Effectiveness of Titanium Occlusive Barriers in Guided Bone Regeneration: A Prospective Analysis of Vertical and Horizontal Bone Augmentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Recruitment
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Ethics
2.6. Preoperative Information
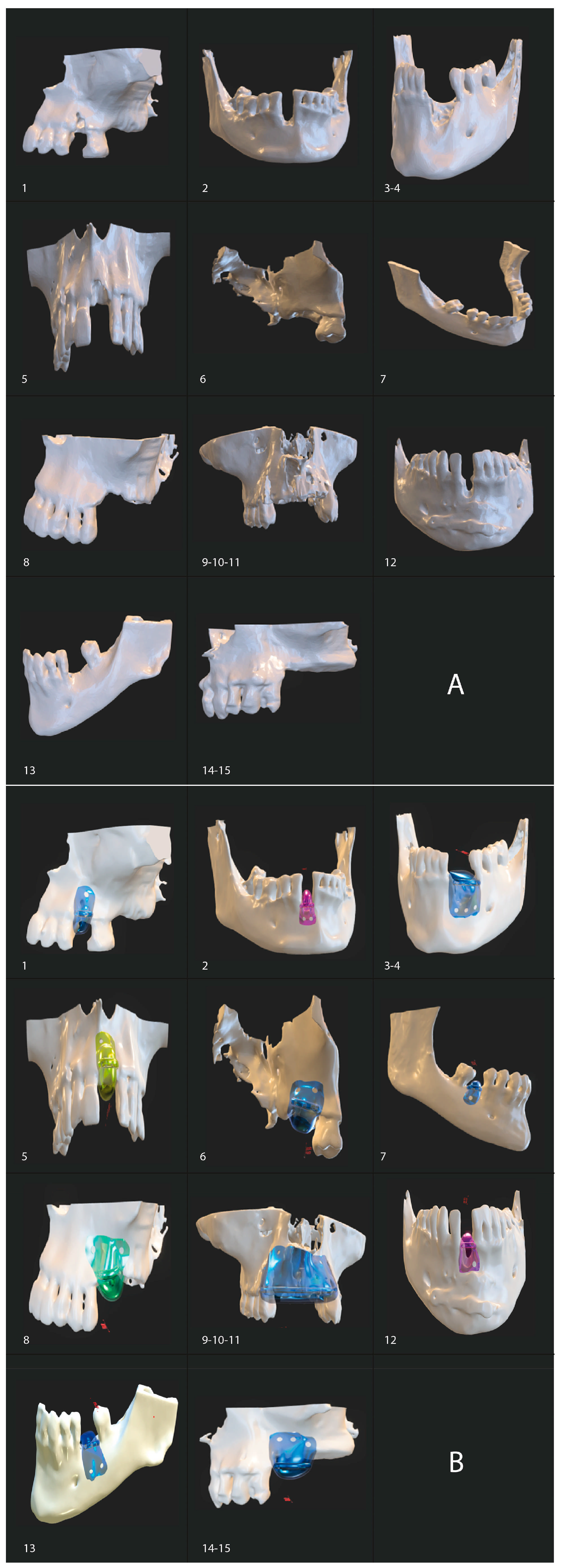
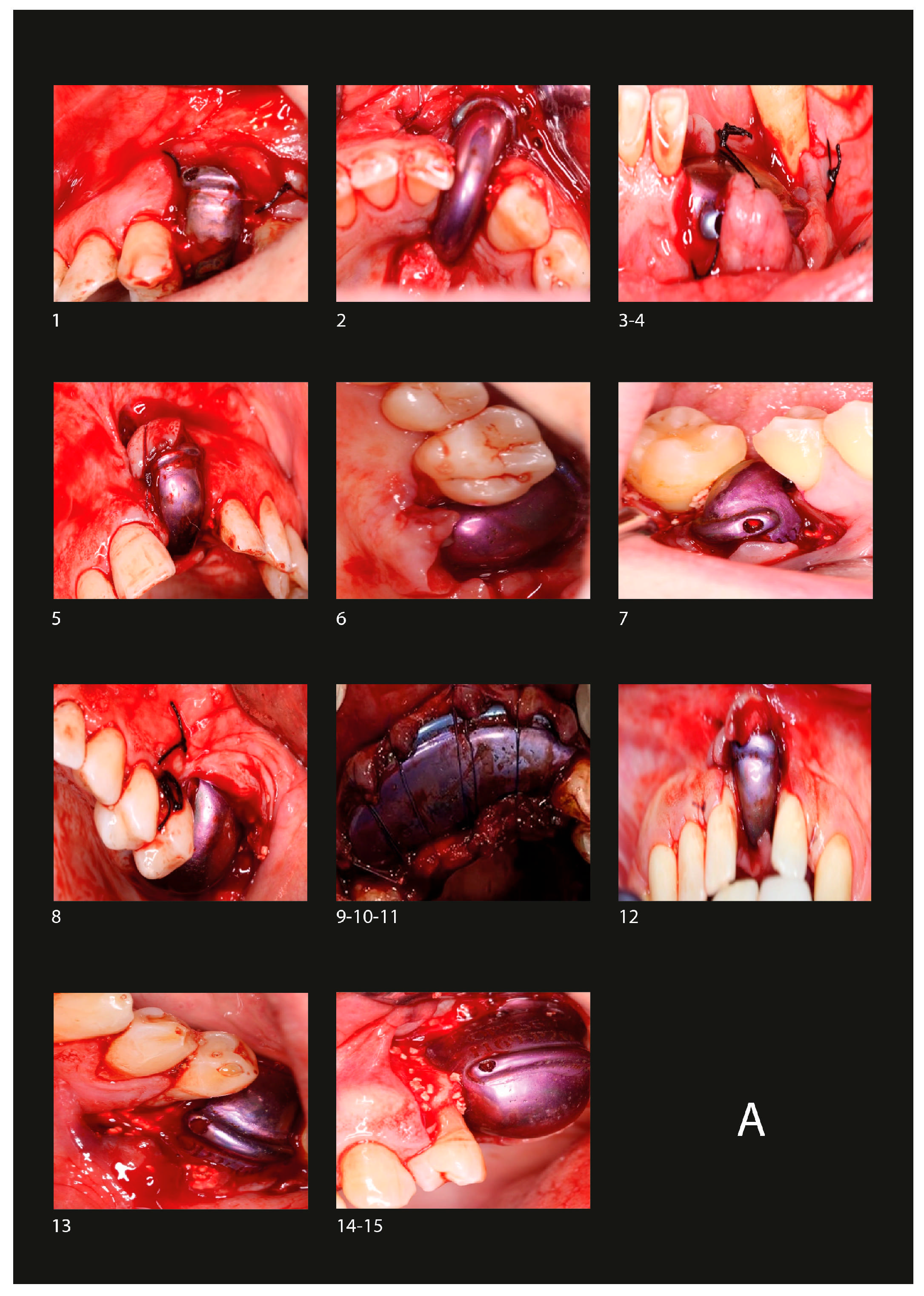
2.7. Surgical Protocol
2.7.1. Barrier Placement
2.7.2. Postoperative Care
2.7.3. Implant Placement
2.8. Variables Analyzed
- A.
- Relationship with bone and soft tissue regeneration
- 1.
- Radiographic Measurements
- 2.
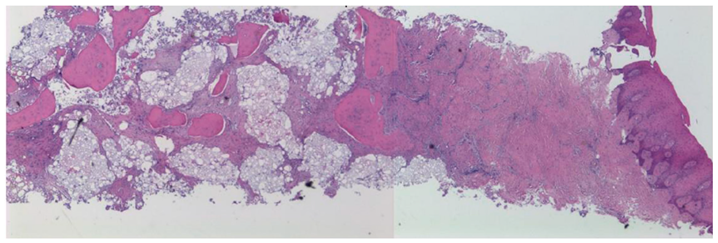
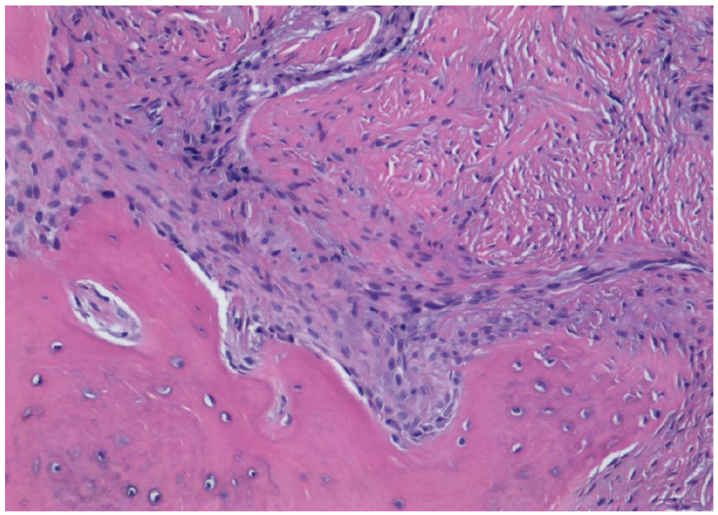
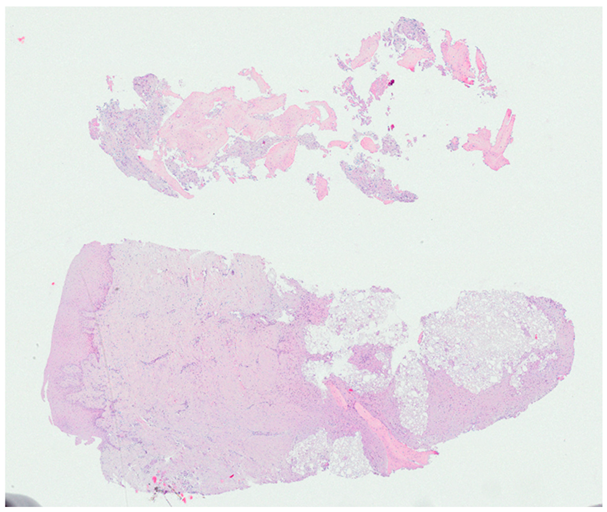
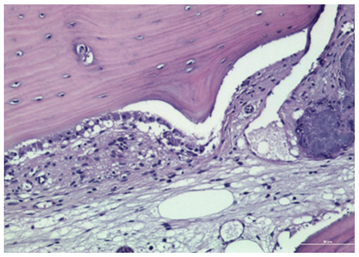
- Histological analysis
- B.
- Relationship with procedure. Complications
- 1.
- Fixation loss
- 2.
- Local infections
2.9. Statistical Analysis
3. Results
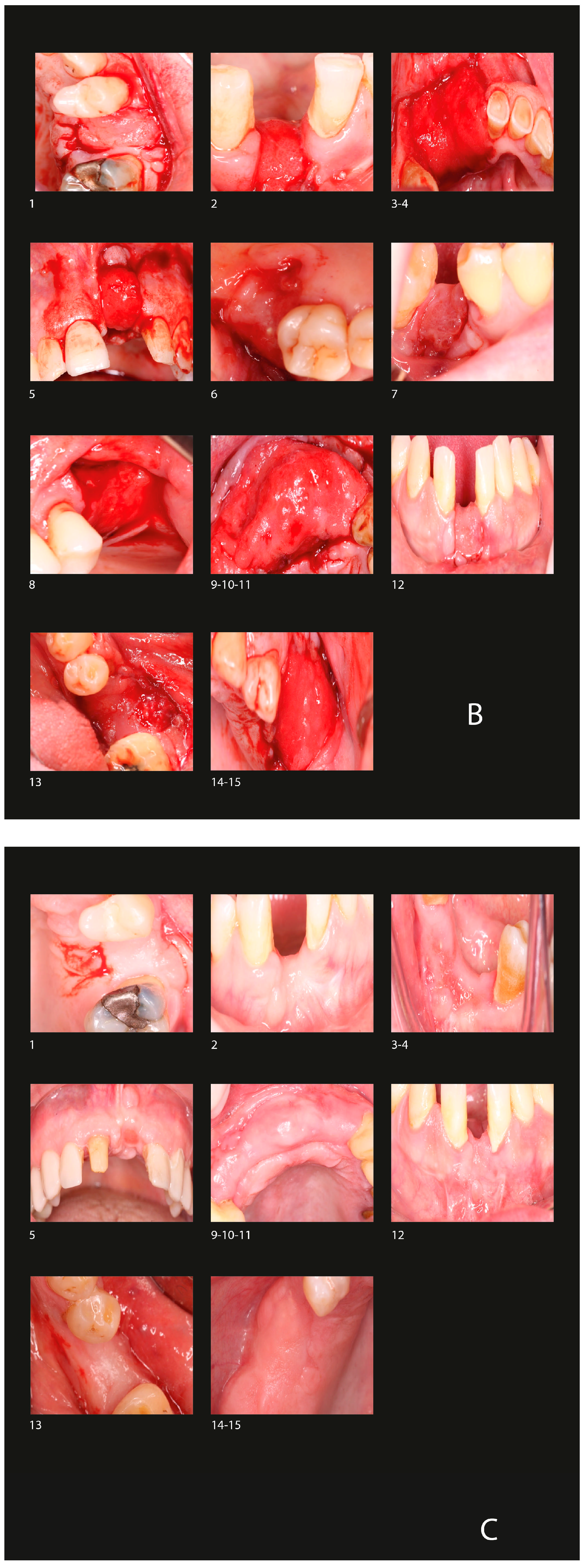
3.1. Radiological Observation
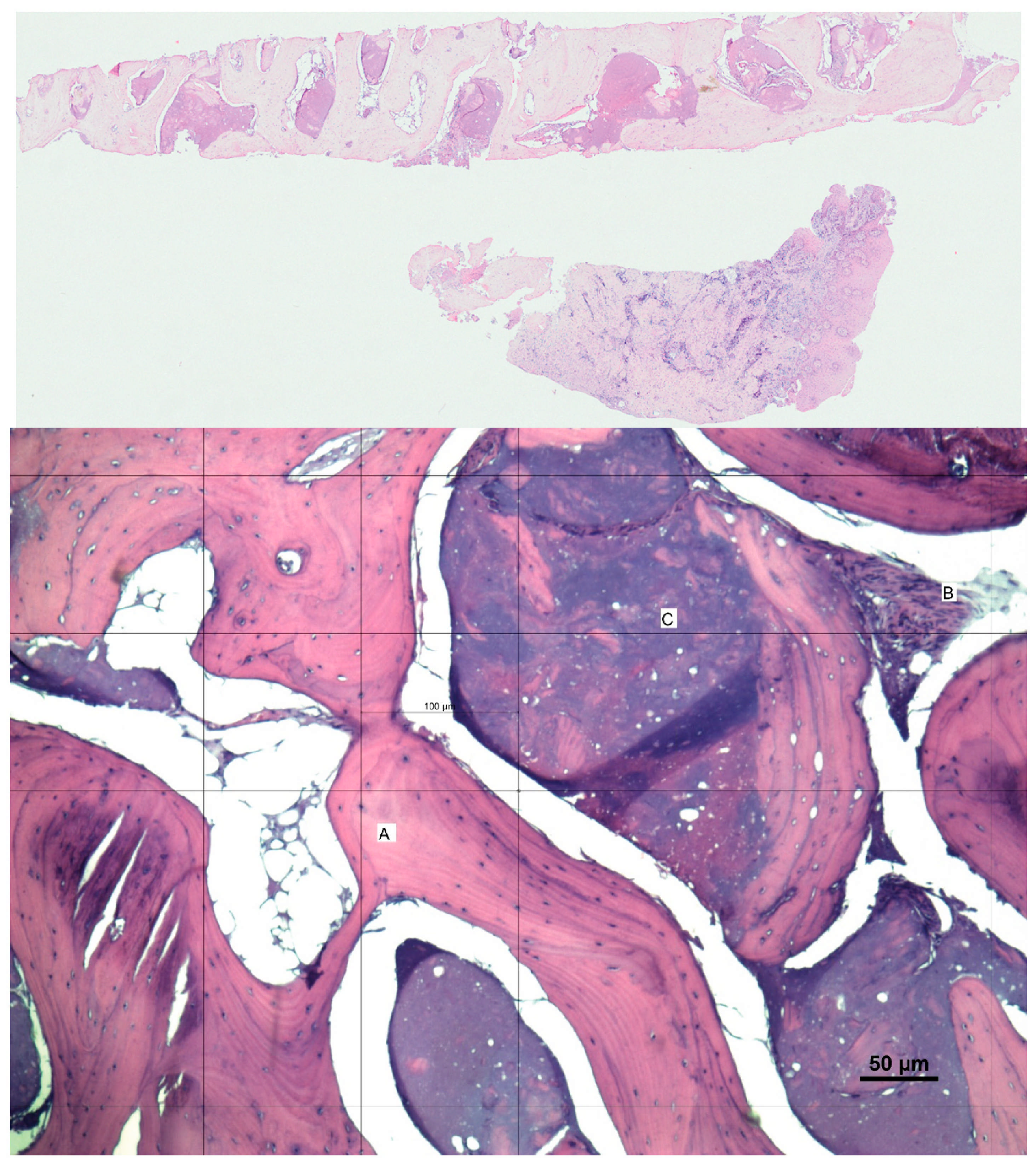
3.2. Histological Observation
3.3. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBCT | cone beam computed tomography |
| GBR | guided bone regeneration |
| STL | stereolithography |
| SSS | sterile saline solution |
| DICOM | Digital Imaging and Communication in Medicine |
| SD | standard deviation |
| IQR | interquartile range |
Appendix A

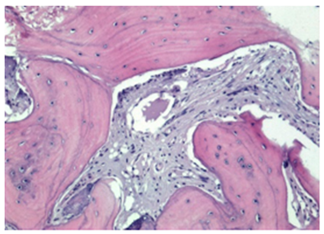
References
- Araújo, M.G.; Silva, C.O.; Misawa, M.; Sukekava, F. Alveolar socket healing: What can we learn? Periodontol. 2000 2015, 68, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G.; Dias, D.R.; Matarazzo, F.M. Anatomical characteristics of the alveolar process and basal bone that have an effect on socket healing. Periodontol. 2000 2023, 93, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Del Fabbro, M.; Khijmatgar, S.; Panda, S.; Ravidà, A.; Tommasato, G.; Sculean, A.; Pesce, P. Dimensional and histomorphometric evaluation of biomaterials used for alveolar ridge preservation: A systematic review and network meta-analysis. Clin. Oral Investig. 2022, 26, 141–158. [Google Scholar] [CrossRef]
- Avila-Ortiz, G.; Chambrone, L.; Vignoletti, F. Effect of alveolar ridge preservation interventions following tooth extraction: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46 (Suppl. 21), 195–223. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Urban, I.; Monje, A.; Kunrath, M.F.; Dahlin, C. Guided bone regeneration in implant dentistry: Basic principle, progress over 35 years, and recent research activities. Periodontol. 2000 2023, 93, 9–25. [Google Scholar] [CrossRef]
- Urban, I.; Montero, E.; Sanz-Sánchez, I.; Palombo, D.; Monje, A.; Tommasato, G.; Chiapasco, M. Minimal invasiveness in vertical ridge augmentation. Periodontol. 2000 2023, 93, 9–25. [Google Scholar] [CrossRef]
- Thoma, D.S.; Naenni, N.; Figuero, E.; Hämmerle, C.H.F.; Schwarz, F.; Jung, R.E.; Sanz-Sánchez, I. Effects of soft tissue augmentation procedures on peri-implant health or disease: A systematic review and meta-analysis. Clin. Oral Implants Res. 2018, 29, 32–49. [Google Scholar] [CrossRef]
- Seyssens, L.; Eghbali, A.; Christiaens, V.; De Bruyckere, T.; Doornewaard, R.; Cosyn, J. A one-year prospective study on alveolar ridge preservation using collagen-enriched deproteinized bovine bone mineral and saddle connective tissue graft: A cone beam computed tomography analysis. Clin. Implant Dent. Relat. Res. 2019, 21, 853–861. [Google Scholar] [CrossRef]
- Sanz-Sánchez, I.; Sanz-Martín, I.; Ortiz-Vigón, A.; Molina, A.; Sanz, M. Complications in bone-grafting procedures: Classification and management. Periodontol. 2000 2022, 88, 86–102. [Google Scholar] [CrossRef]
- Sumida, T.; Otawa, N.; Kamata, Y.U.; Kamakura, S.; Mtsushita, T.; Kitagaki, H.; Mori, S.; Sasaki, K.; Fujibayashi, S.; Takemoto, M.; et al. Custom-made titanium devices as membranes for bone augmentation in implant treatment: Clinical application and the comparison with conventional titanium mesh. J. Craniomaxillofac. Surg. 2015, 43, 2183–2188. [Google Scholar] [CrossRef]
- Seiler, M.; Kämmerer, P.W.; Peetz, M.; Hartmann, A.G. Customized titanium lattice structure in three-dimensional alveolar defect: An initial case letter. J. Oral Implantol. 2018, 44, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, S.; Zhang, T.; Wang, C.; Cai, X. Titanium mesh for bone augmentation in oral implantology: Current application and progress. Int. J. Oral Sci. 2020, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, J.; Du, M.; Zhang, S.; Liu, Y.; Yang, H.; Shi, R.; Guo, Y.; Song, F.; Zhao, Y.; et al. Customized Barrier Membrane (Titanium Alloy, Poly Ether-Ether Ketone and Unsintered Hydroxyapatite/Poly-l-Lactide) for Guided Bone Regeneration. Front. Bioeng. Biotechnol. 2022, 10, 916967. [Google Scholar] [CrossRef] [PubMed]
- Al-Ardah, A.J.; Alqahtani, N.; AlHelal, A.; Goodacre, B.J.; Swamidass, R.; Garbacea, A.; Lozada, J. Using Virtual Ridge Augmentation and 3-Dimensional Printing to Fabricate a Titanium Mesh Positioning Device: A Novel Technique Letter. J. Oral Implantol. 2018, 44, 293–299. [Google Scholar] [CrossRef]
- Milillo, L.; Petruzzi, M. Guided bone regeneration with occlusive titanium barrier: A case report and clinical considerations. Biomimetics 2023, 8, 106. [Google Scholar] [CrossRef]
- Van Steenberghe, D.; Johansson, C.; Quirynen, M.; Molly, L.; Albrektsson, T.; Naert, I. Bone augmentation by means of a stiff occlusive titanium barrier. Clin. Oral Implants Res. 2003, 14, 63–71. [Google Scholar] [CrossRef]
- Ezirganlı, Ş.; Polat, S.; Barış, E.; Tatar, İ.; Çelik, H.H. Comparative investigation of the effects of different materials used with a titanium barrier on new bone formation. Clin. Oral Implants Res. 2013, 24, 312–319. [Google Scholar] [CrossRef]
- de Sousa Gomes, P.; Daugela, P.; Poskevicius, L.; Mariano, L.; Fernandes, M.H. Molecular and cellular aspects of socket healing in the absence and presence of graft materials and autologous platelet concentrates: A focused review. J. Oral Maxillofac. Res. 2019, 10, e2. [Google Scholar] [CrossRef]
- Melcher, A.H.; Dreyer, C.J. Protection of the blood clot in healing circumscribed bone defects. J. Bone Jt. Surg. Br. 1962, 44-B, 424–430. [Google Scholar]
- Milillo, L.; Cinone, F.; Lo Presti, F.; Lauritano, D.; Petruzzi, M. The role of blood clot in guided bone regeneration: Biological considerations and clinical applications with titanium foil. Materials 2021, 14, 6642. [Google Scholar] [CrossRef]
- Lu, H.; Zhou, Y.; Ma, Y.; Xiao, L.; Ji, W.; Zhang, Y.; Wang, X. Current application of beta-tricalcium phosphate in bone repair and its mechanism to regulate osteogenesis. Front. Mater. 2021, 8, 698915. [Google Scholar] [CrossRef]
- Wang, H.L.; Boyapati, L. “PASS” principles for predictable bone regeneration. Implant Dent. 2006, 15, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, L.; Terheyden, H. Preoperative assessment and treatment plan. In ITI Treatment Guide: Ridge Augmentation Procedures in Implant Patients: A Staged Approach; Chen, S., Buser, D., Wismeijer, D., Eds.; Quintessence Publishing: New Malden, UK, 2013; Volume 7, pp. 48–49. [Google Scholar]
- Danesh-Sani, S.A.; Tarnow, D.; Yip, J.K.; Mojaver, R. The influence of cortical bone perforation on guided bone regeneration in humans. Int. J. Oral Maxillofac. Surg. 2017, 46, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef]
- Iglesias-Velázquez, Ó.; Serrano Zamora, R.; López-Pintor, R.M.; Tresguerres, F.G.F.; Leco Berrocal, I.; Meniz García, C.; Fernández Tresguerres, I.; Torres García-Denche, J. Periosteal Pocket Flap technique for lateral ridge augmentation: A comparative pilot study versus guided bone regeneration. J. Clin. Exp. Dent. 2023, 15, e357–e365. [Google Scholar] [CrossRef] [PubMed]
- Urban, I.A.; Montero, E.; Amerio, E.; Palombo, D.; Monje, A. Techniques on vertical ridge augmentation: Indications and effectiveness. Periodontol. 2000 2023, 93, 153–182. [Google Scholar] [CrossRef]
- Lim, G.; Lin, G.H.; Monje, A.; Chan, H.L.; Wang, H.L. Wound Healing Complications Following Guided Bone Regeneration for Ridge Augmentation: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implants 2018, 33, 41–50. [Google Scholar] [CrossRef]
- Dickinson, D.P.; Coleman, B.G.; Batrice, N.; Lee, J.; Koli, K.; Pennington, C.; Susin, C.; Wikesjö, U.M. Events of wound healing/regeneration in the canine supraalveolar periodontal defect model. J. Clin. Periodontol. 2013, 40, 527–541. [Google Scholar] [CrossRef]
- Urban, I.A.; Monje, A.; Wang, H.L. Guided bone regeneration in staged vertical and horizontal bone augmentation procedures: A retrospective study of 250 patients. Int. J. Oral Maxillofac. Implants 2013, 28, 131–140. [Google Scholar]
- Khoury, F.; Hanser, T. Mandibular bone block grafts: Diagnosis, instrumentation, harvesting techniques and surgical procedures. Int. J. Periodontics Restor. Dent. 2007, 27, 329–337. [Google Scholar]
- García-Caballero, L.; Gándara, M.; Cepeda-Emiliani, A.; Gallego, R.; Gude, F.; Suárez-Quintanilla, J.; Ramos-Barbosa, I.; Blanco-Carrión, J. Histological and histomorphometric study of human palatal mucosa: Implications for connective tissue graft harvesting. J. Clin. Periodontol. 2023, 50, 784–795. [Google Scholar] [CrossRef]
- Obreja, K.; Ramanauskaite, A.; Begic, A.; Galarraga-Vinueza, M.E.; Parvini, P.; Schwarz, F. The influence of soft-tissue volume grafting on the maintenance of peri-implant tissue health and stability. Int. J. Implant. Dent. 2021, 7, 15. [Google Scholar] [CrossRef]
- Beca, J.M.; Chan, K.K.W.; Naimark, D.M.J.; Pechlivanoglou, P. Impact of limited sample size and follow-up on single event survival extrapolation for health technology assessment: A simulation study. BMC Med. Res. Methodol. 2021, 21, 282. [Google Scholar] [CrossRef]
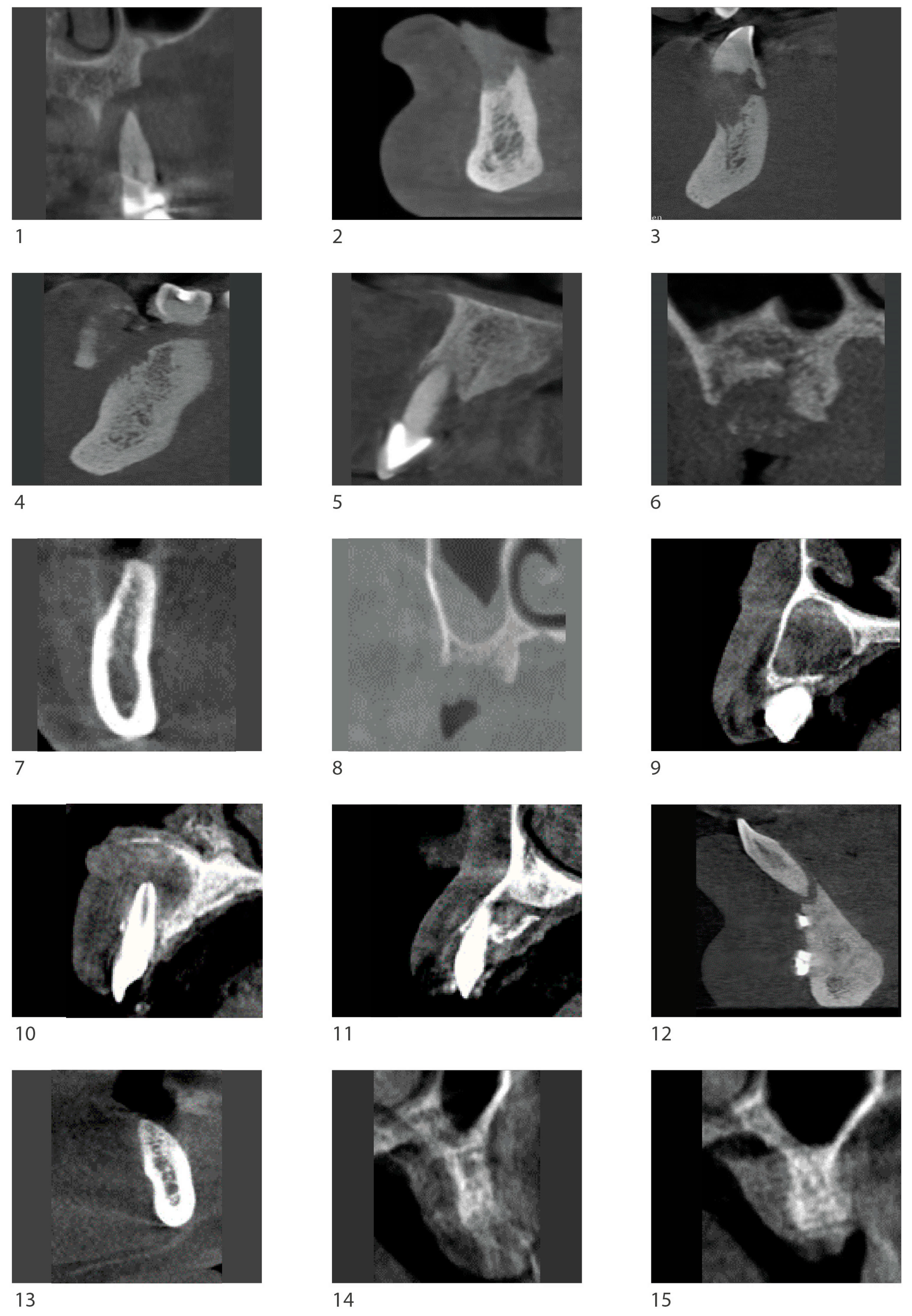
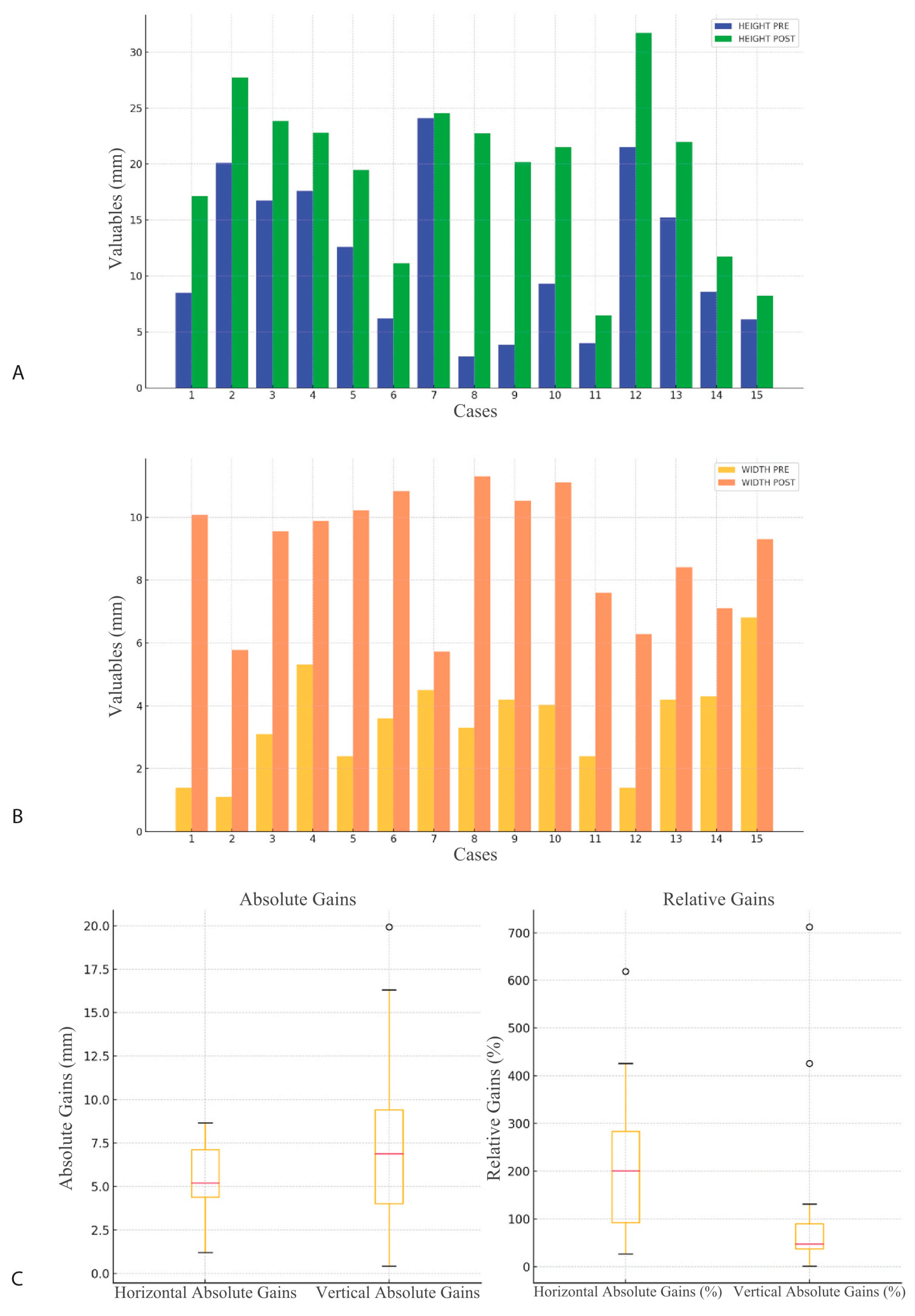
| Observer | Measurement | Pre (mm) | Post (mm) | p-Value |
|---|---|---|---|---|
| Observer 1 | Width | 3.47 (1.57) | 8.91 (1.95) | 0.0000 |
| Observer 1 | Height | 11.81 (6.97) | 19.41 (7.21) | 0.0001 |
| Observer 2 | Width | 3.47 (1.57) | 8.84 (1.98) | 0.0000 |
| Observer 2 | Height | 11.77 (6.86) | 19.34 (7.13) | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leiva-Gea, L.; Sánchez-Palomino, P.; Lendínez-Jurado, A.; Corte-Torres, M.D.; Leiva-Gea, I.; Leiva-Gea, A. Effectiveness of Titanium Occlusive Barriers in Guided Bone Regeneration: A Prospective Analysis of Vertical and Horizontal Bone Augmentation. Biomimetics 2025, 10, 165. https://doi.org/10.3390/biomimetics10030165
Leiva-Gea L, Sánchez-Palomino P, Lendínez-Jurado A, Corte-Torres MD, Leiva-Gea I, Leiva-Gea A. Effectiveness of Titanium Occlusive Barriers in Guided Bone Regeneration: A Prospective Analysis of Vertical and Horizontal Bone Augmentation. Biomimetics. 2025; 10(3):165. https://doi.org/10.3390/biomimetics10030165
Chicago/Turabian StyleLeiva-Gea, Luis, Paulino Sánchez-Palomino, Alfonso Lendínez-Jurado, María Daniela Corte-Torres, Isabel Leiva-Gea, and Antonio Leiva-Gea. 2025. "Effectiveness of Titanium Occlusive Barriers in Guided Bone Regeneration: A Prospective Analysis of Vertical and Horizontal Bone Augmentation" Biomimetics 10, no. 3: 165. https://doi.org/10.3390/biomimetics10030165
APA StyleLeiva-Gea, L., Sánchez-Palomino, P., Lendínez-Jurado, A., Corte-Torres, M. D., Leiva-Gea, I., & Leiva-Gea, A. (2025). Effectiveness of Titanium Occlusive Barriers in Guided Bone Regeneration: A Prospective Analysis of Vertical and Horizontal Bone Augmentation. Biomimetics, 10(3), 165. https://doi.org/10.3390/biomimetics10030165








