Interaction of Manganese-Doped Copper Oxide Nano-Platelets with Cells: Biocompatibility and Anticancer Activity Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Copper Oxide (CuO) and Manganese-Doped Copper Oxide (CuO:Mn) Sample Synthesis
2.3. Characterization Methods
2.4. Cytotoxicity Assays
3. Results
3.1. Characterization of CuO and Manganese-Doped CuO Sample
3.1.1. Structural Characterization
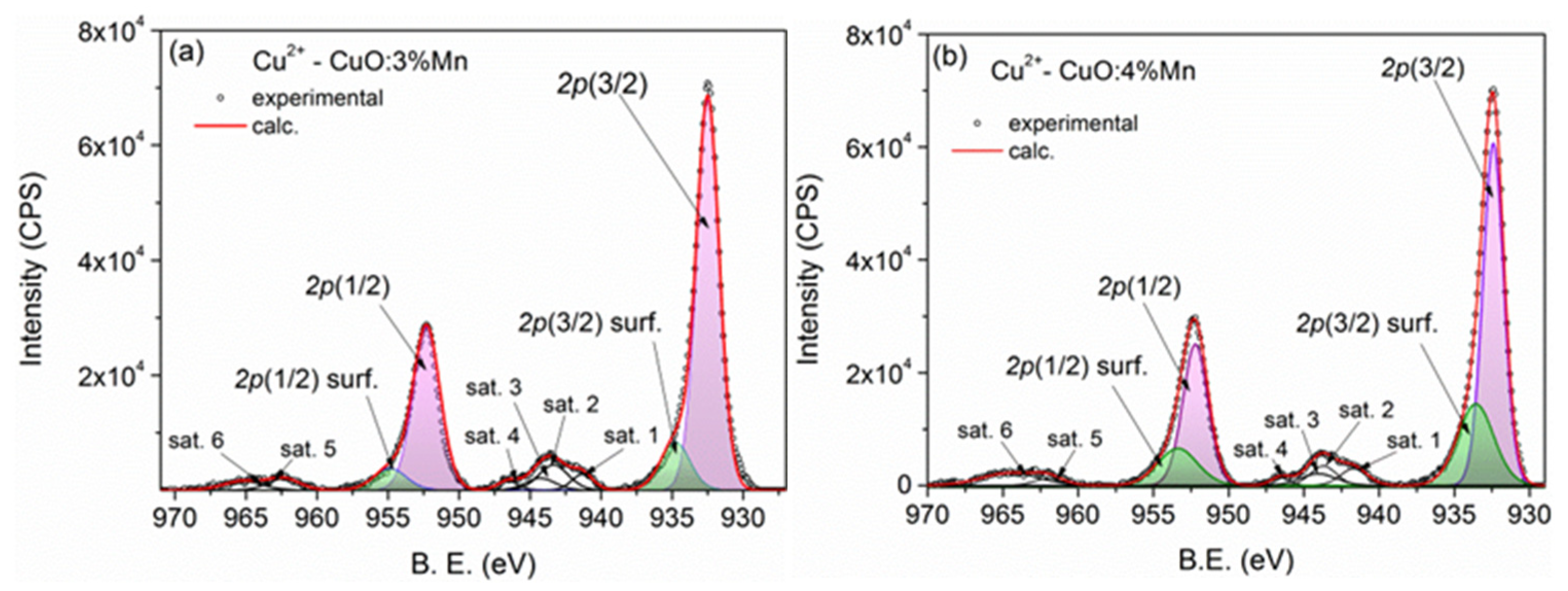
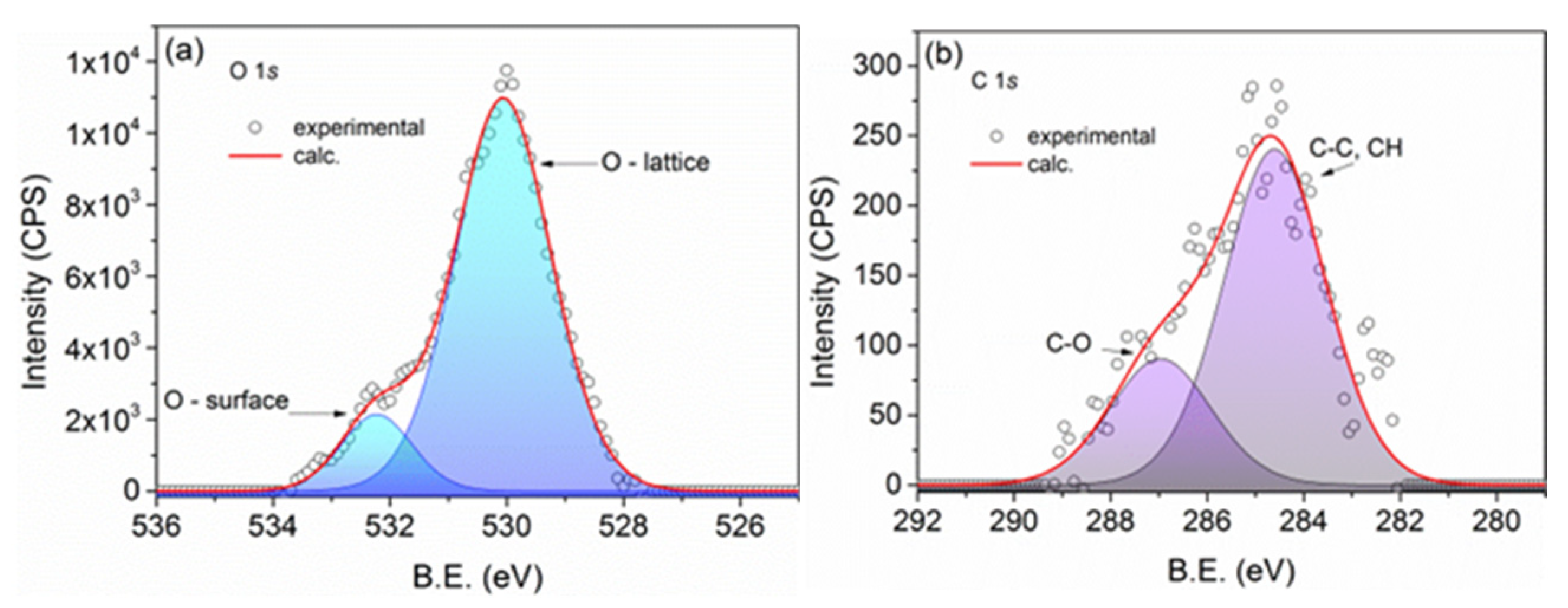
3.1.2. Elemental Composition of the Samples
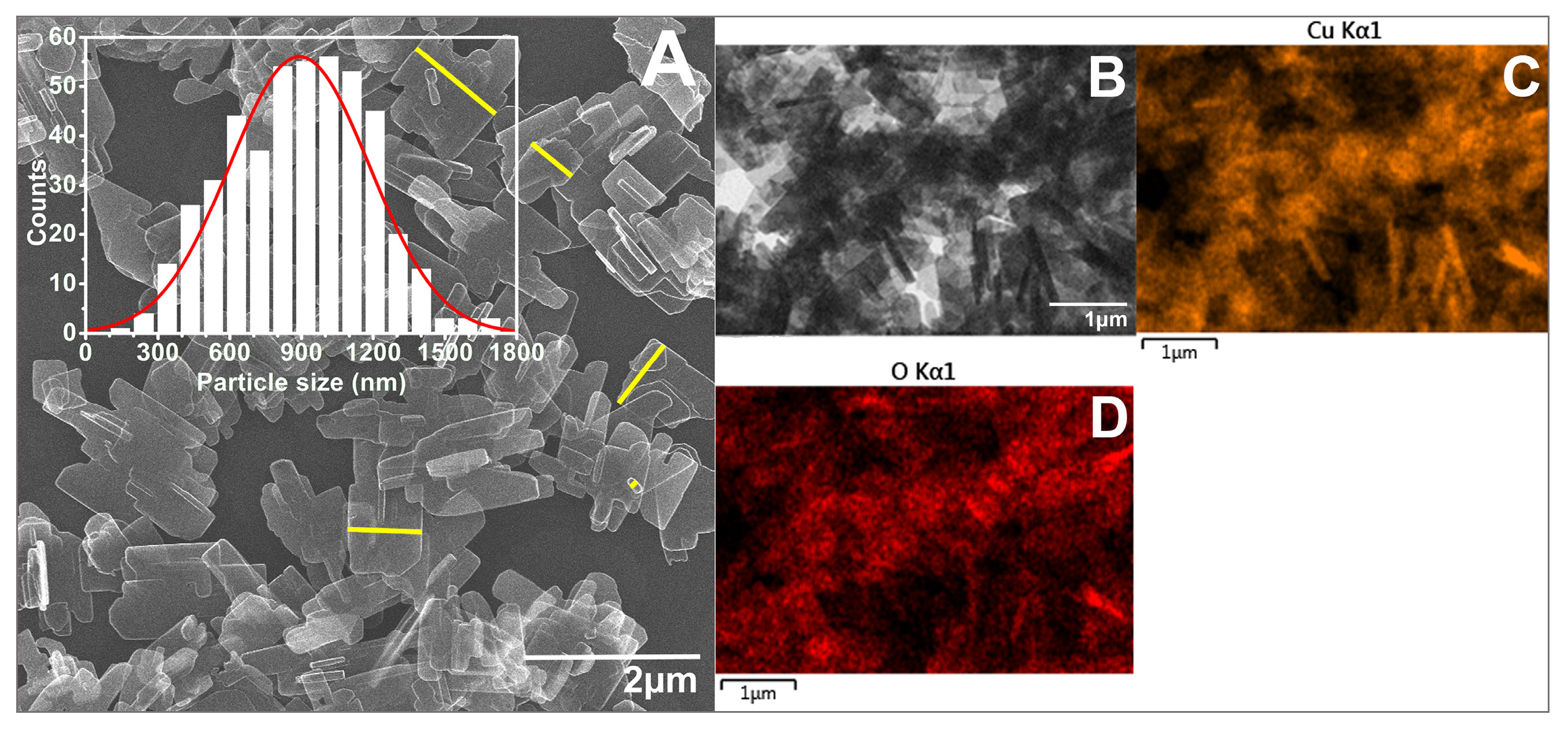
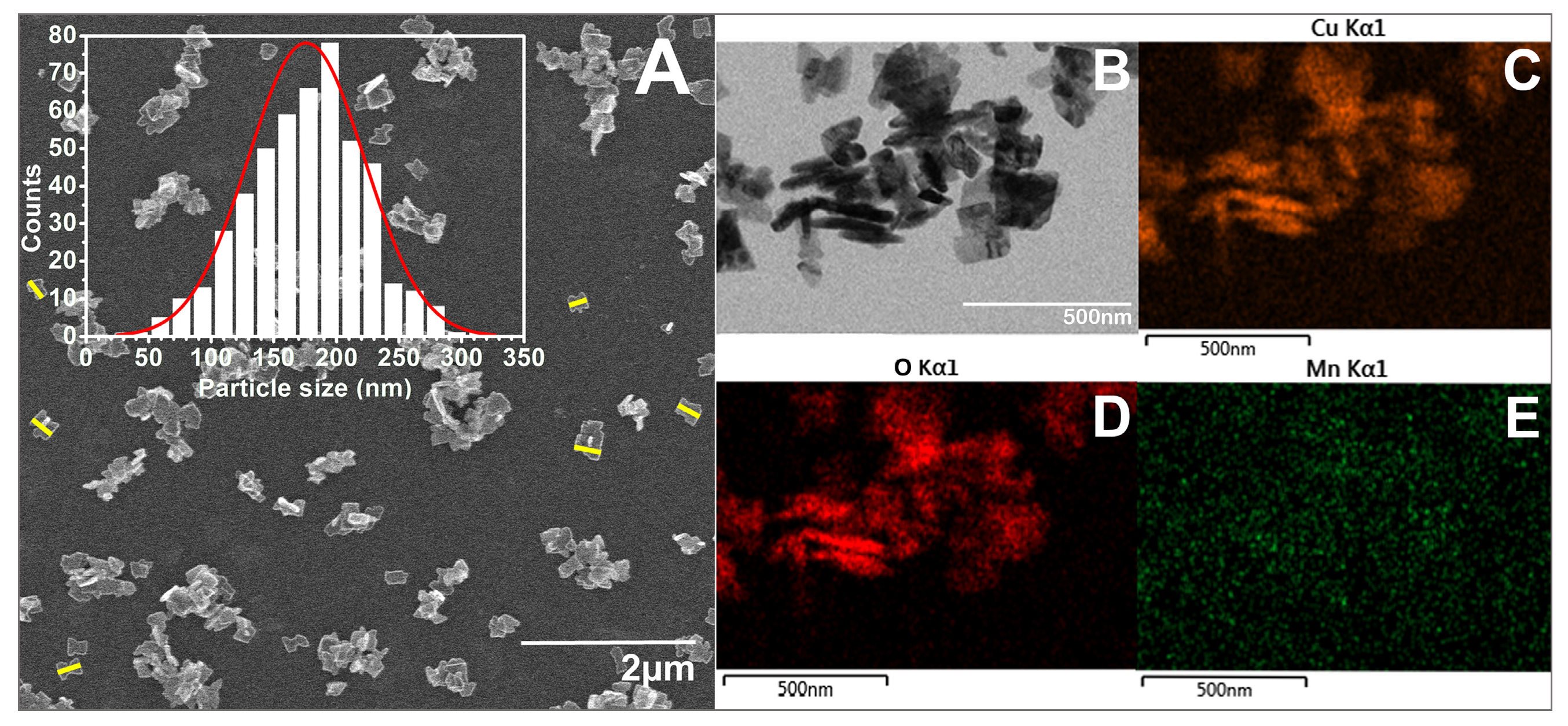
3.1.3. Morphological Characterization
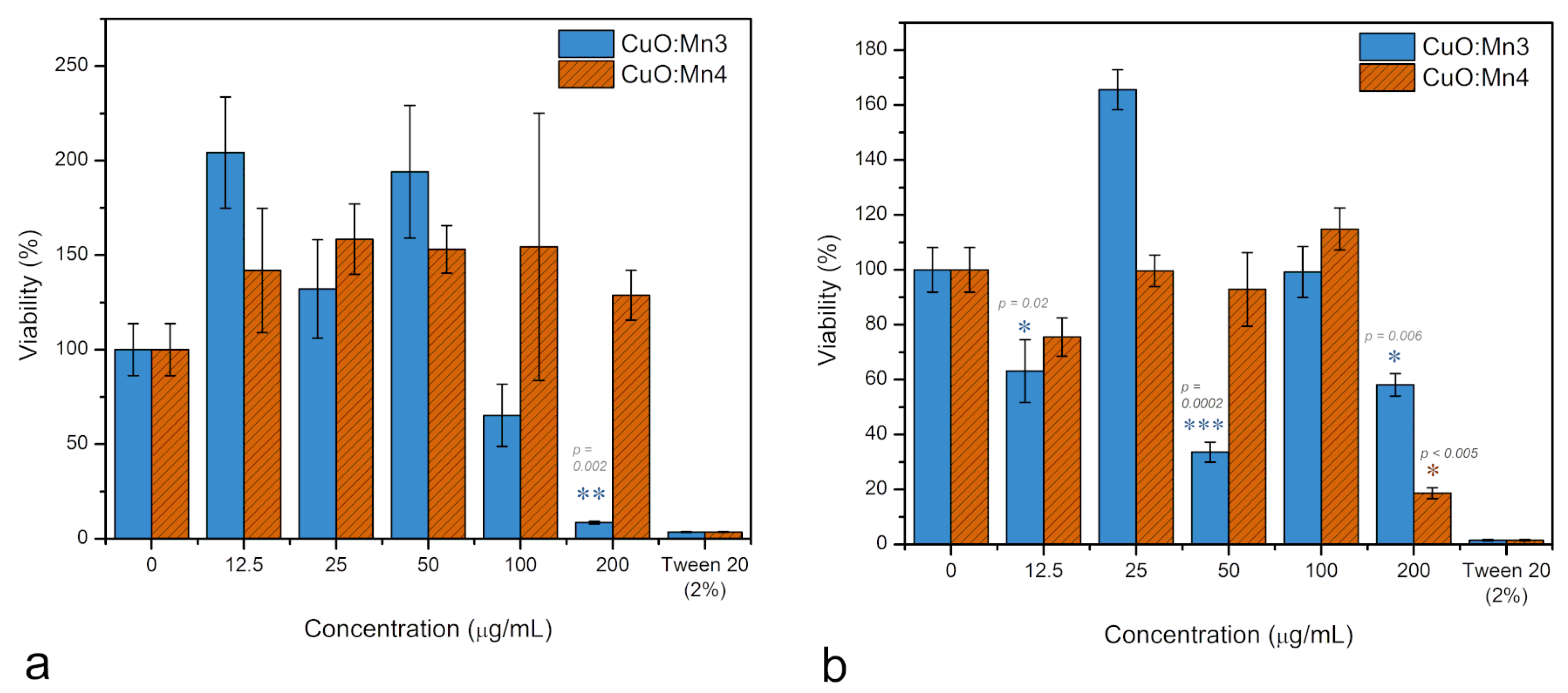
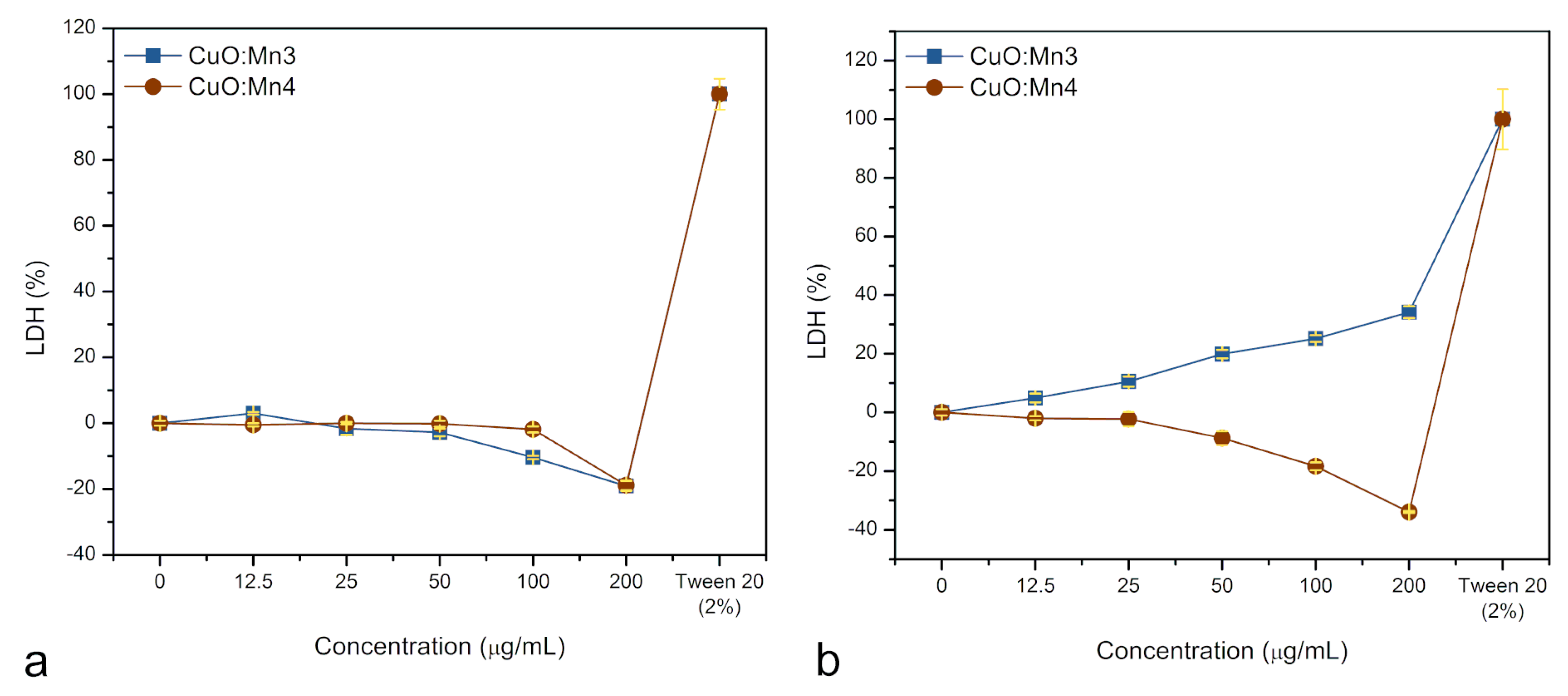
3.1.4. Cytotoxicity Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stefan, M.; Toloman, D.; Popa, A.; Mesaros, A.; Vasile, O.R.; Leostean, C.; Pana, O. Interface Charge Transfer Process in ZnO:Mn/ZnS Nanocomposites. J. Nanoparticle Res. 2016, 18, 59. [Google Scholar] [CrossRef]
- Stefan, M.; Leostean, C.; Pana, O.; Suciu, M.; Popa, A.; Toloman, D.; Macavei, S.; Bele, C.; Tabaran, F.; Barbu-Tudoran, L. Synthesis and Characterization of Fe3O4–ZnS:Mn Nanocomposites for Biomedical Applications. Mater. Chem. Phys. 2021, 264, 124474. [Google Scholar] [CrossRef]
- Râpă, M.; Stefan, M.; Popa, P.A.; Toloman, D.; Leostean, C.; Borodi, G.; Vodnar, D.C.; Wrona, M.; Salafranca, J.; Nerín, C.; et al. Electrospun Nanosystems Based on PHBV and ZnO for Ecological Food Packaging. Polymers 2021, 13, 2123. [Google Scholar] [CrossRef] [PubMed]
- Falamas, A.; Marica, I.; Popa, A.; Toloman, D.; Pruneanu, S.; Pogacean, F.; Nekvapil, F.; Silipas, T.D.; Stefan, M. Size-Dependent Spectroscopic Insight into the Steady-State and Time-Resolved Optical Properties of ZnO Photocatalysts. Mater. Sci. Semicond. Process. 2022, 145, 106644. [Google Scholar] [CrossRef]
- Marica, I.; Nekvapil, F.; Ștefan, M.; Farcău, C.; Falamaș, A. Zinc Oxide Nanostructures for Fluorescence and Raman Signal Enhancement: A Review. Beilstein J. Nanotechnol. 2022, 13, 472–490. [Google Scholar] [CrossRef]
- Ammar, A.U.; Stefan, M.; Macavei, S.G.; Tripon, S.; Pana, O.; Leostean, C.; Vlaicu, I.D.; Rostas, A.M.; Erdem, E. Characterization of Defect Structures in Nanoscaled W-Doped TiO 2 Tested as Supercapacitor Electrode Materials. J. Mater. Sci. Mater. Electron. 2023, 34, 98. [Google Scholar] [CrossRef]
- Sultana, S.; Djaker, N.; Boca-Farcau, S.; Salerno, M.; Charnaux, N.; Astilean, S.; Hlawaty, H.; De La Chapelle, M.L. Comparative Toxicity Evaluation of Flower-Shaped and Spherical Gold Nanoparticles on Human Endothelial Cells. Nanotechnology 2015, 26, 55101. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of Oxidative Stress as an Anticancer Strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- D’Autréaux, B.; Toledano, M.B. ROS as Signalling Molecules: Mechanisms That Generate Specificity in ROS Homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef]
- Cross, C.E. Oxygen Radicals and Human Disease. Ann. Intern. Med. 1987, 107, 526. [Google Scholar] [CrossRef]
- Purohit, V.; Simeone, D.M.; Lyssiotis, C.A. Metabolic Regulation of Redox Balance in Cancer. Cancers 2019, 11, 955. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Mini-Review Free Radicals, Metals and Antioxidants in Oxidative Stress-Induced Cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Taratula, O.; Doddapaneni, B.S.; Schumann, C.; Li, X.; Bracha, S.; Milovancev, M.; Alani, A.W.G.; Taratula, O. Naphthalocyanine-Based Biodegradable Polymeric Nanoparticles for Image-Guided Combinatorial Phototherapy. Chem. Mater. 2015, 27, 6155–6165. [Google Scholar] [CrossRef]
- Jing, Z.; Zhan, J. Fabrication and Gas-Sensing Properties of Porous ZnO Nanoplates. Adv. Mater. 2008, 20, 4547–4551. [Google Scholar] [CrossRef]
- Wang, L.; Huo, M.; Chen, Y.; Shi, J. Tumor Microenvironment-Enabled Nanotherapy. Adv. Healthc. Mater. 2018, 7, e1701156. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Liu, Y.; He, M.; Bu, W. Chemodynamic Therapy: Tumour Microenvironment-Mediated Fenton and Fenton-like Reactions. Angew. Chemie Int. Ed. 2019, 58, 946–956. [Google Scholar] [CrossRef]
- Lin, L.S.; Song, J.; Song, L.; Ke, K.; Liu, Y.; Zhou, Z.; Shen, Z.; Li, J.; Yang, Z.; Tang, W.; et al. Simultaneous Fenton-like Ion Delivery and Glutathione Depletion by MnO2-Based Nanoagent to Enhance Chemodynamic Therapy. Angew. Chemie Int. Ed. 2018, 57, 4902–4906. [Google Scholar] [CrossRef]
- Fu, L.H.; Hu, Y.R.; Qi, C.; He, T.; Jiang, S.; Jiang, C.; He, J.; Qu, J.; Lin, J.; Huang, P. Biodegradable Manganese-Doped Calcium Phosphate Nanotheranostics for Traceable Cascade Reaction-Enhanced Anti-Tumor Therapy. ACS Nano 2019, 13, 13985–13994. [Google Scholar] [CrossRef]
- Liu, Y.; Zhen, W.; Jin, L.; Zhang, S.; Sun, G.; Zhang, T.; Xu, X.; Song, S.; Wang, Y.; Liu, J.; et al. All-in-One Theranostic Nanoagent with Enhanced Reactive Oxygen Species Generation and Modulating Tumor Microenvironment Ability for Effective Tumor Eradication. ACS Nano 2018, 12, 4886–4893. [Google Scholar] [CrossRef]
- Nie, D.; Zhu, Y.; Guo, T.; Yue, M.; Lin, M. Research Advance in Manganese Nanoparticles in Cancer Diagnosis and Therapy. Front. Mater. 2022, 9, 1–15. [Google Scholar] [CrossRef]
- Bayansal, F.; Gülen, Y.; ßahin, B.S.; Kahraman, S.; Çetinkara, H.A. CuO Nanostructures Grown by the SILAR Method: Influence of Pb-Doping on the Morphological, Structural and Optical Properties. J. Alloys Compd. 2014, 619, 378–382. [Google Scholar] [CrossRef]
- Armelao, L.; Barreca, D.; Bertapelle, M.; Bottaro, G.; Sada, C.; Tondello, E. A Sol-Gel Approach to Nanophasic Copper Oxide Thin Films. Thin Solid Film. 2003, 442, 48–52. [Google Scholar] [CrossRef]
- Balamurugan, B.; Mehta, B.R.; Shivaprasad, S.M. Surface-Modified CuO Layer in Size-Stabilized Single-Phase Cu2O Nanoparticles. Appl. Phys. Lett. 2001, 79, 3176–3178. [Google Scholar] [CrossRef]
- Bahoosh, S.G.; Apostolov, A.T.; Apostolova, I.N.; Wesselinowa, J.M. Theory of Phonon Properties in Doped and Undoped CuO Nanoparticles. Phys. Lett. A 2012, 376, 2252–2255. [Google Scholar] [CrossRef]
- Husain, N.; Mahmood, R. Copper(II) generates ROS and RNS, impairs antioxidant system and damages membrane and DNA in human blood cells. Environ. Sci. Pollut. Res. 2019, 26, 20654–20668. [Google Scholar] [CrossRef]
- Kang, D.; Li, Y.; Dai, X.; Li, Z.; Cheng, K.; Song, W.; Yu, D.-G. A Soothing Lavender-Scented Electrospun Fibrous Eye Mask. Molecules 2024, 29, 5461. [Google Scholar] [CrossRef]
- Zuily, L.; Lahrach, N.; Fassler, R.; Genest, O.; Faller, P.; Sénèque, O.; Denis, Y.; Castanié-Cornet, M.; Genevaux, P.; Jakob, U.; et al. Copper Induces Protein Aggregation, a Toxic Process Compensated by Molecular Chaperones. mBio 2022, 13, e03251-21. [Google Scholar] [CrossRef]
- Hassan, I.A.; Parkin, I.P.; Nair, S.P.; Carmalt, C.J. Antimicrobial Activity of Copper and Copper(I) Oxide Thin Films Deposited via Aerosol-Assisted CVD. J. Mater. Chem. B 2014, 2, 2855–2860. [Google Scholar] [CrossRef]
- Santo, C.E.; Morais, P.V.; Grass, G. Isolation and Characterization of Bacteria Resistant to Metallic Copper Surfaces. Appl. Environ. Microbiol. 2010, 76, 1341–1348. [Google Scholar] [CrossRef]
- Hans, M.; Erbe, A.; Mathews, S.; Chen, Y.; Solioz, M.; Mücklich, F. Role of Copper Oxides in Contact Killing of Bacteria. Langmuir 2013, 29, 16160–16166. [Google Scholar] [CrossRef]
- Liu, T.; Xiao, B.; Xiang, F.; Tan, J.; Chen, Z.; Zhang, X.; Wu, C.; Mao, Z.; Luo, G.; Chen, X.; et al. Ultrasmall Copper-Based Nanoparticles for Reactive Oxygen Species Scavenging and Alleviation of Inflammation Related Diseases. Nat. Commun. 2020, 11, 2788. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleki, S.; Aliabadi, A.; Khaksar, S. Unveiling the promising anticancer effect of copper-based compounds: A comprehensive review. J. Cancer Res. Clin. Oncol. 2024, 150, 213. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.W.; Atkins, C.; Pathak, A.; Gilbert, B.E.; Noah, J.W. Benzimidazole analogs inhibit respiratory syncytial virus G protein function. Antiviral Res. 2015, 121, 31–38. [Google Scholar] [CrossRef]
- Devaraji, M.; Thanikachalam, P.V.; Elumalai, K. The potential of copper oxide nanoparticles in nanomedicine: A comprehensive review. Biotechnol. Notes. 2024, 5, 80–99. [Google Scholar] [CrossRef]
- Mahmood, R.I.; Kadhim, A.A.; Ibraheem, S.; Albukhaty, S.; Mohammed-Salih, H.S.; Abbas, R.H.; Jabir, M.S.; Mohammed, M.K.A.; Nayef, U.M.; AlMalki, F.A.; et al. Biosynthesis of copper oxide nanoparticles mediated Annona muricata as cytotoxic and apoptosis inducer factor in breast cancer cell lines. Sci. Rep. 2022, 12, 16165. [Google Scholar] [CrossRef]
- Khalid, A.; Ahmad, P.; Alharthi, A.I.; Muhammad, S.; Khandaker, M.U.; Rehman, M.; Faruque, M.R.I.; Din, I.U.; Alotaibi, M.A.; Alzimami, K.; et al. Structural, Optical and Antibacterial Efficacy of Pure and Zinc-Doped Copper Oxide against Pathogenic Bacteria. Nanomaterials 2021, 11, 451. [Google Scholar] [CrossRef] [PubMed]
- Guţoiu, S.; Pogăcean, F.; Măgeruşan, L.; Miclăuş, M.O.; Grad, O.; Pană, I.-O.; Pruneanu, S. Enhancement of Dopamine Electrochemical Detection with Manganese Doped Crystalline Copper Oxide. Coatings 2023, 13, 1014. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ. National Institutes of Health, Bethesda, Maryland, USA, 1997–2018. Available online: https://ImageJ.net/ij/ (accessed on 19 September 2023).
- Ganea, I.-V.; Nan, A.; Ciorîță, A.; Turcu, R.; Baciu, C. Responsiveness assessment of cell cultures exposed to poly(tartaric acid) and its corresponding magnetic nanostructures. J. Mol. Struct. 2022, 1248, 131459. [Google Scholar] [CrossRef]
- Accelrys’ Materials Studio: A Comprehensive Materials Modeling and Simulation Application|CHEManager. Available online: https://www.chemanager-online.com/en/products/information-technology/accelrys-materials-studio-comprehensive-materials-modeling-and-simul (accessed on 19 September 2023).
- Pop, V.; Gutoiu, S.; Dorolti, E.; Leostean, C.; Isnard, O.; Chicinas, I.; Pana, O. The Influence of Milling and Annealing on the Structural and Magnetic Behavior of Nd2Fe14B/α-Fe Magnetic Nanocomposite. J. Alloys Compd. 2013, 581, 821–827. [Google Scholar] [CrossRef]
- Leostean, C.; Pana, O.; Turcu, R.; Soran, M.L.; Macavei, S.; Chauvet, O.; Payen, C. Comparative Study of Core-Shell Iron/Iron Oxide Gold Covered Magnetic Nanoparticles Obtained in Different Conditions. J. Nanoparticle Res. 2011, 13, 6181–6192. [Google Scholar] [CrossRef]
- Koiri, R.K.; Trigun, S.K.; Dubey, S.K.; Singh, S.; Mishra, L. Metal Cu(II) and Zn(II) bipyridyls as inhibitors of lactate dehydrogenase. Biometals 2008, 21, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Moschini, E.; Gualtieri, M.; Colombo, M.; Fascio, U.; Camatini, M.; Mantecca, P. The Modality of Cell-Particle Interactions Drives the Toxicity of Nanosized CuO and TiO2 in Human Alveolar Epithelial Cells. Toxicol. Lett. 2013, 222, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Moschini, E.; Colombo, G.; Chirico, G.; Capitani, G.; Dalle-Donne, I.; Mantecca, P. Biological Mechanism of Cell Oxidative Stress and Death during Short-Term Exposure to Nano CuO. Sci. Rep. 2023, 13, 2326. [Google Scholar] [CrossRef]
- Wongrakpanich, A.; Mudunkotuwa, I.A.; Geary, S.M.; Morris, A.S.; Mapuskar, K.A.; Spitz, D.R.; Grassian, V.H.; Salem, A.K. Size-Dependent Cytotoxicity of Copper Oxide Nanoparticles in Lung Epithelial Cells. Environ. Sci. Nano 2016, 3, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, N.; Zhao, J.; White, J.C.; Qu, P.; Xing, B. CuO Nanoparticle Interaction with Human Epithelial Cells: Cellular Uptake, Location, Export, and Genotoxicity. Chem. Res. Toxicol. 2012, 25, 1512–1521. [Google Scholar] [CrossRef]
- Di Bucchianico, S.; Fabbrizi, M.R.; Misra, S.K.; Valsami-Jones, E.; Berhanu, D.; Reip, P.; Bergamaschi, E.; Migliore, L. Multiple Cytotoxic and Genotoxic Effects Induced in Vitro by Differently Shaped Copper Oxide Nanomaterials. Mutagenesis 2013, 28, 287–299. [Google Scholar] [CrossRef]
- Sobańska, Z.; Roszak, J.; Kowalczyk, K.; Stępnik, M. Applications and Biological Activity of Nanoparticles of Manganese and Manganese Oxides in in Vitro and in Vivo Models. Nanomaterials 2021, 11, 1084. [Google Scholar] [CrossRef]
- Fernández-Pampín, N.; González Plaza, J.J.; García-Gómez, A.; Peña, E.; Rumbo, C.; Barros, R.; Martel-Martín, S.; Aparicio, S.; Tamayo-Ramos, J.A. Toxicology Assessment of Manganese Oxide Nanomaterials with Enhanced Electrochemical Properties Using Human in Vitro Models Representing Different Exposure Routes. Sci. Rep. 2022, 12, 20991. [Google Scholar] [CrossRef]
- Tootoonchi, M.H.; Hashempour, M.; Blackwelder, P.L.; Fraker, C.A. Manganese Oxide Particles as Cytoprotective, Oxygen Generating Agents. Acta Biomater. 2017, 59, 327–337. [Google Scholar] [CrossRef]
- Urandur, S.; Banala, V.T.; Shukla, R.P.; Gautam, S.; Marwaha, D.; Rai, N.; Sharma, M.; Sharma, S.; Ramarao, P.; Mishra, P.R. Theranostic Lyotropic Liquid Crystalline Nanostructures for Selective Breast Cancer Imaging and Therapy. Acta Biomater. 2020, 113, 522–540. [Google Scholar] [CrossRef]
- Wu, J. Personalized Medicine the Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjug. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Armenta, F.J.; Valdez-Olmos, U.F.; Arvizu-Flores, A.A.; Ayala-Zavala, J.F.; Ochoa-Leyva, A.; Lopez-Zavala, A.A. Metal Ions and Chemical Modification Reagents Inhibit the Enzymatic Activity of Lecithin-Dependent Hemolysin from Vibrio parahaemolyticus. Toxins 2022, 14, 609. [Google Scholar] [CrossRef] [PubMed]




| Sample | Degree of Crystallinity (%) |
|---|---|
| CuO | 90.44 |
| CuO:Mn1 | 89.30 |
| CuO:Mn3 | 88.61 |
| CuO:Mn4 | 90.73 |
| Sample | Hydrodynamic Diameter (nm) | PDI |
|---|---|---|
| CuO | 805.26 ± 49.68 | 0.182 |
| CuO:Mn1 | 258.73 ± 25.31 | 0.328 |
| CuO:Mn3 | 120.94 ± 28.10 | 0.507 |
| CuO:Mn4 | 392.66 ± 26.51 | 0.049 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pană, I.-O.; Ciorîță, A.; Boca, S.; Guțoiu, S.; Kacso, I.; Miclăuș, M.O.; Grad, O.; Gherman, A.M.R.; Leostean, C.; Suciu, M. Interaction of Manganese-Doped Copper Oxide Nano-Platelets with Cells: Biocompatibility and Anticancer Activity Assessment. Biomimetics 2025, 10, 203. https://doi.org/10.3390/biomimetics10040203
Pană I-O, Ciorîță A, Boca S, Guțoiu S, Kacso I, Miclăuș MO, Grad O, Gherman AMR, Leostean C, Suciu M. Interaction of Manganese-Doped Copper Oxide Nano-Platelets with Cells: Biocompatibility and Anticancer Activity Assessment. Biomimetics. 2025; 10(4):203. https://doi.org/10.3390/biomimetics10040203
Chicago/Turabian StylePană, Ioan-Ovidiu, Alexandra Ciorîță, Sanda Boca, Simona Guțoiu, Irina Kacso, Maria Olimpia Miclăuș, Oana Grad, Ana Maria Raluca Gherman, Cristian Leostean, and Maria Suciu. 2025. "Interaction of Manganese-Doped Copper Oxide Nano-Platelets with Cells: Biocompatibility and Anticancer Activity Assessment" Biomimetics 10, no. 4: 203. https://doi.org/10.3390/biomimetics10040203
APA StylePană, I.-O., Ciorîță, A., Boca, S., Guțoiu, S., Kacso, I., Miclăuș, M. O., Grad, O., Gherman, A. M. R., Leostean, C., & Suciu, M. (2025). Interaction of Manganese-Doped Copper Oxide Nano-Platelets with Cells: Biocompatibility and Anticancer Activity Assessment. Biomimetics, 10(4), 203. https://doi.org/10.3390/biomimetics10040203








