related Peptide Loaded Biopolymers for Osteoporotic Bone Defect Repair. Mater. Des. 2024, 244, 113179. [Google Scholar] [CrossRef]
- Tonbul, H.; Ultav, G. Improving the Doxorubicin Loading to PLGA Nanoparticles with TOPSIS-Based Taguchi Design Approach: Effect of the Water Phase. J. Pharm. Innov. 2024, 19, 58. [Google Scholar] [CrossRef]
- Xue, L.; Ding, J.; Liu, Y.; Ma, Y.; Yang, C.; Wang, W.; Wang, Y. Strategies and Methods of Nanocrystal Technology for Targeting Drug Delivery. J. Nanoparticle Res. 2024, 26, 114. [Google Scholar] [CrossRef]
- Cheng, X.; Xie, Q.; Sun, Y. Advances in Nanomaterial-Based Targeted Drug Delivery Systems. Front. Bioeng. Biotechnol. 2023, 11, 1177151. [Google Scholar] [CrossRef]
- Dobrzyńska-Mizera, M.; Dodda, J.M.; Liu, X.; Knitter, M.; Oosterbeek, R.N.; Salinas, P.; Pozo, E.; Ferreira, A.M.; Sadiku, E.R. Engineering of Bioresorbable Polymers for Tissue Engineering and Drug Delivery Applications. Adv. Healthc. Mater. 2024, 13, 2401674. [Google Scholar] [CrossRef]
- Moghanian, A.; Asadi, P.; Akbari, M.; Mohammad Aliha, M.R.; Kizilkurtlu, A.A.; Akpek, A.; Safaee, S. New Trends in 3D and 4D Printed Dental and Orthopedic Implants: Methods, Applications and Future Directions. Bioprinting 2025, 48, e00406. [Google Scholar] [CrossRef]
- Singh, J.; Nayak, P. pH-Responsive Polymers for Drug Delivery: Trends and Opportunities. J. Polym. Sci. 2023, 61, 2828–2850. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, F.; Wang, Y.; Johnston, A.P.R.; Duffin, R.N.; Andrews, P.C.; Ritchie, C.; Such, G.K. Facile Construction of Polyoxometalate-Polymer Hybrid Nanoparticles with pH/Redox Dual-Responsiveness. Chem. Sci. 2025, 16, 288–296. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, Biodegradation and Biomedical Applications of Poly(Lactic Acid)/Poly(Lactic-Co-Glycolic Acid) Micro and Nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Chopra, V.; Fuentes-Velasco, V.; Nacif-Lopez, S.R.; Melendez-Malpicca, J.; Mendez-Hernandez, A.S.; Ramos-Mendez-Iris, L.F.; Arroyo-Jimenez, D.A.; Reyes-Segura, D.G.; Gonzalez-Y-Mendoza, P.; Sanchez-Hernandez, K.A.; et al. Advancements in 3D-4D Printing of Hydroxyapatite Composites for Bone Tissue Engineering. Ceram. Int. 2024, 50 Pt B, 38819–38840. [Google Scholar] [CrossRef]
- Serrano, D.R.; Kara, A.; Yuste, I.; Luciano, F.C.; Ongoren, B.; Anaya, B.J.; Molina, G.; Diez, L.; Ramirez, B.I.; Ramirez, I.O.; et al. 3D Printing Technologies in Personalized Medicine, Nanomedicines, and Biopharmaceuticals. Pharmaceutics 2023, 15, 313. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, H.; Esworthy, T.; Mei, D.; Wang, Y.; Zhang, L.G. Emerging 4D Printing Strategies for Next-Generation Tissue Regeneration and Medical Devices. Adv. Mater. 2022, 34, 2109198. [Google Scholar] [CrossRef] [PubMed]
- Yarali, E.; Mirzaali, M.J.; Ghalayaniesfahani, A.; Accardo, A.; Diaz-Payno, P.J.; Zadpoor, A.A. 4D Printing for Biomedical Applications. Adv. Mater. 2024, 36, 2402301. [Google Scholar] [CrossRef]
- Soleymani, S.; Naghib, S.M. 3D and 4D Printing Hydroxyapatite-Based Scaffolds for Bone Tissue Engineering and Regeneration. Heliyon 2023, 9, e19363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, S.; Zhu, M.; Zhu, Y.; Zhang, Y.; Liu, Z.; Zhang, C. 3D-Printed Magnetic Fe3O4/MBG/PCL Composite Scaffolds with Multifunctionality of Bone Regeneration, Local Anticancer Drug Delivery and Hyperthermia. J. Mater. Chem. B 2014, 2, 7583–7595. [Google Scholar] [CrossRef]
- Dai, W.; Guo, H.; Gao, B.; Ruan, M.; Xu, L.; Wu, J.; Brett Kirk, T.; Xu, J.; Ma, D.; Xue, W. Double Network Shape Memory Hydrogels Activated by Near-Infrared with High Mechanical Toughness, Nontoxicity, and 3D Printability. Chem. Eng. J. 2019, 356, 934–949. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, K.; Gong, T.; Song, J.; Bao, C.; Luo, E.; Weng, J.; Zhou, S. Delivery of Growth Factors Using a Smart Porous Nanocomposite Scaffold to Repair a Mandibular Bone Defect. Biomacromolecules 2014, 15, 1019–1030. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Wu, Y.; Luo, T. Efficacy and Safety of Sequential Therapy for Primary Osteoporosis with Bone Formation Promoters Followed by Bone Resorption Inhibitors: A Meta-Analysis. J. Orthop. Surg. Res. 2025, 20, 147. [Google Scholar] [CrossRef]
- Clogston, J.D.; Foss, W.; Harris, D.; Oberoi, H.; Pan, J.; Pu, E.; Guzmán, E.A.T.; Walter, K.; Brown, S.; Soo, P.L. Current State of Nanomedicine Drug Products: An Industry Perspective. J. Pharm. Sci. 2024, 113, 3395–3405. [Google Scholar] [CrossRef]
- Kametani, Y.; Ito, R.; Manabe, Y.; Kulski, J.K.; Seki, T.; Ishimoto, H.; Shiina, T. PBMC-Engrafted Humanized Mice Models for Evaluating Immune-Related and Anticancer Drug Delivery Systems. Front. Mol. Biosci. 2024, 11, 1447315. [Google Scholar] [CrossRef] [PubMed]
- Loisios-Konstantinidis, I.; Hens, B.; Mitra, A.; Kim, S.; Chiann, C.; Cristofoletti, R. Using Physiologically Based Pharmacokinetic Modeling to Assess the Risks of Failing Bioequivalence Criteria: A Tale of Two Ibuprofen Products. AAPS J. 2020, 22, 113. [Google Scholar] [CrossRef] [PubMed]
- Skidmore, S.; Barker, R.A. Challenges in the Clinical Advancement of Cell Therapies for Parkinson’s Disease. Nat. Biomed. Eng. 2023, 7, 370–386. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Shih, C.-J.; Bao, Y. Advances in 4D Printing of Biodegradable Photopolymers. Responsive Mater. 2024, 2, e20240008. [Google Scholar] [CrossRef]
- Kim, D.; Kim, K.H.; Yang, Y.S.; Jang, K.S.; Jeon, S.; Jeong, J.H.; Park, S.A. 4D Printing and Simulation of Body Temperature-Responsive Shape-Memory Polymers for Advanced Biomedical Applications. IJB 2024, 10, 3035. [Google Scholar] [CrossRef]
- Narupai, B.; Smith, P.T.; Nelson, A. 4D Printing of Multi-Stimuli Responsive Protein-Based Hydrogels for Autonomous Shape Transformations. Adv. Funct. Mater. 2021, 31, 2011012. [Google Scholar] [CrossRef]
- Mallakpour, S.; Tabesh, F.; Hussain, C.M. A New Trend of Using Poly(Vinyl Alcohol) in 3D and 4D Printing Technologies: Process and Applications. Adv. Colloid. Interface Sci. 2022, 301, 102605. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, F.; Jiang, M.; Liu, Y.; Yuan, H.; Leng, J. Programmable 4D Printing of Photoactive Shape Memory Composite Structures. ACS Appl. Mater. Interfaces 2022, 14, 42568–42577. [Google Scholar] [CrossRef]
| Classification | Drug Type | Representative Drugs | Adverse Reactions |
|---|---|---|---|
| Bone-resorption-inhibiting drugs | Bisphosphonates | Alendronate sodium | Skeletal muscle pain |
| Calcitonin | Salmon calcitonin | Allergic reactions, hypocalcemia | |
| Estrogenic | Raloxifene | Thrombocytopenia, gastrointestinal reactions | |
| Monoclonal antibodies | Denosumab | Cardiovascular injuries | |
| Bone-formation-promoting drugs | Parathyroid hormone-like compounds | Teriparatide | Orthostatic hypotension, tingling sensation in the extremities |
| Fluoride | Disodium fluorophosphate | High concentrations are likely to cause bone cancer and neuroarthritis | |
| Statins | Simvastatin | Liver injury | |
| Androgen | Testosterone | Cardiovascular and cerebrovascular injuries | |
| Strontium salt | Strontium ranelate | Myocardial infarction, thromboembolism, DRESS syndrome | |
| Bone nutrition drugs | Calcium | Calcium carbonate | Constipation, hypercalcemia |
| Vitamin D and its derivatives | Vitamin D3/cholecalciferol | Hypercalcemia | |
| Vitamin K | Osteotriol/calcitriol | Hepatotoxicity, hemolytic anemia |
| 3D Printing Category | 3D Printing Techniques | Advantages | Disadvantages | Common Applications |
| Extrusion-based 3D Printing | Fused deposition modeling | More compatible materials; Adjustable pore size; Convenience and low cost | Low resolution; Increased material shear due to the extrusion process; Low efficiency | Surgical simulation model |
| Bioprinting | Maintaining of biological activity; Precise control of the porous structure; Multimaterial printing | Lower mechanical strength; Low efficiency; Low precision; Limited materials; Acidic degradation products | Orthopedic tissue engineering | |
| Powder Bed Fusion | Selective laser sintering | Powdered materials; High-resolution data; High material utilization | High temperatures affect the biological activity of materials; High cost; Rough surface | Implants |
| Electron beam melting | Manufacturing efficiency; High-vacuum environment | Cost and complexity; Material limitations; Rough surface; Low accuracy | Implants | |
| Material Jetting | Inkjet printing | High resolution; Multi-material simultaneous printing; Low cost | Higher material viscosity required; Lower stability | Orthopedic tissue engineering |
| Vat Photopolymerization | Stereolithography | High accuracy; High precision; Good surface quality | Low efficiency; Limited materials | Surgical guide plate and guide |
| Digital light processing | Rapid generation; High accuracy; High precision; Good surface quality | Limited materials | Surgical guide plate and guide |
| Physical Features | Drug Delivery Requirements | Mechanical Properties | Biological Aspects |
|---|---|---|---|
| Adequate pore size | Suitable drug release profile | Mechanical compatibility | Biofunctional |
| High porosity | Drug decomposition protector | Mechanical integrity | Biocompatible |
| High pore interconnectivity | Mechanical strength | Biodegradable | |
| Wettability | Osteoinductive | ||
| Osteoconductive | |||
| Osteogenic | |||
| Osteointegration |
| Physical | Chemical | Biological | Cell-Based |
|---|---|---|---|
| Heat | Solvents | Enzymes | Cell contractile forces |
| Light | Moisture | Glucose | Cell proliferation |
| Electric field | pH | Oligonucleotides | |
| Magnetic field | Ions | ||
| Ultrasound | Redox | ||
| Microwave | |||
| Mechanical forces |
| Stimulus Type | Mechanism of Action | Biological Effects |
|---|---|---|
| Photoresponsive | Infrared laser induces photophysical effects; modulates mitochondrial ATP synthesis | Enhances cellular metabolism; promotes angiogenesis and bone regeneration |
| Magnetic Field | Fe3O4 NPs generate localized heat under magnetic fields (magnetic hyperthermia) | Improves osteogenic differentiation; may also assist in tumor ablation in bone cancer contexts |
| Ultrasound-Responsive | Enhances VEGF-A mRNA expression and chondrocyte proliferation | Accelerates bone matrix maturation; stimulates mineralization |
| Electroresponsive | Electrical currents stimulate BMSCs and upregulate BMPs and cytokines | Promotes BMSC proliferation/differentiation; boosts bone formation through calcium–calmodulin signaling |
| Piezoelectricity | Mechanical stress induces electric charges in scaffolds or bone tissue | Supports stem cell proliferation and osteogenic differentiation |
| Mechanical Stimuli | Mechanical strain activates PI3K/Akt and other pathways | Promotes early osteogenic differentiation of stem cells; improves regeneration in osteoporotic bone |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Codrea, C.I.; Fruth, V. Three-Dimensionally Printed Scaffolds and Drug Delivery Systems in Treatment of Osteoporosis. Biomimetics 2025, 10, 429. https://doi.org/10.3390/biomimetics10070429
Codrea CI, Fruth V. Three-Dimensionally Printed Scaffolds and Drug Delivery Systems in Treatment of Osteoporosis. Biomimetics. 2025; 10(7):429. https://doi.org/10.3390/biomimetics10070429
Chicago/Turabian StyleCodrea, Cosmin Iulian, and Victor Fruth. 2025. "Three-Dimensionally Printed Scaffolds and Drug Delivery Systems in Treatment of Osteoporosis" Biomimetics 10, no. 7: 429. https://doi.org/10.3390/biomimetics10070429
APA StyleCodrea, C. I., & Fruth, V. (2025). Three-Dimensionally Printed Scaffolds and Drug Delivery Systems in Treatment of Osteoporosis. Biomimetics, 10(7), 429. https://doi.org/10.3390/biomimetics10070429








