Myoelectric Control in Rehabilitative and Assistive Soft Exoskeletons: A Comprehensive Review of Trends, Challenges, and Integration with Soft Robotic Devices
Abstract
1. Introduction
2. Methodological Approach
2.1. Search Strategy
- Soft Robotics: “soft robotics”, “soft exoskeletons”, “control of soft robots”, “biomimetic robotics”.
- Exoskeletons: “rehabilitation and assistance exoskeletons”, “soft exoskeletons”, “elbow exoskeletons”, “control of exoskeletons”.
- Myoelectric Control: “myoelectric control”, “surface electromyography”, “sEMG control”, “motion intention estimation”, “sEMG joint torque estimation”, “sEMG joint position estimation”.
- TITLE-ABS-KEY(Control AND (electromyography OR “surface electromyography” OR myoelectric OR emg OR semg) AND (orthesis OR exoskeleton OR robot* OR assit*))
2.2. Inclusion and Exclusion Criteria
- Articles from 2012 to 2025 were included.
- Research involving systems for rehabilitation or assistance of the upper and lower limbs, primarily systems focused on the elbow or knee.
- Both rigid and soft systems were included, although the focus was on soft robotic exoskeletons.
- Articles that integrated control strategies with rehabilitation and assistance exoskeletons.
- Articles with knee systems were considered because the knee is a similar joint to the elbow, making some systems identical for both joints.
- Articles on the processing and selection of EMG signal features.
- Articles that lacked full-text access or were not peer-reviewed.
- Articles that focused solely on hardware design.
- Articles focused on gesture recognition in the hand were excluded.
2.3. Data Filtering and Selection
3. Theoretical Framework and State-of-the-Art in Myoelectric Control Strategies for Soft Robotic Exoskeletons
3.1. Robotic Assistance and Rehabilitation Systems
- Passive Therapy: This form of therapy does not require any effort from the patient during rehabilitation movements. It is typically used in the early stages of rehabilitation or when there is no voluntary response in the affected joint or limb (e.g., post-stroke) [33,34]. The therapy involves repeatedly moving the joint in specific trajectories, effectively reducing spasms and preventing muscle atrophy in the involved joint [35].
- Active Therapy: This type of therapy allows patients to perform some voluntary movements in their affected joints, though they may still lack strength or efficiency. It can be divided into active–assistive therapy and active–resistive therapy. In active–assistive therapy, the patient attempts to move the affected limb voluntarily. At the same time, the robotic device provides external force assistance [36], improving the range of motion of the joint [34]. In active–resistive therapy, the patient tries to perform voluntary movements while the robotic device generates resistance [35], helping to gradually increase muscle strength in the treated joint [34].
- Bilateral Therapy: In this therapy, the affected limb mirrors the movements of the functional limb (mirror therapy). Some exoskeleton systems support this type of therapy, which is commonly applied in stroke recovery [34].
3.2. Soft Robotics Exoskeletons
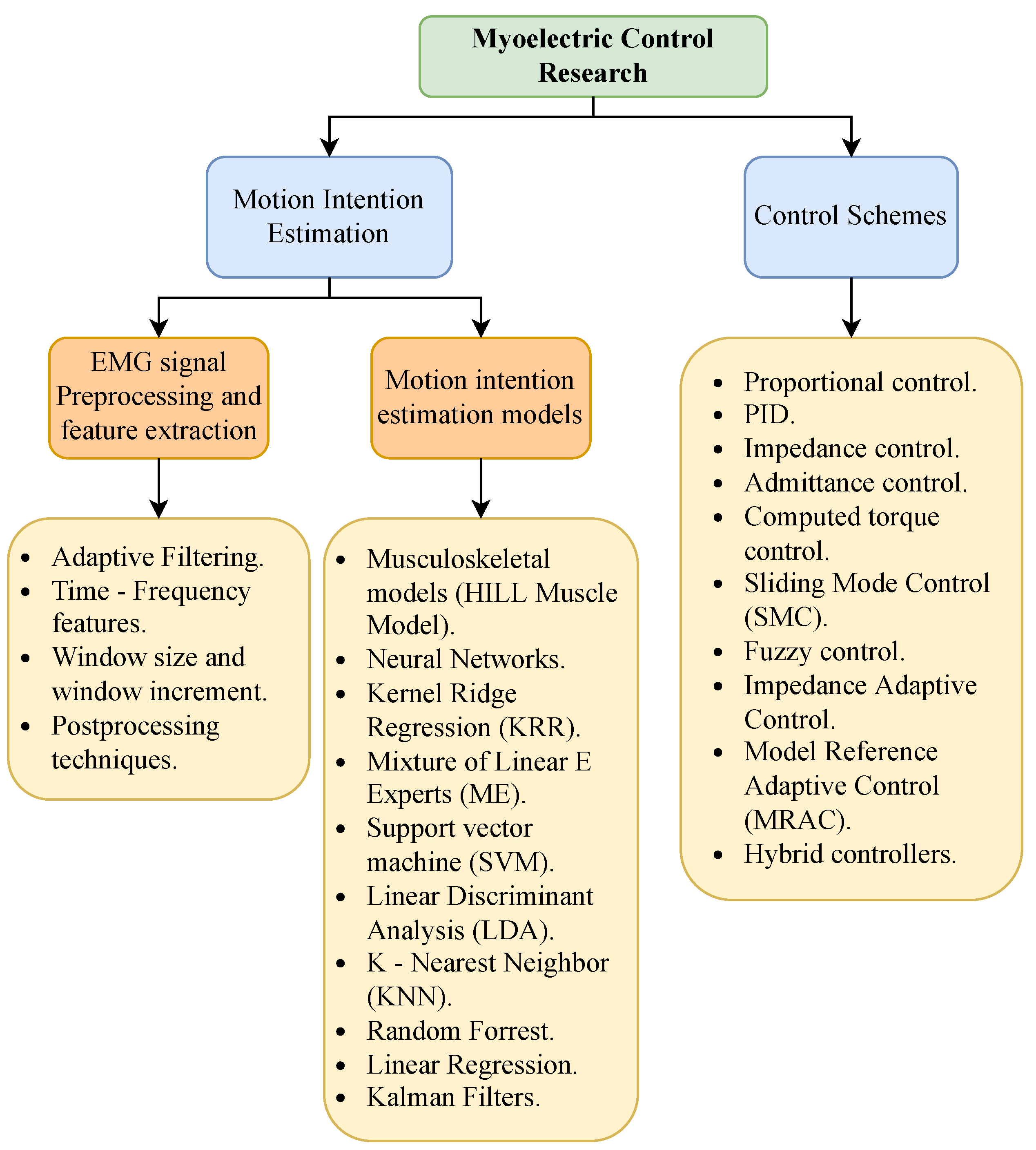
3.3. Myoelectric Control
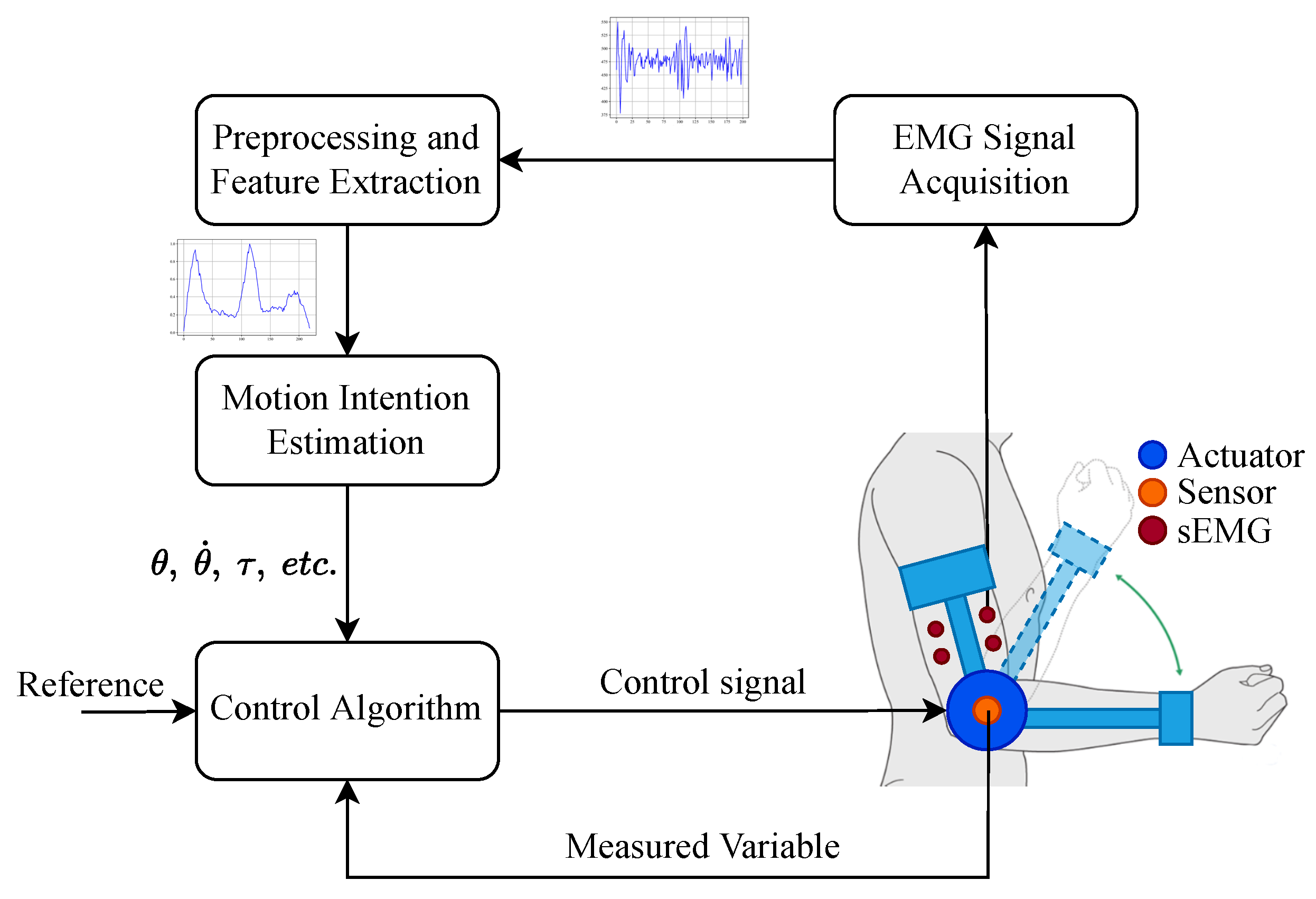
3.3.1. sEMG Acquisition
3.3.2. sEMG Preprocessing and Feature Extraction
Time-Domain Features
Frequency-Domain Features
Time–Frequency-Domain Features
3.3.3. sEMG Motion Intention Estimation
Model-Based Interfaces: EMG Musculoskeletal Models
Data-Driven Interfaces: Machine Learning Motion Intention Algorithms
3.4. Control Strategies
4. Challenges and Future Directions
- Motion Intention Estimation Algorithms: There are still significant challenges in EMG-based motion intention estimation algorithms, a core component of myoelectric control schemes. These challenges include performance, repeatability, computational cost, and computational delay [129,130]. They primarily stem from two factors. The first is the difficulties present in the sEMG acquisition process, which depends on multiple variables that are hard to reproduce across different acquisition sessions, leading to variations in the acquired EMG signal, as mentioned in Section 3.3.1. The second factor is that the EMG signal morphology changes over time due to phenomena like muscle fatigue [13,24]. These factors require motion intention algorithms to be subject-specific and constantly calibrated to perform well.
- Modeling and Control of Soft Robotic Systems: The recent trend of soft robotics introduces major challenges regarding controlling exoskeletons based on soft systems. This challenge is also due to two primary factors. The first is the difficulty in modeling soft systems because of parameter uncertainties and the possibility of having infinite degrees of freedom, making it hard to implement traditional model-based control strategies for these systems. The second factor is that, given the deformable and soft nature of these devices, traditional position sensors are often unsuitable, making it difficult to measure the control variable in soft systems. This issue is evident in soft exoskeletons, where the joint position should be measured without aligning the joint axis with the sensor to avoid user discomfort [131,132,133,134].
- Validation and Evaluation of Myoelectric Controllers: Although many studies have proposed EMG motion intention estimation algorithms for myoelectric control schemes, most of these investigations have been limited to validating the algorithms using only static data and simulations. However, it has been found that models that perform well on static data do not necessarily exhibit good performance during real-time implementations [22,135]. This causes uncertainties regarding whether EMG motion intention algorithms are suitable for online operation.
5. Key Findings
- Innovative Design of Soft Robotics ExoskeletonsIntegrating soft materials in robotic exoskeletons has revolutionized the field by enhancing adaptability and comfort. Soft robotic exoskeletons have demonstrated significant potential in conforming to human body contours and providing better assistance in rehabilitation and daily activities. Their ability to provide effective rehabilitation therapies and help in movement assistance tasks without causing discomfort to the user marks a significant advancement over traditional rigid exoskeletons.
- Integration of Soft Actuation MethodsThe development of various actuation methods, including pneumatic actuators, cable-driven systems, and shape-memory alloys, has expanded the capabilities of soft robotic exoskeletons. These advanced actuation techniques contribute to the flexibility and efficiency of the devices, enabling them to perform a wide range of motions and tasks. The integration of these methods supports the creation of more sophisticated and functional exoskeletons tailored to specific rehabilitation and assistance needs.
- Comparison of Control Strategies in ExoskeletonsControl strategies play a crucial role in exoskeleton design. As shown in Figure 6, adaptive and impedance-based control methods are the most widely used due to their ability to handle uncertainties and human–exoskeleton interactions. These approaches enable more flexible and robust control schemes, particularly in applications requiring real-time adaptability.
- Effectiveness of Myoelectric ControlMyoelectric control strategies have shown promising results in enabling the intuitive control of exoskeletons. These strategies allow exoskeletons to adapt and synchronize with the user’s movements, facilitating more effective rehabilitation and assistance. Using sEMG signals to estimate dynamic variables such as joint position, speed, and torque has proven feasible for achieving seamless human–machine interaction.While myoelectric control has shown significant promise for facilitating intuitive interactions in rehabilitation and assistance exoskeletons, several limitations challenge its commercial application. As mentioned, challenges in the sEMG signal acquisition process, such as electrode placement, skin impedance, and muscle fatigue, can compromise signal consistency and accuracy. This inconsistency is transferred to the controller via the myoelectric interface, often causing delayed or erroneous interpretations of the user’s motion intent, which can be particularly problematic in clinical settings where precise timing and movement coordination are essential. Furthermore, in patients with neuromuscular impairments, the diminished or irregular electrical activity of muscles can further degrade control performance, thus limiting the effectiveness of the interface. This is one of the reasons why myoelectric control interfaces are, for the most part, still limited to controlled testing environments.Additionally, current myoelectric interfaces face challenges related to environmental interference and morphological changes in the signal, which complicate signal processing and may require extensive calibration. Such recalibrations can be time-consuming and require frequent adjustments to accommodate day-to-day physiological changes or activity-induced variations in the sEMG signal. To address these limitations, future research will integrate complementary sensing modalities, such as inertial measurement units (IMUs) or mechanomyography (MMG), and develop advanced signal processing algorithms that enhance noise reduction and improve interpretability. These approaches could produce a more robust, reliable, and user-adaptive control system that optimizes the performance of rehabilitation and assistance exoskeletons.
- Machine Learning Models for Motion Intention EstimationData-driven methods based on machine learning techniques are gaining traction in motion intention estimation. These methods leverage large datasets to train models that can accurately predict user intentions in real time. By utilizing machine learning algorithms, these data-driven approaches enhance the adaptability and precision of myoelectric control schemes, making them more practical for a diverse range of users without requiring long calibration processes.
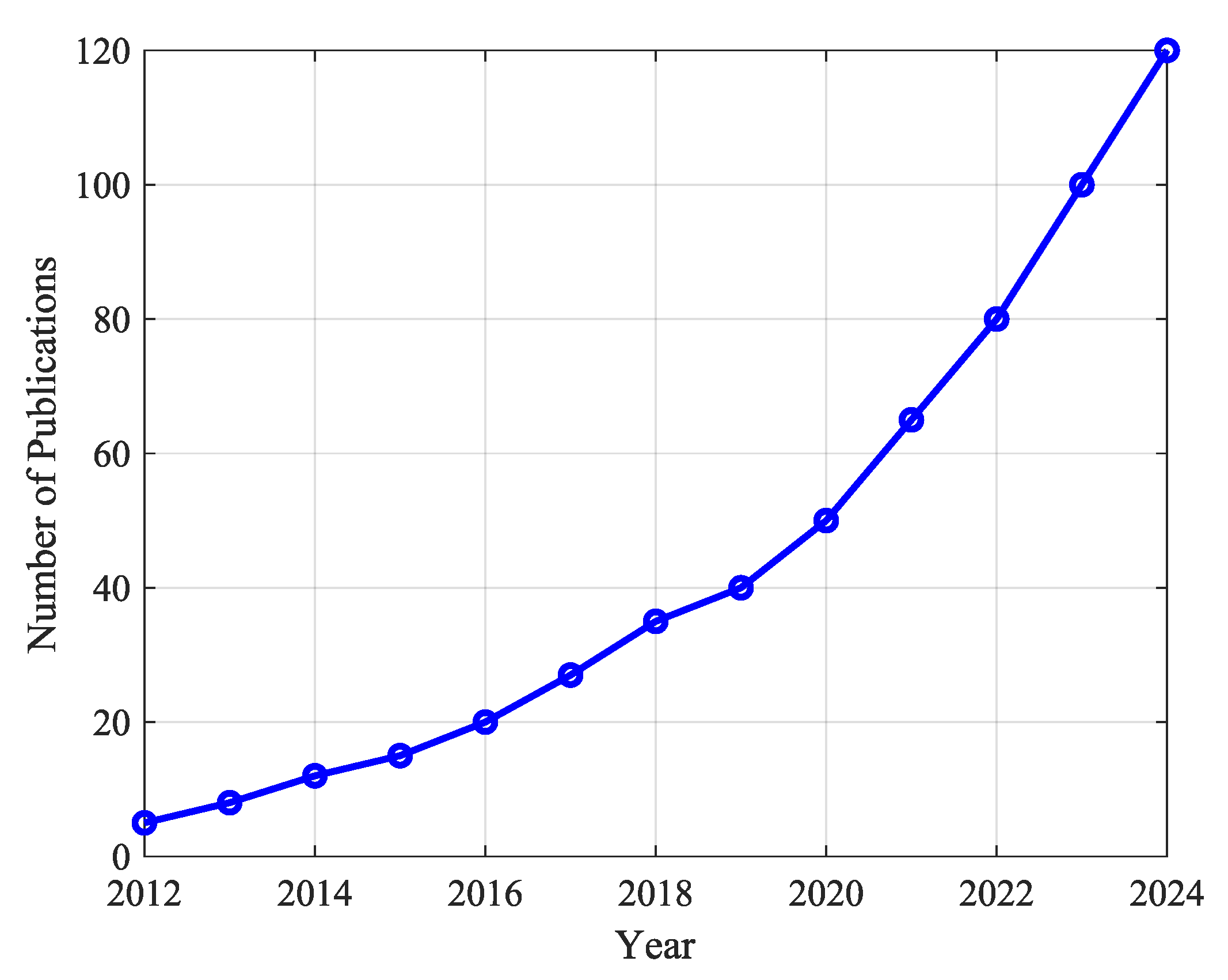
- Trends in Soft Robotics Exoskeleton PublicationsThe increasing research interest in soft robotic exoskeletons is evident in the publication trends over the last decade, as depicted in Figure 7. The number of publications in this area has grown significantly since 2012, demonstrating a strong research focus on improving adaptability and usability in rehabilitation and assistive robotics.
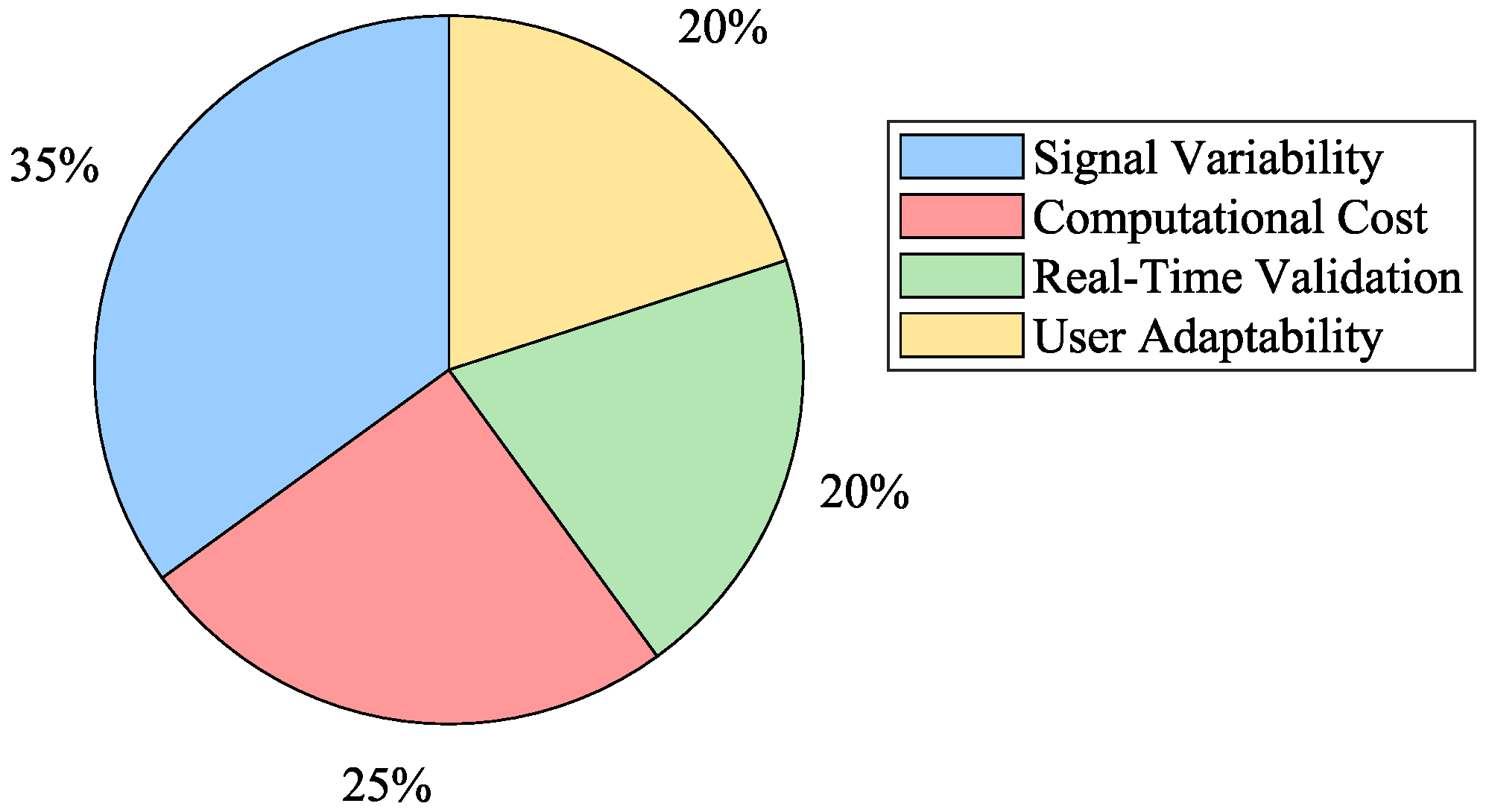
- Challenges in Motion Intention EstimationDespite the advances in myoelectric control, significant challenges remain in motion intention estimation algorithms. Performance, repeatability, computational cost, and delay are critical issues that must be addressed. The variability in sEMG signal acquisition and the changes in signal morphology due to muscle fatigue necessitate ongoing calibration and adaptation of motion intention algorithms to ensure their accuracy and reliability. Figure 8 summarizes the primary obstacles encountered in myoelectric control implementation.
- Adaptive Control StrategiesAdaptive controllers have emerged as a robust solution for dealing with the complexities and uncertainties inherent in exoskeleton dynamics. These controllers, which do not require precise knowledge of the system’s dynamics, have shown fast convergence, accurate trajectory tracking, and effective parameter handling. The application of adaptive control strategies, particularly in soft robotic exoskeletons, holds great promise for enhancing performance and user experience. This is due to their ability to overcome the challenge of modeling and parameter tuning for controlling soft robotic exoskeletons. These strategies allow the controllers to adapt to the nonlinear, variable nature of soft actuators, and they can adjust to different users with varying anatomic and kinematic parameters; in particular, this is important as it facilitates the operation of the exoskeleton.Despite their advantages, adaptive control strategies face some important drawbacks. As mentioned, they are generally computationally expensive, which might limit their implementation in portable devices with limited hardware capabilities. They often require extended calibration and learning phases, which can delay deployment and may introduce transient instabilities. Moreover, their inherent sensitivity to parameter variations can lead to performance degradation over time, further challenging the consistency essential for safe and effective operation.To address these drawbacks, several strategies could be implemented. First, algorithm optimization techniques could be used to reduce computational overhead. Second, using model-free learning strategies, for example, based on reinforcement learning, can minimize reliance on precise system dynamics while maintaining adaptability. Third, implementing pre-trained models that integrate generalized motion data could reduce calibration time. Pairing this with user-specific fine-tuning could enhance system reliability and shorten deployment phases. Lastly, continuous real-time testing during usage could facilitate automatic recalibration, enhancing the device’s adaptability to parameter changes.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beckerle, P.; Salvietti, G.; Unal, R.; Prattichizzo, D.; Rossi, S.; Castellini, C.; Hirche, S.; Endo, S.; Amor, H.B.; Ciocarlie, M.; et al. A Human–Robot Interaction Perspective on Assistive and Rehabilitation Robotics. Front. Neurorobot. 2017, 11, 24. [Google Scholar] [CrossRef]
- Chu, C.Y.; Patterson, R.M. Soft robotic devices for hand rehabilitation and assistance: A narrative review. J. NeuroEngineering Rehabil. 2018, 15, 9. [Google Scholar] [CrossRef]
- du Plessis, T.; Djouani, K.; Oosthuizen, C. A Review of Active Hand Exoskeletons for Rehabilitation and Assistance. Robotics 2021, 10, 40. [Google Scholar] [CrossRef]
- Masia, L.; Xiloyannis, M.; Khanh, D.B.; Wilson, A.C.; Contu, S.; Yongtae, K.G. Actuation for robot-aided rehabilitation: Design and control strategies. In Rehabilitation Robotics; Elsevier: London, UK, 2018; pp. 47–61. [Google Scholar] [CrossRef]
- Denève, A.; Moughamir, S.; Afilal, L.; Zaytoon, J. Control system design of a 3-DOF upper limbs rehabilitation robot. Comput. Methods Programs Biomed. 2008, 89, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Park, G.; Kim, H.Y.; Lee, J.Y.; Ham, Y.; Hwang, D.; Kwon, S.; Shin, J.H. A comparison of the effects and usability of two exoskeletal robots with and without robotic actuation for upper extremity rehabilitation among patients with stroke: A single-blinded randomised controlled pilot study. J. NeuroEngineering Rehabil. 2020, 17, 137. [Google Scholar] [CrossRef] [PubMed]
- Duret, C.; Grosmaire, A.G.; Krebs, H.I. Robot-assisted therapy in upper extremity hemiparesis: Overview of an evidence-based approach. Front. Neurol. 2019, 10, 412. [Google Scholar] [CrossRef]
- Chiaradia, D.; Tiseni, L.; Xiloyannis, M.; Solazzi, M.; Masia, L.; Frisoli, A. An Assistive Soft Wrist Exosuit for Flexion Movements With an Ergonomic Reinforced Glove. Front. Robot. AI 2021, 7, 182. [Google Scholar] [CrossRef]
- Gull, M.A.; Bai, S.; Bak, T. A Review on Design of Upper Limb Exoskeletons. Robotics 2020, 9, 16. [Google Scholar] [CrossRef]
- Lu, L.; Wu, Q.; Chen, X.; Shao, Z.; Chen, B.; Wu, H. Development of a sEMG-based torque estimation control strategy for a soft elbow exoskeleton. Robot. Auton. Syst. 2019, 111, 88–98. [Google Scholar] [CrossRef]
- Nam, C.; Rong, W.; Li, W.; Cheung, C.; Ngai, W.; Cheung, T.; Pang, M.; Li, L.; Hu, J.; Wai, H.; et al. An Exoneuromusculoskeleton for Self-Help Upper Limb Rehabilitation After Stroke. Soft Robot. 2022, 9, 14–35. [Google Scholar] [CrossRef]
- Camardella, C.; Barsotti, M.; Buongiorno, D.; Frisoli, A.; Bevilacqua, V. Towards online myoelectric control based on muscle synergies-to-force mapping for robotic applications. Neurocomputing 2021, 452, 768–778. [Google Scholar] [CrossRef]
- Ison, M.; Artemiadis, P. The role of muscle synergies in myoelectric control: Trends and challenges for simultaneous multifunction control. J. Neural Eng. 2014, 11, 051001. [Google Scholar] [CrossRef] [PubMed]
- Úbeda, A.; Azorín, J.M.; Farina, D.; Sartori, M. Estimation of neuromuscular primitives from EEG slow cortical potentials in incomplete spinal cord injury individuals for a new class of brain-machine interfaces. Front. Comput. Neurosci. 2018, 12, 3. [Google Scholar] [CrossRef]
- Villa-Parra, A.C.; Delisle-Rodríguez, D.; López-Delis, A.; Bastos-Filho, T.; Sagaró, R.; Frizera-Neto, A. Towards a Robotic Knee Exoskeleton Control Based on Human Motion Intention through EEG and sEMGsignals. Procedia Manuf. 2015, 3, 1379–1386. [Google Scholar] [CrossRef]
- Ibitoye, M.O.; Hamzaid, N.A.; Zuniga, J.M.; Abdul Wahab, A.K. Mechanomyography and muscle function assessment: A review of current state and prospects. Clin. Biomech. 2014, 29, 691–704. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, C. A preliminary study of classification of upper limb motions and forces based on mechanomyography. Med. Eng. Phys. 2020, 81, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Godiyal, A.K.; Singh, U.; Anand, S.; Joshi, D. Analysis of force myography based locomotion patterns. Measurement 2019, 140, 497–503. [Google Scholar] [CrossRef]
- Prakash, A.; Sharma, N.; Sharma, S. An affordable transradial prosthesis based on force myography sensor. Sens. Actuators A Phys. 2021, 325, 112699. [Google Scholar] [CrossRef]
- Xiao, Z.G.; Menon, C. Performance of Forearm FMG and sEMG for Estimating Elbow, Forearm and Wrist Positions. J. Bionic Eng. 2017, 14, 284–295. [Google Scholar] [CrossRef]
- Merletti, R.; Farina, D. Surface Electromyography: Physiology, Engineering and Applications; Wiley-IEEE Press: Hoboken, NJ, USA, 2016; pp. 1–570. [Google Scholar] [CrossRef]
- Bi, L.; Feleke, A.; Guan, C. A review on EMG-based motor intention prediction of continuous human upper limb motion for human-robot collaboration. Biomed. Signal Process. Control 2019, 51, 113–127. [Google Scholar] [CrossRef]
- Igual, C.; Pardo, L.A.; Hahne, J.M.; Igual, J. Myoelectric Control for Upper Limb Prostheses. Electronics 2019, 8, 1244. [Google Scholar] [CrossRef]
- Singh, R.M.; Chatterji, S. Trends and Challenges in EMG Based Control Scheme of Exoskeleton Robots—A Review. Int. J. Sci. Eng. Res. 2012, 3, 1–8. [Google Scholar]
- Lowe, B.D.; Billotte, W.G.; Peterson, D.R. ASTM F48 Formation and Standards for Industrial Exoskeletons and Exosuits. IISE Trans. Occup. Ergon. Hum. Factors 2019, 7, 230–236. [Google Scholar] [CrossRef]
- Bardi, E.; Gandolla, M.; Braghin, F.; Resta, F.; Pedrocchi, A.L.; Ambrosini, E. Upper limb soft robotic wearable devices: A systematic review. J. NeuroEngineering Rehabil. 2022, 19, 87. [Google Scholar] [CrossRef]
- Bogue, R. Exoskeletons—A review of industrial applications. Ind. Robot 2018, 45, 585–590. [Google Scholar] [CrossRef]
- Gandolla, M.; Dalla Gasperina, S.; Longatelli, V.; Manti, A.; Aquilante, L.; D’Angelo, M.G.; Biffi, E.; Diella, E.; Molteni, F.; Rossini, M.; et al. An assistive upper-limb exoskeleton controlled by multi-modal interfaces for severely impaired patients: Development and experimental assessment. Robot. Auton. Syst. 2021, 143, 103822. [Google Scholar] [CrossRef]
- Kapsalyamov, A.; Hussain, S.; Jamwal, P.K. State-of-the-art assistive powered upper limb exoskeletons for elderly. IEEE Access 2020, 8, 178991–179001. [Google Scholar] [CrossRef]
- Park, S.J.; Park, C.H. Suit-type Wearable Robot Powered by Shape-memory-alloy-based Fabric Muscle. Sci. Rep. 2019, 9, 9157. [Google Scholar] [CrossRef]
- Thompson, N.; Sinha, A.; Krishnan, G. Characterizing architectures of soft pneumatic actuators for a cable-driven shoulder exoskeleton. In Proceedings of the IEEE International Conference on Robotics and Automation, Montreal, QC, Canada, 20–24 May 2019; pp. 570–576. [Google Scholar] [CrossRef]
- Gassert, R.; Dietz, V. Rehabilitation robots for the treatment of sensorimotor deficits: A neurophysiological perspective. J. NeuroEngineering Rehabil. 2018, 15, 46. [Google Scholar] [CrossRef]
- Proietti, T.; Crocher, V.; Roby-Brami, A.; Jarrasse, N. Upper-limb robotic exoskeletons for neurorehabilitation: A review on control strategies. IEEE Rev. Biomed. Eng. 2016, 9, 4–14. [Google Scholar] [CrossRef]
- Qassim, H.M.; Wan Hasan, W.Z. A Review on Upper Limb Rehabilitation Robots. Appl. Sci. 2020, 10, 6976. [Google Scholar] [CrossRef]
- Ren, Y.; Kang, S.H.; Park, H.S.; Wu, Y.N.; Zhang, L.Q. Developing a multi-joint upper limb exoskeleton robot for diagnosis, therapy, and outcome evaluation in neurorehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 21, 490–499. [Google Scholar] [CrossRef]
- Ueda, J.; Ming, D.; Krishnamoorthy, V.; Shinohara, M.; Ogasawara, T. Individual muscle control using an exoskeleton robot for muscle function testing. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 339–350. [Google Scholar] [CrossRef]
- Shi, Y.; Dong, W.; Lin, W.; Gao, Y. Soft Wearable Robots: Development Status and Technical Challenges. Sensors 2022, 22, 7584. [Google Scholar] [CrossRef] [PubMed]
- Aliman, N.; Ramli, R.; Haris, S.M. Design and development of lower limb exoskeletons: A survey. Robot. Auton. Syst. 2017, 95, 102–116. [Google Scholar] [CrossRef]
- Nordin, M.; Frankel, H.V. Basic Biomechanics of the Musculoskeletal System, 4th ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2012; p. 454. [Google Scholar]
- Gorgey, A.S. Robotic exoskeletons: The current pros and cons. World J. Orthop. 2018, 9, 112–119. [Google Scholar] [CrossRef]
- Trivedi, D.; Rahn, C.D.; Kier, W.M.; Walker, I.D. Soft robotics: Biological inspiration, state of the art, and future research. Appl. Bionics Biomech. 2008, 5, 99–117. [Google Scholar] [CrossRef]
- Laschi, C.; Mazzolai, B.; Cianchetti, M. Soft robotics: Technologies and systems pushing the boundaries of robot abilities. Am. Assoc. Adv. Sci. 2016, 1, eaah3690. [Google Scholar]
- Laschi, C.; Rossiter, J.; Iida, F.; Cianchetti, M.; Margheri, L. Soft Robotics: Trends, Applications and Challenges. In Proceedings of the Soft Robotics Week, Livorno, Italy, 25–30 April 2016, Biosystems & Biorobotics; Laschi, C., Rossiter, J., Iida, F., Cianchetti, M., Margheri, L., Eds.; Springer: Cham, Switzerland, 2017; Volume 17. [Google Scholar] [CrossRef]
- Rossiter, J.; Hauser, H. Soft Robotics—The Next Industrial Revolution? [Industrial Activities]. IEEE Robot. Autom. Mag. 2016, 23, 17–20. [Google Scholar] [CrossRef]
- Rus, D.; Tolley, M.T. Design, fabrication and control of soft robots. Nature 2015, 521, 467–475. [Google Scholar] [CrossRef]
- Michalos, G.; Makris, S.; Tsarouchi, P.; Guasch, T.; Kontovrakis, D.; Chryssolouris, G. Design Considerations for Safe Human-robot Collaborative Workplaces. Procedia CIRP 2015, 37, 248–253. [Google Scholar] [CrossRef]
- Majidi, C. Soft Robotics: A Perspective—Current Trends and Prospects for the Future. Soft Robot. 2013, 1, 1. [Google Scholar] [CrossRef]
- Ang, B.W.; Yeow, C.H. Design and Modeling of a High Force Soft Actuator for Assisted Elbow Flexion. IEEE Robot. Autom. Lett. 2020, 5, 3731–3736. [Google Scholar] [CrossRef]
- Thalman, C.M.; Lam, Q.P.; Nguyen, P.H.; Sridar, S.; Polygerinos, P. A Novel Soft Elbow Exosuit to Supplement Bicep Lifting Capacity. In Proceedings of the IEEE International Conference on Intelligent Robots and Systems, Madrid, Spain, 1–5 October 2018; pp. 6965–6971. [Google Scholar] [CrossRef]
- Zuccon, G.; Bottin, M.; Ceccarelli, M.; Rosati, G. Design and Performance of an Elbow Assisting Mechanism. Machines 2020, 8, 68. [Google Scholar] [CrossRef]
- Wei, W.; Qu, Z.; Wang, W.; Zhang, P.; Hao, F. Design on the bowden cable-driven upper limb soft exoskeleton. Appl. Bionics Biomech. 2018, 2018, 1925694. [Google Scholar] [CrossRef]
- Koo, I.; Yun, C.; Costa, M.V.; Scognamiglio, J.V.; Yangali, T.A.; Park, D.; Cho, K.J. Development of a meal assistive exoskeleton made of soft materials for polymyositis patients. In Proceedings of the IEEE International Conference on Intelligent Robots and Systems, Chicago, IL, USA, 14–18 September 2014; pp. 542–547. [Google Scholar] [CrossRef]
- Park, D.; Koo, I.; Cho, K.J. Evaluation of an improved soft meal assistive exoskeleton with an adjustable weight-bearing system for people with disability. In Proceedings of the IEEE International Conference on Rehabilitation Robotics, Singapore, 11–14 August 2015; pp. 79–84. [Google Scholar] [CrossRef]
- Jeong, J.; Yasir, I.B.; Han, J.; Park, C.H.; Bok, S.K.; Kyung, K.U. Design of Shape Memory Alloy-Based Soft Wearable Robot for Assisting Wrist Motion. Appl. Sci. 2019, 9, 4025. [Google Scholar] [CrossRef]
- Copaci, D.; Serrano, D.; Moreno, L.; Blanco, D. A High-Level Control Algorithm Based on sEMG Signalling for an Elbow Joint SMA Exoskeleton. Sensors 2018, 18, 2522. [Google Scholar] [CrossRef]
- Xiloyannis, M.; Alicea, R.; Georgarakis, A.M.; Haufe, F.L.; Wolf, P.; Masia, L.; Riener, R. Soft Robotic Suits: State of the Art, Core Technologies, and Open Challenges. IEEE Trans. Robot. 2021, 38, 1343–1362. [Google Scholar] [CrossRef]
- Thalman, C.; Artemiadis, P. A review of soft wearable robots that provide active assistance: Trends, common actuation methods, fabrication, and applications. Wearable Technol. 2020, 1, e3. [Google Scholar] [CrossRef]
- Criswell, E. CRAM’s Introduction to Surface Electromyography, 2nd ed.; Jones and Bartlett: Sudbury, MA, USA, 2011; pp. 1–412. [Google Scholar]
- Kutz, M. Biomedical Engineering and Design Handbook Volume 1: Fundamentals, 2nd ed.; McGraw-Hill: New York, NY, USA, 2009. [Google Scholar]
- Simao, M.; Mendes, N.; Gibaru, O.; Neto, P. A Review on Electromyography Decoding and Pattern Recognition for Human-Machine Interaction. IEEE Access 2019, 7, 39564–39582. [Google Scholar] [CrossRef]
- Smith, L.H.; Hargrove, L.J.; Lock, B.A.; Kuiken, T.A. Determining the optimal window length for pattern recognition-based myoelectric control: Balancing the competing effects of classification error and controller delay. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Asogbon, M.G.; Samuel, O.W.; Jiang, Y.; Wang, L.; Geng, Y.; Sangaiah, A.K.; Chen, S.; Fang, P.; Li, G. Appropriate Feature Set and Window Parameters Selection for Efficient Motion Intent Characterization towards Intelligently Smart EMG-PR System. Symmetry 2020, 12, 1710. [Google Scholar] [CrossRef]
- Englehart, K.; Hudgins, B. A Robust, Real-Time Control Scheme for Multifunction Myoelectric Control. IEEE Trans. Biomed. Eng. 2003, 50, 848–854. [Google Scholar] [CrossRef]
- Samuel, O.W.; Asogbon, M.G.; Geng, Y.; Al-Timemy, A.H.; Pirbhulal, S.; Ji, N.; Chen, S.; Fang, P.; Li, G. Intelligent EMG pattern recognition control method for upper-limb multifunctional prostheses: Advances, current challenges, and future prospects. IEEE Access 2019, 7, 10150–10165. [Google Scholar] [CrossRef]
- Hofmann, D.; Jiang, N.; Vujaklija, I.; Farina, D. Bayesian Filtering of Surface EMG for Accurate Simultaneous and Proportional Prosthetic Control. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Stachaczyk, M.; Farokh Atashzar, S.; Farina, D. Adaptive Spatial Filtering of High-Density EMG for Reducing the Influence of Noise and Artefacts in Myoelectric Control. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1511–1517. [Google Scholar] [CrossRef]
- Leserri, D.; Grimmelsmann, N.; Mechtenberg, M.; Meyer, H.G.; Schneider, A. Evaluation of sEMG Signal Features and Segmentation Parameters for Limb Movement Prediction Using a Feedforward Neural Network. Mathematics 2022, 10, 932. [Google Scholar] [CrossRef]
- Sartori, M.; Reggiani, M.; Pagello, E.; Lloyd, D.G. Modeling the human knee for assistive technologies. IEEE Trans. Biomed. Eng. 2012, 59, 2642–2649. [Google Scholar] [CrossRef]
- Sartori, M.; Maculan, M.; Pizzolato, C.; Reggiani, M.; Farina, D. Modeling and simulating the neuromuscular mechanisms regulating ankle and knee joint stiffness during human locomotion. J. Neurophysiol. 2015, 114, 2509–2527. [Google Scholar] [CrossRef]
- Buongiorno, D.; Barsotti, M.; Barone, F.; Bevilacqua, V.; Frisoli, A. A Linear Approach to Optimize an EMG-Driven Neuromusculoskeletal Model for Movement Intention Detection in Myo-Control: A Case Study on Shoulder and Elbow Joints. Front. Neurorobot. 2018, 12, 74. [Google Scholar] [CrossRef]
- Durandau, G.; Farina, D.; Sartori, M. Robust Real-Time Musculoskeletal Modeling Driven by Electromyograms. IEEE Trans. Biomed. Eng. 2017, 65, 556–564. [Google Scholar] [CrossRef]
- Hayashibe, M.; Guiraud, D. Voluntary EMG-to-force estimation with a multi-scale physiological muscle model. BioMed. Eng. Online 2013, 12, 86. [Google Scholar] [CrossRef]
- Cavallaro, E.E.; Rosen, J.; Perry, J.C.; Burns, S. Real-time myoprocessors for a neural controlled powered exoskeleton arm. IEEE Trans. Biomed. Eng. 2006, 53, 2387–2396. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.G.; Besier, T.F. An EMG-driven musculoskeletal model to estimate muscle forces and knee joint moments in vivo. J. Biomech. 2003, 36, 765–776. [Google Scholar] [CrossRef]
- Pau, J.W.; Xie, S.S.; Pullan, A.J. Neuromuscular interfacing: Establishing an EMG-driven model for the human elbow joint. IEEE Trans. Biomed. Eng. 2012, 59, 2586–2593. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.K.; Muceli, S.; Rodrigues, C.; Megia-Garcia, A.; Pascual Valdunciel, A.; Del-Ama, A.J.; Gil-Agudo, A.; Moreno, J.C.; Barroso, F.; Pons, J.L.; et al. Intramuscular EMG-driven Musculoskeletal Modelling: Towards Implanted Muscle Interfacing in Spinal Cord Injury Patients. IEEE Trans. Biomed. Eng. 2021, 69, 63–74. [Google Scholar] [CrossRef]
- Durandau, G.; Farina, D.; Asín-Prieto, G.; Dimbwadyo-Terrer, I.; Lerma-Lara, S.; Pons, J.L.; Moreno, J.C.; Sartori, M. Voluntary control of wearable robotic exoskeletons by patients with paresis via neuromechanical modeling. J. NeuroEngineering Rehabil. 2019, 16, 91. [Google Scholar] [CrossRef]
- Durandau, G.; Sartori, M.; Bortole, M.; Moreno, J.C.; Pons, J.L.; Farina, D. EMG-driven models of human-machine interaction in individuals wearing the H2 exoskeleton. IFAC-PapersOnLine 2016, 49, 200–203. [Google Scholar] [CrossRef]
- Adewuyi, A.A.; Hargrove, L.J.; Kuiken, T.A. Evaluating EMG Feature and classifier selection for application to Partial-hand Prosthesis control. Front. Neurorobot. 2016, 10, 15. [Google Scholar] [CrossRef]
- Liu, Y.; Bi, J.W.; Fan, Z.P. Multi-class sentiment classification: The experimental comparisons of feature selection and machine learning algorithms. Expert Syst. Appl. 2017, 80, 323–339. [Google Scholar] [CrossRef]
- Lorrain, T.; Jiang, N.; Farina, D. Influence of the training set on the accuracy of surface EMG classification in dynamic contractions for the control of multifunction prostheses. J. NeuroEngineering Rehabil. 2011, 8, 25. [Google Scholar] [CrossRef]
- Hargrove, L.J.; Simon, A.M.; Young, A.J.; Lipschutz, R.D.; Finucane, S.B.; Smith, D.G.; Kuiken, T.A. Robotic Leg Control with EMG Decoding in an Amputee with Nerve Transfers. N. Engl. J. Med. 2013, 369, 1237–1242. [Google Scholar] [CrossRef]
- Spanias, J.A.; Simon, A.M.; Finucane, S.B.; Perreault, E.J.; Hargrove, L.J. Online adaptive neural control of a robotic lower limb prosthesis. J. Neural Eng. 2018, 15, 016015. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Samuel, O.W.; Zhang, X.; Wang, H.; Fang, P.; Li, G. A motion-classification strategy based on sEMG-EEG signal combination for upper-limb amputees. J. NeuroEngineering Rehabil. 2017, 14, 2. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Sun, F.; Yang, C.; Xie, Q.; Zhang, W. SEMG-based joint force control for an upper-limb power-assist exoskeleton robot. IEEE J. Biomed. Health Inform. 2014, 18, 1043–1050. [Google Scholar] [CrossRef]
- Kopke, J.V.; Hargrove, L.J.; Ellis, M.D. Applying LDA-based pattern recognition to predict isometric shoulder and elbow torque generation in individuals with chronic stroke with moderate to severe motor impairment. J. NeuroEngineering Rehabil. 2019, 16, 35. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Li, G.; Zhou, P. EMG feature assessment for myoelectric pattern recognition and channel selection: A study with incomplete spinal cord injury. Med Eng. Phys. 2014, 36, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Lujan-Moreno, G.A.; Howard, P.R.; Rojas, O.G.; Montgomery, D.C. Design of experiments and response surface methodology to tune machine learning hyperparameters, with a random forest case-study. Expert Syst. Appl. 2018, 109, 195–205. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Z.; Li, G.; Yuan, P.; Yang, C.; Song, R. Development of Sensory-Motor Fusion-Based Manipulation and Grasping Control for a Robotic Hand-Eye System. IEEE Trans. Syst. Man Cybern. Syst. 2017, 47, 1169–1180. [Google Scholar] [CrossRef]
- Smith, L.H.; Kuiken, T.A.; Hargrove, L.J. Real-time simultaneous and proportional myoelectric control using intramuscular EMG. J. Neural Eng. 2014, 11, 066013. [Google Scholar] [CrossRef]
- Hahne, J.M.; Bießmann, F.; Jiang, N.; Rehbaum, H.; Farina, D.; Meinecke, F.C.; Muller, K.R.; Parra, L.C. Linear and nonlinear regression techniques for simultaneous and proportional myoelectric control. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 269–279. [Google Scholar] [CrossRef]
- Gui, K.; Liu, H.; Zhang, D. A Practical and Adaptive Method to Achieve EMG-Based Torque Estimation for a Robotic Exoskeleton. IEEE/ASME Trans. Mechatron. 2019, 24, 483–494. [Google Scholar] [CrossRef]
- Barsotti, M.; Dupan, S.; Vujaklija, I.; Došen, S.; Frisoli, A.; Farina, D. Online Finger Control Using High-Density EMG and Minimal Training Data for Robotic Applications. IEEE Robot. Autom. Lett. 2019, 4, 217–223. [Google Scholar] [CrossRef]
- Zhang, L.; Long, J.; Zhao, R.; Cao, H.; Zhang, K. Estimation of the Continuous Pronation—Supination Movement by Using Multichannel EMG Signal Features and Kalman Filter: Application to Control an Exoskeleton. Front. Bioeng. Biotechnol. 2022, 9, 1487. [Google Scholar] [CrossRef]
- Ogata, K. Modern Control Engineering, 5th ed.; Pearson: London, UK, 2010. [Google Scholar]
- Khalil, H. Nonlinear Systems, 3rd ed.; Pearson: London, UK, 2001. [Google Scholar]
- Aschepkov, L.T.; Dolgy, D.V.; Kim, T.; Agarwal, R.P. Optimal Control; Springer: Cham, Switzerland, 2016; p. 209. [Google Scholar] [CrossRef]
- Ioannou, P.A.; Sun, J. Robust Adaptive Control; Dover, Ed.; Dover Publications: Mineola, NY, USA, 2012. [Google Scholar]
- Nguyen, N.T. Model-Reference Adaptive Control: A Primer, 1st ed.; Advanced Textbooks in Control and Signal Processing; Springer: Cham, Switzerland, 2018; p. 444. [Google Scholar] [CrossRef]
- Xu, W.; Chu, B.; Rogers, E. Iterative learning control for robotic-assisted upper limb stroke rehabilitation in the presence of muscle fatigue. Control Eng. Pract. 2014, 31, 63–72. [Google Scholar] [CrossRef]
- Yu, H.; Choi, I.S.; Han, K.L.; Choi, J.Y.; Chung, G.; Suh, J. Development of a upper-limb exoskeleton robot for refractory construction. Control Eng. Pract. 2018, 72, 104–113. [Google Scholar] [CrossRef]
- Liu, G.; Sun, N.; Liang, D.; Chen, Y.; Yang, T.; Fang, Y. Neural network-based adaptive command filtering control for pneumatic artificial muscle robots with input uncertainties. Control Eng. Pract. 2022, 118, 104960. [Google Scholar] [CrossRef]
- Song, X.; Wu, C.; Stojanovic, V.; Song, S. 1 bit encoding–decoding-based event-triggered fixed-time adaptive control for unmanned surface vehicle with guaranteed tracking performance. Control Eng. Pract. 2023, 135, 105513. [Google Scholar] [CrossRef]
- Song, X.; Sun, P.; Song, S.; Stojanovic, V. Finite-time adaptive neural resilient DSC for fractional-order nonlinear large-scale systems against sensor-actuator faults. Nonlinear Dyn. 2023, 111, 12181–12196. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, W.; Na, J.; Zhang, D.; Hämäläinen, T.T.; Stojanovic, V.; Lewis, F.L. Value iteration and adaptive optimal output regulation with assured convergence rate. Control Eng. Pract. 2022, 121, 105042. [Google Scholar] [CrossRef]
- Liu, G.; Sun, N.; Yang, T.; Liu, Z.; Fang, Y. Equivalent-input-disturbance rejection-based adaptive motion control for pneumatic artificial muscle arms via hysteresis compensation models. Control Eng. Pract. 2023, 138, 105609. [Google Scholar] [CrossRef]
- Lenzi, T.; De Rossi, S.M.M.; Vitiello, N.; Carrozza, M.C. Intention-based EMG control for powered exoskeletons. IEEE Trans. Biomed. Eng. 2012, 59, 2180–2190. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, Q.; Chen, B.; Zhao, Z. Design and Voluntary Control of Variable Stiffness Exoskeleton Based on sEMG Driven Model. IEEE Robot. Autom. Lett. 2022, 7, 5787–5794. [Google Scholar] [CrossRef]
- Zhao, C. Control design of upper limb rehabilitation exoskeleton robot based on long and short-term memory network. J. Physics: Conf. Ser. 2021, 1986, 012134. [Google Scholar] [CrossRef]
- Yao, S.; Zhuang, Y.; Li, Z.; Song, R. Adaptive admittance control for an ankle exoskeleton using an EMG-driven musculoskeletal model. Front. Neurorobot. 2018, 12, 16. [Google Scholar] [CrossRef]
- da Silva, L.D.L.; Pereira, T.F.; Leithardt, V.R.Q.; Seman, L.O.; Zeferino, C.A. Hybrid Impedance-Admittance Control for Upper Limb Exoskeleton Using Electromyography. Appl. Sci. 2020, 10, 7146. [Google Scholar] [CrossRef]
- Kiguchi, K.; Hayashi, Y. An EMG-based control for an upper-limb power-assist exoskeleton robot. IEEE Trans. Syst. Man, Cybern. Part B Cybern. 2012, 42, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Li, Z.; Li, G.; Yang, C. EMG-Based Neural Network Control of an Upper-Limb Power-Assist Exoskeleton Robot. In Proceedings of the 10th International Symposium on Neural Networks, ISNN 2013, Dalian, China, 4–6 July 2013; pp. 204–211. [Google Scholar] [CrossRef]
- Wei, Q.; Li, Z.; Zhao, K.; Kang, Y.; Su, C.Y. Synergy-Based Control of Assistive Lower-Limb Exoskeletons by Skill Transfer. IEEE/ASME Trans. Mechatron. 2020, 25, 705–715. [Google Scholar] [CrossRef]
- Ye, W.; Li, Z.; Su, C.Y. Development and human-like control of an upper limb rehabilitation exoskeleton using sEMG bio-feedback. In Proceedings of the 2012 IEEE International Conference on Mechatronics and Automation, ICMA 2012, Chengdu, China, 5–8 August 2012; pp. 2077–2082. [Google Scholar] [CrossRef]
- Ye, W.; Li, Z.; Yang, C.; Chen, F.; Su, C.Y. Motion Detection Enhanced Control of an Upper Limb Exoskeleton Robot for Rehabilitation Training. Int. J. Humanoid Robot. 2017, 14, 1650031. [Google Scholar] [CrossRef]
- Li, Z.; Xu, C.; Wei, Q.; Shi, C.; Su, C.Y. Human-Inspired Control of Dual-Arm Exoskeleton Robots with Force and Impedance Adaptation. IEEE Trans. Syst. Man, Cybern. Syst. 2020, 50, 5296–5305. [Google Scholar] [CrossRef]
- Yang, C.; Zeng, C.; Fang, C.; He, W.; Li, Z. A DMPs-Based Framework for Robot Learning and Generalization of Humanlike Variable Impedance Skills. IEEE/ASME Trans. Mechatron. 2018, 23, 1193–1203. [Google Scholar] [CrossRef]
- Peternel, L.; Noda, T.; Petrič, T.; Ude, A.; Morimoto, J.; Babič, J. Adaptive Control of Exoskeleton Robots for Periodic Assistive Behaviours Based on EMG Feedback Minimisation. PLoS ONE 2016, 11, e0148942. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Alvarez, R.; Hernandez, J.H.; Matehuala-Moran, I.; Alfaro-Ponce, M.; Lopez-Gutierrez, R.; Salazar, S.; Lozano, R. Assistive robotic exoskeleton using recurrent neural networks for decision taking for the robust trajectory tracking. Expert Syst. Appl. 2022, 193, 116482. [Google Scholar] [CrossRef]
- Riani, A.; Madani, T.; Benallegue, A.; Djouani, K. Adaptive integral terminal sliding mode control for upper-limb rehabilitation exoskeleton. Control Eng. Pract. 2018, 75, 108–117. [Google Scholar] [CrossRef]
- Jabbari Asl, H.; Katagiri, K.; Narikiyo, T.; Yamashita, M.; Kawanishi, M. Augmenting human power by assistive robots: Application of adaptive neural networks. Control Eng. Pract. 2021, 110, 104769. [Google Scholar] [CrossRef]
- Toro-Ossaba, A.; Tejada, J.C.; Rúa, S.; Núñez, J.D.; Peña, A. Myoelectric Model Reference Adaptive Control with Adaptive Kalman Filter for a soft elbow exoskeleton. Control Eng. Pract. 2024, 142, 105774. [Google Scholar] [CrossRef]
- Souza, R.S.; de Castro Martins, T.; Furtado, G.P.; Forner-Cordero, A. Model Reference Adaptive Impedance Controller Design For Modular Exoskeleton. IFAC-PapersOnLine 2018, 51, 345–349. [Google Scholar] [CrossRef]
- Lv, X.; Han, J.; Yang, C.; Cong, D. Model reference adaptive impedance control in lower limbs rehabilitation robot. In Proceedings of the 2017 IEEE International Conference on Information and Automation, ICIA 2017, Macau, China, 18–21 July 2017; pp. 254–259. [Google Scholar] [CrossRef]
- Sharifi, M.; Behzadipour, S.; Vossoughi, G.R. Model reference adaptive impedance control of rehabilitation robots in operational space. In Proceedings of the IEEE RAS and EMBS International Conference on Biomedical Robotics and Biomechatronics, Rome, Italy, 24–27 June 2012; pp. 1698–1703. [Google Scholar] [CrossRef]
- Abu-Dakka, F.J.; Saveriano, M. Variable Impedance Control and Learning—A Review. Front. Robot. AI 2020, 7, 590681. [Google Scholar] [CrossRef]
- Al-Shuka, H.F.; Leonhardt, S.; Zhu, W.H.; Song, R.; Ding, C.; Li, Y. Active impedance control of bioinspired motion robotic manipulators: An overview. Appl. Bionics Biomech. 2018, 2018, 8203054. [Google Scholar] [CrossRef] [PubMed]
- Jaber, H.A.; Rashid, M.T.; Fortuna, L. Online myoelectric pattern recognition based on hybrid spatial features. Biomed. Signal Process. Control 2021, 66, 102482. [Google Scholar] [CrossRef]
- Mendes Souza, G.C.; Moreno, R.L. Netlab MLP—Performance Evaluation for Pattern Recognition in Myoletric Signal. In Procedia Computer Science; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 130, pp. 932–938. [Google Scholar] [CrossRef]
- Hao, Y.; Visell, Y. Beyond Soft Hands: Efficient Grasping With Non-Anthropomorphic Soft Grippers. Front. Robot. AI 2021, 0, 210. [Google Scholar] [CrossRef]
- Trumic, M.; Della Santina, C.; Jovanovic, K.; Fagiolini, A. Adaptive Control of Soft Robots Based on an Enhanced 3D Augmented Rigid Robot Matching. In Proceedings of the American Control Conference, Virtual Conference, 25–28 May 2021; pp. 4991–4996. [Google Scholar] [CrossRef]
- Thuruthel, T.G.; Ansari, Y.; Falotico, E.; Laschi, C. Control Strategies for Soft Robotic Manipulators: A Survey. Soft Robot. 2018, 5, 149–163. [Google Scholar] [CrossRef]
- Della Santina, C.; Katzschmann, R.K.; Bicchi, A.; Rus, D. Dynamic control of soft robots interacting with the environment. In Proceedings of the 2018 IEEE International Conference on Soft Robotics, RoboSoft 2018, Livorno, Italy, 24–28 April 2018; pp. 46–53. [Google Scholar] [CrossRef]
- Simon, A.M.; Hargrove, L.J.; Lock, B.A.; Kuiken, T.A. A decision-based velocity ramp for minimizing the effect of misclassifications during real-time pattern recognition control. IEEE Trans. Biomed. Eng. 2011, 58, 2360–2368. [Google Scholar] [CrossRef]
- Ameri, A.; Akhaee, M.A.; Scheme, E.; Englehart, K. Real-time, simultaneous myoelectric control using a convolutional neural network. PLoS ONE 2018, 13, e0203835. [Google Scholar] [CrossRef]
- Hwang, H.J.; Hahne, J.M.; Müller, K.R. Real-time robustness evaluation of regression based myoelectric control against arm position change and donning/doffing. PLoS ONE 2017, 12, e0186318. [Google Scholar] [CrossRef]
- Zaim, T.; Abdel-Hadi, S.; Mahmoud, R.; Khandakar, A.; Rakhtala, S.M.; Chowdhury, M.E.H. Machine Learning- and Deep Learning-Based Myoelectric Control System for Upper Limb Rehabilitation Utilizing EEG and EMG Signals: A Systematic Review. Bioengineering 2025, 12, 144. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Fang, X.; Wen, L.; Zhang, Y.; Wang, J. Data-Driven Model-Free Adaptive Containment Control for Uncertain Rehabilitation Exoskeleton Robots with Input Constraints. Actuators 2024, 13, 382. [Google Scholar] [CrossRef]
- Saeed, M.T.; Gul, J.Z.; Kausar, Z.; Mughal, A.M.; Ud Din, Z.M.; Qin, S. Design of Model-Based and Model-Free Robust Control Strategies for Lower Limb Rehabilitation Exoskeletons. Appl. Sci. 2022, 12, 3973. [Google Scholar] [CrossRef]
- Radac, M.B.; Borlea, A.I. Virtual State Feedback Reference Tuning and Value Iteration Reinforcement Learning for Unknown Observable Systems Control. Energies 2021, 14, 1006. [Google Scholar] [CrossRef]
- Radac, M.B.; Lala, T. Hierarchical Cognitive Control for Unknown Dynamic Systems Tracking. Mathematics 2021, 9, 2752. [Google Scholar] [CrossRef]
- Radac, M.B. Trajectory Tracking within a Hierarchical Primitive-Based Learning Approach. Entropy 2022, 24, 889. [Google Scholar] [CrossRef]
- Rose, L.; Bazzocchi, M.C.; Nejat, G. A model-free deep reinforcement learning approach for control of exoskeleton gait patterns. Robotica 2022, 40, 2189–2214. [Google Scholar] [CrossRef]
- Fu, J.; Choudhury, R.; Hosseini, S.M.; Simpson, R.; Park, J.H. Myoelectric Control Systems for Upper Limb Wearable Robotic Exoskeletons and Exosuits—A Systematic Review. Sensors 2022, 22, 8134. [Google Scholar] [CrossRef] [PubMed]




| Domain | Description | Features |
|---|---|---|
| Time-Domain Features | Focus on analyzing the amplitude of the EMG signal. Simple, computationally inexpensive, but sensitive to noise. | Root Mean Square (RMS). Mean Absolute Value (MAV). Waveform Length (WL). Linear Envelope (LE). Zero Crossing (ZC). Slope Sign Changes. |
| Frequency-Domain Features | Focus on the rate of muscle activation. Computationally expensive and with high variance. | Power Spectral Moments. Power Spectral Density (PSD). Median Frequency (MDF). Mean Frequency (MNF). Short-Time Fourier Transform. |
| Time–Frequency-Domain Features | Allow identification of both transient and steady-state patterns in the sEMG signal. Computationally expensive. | Wavelet Packet Transform. Discrete Wavelet Transform. |
| Strategy | Pros | Cons | Real-World Constraints/ Implementation Considerations |
|---|---|---|---|
| Model-Based Interfaces |
|
|
|
| Data-Driven Interfaces |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toro-Ossaba, A.; Tejada, J.C.; Sanin-Villa, D. Myoelectric Control in Rehabilitative and Assistive Soft Exoskeletons: A Comprehensive Review of Trends, Challenges, and Integration with Soft Robotic Devices. Biomimetics 2025, 10, 214. https://doi.org/10.3390/biomimetics10040214
Toro-Ossaba A, Tejada JC, Sanin-Villa D. Myoelectric Control in Rehabilitative and Assistive Soft Exoskeletons: A Comprehensive Review of Trends, Challenges, and Integration with Soft Robotic Devices. Biomimetics. 2025; 10(4):214. https://doi.org/10.3390/biomimetics10040214
Chicago/Turabian StyleToro-Ossaba, Alejandro, Juan C. Tejada, and Daniel Sanin-Villa. 2025. "Myoelectric Control in Rehabilitative and Assistive Soft Exoskeletons: A Comprehensive Review of Trends, Challenges, and Integration with Soft Robotic Devices" Biomimetics 10, no. 4: 214. https://doi.org/10.3390/biomimetics10040214
APA StyleToro-Ossaba, A., Tejada, J. C., & Sanin-Villa, D. (2025). Myoelectric Control in Rehabilitative and Assistive Soft Exoskeletons: A Comprehensive Review of Trends, Challenges, and Integration with Soft Robotic Devices. Biomimetics, 10(4), 214. https://doi.org/10.3390/biomimetics10040214








