A Comprehensive Review on Bioprinted Graphene-Based Material (GBM)-Enhanced Scaffolds for Nerve Guidance Conduits
Abstract
1. Introduction
2. Graphene-Based Materials (GBMs)
2.1. Classification
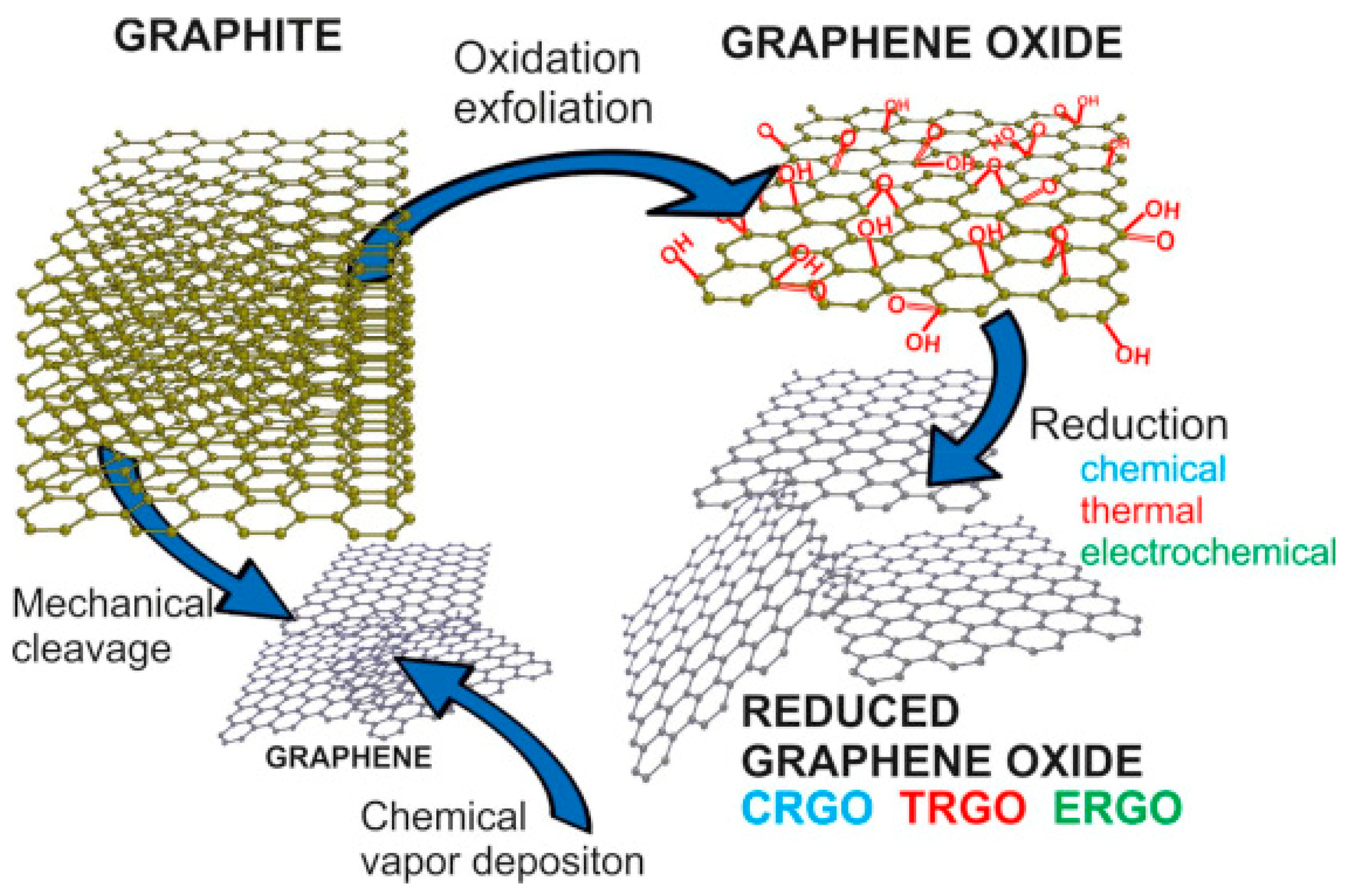
2.2. Synthesis of Graphene
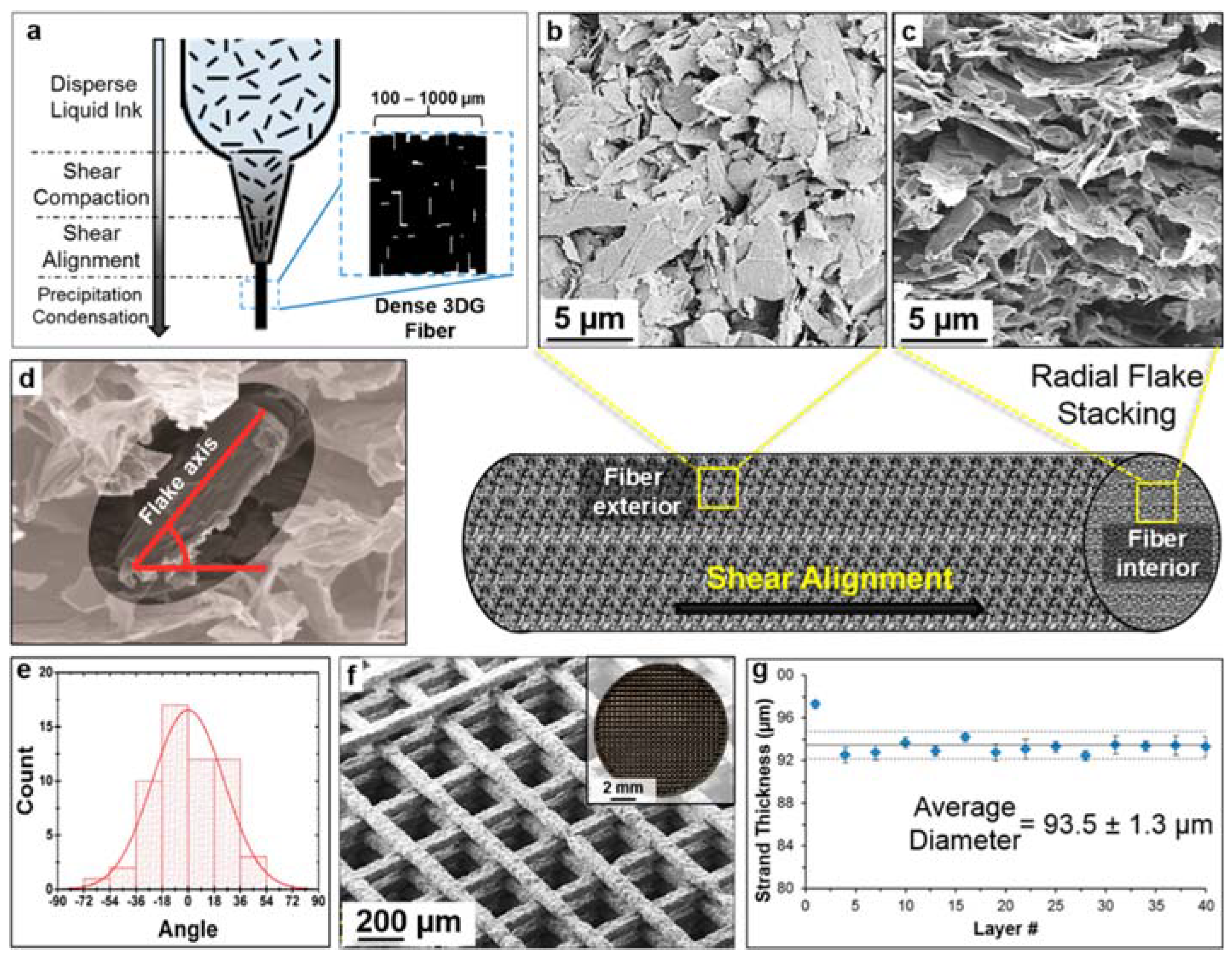
2.2.1. “Top-Down” Approach
2.2.2. “Bottom-Up” Approach
2.3. Properties of Graphene-Based Materials
2.3.1. Mechanical Properties
2.3.2. Electrical Properties
2.3.3. Cytotoxicity Properties
| Materials | Synthesized Method | Average Lateral Dimensions (nm) | In Vivo/In Vitro Models | Dosage (μg/mL) | Results | References |
|---|---|---|---|---|---|---|
| PG | CVD | 50~1500 | Human dermal fibroblasts (HDFs) and L-929 fibroblast cells | 0~500 | Graphene showed a dose-dependent toxicity, and viability of HDFs were 80% with exposure of 15.6 μg/mL of Graphene for 24 h. No significant cytotoxicity of graphene towards L-929 was observed even at 200 μg/mL | [83] |
| PG | CVD | N.A. | L929 fibroblasts cells | N.A. | Compared to control group, 113.5% of cell proliferation was found on graphene coated glass, demonstrating that graphene not only is non-toxic to L929, but also promotes its cell proliferation. | [84] |
| Nitrogen doped graphene | Electrochemical exfoliation of graphite rods | ~1000 | Endothelial cells (HUVEC) and tumor–colorectal adenocarcinoma cells (DLD1) | 0~250 | Exposure of HUVEC cells to N-graphene with 2.33 wt% and 2.56 wt% caused decrease of cell viability; N-graphene has less impact on DLD1 cell viability. | [98] |
| GO | Hummer’s method | ~500 | Hela cells | 0~100 | Cell viability was higher than 80% and cells maintained a normal morphology after 48 h cocultured with 100 μg/mL GO. | [85] |
| GO | N.A. | ~1550 nm for mono-layered GO and ~120 nm for multi-layered (15~20) GO | Dendritic cell (DC2.4) | 0~100 | Both GOs induced ROS and mono-layered GO has less effects on cell viability but caused a significant change in cell morphology. | [88] |
| Multi-layered GO | Hummer’s method | N.A. | Human Erythrocytes and Skin Fibroblasts cells | 0~200 | Smaller-size GO showed lower hemolytic activity compared to larger-size GO. GO showed dose-dependent effect on viability of human skin fibroblasts cells. Viability was about 80% after 24 h exposure to 200 μg/mL GO. | [89] |
| Mono-layered rGO | Hydrothermal reduction | N.A. | Human Erythrocytes and Skin Fibroblasts cells | 0~200 | rGO showed low hemolytic activity due to low oxygen contents and aqueous aggregations. rGO showed dose-dependent effect on viability of human skin fibroblasts cells. Viability was about 80% after 24 h exposure to 12.5 μg/mL rGO. Its high toxicity was caused by aggregation. | [89] |
| rGO | Hydrazine hydrate reduction | N.A. | Human liver cells (HepG2) | 0~300 | rGO did not induce toxicity to HepG2 cells up to concentration of 50 μg/mL | [99] |
| rGO | Hydrazine hydrate reduction | ~500 | Human lung epithelial carcinoma cells (A549) | 0~400 | Hydrazine hydrate reduced GO exhibited a dose-dependent toxic effects, with a maximum of 70% cytotoxicity detected at 400 μg/mL. | [87] |
| rGO | Ascorbic acid reduction | ~500 | Human lung epithelial carcinoma cells (A549) | 0~400 | Increased protein leakage and disruption of membrane were observed in ascorbic acid reduced GO due to sharp protrusions caused during reduction. | [87] |
3. Three-Dimensional-Printed GBM-Based Nerve Guidance Conduits
3.1. Nerve Guidance Conduits
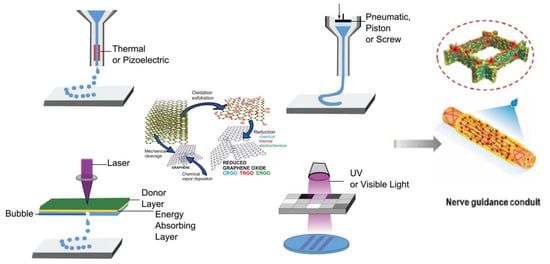
3.2. Three-Dimensional Bioprinting Technology
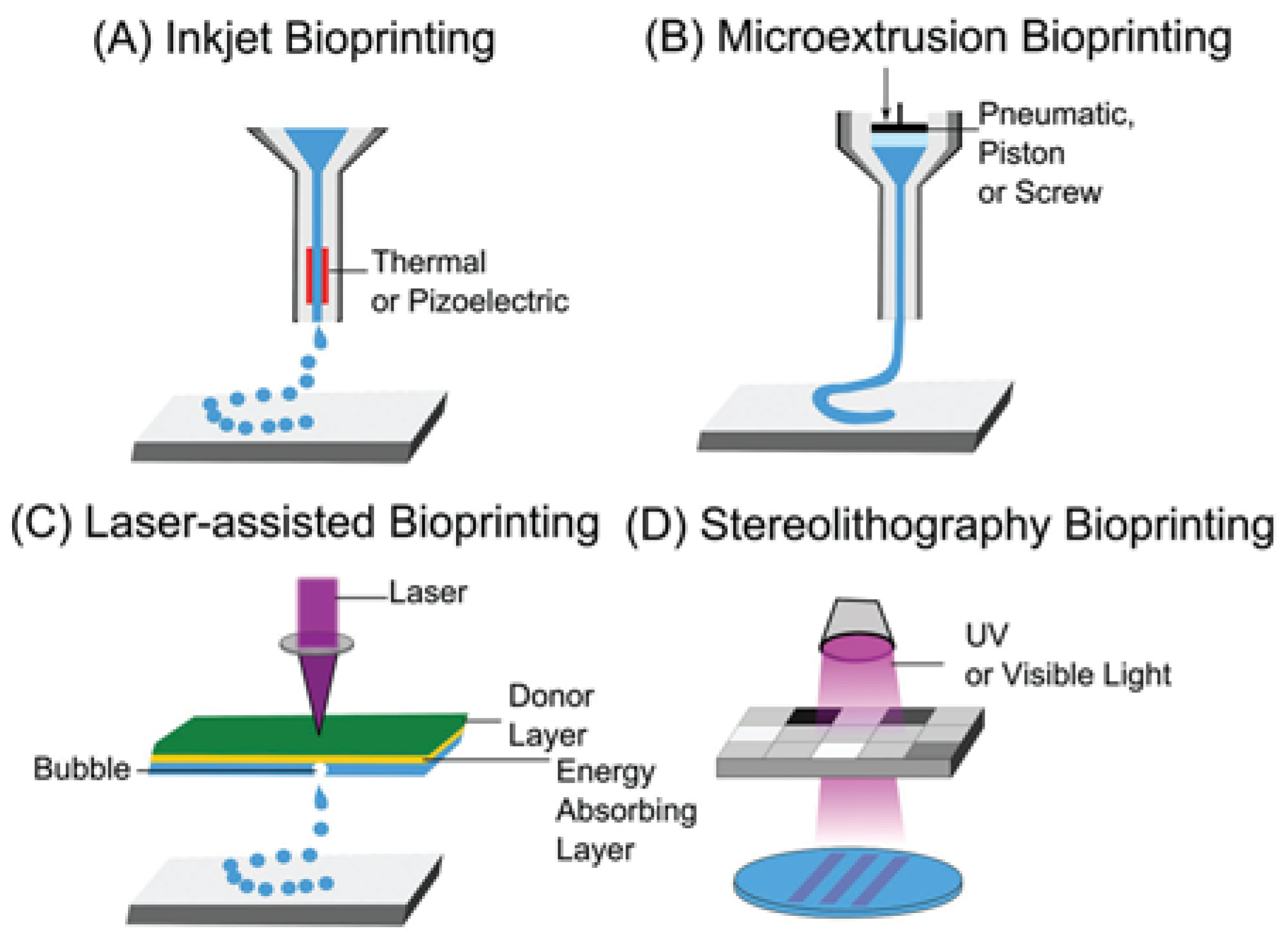
3.2.1. Extrusion-Based 3D Bioprinting
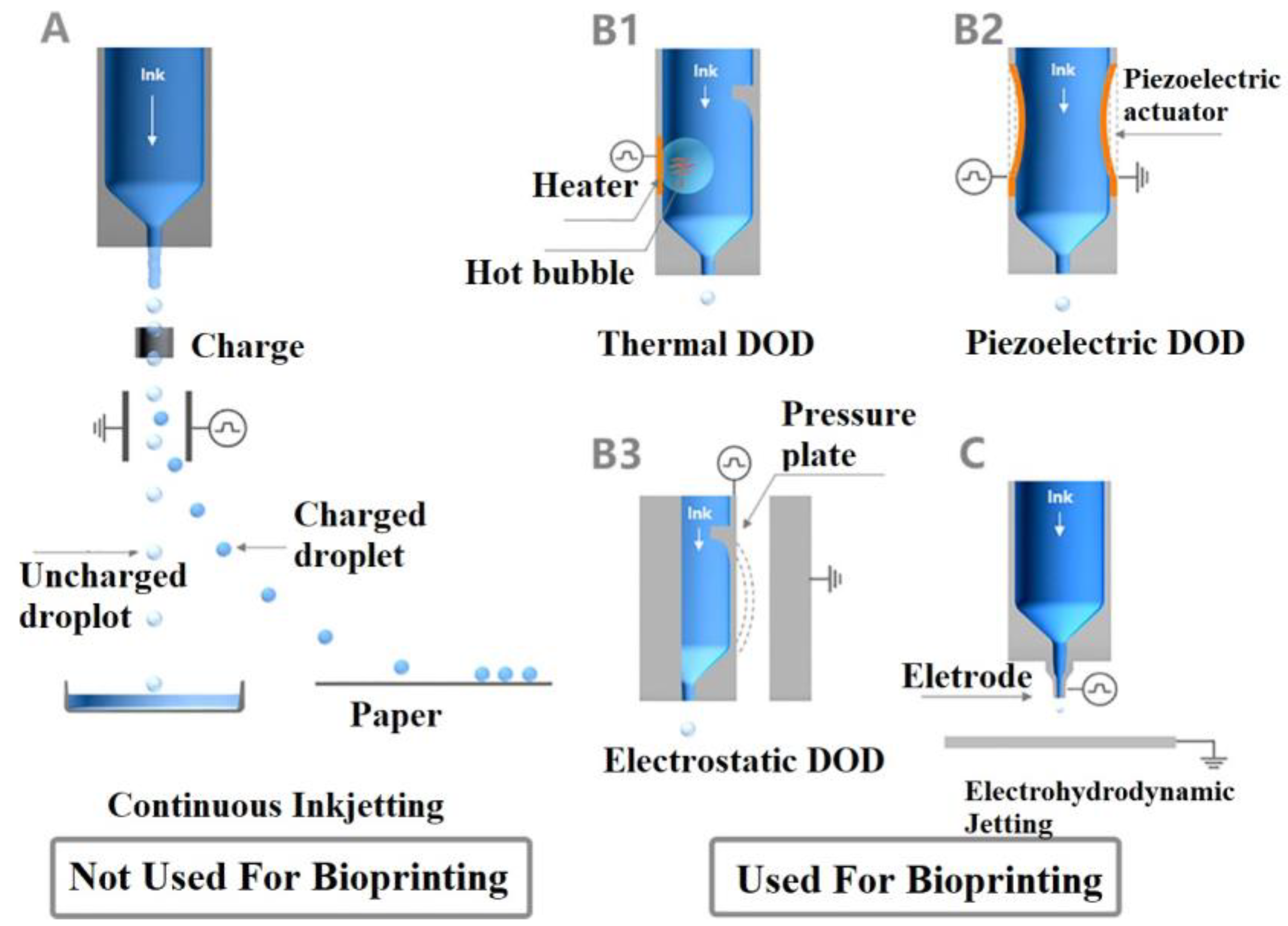
3.2.2. Inkjet-Based 3D Bioprinting
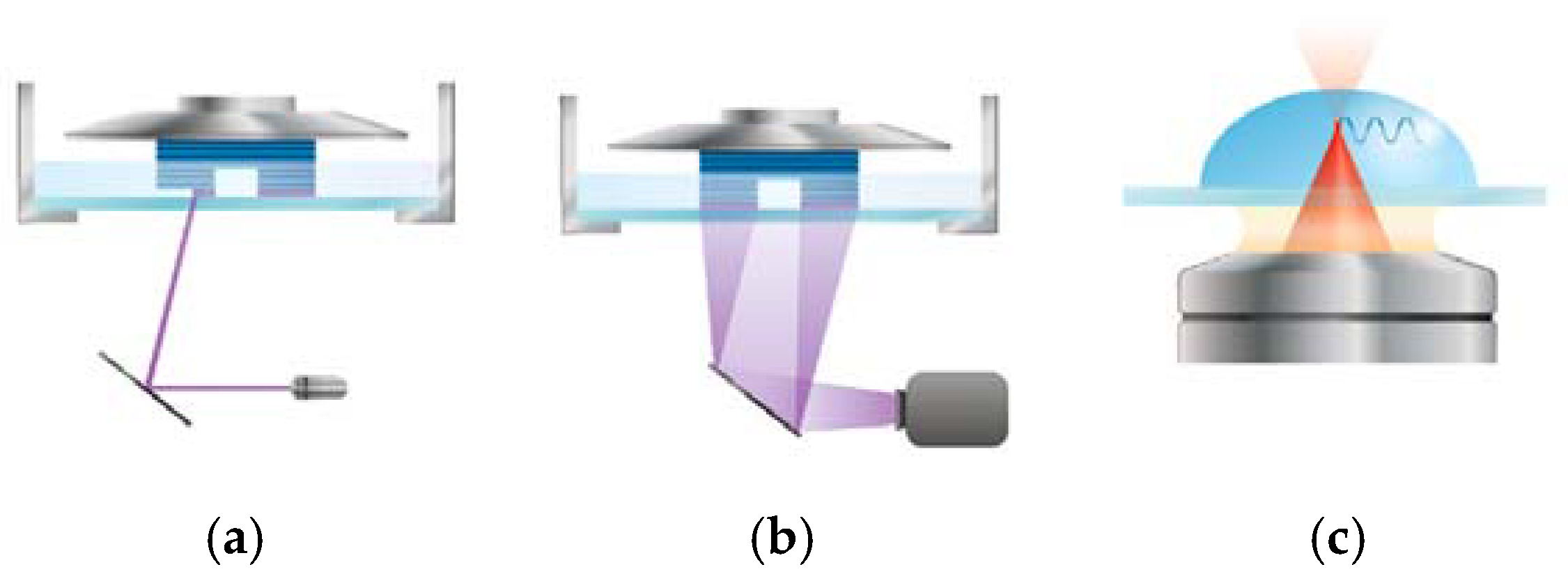
3.2.3. Light-Based 3D Bioprinting
3.2.4. Combination of 3D Printing and Electrospinning Techniques
3.3. Design Strategies of 3D-Printed NGCs
3.3.1. Material Composition and Hybrid Structures
3.3.2. Structural Design for Cell Growth and Guidance
3.3.3. Electrical Conductivity for Enhanced Nerve Regeneration
| Materials of NGCs | Fabrication Technique | Electrical Conductivity () | Cell Behaviors and Axon Formation Results | Reference |
|---|---|---|---|---|
| 5% PEGylated GO-PU | SLA | N.A. | [142] | |
| Porous rGO/PCL/ Mel fiber | DIW | Axon formation was observed in a Sprague–Dawley rat model, with an average diameter of 4.98 ± 2.24 μm and a thickness of 0.59 ± 0.36 μm, comparable to that of autografted NGCs. | [144] | |
| PDA/RGD-SG/PCL | Hybrid technique (casting and 3D printing) | 0.892 | In vitro results showed that SC neural expressions were improved, and in vivo results exhibited functional sciatic nerve recovery and axon regrowth. | [153] |
| PDA/RGD-MG/PCL | Hybrid technique (casting and 3D printing) | 0.637 | In vitro results showed that SC neural expressions were higher than PDA/RGD/PCL but slightly lower than PDA/RGD-SG/PCL, and in vivo results exhibited functional sciatic nerve recovery and axon regrowth. | [153] |
| GO/PCL | Hybrid technique (injection molding and 3D printing) | In vivo results showed that GO/PCL NGCs exhibited a significantly greater total number, area, diameter, and thickness of regenerated nerves and myelinated axons compared to the PCL group, similar to autograft NGCs at 18 weeks post-surgery. | [154] | |
| rGO/PCL | EHD | 0.135 | The addition of rGO results in softer scaffolds support neural differentiation of PC 12 cells | [157] |
| rGO/Silk Fibroin | DLP | 6.5 | In vitro results showed the printed NGCs promote the proliferation of Neuro2a cells and exhibit neurogenic activity by inducing neuronal differentiation in neuroblastoma cells. | [171] |
| GO/GelMA | DLP | 15 | Neuro2a cells exhibit more pronounced neurite growth when encapsulated in GO/GelMA compared to when treated with differentiation induction media. | [170] |
| Graphene-PLA-PCL | FDM | N.A. | [169] | |
| 20% Graphene-PLG | DIW | Human MSCs (hMSCs) exhibited a confluent, sheet-like morphology, indicating characteristic of adherent cell types, such as fibroblasts | [172] | |
| 60% Graphene-PLG | DIW | Human MSCs exhibited axon-like extensions and features on day 14 | [172] |
4. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2P-SL | Two-photon stereolithography |
| CNS | Central nervous system |
| CVD | Chemical vapor deposition |
| DCM | Dichloromethane |
| DIW | Direct ink writing |
| DoD | Drop-on-demand |
| ECM | Extracellular matrix |
| EHD | Electrohydrodynamic |
| FDM | Fused deposition modeling |
| GelMA | Methacrylate anhydride gelatin |
| GF | Graphene foam |
| GO | Graphene oxide |
| GBMs | Graphene-based materials |
| HOPG | Highly ordered pyrolytic graphite |
| LDH | Lactate dehydrogenase |
| LPE | Liquid-phase exfoliation |
| MEW | Melt electrowriting |
| MG | Multi-layered graphene |
| MSCs | Mesenchymal stem cells |
| NGCs | Nerve guidance conduits |
| PCL | poly(caprolactone) |
| PECVD | Plasma-enhanced chemical vapor deposition |
| PG | Pristine graphene |
| PLG | Polylactide-co-glycolide |
| PNS | Peripheral nervous system |
| rGO | Reduced graphene oxide |
| ROS | Reactive oxygen species |
| SCs | Schwann cells |
| SG | Single-layered graphene |
| SLA | Stereolithography |
References
- Sachanandani, N.F.; Pothula, A.; Tung, T.H. Nerve gaps. Plast. Reconstr. Surg. 2014, 133, 313–319. [Google Scholar] [PubMed]
- Rebowe, R.; Rogers, A.; Yang, X.; Kundu, S.; Smith, T.L.; Li, Z. Nerve repair with nerve conduits: Problems, solutions, and future directions. J. Hand Microsurg. 2018, 10, 61–65. [Google Scholar]
- Guarino, V.; Cirillo, V.; Ambrosio, L. 6.18 Multicomponent Conduits for Peripheral Nerve Regeneration; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Jansen, K.; Van Der Werff, J.; Van Wachem, P.; Nicolai, J.A.; De Leij, L.; Van Luyn, M.J.B. A hyaluronan-based nerve guide: In vitro cytotoxicity, subcutaneous tissue reactions, and degradation in the rat. Biomaterials 2004, 25, 483–489. [Google Scholar] [PubMed]
- Wang, S.; Wan, A.C.; Xu, X.; Gao, S.; Mao, H.-Q.; Leong, K.W.; Yu, H.J.B. A new nerve guide conduit material composed of a biodegradable poly (phosphoester). Biomaterials 2001, 22, 1157–1169. [Google Scholar] [PubMed]
- Kehoe, S.; Zhang, X.; Boyd, D. Composition–property relationships for an experimental composite nerve guidance conduit: Evaluating cytotoxicity and initial tensile strength. J. Mater. Sci. Mater. Med. 2011, 22, 945–959. [Google Scholar]
- Meek, M.F.; Den Dunnen, W.F. Porosity of the wall of a Neurolac® nerve conduit hampers nerve regeneration. Microsurgery 2009, 29, 473–478. [Google Scholar]
- Houshyar, S.; Bhattacharyya, A.; Shanks, R. Peripheral nerve conduit: Materials and structures. ACS Chem. Neurosci. 2019, 10, 3349–3365. [Google Scholar]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.-E.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-based materials: Synthesis, characterization, properties, and applications. Small 2011, 7, 1876–1902. [Google Scholar]
- Cong, H.-P.; Chen, J.-F.; Yu, S.-H. Graphene-based macroscopic assemblies and architectures: An emerging material system. Chem. Soc. Rev. 2014, 43, 7295–7325. [Google Scholar]
- Seabra, A.B.; Paula, A.J.; De Lima, R.; Alves, O.L.; Durán, N. Nanotoxicity of graphene and graphene oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.C.; Ali, M.; Hasan, E.A.; Wehbe, N.; Alshareef, H.N.; Alsulaiman, D. Smartphone-Interfaced Electrochemical Biosensor for microRNA Detection Based on Laser-Induced Graphene with π–π Stacked Peptide Nucleic Acid Probes. ACS Mater. Lett. 2024, 6, 837–846. [Google Scholar] [CrossRef]
- Itoo, A.M.; Paul, M.; Ghosh, B.; Biswas, S. Polymeric graphene oxide nanoparticles loaded with doxorubicin for combined photothermal and chemotherapy in triple negative breast cancer. Biomater. Adv. 2023, 153, 213550. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Liu, T.; Zhang, Y.; Wang, C.; Xie, B. Ultrasmall graphene oxide for combination of enhanced chemotherapy and photothermal therapy of breast cancer. Colloids Surf. B Biointerfaces 2023, 225, 113288. [Google Scholar] [CrossRef]
- Adamowicz, J.; Pasternak, I.; Kloskowski, T.; Gniadek, M.; Van Breda, S.; Buhl, M.; Balcerczyk, D.; Gagat, M.; Grzanka, D.; Strupinski, W. Development of a conductive biocomposite combining graphene and amniotic membrane for replacement of the neuronal network of tissue-engineered urinary bladder. Sci. Rep. 2020, 10, 5824. [Google Scholar] [CrossRef]
- Wang, C.-H.; Guo, Z.-S.; Pang, F.; Zhang, L.-Y.; Yan, M.; Yan, J.-H.; Li, K.-W.; Li, X.-J.; Li, Y.; Bi, L. Effects of graphene modification on the bioactivation of polyethylene-terephthalate-based artificial ligaments. ACS Appl. Mater. Interfaces 2015, 7, 15263–15276. [Google Scholar] [CrossRef]
- Uehara, T.M.; Paino, I.M.; Santos, F.A.; Scagion, V.P.; Correa, D.S.; Zucolotto, V. Fabrication of random and aligned electrospun nanofibers containing graphene oxide for skeletal muscle cells scaffold. Polym. Adv. Technol. 2020, 31, 1437–1443. [Google Scholar] [CrossRef]
- Sarkar, S.D.; Uddin, M.M.; Roy, C.K.; Hossen, M.J.; Sujan, M.I.; Azam, M.S. Mechanically tough and highly stretchable poly (acrylic acid) hydrogel cross-linked by 2D graphene oxide. RSC Adv. 2020, 10, 10949–10958. [Google Scholar] [CrossRef]
- Yi, W.; Wu, H.; Wang, H.; Du, Q. Interconnectivity of macroporous hydrogels prepared via graphene oxide-stabilized pickering high internal phase emulsions. Langmuir 2016, 32, 982–990. [Google Scholar] [CrossRef]
- Park, J.; Jeon, N.; Lee, S.; Choe, G.; Lee, E.; Lee, J.Y. Conductive hydrogel constructs with three-dimensionally connected graphene networks for biomedical applications. Chem. Eng. J. 2022, 446, 137344. [Google Scholar] [CrossRef]
- Roca, F.G.; Santos, L.G.; Roig, M.M.; Medina, L.M.; Martínez-Ramos, C.; Pradas, M.M.J.B. Novel tissue-engineered multimodular hyaluronic acid-polylactic acid conduits for the regeneration of sciatic nerve defect. Biomedicines 2022, 10, 963. [Google Scholar] [CrossRef] [PubMed]
- Panahi-Joo, Y.; Karkhaneh, A.; Nourinia, A.; Abd-Emami, B.; Negahdari, B.; Renaud, P.; Bonakdar, S.J.B.M. Design and fabrication of a nanofibrous polycaprolactone tubular nerve guide for peripheral nerve tissue engineering using a two-pole electrospinning system. Biomed. Mater. 2016, 11, 025017. [Google Scholar]
- Sun, Y.; Zhang, Y.; Guo, Y.; He, D.; Xu, W.; Fang, W.; Zhang, C.; Zuo, Y.; Zhang, Z. Electrical aligned polyurethane nerve guidance conduit modulates macrophage polarization and facilitates immunoregulatory peripheral nerve regeneration. J. Nanobiotechnol. 2024, 22, 244. [Google Scholar]
- Marino, A.; Tonda-Turo, C.; De Pasquale, D.; Ruini, F.; Genchi, G.; Nitti, S.; Cappello, V.; Gemmi, M.; Mattoli, V.; Ciardelli, G.; et al. Gelatin/nanoceria nanocomposite fibers as antioxidant scaffolds for neuronal regeneration. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 386–395. [Google Scholar]
- Solomevich, S.O.; Oranges, C.M.; Kalbermatten, D.F.; Schwendeman, A.; Madduri, S. Natural polysaccharides and their derivatives as potential medical materials and drug delivery systems for the treatment of peripheral nerve injuries. Carbohydr. Polym. 2023, 315, 120934. [Google Scholar]
- Itai, S.; Suzuki, K.; Kurashina, Y.; Kimura, H.; Amemiya, T.; Sato, K.; Nakamura, M.; Onoe, H. Cell-encapsulated chitosan-collagen hydrogel hybrid nerve guidance conduit for peripheral nerve regeneration. Biomed. Microdevices 2020, 22, 81. [Google Scholar] [CrossRef]
- Larijani, G.; Poostchi, M.; Faridghiasi, F.; Pal Singh Chauhan, N.; Rajaeih, S.; Amini, N.; Simorgh, S. Electrospun PCL/Alginate/Nanoclay Nerve Conduit with Olfactory Ectomesenchymal Stem Cells for Nerve Regeneration. ACS Appl. Bio Mater. 2024, 7, 7522–7534. [Google Scholar]
- Monfette, V.; Choinière, W.; Godbout-Lavoie, C.; Pelletier, S.; Langelier, È.; Lauzon, M.-A. Thermoelectric freeze-casting of biopolymer blends: Fabrication and characterization of large-size scaffolds for nerve tissue engineering applications. J. Funct. Biomater. 2023, 14, 330. [Google Scholar] [CrossRef]
- Berkovitch, Y.; Cohen, T.; Peled, E.; Schmidhammer, R.; Florian, H.; Teuschl, A.H.; Wolbank, S.; Yelin, D.; Redl, H.; Seliktar, D.; et al. Hydrogel composition and laser micropatterning to regulate sciatic nerve regeneration. J. Tissue Eng. Regen. Med. 2018, 12, 1049–1061. [Google Scholar]
- Huang, C.; Ouyang, Y.; Niu, H.; He, N.; Ke, Q.; Jin, X.; Li, D.; Fang, J.; Liu, W.; Fan, C.; et al. Nerve guidance conduits from aligned nanofibers: Improvement of nerve regeneration through longitudinal nanogrooves on a fiber surface. ACS Appl. Mater. Interfaces 2015, 7, 7189–7196. [Google Scholar] [CrossRef]
- Jandyal, A.; Chaturvedi, I.; Wazir, I.; Raina, A.; Haq, M.I.U. 3D printing—A review of processes, materials and applications in industry 4.0. Sustain. Oper. Comput. 2022, 3, 33–42. [Google Scholar]
- Ławkowska, K.; Pokrywczyńska, M.; Koper, K.; Kluth, L.A.; Drewa, T.; Adamowicz, J. Application of graphene in tissue engineering of the nervous system. Int. J. Mol. Sci. 2021, 23, 33. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, T.; Li, Y. 3D printing and bioprinting nerve conduits for neural tissue engineering. Polymers 2020, 12, 1637. [Google Scholar] [CrossRef] [PubMed]
- Bei, H.P.; Yang, Y.; Zhang, Q.; Tian, Y.; Luo, X.; Yang, M.; Zhao, X. Graphene-based nanocomposites for neural tissue engineering. Molecules 2019, 24, 658. [Google Scholar] [CrossRef]
- Song, J.; Lv, B.; Chen, W.; Ding, P.; He, Y. Advances in 3D printing scaffolds for peripheral nerve and spinal cord injury repair. Int. J. Extrem. Manuf. 2023, 5, 032008. [Google Scholar]
- Liu, K.; Yan, L.; Li, R.; Song, Z.; Ding, J.; Liu, B.; Chen, X. 3D printed personalized nerve guide conduits for precision repair of peripheral nerve defects. Adv. Sci. 2022, 9, 2103875. [Google Scholar]
- Liao, W.; Shi, Y.; Li, Z.; Yin, X. Advances in 3D printing combined with tissue engineering for nerve regeneration and repair. J. Nanobiotechnol. 2025, 23, 5. [Google Scholar]
- Shinohara, H.; Tiwari, A. Graphene: An Introduction to the Fundamentals and Industrial Applications; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Novoselov, K.S.; Geim, A.; Morozov, S.; Dubonos, S.; Zhang, Y.; Jiang, D. Room-temperature electric field effect and carrier-type inversion in graphene films. arXiv 2004. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S.T. Graphene oxide, highly reduced graphene oxide, and graphene: Versatile building blocks for carbon-based materials. Small 2010, 6, 711–723. [Google Scholar]
- Mohan, V.B.; Brown, R.; Jayaraman, K.; Bhattacharyya, D. Characterisation of reduced graphene oxide: Effects of reduction variables on electrical conductivity. Mater. Sci. Eng. B 2015, 193, 49–60. [Google Scholar]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on graphene-, graphene oxide-, reduced graphene oxide-based flexible composites: From fabrication to applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Cruz, A.; Ruiz-Hernández, A.R.; Vega-Clemente, J.F.; Luna-Gazcón, D.G.; Campos-Delgado, J. A review of top-down and bottom-up synthesis methods for the production of graphene, graphene oxide and reduced graphene oxide. J. Mater. Sci. 2022, 57, 14543–14578. [Google Scholar]
- Rowley-Neale, S.J.; Randviir, E.P.; Dena, A.S.A.; Banks, C.E. An overview of recent applications of reduced graphene oxide as a basis of electroanalytical sensing platforms. Appl. Mater. Today 2018, 10, 218–226. [Google Scholar]
- Yi, M.; Shen, Z. A review on mechanical exfoliation for the scalable production of graphene. J. Mater. Chem. A 2015, 3, 11700–11715. [Google Scholar]
- Jayasena, B.; Subbiah, S. A novel mechanical cleavage method for synthesizing few-layer graphenes. Nanoscale Res. Lett. 2011, 6, 95. [Google Scholar]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar]
- Li, Z.; Young, R.J.; Backes, C.; Zhao, W.; Zhang, X.; Zhukov, A.A.; Tillotson, E.; Conlan, A.P.; Ding, F.; Haigh, S.J. Mechanisms of liquid-phase exfoliation for the production of graphene. ACS Nano 2020, 14, 10976–10985. [Google Scholar]
- Prekodravac, J.R.; Kepić, D.P.; Colmenares, J.C.; Giannakoudakis, D.A.; Jovanović, S.P. A comprehensive review on selected graphene synthesis methods: From electrochemical exfoliation through rapid thermal annealing towards biomass pyrolysis. J. Mater. Chem. C 2021, 9, 6722–6748. [Google Scholar]
- Eda, G.; Fanchini, G.; Chhowalla, M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3, 270–274. [Google Scholar]
- Phuong Pham, V. Direct Growth of Graphene on Flexible Substrates Towards Flexible Electronics: A Promising Perspective. arxiv 2017, arXiv:1712.09714. [Google Scholar]
- Liu, L.; Zhou, H.; Cheng, R.; Yu, W.J.; Liu, Y.; Chen, Y.; Shaw, J.; Zhong, X.; Huang, Y.; Duan, X. High-yield chemical vapor deposition growth of high-quality large-area AB-stacked bilayer graphene. ACS Nano 2012, 6, 8241–8249. [Google Scholar] [PubMed]
- Shi, Q.; Tokarska, K.; Ta, H.Q.; Yang, X.; Liu, Y.; Ullah, S.; Liu, L.; Trzebicka, B.; Bachmatiuk, A.; Sun, J. Substrate developments for the chemical vapor deposition synthesis of graphene. Adv. Mater. Interfaces 2020, 7, 1902024. [Google Scholar]
- Pang, J.; Mendes, R.G.; Wrobel, P.S.; Wlodarski, M.D.; Ta, H.Q.; Zhao, L.; Giebeler, L.; Trzebicka, B.; Gemming, T.; Fu, L. Self-terminating confinement approach for large-area uniform monolayer graphene directly over Si/SiOx by chemical vapor deposition. ACS Nano 2017, 11, 1946–1956. [Google Scholar] [PubMed]
- Chen, Z.; Ci, H.; Tan, Z.; Dou, Z.; Chen, X.-d.; Liu, B.; Liu, R.; Lin, L.; Cui, L.; Gao, P. Growth of 12-inch uniform monolayer graphene film on molten glass and its application in PbI 2-based photodetector. Nano Res. 2019, 12, 1888–1893. [Google Scholar]
- Zhang, L.; Shi, Z.; Wang, Y.; Yang, R.; Shi, D.; Zhang, G. Catalyst-free growth of nanographene films on various substrates. Nano Res. 2011, 4, 315–321. [Google Scholar]
- Aleemardani, M.; Zare, P.; Seifalian, A.; Bagher, Z.; Seifalian, A.M. Graphene-based materials prove to be a promising candidate for nerve regeneration following peripheral nerve injury. Biomedicines 2021, 10, 73. [Google Scholar] [CrossRef]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. The structure of suspended graphene sheets. Nature 2007, 446, 60–63. [Google Scholar]
- Wu, J.; Lin, H.; Moss, D.J.; Loh, K.P.; Jia, B. Graphene oxide for photonics, electronics and optoelectronics. Nat. Rev. Chem. 2023, 7, 162–183. [Google Scholar]
- Lonkar, S.P.; Deshmukh, Y.S.; Abdala, A.A. Recent advances in chemical modifications of graphene. Nano Res. 2015, 8, 1039–1074. [Google Scholar]
- Hsu, I.-K.; Pettes, M.T.; Bushmaker, A.; Aykol, M.; Shi, L.; Cronin, S.B. Optical absorption and thermal transport of individual suspended carbon nanotube bundles. Nano Lett. 2009, 9, 590–594. [Google Scholar]
- Tavakol, M.; Montazeri, A.; Aboutalebi, S.H.; Asgari, R. Mechanical properties of graphene oxide: The impact of functional groups. Appl. Surf. Sci. 2020, 525, 146554. [Google Scholar] [CrossRef]
- Suk, J.W.; Piner, R.D.; An, J.; Ruoff, R.S. Mechanical properties of monolayer graphene oxide. ACS Nano 2010, 4, 6557–6564. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Navarro, C.; Burghard, M.; Kern, K. Elastic properties of chemically derived single graphene sheets. Nano Lett. 2008, 8, 2045–2049. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Colombo, L.; Gellert, P.; Schwab, M.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Petrone, N.; Dean, C.R.; Meric, I.; Van Der Zande, A.M.; Huang, P.Y.; Wang, L.; Muller, D.; Shepard, K.L.; Hone, J. Chemical vapor deposition-derived graphene with electrical performance of exfoliated graphene. Nano Lett. 2012, 12, 2751–2756. [Google Scholar] [CrossRef]
- Lim, S.; Park, H.; Yamamoto, G.; Lee, C.; Suk, J.W. Measurements of the electrical conductivity of monolayer graphene flakes using conductive atomic force microscopy. Nanomaterials 2021, 11, 2575. [Google Scholar] [CrossRef]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Ri Kim, H.; Song, Y.I. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef]
- Guex, L.G.; Sacchi, B.; Peuvot, K.F.; Andersson, R.L.; Pourrahimi, A.M.; Ström, V.; Farris, S.; Olsson, R.T. Experimental review: Chemical reduction of graphene oxide (GO) to reduced graphene oxide (rGO) by aqueous chemistry. Nanoscale 2017, 9, 9562–9571. [Google Scholar] [CrossRef]
- Rao, S.; Upadhyay, J.; Polychronopoulou, K.; Umer, R.; Das, R. Reduced graphene oxide: Effect of reduction on electrical conductivity. J. Compos. Sci. 2018, 2, 25. [Google Scholar] [CrossRef]
- Mohan, V.B.; Jayaraman, K.; Stamm, M.; Bhattacharyya, D. Physical and chemical mechanisms affecting electrical conductivity in reduced graphene oxide films. Thin Solid Film. 2016, 616, 172–182. [Google Scholar]
- Wang, Y.; Chen, Y.; Lacey, S.D.; Xu, L.; Xie, H.; Li, T.; Danner, V.A.; Hu, L. Reduced graphene oxide film with record-high conductivity and mobility. Mater. Today 2018, 21, 186–192. [Google Scholar] [CrossRef]
- SAFRON, N.S.; ARNOLD, M.S. Characterization of conduction mechanisms relevant to device performance in nanoperforated graphene. Int. J. High Speed Electron. Syst. 2011, 20, 697–706. [Google Scholar]
- Pei, S.; Zhao, J.; Du, J.; Ren, W.; Cheng, H.-M. Direct reduction of graphene oxide films into highly conductive and flexible graphene films by hydrohalic acids. Carbon 2010, 48, 4466–4474. [Google Scholar]
- Hadj Said, M.; Khlifi, A.; Zardi, N.; Gomri, S.; Haj Said, A.; Tounsi, F. Toward the use of graphene oxide-based planar inductors as a transducer for gas sensing applications. Smart Sci. 2024, 12, 293–304. [Google Scholar]
- Syama, S.; Mohanan, P.V. Comprehensive application of graphene: Emphasis on biomedical concerns. Nano-Micro Lett. 2019, 11, 6. [Google Scholar]
- Lu, P.; Zehtab Yazdi, A.; Han, X.X.; Al Husaini, K.; Haime, J.; Waye, N.; Chen, P. Mechanistic insights into the cytotoxicity of graphene oxide derivatives in mammalian cells. Chem. Res. Toxicol. 2020, 33, 2247–2260. [Google Scholar] [CrossRef]
- Conroy, J.; Verma, N.K.; Smith, R.J.; Rezvani, E.; Duesberg, G.S.; Coleman, J.N.; Volkov, Y. Biocompatibility of pristine graphene monolayers, nanosheets and thin films. arXiv 2014, arXiv:1406.2497. [Google Scholar]
- Zhang, Y.; Ali, S.F.; Dervishi, E.; Xu, Y.; Li, Z.; Casciano, D.; Biris, A.S. Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma-derived PC12 cells. ACS Nano 2010, 4, 3181–3186. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, Y.C.; Jin, O.S.; Lee, E.J.; Han, D.-W.; Kang, S.H.; Hong, S.W.; Ahn, J.Y.; Kim, S.H. Cytotoxicity evaluations of pristine graphene and carbon nanotubes in fibroblastic cells. J. Korean Phys. Soc. 2012, 61, 873–877. [Google Scholar]
- Lasocka, I.; Szulc-Dąbrowska, L.; Skibniewski, M.; Skibniewska, E.; Strupinski, W.; Pasternak, I.; Kmieć, H.; Kowalczyk, P. Biocompatibility of pristine graphene monolayer: Scaffold for fibroblasts. Toxicol. In Vitro 2018, 48, 276–285. [Google Scholar] [PubMed]
- Ma, B.; Guo, S.; Nishina, Y.; Bianco, A. Reaction between graphene oxide and intracellular glutathione affects cell viability and proliferation. ACS Appl. Mater. Interfaces 2021, 13, 3528–3535. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Stein, A.J.; Kruger, A.; Leblanc, R.M. Head groups of lipids govern the interaction and orientation between graphene oxide and lipids. J. Phys. Chem. C 2013, 117, 16150–16158. [Google Scholar]
- Dervin, S.; Murphy, J.; Aviles, R.; Pillai, S.C.; Garvey, M. An in vitro cytotoxicity assessment of graphene nanosheets on alveolar cells. Appl. Surf. Sci. 2018, 434, 1274–1284. [Google Scholar]
- Yang, Z.; Pan, Y.; Chen, T.; Li, L.; Zou, W.; Liu, D.; Xue, D.; Wang, X.; Lin, G. Cytotoxicity and immune dysfunction of dendritic cells caused by graphene oxide. Front. Pharmacol. 2020, 11, 1206. [Google Scholar]
- Liao, K.-H.; Lin, Y.-S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar]
- Li, R.; Guiney, L.M.; Chang, C.H.; Mansukhani, N.D.; Ji, Z.; Wang, X.; Liao, Y.-P.; Jiang, W.; Sun, B.; Hersam, M.C. Surface oxidation of graphene oxide determines membrane damage, lipid peroxidation, and cytotoxicity in macrophages in a pulmonary toxicity model. ACS Nano 2018, 12, 1390–1402. [Google Scholar]
- Majeed, W.; Bourdo, S.; Petibone, D.M.; Saini, V.; Vang, K.B.; Nima, Z.A.; Alghazali, K.M.; Darrigues, E.; Ghosh, A.; Watanabe, F. The role of surface chemistry in the cytotoxicity profile of graphene. J. Appl. Toxicol. 2017, 37, 462–470. [Google Scholar]
- Ghosal, K.; Mondal, P.; Bera, S.; Ghosh, S. Graphene family nanomaterials-opportunities and challenges in tissue engineering applications. FlatChem 2021, 30, 100315. [Google Scholar]
- Zha, Y.; Chai, R.; Song, Q.; Chen, L.; Wang, X.; Cheng, G.; Tang, M.; Wang, M. Characterization and toxicological effects of three-dimensional graphene foams in rats in vivo. J. Nanoparticle Res. 2016, 18, 122. [Google Scholar] [CrossRef]
- Sydlik, S.A.; Jhunjhunwala, S.; Webber, M.J.; Anderson, D.G.; Langer, R. In vivo compatibility of graphene oxide with differing oxidation states. ACS Nano 2015, 9, 3866–3874. [Google Scholar] [PubMed]
- Demirel, E.; Karaca, E.; Durmaz, Y.Y. Effective PEGylation method to improve biocompatibility of graphene derivatives. Eur. Polym. J. 2020, 124, 109504. [Google Scholar] [CrossRef]
- Ovcharenko, E.A.; Seifalian, A.; Rezvova, M.A.; Klyshnikov, K.Y.; Glushkova, T.V.; Akenteva, T.N.; Antonova, L.V.; Velikanova, E.A.; Chernonosova, V.S.; Shevelev, G.Y. A new nanocomposite copolymer based on functionalised graphene oxide for development of heart valves. Sci. Rep. 2020, 10, 5271. [Google Scholar] [CrossRef]
- Buinov, A.S.; Kholkhoev, B.C.; Farion, I.A.; Gapich, D.I.; Kuznetsov, V.A.; Burdukovskii, V.F. Conductive graphene-containing biocompatible films. Bull. Mater. Sci. 2024, 47, 132. [Google Scholar]
- Baldea, I.; Olteanu, D.; Filip, G.A.; Pogacean, F.; Coros, M.; Suciu, M.; Tripon, S.C.; Cenariu, M.; Magerusan, L.; Stefan-van Staden, R.-I. Cytotoxicity mechanisms of nitrogen-doped graphene obtained by electrochemical exfoliation of graphite rods, on human endothelial and colon cancer cells. Carbon 2020, 158, 267–281. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Khan, M.M.; Alhadlaq, H.A. Reduced graphene oxide mitigates cadmium-induced cytotoxicity and oxidative stress in HepG2 cells. Food Chem. Toxicol. 2020, 143, 111515. [Google Scholar] [CrossRef]
- Taras, J.S.; Nanavati, V.; Steelman, P. Nerve conduits. J. Hand Ther. 2005, 18, 191–197. [Google Scholar]
- Song, S.; Wang, X.; Wang, T.; Yu, Q.; Hou, Z.; Zhu, Z.; Li, R. Additive manufacturing of nerve guidance conduits for regeneration of injured peripheral nerves. Front. Bioeng. Biotechnol. 2020, 8, 590596. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Dong, Y. Collagen-based biomaterials for tissue engineering. ACS Biomater. Sci. Eng. 2023, 9, 1132–1150. [Google Scholar]
- Yang, Y.; Zhao, Y.; Gu, Y.; Yan, X.; Liu, J.; Ding, F.; Gu, X. Degradation behaviors of nerve guidance conduits made up of silk fibroin in vitro and in vivo. Polym. Degrad. Stab. 2009, 94, 2213–2220. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, M.S.; Jeon, J.; Lee, D.H.; Yang, K.; Cho, S.W.; Han, I.; Yang, H.S. Biodegradable nerve guidance conduit with microporous and micropatterned poly (lactic-co-glycolic acid)-accelerated sciatic nerve regeneration. Macromol. Biosci. 2018, 18, 1800290. [Google Scholar]
- Farzamfar, S.; Salehi, M.; Tavangar, S.M.; Verdi, J.; Mansouri, K.; Ai, A.; Malekshahi, Z.V.; Ai, J. A novel polycaprolactone/carbon nanofiber composite as a conductive neural guidance channel: An in vitro and in vivo study. Prog. Biomater. 2019, 8, 239–248. [Google Scholar] [PubMed]
- Kehoe, S.; Zhang, X.; Boyd, D. FDA approved guidance conduits and wraps for peripheral nerve injury: A review of materials and efficacy. Injury 2012, 43, 553–572. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. (2014). 510(k) Premarket Notification: K132660—Axoguard Nerve Protector. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf13/K132660.pdf (accessed on 25 February 2025).
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of poly (vinyl alcohol) and natural polymers. Polym. Rev. 2018, 58, 247–287. [Google Scholar]
- Yannas, I.V. Tissue and Organ Regeneration in Adults: Extension of the Paradigm to Several Organs; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Terzopoulou, Z.; Zamboulis, A.; Koumentakou, I.; Michailidou, G.; Noordam, M.J.; Bikiaris, D.N. Biocompatible synthetic polymers for tissue engineering purposes. Biomacromolecules 2022, 23, 1841–1863. [Google Scholar]
- Horvath, J.; Horvath, J. A brief history of 3D printing. In Mastering 3d Print; Apress: Berkeley, CA, USA, 2014; pp. 3–10. [Google Scholar]
- Kong, B.; Zhao, Y. 3D bioprinting for biomedical applications. BME Front. 2023, 4, 0010. [Google Scholar] [CrossRef]
- Mota, C.; Puppi, D.; Gazzarri, M.; Bártolo, P.; Chiellini, F. Melt electrospinning writing of three-dimensional star poly (ϵ-caprolactone) scaffolds. Polym. Int. 2013, 62, 893–900. [Google Scholar] [CrossRef]
- Foyt, D.A.; Norman, M.D.; Yu, T.T.; Gentleman, E. Exploiting advanced hydrogel technologies to address key challenges in regenerative medicine. Adv. Healthc. Mater. 2018, 7, 1700939. [Google Scholar]
- Vaezi, M.; Zhong, G.; Kalami, H.; Yang, S. Extrusion-based 3D printing technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds; Elsevier: Amsterdam, The Netherlands, 2018; pp. 235–254. [Google Scholar]
- Yin, X.Y.; Zhang, Y.; Xiao, J.; Moorlag, C.; Yang, J. Monolithic dual-material 3D printing of ionic skins with long-term performance stability. Adv. Funct. Mater. 2019, 29, 1904716. [Google Scholar]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [PubMed]
- Li, X.; Liu, B.; Pei, B.; Chen, J.; Zhou, D.; Peng, J.; Zhang, X.; Jia, W.; Xu, T. Inkjet bioprinting of biomaterials. Chem. Rev. 2020, 120, 10793–10833. [Google Scholar] [PubMed]
- Rayleigh, L. On the instability of jets. Proc. Lond. Math. Soc. 1878, 1, 4–13. [Google Scholar]
- Liu, W.C.; Chou, V.H.Y.; Behera, R.P.; Le Ferrand, H. Magnetically assisted drop-on-demand 3D printing of microstructured multimaterial composites. Nat. Commun. 2022, 13, 5015. [Google Scholar]
- Wu, Y. Electrohydrodynamic jet 3D printing in biomedical applications. Acta Biomater. 2021, 128, 21–41. [Google Scholar]
- Yu, M.; Ahn, K.H.; Lee, S.J. Design optimization of ink in electrohydrodynamic jet printing: Effect of viscoelasticity on the formation of Taylor cone jet. Mater. Des. 2016, 89, 109–115. [Google Scholar]
- Lee, M.; Rizzo, R.; Surman, F.; Zenobi-Wong, M. Guiding lights: Tissue bioprinting using photoactivated materials. Chem. Rev. 2020, 120, 10950–11027. [Google Scholar]
- Goodarzi Hosseinabadi, H.; Dogan, E.; Miri, A.K.; Ionov, L. Digital light processing bioprinting advances for microtissue models. ACS Biomater. Sci. Eng. 2022, 8, 1381–1395. [Google Scholar]
- Sakellari, I.; Gaidukeviciute, A.; Giakoumaki, A.; Gray, D.; Fotakis, C.; Farsari, M.; Vamvakaki, M.; Reinhardt, C.; Ovsianikov, A.; Chichkov, B. Two-photon polymerization of titanium-containing sol–gel composites for three-dimensional structure fabrication. Appl. Phys. A 2010, 100, 359–364. [Google Scholar]
- Lee, K.S.; Yang, D.Y.; Park, S.H.; Kim, R.H. Recent developments in the use of two-photon polymerization in precise 2D and 3D microfabrications. Polym. Adv. Technol. 2006, 17, 72–82. [Google Scholar]
- Malinauskas, M.; Farsari, M.; Piskarskas, A.; Juodkazis, S. Ultrafast laser nanostructuring of photopolymers: A decade of advances. Phys. Rep. 2013, 533, 1–31. [Google Scholar]
- Xie, Z.; Gao, M.; Lobo, A.O.; Webster, T.J. 3D bioprinting in tissue engineering for medical applications: The classic and the hybrid. Polymers 2020, 12, 1717. [Google Scholar] [CrossRef] [PubMed]
- Namhongsa, M.; Daranarong, D.; Sriyai, M.; Molloy, R.; Ross, S.; Ross, G.M.; Tuantranont, A.; Tocharus, J.; Sivasinprasasn, S.; Topham, P.D. Surface-modified polypyrrole-coated PLCL and PLGA nerve guide conduits fabricated by 3D printing and electrospinning. Biomacromolecules 2022, 23, 4532–4546. [Google Scholar] [PubMed]
- Lee, S.-J.; Nowicki, M.; Harris, B.; Zhang, L.G. Fabrication of a highly aligned neural scaffold via a table top stereolithography 3D printing and electrospinning. Tissue Eng. Part A 2017, 23, 491–502. [Google Scholar]
- Yang, D.L.; Faraz, F.; Wang, J.X.; Radacsi, N. Combination of 3D printing and electrospinning techniques for biofabrication. Adv. Mater. Technol. 2022, 7, 2101309. [Google Scholar]
- Mohammadi, M.; Shaegh, S.A.M.; Alibolandi, M.; Ebrahimzadeh, M.H.; Tamayol, A.; Jaafari, M.R.; Ramezani, M. Micro and nanotechnologies for bone regeneration: Recent advances and emerging designs. J. Control. Release 2018, 274, 35–55. [Google Scholar]
- Saniei, H.; Mousavi, S. Surface modification of PLA 3D-printed implants by electrospinning with enhanced bioactivity and cell affinity. Polymer 2020, 196, 122467. [Google Scholar]
- Efimov, A.; Agapova, O.; Safonova, L.; Bobrova, M.; Parfenov, V.; Koudan, E.; Pereira, F.; Bulanova, E.; Mironov, V.; Agapov, I. 3D scanning probe nanotomography of tissue spheroid fibroblasts interacting with electrospun polyurethane scaffold. Express Polym. Lett. 2019, 13, 632–641. [Google Scholar]
- Centola, M.; Rainer, A.; Spadaccio, C.; De Porcellinis, S.; Genovese, J.; Trombetta, M. Combining electrospinning and fused deposition modeling for the fabrication of a hybrid vascular graft. Biofabrication 2010, 2, 014102. [Google Scholar]
- Yoon, Y.; Kim, C.H.; Lee, J.E.; Yoon, J.; Lee, N.K.; Kim, T.H.; Park, S.-H. 3D bioprinted complex constructs reinforced by hybrid multilayers of electrospun nanofiber sheets. Biofabrication 2019, 11, 025015. [Google Scholar]
- Ribeiro, A.; Powell, E.; Leach, J. Neural stem cell differentiation in 2D and 3D microenvironments. In Proceedings of the 26th Southern Biomedical Engineering Conference SBEC 2010, College Park, MD, USA, 30 April–2 May 2010; pp. 422–425. [Google Scholar]
- Yildiz, A.P.Z.; Darici, H.; Yavuz, B.; Abamor, E.S.; Ozdemir, C.; Yasin, M.E.; Bagirova, M.; Allahverdiyev, A.; Karaoz, E. Preparation and characterization of graphene-based 3D biohybrid hydrogel bioink for peripheral neuroengineering. JoVE (J. Vis. Exp.) 2022, 183, e63622. [Google Scholar]
- Blaeser, A.; Duarte Campos, D.F.; Puster, U.; Richtering, W.; Stevens, M.M.; Fischer, H. Controlling shear stress in 3D bioprinting is a key factor to balance printing resolution and stem cell integrity. Adv. Healthc. Mater. 2016, 5, 326–333. [Google Scholar] [PubMed]
- Litowczenko, J.; Wychowaniec, J.K.; Załęski, K.; Marczak, Ł.; Edwards-Gayle, C.J.; Tadyszak, K.; Maciejewska, B.M. Micro/nano-patterns for enhancing differentiation of human neural stem cells and fabrication of nerve conduits via soft lithography and 3d printing. Biomater. Adv. 2023, 154, 213653. [Google Scholar]
- Peng, J.; Li, L.; Nie, Y.; Liu, T.; Song, K. 3D Bio-Printing Fabrication and Properties of Graphene Dispersion-based Hybrid Scaffolds. J. Phys. Conf. Ser. 2020, 1622, 012062. [Google Scholar]
- Farzan, A.; Borandeh, S.; Seppälä, J. Conductive polyurethane/PEGylated graphene oxide composite for 3D-printed nerve guidance conduits. Eur. Polym. J. 2022, 167, 111068. [Google Scholar]
- Bendszus, M.; Wessig, C.; Solymosi, L.; Reiners, K.; Koltzenburg, M. MRI of peripheral nerve degeneration and regeneration: Correlation with electrophysiology and histology. Exp. Neurol. 2004, 188, 171–177. [Google Scholar]
- Zhang, J.; Liu, Z.; Wang, J.; Zhang, Y.; Dong, J.; Gao, J.; Zhang, L.; Wang, J.; Tang, P.; Zhang, Q. 3D Coaxially Printing rGO Aerogel-Based Biocompatible Fiber for Peripheral Nerve Regeneration. Adv. Fiber Mater. 2024, 6, 713–726. [Google Scholar]
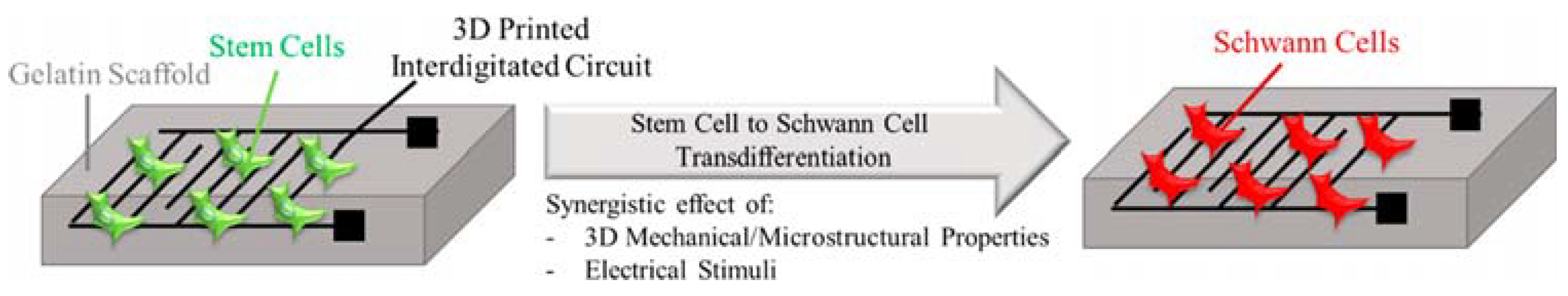
- Uz, M.; Donta, M.; Mededovic, M.; Sakaguchi, D.S.; Mallapragada, S.K. Development of gelatin and graphene-based nerve regeneration conduits using three-dimensional (3D) printing strategies for electrical transdifferentiation of mesenchymal stem cells. Ind. Eng. Chem. Res. 2019, 58, 7421–7427. [Google Scholar]
- Hoveizi, E.J.B.M. Enhancement of nerve regeneration through schwann cell-mediated healing in a 3D printed polyacrylonitrile conduit incorporating hydrogel and graphene quantum dots: A study on rat sciatic nerve injury model. Biomed. Mater. 2023, 19, 015012. [Google Scholar]
- Zhang, X.; Zhang, H.; Zhang, Y.; Huangfu, H.; Yang, Y.; Qin, Q.; Zhang, Y.; Zhou, Y. 3D printed reduced graphene oxide-GelMA hybrid hydrogel scaffolds for potential neuralized bone regeneration. J. Mater. Chem. B 2023, 11, 1288–1301. [Google Scholar]
- Jenq, C.-B.; Coggeshall, R.E. Nerve regeneration through holey silicone tubes. Brain Res. 1985, 361, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Dodla, M.C.; Bellamkonda, R.V. Differences between the effect of anisotropic and isotropic laminin and nerve growth factor presenting scaffolds on nerve regeneration across long peripheral nerve gaps. Biomaterials 2008, 29, 33–46. [Google Scholar] [CrossRef]
- Rodríguez, F.J.; Gómez, N.; Perego, G.; Navarro, X. Highly permeable polylactide-caprolactone nerve guides enhance peripheral nerve regeneration through long gaps. Biomaterials 1999, 20, 1489–1500. [Google Scholar] [CrossRef] [PubMed]
- Vleggeert-Lankamp, C.; De Ruiter, G.; Wolfs, J.; Pego, A.; Van Den Berg, R.; Feirabend, H.; Malessy, M.; Lakke, E. Pores in synthetic nerve conduits are beneficial to regeneration. J. Biomed. Mater. Res. Part A 2007, 80, 965–982. [Google Scholar] [CrossRef] [PubMed]
- Sarazin, P.; Roy, X.; Favis, B.D. Controlled preparation and properties of porous poly (L-lactide) obtained from a co-continuous blend of two biodegradable polymers. Biomaterials 2004, 25, 5965–5978. [Google Scholar] [CrossRef]
- Qian, Y.; Zhao, X.; Han, Q.; Chen, W.; Li, H.; Yuan, W. An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration. Nat. Commun. 2018, 9, 323. [Google Scholar] [CrossRef]
- Qian, Y.; Song, J.; Zhao, X.; Chen, W.; Ouyang, Y.; Yuan, W.; Fan, C. 3D fabrication with integration molding of a graphene oxide/polycaprolactone nanoscaffold for neurite regeneration and angiogenesis. Adv. Sci. 2018, 5, 1700499. [Google Scholar] [CrossRef]
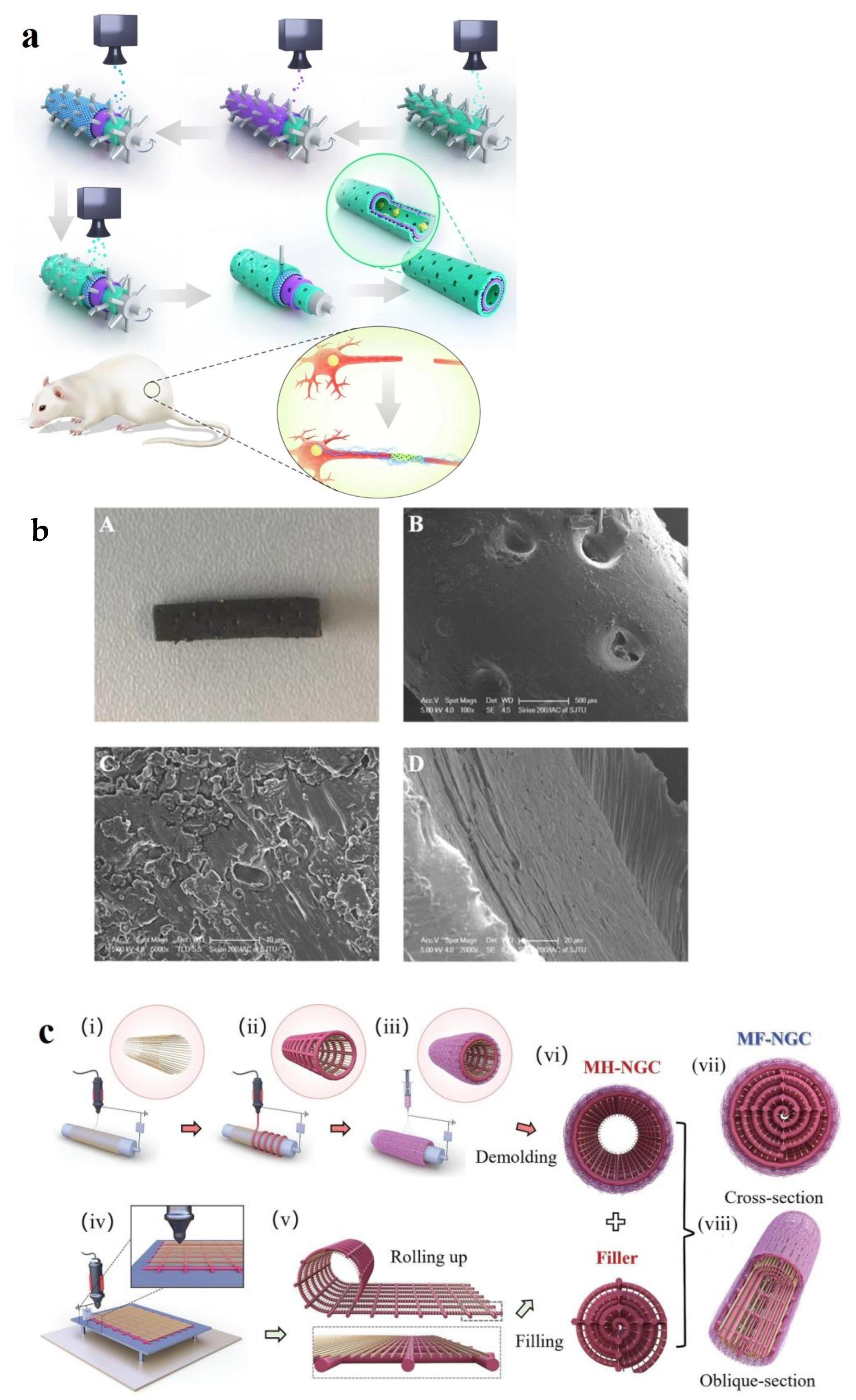
- Fang, Y.; Wang, C.; Liu, Z.; Ko, J.; Chen, L.; Zhang, T.; Xiong, Z.; Zhang, L.; Sun, W. 3D printed conductive multiscale nerve guidance conduit with hierarchical fibers for peripheral nerve regeneration. Adv. Sci. 2023, 10, 2205744. [Google Scholar]
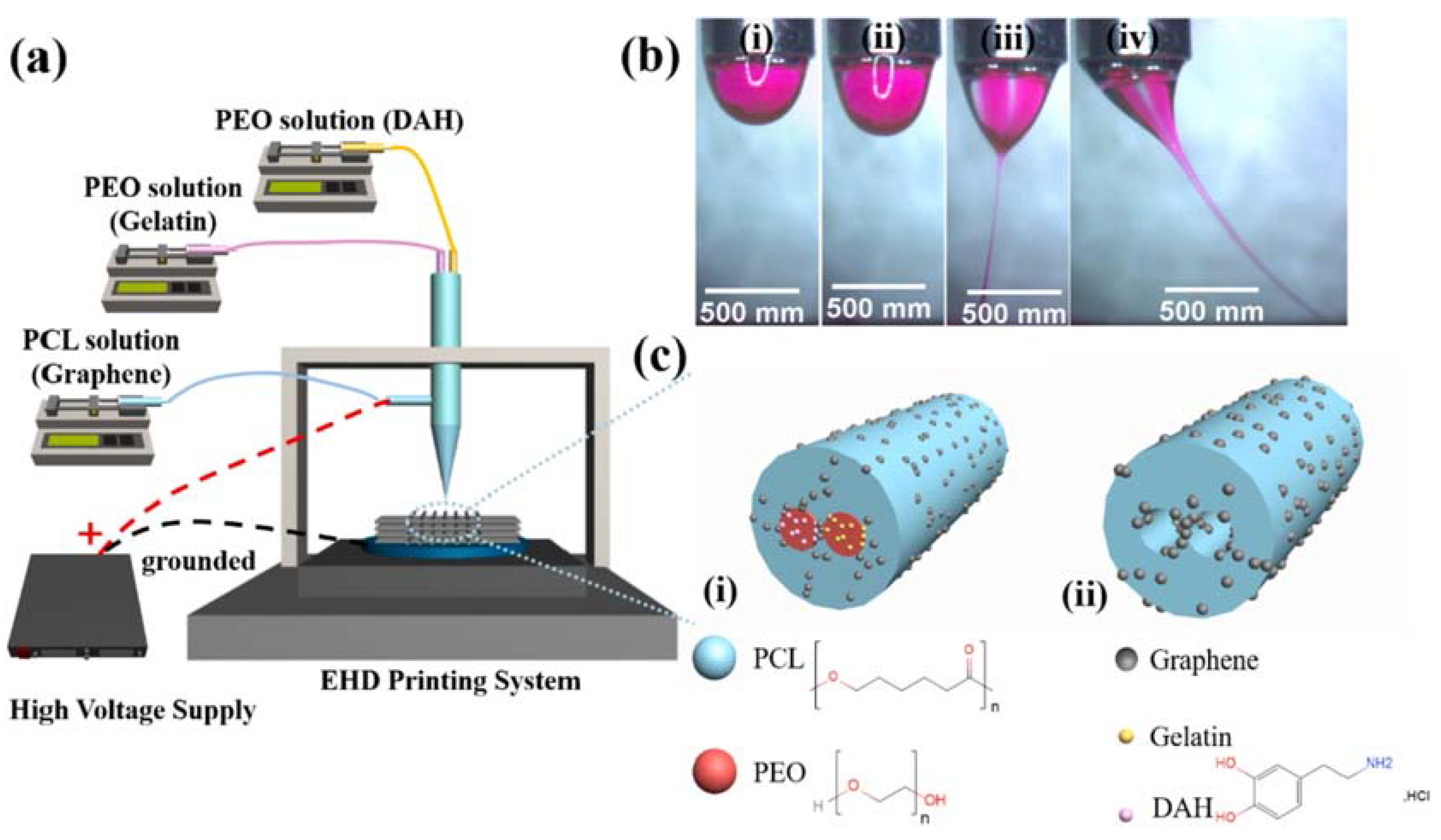
- Wang, B.; Chen, X.; Ahmad, Z.; Huang, J.; Chang, M.-W. 3D electrohydrodynamic printing of highly aligned dual-core graphene composite matrices. Carbon 2019, 153, 285–297. [Google Scholar]
- Vijayavenkataraman, S.; Thaharah, S.; Zhang, S.; Lu, W.F.; Fuh, J.Y.H. 3D-printed PCL/rGO conductive scaffolds for peripheral nerve injury repair. Artif. Organs 2019, 43, 515–523. [Google Scholar]
- Wang, B.; Wu, S.; Ahmad, Z.; Li, J.-s.; Chang, M.-W. Co-printing of vertical axis aligned micron-scaled filaments via simultaneous dual needle electrohydrodynamic printing. Eur. Polym. J. 2018, 104, 81–89. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, H.; Zhang, F.; Wang, X.; Liu, X.; Zhang, Y. Polydopamine doped reduced graphene oxide/mesoporous silica nanosheets for chemo-photothermal and enhanced photothermal therapy. Mater. Sci. Eng. C 2019, 96, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Wang, Y.; Ma, C.; Zheng, W.; Li, L.; Zheng, Y. Electrospinning of PLGA/gelatin randomly-oriented and aligned nanofibers as potential scaffold in tissue engineering. Mater. Sci. Eng. C 2010, 30, 1204–1210. [Google Scholar] [CrossRef]
- Wychowaniec, J.K.; Litowczenko, J.; Tadyszak, K. Fabricating versatile cell supports from nano-and micro-sized graphene oxide flakes. J. Mech. Behav. Biomed. Mater. 2020, 103, 103594. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Lu, C.; Sun, D.; Kang, S.; Wang, X.; Lu, L. Reduced graphene oxide fibers combined with electrical stimulation promote peripheral nerve regeneration. Int. J. Nanomed. 2024, 2341–2357. [Google Scholar] [CrossRef]
- Zhang, D.; Yao, Y.; Duan, Y.; Yu, X.; Shi, H.; Nakkala, J.R.; Zuo, X.; Hong, L.; Mao, Z.; Gao, C. Surface-anchored graphene oxide nanosheets on cell-scale micropatterned poly (d, l-lactide-co-caprolactone) conduits promote peripheral nerve regeneration. ACS Appl. Mater. Interfaces 2020, 12, 7915–7930. [Google Scholar]
- Gasparotto, M.; Bellet, P.; Scapin, G.; Busetto, R.; Rampazzo, C.; Vitiello, L.; Shah, D.I.; Filippini, F. 3D printed graphene-PLA scaffolds promote cell alignment and differentiation. Int. J. Mol. Sci. 2022, 23, 1736. [Google Scholar] [CrossRef]
- Zhang, Z.; Jørgensen, M.L.; Wang, Z.; Amagat, J.; Wang, Y.; Li, Q.; Dong, M.; Chen, M. 3D anisotropic photocatalytic architectures as bioactive nerve guidance conduits for peripheral neural regeneration. Biomaterials 2020, 253, 120108. [Google Scholar] [CrossRef]
- Das, S.R.; Uz, M.; Ding, S.; Lentner, M.T.; Hondred, J.A.; Cargill, A.A.; Sakaguchi, D.S.; Mallapragada, S.; Claussen, J.C. Electrical differentiation of mesenchymal stem cells into Schwann-cell-like phenotypes using inkjet-printed graphene circuits. Adv. Healthc. Mater. 2017, 6, 1601087. [Google Scholar]
- Curti, S.; O’Brien, J. Characteristics and plasticity of electrical synaptic transmission. BMC Cell Biol. 2016, 17, 13. [Google Scholar] [CrossRef]
- Rahman, M.; Mahady Dip, T.; Padhye, R.; Houshyar, S. Review on electrically conductive smart nerve guide conduit for peripheral nerve regeneration. J. Biomed. Mater. Res. Part A 2023, 111, 1916–1950. [Google Scholar]
- Gerdefaramarzi, R.S.; Ebrahimian-Hosseinabadi, M.; Khodaei, M. 3D printed poly (lactic acid)/poly (ε-caprolactone)/graphene nanocomposite scaffolds for peripheral nerve tissue engineering. Arab. J. Chem. 2024, 17, 105927. [Google Scholar]
- Lee, Y.J.; Ajiteru, O.; Lee, J.S.; Lee, O.J.; Choi, K.Y.; Kim, S.H.; Park, C.H. Highly conductive, stretchable, and biocompatible graphene oxide biocomposite hydrogel for advanced tissue engineering. Biofabrication 2024, 16, 045032. [Google Scholar]
- Ajiteru, O.; Sultan, M.T.; Lee, Y.J.; Seo, Y.B.; Hong, H.; Lee, J.S.; Lee, H.; Suh, Y.J.; Ju, H.W.; Lee, O.; et al. A 3D printable electroconductive biocomposite bioink based on silk fibroin-conjugated graphene oxide. Nano Lett. 2020, 20, 6873–6883. [Google Scholar]
- Jakus, A.E.; Secor, E.B.; Rutz, A.L.; Jordan, S.W.; Hersam, M.C.; Shah, R.N. Three-dimensional printing of high-content graphene scaffolds for electronic and biomedical applications. ACS Nano 2015, 9, 4636–4648. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, S.; Wang, J. A Comprehensive Review on Bioprinted Graphene-Based Material (GBM)-Enhanced Scaffolds for Nerve Guidance Conduits. Biomimetics 2025, 10, 213. https://doi.org/10.3390/biomimetics10040213
Su S, Wang J. A Comprehensive Review on Bioprinted Graphene-Based Material (GBM)-Enhanced Scaffolds for Nerve Guidance Conduits. Biomimetics. 2025; 10(4):213. https://doi.org/10.3390/biomimetics10040213
Chicago/Turabian StyleSu, Siheng, and Jilong Wang. 2025. "A Comprehensive Review on Bioprinted Graphene-Based Material (GBM)-Enhanced Scaffolds for Nerve Guidance Conduits" Biomimetics 10, no. 4: 213. https://doi.org/10.3390/biomimetics10040213
APA StyleSu, S., & Wang, J. (2025). A Comprehensive Review on Bioprinted Graphene-Based Material (GBM)-Enhanced Scaffolds for Nerve Guidance Conduits. Biomimetics, 10(4), 213. https://doi.org/10.3390/biomimetics10040213