Using Patient-Specific 3D-Printed C1–C2 Interfacet Spacers for the Treatment of Type 1 Basilar Invagination: A Clinical Case Report
Abstract
1. Introduction
2. Patient Information and Presentation
2.1. Clinical Presentation
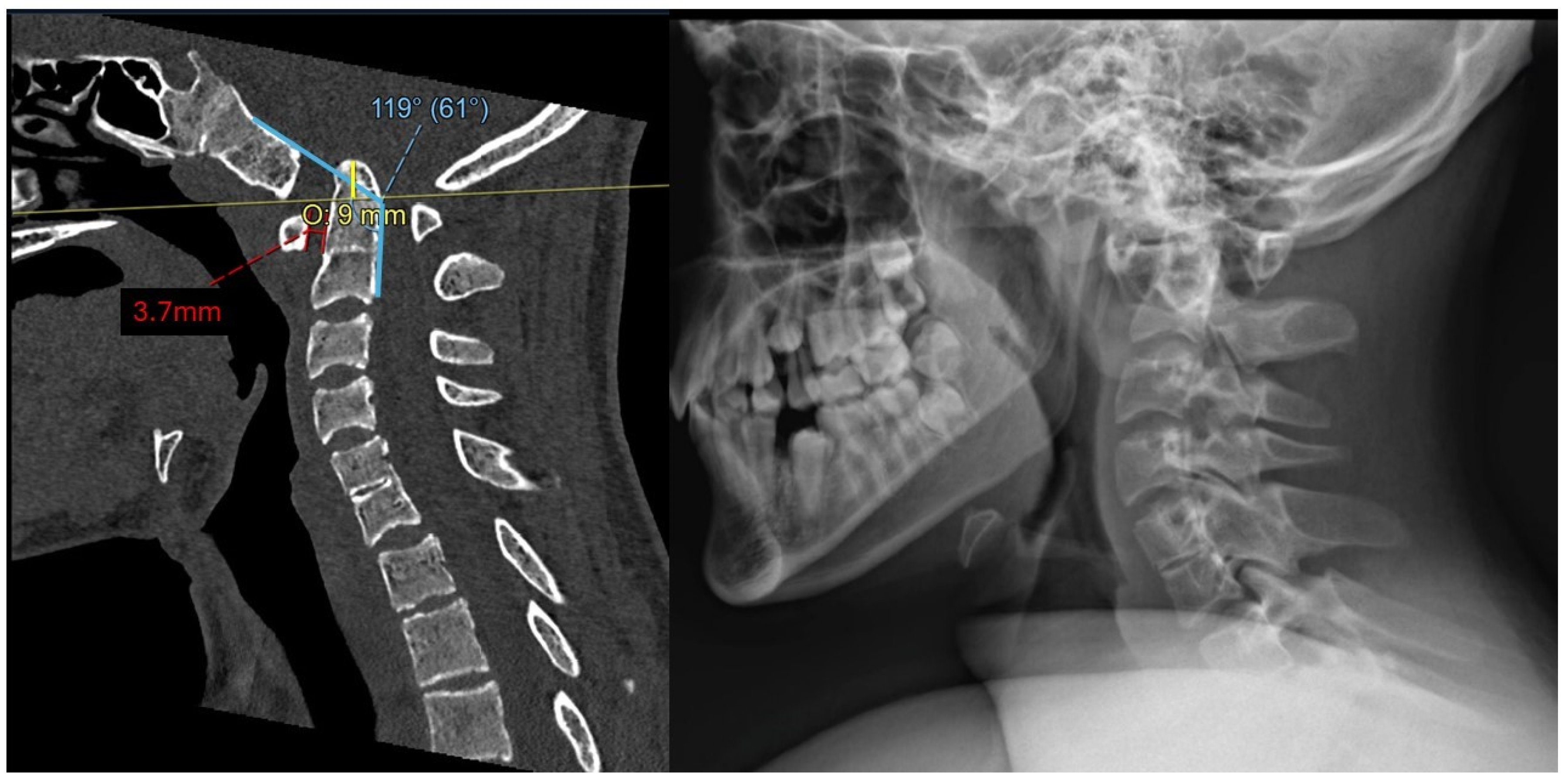
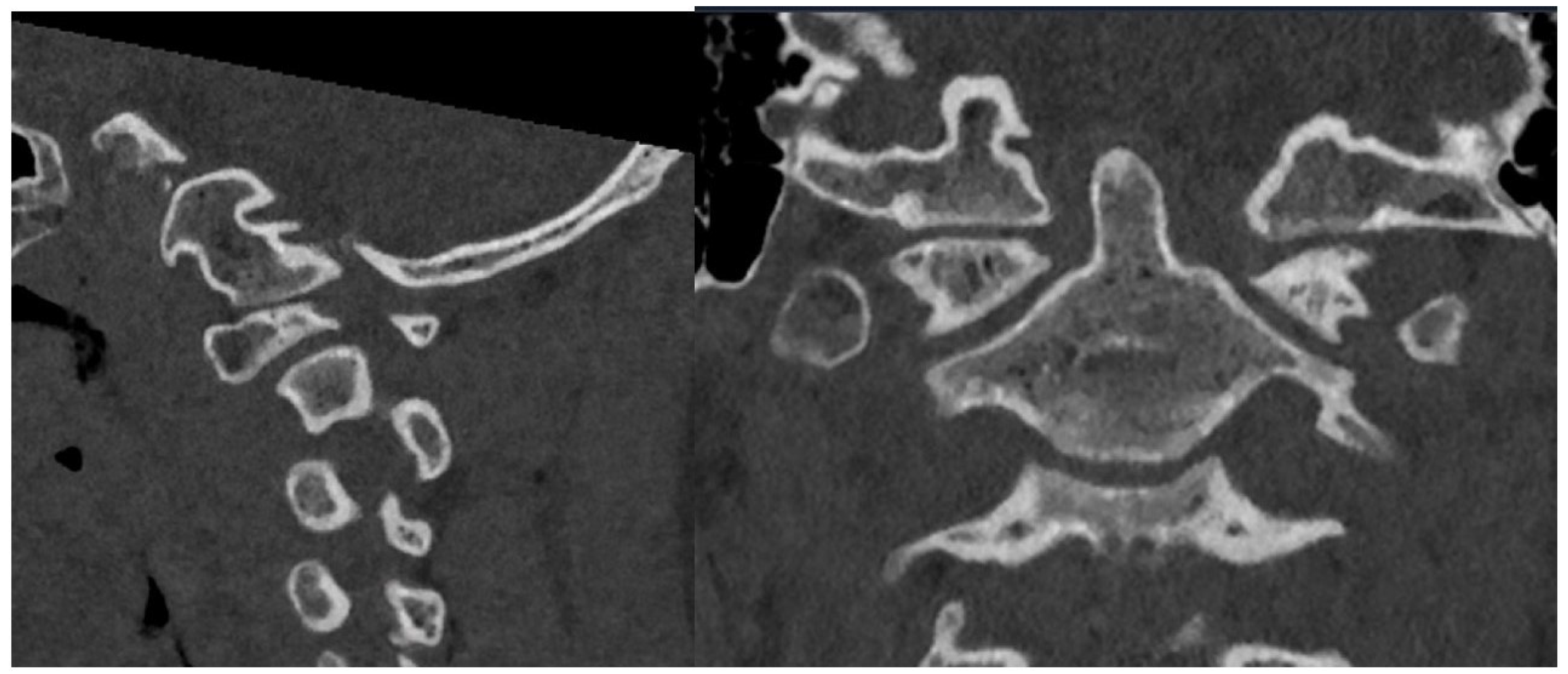
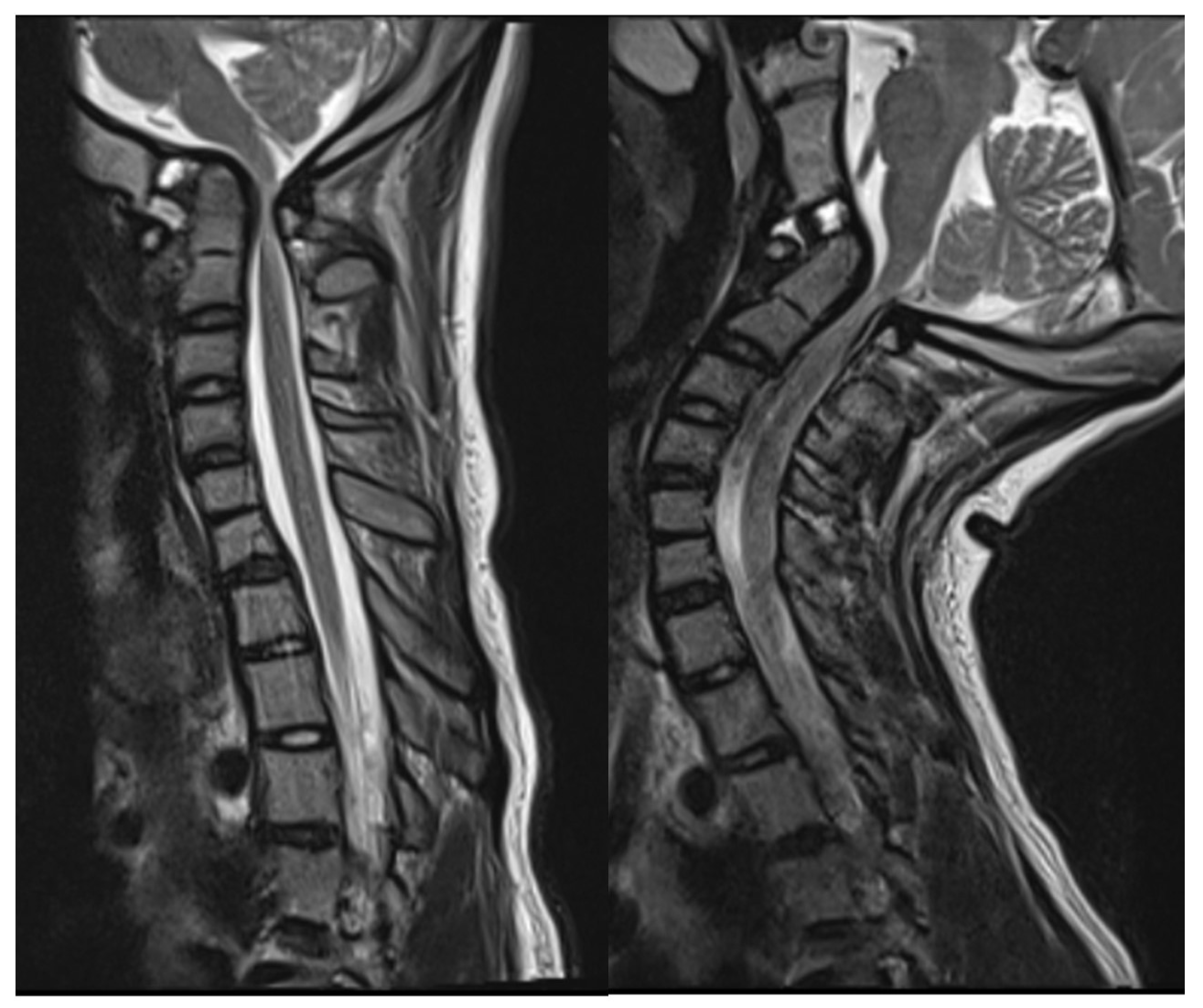
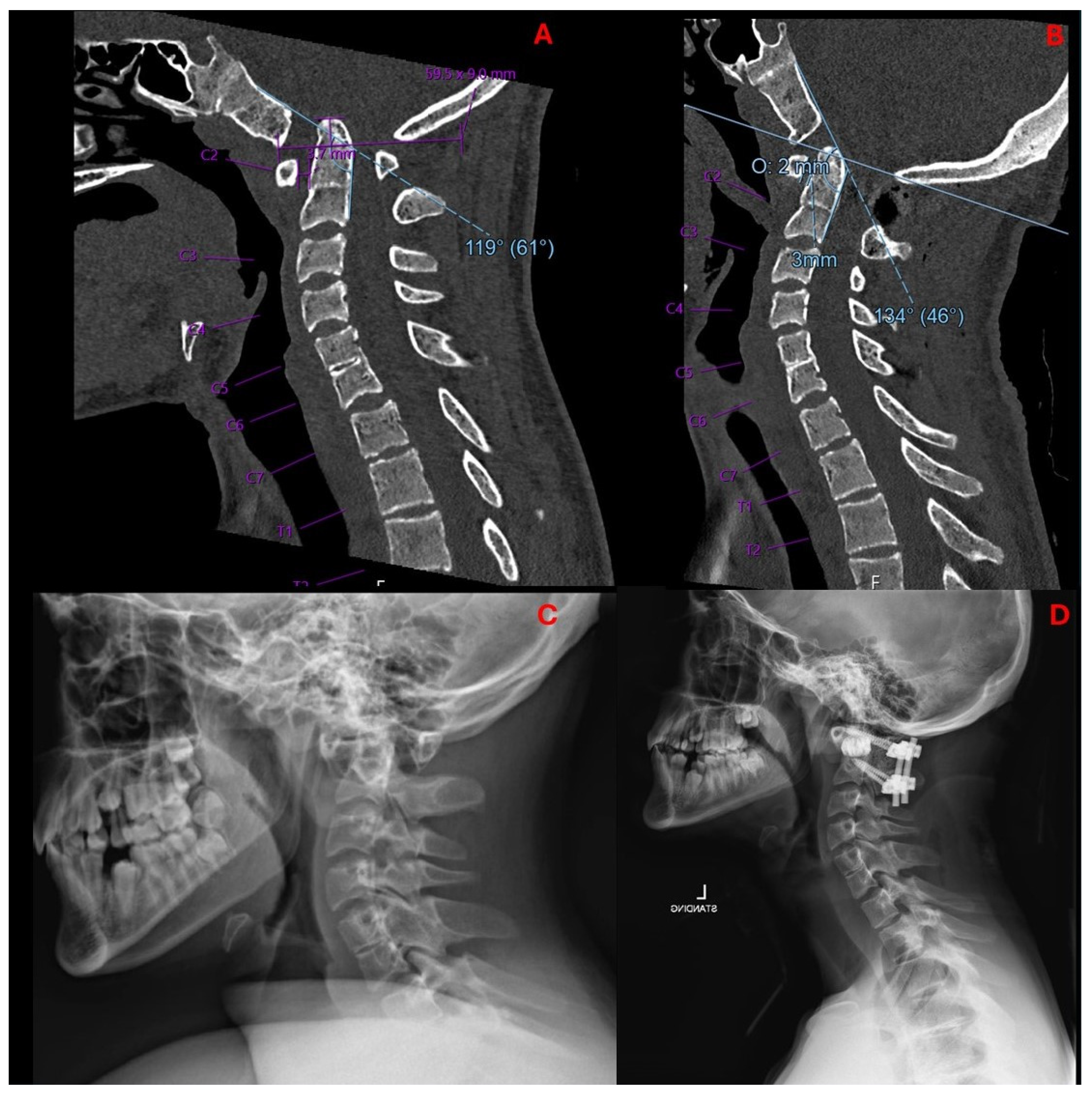
2.2. Imaging and Diagnosis
3. Materials and Methods
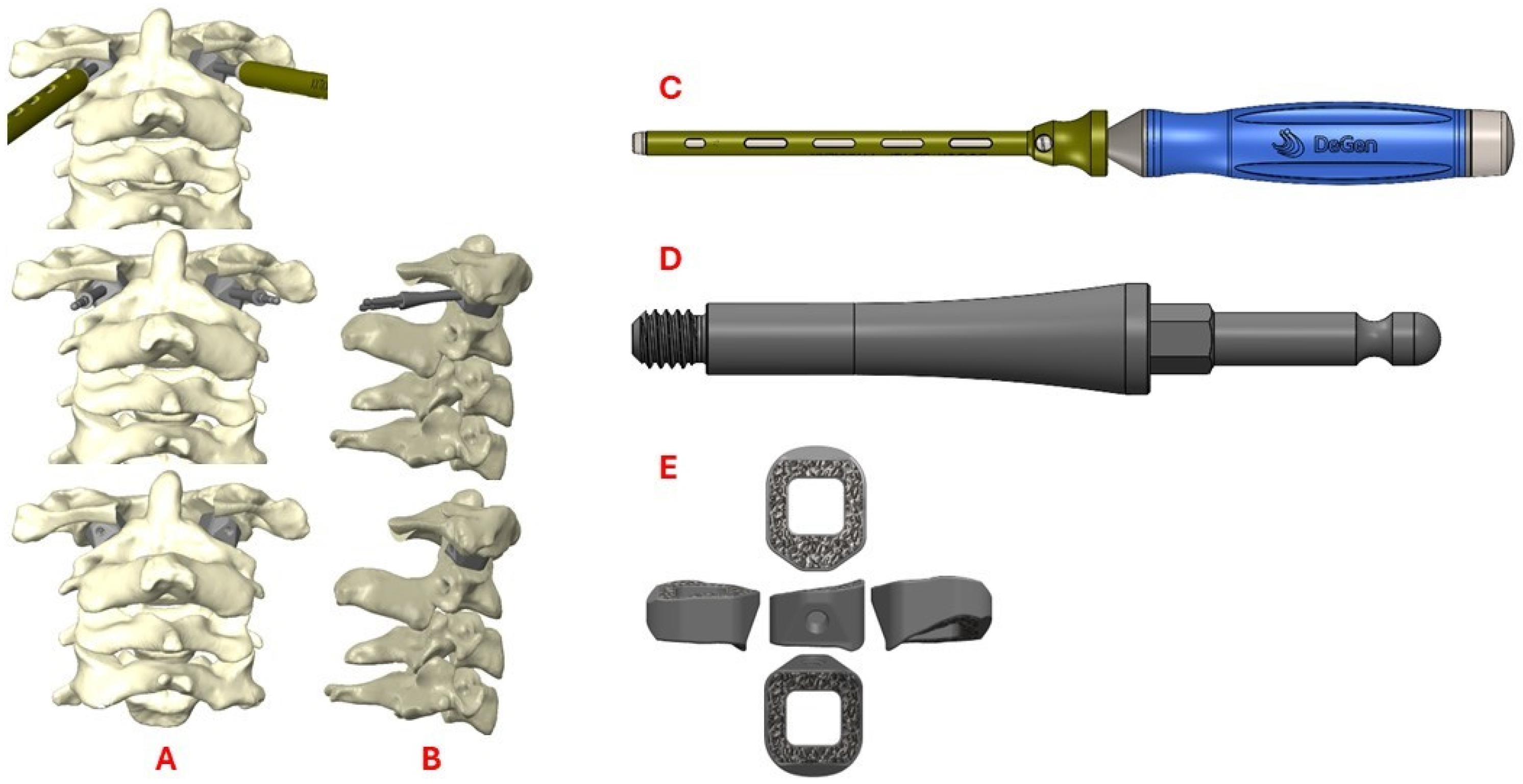
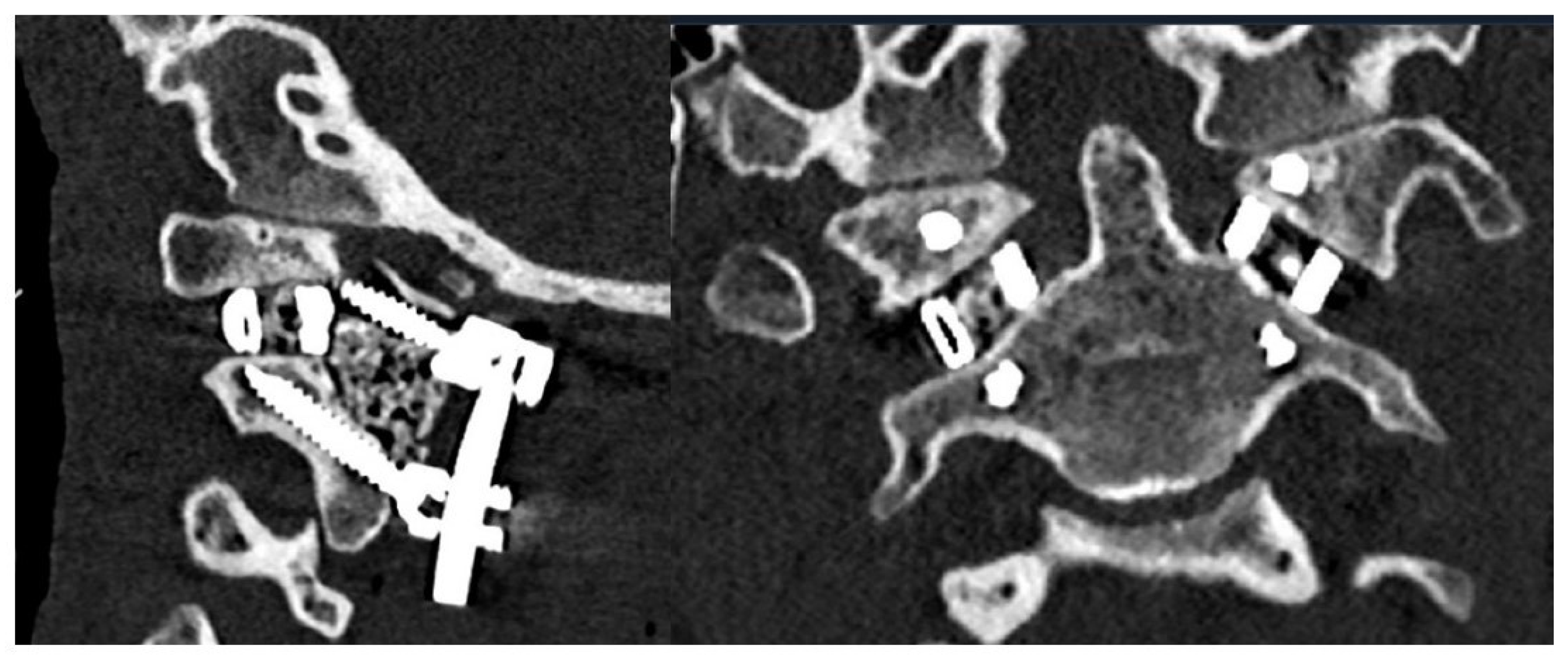
3.1. Preoperative Planning and 3DPI Design
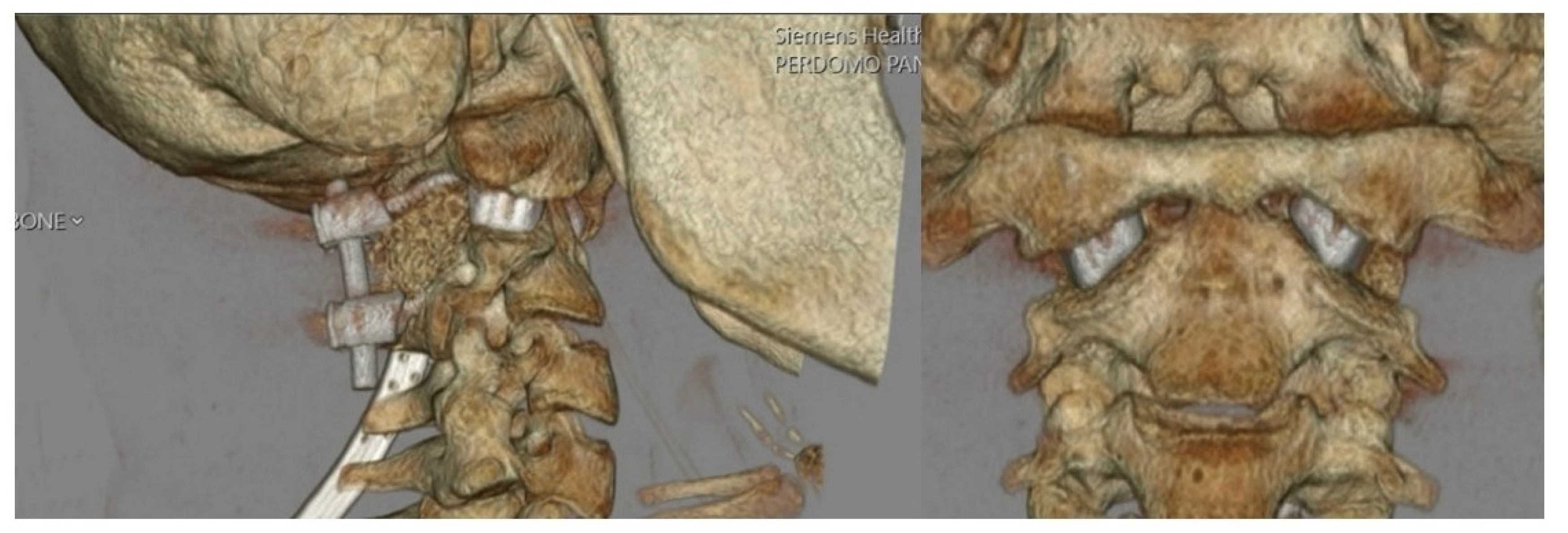
3.2. Surgical Approach
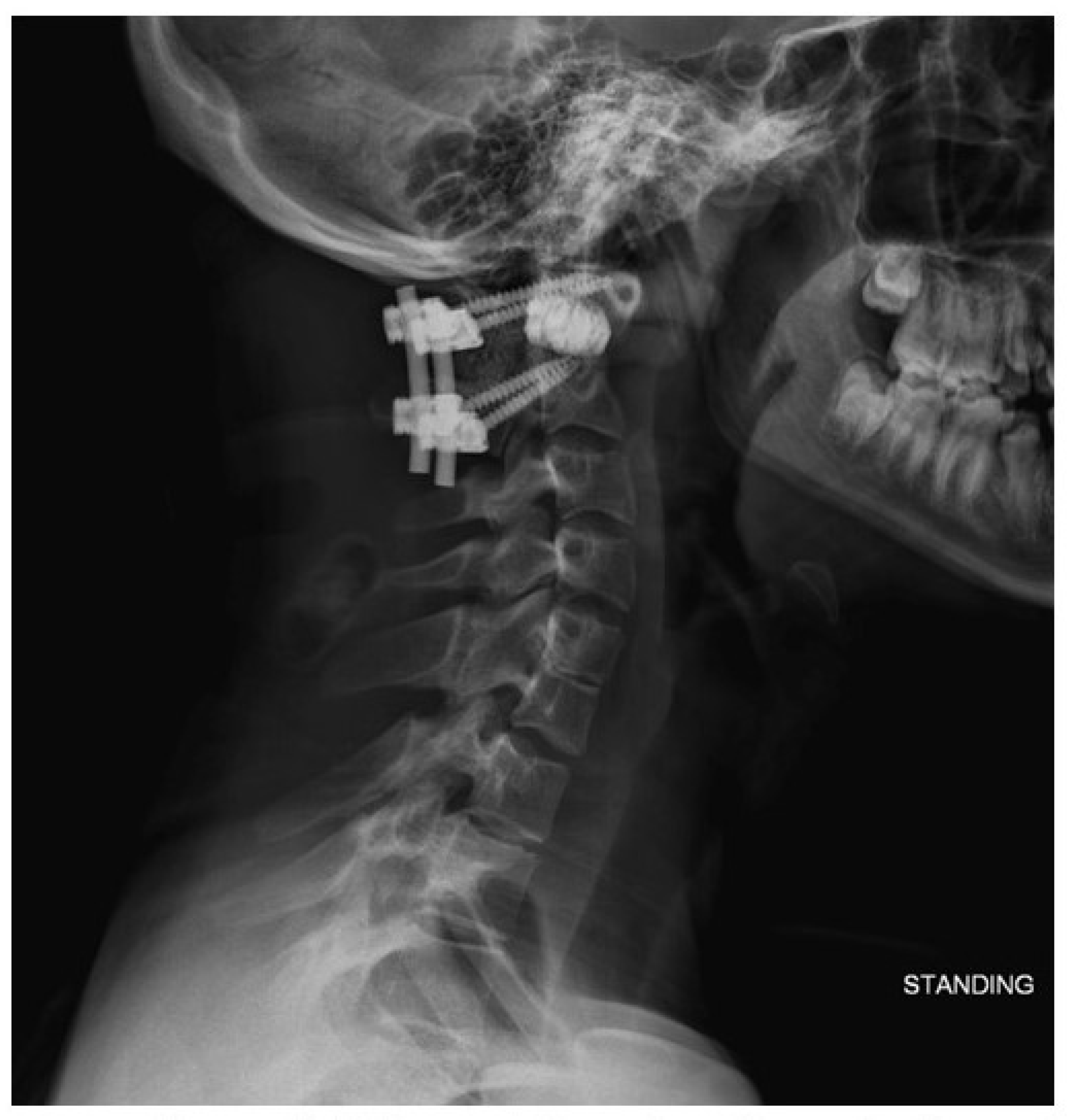
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3DPI | Three-Dimensional Printed Custom Implants |
| ADI | Atlantodental Interval |
| BI | Basilar Invagination |
| BMP-2 | Bone Morphogenic Protein 2 |
| CAD | Computer-Aided Design |
| CT | Computed Tomography |
| CVJ | Craniovertebral Junction |
| CXA | Clivo-axial Angle |
| DICOM | Digital Imaging and Communications in Medicine |
| MDPI | Multidisciplinary Digital Publishing Institute |
| MEP | Motor Evoked Potential |
| MLV | McRae’s Line Violation |
| MRI | Magnetic Resonance Imaging |
| SSEP | Somatosensory Evoked Potential |
References
- Donnally, I.C.; Munakomi, S.; Varacallo, M.A. Basilar Invagination. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Goel, A.; Bhatjiwale, M.; Desai, K. Basilar invagination: A study based on 190 surgically treated patients. J. Neurosurg. 1998, 88, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Botelho, R.V.; Ferreira, E.D. Angular craniometry in craniocervical junction malformation. Neurosurg. Rev. 2013, 36, 603–610, discussion 610. [Google Scholar] [CrossRef] [PubMed]
- Klekamp, J. Surgery for basilar invagination with and without Chiari I malformation. Neurosurg. Focus. 2023, 54, E11. [Google Scholar] [CrossRef] [PubMed]
- Joitescu, I.; Amelot, A.; Lot, G.; Aghakhani, N.; Parker, F.; Knafo, S. A Staged Approach for Surgical Management of Basilar Invagination. Oper. Neurosurg. 2024, 27, 424–430. [Google Scholar] [CrossRef]
- Zou, X.; Ouyang, B.; Yang, H.; Wang, B.; Ge, S.; Chen, Y.; Ni, L.; Zhang, S.; Xia, H.; Yang, J.; et al. Surgical treatment for basilar invagination with irreducible atlantoaxial dislocation: Transoral atlantoaxial reduction plate fixation vs occipitocervical fixation. BMC Musculoskelet. Disord. 2020, 21, 825. [Google Scholar] [CrossRef]
- Botelho, R.V.; Alves, O.L.; Sá Carneiro, G.; Chen, Z.; Yaman, O.; Parthiban, J.; Visocchi, M.; Klekamp, J.; Goel, A.; Zileli, M. Surgical Treatment of Basilar Invagination: WFNS Spine Committee Recommendations. Spine 2025, 50, 751–759. [Google Scholar] [CrossRef]
- Patel, R.; Solanki, A.M.; Acharya, A. Surgical outcomes of posterior occipito-cervical decompression and fusion for basilar invagination: A prospective study. J. Clin. Orthop. Trauma 2021, 13, 127–133. [Google Scholar] [CrossRef]
- Sheha, E.D.; Gandhi, S.D.; Colman, M.W. 3D printing in spine surgery. Ann. Transl. Med. 2019, 7 (Suppl. S5), S164. [Google Scholar] [CrossRef]
- Yahanda, A.T.; Barot, K.; Ruiz-Cardozo, M.A.; Pet, M.A.; English, I.; Ohman, J.W.; Sanchez, L.A.; Hunt, S.R.; Brogan, D.M.; O’Keefe, R.J.; et al. Rapid Manufacturing, Regulatory Approval, and Utilization of Patient-specific 3D-Printed Titanium Implants for Complex Multistage Spinal Surgeries. Glob. Spine J. 2025, 21925682251321787. [Google Scholar] [CrossRef]
- Ruiz-Cardozo, M.A.; Trevino, G.; Pando, A.; Brehm, S.; Olufawo, M.; Barot, K.; Carey-Ewend, A.; Yahanda, A.T.; Perdomo-Pantoja, A.; Jauregui, J.J.; et al. Rapid Implementation of a 3-Dimensional–Printed Patient-Specific Titanium Sacrum Implant for Severe Neuropathic Spinal Arthropathy and Guide to Compassionate US Regulatory Approval. Oper. Neurosurg. 2023, 25, 469–477. [Google Scholar] [CrossRef]
- Jian, Q.; Qin, S.; Hou, Z.; Zhao, X.; Liang, C.; Fan, T. Individualized C1-2 intra-articular three-dimensional printed porous titanium alloy cage for craniovertebral deformity. J. Orthop. Surg. Res. 2024, 19, 569. [Google Scholar] [CrossRef] [PubMed]
- Mensah, E.O.; Chalif, J.I.; Baker, J.G.; Chalif, E.; Biundo, J.; Groff, M.W. Challenges in Contemporary Spine Surgery: A Comprehensive Review of Surgical, Technological, and Patient-Specific Issues. J. Clin. Med. 2024, 13, 5460. [Google Scholar] [CrossRef] [PubMed]
- Molina, C.A.; Dibble, C.F.; Lo, S.L.; Witham, T.; Sciubba, D.M. Augmented reality-mediated stereotactic navigation for execution of en bloc lumbar spondylectomy osteotomies. J. Neurosurg. Spine 2021, 34, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Kann, M.R.; Ruiz-Cardozo, M.A.; Brehm, S.; Bui, T.; Joseph, K.; Barot, K.; Trevino, G.; Carey-Ewend, A.; Singh, S.P.; De La Paz, M.; et al. Utilization of Augmented Reality Head-Mounted Display for the Surgical Management of Thoracolumbar Spinal Trauma. Medicina 2024, 60, 281. [Google Scholar] [CrossRef]
- Hajnal, B.; Pokorni, A.J.; Turbucz, M.; Bereczki, F.; Bartos, M.; Lazary, A.; Eltes, P.E. Clinical applications of 3D printing in spine surgery: A systematic review. Eur. Spine J. 2025, 34, 454–471. [Google Scholar] [CrossRef]
- Wallace, N.; Schaffer, N.E.; Aleem, I.S.; Patel, R. 3D-printed Patient-specific Spine Implants: A Systematic Review. Clin. Spine Surg. 2020, 33, 400–407. [Google Scholar] [CrossRef]
- Neuman, B.J.; Harris, A.B.; Klineberg, E.O.; Hostin, R.A.; Protopsaltis, T.S.; Passias, P.G.; Gum, J.L.; Hart, R.A.; Kelly, M.P.; Daniels, A.H.; et al. Defining a Surgical Invasiveness Threshold for Increased Risk of a Major Complication Following Adult Spinal Deformity Surgery. Spine 2021, 46, 931–938. [Google Scholar] [CrossRef]
- Cizik, A.M.; Lee, M.J.; Martin, B.I.; Bransford, R.J.; Bellabarba, C.; Chapman, J.R.; Mirza, S.K. Using the spine surgical invasiveness index to identify risk of surgical site infection: A multivariate analysis. J. Bone Jt. Surg. Am. 2012, 94, 335–342. [Google Scholar] [CrossRef]
- Chatain, G.P.; Chee, K.; Finn, M. Review of transoral odontoidectomy. Where do we stand? Technical note and a single-center experience. Interdiscip. Neurosurg. 2022, 29, 101549. [Google Scholar] [CrossRef]
- Goel, A.; Shah, A.; Jadhav, M.; Nama, S. Distraction of facets with intraarticular spacers as treatment for lumbar canal stenosis: Report on a preliminary experience with 21 cases. J. Neurosurg. Spine 2013, 19, 672–677. [Google Scholar] [CrossRef]
- Niu, G.; Chen, H.; Liu, L.; Zhou, G.; Zhou, Q.; Li, C.; Dai, J.; Nie, H.; Bai, J.; Zhang, J. Surgical treatment for upper cervical deformity with atlantoaxial joint dislocation using individualized 3D printing occipitocervical fusion instrument: A case report and literature review. Medicine 2021, 100, e25202. [Google Scholar] [CrossRef] [PubMed]
- Parisien, A.; Wai, E.K.; ElSayed, M.S.A.; Frei, H. Subsidence of Spinal Fusion Cages: A Systematic Review. Int. J. Spine Surg. 2022, 16, 1103–1118. [Google Scholar] [CrossRef] [PubMed]
- Cofano, F.; Sciarrone, G.J.; Pecoraro, M.F.; Marengo, N.; Ajello, M.; Penner, F.; Petrone, S.; Ducati, A.; Zenga, F.; Musso, C.; et al. Cervical Interfacet Spacers to Promote Indirect Decompression and Enhance Fusion in Degenerative Spine: A Review. World Neurosurg. 2019, 126, 447–452. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, T.T.; Yahanda, A.T.; Joseph, K.; Ruiz-Cardozo, M.; de Monaco, B.A.; Perdomo-Pantoja, A.; Koleske, J.P.; McEvoy, S.D.; Molina, C.A. Using Patient-Specific 3D-Printed C1–C2 Interfacet Spacers for the Treatment of Type 1 Basilar Invagination: A Clinical Case Report. Biomimetics 2025, 10, 408. https://doi.org/10.3390/biomimetics10060408
Bui TT, Yahanda AT, Joseph K, Ruiz-Cardozo M, de Monaco BA, Perdomo-Pantoja A, Koleske JP, McEvoy SD, Molina CA. Using Patient-Specific 3D-Printed C1–C2 Interfacet Spacers for the Treatment of Type 1 Basilar Invagination: A Clinical Case Report. Biomimetics. 2025; 10(6):408. https://doi.org/10.3390/biomimetics10060408
Chicago/Turabian StyleBui, Tim T., Alexander T. Yahanda, Karan Joseph, Miguel Ruiz-Cardozo, Bernardo A. de Monaco, Alexander Perdomo-Pantoja, Joshua P. Koleske, Sean D. McEvoy, and Camilo A. Molina. 2025. "Using Patient-Specific 3D-Printed C1–C2 Interfacet Spacers for the Treatment of Type 1 Basilar Invagination: A Clinical Case Report" Biomimetics 10, no. 6: 408. https://doi.org/10.3390/biomimetics10060408
APA StyleBui, T. T., Yahanda, A. T., Joseph, K., Ruiz-Cardozo, M., de Monaco, B. A., Perdomo-Pantoja, A., Koleske, J. P., McEvoy, S. D., & Molina, C. A. (2025). Using Patient-Specific 3D-Printed C1–C2 Interfacet Spacers for the Treatment of Type 1 Basilar Invagination: A Clinical Case Report. Biomimetics, 10(6), 408. https://doi.org/10.3390/biomimetics10060408





