Smart Nanocarriers in Cosmeceuticals Through Advanced Delivery Systems
Abstract
1. Introduction
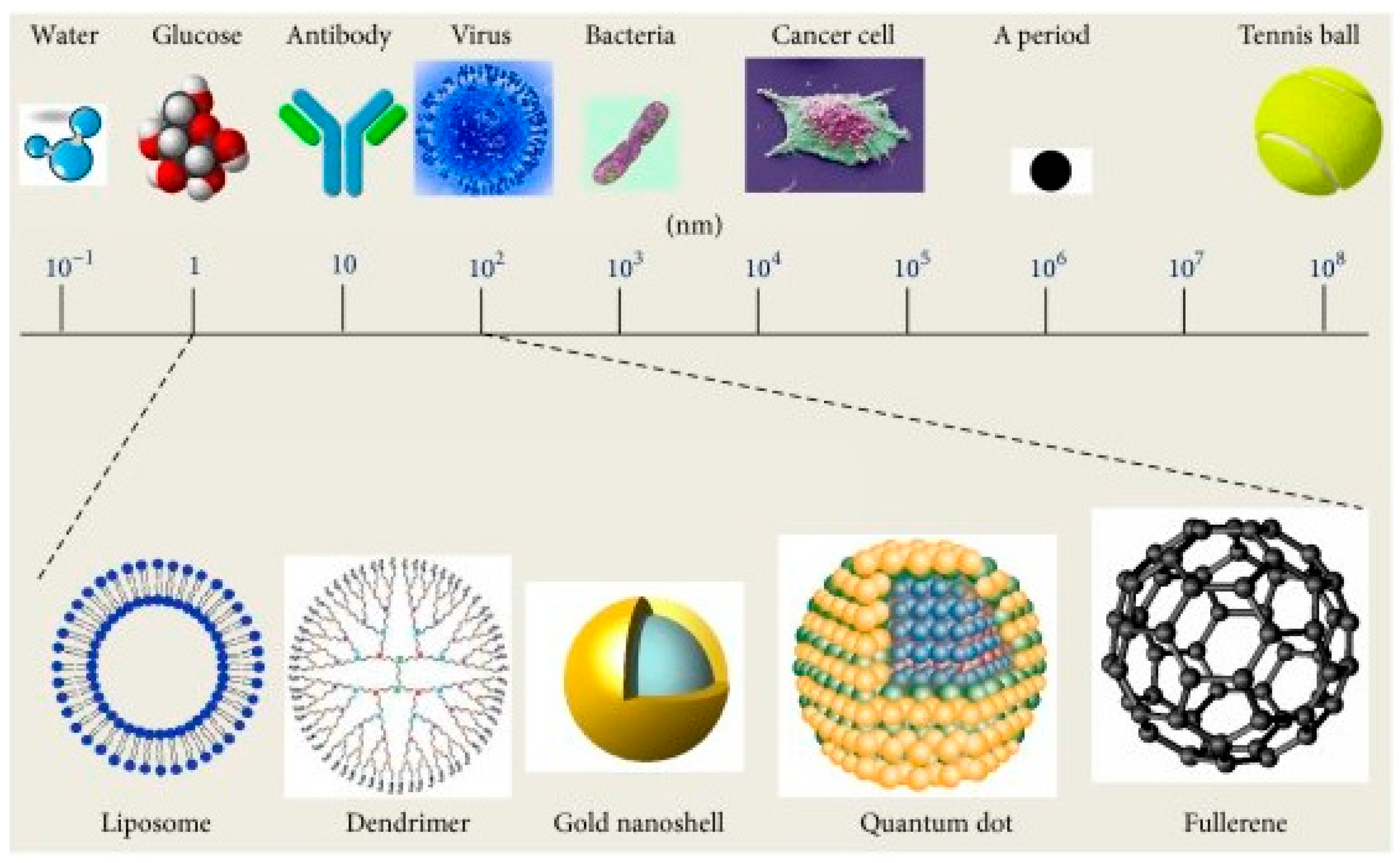
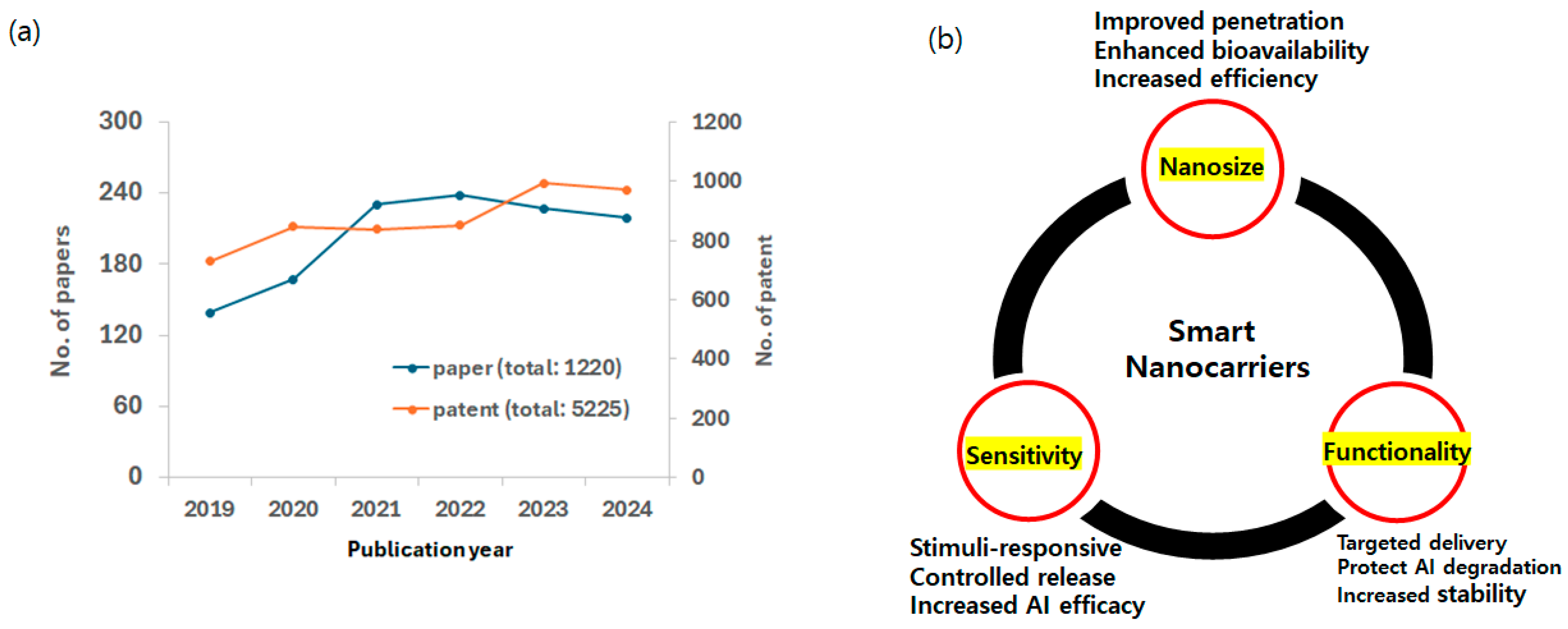
2. Smart Nanomaterials
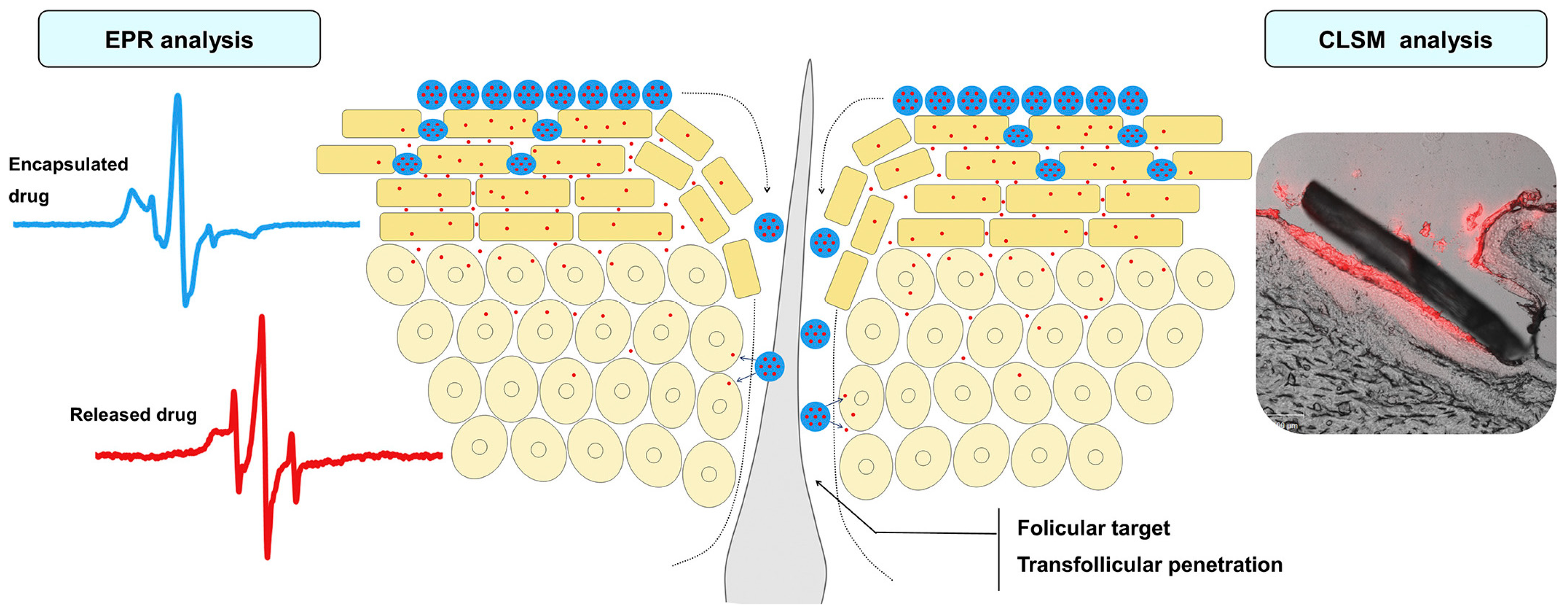
2.1. Thermosensitive Nanocarriers
| Stimuli | Mechanism (Mode of Action) | Nanocarrier | Applications | Reference |
|---|---|---|---|---|
| Thermo | Structural change | SLNs Poloxamers (or Pluronics) nanogels Polyglycerol-based nanogels Porous silica nanogels Polyether-based nanogels Medecassoside liposomes | Dermal delivery TDDS for skin infection treatment Topical drug delivery carrier Skin penetration enhancer Dermal delivery of biomolecules Treatment of inflammatory skin Topical drug delivery Wound healing | [36,38,46,47,48,49,50,51,52,53,54] |
| pH | Electrostatic repulsion | PLGA-based nanoparticles Eudragit E100 Cellulose phthalates NPs Eudragit L100 | Treatment of atopic dermatitis Skin care Dermal carriers Dermal carriers | [32,55,56] |
| Redox | Cleavage of specific bonds | PEG-block-PLA nanoparticles | Topical application of retinol | [57] |
| Enzyme | Cleavage of specific peptide sequences | PCL nanofiber patch | Wound healing | [58] |
| Electro | Structural change | Carbon nanotubes | Transdermal delivery system | [59] |
| Multi-stimuli | Temp/pH Redox/pH | PNIPAM-co-AAc nanogels PNIPAM-based microgels Liposome modified with acrylic polymers Liposomal sludge | Topical caffeine delivery Dermal delivery of biomolecules Delivery of cosmetic agents Transdermal delivery | [60,61,62,63] |
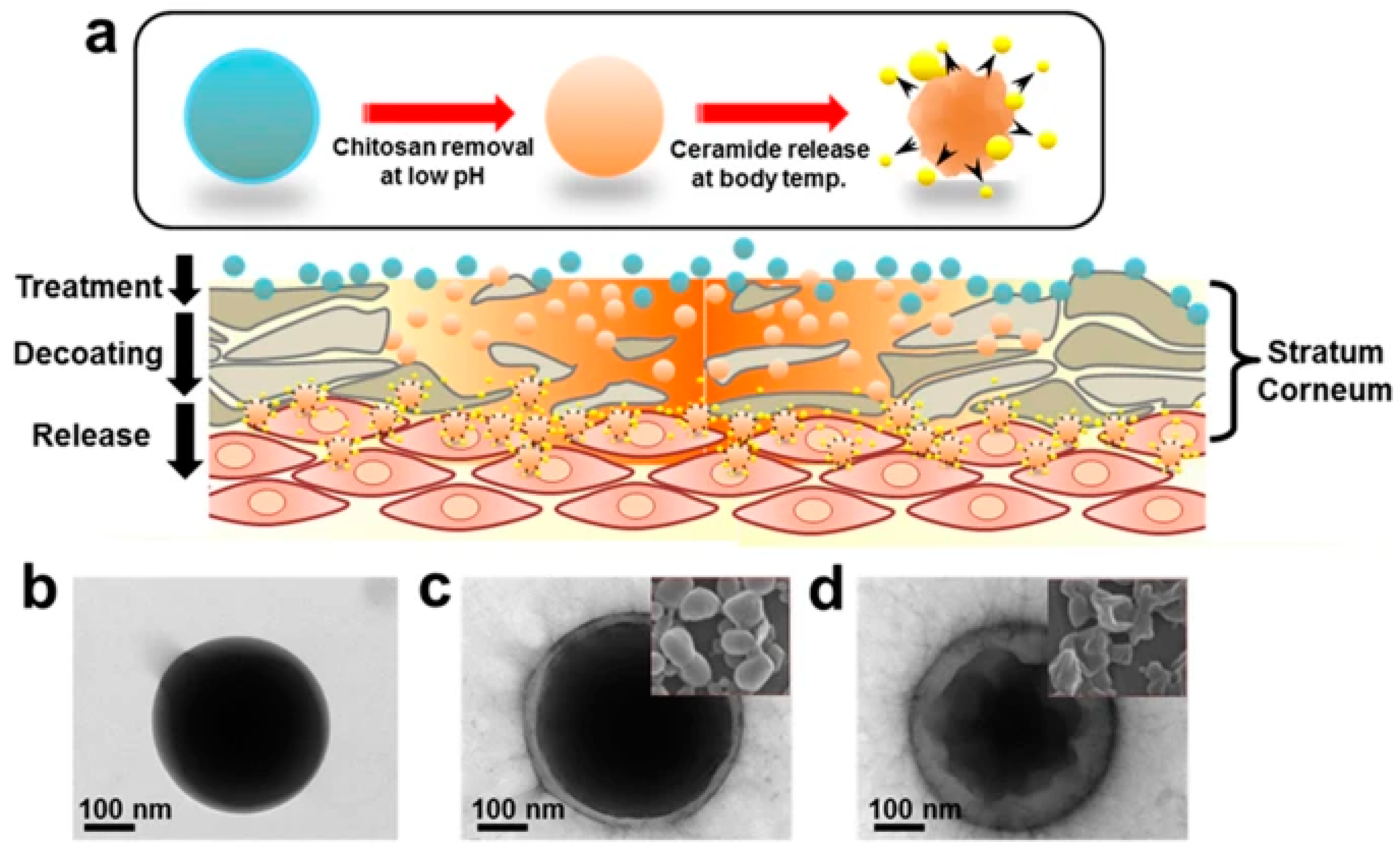
2.2. pH-Sensitive Nanocarriers
2.3. Other Stimuli-Sensitive Nanocarriers
2.4. Multiple Stimuli-Sensitive Systems
3. Biocompatibility of Nanocarriers
3.1. Nanomaterials
3.2. Regulatory Aspects
3.3. Biocompatibility Testing
3.3.1. In Vitro Testing
3.3.2. In Vivo Testing and Clinical Trial
4. Challenges and Future Perspectives
Funding
Conflicts of Interest
Abbreviations
| USPTO | United States Patent and Trademark Office |
| TDDS | transdermal drug delivery system |
| SLNs | solid lipid nanoparticles |
| PNIPAM | poly(N-isopropyl acrylamide |
| LCST | lower critical solution temperature |
| PEG | poly(ethylene glycol) |
| PLA | polylactic acid |
| PCL | polycaprolactone |
| AAc | acrylic acid |
| VPTT | volume phase transition temperature |
| NIPMAM | N-isopropylmethacryl amide |
| dPG | dendritic polyglycerol |
| NCs | nanocapsules |
| NPs | nanoparticles |
| OEG | oligo ethylene glycol |
| MSNs | mesoporous silica nanoparticles |
| PEGDMA | poly(ethylene glycol) methacrylate |
| PVCL | poly(N-viylcaprolactam |
| tNGs | thermosensitive nanogels |
| AD | atopic dermatitis |
| EGF | epidermal growth factor |
| MMP | matrix metalloproteinase |
| GSH | glutathione |
| NNI | National Nanotechnology Initiative |
| EC | European Committee |
| SCCS | Scientific Committee on Consumer Safety |
| EUON | European Observatory for Nanomaterials |
| NTF | Nanotechnology Task Force |
| PCPC | Personal Care Products Council |
References
- Gupta, V.; Mohapatra, S.; Mishra, H.; Farooq, U.; Kumar, K.; Ansari, M.J.; Aldawsari, M.F.; Alalaiwe, A.S.; Mirza, M.A.; Iqbal, Z. Nanotechnology in Cosmetics and Cosmeceuticals—A Review of Latest Advancements. Gels 2022, 8, 173. [Google Scholar] [CrossRef]
- Aziz, Z.A.A.; Mohd-Nasir, H.; Ahmad, A.; Mohd Setapar, S.H.; Peng, W.L.; Chuo, S.C.; Khatoon, A.; Umar, K.; Yaqoob, A.A.; Mohamad Ibrahim, M.N. Role of Nanotechnology for Design and Development of Cosmeceutical: Application in Makeup and Skin Care. Front. Chem. 2019, 7, 739. [Google Scholar] [CrossRef]
- Zhou, H.; Luo, D.; Chen, D.; Tan, X.; Bai, X.; Liu, Z.; Yang, X.; Liu, W. Current Advances of Nanocarrier Technology-Based Active Cosmetic Ingredients for Beauty Applications. Clin. Cosmet. Investig. Dermatol. 2021, 14, 867–887. [Google Scholar] [CrossRef] [PubMed]
- Martel-Estrada, S.; Morales-Cardona, A.; Vargas-Requena, C.; Rubio-Lara, J.; Martínez-Pérez, C.; Jimenez-Vega, F. Delivery systems in nanocosmeceuticals. Rev. Adv. Mater. Sci. 2022, 61, 901–930. [Google Scholar] [CrossRef]
- Golubovic-Liakopoulos, N.; Simon, S.R.; Shah, B. Nanotechnology use with cosmeceuticals. Semin. Cutan. Med. Surg. 2011, 30, 176–180. [Google Scholar] [CrossRef]
- Mohammed, Y.; Holmes, A.; Kwok, P.C.L.; Kumeria, T.; Namjoshi, S.; Imran, M.; Matteucci, L.; Ali, M.; Tai, W.T.; Benson, H.A.E.; et al. Advances and future perspectives in epithelial drug delivery. Adv. Drug Deliv. Rev. 2022, 186, 114293. [Google Scholar] [CrossRef]
- Puglia, C.; Santonocito, D. Cosmeceuticals: Nanotechnology-Based Strategies for the Delivery of Phytocompounds. Curr. Pharm. Des. 2019, 25, 2314–2322. [Google Scholar] [CrossRef]
- Marianecci, C.; Di Marzio, L.; Rinaldi, F.; Celia, C.; Paolino, D.; Alhaique, F.; Esposito, S.; Carafa, M. Niosomes from 80s to present: The state of the art. Adv. Colloid. Interface Sci. 2014, 205, 187–206. [Google Scholar] [CrossRef]
- Katz, L.; Dewan, K.; Bronaugh, R. Nanotechnology in cosmetics. Food Chem. Toxicol. 2015, 85, 127–137. [Google Scholar] [CrossRef]
- Mihranyan, A.; Ferraz, N.; Stromme, M. Current status and future prospects of nanotechnology in cosmetics. Prog. Mater. Sci. 2012, 57, 875–910. [Google Scholar] [CrossRef]
- Raszewska-Famielec, M.; Flieger, J. Nanoparticles for Topical Application in the Treatment of Skin Dysfunctions-An Overview of Dermo-Cosmetic and Dermatological Products. Int. J. Mol. Sci. 2022, 23, 15980. [Google Scholar] [CrossRef] [PubMed]
- Sethi, M.; Rana, R.; Sambhakar, S.; Chourasia, M. Nanocosmeceuticals: Trends and Recent Advancements in Self Care. Aaps Pharmscitech 2024, 25, 51. [Google Scholar] [CrossRef]
- Pareek, A.; Kapoor, S.K.; Yadav, S.; Rashid, M.; Fareed, M.S.; Akhter, G.; Muteeb, M.; Gupta, M.; Prajapati, B.G. Advancing lipid nanoparticles: A pioneering technology in cosmetic and dermatological treatments. Colloid. Interface Sci. Commun. 2025, 64, 100814. [Google Scholar] [CrossRef]
- Fytianos, G.; Rahdar, A.; Kyzas, G. Nanomaterials in Cosmetics: Recent Updates. Nanomaterials 2020, 10, 979. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Bansal, R.; Jindal, N.; Jindal, A. Nanocarriers and nanoparticles for skin care and dermatological treatments. Indian. Dermatol. Online J. 2013, 4, 267–272. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Coelho, C.; Teixeira, J.A.; Ferreira-Santos, P.; Botelho, C.M. Nanocarriers as Active Ingredients Enhancers in the Cosmetic Industry-The European and North America Regulation Challenges. Molecules 2022, 27, 1669. [Google Scholar] [CrossRef]
- Antunes, A.F.; Pereira, P.; Reis, C.; Rijo, P. Nanosystems for Skin Delivery: From Drugs to Cosmetics. Curr. Drug Metab. 2017, 18, 412–425. [Google Scholar] [CrossRef]
- Khezri, K.; Saeedi, M.; Dizaj, S. Application of nanoparticles in percutaneous delivery of active ingredients in cosmetic preparations. Biomed. Pharmacother. 2018, 106, 1499–1505. [Google Scholar] [CrossRef]
- Piluk, T.; Faccio, G.; Letsiou, S.; Liang, R.; Freire-Gormaly, M. A critical review investigating the use of nanoparticles in cosmetic skin products. Environ. Sci.-Nano 2024, 11, 3674–3692. [Google Scholar] [CrossRef]
- Maghraby, Y.R.; Ibrahim, A.H.; El-Shabasy, R.M.; Azzazy, H.M.E.-S. Overview of Nanocosmetics with Emphasis on those Incorporating Natural Extracts. ACS Omega 2024, 9, 36001–36022. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.I.; Glaser, D.A. Cosmeceuticals: The new medicine of beauty. Mo. Med. 2011, 108, 60–63. [Google Scholar] [PubMed]
- Goyal, A.; Sharma, J.; Kaur, S.; Kumari, M.; Garg, R.K.; Sindhu, M.H.; Rahman, M.F.; Akhtar, P.; Tagde, A.; Najda, B.; et al. Bioactive-Based Cosmeceuticals: An Update on Emerging Trends. Molecules 2022, 27, 828. [Google Scholar] [CrossRef]
- Pandey, A.; Jatana, G.K.; Sonthalia, S. Cosmeceuticals. In StatPearls; StatPearls Publishing: Treasure Island: FL, USA, 2025. [Google Scholar]
- Manela-Azulay, M.; Bagatin, E. Cosmeceuticals vitamins. Clin. Dermatol. 2009, 27, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.S.; Bawiskar, D.; Wagh, V. Nanocosmetics and Skin Health: A Comprehensive Review of Nanomaterials in Cosmetic Formulations. Cureus 2024, 16, e52754. [Google Scholar] [CrossRef]
- Pradhan, N.; Singh, S.; Ojha, N.; Shrivastava, A.; Barla, A.; Rai, V.; Bose, S. Facets of Nanotechnology as Seen in Food Processing, Packaging, and Preservation Industry. BioMed Research Int. 2015, 1, 365672. [Google Scholar] [CrossRef]
- Vinardell, M.P.; Mitjans, M. Nanocarriers for Delivery of Antioxidants on the Skin. Cosmetics 2015, 2, 342–354. [Google Scholar] [CrossRef]
- Van Gheluwe, L.; Chourpa, I.; Gaigne, C.; Munnier, E. Polymer-Based Smart Drug Delivery Systems for Skin Application and Demonstration of Stimuli-Responsiveness. Polymers 1285, 2021, 13. [Google Scholar] [CrossRef]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Klee, S.; Farwick, M.; Lersch, P. Triggered release of sensitive active ingredients upon response to the skin’s natural pH. Colloids Surf. a-Physicochem. Eng. Asp. 2009, 338, 162–166. [Google Scholar] [CrossRef]
- Nastiti, C.; Ponto, T.; Mohammed, Y.; Roberts, M.S.; Benson, H.A.E. Novel Nanocarriers for Targeted Topical Skin Delivery of the Antioxidant Resveratrol. Pharmaceutics 2020, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Elkhoury, K.; Koçak, P.; Kang, A.; Arab-Tehrany, E.; Ward, J.E.; Shin, S.R. Engineering Smart Targeting Nanovesicles and Their Combination with Hydrogels for Controlled Drug Delivery. Pharmaceutics 2020, 12, 849. [Google Scholar] [CrossRef]
- Parhi, R. Development and optimization of pluronic® F127 and HPMC based thermosensitive gel for the skin delivery of metoprolol succinate. J. Drug Deliv. Sci. Technol. 2016, 36, 23–33. [Google Scholar] [CrossRef]
- Rancan, F.; Giulbudagian, M.; Jurisch, J.; Blume-Peytavi, U.; Calderón, M.; Vogt, A. Drug delivery across intact and disrupted skin barrier: Identification of cell populations interacting with penetrated thermoresponsive nanogels. Eur. J. Pharm. Biopharm. 2017, 116, 4–11. [Google Scholar] [CrossRef]
- op’t Veld, R.C.; van den Boomen, O.I.; Lundvig, D.M.; Bronkhorst, E.M.; Kouwer, P.H.; Jansen, J.A.; Middelkoop, E.; Von den Hoff, J.W.; Rowan, A.E.; Wagener, F.A.D.T.G. Thermosensitive biomimetic polyisocyanopeptide hydrogels may facilitate wound repair. Biomaterials 2018, 181, 392–401. [Google Scholar] [CrossRef]
- Asadian-Birjand, M.; Bergueiro, J.; Rancan, F.; Cuggino, J.C.; Mutihac, R.C.; Achazi, K.; Dernedde, J.; Blume-Peytayi, U.; Vogt, A.; Calderón, M. Engineering thermoresponsive polyether-based nanogels for temperature dependent skin penetration. Polym. Chem. 2015, 6, 5827–5831. [Google Scholar] [CrossRef]
- Kang, M.; Hong, S.; Kim, J. Release property of microgels formed by electrostatic interaction between poly(N-isopropylacrylamide-co-methacrylic acid) and poly(N-isopropylacrylamide-co-dimethylaminoethylmethacrylate). J. Appl. Polym. Sci. 2012, 125, 1993–1999. [Google Scholar] [CrossRef]
- Ferreira, N.; Ferreira, L.; Cardoso, V.; Boni, F.; Souza, A.; Gremiao, M. Recent advances in smart hydrogels for biomedical applications: From self-assembly to functional approaches. Eur. Polym. J. 2018, 99, 117–133. [Google Scholar] [CrossRef]
- Neumann, M.; di Marco, G.; Iudin, D.; Viola, M.; van Nostrum, C.F.; van Ravensteijn, B.G.; Vermonden, T. Stimuli-Responsive Hydrogels: The Dynamic Smart Biomaterials of Tomorrow. Macromolecules 2023, 56, 8377–8392. [Google Scholar] [CrossRef]
- Gandhi, A.; Paul, A.; Sen, S.; Sen, K. Studies on thermoresponsive polymers: Phase behaviour, drug delivery and biomedical applications. Asian J. Pharm. Sci. 2015, 10, 99–107. [Google Scholar] [CrossRef]
- Ghosh, D.D.; Chakrabarti, G. Applications of Targeted Nano Drugs and Delivery Systems. In Thermoresponsive Drug Delivery Systems, Characterization and Application; Ghosh, D.D., Chakrabarti, G., Eds.; Elsevier: Oxford, UK, 2019; pp. 133–155. [Google Scholar]
- Ding, Y.; Pyo, S.M.; Müller, R.H. smartLipids(®) as third solid lipid nanoparticle generation—Stabilization of retinol for dermal application. Pharmazie 2017, 72, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Schäfer-Korting, M.; Mehnert, W.; Korting, H.C. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv. Drug. Deliv. Rev. 2007, 59, 427–443. [Google Scholar] [CrossRef]
- Kang, J.H.; Chon, J.; Kim, Y.I.; Lee, H.J.; Oh, D.W.; Lee, H.G.; Han, C.S.; Kim, D.W.; Park, C.W. Preparation and evaluation of tacrolimus-loaded thermosensitive solid lipid nanoparticles for improved dermal distribution. Int. J. Nanomed. 2019, 14, 5381–5396. [Google Scholar] [CrossRef]
- Brugués, A.; Naveros, B.; Campmany, A.; Pastor, P.; Saladrigas, R.; Lizandra, C. Developing cutaneous applications of paromomycin entrapped in stimuli-sensitive block copolymer nanogel dispersions. Nanomedicine 2015, 10, 227–240. [Google Scholar] [CrossRef]
- Witting, M.; Molina, M.; Obst, K.; Plank, R.; Eckl, K.M.; Hennies, H.C.; Calderón, M.; Frieß, W.; Hedtrich, S. Thermosensitive dendritic polyglycerol-based nanogels for cutaneous delivery of biomacromolecules. Nanomed.-Nanotechnol. Biol. Med. 2015, 11, 1179–1187. [Google Scholar] [CrossRef]
- Gerecke, C.; Edlich, A.; Giulbudagian, M.; Schumacher, F.; Zhang, N.; Said, A.; Yealland, G.; Lohan, S.B.; Neumann, F.; Meinke, M.C.; et al. Biocompatibility and characterization of polyglycerol-based thermoresponsive nanogels designed as novel drug-delivery systems and their intracellular localization in keratinocytes. Nanotoxicology 2017, 11, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Blanco, E.R.; Rancan, F.; Klossek, A.; Nissen, J.H.; Hoffmann, L.; Bergueiro, J.; Riedel, S.; Vogt, A.; Rühl, E.; Calderón, M. Polyglycerol-Based Thermoresponsive Nanocapsules Induce Skin Hydration and Serve as a Skin Penetration Enhancer. Acs Appl. Mater. Interfaces 2020, 12, 30136–30144. [Google Scholar] [CrossRef] [PubMed]
- Ugazio, E.; Gastaldi, L.; Brunella, V.; Scalarone, D.; Jadhav, S.A.; Oliaro-Bosso, S.; Zonari, D.; Berlier, G.; Miletto, I.; Sapino, S. Thermoresponsive mesoporous silica nanoparticles as a carrier for skin delivery of quercetin. Int. J. Pharm. 2016, 511, 446–454. [Google Scholar] [CrossRef]
- Jadhav, S.; Scalarone, D.; Brunella, V.; Ugazio, E.; Sapino, S.; Berlier, G. Thermoresponsive copolymer-grafted SBA-15 porous silica particles for temperature-triggered topical delivery systems. Express Polym. Lett. 2017, 11, 96–105. [Google Scholar] [CrossRef]
- Giulbudagian, M.; Hönzke, S.; Bergueiro, J.; Işık, D.; Schumacher, F.; Saeidpour, S.; Lohan, S.B.; Meinke, M.C.; Teutloff, C.; Schäfer-Korting, M.; et al. Enhanced topical delivery of dexamethasone by β-cyclodextrin decorated thermoresponsive nanogels. Nanoscale 2018, 10, 469–479. [Google Scholar] [CrossRef]
- Liu, M.; Chen, W.; Zhang, X.; Su, P.; Yue, F.; Zeng, S.; Du, S. Improved surface adhesion and wound healing effect of madecassoside liposomes modified by temperature -responsive PEG-PCL-PEG copolymers. Eur. J. Pharm. Sci. 2020, 151, 105373. [Google Scholar] [CrossRef]
- Jung, S.M.; Yoon, G.H.; Lee, H.C.; Jung, M.H.; Yu, S.I.; Yeon, S.J.; Min, S.K.; Kwon, Y.S.; Hwang, J.H.; Shin, H.S. Thermodynamic Insights and Conceptual Design of Skin-Sensitive Chitosan Coated Ceramide/PLGA Nanodrug for Regeneration of Stratum Corneum on Atopic Dermatitis. Sci. Rep. 2015, 5, 18089. [Google Scholar] [CrossRef]
- Sahle, F.; Gerecke, C.; Kleuser, B.; Bodmeier, R. Formulation and comparative in vitro evaluation of various dexamethasone-loaded pH-sensitive polymeric nanoparticles intended for dermal applications. Int. J. Pharm. 2017, 516, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Van Gheluwe, L.; Buchy, E.; Chourpa, I.; Munnier, E. Three-Step Synthesis of a Redox-Responsive Blend of PEG-block-PLA and PLA and Application to the Nanoencapsulation of Retinol. Polymers 2020, 12, 10. [Google Scholar] [CrossRef]
- Kim, S.E.; Lee, P.W.; Pokorski, J.K. Biologically Triggered Delivery of EGF from Polymer Fiber Patches. ACS Macro Lett. 2017, 6, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Im, J.S.; Bai, B.; Lee, Y.S. The effect of carbon nanotubes on drug delivery in an electro-sensitive transdermal drug delivery system. Biomaterials 2010, 31, 1414–1419. [Google Scholar] [CrossRef]
- Samah, N.A.; Heard, C. Enhanced in vitro transdermal delivery of caffeine using a temperature- and pH-sensitive nanogel, poly(NIPAM-co-AAc). Int. J. Pharm. 2013, 453, 630–640. [Google Scholar] [CrossRef]
- Lopez, V.; Hadgraft, J.; Snowden, M. The use of colloidal microgels as a (trans)dermal drug delivery system. Int. J. Pharm. 2005, 292, 137–147. [Google Scholar] [CrossRef]
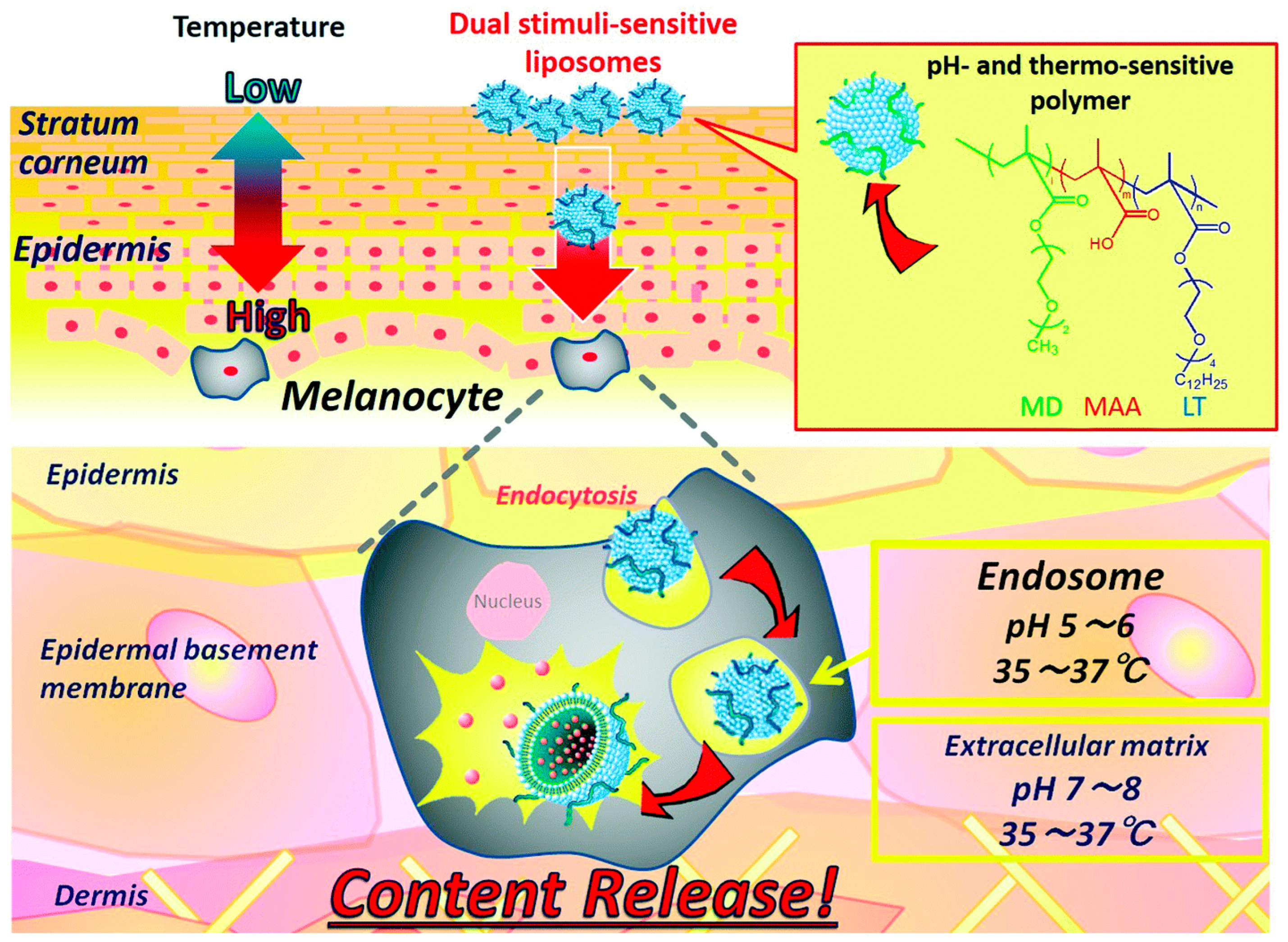
- Yamazaki, N.; Sugimoto, T.; Fukushima, M.; Teranishi, R.; Kotaka, A.; Shinde, C.; Kumei, T.; Sumida, Y.; Munekata, Y.; Maruyama, K.I.; et al. Dual-stimuli responsive liposomes using pH- and temperature-sensitive polymers for controlled transdermal delivery. Polym. Chem. 2017, 8, 1507–1518. [Google Scholar] [CrossRef]
- Mavuso, S.; Marimuthu, T.; Kumar, P.; Kondiah, P.P.; du Toit, L.C.; Choonara, Y.E.; Pillay, V. In Vitro, Ex Vivo, and In Vivo Evaluation of a Dual pH/Redox Responsive Nanoliposomal Sludge for Transdermal Drug Delivery. J. Pharm. Sci. 2018, 107, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Pelegrino, M.; de Araújo, D.; Seabra, A. S-nitrosoglutathione-containing chitosan nanoparticles dispersed in Pluronic F-127 hydrogel: Potential uses in topical applications. J. Drug Deliv. Sci. Technol. 2018, 43, 211–220. [Google Scholar] [CrossRef]
- Zavgorodnya, O.; Carmona-Moran, C.; Kozlovskaya, V.; Liu, F.; Wick, T.; Kharlampieva, E. Temperature-responsive nanogel multilayers of poly(N-vinylcaprolactam) for topical drug delivery. J. Colloid. Interface Sci. 2017, 506, 589–602. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, H.; Cheng, S.; Zhai, G.; Shen, C. Development of curcumin loaded nanostructured lipid carrier based thermosensitive in situ gel for dermal delivery. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 356–362. [Google Scholar] [CrossRef]
- Eberlein-König, B.; Schäfer, T.; Huss-Marp, J.; Darsow, U.; Möhrenschlager, M.; Herbert, O.; Abeck, D.; Krämer, U.; Behrendt, H.; Ring, J. Skin surface pH, stratum corneum hydration, trans-epidermal water loss and skin roughness related to atopic eczema and skin dryness in a population of primary school children. Acta Derm. Venereol. 2000, 80, 188–191. [Google Scholar] [CrossRef]
- Schürer, N. pH and Acne. Curr. Probl. Dermatol. 2018, 54, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Eslami, M.; Sahandi-Zangabad, P.; Mirab, F.; Farajisafiloo, N.; Shafaei, Z.; Ghosh, D.; Bozorgomid, M.; Dashkhaneh, F.; Hamblin, M.R. pH-Sensitive stimulus-responsive nanocarriers for targeted delivery of therapeutic agents. Wiley Interdiscip. Rev.-Nanomed. Nanobiotechnol. 2016, 8, 696–716. [Google Scholar] [CrossRef]
- Aghdam, M.A.; Bagheri, R.; Mosafer, J.; Baradaran, B.; Hashemzaei, M.; Baghbanzadeh, A.; de la Guardia, M.; Mokhtarzadeh, A. Recent advances on thermosensitive and pH-sensitive liposomes employed in controlled release. J. Control. Release 2019, 315, 1–22. [Google Scholar] [CrossRef]
- Banerjee, I.; Mishra, D.; Das, T.; Maiti, T. Wound pH-Responsive Sustained Release of Therapeutics from a Poly(NIPAAm-co-AAc) Hydrogel. J. Biomater. Sci.-Polym. Ed. 2012, 23, 111–132. [Google Scholar] [CrossRef]
- Jeong, H.; Nam, S.; Song, J.; Park, S. Synthesis and physicochemical properties of pH-sensitive hydrogel based on carboxymethyl chitosan/2-hydroxyethyl acrylate for transdermal delivery of nobiletin. J. Drug Deliv. Sci. Technol. 2019, 51, 194–203. [Google Scholar] [CrossRef]
- Soriano-Ruiz, J.L.; Calpena-Campmany, A.C.; Silva-Abreu, M.; Halbout-Bellowa, L.; Bozal-de Febrer, N.; Rodriguez-Lagunas, M.J.; Clares-Naveros, B. Design and evaluation of a multifunctional thermosensitive poloxamer-chitosan-hyaluronic acid gel for the treatment of skin burns. Int. J. Biol. Macromol. 2020, 142, 412–422. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.; Farokhzad, O. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Vecchio, D.; Li, J.; Zhu, J.; Zhang, Q.; Fu, V.; Li, J.; Thamphiwatana, S.; Lu, D.; Zhang, L. Hydrogel containing nanoparticle-stabilized liposomes for topical antimicrobial delivery. ACS Nano 2014, 8, 2900–2907. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Lai, T.C.; Kwon, G.S.; Sako, K. pH- and ion-sensitive polymers for drug delivery. Expert. Opin. Drug Deliv. 2013, 10, 1497–1513. [Google Scholar] [CrossRef]
- Dong, P.; Sahle, S.B.; Lohan, S.; Saeidpour, S.; Albrecht, C.; Teutloff, R.; Bodmeier, M.; Unbehauen, C.; Wolff, R.; Haag, J.; et al. pH-sensitive Eudragit® L 100 nanoparticles promote cutaneous penetration and drug release on the skin. J. Control. Release 2019, 295, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Rizi, K.; Green, R.; Donaldson, M.; Williams, A. Using pH Abnormalities in Diseased Skin to Trigger and Target Topical Therapy. Pharm. Res. 2011, 28, 2589–2598. [Google Scholar] [CrossRef]
- Shields IV, C.W.; White, J.P.; Osta, E.G.; Patel, J.; Rajkumar, S.; Kirby, N.; Therrien, J.P.; Zauscher, S. Encapsulation and controlled release of retinol from silicone particles for topical delivery. J. Control. Release 2018, 278, 37–48. [Google Scholar] [CrossRef]
- Kang, Y.; Zhang, S.X.; Wang, G.Q.; Yan, Z.W.; Wu, G.Y.; Tang, L.; Wang, W. Nanocarrier-Based Transdermal Drug Delivery Systems for Dermatological Therapy. Pharmaceutics 2024, 16, 1384. [Google Scholar] [CrossRef]
- Ahmad, U.; Ahmad, Z.; Khan, A.A.; Akhtar, J.; Singh, S.P.; Ahmad, F.J. Strategies in Development and Delivery of Nanotechnology Based Cosmetic Products. Drug. Res. 2018, 68, 545–552. [Google Scholar] [CrossRef]
- Kim, A.R.; Lee, S.L.; Park, S.N. Properties and in vitro drug release of pH- and temperature-sensitive double cross-linked interpenetrating polymer network hydrogels based on hyaluronic acid/poly (N-isopropylacrylamide) for transdermal delivery of luteolin. Int. J. Biol. Macromol. 2018, 118 Pt A, 731–740. [Google Scholar] [CrossRef]
- Hubbs, A.; Mercer, R.R.; Benkovic, S.; Harkema, J.; Sriram, K.; Schwegler-Berry, D.; Goravanahally, M.; Nurkiewicz, T.; Castranova, V.; Sargent, L.M. Nanotoxicology-A Pathologist’s Perspective. Toxicol. Pathol. 2011, 39, 301–324. [Google Scholar] [CrossRef]
- Mogharabi, M.; Abdollahi, M.; Faramarzi, M. Toxicity of nanomaterials; an undermined issue. Daru-J. Pharm. Sci. Editor. Mater. 2014, 22, 59. [Google Scholar] [CrossRef]
- Ferraris, C.; Rimicci, C.; Garelli, S.; Ugazio, E.; Battaglia, L. Nanosystems in Cosmetic Products: A Brief Overview of Functional, Market, Regulatory and Safety Concerns. Pharmaceutics 2021, 13, 1408. [Google Scholar] [CrossRef]
- Schneider, S.L.; Lim, H.W. A review of inorganic UV filters zinc oxide and titanium dioxide. Photodermatol. Photoimmunol. Photomed. 2019, 35, 442–446. [Google Scholar] [CrossRef]
- Melo, A.; Amadeu, M.; Lancellotti, M.; de Hollanda, L.; Machado, D. The role of nanomaterials in cosmetics: National and international legislative aspects. Quim. Nova 2015, 38, 599–603. [Google Scholar] [CrossRef]
- Ajazzuddin, M.; Jeswani, G.; Jha, A.K. Nanocosmetics: Past, Present and Future Trends. Recent Pat. Nanomed. 2015, 5, 3–11. [Google Scholar] [CrossRef]
- Guidance, D. Guidance for Industry Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology. Biotechnol. Law. Rep. 2011, 30, 613–616. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, Mr17–Mr71. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Gebel, T.; Foth, H.; Damm, G.; Freyberger, A.; Kramer, P.J.; Lilienblum, W.; Röhl, C.; Schupp, T.; Weiss, C.; Wollin, K.M.; et al. Manufactured nanomaterials: Categorization and approaches to hazard assessment. Arch. Toxicol. 2014, 88, 2191–2211. [Google Scholar] [CrossRef]
- Chan, W.W.; Glotzer, Y.; Gogotsi, J.H.; Hafner, P.T.; Hammond, M.C.; Hersam, A.; Javey, C.R.; Kagan, A.; Khademhosseini, N.A.; Kotov, S.T.; et al. Grand Plans for Nano. ACS Nano 2015, 9, 11503–11505. [Google Scholar] [CrossRef]
- Shokri, J. Nanocosmetics: Benefits and risks. BioImpacts 2017, 7, 207–208. [Google Scholar] [CrossRef]
- Schneider, M.; Stracke, F.; Hansen, S.; Schaefer, U.F. Nanoparticles and their interactions with the dermal barrier. Dermatoendocrinol 2009, 1, 197–206. [Google Scholar] [CrossRef]
- Wang, M.; Lai, X.; Shao, L.; Li, L. Evaluation of immunoresponses and cytotoxicity from skin exposure to metallic nanoparticles. Int. J. Nanomed. 2018, 13, 4445–4459. [Google Scholar] [CrossRef]
- Groso, A.; Petri-Fink, A.; Magrez, A.; Riediker, M.; Meyer, T. Management of nanomaterials safety in research environment. Part. Fibre Toxicol. 2010, 7, 40. [Google Scholar] [CrossRef]
- Drasler, B.; Sayre, P.; Steinhäuser, K.; Petri-Fink, A.; Rothen-Rutishauser, B. In vitro approaches to assess the hazard of nanomaterials (vol 8, pg 99, 2017). Nanoimpact 2018, 9, 51. [Google Scholar] [CrossRef]
- Lahkar, S.; Das, M.K. Smart Lipid Nanoparticles for Cosmetic Use; Academic Press: Amsterdam, The Netherlands, 2022; pp. 307–317. [Google Scholar]
- Neupane, R.; Boddu, S.H.S.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to Biological Skin in Permeation Studies: Current Trends and Possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef]
- Kazem, S.; Linssen, E.C.; Gibbs, S. Skin metabolism phase I and phase II enzymes in native and reconstructed human skin: A short review. Drug Discov. Today 2019, 24, 1899–1910. [Google Scholar] [CrossRef]
- Santos, A.C.; Morais, F.; Simões, A.; Pereira, I.; Sequeira, J.A.; Pereira-Silva, M.; Veiga, F.; Ribeiro, A. Nanotechnology for the development of new cosmetic formulations. Expert Opin. Drug Deliv. 2019, 16, 313–330. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Yan, B. Nanotoxicity Overview: Nano-Threat to Susceptible Populations. Int. J. Mol. Sci. 2014, 15, 3671–3697. [Google Scholar] [CrossRef]
- Lee, J.H.; Yeo, Y. Controlled drug release from pharmaceutical nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Gupta, M.; Agrawal, U.; Vyas, S.P. Nanocarrier-based topical drug delivery for the treatment of skin diseases. Expert. Opin. Drug Deliv. 2012, 9, 783–804. [Google Scholar] [CrossRef]
- Phan, H.T.; Haes, A.J. What Does Nanoparticle Stability Mean? J. Phys. Chem. C 2019, 123, 16495–16507. [Google Scholar] [CrossRef]
- Akombaetwa, N.; Ilangala, A.B.; Thom, L.; Memvanga, P.B.; Witika, B.A.; Buya, A.B. Current Advances in Lipid Nanosystems Intended for Topical and Transdermal Drug Delivery Applications. Pharmaceutics 2023, 15, 2. [Google Scholar] [CrossRef]
- John, R.; Monpara, J.; Swaminathan, S.; Kalhapure, R. Chemistry and Art of Developing Lipid Nanoparticles for Biologics Delivery: Focus on Development and Scale-Up. Pharmaceutics 2024, 16, 131. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, K.H. Future value and direction of cosmetics in the era of metaverse. J. Cosmet. Dermatol. 2022, 21, 4176–4183. [Google Scholar] [CrossRef]
- Tarar, A.A.; Mohammad, U.; Srivastava, S.K. Wearable Skin Sensors and Their Challenges: A Review of Transdermal, Optical, and Mechanical Sensors. Biosensors 2020, 10, 56. [Google Scholar] [CrossRef]
- Elder, A.; Cappelli, M.; Ring, C.; Saedi, N. Artificial intelligence in cosmetic dermatology: An update on current trends. Clin. Dermatol. 2024, 42, 216–220. [Google Scholar] [CrossRef]
- Kania, B.; Montecinos, K.; Goldberg, D. Artificial intelligence in cosmetic dermatology. J. Cosmet. Dermatol. 2024, 23, 3305–3311. [Google Scholar] [CrossRef]
- Vatiwutipong, P.; Vachmanus, S.; Noraset, T.; Tuarob, S. Artificial Intelligence in Cosmetic Dermatology: A Systematic Literature Review. IEEE Access 2023, 11, 71407–71425. [Google Scholar] [CrossRef]

| EU | US | |
|---|---|---|
| Regulating organizations |
|
|
| Regulatory guideline |
|
|
| Notification and labeling |
|
|
| Safety guidance |
|
|
| Definition review |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J. Smart Nanocarriers in Cosmeceuticals Through Advanced Delivery Systems. Biomimetics 2025, 10, 217. https://doi.org/10.3390/biomimetics10040217
Kim J. Smart Nanocarriers in Cosmeceuticals Through Advanced Delivery Systems. Biomimetics. 2025; 10(4):217. https://doi.org/10.3390/biomimetics10040217
Chicago/Turabian StyleKim, Jinku. 2025. "Smart Nanocarriers in Cosmeceuticals Through Advanced Delivery Systems" Biomimetics 10, no. 4: 217. https://doi.org/10.3390/biomimetics10040217
APA StyleKim, J. (2025). Smart Nanocarriers in Cosmeceuticals Through Advanced Delivery Systems. Biomimetics, 10(4), 217. https://doi.org/10.3390/biomimetics10040217







