Nanotopographical Features of Polymeric Nanocomposite Scaffolds for Tissue Engineering and Regenerative Medicine: A Review
Abstract
1. Introduction
2. Overview of Polymeric Nanocomposite Scaffolds
3. Cellular Responses to Nanotopography of Polymeric Nanocomposite Scaffolds
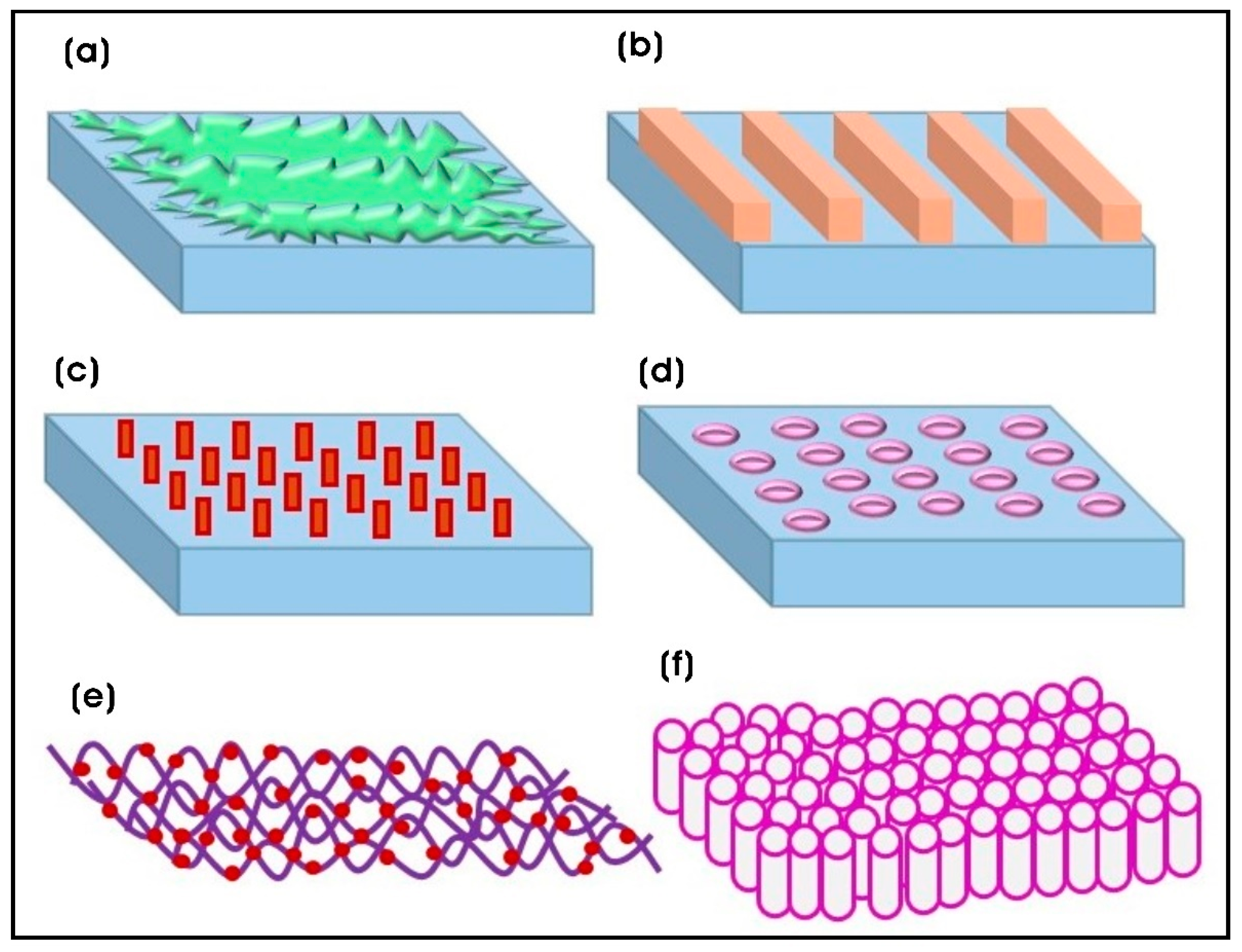
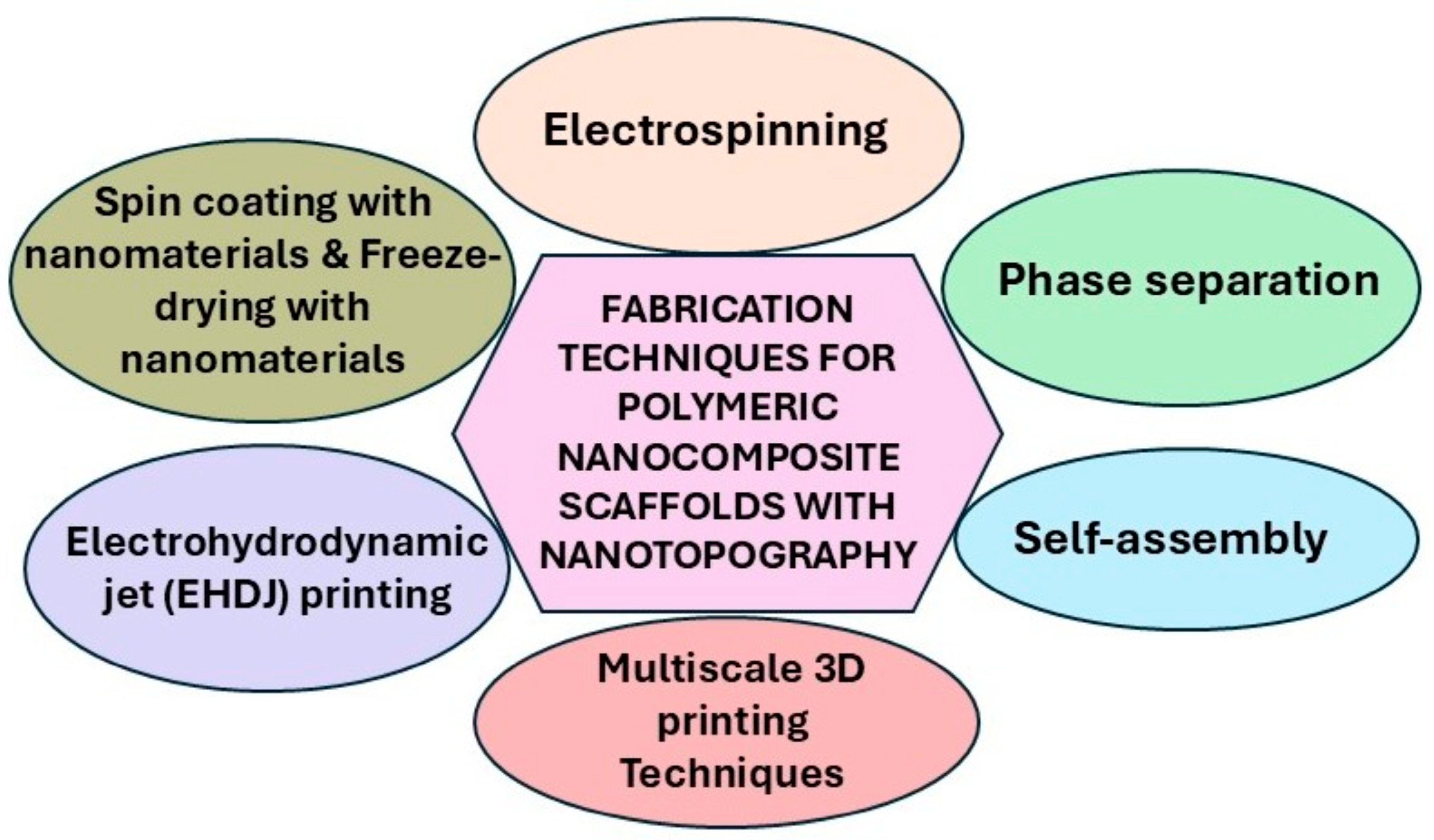
4. Fabrication Techniques of Polymeric Nanocomposite Scaffolds with Nanotopographies
| Fabrication Technique | Nanotopographical Dimension | Advantages | References |
|---|---|---|---|
| Electrohydrodynamic jet (EHDJ) printing | ~100 nm–1 µm | Precise deposition of biomaterials and nanoparticles, tunable feature sizes | [55] |
| Electrospinning | Nanofibers: 50–500 diameter | Mimics native ECM architecture; high surface area-to-volume ratio | [59] |
| Phase separation | Nanopores: 50–200 nm | Produces porous, interconnected structures | [67] |
| Self-assembly | Nanodomains: 10–100 nm | Mimics natural ECM at the nanoscale; tailorable surface chemistry | [70,74] |
| 3D printing (two-photon polymerization) | ~100 nm | Fabricate complex 3D structures, with high spatial control with porosity and architecture | [63] |
| Layer-by-layer (LbL) assembly | Nanolayers: ~1–10 nm/layer | Molecular-level control, functional multilayer coatings, versatile biomolecule incorporation | [66] |
| Spin coating with nanomaterials | Nanoroughness: 10–100 nm | Tunable surface roughness | [85] |
| Freeze-drying with nanomaterials | Nanoscale structures | High porosity, nanoroughness | [86] |
5. Applications of Nanotopography of Polymeric Composite Scaffolds in Tissue Engineering and Regenerative Medicine
5.1. Organic Nanomaterial-Modified Topographies
5.2. Inorganic Nanomaterial-Modified Topographies
| Biomaterial/Nanocomposite | Nanotopographical Features | Biological Applications | Reference |
|---|---|---|---|
| Organic nanomaterial-modified topographies | |||
| hAME-encapsulated dextran/chitosan nanoparticles decorated on the polycaprolactone (PCL) nanofibrous scaffold–PVA-based hydrogel | Dextran/chitosan; spherical (d = 212 nm) | Corneal transplantation | [92] |
| Berberine-loaded chitosan nanoparticles (BerNChs) in alginate-chitosan (Alg-Ch) hydrogel (Alg-Ch/BerNChs) | Spherical; chitosan NPs (NCh) (d = 214 ± 42 nm); BerNChs (d = 252 nm) | Regeneration of injured spinal cord using endometrial stem cells and neural tissue engineering | [93] |
| Chitosan scaffolds with chitosan and ZnO nanoparticles | Spherical; chitosan NPs (d = 11.98 nm); ZnO NPs (d = 20 nm) | Tissue engineering and regenerative medicine | [94] |
| Collagen–nanohydroxyapatite (coll-nHAp) scaffolds with miR-26a NPs complexed with cell-penetrating peptide (RALA) | miR-26a cell-penetrating peptide (RALA) nanoparticle; spherical (z-average size: <200 nm up to 40 °C) | Critical-sized calvarial bone defect repair and bone tissue engineering | [102] |
| PCL–gelatin/collagen nanofibers or collagen nanoparticles | PCL–gelatin/collagen nanofiber (121 ± 28 nm); PCL–gelatin/collagen nanoparticles (141 ± 52 nm) | Skin tissue engineering and regenerative medicine | [103] |
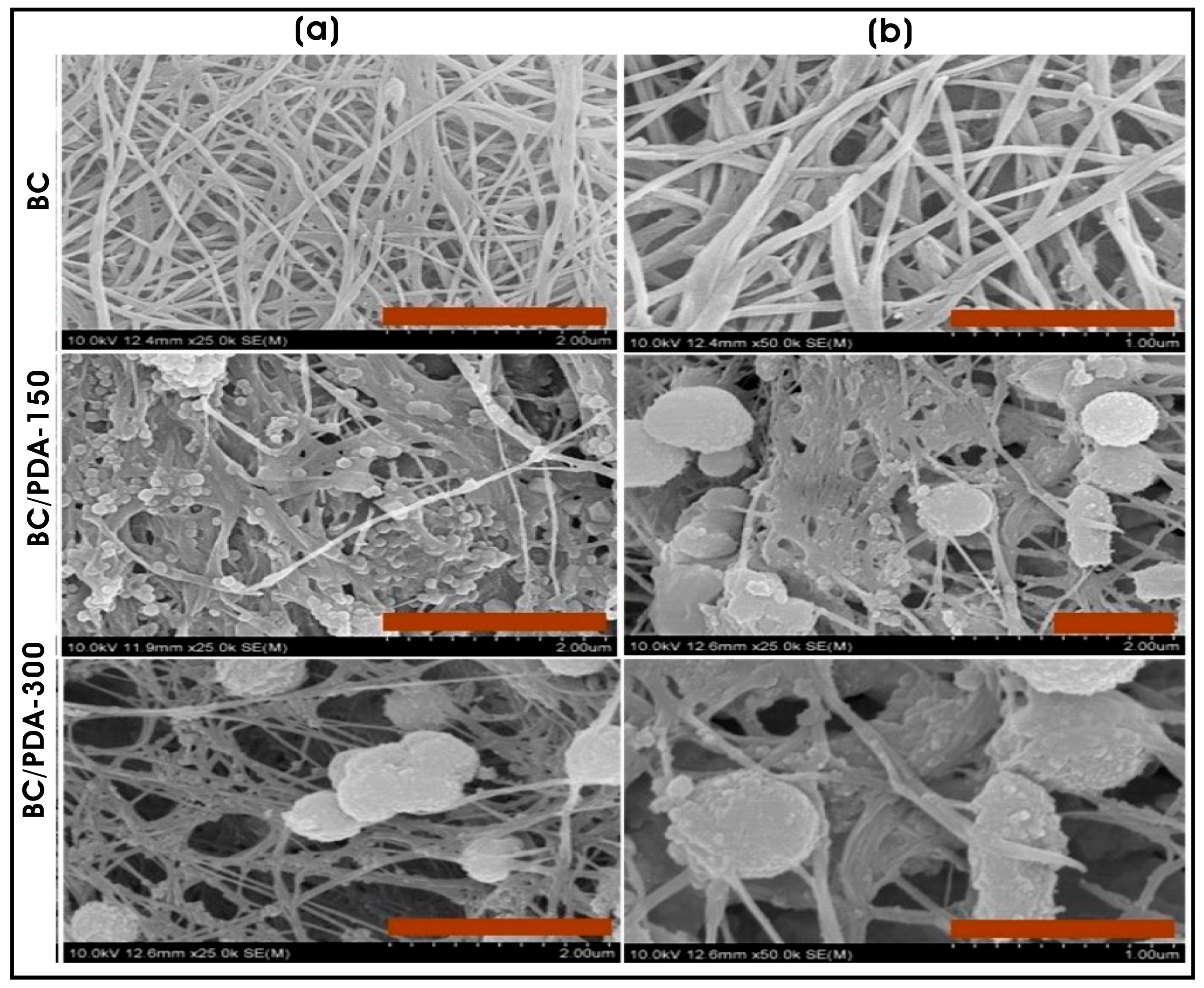
| Bacterial cellulose hydrogel coated with polydopamine (PDA) micro/nanospheres | Polydopamine (PDA) micro-/nanospheres; spherical (0.65 ± 0.14 µm) | Skin tissue engineering and regenerative medicine | [104] |
| Porous zirconia scaffold coated with calcium phosphate and loaded with gentamicin-encapsulated PLGA nanoparticles | Gentamicin-loaded PLGA nanoparticles; spherical; #empty PLGA NPs (100–320 nm; average d = 214.6 ± 14 nm); gentamicin-loaded NPs (120–340 nm) | Bone tissue repair and Bone tissue engineering | [108] |
| PCL microfibrous scaffold with co-sprayed collagen and PPy NPs | Polypyrrole nanoparticles (PPy NPs); spherical (~70 nm) | Neural tissue engineering | [109] |
| PPy NP-embedded collagen–HAMA hybrid hydrogel | PPy NPs; spherical (d = 40–50 nm) | Spinal cord injury (SCI) repair and neural tissue engineering | [110] |
| CA-LNP-loaded SiO2-doped tricalcium phosphate (TCP) scaffold | Carvacrol-loaded lipid nanoparticles (CA-LNPs); spherical (~129 nm) | Bone tissue engineering | [111] |
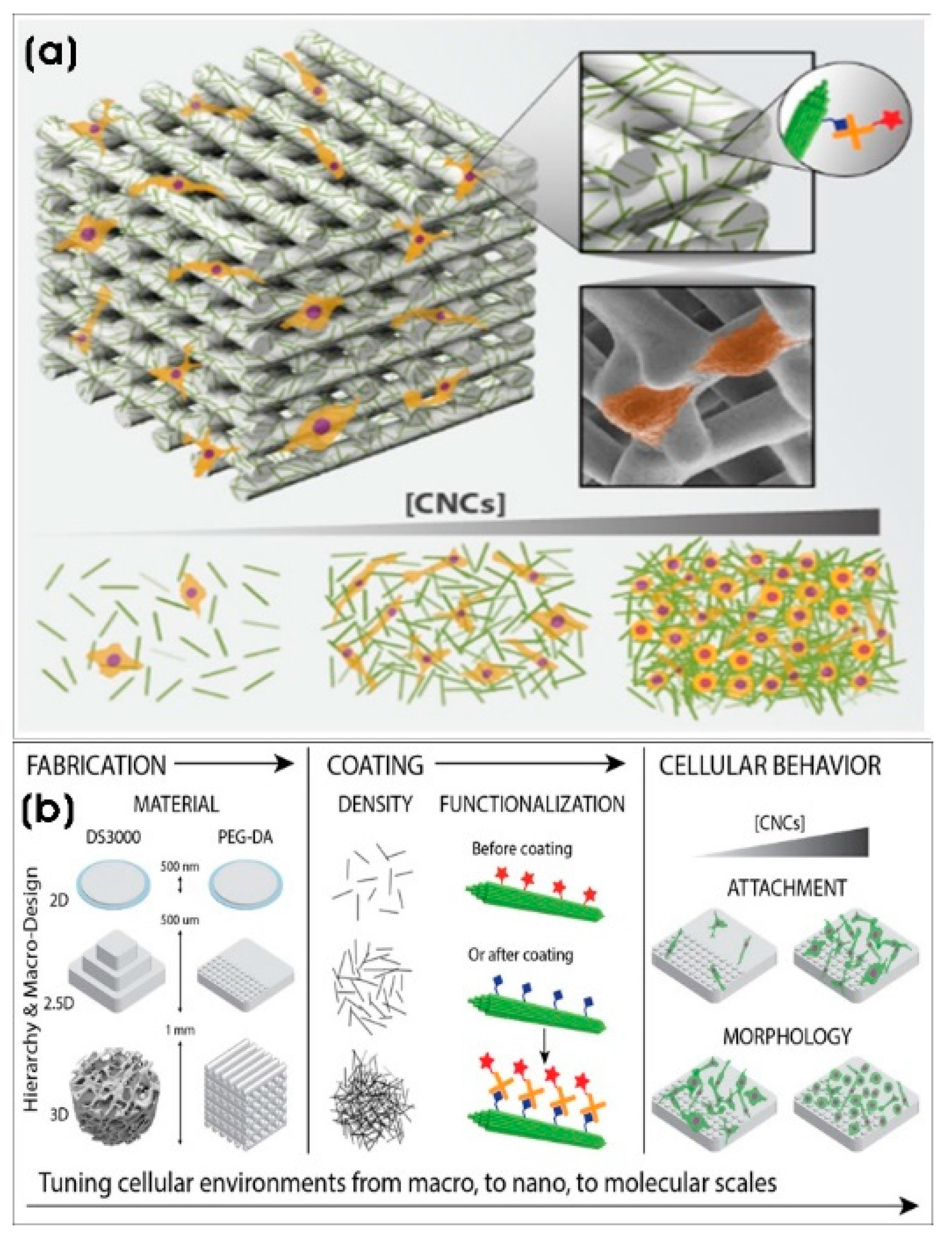
| DS3000 and poly(ethylene glycol)diacrylate (PEG-DA) 3D-printed scaffolds with CNC coating | Cellulose nanocrystals (CNCs) (length × width; 100–200 nm × 5–20 nm) | Tissue engineering and regenerative medicine | [114] |
| Inorganic nanomaterial-modified topographies | |||
| PCL/PLA scaffold by 3D printing technique | Strontium doped bredigite nanoparticles (Bre-Sr); spherical (<200 nm) | Bone tissue engineering | [11] |
| Poly-L/D-lactide (PLDLA) copolymer scaffold containing BTNP | Al2O3- and SiO2-coated barium titanate nanoparticles (BTNPs); 50–80 nm (round, oval, and angular-shaped) | Bone tissue engineering | [120] |
| MgO-xAg nanocomposite | MgO (34.2 nm); nano-lamellae MgO-10Cu (101.2 nm length × 9.6 nm thickness) | Bone tissue engineering | [117] |
| Poly(L-lactic acid) (PLLA) scaffold with GO@SiO2 | SiO2 nanoparticles on graphene oxide nanosheets (GO@SiO2) | Bone tissue engineering | [121] |
| Glass surface | Cluster-assembled ns-TiO2; 20.2 ± 0.5 nm rms roughness (50 nm film thickness) and 29.1 ± 1.0 nm rms roughness (200 nm film thickness) | Nerve regeneration | [159] |
| Gelatin/Fe3O4 composite scaffold | Fe3O4-citrate nanoparticles; flower-like shape (average size of 29.6 ± 3.9 nm) | Cancer therapy and adipose tissue regeneration | [152] |
| PLLA fibrous scaffold grafted with SPIONs | Superparamagnetic IONPs (SPIONs); average number of aggregates of 6 ± 4 per 100 µm2, and the average size of the aggregates was 0.52 ± 0.54 µm2 | Axonal regeneration and neural tissue engineering | [153] |
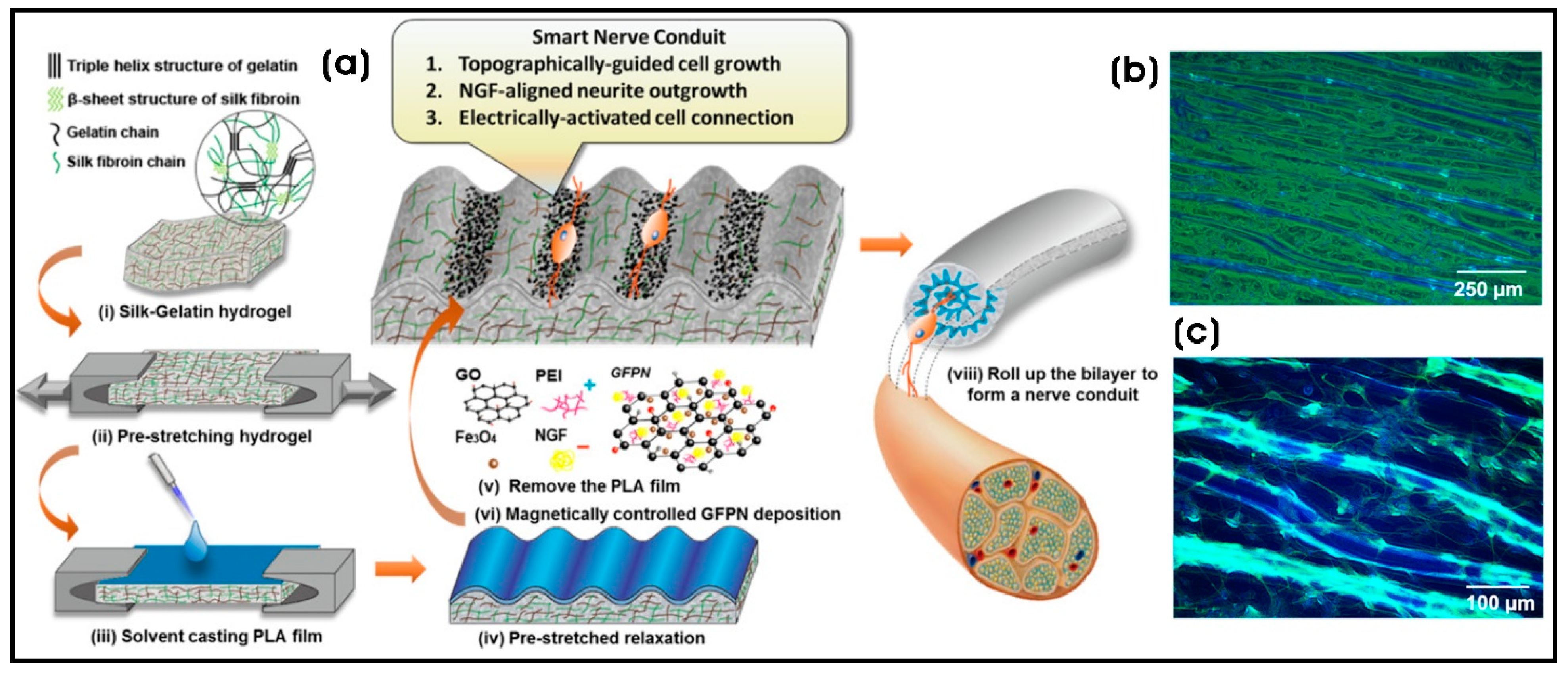
| Silk–gelatin (SG)/polylactic acid (PLA) bilayer nanocomposite | Nerve growth factor-incorporated IONP–graphene nanoparticles (GFPN)-PEI; Fe3O4 NPs (5–10 nm) distributed on the sheet-like structural rGO | Nerve regeneration and next-generation nerve conduits | [154] |
| Collagen film with PEG-capped paramagnetic IONPs (collagen/PEG@IOPs film) | PEG@IONPs; Spherical (80 nm in diameter) | Tissue engineering and regenerative medicine | [155] |
| Alginate/magnetic short nanofiber composite hydrogel | Superparamagnetic Fe3O4 nanoparticles (SPION); M.SNF of 222 ± 64 nm containing spherical SPIONs (10 ± 2 nm) | Nerve regeneration | [157] |
| Chitosan-ZnO NPs composite conduit (CZON) | ZnO NPs; spherical (30 nm) | Nerve regeneration and nerve conduits | [142] |
| PCL/ZnO NPs scaffold | ZnO NPs; spherical (30–80 nm) | Nerve regeneration and nerve conduits | [143] |
| Poly-ɛ-caprolactone composite nanofibers with ZnO NPs coated with PDA and QK peptides (PCL@Z/P/QK) | ZnO NPs | Orthopedic implants and bone repair and bone tissue engineering | [144] |
| Gelatin/nanoceria nanocomposite fibers | Nanoceria (CeO2 nanoparticles); nanoparticle dispersion (d < 5 nm) | Neuronal tissue engineering and regenerative medicine | [149] |
| PLA microfiber with streptomycin-loaded hydroxyapatite nanoparticles | Nanohydroxyapatite (nHAp); nanorod (diameter × length; 20–50 nm × 50–150 nm) | Tissue engineering and regenerative medicine | [125] |
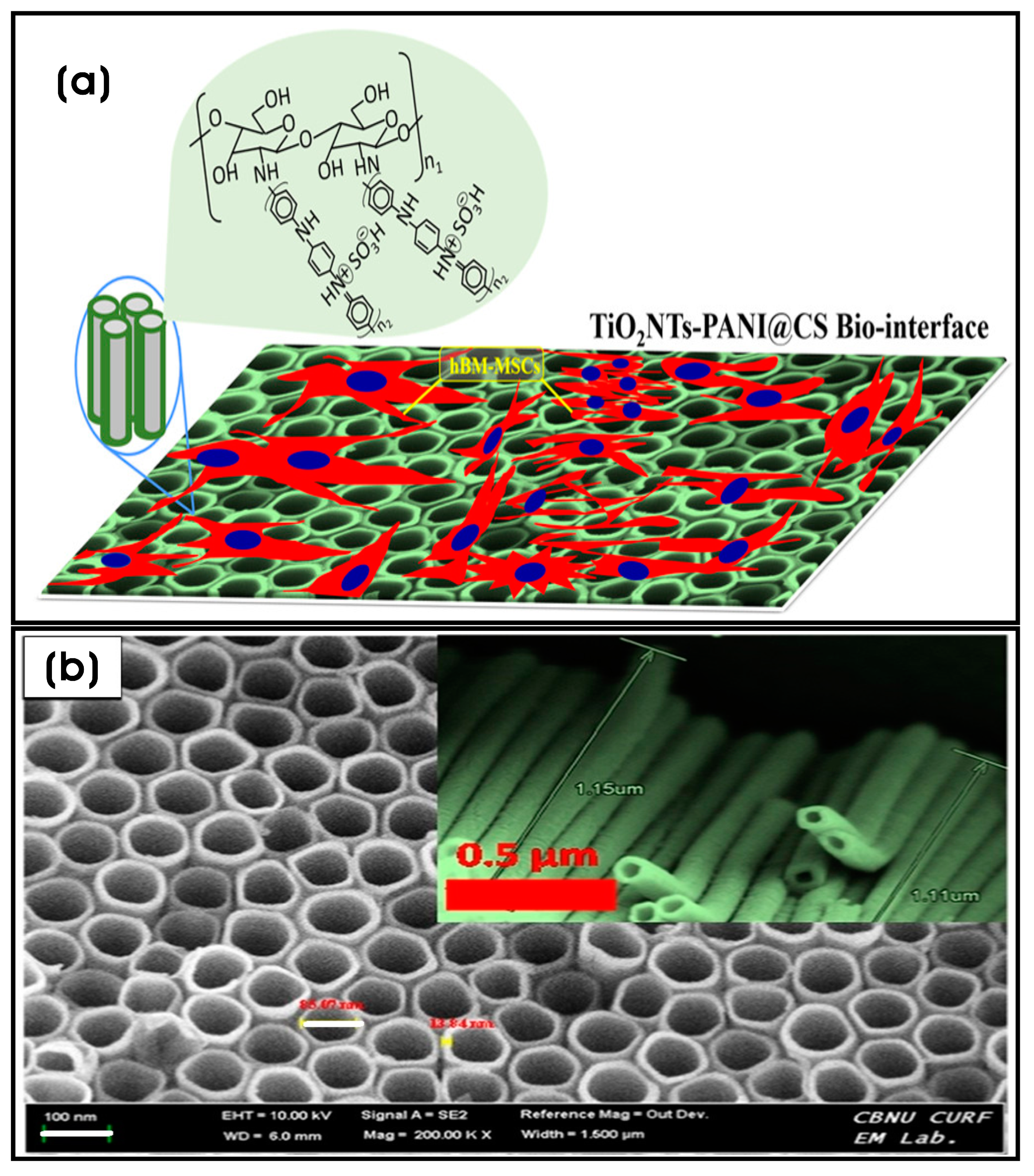
| Chitosan-crosslinked polyaniline nanonets coated with titanium nanotubes (TiO2NTs-PANI@CS) | TiO2 NTs; TiO2 NTs (tube wall thickness × length × inner diameter; 13.84 × 1.15 × 85.07 nm) | Bone tissue engineering | [161] |
| Hydroxyapatite/Polycaprolactone nanoparticles (HAp/PCL NPs) | Hydroxyapatite NPs; rod-like morphology (length × width; 92.18 ± 18.21 nm × 30.3 ± 4.7 nm) | Bone tissue engineering | [124] |
| Poly-ɛ-caprolactone (PCL) nanofibrous membrane loaded with tantalum (Ta)/whitlockite (WH) nanoparticles (PCL/Ta/WH) | Tantalum (Ta) (Ta NPs; 50 nm) and whitlockite (WH) nanoparticles | Bone repair and tissue engineering | [129] |
| PCL/nanoparticulate willemite (npW) composite scaffold (3D printing) | Silicium dioxide (SiO2) (d = 5–20 nm); zinc oxide (ZnO) (<100 nm); willemite (Zn2SiO4) nanoparticle (spherical, d = 20–70 nm) | Osteonecrosis defects and steroid-associated osteonecrosis | [136] |
| Glass surface coated with Ag, Au, or ZnO NPs | AgNPs or AuNPs or ZnO NPs; particle diameters (AgNPs: 110 ± 40 nm; AuNPs: 100 ± 40 nm; ZnONPs: 115 ± 45 nm) | Nerve regeneration and regenerative medicine | [163] |
| PEG hydrogel/Ag nanowire composite micropattern-based sensor on a flexible PET film | Ag nanowires on polyethylene terephthalate (PET) film | Neural implant or graft and neural stem cell therapy | [165] |
| AuNPs-PDA@PLGA/Lys-g-GO composite scaffolds | L-lysine-grafted GO (Lys-g-GO) (wrinkled surface) NPs and AuNP–Polydopamine (AuNPs-PDA) NPs | Bone defect treatment and bone tissue engineering | [167] |
| PLGA nanofibrous conduit functionalized with laminin containing BDNF and AuNPs-CNPs | BDNF- and AuNP-encapsulated chitosan nanoparticles (BDNF/AuNPs-CNPs); BDNF/AuNP-encapsulated CNPs (d = 77.8 ± 2.05 µm) | Nerve regeneration and neurological repair | [169] |
| Theranostic AuNP-encapsulated PLGA microspheres embedded in the nanofibrous structure | Gold nanoparticle (AuNP) core, a conjugated folic acid (FA)–chitosan (CS) polymeric shell; spherical AuNPs (d = 60–110 nm) | Tissue regeneration and postoperative cancer management | [170] |
| Micro/nano-channeled PCL/PLGA film scaffold surface decorated with IKVAV peptide/AuNPs | Surface decorated with IKVAV pentapeptide/AuNPs; spherical AuNPs (d = 50 nm) | Nerve regeneration and neural tissue engineering | [171] |
| Gelatin methacrylolyl (GelMa) with MXene or AuNPs nanocomposite conductive bioink for 3D printing | AuNPs and MXene (titanium carbide; Ti3C2Tx) nanosheets; MXene (Ti3C2Tx) nanosheets (lateral size × thickness; 2–3 µm × 3–4 nm (3–4 layers); spherical AuNPs (d = 50 nm) | Skeletal muscle tissue engineering | [172] |
| Polyethyleneimine (PEI)-coated cover glass with adsorbed gold nanoparticles (GNPs) | AuNPs; spherical (d = 39 nm) | Nerve regeneration and neural tissue engineering | [173] |
| PCL/chitosan nanofibers (d = 114–180 nm with varying chitosan concentrations) | AuNPs; spherical (d = 175 ± 69 nm) | Peripheral nerve regeneration and neural tissue engineering | [175] |
| PDA-gold/PCL nanocomposite channels (nerve conduit) | AuNPs and polydopamine | Peripheral nerve regeneration and neural tissue engineering | [180] |
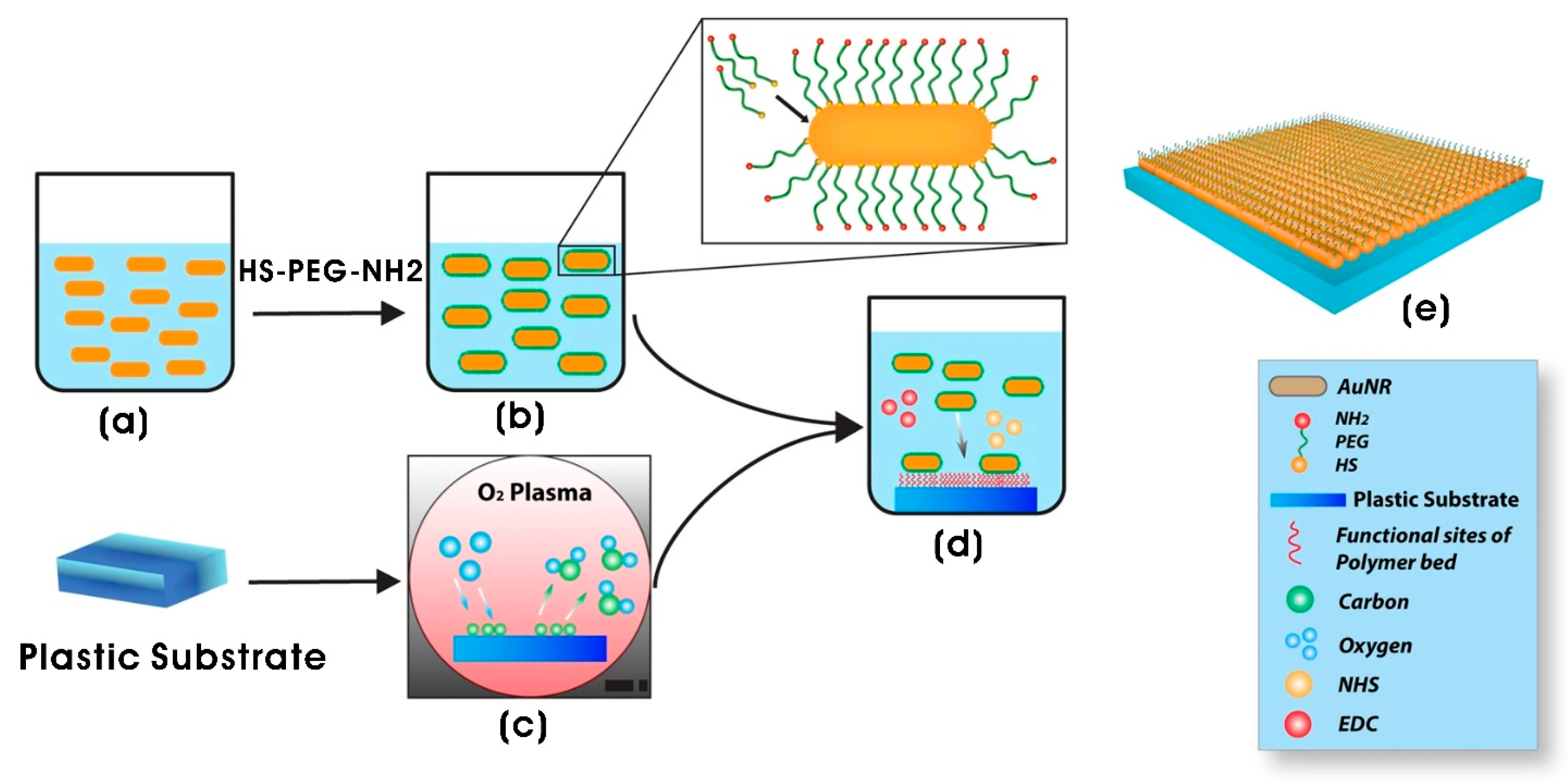
| AuNR-functionalized polyethylene glycol (PEG) (AuNRs-SH-PEG-NH2) composite (2D) system | Gold nanorods (AuNRs); AuNRs (12 nm diameter; 36 nm length) | Neural tissue engineering | [182] |
| PCL/gelatin nanofiber scaffolds (average fiber diameter of 260 ± 70 nm) | AuNPs (d = 10 nm) | Neuronal tissue engineering and neural regeneration | [184] |
| Alginate–chitosan (Alg/Cs) scaffolds with AuNPs | AuNPs; spherical (d = ~32 nm) | Tissue engineering and regenerative medicine | [16] |
| PCL-AuNPs and PCL-Au-PEG 3D scaffolds | AuNPs; spherical (d = 15.65 ± 6.41 nm) | Skeletal muscle tissue engineering | [189] |
6. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Huang, G.; Li, F.; Zhao, X.; Ma, Y.; Li, Y.; Lin, M.; Jin, G.; Lu, T.J.; Genin, G.M.; Xu, F. Functional and biomimetic materials for engineering of the three-dimensional cell microenvironment. Chem. Rev. 2017, 117, 12764–12850. [Google Scholar] [CrossRef]
- Yang, F.; Neeley, W.L.; Moore, M.J.; Karp, J.M.; Shukla, A.; Langer, R. Tissue engineering: The therapeutic strategy of the twenty-first century. In Nanotechnology and Tissue Engineering: The Scaffold; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2008; pp. 3–32. [Google Scholar]
- Murtuza, B.; Nichol, J.W.; Khademhosseini, A. Micro-and nanoscale control of the cardiac stem cell niche for tissue fabrication. Tissue Eng. Part B Rev. 2009, 15, 443–454. [Google Scholar] [CrossRef]
- Monteiro, N.; Fangueiro, J.; Reis, R.; Neves, N. Replication of natural surface topographies to generate advanced cell culture substrates. Bioact. Mater. 2023, 28, 337–347. [Google Scholar] [CrossRef]
- LaPointe, V.L.; Fernandes, A.T.; Bell, N.C.; Stellacci, F.; Stevens, M.M. Nanoscale topography and chemistry affect embryonic stem cell self-renewal and early differentiation. Adv. Healthc. Mater. 2013, 2, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, K.; Thomas, S.; Kalarikkal, N.; Gnanamani, A. Collagen coated electrospun polycaprolactone (PCL) with titanium dioxide (TiO2) from an environmentally benign solvent: Preliminary physico-chemical studies for skin substitute. J. Polym. Res. 2014, 21, 410. [Google Scholar] [CrossRef]
- Kandhasamy, S.; Perumal, S.; Madhan, B.; Umamaheswari, N.; Banday, J.A.; Perumal, P.T.; Santhanakrishnan, V.P. Synthesis and fabrication of collagen-coated ostholamide electrospun nanofiber scaffold for wound healing. ACS Appl. Mater. Interfaces 2017, 9, 8556–8568. [Google Scholar] [CrossRef]
- Özdabak Sert, A.e.B.; Bittrich, E.; Uhlmann, P.; Kok, F.N.; Kılıç, A. Monitoring Cell Adhesion on Polycaprolactone–Chitosan Films with Varying Blend Ratios by Quartz Crystal Microbalance with Dissipation. ACS Omega 2023, 8, 17017–17027. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yu, H.; Li, G.; Wang, Y.; Liu, L. Facile modulation of cell adhesion to a poly (ethylene glycol) diacrylate film with incorporation of polystyrene nano-spheres. Biomed. Microdevices 2016, 18, 107. [Google Scholar] [CrossRef]
- Nadi, A.; Khodaei, M.; Javdani, M.; Mirzaei, S.A.; Soleimannejad, M.; Tayebi, L.; Asadpour, S. Fabrication of functional and nano-biocomposite scaffolds using strontium-doped bredigite nanoparticles/polycaprolactone/poly lactic acid via 3D printing for bone regeneration. Int. J. Biol. Macromol. 2022, 219, 1319–1336. [Google Scholar] [CrossRef]
- Khurana, I.; Allawadhi, P.; Neeradi, D.; Banothu, A.K.; Thalugula, S.; Naik, R.R.; Packirisamy, G.; Bharani, K.K.; Khurana, A. Introduction to nanoengineering and nanotechnology for biomedical applications. In Emerging Nanotechnologies for Medical Applications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–34. [Google Scholar]
- Gaetani, R.; Chimenti, I. 3D cultures for modelling the microenvironment: Current research trends and applications. Int. J. Mol. Sci. 2023, 24, 11109. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, J.; Zhou, Z.; Zhou, S.; Hu, Z. Micro-/nano-scales direct cell behavior on biomaterial surfaces. Molecules 2018, 24, 75. [Google Scholar] [CrossRef]
- Pamuła, E.; De Cupere, V.; Dufrêne, Y.F.; Rouxhet, P.G. Nanoscale organization of adsorbed collagen: Influence of substrate hydrophobicity and adsorption time. J. Colloid Interface Sci. 2004, 271, 80–91. [Google Scholar] [CrossRef]
- Viveros-Moreno, N.G.; Garcia-Lorenzana, M.; Peña-Mercado, E.; García-Sanmartín, J.; Narro-Íñiguez, J.; Salazar-García, M.; Huerta-Yepez, S.; Sanchez-Gomez, C.; Martínez, A.; Beltran-Vargas, N.E. In vivo biocompatibility testing of nanoparticle-functionalized alginate–chitosan scaffolds for tissue engineering applications. Front. Bioeng. Biotechnol. 2023, 11, 1295626. [Google Scholar] [CrossRef]
- Woo, K.M.; Chen, V.J.; Ma, P.X. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J. Biomed. Mater. Res. Part A 2003, 67, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.; Müller, R.; Borgmann, S.; Brocker, E.-B.; Friedl, P. Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood 2003, 102, 3262–3269. [Google Scholar] [CrossRef]
- Dent, E.W.; Gertler, F.B. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron 2003, 40, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Novoseletskaya, E.S.; Evdokimov, P.V.; Efimenko, A.Y. Extracellular matrix-induced signaling pathways in mesenchymal stem/stromal cells. Cell Commun. Signal. 2023, 21, 244. [Google Scholar] [CrossRef]
- Bettinger, C.J.; Langer, R.; Borenstein, J.T. Engineering substrate topography at the micro-and nanoscale to control cell function. Angew. Chem. Int. Ed. 2009, 48, 5406–5415. [Google Scholar] [CrossRef]
- Kriparamanan, R.; Aswath, P.; Zhou, A.; Tang, L.; Nguyen, K.T. Nanotopography: Cellular responses to nanostructured materials. J. Nanosci. Nanotechnol. 2006, 6, 1905–1919. [Google Scholar] [CrossRef]
- Saydé, T.; El Hamoui, O.; Alies, B.; Gaudin, K.; Lespes, G.; Battu, S. Biomaterials for three-dimensional cell culture: From applications in oncology to nanotechnology. Nanomaterials 2021, 11, 481. [Google Scholar] [CrossRef] [PubMed]
- Koegler, P.; Clayton, A.; Thissen, H.; Santos, G.N.C.; Kingshott, P. The influence of nanostructured materials on biointerfacial interactions. Adv. Drug Deliv. Rev. 2012, 64, 1820–1839. [Google Scholar] [CrossRef]
- Nurkesh, A.; Jaguparov, A.; Jimi, S.; Saparov, A. Recent advances in the controlled release of growth factors and cytokines for improving cutaneous wound healing. Front. Cell Dev. Biol. 2020, 8, 638. [Google Scholar] [CrossRef]
- Zhu, L.; Luo, D.; Liu, Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int. J. Oral Sci. 2020, 12, 6. [Google Scholar] [CrossRef]
- Paul, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Carbon-based polymer nanocomposites for high-performance applications. Polymers 2020, 12, 872. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.-Y.; Baek, J.-B. Nanocomposites derived from polymers and inorganic nanoparticles. Materials 2010, 3, 3654–3674. [Google Scholar] [CrossRef]
- Darwish, M.S.; Mostafa, M.H.; Al-Harbi, L.M. Polymeric nanocomposites for environmental and industrial applications. Int. J. Mol. Sci. 2022, 23, 1023. [Google Scholar] [CrossRef]
- Njuguna, J.; Ansari, F.; Sachse, S.; Rodriguez, V.M.; Siqqique, S.; Zhu, H. Nanomaterials, nanofillers, and nanocomposites: Types and properties. In Health and Environmental Safety of Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–37. [Google Scholar]
- Vedarethinam, V.; Arun, C. Nanocomposites and Nanofillers. In Handbook of Nanofillers; Springer: Cham, Swizerland, 2024; pp. 1–18. [Google Scholar]
- Perez-Puyana, V.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Polymer-based scaffolds for soft-tissue engineering. Polymers 2020, 12, 1566. [Google Scholar] [CrossRef]
- Stevens, M.M.; George, J.H. Exploring and engineering the cell surface interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef]
- Kadler, K.E.; Hill, A.; Canty-Laird, E.G. Collagen fibrillogenesis: Fibronectin, integrins, and minor collagens as organizers and nucleators. Curr. Opin. Cell Biol. 2008, 20, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Schwarzbauer, J.E.; DeSimone, D.W. Fibronectins, their fibrillogenesis, and in vivo functions. Cold Spring Harb. Perspect. Biol. 2011, 3, a005041. [Google Scholar] [CrossRef]
- Muiznieks, L.D.; Keeley, F.W. Molecular assembly and mechanical properties of the extracellular matrix: A fibrous protein perspective. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1832, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Abrams, G.; Goodman, S.; Nealey, P.; Franco, M.; Murphy, C. Nanoscale topography of the basement membrane underlying the corneal epithelium of the rhesus macaque. Cell Tissue Res. 2000, 299, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Walker, M.; Xiao, Y.; Donnelly, H.; Dalby, M.J.; Salmeron-Sanchez, M. The influence of nanotopography on cell behaviour through interactions with the extracellular matrix—A review. Bioact. Mater. 2022, 15, 145–159. [Google Scholar] [CrossRef]
- Pang, X.; He, X.; Qiu, Z.; Zhang, H.; Xie, R.; Liu, Z.; Gu, Y.; Zhao, N.; Xiang, Q.; Cui, Y. Targeting integrin pathways: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 1. [Google Scholar] [CrossRef]
- Malcor, J.-D.; Mallein-Gerin, F. Biomaterial functionalization with triple-helical peptides for tissue engineering. Acta Biomater. 2022, 148, 1–21. [Google Scholar] [CrossRef]
- Kim, H.N.; Jiao, A.; Hwang, N.S.; Kim, M.S.; Kang, D.H.; Kim, D.-H.; Suh, K.-Y. Nanotopography-guided tissue engineering and regenerative medicine. Adv. Drug Deliv. Rev. 2013, 65, 536–558. [Google Scholar] [CrossRef]
- Janson, I.A.; Putnam, A.J. Extracellular matrix elasticity and topography: Material-based cues that affect cell function via conserved mechanisms. J. Biomed. Mater. Res. Part A 2015, 103, 1246–1258. [Google Scholar] [CrossRef]
- Kuo, C.W.; Chueh, D.-Y.; Chen, P. Investigation of size–dependent cell adhesion on nanostructured interfaces. J. Nanobiotechnol. 2014, 12, 54. [Google Scholar] [CrossRef]
- Dalby, M.; Giannaras, D.; Riehle, M.; Gadegaard, N.; Affrossman, S.; Curtis, A. Rapid fibroblast adhesion to 27 nm high polymer demixed nano-topography. Biomaterials 2004, 25, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Klausen, L.H.; Zhang, W.; Jahed, Z.; Tsai, C.-T.; Li, T.L.; Cui, B. Nanoscale surface topography reduces focal adhesions and cell stiffness by enhancing integrin endocytosis. Nano Lett. 2021, 21, 8518–8526. [Google Scholar] [CrossRef]
- Flemming, R.G.; Murphy, C.J.; Abrams, G.A.; Goodman, S.L.; Nealey, P.F. Effects of synthetic micro-and nano-structured surfaces on cell behavior. Biomaterials 1999, 20, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Jung, K.; Ko, E.; Kim, J.; Park, K.I.; Kim, J.; Cho, S.-W. Nanotopographical manipulation of focal adhesion formation for enhanced differentiation of human neural stem cells. ACS Appl. Mater. Interfaces 2013, 5, 10529–10540. [Google Scholar] [CrossRef]
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.J.; Marei, H.E.-S. Nanoparticles in tissue engineering: Applications, challenges and prospects. Int. J. Nanomed. 2018, 13, 5637–5655. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Rahaman, M.N.; Zhou, N.; Huang, W.; Wang, D.; Zhang, L.; Li, H. In vitro study on different cell response to spherical hydroxyapatite nanoparticles. J. Biomater. Appl. 2008, 23, 37–50. [Google Scholar]
- Wijerathne, H.S.; Yan, D.; Zeng, B.; Xie, Y.; Hu, H.; Wickramaratne, M.N.; Han, Y. Effect of nano-hydroxyapatite on protein adsorption and cell adhesion of poly (lactic acid)/nano-hydroxyapatite composite microspheres. SN Appl. Sci. 2020, 2, 722. [Google Scholar] [CrossRef]
- Chi, M.; Li, N.; Cui, J.; Karlin, S.; Rohr, N.; Sharma, N.; Thieringer, F.M. Biomimetic, mussel-inspired surface modification of 3D-printed biodegradable polylactic acid scaffolds with nano-hydroxyapatite for bone tissue engineering. Front. Bioeng. Biotechnol. 2022, 10, 989729. [Google Scholar] [CrossRef]
- Schexnailder, P.J.; Gaharwar, A.K.; Bartlett II, R.L.; Seal, B.L.; Schmidt, G. Tuning cell adhesion by incorporation of charged silicate nanoparticles as cross-linkers to polyethylene oxide. Macromol. Biosci. 2010, 10, 1416–1423. [Google Scholar] [CrossRef]
- Eftekhari, B.S.; Eskandari, M.; Janmey, P.A.; Samadikuchaksaraei, A.; Gholipourmalekabadi, M. Surface topography and electrical signaling: Single and synergistic effects on neural differentiation of stem cells. Adv. Funct. Mater. 2020, 30, 1907792. [Google Scholar] [CrossRef]
- Ul Hassan, R.; Sharipov, M.; Ryu, W. Electrohydrodynamic (EHD) printing of nanomaterial composite inks and their applications. Micro Nano Syst. Lett. 2024, 12, 2. [Google Scholar] [CrossRef]
- Wu, Y. Electrohydrodynamic jet 3D printing in biomedical applications. Acta Biomater. 2021, 128, 21–41. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Wang, X.; Leng, B.; Zhan, N.; Liu, H.; Wang, S.; Lu, Y.; Sun, J.; Huang, D. Engineered nanotopography on the microfibers of 3D-printed PCL scaffolds to modulate cellular responses and establish an in vitro tumor model. ACS Appl. Bio Mater. 2021, 4, 1381–1394. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, D.; Zhao, K.; Song, K.; Liang, J. Electrohydrodynamic jet 3D printing of PCL/PVP composite scaffold for cell culture. Talanta 2020, 211, 120750. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Keirouz, A.; Wang, Z.; Reddy, V.S.; Nagy, Z.K.; Vass, P.; Buzgo, M.; Ramakrishna, S.; Radacsi, N. The history of electrospinning: Past, present, and future developments. Adv. Mater. Technol. 2023, 8, 2201723. [Google Scholar] [CrossRef]
- SalehHudin, H.S.; Mohamad, E.N.; Afifi, A.M.; Mahadi, W.N.L.W. Simulation and experimental study of parameters in multiple-nozzle electrospinning: Effects of voltage and nozzle configuration on the electric field and electrospun jet attributes. J. Manuf. Process. 2023, 85, 544–555. [Google Scholar] [CrossRef]
- Patil, D.; Kumari, S.; Chatterjee, K. Bioinspired nanotopography on 3D printed tissue scaffold to impart mechanobactericidal and osteogenic activities. Colloids Surf. B Biointerfaces 2023, 228, 113401. [Google Scholar] [CrossRef]
- Zieliński, P.S.; Gudeti, P.K.R.; Rikmanspoel, T.; Włodarczyk-Biegun, M.K. 3D printing of bio-instructive materials: Toward directing the cell. Bioact. Mater. 2023, 19, 292–327. [Google Scholar] [CrossRef]
- Aazmi, A.; Zhang, D.; Mazzaglia, C.; Yu, M.; Wang, Z.; Yang, H.; Huang, Y.Y.S.; Ma, L. Biofabrication methods for reconstructing extracellular matrix mimetics. Bioact. Mater. 2024, 31, 475–496. [Google Scholar] [CrossRef] [PubMed]
- Yavari, S.A.; Croes, M.; Akhavan, B.; Jahanmard, F.; Eigenhuis, C.; Dadbakhsh, S.; Vogely, H.; Bilek, M.; Fluit, A.; Boel, C. Layer by layer coating for bio-functionalization of additively manufactured meta-biomaterials. Addit. Manuf. 2020, 32, 100991. [Google Scholar]
- Wang, Z.; Qiu, W.; Zhang, Q. Constructing phase separation in polymer gels: Strategies, functions and applications. Prog. Polym. Sci. 2024, 154, 101847. [Google Scholar] [CrossRef]
- Schaub, N.J.; Britton, T.; Rajachar, R.; Gilbert, R.J. Engineered nanotopography on electrospun PLLA microfibers modifies RAW 264.7 cell response. ACS Appl. Mater. Interfaces 2013, 5, 10173–10184. [Google Scholar] [CrossRef]
- Kocourková, K.; Kadleckova, M.; Wrzecionko, E.; Mikulka, F.; Knechtova, E.; Černá, P.; Humenik, M.; Minarik, A. Silk Fibroin Surface Engineering Using Phase Separation Approaches for Enhanced Cell Adhesion and Proliferation. ACS Appl. Mater. Interfaces 2025, 17, 13702–13712. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Boncheva, M. Beyond molecules: Self-assembly of mesoscopic and macroscopic components. Proc. Natl. Acad. Sci. USA 2002, 99, 4769–4774. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bhushan, P.; Bhattacharya, S. Fabrication of nanostructures with bottom-up approach and their utility in diagnostics, therapeutics, and others. In Environmental, Chemical and Medical Sensors; Springer: Cham, Swizerland, 2017; pp. 167–198. [Google Scholar]
- Ariga, K.; Hill, J.P.; Lee, M.V.; Vinu, A.; Charvet, R.; Acharya, S. Challenges and breakthroughs in recent research on self-assembly. Sci. Technol. Adv. Mater. 2008, 9, 014109. [Google Scholar] [CrossRef]
- Hu, X.-H.; Xiong, S. Fabrication of nanodevices through block copolymer self-assembly. Front. Nanotechnol. 2022, 4, 762996. [Google Scholar] [CrossRef]
- Zhang, S. Self-assembling peptides: From a discovery in a yeast protein to diverse uses and beyond. Protein Sci. 2020, 29, 2281–2303. [Google Scholar] [CrossRef]
- Xiang, X.; Ding, X.; Moser, T.; Gao, Q.; Shokuhfar, T.; Heiden, P.A. Peptide-directed self-assembly of functionalized polymeric nanoparticles. Part II: Effects of nanoparticle composition on assembly behavior and multiple drug loading ability. Macromol. Biosci. 2015, 15, 568–582. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, R.; He, J.; Liu, Q.; Zhang, Y.; Wang, Y.; Liu, L.; Song, M.; Chen, F. A Recombinant Human Collagen and RADA-16 Fusion Protein Promotes Hemostasis and Rapid Wound Healing. ACS Appl. Bio Mater. 2024, 8, 236–251. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wang, H.; Huang, F.; Li, S.; Li, M.; Deng, W.; Chen, W. Investigation on the effects and mechanisms of novel peptide nanofiber gel to promote wound healing of deep second-degree burns in mice. Int. J. Biol. Macromol. 2025, 292, 139221. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, M.; Nakamura, R.; Kishimoto, Y.; Yurie, H.; Hayashi, Y.; Kaba, S.; Ohnishi, H.; Yamashita, M.; Tateya, I.; Omori, K. Recurrent laryngeal nerve regeneration using a self-assembling peptide hydrogel. Laryngoscope 2020, 130, 2420–2427. [Google Scholar] [CrossRef]
- Zhang, S.; Ellis-Behnke, R.; Zhao, X.; Spirio, L. PuraMatrix: Self-assembling peptide nanofiber scaffolds. Growth 2005, 15, 23. [Google Scholar]
- Akiyama, N.; Yamamoto-Fukuda, T.; Takahashi, H.; Koji, T. In situ tissue engineering with synthetic self-assembling peptide nanofiber scaffolds, PuraMatrix, for mucosal regeneration in the rat middle-ear. Int. J. Nanomed. 2013, 8, 2629–2640. [Google Scholar] [CrossRef]
- Haines-Butterick, L.; Rajagopal, K.; Branco, M.; Salick, D.; Rughani, R.; Pilarz, M.; Lamm, M.S.; Pochan, D.J.; Schneider, J.P. Controlling hydrogelation kinetics by peptide design for three-dimensional encapsulation and injectable delivery of cells. Proc. Natl. Acad. Sci. USA 2007, 104, 7791–7796. [Google Scholar] [CrossRef]
- Schneible, J.D.; Shi, K.; Young, A.T.; Ramesh, S.; He, N.; Dowdey, C.E.; Dubnansky, J.M.; Lilova, R.L.; Gao, W.; Santiso, E. Modified gaphene oxide (GO) particles in peptide hydrogels: A hybrid system enabling scheduled delivery of synergistic combinations of chemotherapeutics. J. Mater. Chem. B 2020, 8, 3852–3868. [Google Scholar] [CrossRef]
- Worthington, P.; Drake, K.M.; Li, Z.; Napper, A.D.; Pochan, D.J.; Langhans, S.A. Implementation of a high-throughput pilot screen in peptide hydrogel-based three-dimensional cell cultures. SLAS Discov. 2019, 24, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Sola, A.; Bertacchini, J.; D’Avella, D.; Anselmi, L.; Maraldi, T.; Marmiroli, S.; Messori, M. Development of solvent-casting particulate leaching (SCPL) polymer scaffolds as improved three-dimensional supports to mimic the bone marrow niche. Mater. Sci. Eng. C 2019, 96, 153–165. [Google Scholar] [CrossRef]
- Fortunati, E.; Mattioli, S.; Armentano, I.; Kenny, J.M. Spin coated cellulose nanocrystal/silver nanoparticle films. Carbohydr. Polym. 2014, 113, 394–402. [Google Scholar] [CrossRef]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Metavarayuth, K.; Sitasuwan, P.; Zhao, X.; Lin, Y.; Wang, Q. Influence of surface topographical cues on the differentiation of mesenchymal stem cells in vitro. ACS Biomater. Sci. Eng. 2016, 2, 142–151. [Google Scholar] [CrossRef]
- Teo, B.K.K.; Wong, S.T.; Lim, C.K.; Kung, T.Y.; Yap, C.H.; Ramagopal, Y.; Romer, L.H.; Yim, E.K. Nanotopography modulates mechanotransduction of stem cells and induces differentiation through focal adhesion kinase. ACS Nano 2013, 7, 4785–4798. [Google Scholar] [CrossRef] [PubMed]
- Werner, M.; Kurniawan, N.A.; Bouten, C.V. Cellular geometry sensing at different length scales and its implications for scaffold design. Materials 2020, 13, 963. [Google Scholar] [CrossRef] [PubMed]
- Refaaq, F.; Chen, X.; Pang, S. Effects of topographical guidance cues on osteoblast cell migration. Sci. Rep. 2020, 10, 20003. [Google Scholar] [CrossRef]
- Mutlu, N.; Liverani, L.; Kurtuldu, F.; Galusek, D.; Boccaccini, A.R. Zinc improves antibacterial, anti-inflammatory and cell motility activity of chitosan for wound healing applications. Int. J. Biol. Macromol. 2022, 213, 845–857. [Google Scholar] [CrossRef]
- Bakhshandeh, H.; Atyabi, F.; Soleimani, M.; Taherzadeh, E.S.; Shahhoseini, S.; Cohan, R.A. Biocompatibility improvement of artificial cornea using chitosan-dextran nanoparticles containing bioactive macromolecules obtained from human amniotic membrane. Int. J. Biol. Macromol. 2021, 169, 492–499. [Google Scholar] [CrossRef]
- Mahya, S.; Ai, J.; Shojae, S.; Khonakdar, H.A.; Darbemamieh, G.; Shirian, S. Berberine loaded chitosan nanoparticles encapsulated in polysaccharide-based hydrogel for the repair of spinal cord. Int. J. Biol. Macromol. 2021, 182, 82–90. [Google Scholar] [CrossRef]
- Angarita, J.E.V.; Insuasty, D.; Castro, J.I.; Valencia-Llano, C.H.; Zapata, P.A.; Delgado-Ospina, J.; Navia-Porras, D.P.; Albis, A.; Grande-Tovar, C.D. Biological activity of lyophilized chitosan scaffolds with inclusion of chitosan and zinc oxide nanoparticles. RSC Adv. 2024, 14, 13565–13582. [Google Scholar] [CrossRef]
- Poliseno, L.; Tuccoli, A.; Mariani, L.; Evangelista, M.; Citti, L.; Woods, K.; Mercatanti, A.; Hammond, S.; Rainaldi, G. MicroRNAs modulate the angiogenic properties of HUVECs. Blood 2006, 108, 3068–3071. [Google Scholar] [CrossRef]
- Mencía Castaño, I.; Curtin, C.M.; Duffy, G.P.; O’Brien, F.J. Next generation bone tissue engineering: Non-viral miR-133a inhibition using collagen-nanohydroxyapatite scaffolds rapidly enhances osteogenesis. Sci. Rep. 2016, 6, 27941. [Google Scholar] [CrossRef] [PubMed]
- Mencía Castaño, I.; Curtin, C.M.; Duffy, G.P.; O’Brien, F.J. Harnessing an inhibitory role of miR-16 in osteogenesis by human mesenchymal stem cells for advanced scaffold-based bone tissue engineering. Tissue Eng. Part A 2019, 25, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Feng, C.; Quan, J.; Wang, Z.; Wei, W.; Zang, S.; Kang, S.; Hui, G.; Chen, X.; Wang, Q. In situ controlled release of stromal cell-derived factor-1α and antimiR-138 for on-demand cranial bone regeneration. Carbohydr. Polym. 2018, 182, 215–224. [Google Scholar] [CrossRef]
- Wang, S.; Aurora, A.B.; Johnson, B.A.; Qi, X.; McAnally, J.; Hill, J.A.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell 2008, 15, 261–271. [Google Scholar] [CrossRef]
- Fasanaro, P.; D’Alessandra, Y.; Di Stefano, V.; Melchionna, R.; Romani, S.; Pompilio, G.; Capogrossi, M.C.; Martelli, F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J. Biol. Chem. 2008, 283, 15878–15883. [Google Scholar] [CrossRef] [PubMed]
- Chambers, P.; Ziminska, M.; Elkashif, A.; Wilson, J.; Redmond, J.; Tzagiollari, A.; Ferreira, C.; Balouch, A.; Bogle, J.; Donahue, S.W. The osteogenic and angiogenic potential of microRNA-26a deliveredviaa non-viral delivery peptide for bone repair. J. Ophthalmol. Clin. Res. 2023, 362, 489–501. [Google Scholar]
- Sadowska, J.M.; Ziminska, M.; Ferreira, C.; Matheson, A.; Balouch, A.; Bogle, J.; Wojda, S.; Redmond, J.; Elkashif, A.; Dunne, N. Development of miR-26a-activated scaffold to promote healing of critical-sized bone defects through angiogenic and osteogenic mechanisms. Biomaterials 2023, 303, 122398. [Google Scholar] [CrossRef]
- Ziaei Amiri, F.; Pashandi, Z.; Lotfibakhshaiesh, N.; Mirzaei-Parsa, M.J.; Ghanbari, H.; Faridi-Majidi, R. Cell attachment effects of collagen nanoparticles on crosslinked electrospun nanofibers. Int. J. Artif. Organs 2021, 44, 199–207. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Bhaskar, R.; Sudhakar, K.; Nam, D.H.; Han, S.S. Polydopamine-Functionalized Bacterial Cellulose as Hydrogel Scaffolds for Skin Tissue Engineering. Gels 2023, 9, 656. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Bhaskar, R.; Han, S.S. Leveraging the nanotopography of filamentous fungal chitin-glucan nano/microfibrous spheres (FNS) coated with collagen (type I) for scaffolded fibroblast spheroids in regenerative medicine. Tissue Cell 2025, 93, 102734. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Zo, S.M.; Han, S.S. Novel biomimetic chitin-glucan polysaccharide nano/microfibrous fungal-scaffolds for tissue engineering applications. Int. J. Biol. Macromol. 2020, 149, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.O.; Allo, B.A.; Klassen, R.; Hutter, J.L.; Dixon, S.J.; Rizkalla, A.S. Control of surface topography in biomimetic calcium phosphate coatings. Langmuir 2012, 28, 3871–3880. [Google Scholar] [CrossRef] [PubMed]
- Pudełko, I.; Moskwik, A.; Kwiecień, K.; Kriegseis, S.; Krok-Borkowicz, M.; Schickle, K.; Ochońska, D.; Dobrzyński, P.; Brzychczy-Włoch, M.; Gonzalez-Julian, J. Porous Zirconia Scaffolds Functionalized with Calcium Phosphate Layers and PLGA Nanoparticles Loaded with Hydrophobic Gentamicin. Int. J. Mol. Sci. 2023, 24, 8400. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wu, C.; Chen, S.; Qiao, Z.; Borovskikh, P.; Shchegolkov, A.; Chen, L.; Wei, D.; Sun, J.; Fan, H. Combining electrospinning and electrospraying to prepare a biomimetic neural scaffold with synergistic cues of topography and electrotransduction. ACS Appl. Bio Mater. 2020, 3, 5148–5159. [Google Scholar] [CrossRef]
- Wu, C.; Chen, S.; Zhou, T.; Wu, K.; Qiao, Z.; Zhang, Y.; Xin, N.; Liu, X.; Wei, D.; Sun, J. Antioxidative and conductive nanoparticles-embedded cell niche for neural differentiation and spinal cord injury repair. ACS Appl. Mater. Interfaces 2021, 13, 52346–52361. [Google Scholar] [CrossRef]
- Dahiya, A.; Chaudhari, V.S.; Kushram, P.; Bose, S. 3D Printed SiO2–Tricalcium Phosphate Scaffolds Loaded with Carvacrol Nanoparticles for Bone Tissue Engineering Application. J. Med. Chem. 2023, 67, 2745–2757. [Google Scholar] [CrossRef]
- Vu, A.A.; Burke, D.A.; Bandyopadhyay, A.; Bose, S. Effects of surface area and topography on 3D printed tricalcium phosphate scaffolds for bone grafting applications. Addit. Manuf. 2021, 39, 101870. [Google Scholar] [CrossRef]
- Qi, Y.; Guo, Y.; Liza, A.A.; Yang, G.; Sipponen, M.H.; Guo, J.; Li, H. Nanocellulose: A review on preparation routes and applications in functional materials. Cellulose 2023, 30, 4115–4147. [Google Scholar] [CrossRef]
- Babi, M.; Riesco, R.; Boyer, L.; Fatona, A.; Accardo, A.; Malaquin, L.; Moran-Mirabal, J. Tuning the nanotopography and chemical functionality of 3d printed scaffolds through cellulose nanocrystal coatings. ACS Appl. Bio Mater. 2021, 4, 8443–8455. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 38. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, X.; Luo, J.; Zheng, X.; Wang, H.; Wang, J.; Xi, Z.; Liao, X.; Machuki, J.O.a.; Guo, K. Novel hierarchical nitrogen-doped multiwalled carbon nanotubes/cellulose/nanohydroxyapatite nanocomposite as an osteoinductive scaffold for enhancing bone regeneration. ACS Biomater. Sci. Eng. 2018, 5, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yang, S.; Xiao, J.; Ouyang, Z.; Yang, M.; Zhang, M.; Zhao, D.; Huang, Q. Facile synthesis of multi-functional nano-composites by precise loading of Cu2+ onto MgO nano-particles for enhanced osteoblast differentiation, inhibited osteoclast formation and effective bacterial killing. Mater. Sci. Eng. C 2021, 130, 112442. [Google Scholar] [CrossRef]
- Yi, B.; Xu, Q.; Liu, W. An overview of substrate stiffness guided cellular response and its applications in tissue regeneration. Bioact. Mater. 2022, 15, 82–102. [Google Scholar] [CrossRef] [PubMed]
- Makaremi, S.; Luu, H.; Boyle, J.P.; Zhu, Y.; Cerson, C.; Bowdish, D.M.; Moran-Mirabal, J.M. The topography of silica films modulates primary macrophage morphology and function. Adv. Mater. Interfaces 2019, 6, 1900677. [Google Scholar] [CrossRef]
- Kemppi, H.; Finnilä, M.; Lorite, G.; Nelo, M.; Juuti, J.; Kokki, M.; Kokki, H.; Räsänen, J.; Mobasheri, A.; Saarakkala, S. Design and development of poly-L/D-lactide copolymer and barium titanate nanoparticle 3D composite scaffolds using breath figure method for tissue engineering applications. Colloids Surf. B Biointerfaces 2021, 199, 111530. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, F.; Shuai, Y.; Peng, S.; Chen, S.; Deng, Y.; Feng, P. Silicon dioxide nanoparticles decorated on graphene oxide nanosheets and their application in poly (l-lactic acid) scaffold. J. Adv. Res. 2023, 48, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liang, L.; Liu, L.; Yin, Y.; Liu, Y.; Lei, G.; Zhou, K.; Huang, Q.; Wu, H. Using MgO nanoparticles as a potential platform to precisely load and steadily release Ag ions for enhanced osteogenesis and bacterial killing. Mater. Sci. Eng. C 2021, 119, 111399. [Google Scholar] [CrossRef]
- Rezaei, A.; Mohammadi, M. In vitro study of hydroxyapatite/polycaprolactone (HA/PCL) nanocomposite synthesized by an in situ sol–gel process. Mater. Sci. Eng. C 2013, 33, 390–396. [Google Scholar] [CrossRef]
- El-Habashy, S.E.; Eltaher, H.M.; Gaballah, A.; Zaki, E.I.; Mehanna, R.A.; El-Kamel, A.H. Hybrid bioactive hydroxyapatite/polycaprolactone nanoparticles for enhanced osteogenesis. Mater. Sci. Eng. C 2021, 119, 111599. [Google Scholar] [CrossRef]
- Kadkhodaie-Elyaderani, A.; de Lama-Odría, M.d.C.; Rivas, M.; Martínez-Rovira, I.; Yousef, I.; Puiggalí, J.; Del Valle, L.J. Medicated scaffolds prepared with hydroxyapatite/streptomycin nanoparticles encapsulated into polylactide microfibers. Int. J. Mol. Sci. 2022, 23, 1282. [Google Scholar] [CrossRef]
- Zhukova, Y.; Skorb, E.V. Cell guidance on nanostructured metal based surfaces. Adv. Healthc. Mater. 2017, 6, 1600914. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A. Magnesium whitlockite–omnipresent in pathological mineralisation of soft tissues but not a significant inorganic constituent of bone. Acta Biomater. 2021, 125, 72–82. [Google Scholar] [CrossRef]
- Hughes, J.M.; Jolliff, B.L.; Rakovan, J. The crystal chemistry of whitlockite and merrillite and the dehydrogenation of whitlockite to merrillite. Am. Mineral. 2008, 93, 1300–1305. [Google Scholar] [CrossRef]
- Zhang, K.; Hu, H.; Sun, Y.; Nan, J.; Liu, W.; Lei, P.; Hu, Y. The bio-functionalized membrane loaded with Ta/WH nanoparticles promote bone regeneration through neurovascular coupling. Colloids Surf. B Biointerfaces 2023, 230, 113506. [Google Scholar] [CrossRef]
- Franco-Urquiza, E.A. Clay-based polymer nanocomposites: Essential work of fracture. Polymers 2021, 13, 2399. [Google Scholar] [CrossRef]
- Timmins, R.L.; Kumar, A.; Röhrl, M.; Havlíček, K.; Agarwal, S.; Breu, J. High barrier nanocomposite film with accelerated biodegradation by clay swelling induced fragmentation. Macromol. Mater. Eng. 2022, 307, 2100727. [Google Scholar] [CrossRef]
- Islam, H.B.M.Z.; Susan, M.A.B.H.; Imran, A.B. High-strength potato starch/hectorite clay-based nanocomposite film: Synthesis and characterization. Iran. Polym. J. 2021, 30, 513–521. [Google Scholar] [CrossRef]
- Rebitski, E.P.; Darder, M.; Carraro, R.; Aranda, P.; Ruiz-Hitzky, E. Chitosan and pectin core–shell beads encapsulating metformin–clay intercalation compounds for controlled delivery. New J. Chem. 2020, 44, 10102–10110. [Google Scholar] [CrossRef]
- Masood, F.; Haider, H.; Yasin, T. Sepiolite/poly-3-hydroxyoctanoate nanocomposites: Effect of clay content on physical and biodegradation properties. Appl. Clay Sci. 2019, 175, 130–138. [Google Scholar] [CrossRef]
- Halabian, R.; Moridi, K.; Korani, M.; Ghollasi, M. Composite nanoscaffolds modified with bio-ceramic nanoparticles (Zn2SiO4) prompted osteogenic differentiation of human induced pluripotent stem cells. Int. J. Mol. Cell. Med. 2019, 8, 24. [Google Scholar]
- Bardeei, L.K.; Seyedjafari, E.; Hossein, G.; Nabiuni, M.; Ara, M.H.M.; Salber, J. Regeneration of bone defects in a rabbit femoral osteonecrosis model using 3D-printed poly (epsilon-caprolactone)/nanoparticulate willemite composite scaffolds. Int. J. Mol. Sci. 2021, 22, 10332. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xia, P.; Pan, S.; Qi, Z.; Fu, C.; Yu, Z.; Kong, W.; Chang, Y.; Wang, K.; Wu, D. The advances of ceria nanoparticles for biomedical applications in orthopaedics. Int. J. Nanomed. 2020, 2020, 7199–7214. [Google Scholar] [CrossRef] [PubMed]
- Burdușel, A.-C.; Gherasim, O.; Andronescu, E.; Grumezescu, A.M.; Ficai, A. Inorganic nanoparticles in bone healing applications. Pharmaceutics 2022, 14, 770. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, Y.; Xiong, S.; Zhou, Y.; Xiao, L.; Ma, Y.; Xiao, Y.; Wang, X. Modulatory Role of Silver Nanoparticles and Mesenchymal Stem Cell–Derived Exosome-Modified Barrier Membrane on Macrophages and Osteogenesis. Front. Chem. 2021, 9, 699802. [Google Scholar] [CrossRef]
- Seisenbaeva, G.A.; Fromell, K.; Vinogradov, V.V.; Terekhov, A.N.; Pakhomov, A.V.; Nilsson, B.; Ekdahl, K.N.; Vinogradov, V.V.; Kessler, V.G. Dispersion of TiO2 nanoparticles improves burn wound healing and tissue regeneration through specific interaction with blood serum proteins. Sci. Rep. 2017, 7, 15448. [Google Scholar] [CrossRef]
- Kapat, K.; Shubhra, Q.T.; Zhou, M.; Leeuwenburgh, S. Piezoelectric nano-biomaterials for biomedicine and tissue regeneration. Adv. Funct. Mater. 2020, 30, 1909045. [Google Scholar] [CrossRef]
- Iman, M.; Araghi, M.; Panahi, Y.; Mohammadi, R. Effects of chitosan-zinc oxide nanocomposite conduit on transected sciatic nerve: An animal model study. Bull. Emerg. Trauma 2017, 5, 240. [Google Scholar] [CrossRef][Green Version]
- Qian, Y.; Cheng, Y.; Song, J.; Xu, Y.; Yuan, W.E.; Fan, C.; Zheng, X. Mechano-informed biomimetic polymer scaffolds by incorporating self-powered zinc oxide nanogenerators enhance motor recovery and neural function. Small 2020, 16, 2000796. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Dai, Z.; Hu, H.; Liu, Z.; Liu, W. Surface-modified electrospun poly-ε-caprolactone incorporating ZnO NPs and QK peptide to repair bone defect via osteogenesis, angiogenesis and antibacterial. Colloids Surf. B: Biointerfaces 2025, 246, 114388. [Google Scholar] [CrossRef]
- Rzigalinski, B.A.; Carfagna, C.S.; Ehrich, M. Cerium oxide nanoparticles in neuroprotection and considerations for efficacy and safety. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1444. [Google Scholar] [CrossRef]
- Ciofani, G.; Genchi, G.G.; Liakos, I.; Cappello, V.; Gemmi, M.; Athanassiou, A.; Mazzolai, B.; Mattoli, V. Effects of cerium oxide nanoparticles on PC12 neuronal-like cells: Proliferation, differentiation, and dopamine secretion. Pharm. Res. 2013, 30, 2133–2145. [Google Scholar] [CrossRef] [PubMed]
- Allu, I.; Kumar Sahi, A.; Kumari, P.; Sakhile, K.; Sionkowska, A.; Gundu, S. A brief review on cerium oxide (CeO2NPs)-based scaffolds: Recent advances in wound healing applications. Micromachines 2023, 14, 865. [Google Scholar] [CrossRef] [PubMed]
- Polo, Y.; Luzuriaga, J.; de Langarica, S.G.; Pardo-Rodríguez, B.; Martínez-Tong, D.E.; Tapeinos, C.; Manero-Roig, I.; Marin, E.; Muñoz-Ugartemendia, J.; Ciofani, G. Self-assembled three-dimensional hydrogels based on graphene derivatives and cerium oxide nanoparticles: Scaffolds for co-culture of oligodendrocytes and neurons derived from neural stem cells. Nanoscale 2023, 15, 4488–4505. [Google Scholar] [CrossRef]
- Marino, A.; Tonda-Turo, C.; De Pasquale, D.; Ruini, F.; Genchi, G.; Nitti, S.; Cappello, V.; Gemmi, M.; Mattoli, V.; Ciardelli, G. Gelatin/nanoceria nanocomposite fibers as antioxidant scaffolds for neuronal regeneration. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 386–395. [Google Scholar] [CrossRef]
- Xiang, J.-Y.; Kang, L.; Li, Z.-M.; Tseng, S.-L.; Wang, L.-Q.; Li, T.-H.; Li, Z.-J.; Huang, J.-Z.; Yu, N.-Z.; Long, X. Biological scaffold as potential platforms for stem cells: Current development and applications in wound healing. World J. Stem Cells 2024, 16, 334. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, V.; Beheshtizadeh, N.; Gharibshahian, M.; Sabouri, L.; Varzandeh, M.; Rezaei, N. Recent advances in regenerative medicine strategies for cancer treatment. Biomed. Pharmacother. 2021, 141, 111875. [Google Scholar] [CrossRef]
- Sun, R.; Chen, H.; Zheng, J.; Yoshitomi, T.; Kawazoe, N.; Yang, Y.; Chen, G. Composite scaffolds of gelatin and Fe3O4 nanoparticles for magnetic hyperthermia-based breast cancer treatment and adipose tissue regeneration. Adv. Healthc. Mater. 2023, 12, 2202604. [Google Scholar] [CrossRef]
- Funnell, J.L.; Ziemba, A.M.; Nowak, J.F.; Awada, H.; Prokopiou, N.; Samuel, J.; Guari, Y.; Nottelet, B.; Gilbert, R.J. Assessing the combination of magnetic field stimulation, iron oxide nanoparticles, and aligned electrospun fibers for promoting neurite outgrowth from dorsal root ganglia in vitro. Acta Biomater. 2021, 131, 302–313. [Google Scholar] [CrossRef]
- Lin, C.-C.; Chang, J.-J.; Yung, M.-C.; Huang, W.-C.; Chen, S.-Y. Spontaneously micropatterned silk/gelatin scaffolds with topographical, biological, and electrical stimuli for neuronal regulation. ACS Biomater. Sci. Eng. 2020, 6, 1144–1153. [Google Scholar] [CrossRef]
- Bonfrate, V.; Manno, D.; Serra, A.; Salvatore, L.; Sannino, A.; Buccolieri, A.; Serra, T.; Giancane, G. Enhanced electrical conductivity of collagen films through long-range aligned iron oxide nanoparticles. J. Colloid Interface Sci. 2017, 501, 185–191. [Google Scholar] [CrossRef]
- Friedrich, R.P.; Cicha, I.; Alexiou, C. Iron oxide nanoparticles in regenerative medicine and tissue engineering. Nanomaterials 2021, 11, 2337. [Google Scholar] [CrossRef]
- Karimi, S.; Bagher, Z.; Najmoddin, N.; Simorgh, S.; Pezeshki-Modaress, M. Alginate-magnetic short nanofibers 3D composite hydrogel enhances the encapsulated human olfactory mucosa stem cells bioactivity for potential nerve regeneration application. Int. J. Biol. Macromol. 2021, 167, 796–806. [Google Scholar] [CrossRef]
- He, X.; Zhang, G.; Wang, X.; Hang, R.; Huang, X.; Qin, L.; Tang, B.; Zhang, X. Biocompatibility, corrosion resistance and antibacterial activity of TiO2/CuO coating on titanium. Ceram. Int. 2017, 43, 16185–16195. [Google Scholar] [CrossRef]
- Tamplenizza, M.; Lenardi, C.; Maffioli, E.; Nonnis, S.; Negri, A.; Forti, S.; Sogne, E.; De Astis, S.; Matteoli, M.; Schulte, C. Nitric oxide synthase mediates PC12 differentiation induced by the surface topography of nanostructured TiO2. J. Nanobiotechnol. 2013, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Baltatu, M.S.; Vizureanu, P.; Sandu, A.V.; Solcan, C.; Hritcu, L.D.; Spataru, M.C. Research Progress of Titanium-Based Alloys for Medical Devices. Biomedicines 2023, 11, 2997. [Google Scholar] [CrossRef] [PubMed]
- Kandel, R.; Jang, S.R.; Shrestha, S.; Lee, S.Y.; Shrestha, B.K.; Park, C.H.; Kim, C.S. Biomimetic cell–substrate of chitosan-cross-linked polyaniline patterning on TiO2 nanotubes enables hBM-MSCs to differentiate the osteoblast cell type. ACS Appl. Mater. Interfaces 2021, 13, 47100–47117. [Google Scholar] [CrossRef]
- Alon, N.; Miroshnikov, Y.; Perkas, N.; Nissan, I.; Gedanken, A.; Shefi, O. Silver nanoparticles promote neuronal growth. Procedia Eng. 2013, 59, 25–29. [Google Scholar] [CrossRef]
- Alon, N.; Miroshnikov, Y.; Perkas, N.; Nissan, I.; Gedanken, A.; Shefi, O. Substrates coated with silver nanoparticles as a neuronal regenerative material. Int. J. Nanomed. 2014, 9, 23–31. [Google Scholar]
- Dayem, A.A.; Kim, B.; Gurunathan, S.; Choi, H.Y.; Yang, G.; Saha, S.K.; Han, D.; Han, J.; Kim, K.; Kim, J.H. Biologically synthesized silver nanoparticles induce neuronal differentiation of SH-SY5Y cells via modulation of reactive oxygen species, phosphatases, and kinase signaling pathways. Biotechnol. J. 2014, 9, 934–943. [Google Scholar] [CrossRef]
- Lee, J.M.; Moon, J.Y.; Kim, T.H.; Lee, S.W.; Ahrberg, C.D.; Chung, B.G. Conductive hydrogel/nanowire micropattern-based sensor for neural stem cell differentiation. Sens. Actuators B Chem. 2018, 258, 1042–1050. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Pan, Z.; Liu, Y. Advanced bioactive nanomaterials for biomedical applications. Exploration 2021, 1, 20210089. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Jiang, Y.; Yang, X.; Wang, Y.; Ji, W.; Jia, G. Mussel-inspired gold nanoparticle and PLGA/L-lysine-g-graphene oxide composite scaffolds for bone defect repair. Int. J. Nanomed. 2021, 2021, 6693–6718. [Google Scholar] [CrossRef]
- Raspa, A.; Marchini, A.; Pugliese, R.; Mauri, M.; Maleki, M.; Vasita, R.; Gelain, F. A biocompatibility study of new nanofibrous scaffolds for nervous system regeneration. Nanoscale 2016, 8, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.; Jahromi, M.; Vatankhah, E.; Seyedebrahimi, R. Differential effects of rat ADSCs encapsulation in fibrin matrix and combination delivery of BDNF and Gold nanoparticles on peripheral nerve regeneration. BMC Neurosci. 2021, 22, 50. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhao, Q.; Zheng, L.w.; Wang, M. Multifunctional Nanofibrous Scaffolds Capable of Localized Delivery of Theranostic Nanoparticles for Postoperative Cancer Management. Adv. Healthc. Mater. 2023, 12, e2302484. [Google Scholar] [CrossRef]
- Aydeger, A.; Aysit, N.; Baydas, G.; Cakici, C.; Erim, U.C.; Arpa, M.D.; Ozcicek, I. Design of IKVAV peptide/gold nanoparticle decorated, micro/nano-channeled PCL/PLGA film scaffolds for neuronal differentiation and neurite outgrowth. Biomater. Adv. 2023, 152, 213472. [Google Scholar] [CrossRef]
- Boularaoui, S.; Shanti, A.; Lanotte, M.; Luo, S.; Bawazir, S.; Lee, S.; Christoforou, N.; Khan, K.A.; Stefanini, C. Nanocomposite conductive bioinks based on low-concentration GelMA and MXene nanosheets/gold nanoparticles providing enhanced printability of functional skeletal muscle tissues. ACS Biomater. Sci. Eng. 2021, 7, 5810–5822. [Google Scholar] [CrossRef]
- Adel, M.; Zahmatkeshan, M.; Johari, B.; Kharrazi, S.; Mehdizadeh, M.; Bolouri, B.; Rezayat, S.M. Investigating the effects of electrical stimulation via gold nanoparticles on in vitro neurite outgrowth: Perspective to nerve regeneration. Microelectron. Eng. 2017, 173, 1–5. [Google Scholar] [CrossRef]
- Sharifi, M.; Farahani, M.K.; Salehi, M.; Atashi, A.; Alizadeh, M.; Kheradmandi, R.; Molzemi, S. Exploring the Physicochemical, Electroactive, and Biodelivery Properties of Metal Nanoparticles on Peripheral Nerve Regeneration. ACS Biomater. Sci. Eng. 2022, 9, 106–138. [Google Scholar] [CrossRef]
- Saderi, N.; Rajabi, M.; Akbari, B.; Firouzi, M.; Hassannejad, Z. Fabrication and characterization of gold nanoparticle-doped electrospun PCL/chitosan nanofibrous scaffolds for nerve tissue engineering. J. Mater. Sci. Mater. Med. 2018, 29, 134. [Google Scholar] [CrossRef]
- Bowman, A.B.; Kwakye, G.F.; Hernández, E.H.; Aschner, M. Role of manganese in neurodegenerative diseases. J. Trace Elem. Med. Biol. 2011, 25, 191–203. [Google Scholar] [CrossRef]
- Underwood, E. Trace Elements in Human and Animal Nutrition; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Bhang, S.H.; Han, J.; Jang, H.-K.; Noh, M.-K.; La, W.-G.; Yi, M.; Kim, W.-S.; Kwon, Y.K.; Yu, T.; Kim, B.-S. pH-triggered release of manganese from MnAu nanoparticles that enables cellular neuronal differentiation without cellular toxicity. Biomaterials 2015, 55, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Zangene, E.; Manouchehri, S.; Amirabad, L.M.; Baheiraei, N.; Hadjighasem, M.R.; Farokhi, M.; Ganjali, M.R.; Walker, B.W.; Saeb, M.R. Conductive biomaterials as nerve conduits: Recent advances and future challenges. Appl. Mater. Today 2020, 20, 100784. [Google Scholar] [CrossRef]
- Qian, Y.; Song, J.; Zheng, W.; Zhao, X.; Ouyang, Y.; Yuan, W.; Fan, C. 3D manufacture of gold nanocomposite channels facilitates neural differentiation and regeneration. Adv. Funct. Mater. 2018, 28, 1707077. [Google Scholar] [CrossRef]
- Wang, S.; Guan, S.; Zhu, Z.; Li, W.; Liu, T.; Ma, X. Hyaluronic acid doped-poly (3, 4-ethylenedioxythiophene)/chitosan/gelatin (PEDOT-HA/Cs/Gel) porous conductive scaffold for nerve regeneration. Mater. Sci. Eng. C 2017, 71, 308–316. [Google Scholar] [CrossRef]
- Alghazali, K.M.; Newby, S.D.; Nima, Z.A.; Hamzah, R.N.; Watanabe, F.; Bourdo, S.E.; Masi, T.J.; Stephenson, S.M.; Anderson, D.E.; Dhar, M.S. Functionalized gold nanorod nanocomposite system to modulate differentiation of human mesenchymal stem cells into neural-like progenitors. Sci. Rep. 2017, 7, 16654. [Google Scholar] [CrossRef]
- Paviolo, C.; Haycock, J.W.; Yong, J.; Yu, A.; McArthur, S.L.; Stoddart, P.R. Plasmonic properties of gold nanoparticles can promote neuronal activity. In Proceedings of the Optical Interactions with Tissue and Cells XXIV, San Francisco, CA, USA, 4–5 February 2013; pp. 63–73. [Google Scholar]
- Baranes, K.; Shevach, M.; Shefi, O.; Dvir, T. Gold nanoparticle-decorated scaffolds promote neuronal differentiation and maturation. Nano Lett. 2016, 16, 2916–2920. [Google Scholar] [CrossRef]
- Shevach, M.; Fleischer, S.; Shapira, A.; Dvir, T. Gold nanoparticle-decellularized matrix hybrids for cardiac tissue engineering. Nano Lett. 2014, 14, 5792–5796. [Google Scholar] [CrossRef]
- Somasuntharam, I.; Yehl, K.; Carroll, S.L.; Maxwell, J.T.; Martinez, M.D.; Che, P.-L.; Brown, M.E.; Salaita, K.; Davis, M.E. Knockdown of TNF-α by DNAzyme gold nanoparticles as an anti-inflammatory therapy for myocardial infarction. Biomaterials 2016, 83, 12–22. [Google Scholar] [CrossRef]
- Maharjan, B.; Kumar, D.; Awasthi, G.P.; Bhattarai, D.P.; Kim, J.Y.; Park, C.H.; Kim, C.S. Synthesis and characterization of gold/silica hybrid nanoparticles incorporated gelatin methacrylate conductive hydrogels for H9C2 cardiac cell compatibility study. Compos. Part B Eng. 2019, 177, 107415. [Google Scholar] [CrossRef]
- Beltran-Vargas, N.E.; Peña-Mercado, E.; Sánchez-Gómez, C.; Garcia-Lorenzana, M.; Ruiz, J.-C.; Arroyo-Maya, I.; Huerta-Yepez, S.; Campos-Terán, J. Sodium alginate/chitosan scaffolds for cardiac tissue engineering: The influence of its three-dimensional material preparation and the use of gold nanoparticles. Polymers 2022, 14, 3233. [Google Scholar] [CrossRef] [PubMed]
- Beldjilali-Labro, M.; Jellali, R.; Brown, A.D.; Garcia Garcia, A.; Lerebours, A.; Guenin, E.; Bedoui, F.; Dufresne, M.; Stewart, C.; Grosset, J.-F. Multiscale-engineered muscle constructs: PEG hydrogel micro-patterning on an electrospun PCL mat functionalized with gold nanoparticles. Int. J. Mol. Sci. 2021, 23, 260. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narayanan, K.B. Nanotopographical Features of Polymeric Nanocomposite Scaffolds for Tissue Engineering and Regenerative Medicine: A Review. Biomimetics 2025, 10, 317. https://doi.org/10.3390/biomimetics10050317
Narayanan KB. Nanotopographical Features of Polymeric Nanocomposite Scaffolds for Tissue Engineering and Regenerative Medicine: A Review. Biomimetics. 2025; 10(5):317. https://doi.org/10.3390/biomimetics10050317
Chicago/Turabian StyleNarayanan, Kannan Badri. 2025. "Nanotopographical Features of Polymeric Nanocomposite Scaffolds for Tissue Engineering and Regenerative Medicine: A Review" Biomimetics 10, no. 5: 317. https://doi.org/10.3390/biomimetics10050317
APA StyleNarayanan, K. B. (2025). Nanotopographical Features of Polymeric Nanocomposite Scaffolds for Tissue Engineering and Regenerative Medicine: A Review. Biomimetics, 10(5), 317. https://doi.org/10.3390/biomimetics10050317






