The Effects of the Substrate Length and Cultivation Time on the Physical and Mechanical Properties of Mycelium-Based Cushioning Materials from Salix psammophila and Peanut Straw
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Mycelium-Based Cushioning Materials
2.3. Morphological Characterization of Mycelium-Based Cushioning Materials
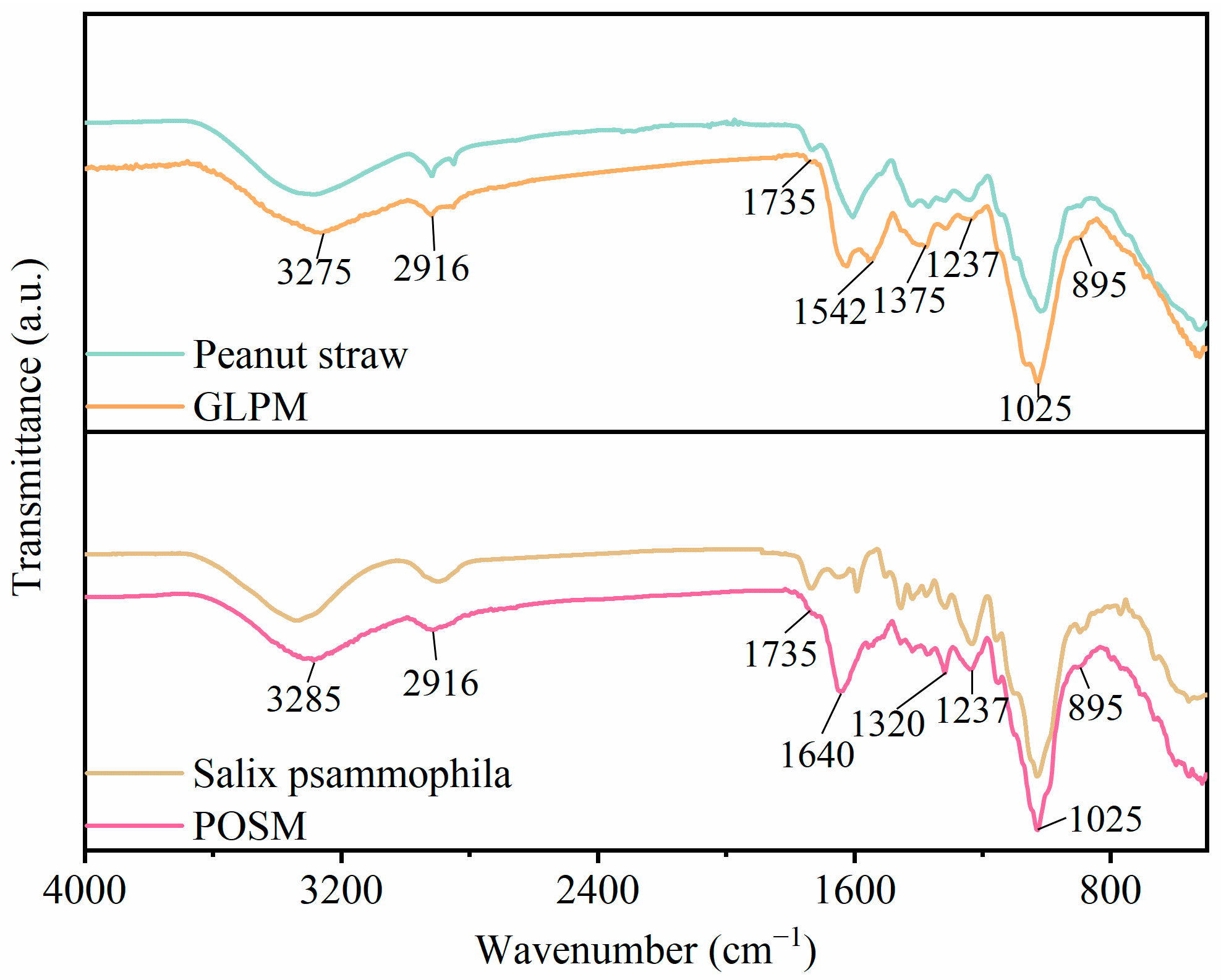
2.4. Infrared Spectroscopy Testing
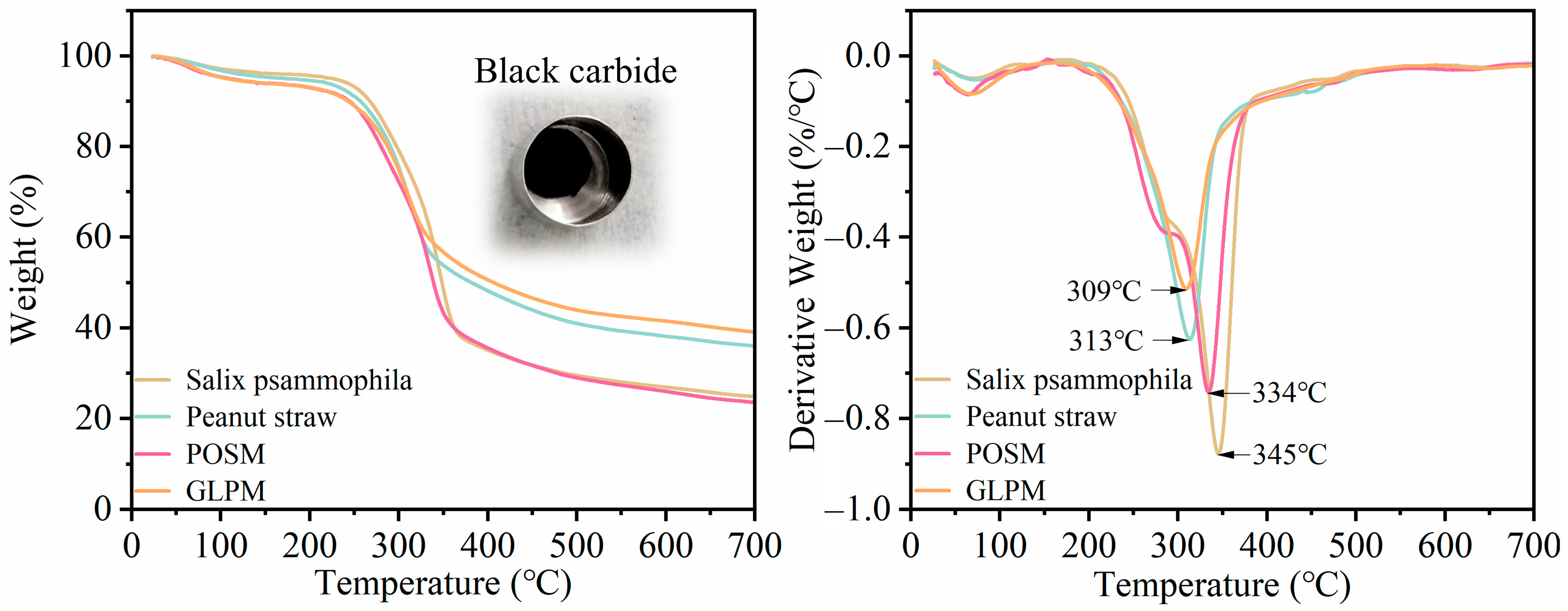
2.5. Thermal Stability Testing
2.6. Density
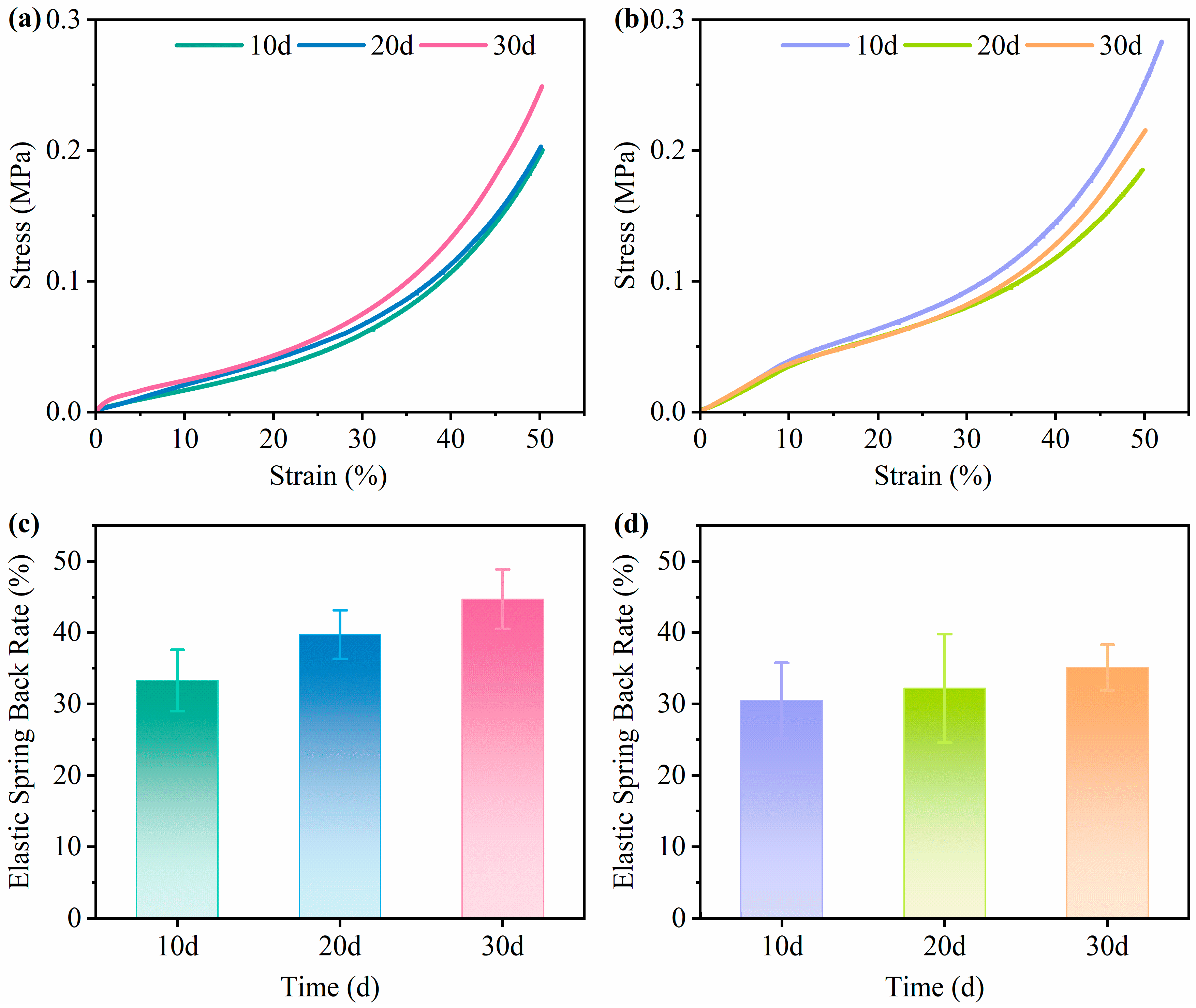
2.7. Compressive Strength Testing
2.8. Elastic Spring Back Rate Testing
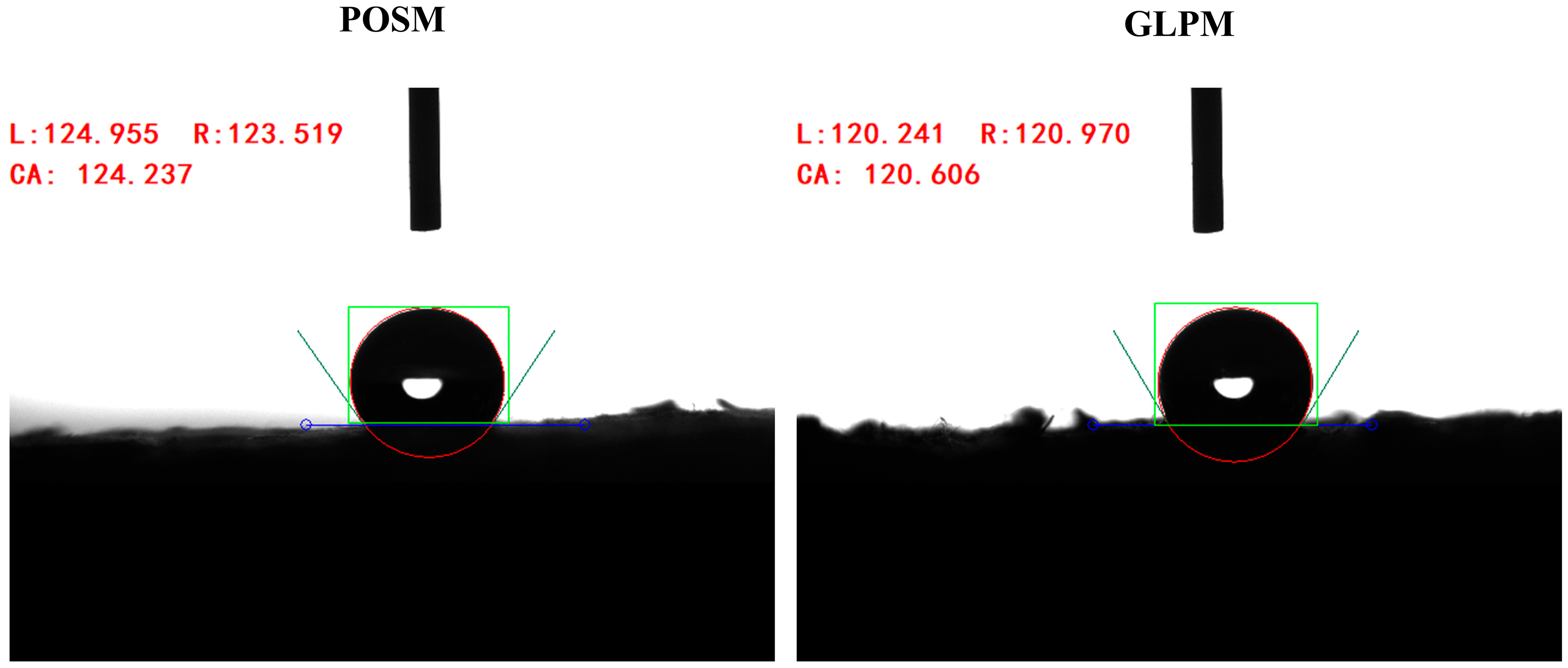
2.9. Water Contact Angle Measurement
2.10. Thermal Conductivity Measurement
2.11. Statistical Analysis
3. Results and Discussion
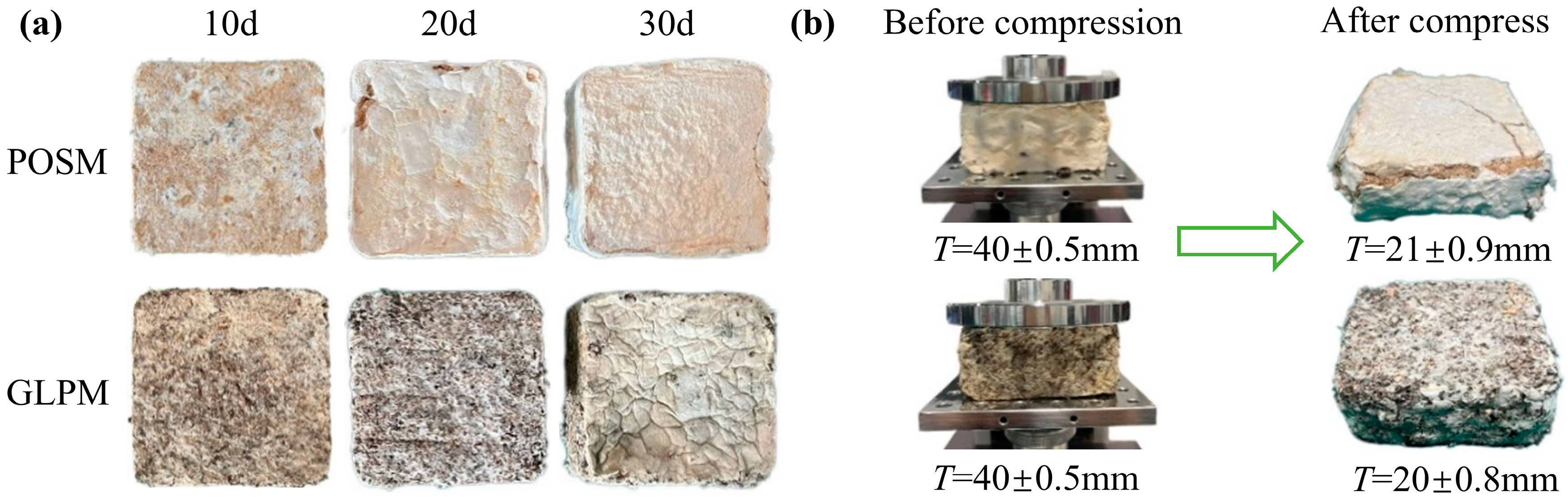
3.1. Morphological Analysis of Mycelium-Based Cushioning Materials
3.2. Sample Functional Group Analysis
3.3. Thermal Performance Analysis
3.4. Mechanical Performance Analysis
3.4.1. Effects of Cultivation Time on the Performance of Mycelium-Based Cushioning Materials
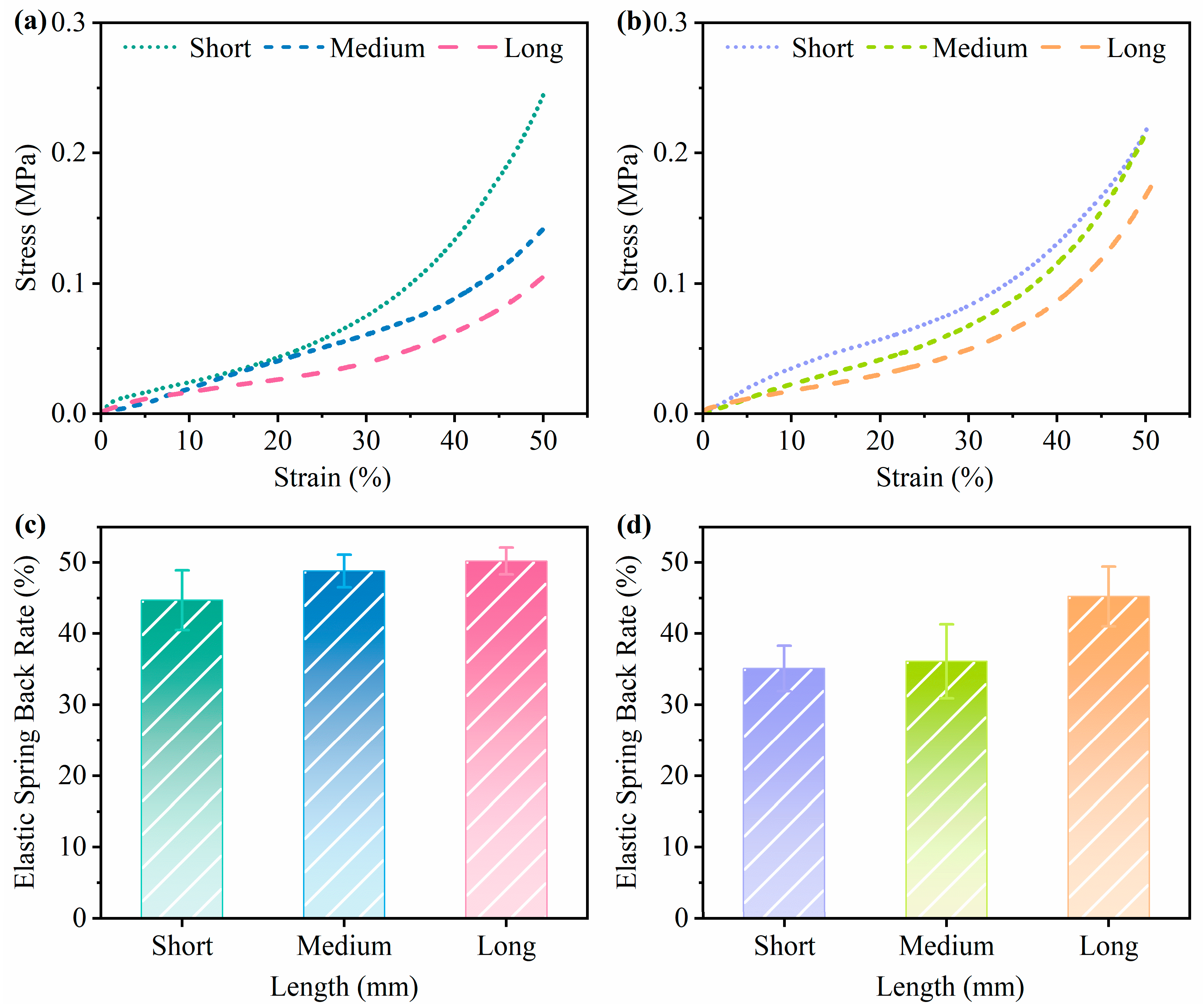
3.4.2. Effects of Substrate Length on the Performance of Mycelium-Based Cushioning Materials
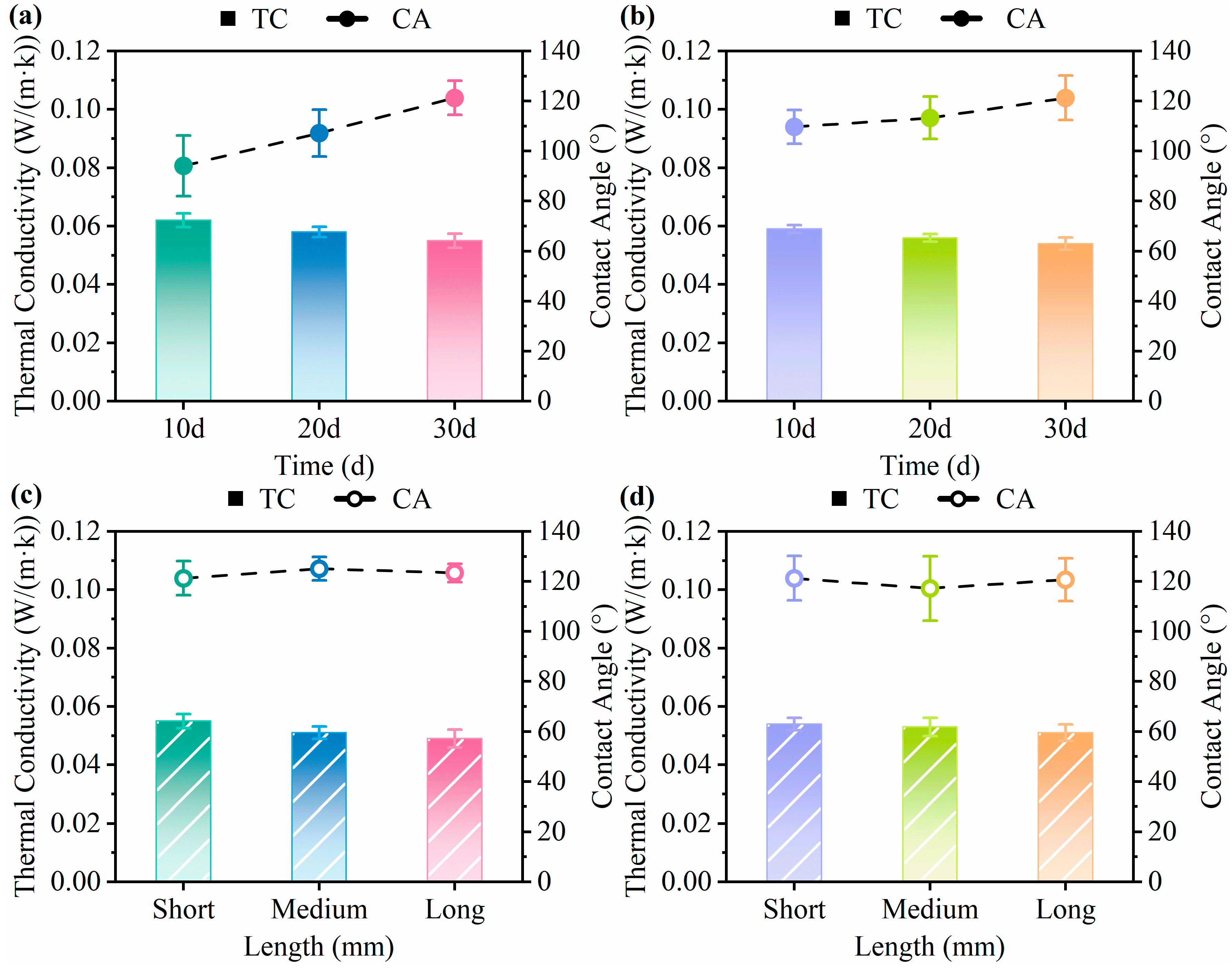
3.5. Water Contact Angle and Thermal Conductivity Analysis
4. Conclusions
- Mycelium-based cushioning material performance varies with the lignocellulose type and fungal species, with POSM outperforming GLPM overall.
- Mycelium-based cushioning materials exhibit thermal degradation profiles similar to those of synthetic foams and relatively high thermal stability.
- Longer cultivation improved the physical and mechanical properties, particularly surface hydrophobicity, as a result of the development of a mycelial biofilm. Overall, incubating the fungi for 30 days yielded superior performance. However, further cultivation could degrade the mechanical strength.
- Increasing the substrate length improved most performance metrics of the mycelium-based cushioning materials. However, samples made with larger substrate particles exhibited lower compressive strengths.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, J.; Liu, H.; Shen, D.; Gou, J.; Meng, X.; Sun, X. Degradable Foaming Material and Its Application in Cushion Packaging. Green Packag. 2017, 2, 41–47. [Google Scholar] [CrossRef]
- Huang, D.; Cai, X. Overview on Cushioning Packaging Materials for Refrigerators. Electr. Appl. 2020, 63, 31–35. [Google Scholar]
- Liu, J.; Zhao, X.; Weng, Y. Development and Application of Biobased Materials for Food Packaging. Packag. Eng. 2023, 44, 19–26. [Google Scholar] [CrossRef]
- Elkaliny, N.E.; Alzamel, N.M.; Moussa, S.H.; Elodamy, N.I.; Madkor, E.A.; Ibrahim, E.M.; Elshobary, M.E.; Ismail, G.A. Macroalgae Bioplastics: A Sustainable Shift to Mitigate the Ecological Impact of Petroleum-Based Plastics. Polymers 2024, 16, 1246. [Google Scholar] [CrossRef]
- Froese, R. Insulating properties of styrofoam boxes used for transporting live fish. Aquaculture 1998, 159, 283–292. [Google Scholar] [CrossRef]
- Lopez Nava, J.A.; Mendez Gonzalez, J.; Ruelas Chacon, X.; Najera Luna, J.A. Assessment of Edible Fungi and Films Bio-Based Material Simulating Expanded Polystyrene. Mater. Manuf. Process. 2016, 31, 1085–1090. [Google Scholar] [CrossRef]
- Wan, Z.; Han, S.; Song, W. Research progress on application of polyolefin elastomers in resin modification. Petrochem. Technol. 2025, 54, 262–268. [Google Scholar]
- Amaraweera, S.M.; Gunathilake, C.; Gunawardene, O.H.P.; Dassanayake, R.S.; Fernando, N.M.L.; Wanninayaka, D.B.; Rajapaksha, S.M.; Manamperi, A.; Gangoda, M.; Manchanda, A.; et al. Preparation and Characterization of Dual-Modified Cassava Starch-Based Biodegradable Foams for Sustainable Packaging Applications. ACS Omega 2022, 7, 19579–19590. [Google Scholar] [CrossRef]
- Sivaprasad, S.; Byju, S.K.; Prajith, C.; Shaju, J.; Rejeesh, C.R. Development of a novel mycelium bio-composite material to substitute for polystyrene in packaging applications. In Proceedings of the International Conference on Sustainable Materials, Manufacturing and Renewable Technologies (I-SMaRT), Electr Network, Amsterdam, The Netherlands, 22–23 April 2021; pp. 5038–5044. [Google Scholar]
- Ramesh, M.; Palanikumar, K.; Reddy, K.H. Plant fibre based bio-composites: Sustainable and renewable green materials. Renew. Sust. Energ. Rev. 2017, 79, 558–584. [Google Scholar] [CrossRef]
- Pavel, S.; Supinit, V. Bangladesh invented bioplastic jute poly bag and international market potentials. Open J. Bus. Manag. 2017, 05, 624–640. [Google Scholar] [CrossRef]
- Thapliyal, D.; Karale, M.; Diwan, V.; Kumra, S.; Arya, R.K.; Verros, G.D. Current Status of Sustainable Food Packaging Regulations: Global Perspective. Sustainability 2024, 16, 5554. [Google Scholar] [CrossRef]
- Wu, Y.; Tang, R.; Guo, A.; Tao, X.; Hu, Y.; Sheng, X.; Qu, P.; Wang, S.; Li, J.; Li, F. Enhancing Starch-Based Packaging Materials: Optimization of Plasticizers and Process Parameters. Materials 2023, 16, 5953. [Google Scholar] [CrossRef]
- Li, J.; He, Z.; Song, W.; Sun, B.; Zhao, Y.; Zhang, Q.; Ma, X. Preparation of Hydrophobic Plant Fiber Active Foam Buffer Packaging Materials. Packag. Eng. 2024, 45, 12–18. [Google Scholar] [CrossRef]
- Peng, L.; Yi, J.; Yang, X.; Xie, J.; Chen, C. Development and characterization of mycelium bio-composites by utilization of different agricultural residual byproducts. J. Bioresour. Bioprod. 2023, 8, 78–89. [Google Scholar] [CrossRef]
- Jones, M.; Huynh, T.; Dekiwadia, C.; Daver, F.; John, S. Mycelium composites: A review of engineering characteristics and growth kinetics. J. Bionanoscience 2017, 11, 241–257. [Google Scholar] [CrossRef]
- Arifin, Y.H.; Yusuf, Y. Mycelium Fibers as New Resource For Environmental Sustainability. In Proceedings of the Malaysian-Technical-Universities Conference on Engineering and Technology (MUCET), Kangar Perlis, Malaysia, 20–21 November 2012; pp. 504–508. [Google Scholar]
- Cerimi, K.; Akkaya, K.C.; Pohl, C.; Schmidt, B.; Neubauer, P. Fungi as source for new bio-based materials: A patent review. Fungal Biol. Biotechnol. 2019, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Park, D.; Qin, Z. Material Function of Mycelium-Based Bio-Composite: A Review. Front. Mater. 2021, 8, 737377. [Google Scholar] [CrossRef]
- Aiduang, W.; Jatuwong, K.; Luangharn, T.; Jinanukul, P.; Thamjaree, W.; Teeraphantuvat, T.; Waroonkun, T.; Lumyong, S. A Review Delving into the Factors Influencing Mycelium-Based Green Composites (MBCs) Production and Their Properties for Long-Term Sustainability Targets. Biomimetics 2024, 9, 337. [Google Scholar] [CrossRef]
- Enarevba, D.R.; Haapala, K.R. A comparative life cycle assessment of expanded polystyrene and mycelium packaging box inserts. Procedia CIRP 2023, 116, 654–659. [Google Scholar] [CrossRef]
- McBee, R.M.; Lucht, M.; Mukhitov, N.; Richardson, M.; Srinivasan, T.; Meng, D.; Chen, H.; Kaufman, A.; Reitman, M.; Munck, C. Engineering living and regenerative fungal–bacterial biocomposite structures. Nat. Mater. 2022, 21, 471–478. [Google Scholar] [CrossRef]
- Verma, N.; Jujjavarapu, S.E.; Mahapatra, C. Green sustainable biocomposites: Substitute to plastics with innovative fungal mycelium based biomaterial. J. Environ. Chem. Eng. 2023, 11, 110396. [Google Scholar] [CrossRef]
- Irbe, I.; Loris, G.D.; Filipova, I.; Andze, L.; Skute, M. Characterization of self-growing biomaterials made of fungal mycelium and various lignocellulose-containing ingredients. Materials 2022, 15, 7608. [Google Scholar] [CrossRef]
- Pickering, K.L.; Efendy, M.A.; Le, T.M. A review of recent developments in natural fibre composites and their mechanical performance. Compos. Pt. A-Appl. Sci. Manuf. 2016, 83, 98–112. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, J.; Wu, G.; Song, P.; Du, G. A review of research on hemicellulose/lignin and its derivatives as 3D printing materials. New Chem. Mater. 2023, 51, 244–249. [Google Scholar] [CrossRef]
- Amstislavski, P.; Pöhler, T.; Valtonen, A.; Wikström, L.; Harlin, A.; Salo, S.; Jetsu, P.; Szilvay, G.R. Low-density, water-repellent, and thermally insulating cellulose-mycelium foams. Cellulose 2024, 31, 8769–8785. [Google Scholar] [CrossRef]
- Cai, J.; Han, J.; Ge, F.; Lin, Y.; Pan, J.; Ren, A.J.C.; Materials, B. Development of impact-resistant mycelium-based composites (MBCs) with agricultural waste straws. Constr. Build. Mater. 2023, 389, 131730. [Google Scholar] [CrossRef]
- Jones, M.; Mautner, A.; Luenco, S.; Bismarck, A.; John, S. Engineered mycelium composite construction materials from fungal biorefineries: A critical review. Mater. Des. 2020, 187, 108397. [Google Scholar] [CrossRef]
- Schritt, H.; Vidi, S.; Pleissner, D. Spent mushroom substrate and sawdust to produce mycelium-based thermal insulation composites. J. Clean Prod. 2021, 313, 127910. [Google Scholar] [CrossRef]
- Appels, F.V.W.; Camere, S.; Montalti, M.; Karana, E.; Jansen, K.M.B.; Dijksterhuis, J.; Krijgsheld, P.; Wosten, H.A.B. Fabrication factors influencing mechanical, moisture- and water-related properties of mycelium-based composites. Mater. Des. 2019, 161, 64–71. [Google Scholar] [CrossRef]
- Kuribayashi, T.; Lankinen, P.; Hietala, S.; Mikkonen, K.S. Dense and continuous networks of aerial hyphae improve flexibility and shape retention of mycelium composite in the wet state. Compos. Pt. A-Appl. Sci. Manuf. 2022, 152, 106688. [Google Scholar] [CrossRef]
- Bruscato, C.; Malvessi, E.; Brandalise, R.N.; Camassola, M. High performance of macrofungi in the production of mycelium-based biofoams using sawdust—Sustainable technology for waste reduction. J. Clean Prod. 2019, 234, 225–232. [Google Scholar] [CrossRef]
- Aiduang, W.; Kumla, J.; Srinuanpan, S.; Thamjaree, W.; Lumyong, S.; Suwannarach, N. Mechanical, Physical, and Chemical Properties of Mycelium-Based Composites Produced from Various Lignocellulosic Residues and Fungal Species. J. Fungi 2022, 8, 1125. [Google Scholar] [CrossRef]
- Elsacker, E.; Vandelook, S.; Brancart, J.; Peeters, E.; De Laet, L. Mechanical, physical and chemical characterisation of mycelium-based composites with different types of lignocellulosic substrates. PLoS ONE 2019, 14, e0213954. [Google Scholar] [CrossRef] [PubMed]
- Tacer-Caba, Z.; Varis, J.J.; Lankinen, P.; Mikkonen, K.S. Comparison of novel fungal mycelia strains and sustainable growth substrates to produce humidity-resistant biocomposites. Mater. Des. 2020, 192, 108728. [Google Scholar] [CrossRef]
- Liu, W.; Lu, J. Preparation of activated carbon with high specific surface area from Salix psammophila. New Chem. Mater. 2021, 49, 174–177. [Google Scholar] [CrossRef]
- Zhong, L.; Li, G.; Chen, G.; Wang, Y.; Chen, H.; WU, W.; Li, J.; Song, Y.; Yan, B. Research progress on the distribution characteristics of crop straws and the preparation and application of straw carbon-based fertilizers in China. J. Agric. Resour. Environ. 2022, 39, 575–585. [Google Scholar] [CrossRef]
- GB/T 8168-2008; Testing Method of Static Compression for Packaging Cushioning Materials. Standardization Administration of China (SAC): Beijing, China, 2008.
- GB/T 30693-2014; Measurement of Water Contact Angles of Plastic Films. Standardization Administration of China (SAC): Beijing, China, 2014.
- Hoa, H.T.; Wang, C.-L. The Effects of Temperature and Nutritional Conditions on Mycelium Growth of Two Oyster Mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2015, 43, 14–23. [Google Scholar] [CrossRef]
- Angelova, G.; Brazkova, M.; Stefanova, P.; Blazheva, D.; Vladev, V.; Petkova, N.; Slavov, A.; Denev, P.; Karashanova, D.; Zaharieva, R.; et al. Waste Rose Flower and Lavender Straw Biomass-An Innovative Lignocellulose Feedstock for Mycelium Bio-Materials Development Using Newly Isolated Ganoderma resinaceum GA1M. J. Fungi 2021, 7, 866. [Google Scholar] [CrossRef]
- Powrie, W.D.; Wu, C.H.; Molund, V.P. Browning reaction systems as sources of mutagens and antimutagens. Environ. Health Perspect. 1986, 67, 47–54. [Google Scholar] [CrossRef]
- Gelbrich, J.; Mai, C.; Militz, H. Evaluation of bacterial wood degradation by Fourier Transform Infrared (FTIR) measurements. J. Cult. Herit. 2012, 13, S135–S138. [Google Scholar] [CrossRef]
- Castro-Alves, V.C.; Gomes, D.; Menolli Jr, N.; Sforça, M.L.; do Nascimento, J.R.O. Characterization and immunomodulatory effects of glucans from Pleurotus albidus, a promising species of mushroom for farming and biomass production. Int. J. Biol. Macromol. 2017, 95, 215–223. [Google Scholar] [CrossRef]
- Alemu, D.; Tafesse, M.; Gudetta Deressa, Y. Production of mycoblock from the mycelium of the fungus Pleurotus ostreatus for use as sustainable construction materials. Adv. Mater. Sci. Eng. 2022, 2022, 2876643. [Google Scholar] [CrossRef]
- Haneef, M.; Ceseracciu, L.; Canale, C.; Bayer, I.S.; Heredia-Guerrero, J.A.; Athanassiou, A. Advanced Materials From Fungal Mycelium: Fabrication and Tuning of Physical Properties. Sci Rep 2017, 7, srep41292. [Google Scholar] [CrossRef]
- Elsacker, E.; Vandelook, S.; Van Wylick, A.; Ruytinx, J.; De Laet, L.; Peeters, E. A comprehensive framework for the production of mycelium-based lignocellulosic composites. Sci. Total Environ. 2020, 725, 138431. [Google Scholar] [CrossRef]
- Lankiewicz, T.S.; Choudhary, H.; Gao, Y.; Amer, B.; Lillington, S.P.; Leggieri, P.A.; Brown, J.L.; Swift, C.L.; Lipzen, A.; Na, H.; et al. Lignin deconstruction by anaerobic fungi. Nat. Microbiol. 2023, 8, 596–610. [Google Scholar] [CrossRef]
- Aiduang, W.; Jatuwong, K.; Jinanukul, P.; Suwannarach, N.; Kumla, J.; Thamjaree, W.; Teeraphantuvat, T.; Waroonkun, T.; Oranratmanee, R.; Lumyong, S. Sustainable Innovation: Fabrication and Characterization of Mycelium-Based Green Composites for Modern Interior Materials Using Agro-Industrial Wastes and Different Species of Fungi. Polymers 2024, 16, 550. [Google Scholar] [CrossRef]
- Ahmadi, H.; O’Keefe, A.; Bilek, M.A.; Korehei, R.; Sella Kapu, N.; Martinez, M.D.; Olson, J.A. Investigation of properties and applications of cellulose-mycelium foam. J. Mater. Sci. 2022, 57, 10167–10178. [Google Scholar] [CrossRef]
- Borsoi, C.; Scienza, L.C.; Zattera, A.J. Characterization of composites based on recycled expanded polystyrene reinforced with curaua fibers. J. Appl. Polym. Sci. 2012, 128, 653–659. [Google Scholar] [CrossRef]
- Jones, M.; Bhat, T.; Huynh, T.; Kandare, E.; Yuen, R.; Wang, C.H.; John, S. Waste-derived low-cost mycelium composite construction materials with improved fire safety. Fire Mater. 2018, 42, 816–825. [Google Scholar] [CrossRef]
- Liang, Y.; Xue, Z. Research on low temperature pyrolysis of Salix. For. Grassl. Mach. 2014, 25, 48–50+58. [Google Scholar] [CrossRef]
- Pan, Q.; Wu, D.; Shan, W.; Xiang, H. Preparation Technology and Optimization of Peanut Straw Fiberboard without Adhesive. Packag. Eng. 2020, 41, 127–134. [Google Scholar] [CrossRef]
- Majib, N.M.; Sam, S.T.; Yaacob, N.D.; Rohaizad, N.M.; Tan, W.K. Characterization of fungal foams from edible mushrooms using different agricultural wastes as substrates for packaging material. Polymers 2023, 15, 873. [Google Scholar] [CrossRef]
- Manan, S.; Ullah Muhammad, W.; Ul-Islam, M.; Atta Omar, M.; Yang, G. Synthesis and Applications of Fungal Mycelium-based Advanced Functional Materials. J. Bioresour. Bioprod. 2021, 6, 1–10. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Z.; Zhang, R.; Peng, Y.; Wang, M.; Cao, J. Lightweight, thermal insulation, hydrophobic mycelium composites with hierarchical porous structure: Design, manufacture and applications. Compos. Part B Eng. 2023, 266, 111003. [Google Scholar] [CrossRef]
- Ghazvinian, A.; Farrokhsiar, P.; Vieira, F.; Pecchia, J.; Gursoy, B. Mycelium-Based Bio-Composites For Architecture: Assessing the Effects of Cultivation Factors on Compressive Strength. In Proceedings of the 37th Conference on Education-and-Research-in-Computer-Aided-Architectural-Design-in-Europe (eCAADe)/23rd Conference of the Iberoamerican-Society-Digital-Graphics (SIGraDi), Univ Porto, Fac Architecture, Porto, Portugal, 11–13 September 2019; pp. 505–514. [Google Scholar]
- Aiduang, W.; Chanthaluck, A.; Kumla, J.; Jatuwong, K.; Srinuanpan, S.; Waroonkun, T.; Oranratmanee, R.; Lumyong, S.; Suwannarach, N. Amazing Fungi for Eco-Friendly Composite Materials: A Comprehensive Review. J. Fungi 2022, 8, 842. [Google Scholar] [CrossRef]
- Soh, E.; Chew, Z.Y.; Saeidi, N.; Javadian, A.; Hebel, D.; Le Ferrand, H. Development of an extrudable paste to build mycelium-bound composites. Mater. Des. 2020, 195, 109058. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, T.R. Contact angle and wetting properties. In Surface Science Techniques; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3–34. [Google Scholar]
- Zabihzadeh, S.M. Water uptake and flexural properties of natural filler/HDPE composites. BioResources 2010, 5, 316–323. [Google Scholar] [CrossRef]
- Sammer, D.; Krause, K.; Gube, M.; Wagner, K.; Kothe, E. Hydrophobins in the Life Cycle of the Ectomycorrhizal Basidiomycete Tricholoma vaccinum. PLoS ONE 2016, 11, e0167773. [Google Scholar] [CrossRef]
- Wessels, J.G.H. Hydrophobins: Proteins that change the nature of the fungal surface. In Advances in Microbial Physiology; Poole, R.K., Ed.; Advances in Microbial Physiology; Elsevier: Amsterdam, The Netherlands, 1997; Volume 38, pp. 1–45. [Google Scholar]
- Sun, W.; Tajvidi, M.; Howell, C.; Hunt, C.G. Functionality of Surface Mycelium Interfaces in Wood Bonding. ACS Appl. Mater. Interfaces 2020, 12, 57431–57440. [Google Scholar] [CrossRef] [PubMed]
- Izan, N.L.M.; Bahrin, E.K.; Yusoff, M.Z.M.; Simarani, K.; Sharip, N.S.; Ariffin, H. Sustainable utilisation of oil palm empty fruit bunch and Perenniporia subtephropora for eco-friendly mycelium-based biofoam. Biocatal. Agric. Biotechnol. 2024, 62, 103436. [Google Scholar] [CrossRef]
- Yan, W.; Yu, L.; Cao, C.; Shi, T. Study on fire performance of mycelium bio-foam. Fire Sci. Technol. 2021, 40, 1239–1242. [Google Scholar]
- Yan, M.; Fu, Y.; Pan, Y.; Cheng, X.; Gong, L.; Zhou, Y.; Ahmed, H.; Zhang, H. Highly elastic and fatigue resistant wood/silica composite aerogel operated at extremely low temperature. Compos. Part B Eng. 2022, 230, 109496. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, F.; Still, B.; White, M.; Amstislavski, P. Physical and Mechanical Properties of Fungal Mycelium-Based Biofoam. J. Mater. Civ. Eng. 2017, 29, 04017030. [Google Scholar] [CrossRef]


| Time (d) | POSM | GLPM | ||
|---|---|---|---|---|
| Density (g/cm3) | Compression Strength (MPa) | Density (g/cm3) | Compression Strength (MPa) | |
| 10 | 0.151 ± 0.0057 | 0.19 ± 0.012 | 0.153 ± 0.0037 | 0.27 ± 0.012 |
| 20 | 0.157 ± 0.0047 | 0.20 ± 0.022 | 0.155 ± 0.0042 | 0.17 ± 0.022 |
| 30 | 0.164 ± 0.0067 | 0.25 ± 0.017 | 0.163 ± 0.0060 | 0.21 ± 0.030 |
| Length (mm) | POSM | GLPM | ||
|---|---|---|---|---|
| Density (g/cm3) | Compression Strength (MPa) | Density (g/cm3) | Compression Strength (MPa) | |
| Short (2–4) | 0.164 ± 0.0067 | 0.25 ± 0.017 | 0.163 ± 0.0060 | 0.21 ± 0.030 |
| Medium (4–6) | 0.152 ± 0.0047 | 0.14 ± 0.020 | 0.158 ± 0.0074 | 0.20 ± 0.023 |
| Long (6–8) | 0.132 ± 0.0058 | 0.10 ± 0.035 | 0.133 ± 0.0038 | 0.17 ± 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Chen, S.; Wu, J.; Cai, Z.; Zhang, Y.; Na, R.; Lv, H.; He, C.; Wu, T.; Wang, X. The Effects of the Substrate Length and Cultivation Time on the Physical and Mechanical Properties of Mycelium-Based Cushioning Materials from Salix psammophila and Peanut Straw. Biomimetics 2025, 10, 371. https://doi.org/10.3390/biomimetics10060371
Song X, Chen S, Wu J, Cai Z, Zhang Y, Na R, Lv H, He C, Wu T, Wang X. The Effects of the Substrate Length and Cultivation Time on the Physical and Mechanical Properties of Mycelium-Based Cushioning Materials from Salix psammophila and Peanut Straw. Biomimetics. 2025; 10(6):371. https://doi.org/10.3390/biomimetics10060371
Chicago/Turabian StyleSong, Xiaowen, Shuoye Chen, Jianxin Wu, Ziyi Cai, Yanfeng Zhang, Risu Na, He Lv, Cong He, Tingting Wu, and Xiulun Wang. 2025. "The Effects of the Substrate Length and Cultivation Time on the Physical and Mechanical Properties of Mycelium-Based Cushioning Materials from Salix psammophila and Peanut Straw" Biomimetics 10, no. 6: 371. https://doi.org/10.3390/biomimetics10060371
APA StyleSong, X., Chen, S., Wu, J., Cai, Z., Zhang, Y., Na, R., Lv, H., He, C., Wu, T., & Wang, X. (2025). The Effects of the Substrate Length and Cultivation Time on the Physical and Mechanical Properties of Mycelium-Based Cushioning Materials from Salix psammophila and Peanut Straw. Biomimetics, 10(6), 371. https://doi.org/10.3390/biomimetics10060371






