Biomimetic Phantoms in X-Ray-Based Radiotherapy Research: A Narrative Review
Abstract
1. Introduction
2. Virtual Phantoms in Radiotherapy Research
3. Physical Phantoms and Mimicry
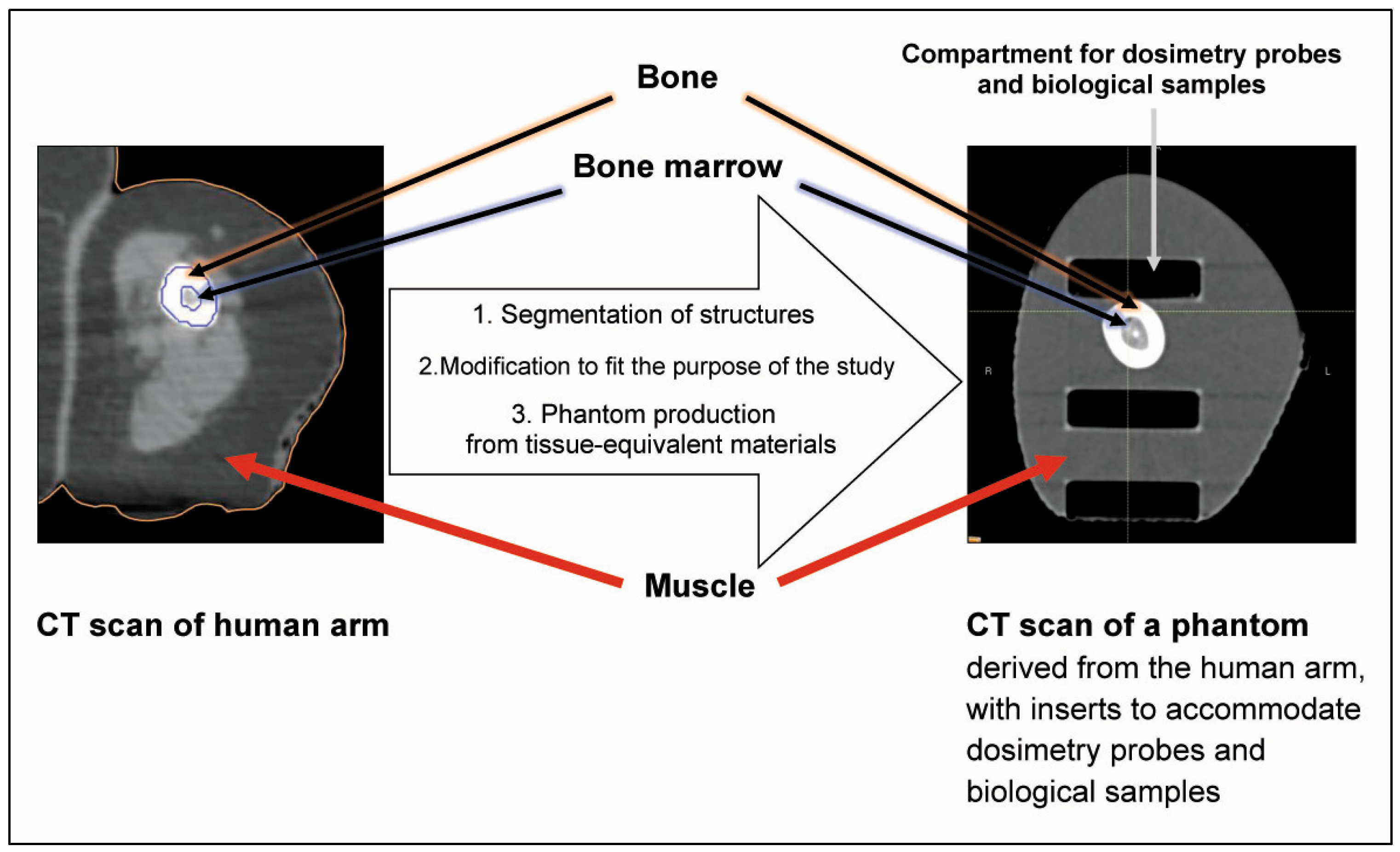
3.1. Anatomic Mimicry
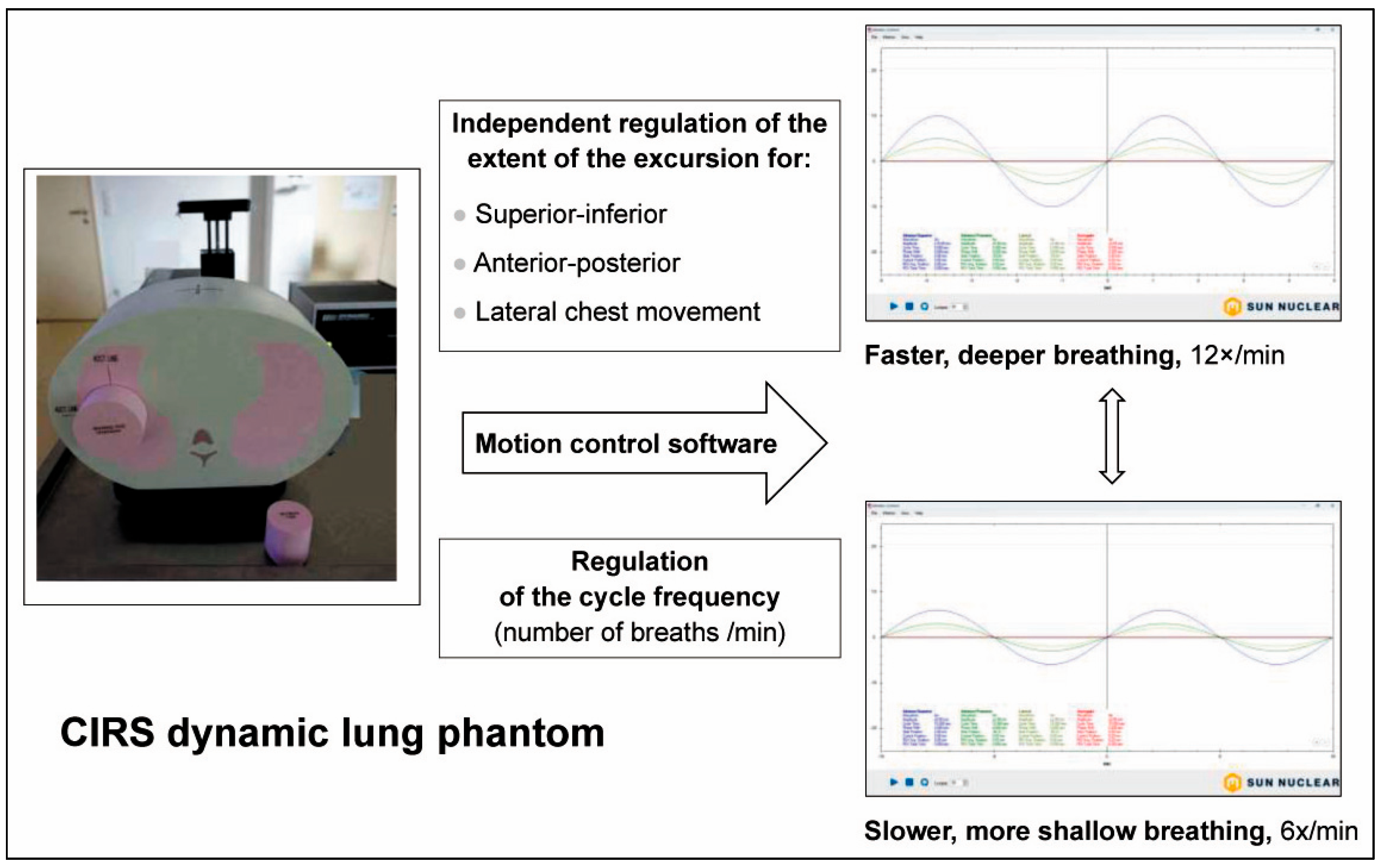
3.2. Biomechanical Mimicry
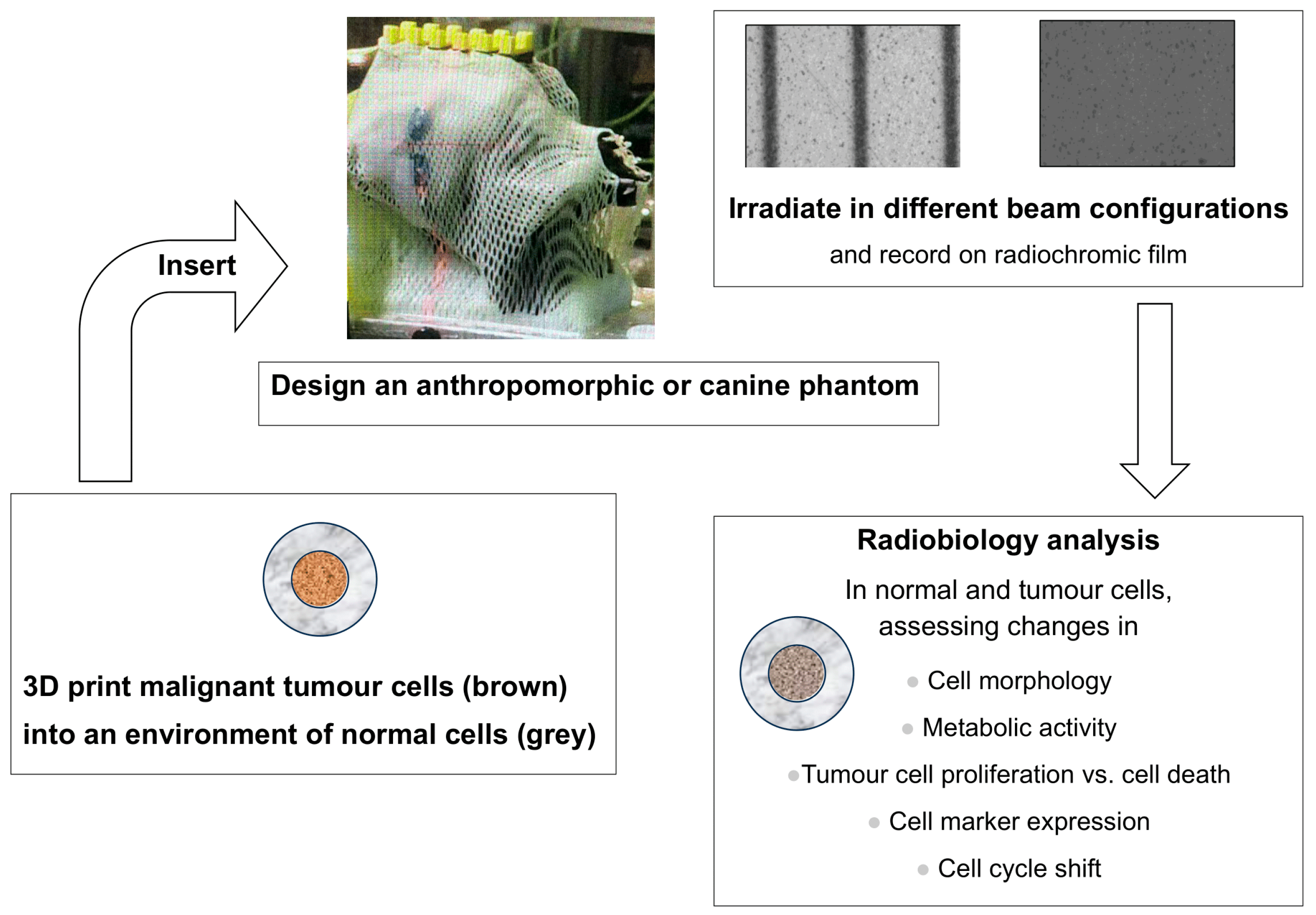
3.3. Simulating the Tumour Microenvironment
4. Which Phantom Serves Best?
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAPM | American Association of Physicists in Medicine |
| ABS | Acrylonitrile butadiene styrene |
| CT | Computed tomography |
| HU | Hounsfield units |
| ICRU | International Commission on Radiation Units and Measurements |
| IGRT | Image-guided radiotherapy |
| MRI | Magnetic resonance imaging |
| MRT | Microbeam radiotherapy |
| PET-CT | Positron emission tomography, combined with a CT scan |
| PVA | Polyvinyl alcohol |
| QA | Quality assurance |
| SFRT | Spatially fractionated radiotherapy |
| TME | Tumour microenvironment |
| TPS | Treatment planning system |
References
- Smith, K.; Blasi, O.; Casey, D.; Chan, M.; Dieterich, S.; Dresser, S.; Kennedy, C.; Kielar, K.; Lowenstein, J.; Pawlicki, T.; et al. AAPM Task Group Report 332: Verification of Vendor-Provided Data, Tools, and Test Procedures in Radiotherapy. Med. Phys. 2025, 52, 3509–3527. [Google Scholar] [CrossRef]
- Brock, K.K.; Mutic, S.; McNutt, T.R.; Li, H.; Kessler, M.L. Use of image registration and fusion algorithms and techniques in radiotherapy: Report of the AAPM Radiation Therapy Committee Task Group No. 132. Med. Phys. 2017, 44, e43–e76. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Technical Specifications of Radiotherapy Equipment for Cancer Treatment; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-001998-0. [Google Scholar]
- Kawrakow, I.; Fippel, M.; Friedrich, K. 3D electron dose calculation using a voxel based Monte Carlo algorithm (VMC). Med. Phys. 1996, 23, 445–457. [Google Scholar] [CrossRef]
- Evans, J.F.; Blue, T.E.; Gupta, N. Absorbed dose estimates to structures of the brain and head using a high-resolution voxel-based head phantom. Med. Phys. 2001, 28, 780–786. [Google Scholar] [CrossRef]
- Fanti, V.; Marzeddu, R.; Massazza, G.; Randaccio, P.; Brunetti, A.; Golosio, B. A simulator for X-ray images. Radiat. Prot. Dosim. 2005, 114, 350–354. [Google Scholar] [CrossRef]
- Ferrauche, G.; Tramontin, G.; Massera, R.T.; Tomal, A. Impact of fibroglandular tissue distribution and breast shape in voxelized breast models for dosimetry in mammography. Phys. Med. Biol. 2023, 68, 074003. [Google Scholar] [CrossRef]
- Cravo Sá, A.; Barateiro, A.; Bednarz, B.; Borges, C.; Pereira, J.; Baptista, M.; Pereira, M.; Zarza-Moreno, M.; Almeida, P.; Vaz, P.; et al. Assessment of out-of-field doses in radiotherapy treatments of paediatric patients using Monte Carlo methods and measurements. Phys. Med. 2020, 71, 53–61. [Google Scholar] [CrossRef]
- Baptista, M.; Di Maria, S.; Vieira, S.; Santos, J.; Pereira, J.; Pereira, M.; Vaz, P. Dosimetric assessment of the exposure of radiotherapy patients due to cone-beam CT procedures. Radiat. Environ. Biophys. 2019, 58, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Snyder, W.S.; Fisher, H.L., Jr.; Ford, M.R.; Warner, G.G. Estimates of absorbed fractions for monoenergetic photon sources uniformly distributed in various organs of a heterogeneous phantom. J. Nucl. Med. 1969, 10 (Suppl. S3), 7–52. [Google Scholar] [PubMed]
- Han, M.C.; Kim, J.; Hong, C.S.; Chang, K.H.; Han, S.C.; Park, K.; Kim, D.W.; Kim, H.; Chang, J.S.; Kim, J.; et al. Performance evaluation of deformable image registration algorithms using computed tomography of multiple lung metastases. Technol. Cancer Res. Treat. 2022, 21, 15330338221078464. [Google Scholar] [CrossRef]
- Al-Zein, A.; Naim, R.H.; Jalbout, W.; Shahine, B.H. Performance evaluation and quantitative comparison of two 4DCT imaging respiratory systems using deformable image registration. J. Appl. Clin. Med. Phys. 2025, 26, e70279. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Xiangyu, E.; Lv, M.; Zeng, S.; Feng, Y.; Shen, W.; Guan, W.; Zhang, Y.; Zhao, R.; Yu, J. Deep learning-based synthetic CT for dosimetric monitoring of combined conventional radiotherapy and lattice boost in large lung tumors. Radiat. Oncol. 2025, 20, 12. [Google Scholar] [CrossRef]
- Chamunyonga, C.; Burbery, J.; Caldwell, P.; Rutledge, P.; Fielding, A.; Crowe, S. Utilising the Virtual Environment for Radiotherapy Training System to Support Undergraduate Teaching of IMRT, VMAT, DCAT Treatment Planning, and QA Concepts. J. Med. Imaging Radiat. Sci. 2018, 49, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Hindley, N.; Keall, P.J. An open-source deep learning framework for respiratory motion monitoring and volumetric imaging during radiation therapy. Med. Phys. 2025, 52, e18015. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, Z.; Wu, H.; Li, C.; Liu, H.; Zhang, Y. Deep learning-based synthetization of real-time in-treatment 4D images using surface motion and pretreatment images: A proof-of-concept study. Med. Phys. 2022, 49, 7016–7024. [Google Scholar] [CrossRef]
- Bolch, W.; Lee, C.; Wayson, M.; Johnson, P. Hybrid computational phantoms for medical dose reconstruction. Radiat. Environ. Biophys. 2010, 49, 155–168. [Google Scholar] [CrossRef]
- Ahmad, R.; Cantwell, J.; Borrelli, C.; Lim, P.; D’Souza, D.; Gaze, M.N.; Moinuddin, S.; Gains, J.; Veiga, C. Development of age-specific population-based paediatric computational phantoms for image-based data mining and other radiotherapy applications. Biomed. Phys. Eng. Express 2024, 11, 015001. [Google Scholar] [CrossRef]
- Lee, C.; Lee, C.; Park, S. –H.; Lee, J.–K. Development of the two Korean adult tomographic computational phantoms for organ dosimetry. Med. Phys. 2006, 33, 380–390. [Google Scholar] [CrossRef]
- Chu, P.W.; Kofler, C.; Mahendra, M.; Wang, Y.; Chu, C.A.; Stewart, C.; Delman, B.N.; Haas, B.; Lee, C.; Bolch, W.E.; et al. Dose length product to effective dose coefficients in children. Pediatr. Radiol. 2023, 53, 1659–1668. [Google Scholar] [CrossRef]
- Alem-Bezoubiri, A.; Bezoubiri, F.; Speiser, M.; Suleiman, S.A.; Donya, H.; Chami, A.C. Monte Carlo study of organ doses and related secondary cancer risk estimations for patients undergoing prostate radiotherapy: Algerian population-based study. Appl. Radiat. Isot. 2025, 216, 111595. [Google Scholar] [CrossRef]
- Owens, C.A.; Rigaud, B.; Ludmir, E.B.; Gupta, A.C.; Shrestha, S.; Paulino, A.C.; Smith, S.A.; Peterson, C.B.; Kry, S.F.; Lee, C.; et al. Development and validation of a population-based anatomical colorectal model for radiation dosimetry in late effects studies of survivors of childhood cancer. Radiother. Oncol. 2022, 176, 118–126. [Google Scholar] [CrossRef]
- Somerwil, P.C.; Nout, R.A.; Mens, J.M.; Kolkman-Deurloo, I.K.; van Beekhuizen, H.J.; Dankelman, J.; van de Berg, N.J. An anthropomorphic deformable phantom of the vaginal wall and cavity. Biomed. Phys. Eng. Express 2021, 7, 055019. [Google Scholar] [CrossRef]
- Jiang, S.; Yang, S.; Yang, Z.; Zhang, D.; Luan, Y.; Zhou, Z. A Personalized Tumor kV-IORT Navigation System Based on Hybrid Twin. J. Appl. Clin. Med. Phys. 2025, 26, e70243. [Google Scholar] [CrossRef]
- ICRU Report 64: Dosimetry of High-Energy Photon Beams based on Standards of Absorbed Dose to Water. 2001. Available online: https://journals.sagepub.com/toc/crua/1/1 (accessed on 24 September 2025).
- Shariff, M.; Grigo, J.; Masitho, S.; Brandt, T.; Weiß, A.; Lambrecht, U.; Stillkrieg, W.; Lotter, M.; Putz, F.; Fietkau, R.; et al. End to end testing for stereotactic radiotherapy including the development of a multimodality phantom. Z. Med. Phys. 2024, 34, 477–484. [Google Scholar] [CrossRef]
- Kodama, S.; Iijima, K.; Okamoto, H.; Nishioka, S.; Sakasai, T.; Igaki, H.; Itami, J. Performance of a newly designed end to end phantom compatible with magnetic resonance guided radiotherapy systems. Med. Phys. 2021, 48, 7541–7551. [Google Scholar] [CrossRef]
- Singhrao, K.; Fu, J.; Wu, H.H.; Hu, P.; Kishan, A.U.; Chin, R.K.; Lewis, J.H. A novel anthropomorphic multimodality phantom for MRI-based radiotherapy quality assurance testing. Med. Phys. 2020, 47, 1443–1451. [Google Scholar] [CrossRef]
- Steinmann, A.; Alvarez, P.; Lee, H.; Court, L.; Stafford, R.; Sawakuchi, G.; Wen, Z.; Fuller, C.D.; Followill, D. MRIgRT head and neck anthropomorphic QA phantom: Design, development, reproducibility, and feasibility study. Med. Phys. 2020, 47, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Dowling, J.; Pichler, P.; Menk, F.; Rivest-Henault, D.; Lambert, J.; Parker, J.; Arm, J.; Best, L.; Martin, J.; et al. MRI simulation: End-to-end testing for prostate radiation therapy using geometric pelvic MRI phantoms. Phys. Med. Biol. 2015, 60, 3097–3109. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Lakotsenina, E.; Wegner, M.; Hehne, T.; Krause, D.; Hakimeh, D.; Wu, D.; Schültke, E.; Hausmann, F.; Kurreck, J.; et al. Three-Dimensional-Bioprinted Non-Small Cell Lung Cancer Models in a Mouse Phantom for Radiotherapy Research. Int. J. Mol. Sci. 2024, 25, 10268. [Google Scholar] [CrossRef]
- Mei, Y.; Wu, D.; Berg, J.; Tolksdorf, B.; Roehrs, V.; Kurreck, A.; Hiller, T.; Kurreck, J. Generation of a perfusable 3D lung cancer model by digital light processing. Int. J. Mol. Sci. 2023, 24, 6071. [Google Scholar] [CrossRef]
- The Alderson Radiation Therapy Phantom—Radiology Support Devices Inc. Available online: https://rsdphantoms.com/product/the-alderson-radiation-therapy-phantom/ (accessed on 24 April 2025).
- Tillery, H.; Moore, M.; Gallagher, K.J.; Taddei, P.J.; Leuro, E.; Argento, D.; Moffitt, G.; Kranz, M.; Carey, M.; Heymsfield, S.B.; et al. Personalized 3D-printed anthropomorphic whole-body phantom irradiated by protons, photons, and neutrons. Biomed. Phys. Eng. Express. 2022, 8, 027004. [Google Scholar] [CrossRef]
- Tanabe, Y.; Iseri, T.; Onizuka, R.; Ishida, T.; Eto, H.; Nakaichi, M. Improving animal-specific radiotherapy quality assurance for kilovoltage X-ray radiotherapy using a 3D printed dog skull water phantom. Open Vet. J. 2023, 13, 427–432. [Google Scholar] [CrossRef]
- Bajrami, D.; Spano, F.; Wei, K.; Bonmarin, M.; Rossi, R.M. Human skin models in biophotonics: Materials, methods, and applications. Adv. Healthc. Mater. 2025, 14, e2501894. [Google Scholar] [CrossRef]
- Pogue, B.W.; Patterson, M.S. Review of tissue simulating phantoms for optical spectroscopy, imaging and dosimetry. Biomed. Opt. 2006, 11, 041102. [Google Scholar] [CrossRef]
- Bustillo, J.P.O.; Mata, J.L.; Posadas, J.R.D.; Inocencio, E.T.; Rosenfeld, A.B.; Lerch, M.L.F. Characterization evaluation methods of fused deposition modeling and stereolithography additive manufacturing for clinical linear accelerator photon and electron radiotherapy applications. Phys. Med. 2025, 130, 104904. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.M.M.; Buschmann, M.; Breyer, L.; Kuntner, C.; Homolka, P. Tailoring the mass density of 3D printing materials for accurate X-ray imaging simulation by controlled underfilling for radiographic phantoms. Polymers 2024, 16, 1116. [Google Scholar] [CrossRef] [PubMed]
- McGarry, C.K.; Grattan, L.J.; Ivory, A.M.; Leek, F.; Liney, G.P.; Liu, Y.; Miloro, P.; Rai, R.; Robinson, A.P.; Shih, A.J.; et al. Tissue mimicking materials for imaging and therapy phantoms: A review. Phys. Med. Biol. 2020, 65, 23TR01. [Google Scholar] [CrossRef]
- Tino, R.; Yeo, A.; Leary, M.; Brandt, M.; Kron, T. A systematic review on 3D-printed imaging and dosimetry phantoms in radiation therapy. Technol. Cancer Res. Treat. 2019, 18, 1533033819870208. [Google Scholar] [CrossRef]
- Kamomae, T.; Shimizu, H.; Nakaya, T.; Okudaira, K.; Aoyama, T.; Oguchi, H.; Komori, M.; Kawamura, M.; Ohtakara, K.; Monzen, H.; et al. Three-dimensional printer-generated patient-specific phantom for artificial in vivo dosimetry in radiotherapy quality assurance. Phys. Med. 2017, 44, 205–211. [Google Scholar] [CrossRef]
- Frerker, B.; Engels, E.; Paino, J.; Rover, V.; Bustillo, J.P.; Wegner, M.; Cameron, M.; Fiedler, S.; Häusermann, D.; Hildebrandt, G.; et al. Fast and fractionated: Correlation of dose attenuation and the response of human cancer cells in a new anthropomorphic brain phantom. Biomimetics 2025, 10, 440. [Google Scholar] [CrossRef]
- Breslin, T.; Paino, J.; Wegner, M.; Engels, E.; Fiedler, S.; Forrester, H.; Rennau, H.; Bustillo, J.; Cameron, M.; Häusermann, D.; et al. A novel anthropomorphic phantom composed of tissue-equivalent materials for use in experimental radiotherapy: Design, dosimetry and biological pilot study. Biomimetics 2023, 8, 230. [Google Scholar] [CrossRef]
- Wegner, M.; Frenzel, T.; Krause, D.; Gargioni, E. Development and characterization of modular mouse phantoms for end-to-end testing and training in radiobiology experiments. Phys. Med. Biol. 2023, 68. [Google Scholar] [CrossRef]
- Tino, R.B.; Yeo, A.U.; Brandt, M.; Leary, M.; Kron, T. A customizable anthropomorphic phantom for dosimetric verification of 3D-printed lung, tissue, and bone density materials. Med. Phys. 2022, 49, 52–69. [Google Scholar] [CrossRef] [PubMed]
- ICRU Tissues. Available online: https://www.qrm.de/en/products/icru-tissues (accessed on 24 September 2025).
- Ma, X.; Buschmann, M.; Unger, E.; Homolka, P. Classification of X-Ray Attenuation Properties of Additive Manufacturing and 3D Printing Materials Using Computed Tomography From 70 to 140 kVp. Front. Bioeng. Biotechnol. 2021, 9, 763960. [Google Scholar] [CrossRef] [PubMed]
- Mei, K.; Pasyar, P.; Geagan, M.; Liu, L.P.; Shapira, N.; Gang, G.J.; Stayman, J.W.; Noël, P.B. Design and fabrication of 3D-printed patient-specific soft tissue and bone phantoms for CT imaging. Sci. Rep. 2023, 13, 1749. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ptwdosimetry.com/en/products/microdiamond (accessed on 24 September 2025).
- Petasecca, M.; Cullen, A.; Fuduli, I.; Espinoza, A.; Porumb, C.; Stanton, C.; Aldosari, A.H.; Brauer-Krisch, E.; Requardt, H.; Bravin, A.; et al. X-Tream: A novel dosimetry system for Synchrotron Microbeam Radiation Therapy. J. Instrum. 2012, 7, P07022. [Google Scholar] [CrossRef]
- Carlsson Tedgren, A.; Carlsson, G.A. Influence of phantom material and dimensions on experimental 192Ir dosimetry. Med. Phys. 2009, 36, 2228–2235. [Google Scholar] [CrossRef]
- Li, X.; Gou, J.; Santhanam, A.P.; Maiti, C.; Ilegbusi, O.J. Tissue mimicking hydrogel foam materials with mechanical and radiological properties equivalent to human lung. Sci. Rep. 2025, 15, 7471. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, S.; Feng, W. PVA hydrogel properties for biomedical application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1228–1233. [Google Scholar] [CrossRef]
- Malone, A.J.; Cournane, S.; Naydenova, I.G.; Fagan, A.J.; Browne, J.E. Polyvinyl alcohol cryogel based vessel mimicking material for modelling the progression of atherosclerosis. Phys. Med. 2020, 69, 1–8. [Google Scholar] [CrossRef]
- Vanina, A.S.; Lavrova, A.I.; Safonov, D.A.; Sychev, A.V.; Proskurkin, I.S.; Postnikov, E.B. Mimicking marker spread after disruption of the blood–brain barrier with a collagen-based hydrogel phantom. Biomimetics 2024, 9, 667. [Google Scholar] [CrossRef]
- Baker, A.J.A.; Galindo, E.J.; Angelos, J.D.; Salazar, D.K.; Sterritt, S.M.; Willis, A.M.; Tartis, M.S. Mechanical characterization data of polyacrylamide hydrogel formulations and 3D printed PLA for application in human head phantoms. Data Brief 2023, 48, 109114. [Google Scholar] [CrossRef]
- Sunnuclear. Dynamic Thorax Brochure. Available online: https://www.sunnuclear.com/uploads/documents/datasheets/Dynamic-Thorax-Brochure_103024.pdf (accessed on 24 September 2025).
- World Health Organization. Lung Cancer—Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/lung-cancer (accessed on 14 September 2025).
- Wheatley, M.; de Deene, Y. A novel anthropomorphic breathing phantom with a pneumatic MR-safe actuator for tissue deformation studies during MRI and radiotherapy. Phys. Medica 2022, 104, 43–55. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.; Shin, H.; Ji, S.; Park, S.; Kim, J.; Jang, H.; Kang, Y. Development of deformable moving lung phantom to simulate respiratory motion in radiotherapy. Med. Dosim. 2016, 41, 113–117. [Google Scholar] [CrossRef]
- Colvill, E.; Krieger, M.; Bosshard, P.; Steinacher, P.; Rohrer Schnidrig, B.A.; Parkel, T.; Stergiou, I.; Zhang, Y.; Peroni, M.; Safai, S.; et al. Anthropomorphic phantom for deformable lung and liver CT and MR imaging for radiotherapy. Phys. Med. Biol. 2020, 65, 07NT02. [Google Scholar] [CrossRef]
- Mawatari, S.; Oku, Y.; Toyota, M. Comparison of target phase positioning with respiratory motion between four-dimensional CT and four-dimensional cone beam CT: A phantom study. Nihon Hoshasen Gijutsu Gakkai Zasshi 2025, 81. [Google Scholar] [CrossRef]
- Sahin, S.; Ozen, S.K.; Ertan, F.; Sahiner, E. Design and manufacturing of a dynamically deformable liver phantom for radiotherapy. Appl. Radiat. Isot. 2025, 215, 111561. [Google Scholar] [CrossRef]
- Malin, E.J.; Ceritoglu, C.; Abadi, E.; Ratnanather, J.T.; Samei, E.; Segars, W.P. Library of realistic 4D digital beating heart models based on patient CT data. Med. Phys. 2025, 52, e17945. [Google Scholar] [CrossRef]
- Gregg, K.W.; Ruff, C.; Koenig, G.; Penev, K.I.; Shepard, A.; Kreissler, G.; Amatuzio, M.; Owens, C.; Nagpal, P.; Glide-Hurst, C.K. Development and first implementation of a novel multi-modality cardiac motion and dosimetry phantom for radiotherapy applications. Med. Phys. 2024, 51, 7479–7491. [Google Scholar] [CrossRef]
- Ali, I.G.; El Naqa, I. The Biophysics of Flash Radiotherapy: Tools for Measuring Tumor and Normal Tissues Microenvironment. Antioxidants 2025, 14, 899. [Google Scholar] [CrossRef]
- Leung, J.K.; Panchal, R.; Anbalagan, S.; Durie, E.P.; Mansfeld, J.; Somaiah, N. Modulating Redox Biology to Improve Radiation Responses. Cancer J. 2025, 31, e0778. [Google Scholar] [CrossRef]
- Wu, Q.; Ren, W.; Yu, Z.; Dong, E.; Zhang, S.; Xu, R.X. Microfabrication of polydimethylsiloxane phantoms to simulate tumor hypoxia and vascular anomaly. Biomed. Opt. 2015, 20, 121308. [Google Scholar] [CrossRef]
- Liu, L.; Wu, D.; Qian, Z.; Jiang, Y.; You, Y.; Wang, Y.; Zhang, F.; Ning, X.; Mei, J.; Iqbal, J.; et al. Empowering hypoxia to convert cold tumors into hot tumors for breast cancer immunotherapy. Cell Death Discov. 2025, 11, 381. [Google Scholar] [CrossRef]
- Pignatta, S.; Cortesi, M.; Arienti, C.; Zanoni, M.; Cocchi, C.; Sarnelli, A.; Arpa, D.; Piccinini, F.; Tesei, A. Effects of radiotherapy and short-term starvation combination on metastatic and non-tumor cell lines. DNA Repair 2020, 95, 102949. [Google Scholar] [CrossRef]
- McHugh, D.J.; Zhou, F.L.; Wimpenny, I.; Poologasundarampillai, G.; Naish, J.H.; Hubbard Cristinacce, P.L.; Parker, G.J.M. A biomimetic tumor tissue phantom for validating diffusion-weighted MRI measurements. Magn. Reson. Med. 2018, 80, 147–158. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, F.; Qian, Z.; Jiang, Y.; Wu, D.; Liu, L.; Ning, X.; Mei, J.; Chen, D.; Zhang, Y. Targeting collagen to optimize cancer immunotherapy. Exp. Hematol. Oncol. 2025, 14, 101. [Google Scholar] [CrossRef]
- Chavez, L.; Gao, S.; Pandey, V.; Yuan, N.; Ragab, S.; Li, J.; Hepburn, M.S.; Smith, P.; Edelheit, C.; Corr, D.T.; et al. Design and characterization of an optical phantom for mesoscopic multimodal fluorescence lifetime imaging and optical coherence elastography. Biomed. Opt. Express 2025, 16, 1006–1024. [Google Scholar] [CrossRef]
- Cho, G.Y.; Kim, S.; Jensen, J.H.; Storey, P.; Sodickson, D.K.; Sigmund, E.E. A versatile flow phantom for intravoxel incoherent motion MRI. Magn. Reson. Med. 2012, 67, 1710–1720. [Google Scholar] [CrossRef]
- Yu, H.B.; Han, B.J.; Hu, J.Q.; Luo, Y.; Liu, H.Y.; Zhang, X.Y.; Li, Y.; Liu, R.; Hua, B.J. Worldwide research on 3D printing for cancer: A dual-method analysis of bibliometrics and stratified focused thematic. Int. J. Surg. 2025. [Google Scholar] [CrossRef]
- Bustillo, J.P.O.; de Rover, V.; Engels, E.E.M.; Cayley, J.; Cameron, M.; Long, S.; Carolan, M.; Frerker, B.; Lerch, M.L.F.; Schültke, E. A canine head phantom for quality assurance in multiport microbeam radiation therapy: Animal phantom fabrication and dosimetry protocol. Biomed. Phys. Eng. Express 2025, 11, 055001. [Google Scholar] [CrossRef]
- Constantinou, C.; Attix, F.H.; Paliwal, B.R. A solid water phantom material for radiotherapy X-ray and gamma-ray beam calibrations. Med. Phys. 1982, 9, 436–441. [Google Scholar] [CrossRef]
- Ramaseshan, R.; Heydarian, M. Dosimetric evaluation of Plastic Water diagnostic therapy. J. Appl. Clin. Med. Phys. 2008, 9, 2761. [Google Scholar] [CrossRef]
- Gargett, M.A.; Briggs, A.R.; Booth, J.T. Water equivalence of a solid phantom material for radiation dosimetry applications. Phys. Imaging Radiat. Oncol. 2020, 14, 43–47. [Google Scholar] [CrossRef]
- Manchado de Sola, F.; Vilches, M.; Prezado, Y.; Lallena, A.M. Impact of cardiosynchronous brain pulsations on Monte Carlo calculated doses for synchrotron micro- and minibeam radiation therapy. Med. Phys. 2018, 45, 3379–3390. [Google Scholar] [CrossRef]
- Liu, H.H.; Koch, N.; Starkschall, G.; Jacobson, M.; Forster, K.; Liao, Z.; Komaki, R.; Stevens, C.W. Evaluation of internal lung motion for respiratory-gated radiotherapy using MRI: Part II-margin reduction of internal target volume. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 1473–1483. [Google Scholar] [CrossRef]
- Davidson, S.E.; Ibbott, G.S.; Prado, K.L.; Dong, L.; Liao, Z.; Followill, D.S. Accuracy of two heterogeneity dose calculation algorithms for IMRT in treatment plans designed using an anthropomorphic thorax phantom. Med. Phys. 2007, 34, 1850–1857. [Google Scholar] [CrossRef]
- Yadav, N.; Singh, M.; Mishra, S.P.; Ansari, S. Development of an Anthropomorphic Heterogeneous Female Pelvic Phantom and Its Comparison with a Homogeneous Phantom in Advance Radiation Therapy: Dosimetry Analysis. Med. Sci. 2023, 11, 59. [Google Scholar] [CrossRef]
- Fogliata, A.; Cozzi, L. Dose calculation algorithm accuracy for small fields in non-homogeneous media: The lung SBRT case. Phys. Med. 2017, 44, 157–162. [Google Scholar] [CrossRef]
- Monti di Sopra, F.; Keall, P.; Beckham, W. Comparing dose calculation algorithms for an orthovoltage beam in a bone phantom. Australas. Phys. Eng. Sci. Med. 1998, 21, 148–151. [Google Scholar]
- Hoffmann, L.; Linaa, M.B.; Møller, D.S. Independent secondary dose calculation for patient-specific quality assurance: Quantitative benefit of Monte-Carlo and custom beam modeling. J. Appl. Clin. Med. Phys. 2025, 26, e70265. [Google Scholar] [CrossRef]
- Mentzel, F.; Kröninger, K.; Lerch, M.; Nackenhorst, O.; Rosenfeld, A.; Tsoi, A.C.; Weingarten, J.; Hagenbuchner, M.; Guatelli, S. Small beams, fast predictions: A comparison of machine learning dose prediction models for proton minibeam therapy. Med. Phys. 2022, 49, 7791–7801. [Google Scholar] [CrossRef] [PubMed]
| Organic Tissue | Density [47] | Hounsfield Units [47] | Substitute | Density [48] | Hounsfield Units |
|---|---|---|---|---|---|
| Brain | 1.04 | Average 38 White matter 25, Grey matter 40 | ABS, 80–100% infill [48] or PLA or PLA calcium doped infill 50–60% [49] | matched by modifying the percentage of infill | |
| Bone, solid | 1.33–1.68 | 544–1092 | PLA stone PLA chalk PLA 90–100% infill [48] | 1.64 1.39 1.24 | 1063 537 [48] |
| Fat | 0.95 | −75 | ABS, 80–100% infill [48] or PLA or PLA calcium doped infill 50–60% [49] | 1.07 | 30 [48] |
| Soft tissue (muscle) | 1.05 | 43 | PLA or PLA calcium doped, infill 50–60% [49] | matched by modifying the percentage of infill | |
| Water | 1.0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schültke, E. Biomimetic Phantoms in X-Ray-Based Radiotherapy Research: A Narrative Review. Biomimetics 2025, 10, 794. https://doi.org/10.3390/biomimetics10120794
Schültke E. Biomimetic Phantoms in X-Ray-Based Radiotherapy Research: A Narrative Review. Biomimetics. 2025; 10(12):794. https://doi.org/10.3390/biomimetics10120794
Chicago/Turabian StyleSchültke, Elisabeth. 2025. "Biomimetic Phantoms in X-Ray-Based Radiotherapy Research: A Narrative Review" Biomimetics 10, no. 12: 794. https://doi.org/10.3390/biomimetics10120794
APA StyleSchültke, E. (2025). Biomimetic Phantoms in X-Ray-Based Radiotherapy Research: A Narrative Review. Biomimetics, 10(12), 794. https://doi.org/10.3390/biomimetics10120794






