Development and Evaluation of a Novel Upper-Limb Rehabilitation Device Integrating Piano Playing for Enhanced Motor Recovery
Abstract
1. Introduction
2. Materials and Methods
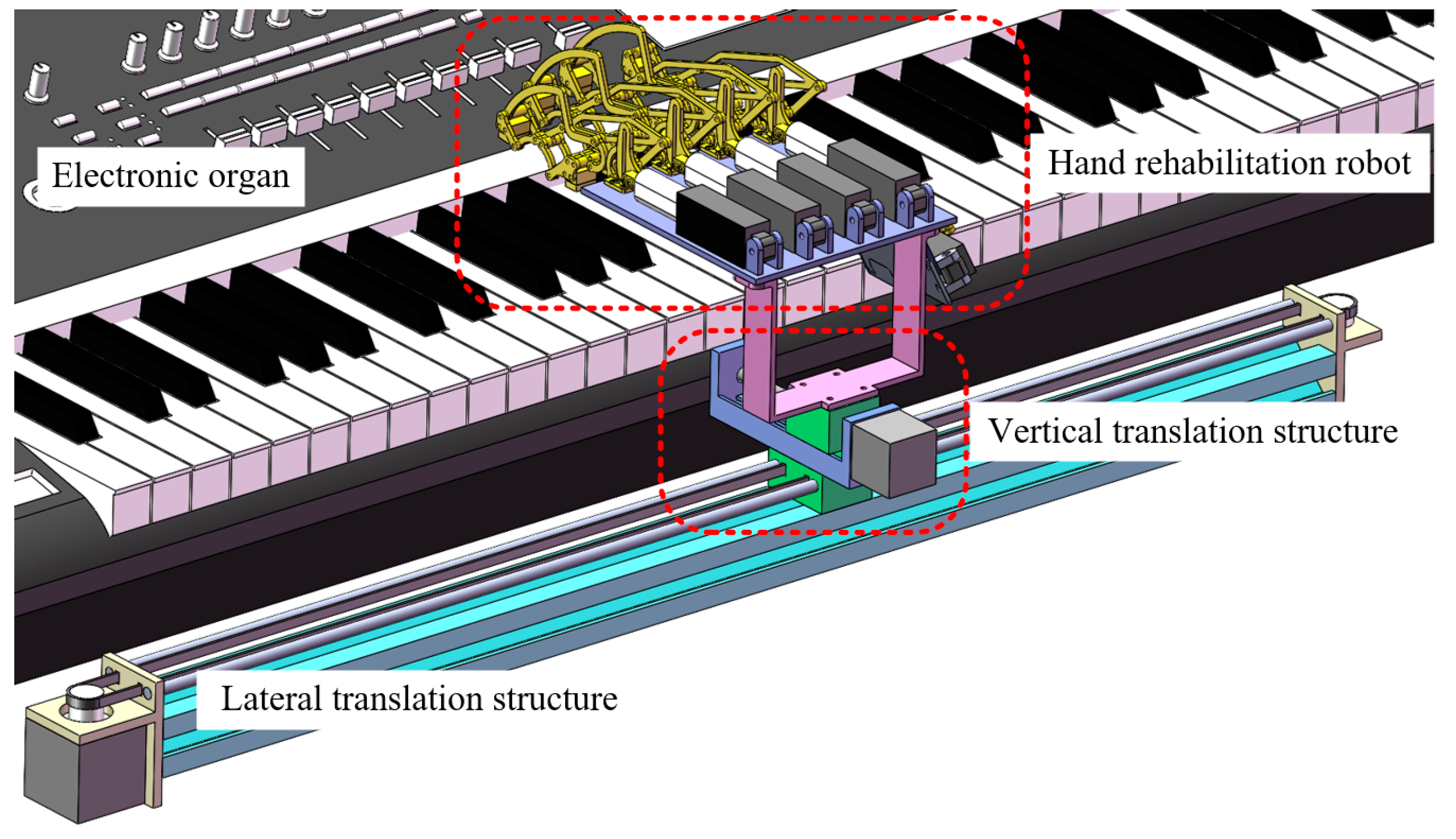
2.1. Mechanical Structure Design
2.1.1. The Horizontal Translation Mechanism
2.1.2. The Vertical Translation Mechanism
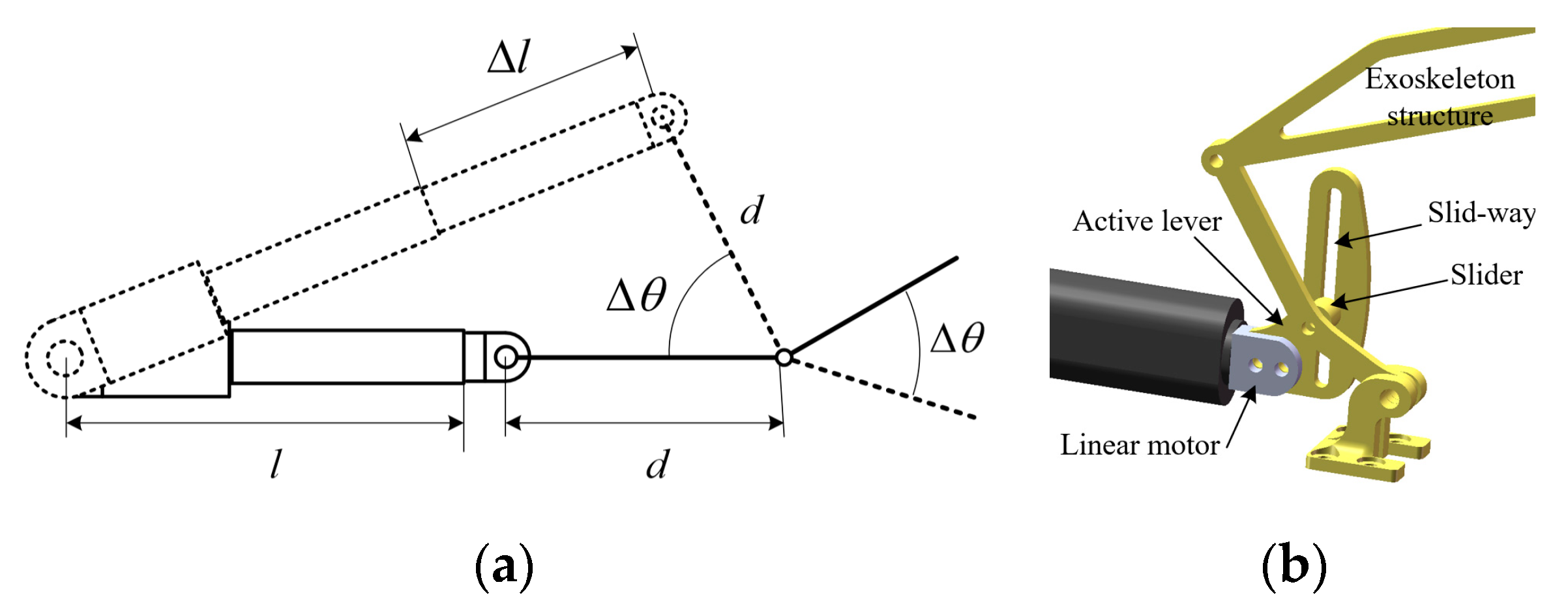
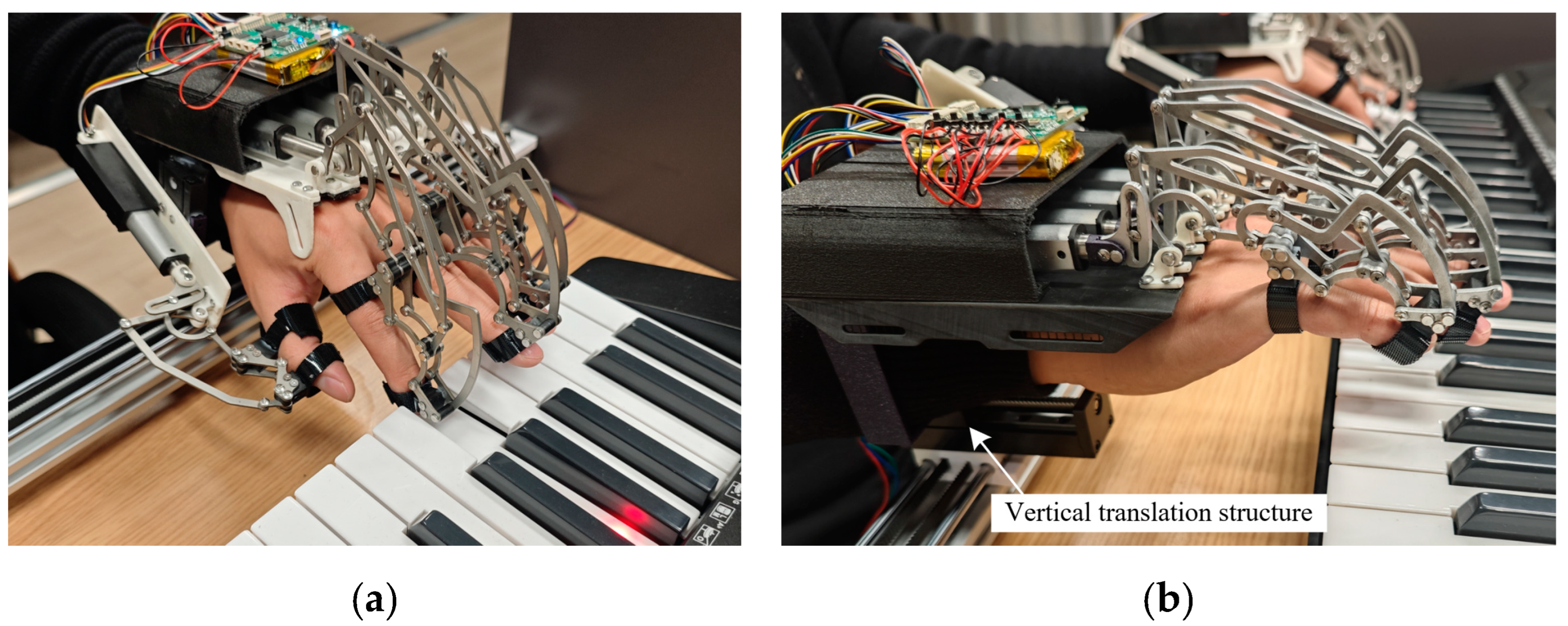
2.1.3. The Hand Rehabilitation Robot
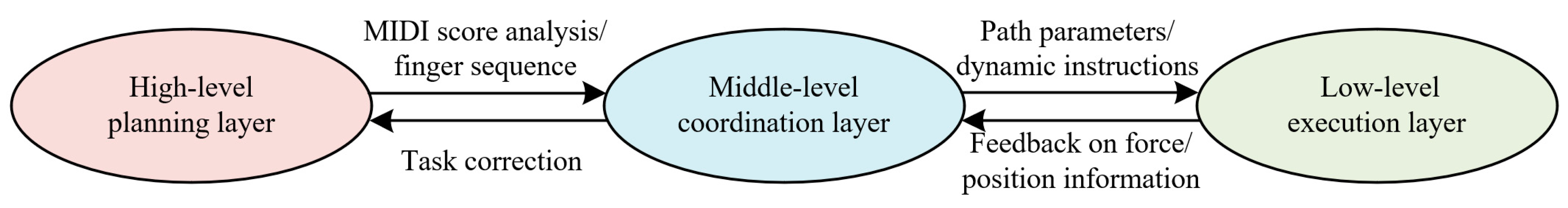
2.2. Control Strategy Design
2.2.1. High-Level Planning Layer: Music-to-Mechanical Action Mapping
- White keys: Width 23.5 mm (C1 to C6, 36 white keys in total).
- Black keys: Width 13 mm, positioned above the gaps between white keys.
- The horizontal position of the nth white key is calculated as:
- Black keys are located between adjacent white keys, with a lateral offset of +9.25 mm from the left white key and a fixed vertical offset of 12 mm.
2.2.2. Mid-Level Coordination Layer: Motion Sequence Planning
2.2.3. Low-Level Execution Layer: High-Precision Motion Control
3. Experiment and Result Analysis
3.1. Comparative Experiment Setup
3.1.1. Experimental Design
3.1.2. Participant Recruitment and Grouping
3.1.3. Evaluation Indicators and Methods
3.1.4. Data Collection and Analysis
3.2. Analysis of Experimental Results
4. Discussion
4.1. Upper-Limb Motor Function Improvement
4.2. Manual Dexterity and Fine Motor Control
4.3. Patient Motivation and Rehabilitation Adherence
4.4. Clinical Implications and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gittler, M.; Davis, A.M. Guidelines for Adult Stroke Rehabilitation and Recovery. JAMA 2018, 319, 820–821. [Google Scholar] [CrossRef] [PubMed]
- Hayward, K.S.; Kramer, S.F.; Dalton, E.J.; Hughes, G.R.; Brodtmann, A.; Churilov, L.; Cloud, G.; Corbett, D.; Jolliffe, L.; Kaffenberger, T.; et al. Timing and Dose of Upper Limb Motor Intervention After Stroke: A Systematic Review. Stroke 2021, 52, 3706–3717. [Google Scholar] [CrossRef]
- Lim, D.Y.-L.; Lai, H.-S.; Yeow, R.C.-H. A Bidirectional Fabric-Based Soft Robotic Glove for Hand Function Assistance in Patients with Chronic Stroke. J. NeuroEng. Rehabil. 2023, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Saini, M.; Kumar, N.; Srivastava, M.V.P.; Mehndiratta, A. Evidence of Neuroplasticity with Robotic Hand Exoskeleton for Post-Stroke Rehabilitation: A Randomized Controlled Trial. J. NeuroEng. Rehabil. 2021, 18, 76. [Google Scholar] [CrossRef]
- Fujioka, T.; Hunt, A.M. Mechanisms of Music Therapy and Music-Based Interventions. In Music Therapy and Music-Based Interventions in Neurology: Perspectives on Research and Practice; Devlin, K., Kang, K., Pantelyat, A., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 9–21. ISBN 978-3-031-47092-9. [Google Scholar]
- Ouendi, N.; Hubaut, R.; Pelayo, S.; Anceaux, F.; Wallard, L. The Rehabilitation Robot: Factors Influencing Its Use, Advantages and Limitations in Clinical Rehabilitation. Disabil. Rehabil. Assist. Technol. 2024, 19, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Morone, G.; Cocchi, I.; Paolucci, S.; Iosa, M. Robot-Assisted Therapy for Arm Recovery for Stroke Patients: State of the Art and Clinical Implication. Expert Rev. Med. Devices 2020, 17, 223–233. [Google Scholar] [CrossRef]
- Weber, L.M.; Stein, J. The Use of Robots in Stroke Rehabilitation: A Narrative Review. NeuroRehabilitation 2018, 43, 99–110. [Google Scholar] [CrossRef]
- Bertomeu-Motos, A.; Blanco, A.; Badesa, F.J.; Barios, J.A.; Zollo, L.; Garcia-Aracil, N. Human Arm Joints Reconstruction Algorithm in Rehabilitation Therapies Assisted by End-Effector Robotic Devices. J. NeuroEng. Rehabil. 2018, 15, 10. [Google Scholar] [CrossRef]
- Rosenthal, O.; Wing, A.M.; Wyatt, J.L.; Punt, D.; Brownless, B.; Ko-Ko, C.; Miall, R.C. Boosting Robot-Assisted Rehabilitation of Stroke Hemiparesis by Individualized Selection of Upper Limb Movements—A Pilot Study. J. NeuroEng. Rehabil. 2019, 16, 42. [Google Scholar] [CrossRef]
- Pandey, S.; Singaravelan, D.R.R. Effects of Music Therapy on Cognitive Function and Mood in Patients with Middle Cerebral Artery Stroke. Int. J. Health Sci. Res. 2019, 9, 151–157. [Google Scholar]
- Fujioka, T.; Dawson, D.R.; Wright, R.; Honjo, K.; Chen, J.L.; Chen, J.J.; Black, S.E.; Stuss, D.T.; Ross, B. The Effects of Music-Supported Therapy on Motor, Cognitive, and Psychosocial Functions in Chronic Stroke. Ann. N. Y. Acad. Sci. 2018, 1423, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Haire, C.M.; Tremblay, L.; Vuong, V.; Patterson, K.K.; Chen, J.L.; Burdette, J.H.; Schaffert, N.; Thaut, M.H. Therapeutic Instrumental Music Training and Motor Imagery in Post-Stroke Upper-Extremity Rehabilitation: A Randomized-Controlled Pilot Study. Arch. Rehabil. Res. Clin. Transl. 2021, 3, 100162. [Google Scholar] [CrossRef] [PubMed]
- Magee, W.L.; Clark, I.; Tamplin, J.; Bradt, J. Music Interventions for Acquired Brain Injury. Cochrane Database Syst. Rev. 2017, 1, CD006787. [Google Scholar] [CrossRef]
- Brancatisano, O.; Baird, A.; Thompson, W.F. Why Is Music Therapeutic for Neurological Disorders? Neurosci. Biobehav. Rev. 2020, 112, 600–615. [Google Scholar] [CrossRef] [PubMed]
- Goebl, W.; Palmer, C. Synchronization of Timing and Motion Among Performing Musicians. Music Percept. 2009, 26, 427–438. [Google Scholar] [CrossRef]
- Zatorre, R.J.; Chen, J.L.; Penhune, V.B. When the Brain Plays Music: Auditory–Motor Interactions in Music Perception and Production. Nat. Rev. Neurosci. 2007, 8, 547–558. [Google Scholar] [CrossRef]
- Pantev, C.; Ross, B.; Fujioka, T.; Trainor, L.J.; Schulte, M.; Schulz, M. Music and Learning-Induced Cortical Plasticity. Ann. N. Y. Acad. Sci. 2003, 999, 438–450. [Google Scholar] [CrossRef]
- Santonja-Medina, C.S.; Marrades-Caballero, E.; Santonja-Medina, F.; Sanz-Mengibar, J.M. Neurologic Music Therapy Improves Participation in Children With Severe Cerebral Palsy. Front. Neurol. 2022, 13, 795533. [Google Scholar] [CrossRef]
- Wan, C.Y.; Schlaug, G. Music Making as a Tool for Promoting Brain Plasticity across the Life Span. Neuroscientist 2010, 16, 566–577. [Google Scholar] [CrossRef]
- Tamir-Ostrover, H.; Hassin-Baer, S.; Fay-Karmon, T.; Friedman, J. Quantifying Changes in Dexterity as a Result of Piano Training in People with Parkinson’s Disease. Sensors 2024, 24, 3318. [Google Scholar] [CrossRef]
- Yu, J.; Zheng, L.; Bai, X.; Du, Y.; Zhang, L.; Xie, P. Analysis and Prediction of Multi-Joint Coupling Torque of Fingers. In Proceedings of the 2019 Intelligent Rehabilitation and Human-machine Engineering Conference (IRHE-2019), Qinhuangdao, China, 18–21 November 2019; pp. 1–6. [Google Scholar]
- Jo, I.; Park, Y.; Lee, J.; Bae, J. A Portable and Spring-Guided Hand Exoskeleton for Exercising Flexion/Extension of the Fingers. Mech. Mach. Theory 2019, 135, 176–191. [Google Scholar] [CrossRef]
- Dhanalakshmy, D.M.; Menon, H.P.; Vinaya, V. Musical Notes to MIDI Conversion. In Proceedings of the 2017 International Conference on Advances in Computing, Communications and Informatics (ICACCI), Udupi, India, 13–16 September 2017; pp. 799–804. [Google Scholar]
- Strayer, H. From Neumes to Notes: The Evolution of Music Notation. Music. Offer. 2013, 4, 1. [Google Scholar] [CrossRef][Green Version]
- Guzmán, J.L.; Hägglund, T.; Visioli, A. Feedforward Compensation for PID Control Loops. In PID Control in the Third Millennium: Lessons Learned and New Approaches; Vilanova, R., Visioli, A., Eds.; Springer: London, UK, 2012; pp. 207–234. ISBN 978-1-4471-2425-2. [Google Scholar]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The Fugl-Meyer Assessment of Motor Recovery after Stroke: A Critical Review of Its Measurement Properties. Neurorehabilit. Neural Repair 2002, 16, 232–240. [Google Scholar] [CrossRef]
- Desrosiers, J.; Bravo, G.; Hébert, R.; Dutil, É.; Mercier, L. Validation of the Box and Block Test as a Measure of Dexterity of Elderly People: Reliability, Validity, and Norms Studies. Arch. Phys. Med. Rehabil. 1994, 75, 751–755. [Google Scholar] [CrossRef]
- Mathiowetz, V.; Volland, G.; Kashman, N.; Weber, K. Adult Norms for the Box and Block Test of Manual Dexterity. Am. J. Occup. Ther. 1985, 39, 386–391. [Google Scholar] [CrossRef]
- White, G.N.; Cordato, D.; O’Rourke, F.; Mendis, R.L.; Ghia, D.; Chan, D. Validation of the Stroke Rehabilitation Motivation Scale: A Pilot Study. Asian J. Gerontol. Geriatr. 2012, 7, 80–87. [Google Scholar]
- Yoshida, T.; Otaka, Y.; Kitamura, S.; Ushizawa, K.; Kumagai, M.; Kurihara, Y.; Yaeda, J.; Osu, R. Development and Validation of New Evaluation Scale for Measuring Stroke Patients’ Motivation for Rehabilitation in Rehabilitation Wards. PLoS ONE 2022, 17, e0265214. [Google Scholar] [CrossRef]
- Temporiti, F.; Mandaresu, S.; Calcagno, A.; Coelli, S.; Bianchi, A.M.; Gatti, R.; Galli, M. Kinematic Evaluation and Reliability Assessment of the Nine Hole Peg Test for Manual Dexterity. J. Hand Ther. 2023, 36, 560–567. [Google Scholar] [CrossRef]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect Size Estimates: Current Use, Calculations, and Interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef]





| Characteristic | Experimental Group (n = 5) | Control Group (n = 5) | p-Value |
|---|---|---|---|
| Age (years) | 59.4 ± 2.2 | 56.2 ± 4.8 | 0.27 |
| Sex (M/F) | 3/2 | 2/3 | 0.63 |
| Course of disease (months) | 6.1 ± 2.2 | 5.9 ± 2.1 | 0.58 |
| FMA-UE (score) | 28.9 ± 2.3 | 28.7 ± 3.1 | 0.92 |
| BBT (score) | 21.2 ± 3.7 | 21.3 ± 3.3 | 0.98 |
| Characteristic | Experimental Group (n = 5) | Control Group (n = 5) | p-value |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Zhang, Y.; Zhang, Y.; Zhang, P.; Yu, J.; Yuan, S. Development and Evaluation of a Novel Upper-Limb Rehabilitation Device Integrating Piano Playing for Enhanced Motor Recovery. Biomimetics 2025, 10, 200. https://doi.org/10.3390/biomimetics10040200
Zhao X, Zhang Y, Zhang Y, Zhang P, Yu J, Yuan S. Development and Evaluation of a Novel Upper-Limb Rehabilitation Device Integrating Piano Playing for Enhanced Motor Recovery. Biomimetics. 2025; 10(4):200. https://doi.org/10.3390/biomimetics10040200
Chicago/Turabian StyleZhao, Xin, Ying Zhang, Yi Zhang, Peng Zhang, Jinxu Yu, and Shuai Yuan. 2025. "Development and Evaluation of a Novel Upper-Limb Rehabilitation Device Integrating Piano Playing for Enhanced Motor Recovery" Biomimetics 10, no. 4: 200. https://doi.org/10.3390/biomimetics10040200
APA StyleZhao, X., Zhang, Y., Zhang, Y., Zhang, P., Yu, J., & Yuan, S. (2025). Development and Evaluation of a Novel Upper-Limb Rehabilitation Device Integrating Piano Playing for Enhanced Motor Recovery. Biomimetics, 10(4), 200. https://doi.org/10.3390/biomimetics10040200





