Synergistic Membrane Disruption of E. coli Tethered Lipid Bilayers by Antimicrobial Lipid Mixtures
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
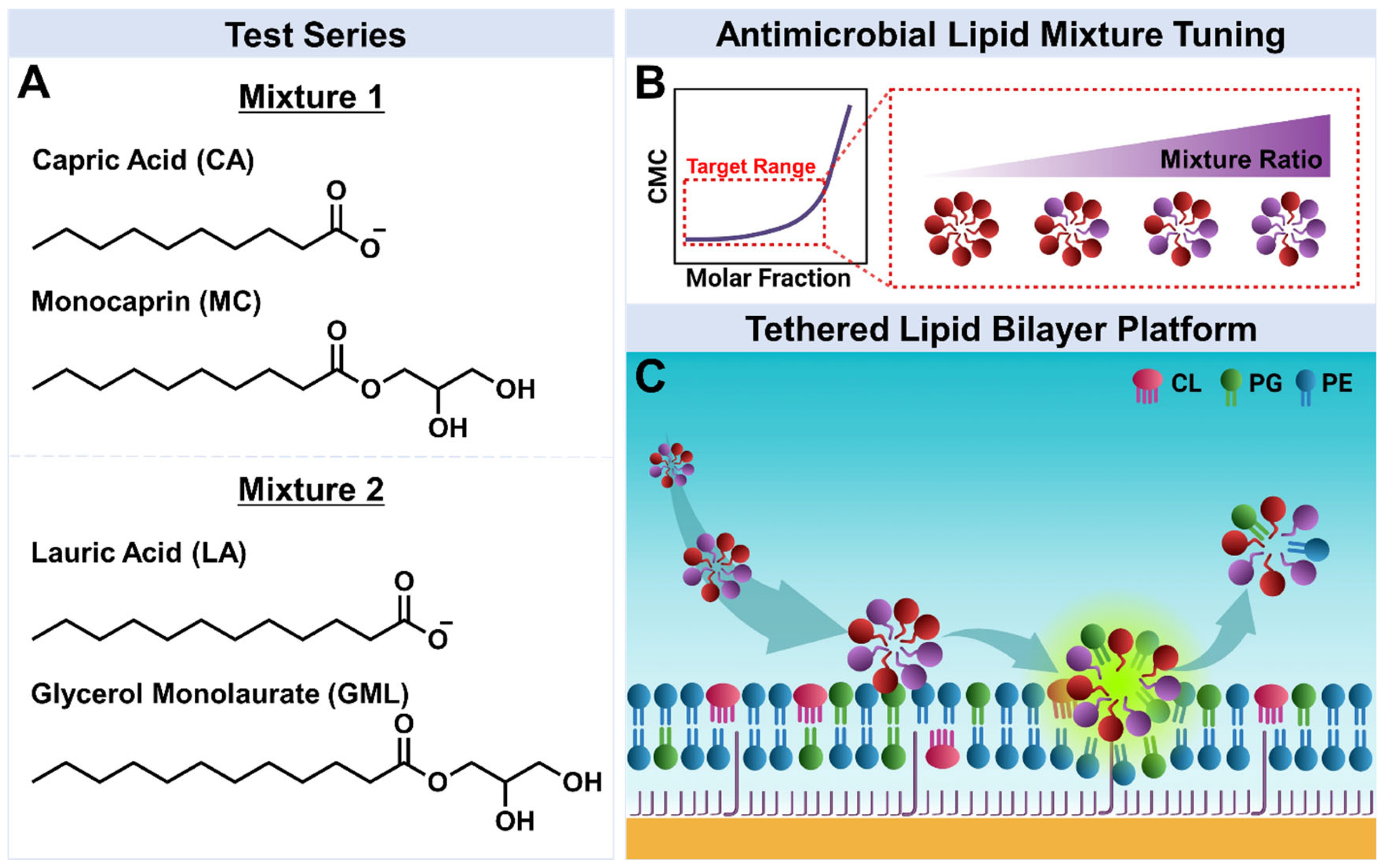
3.1. Experimental Strategy
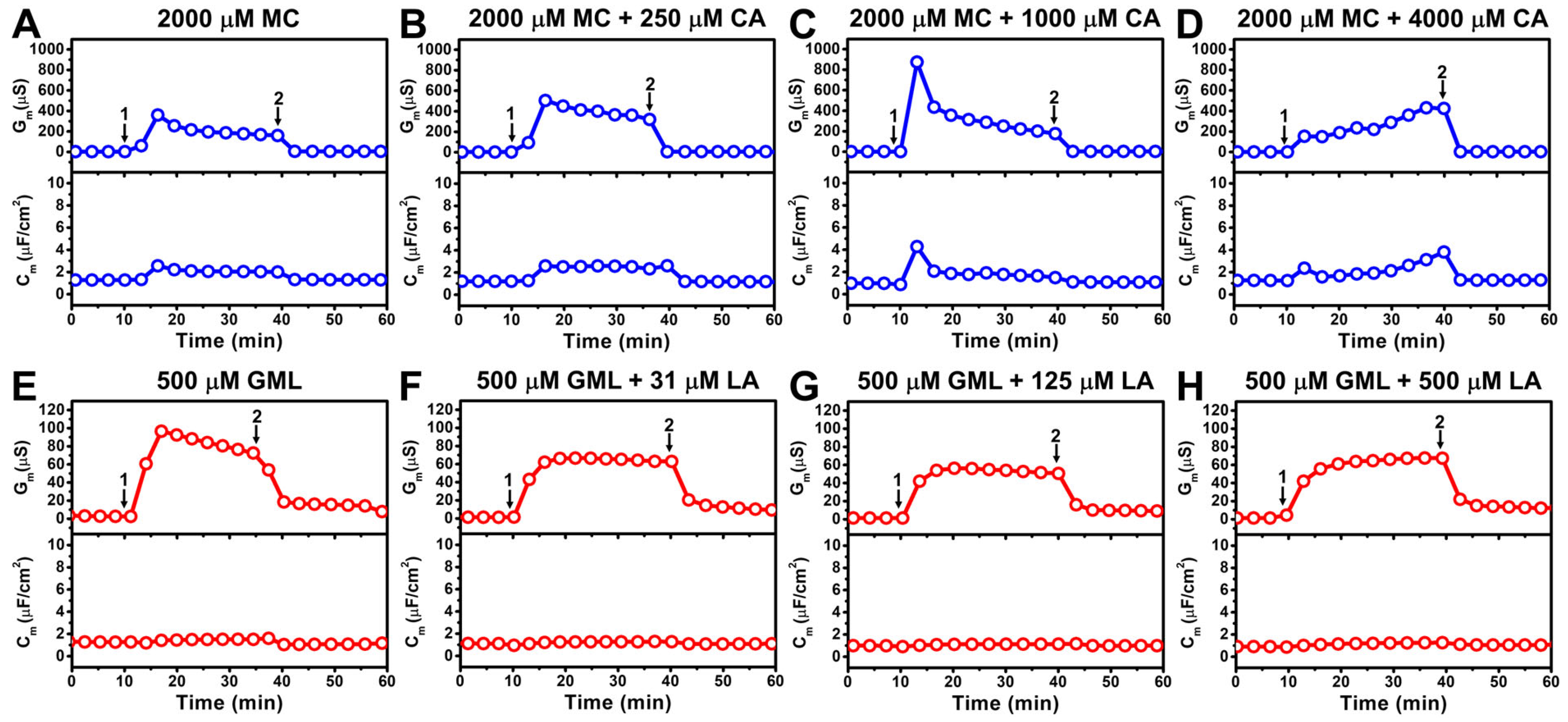
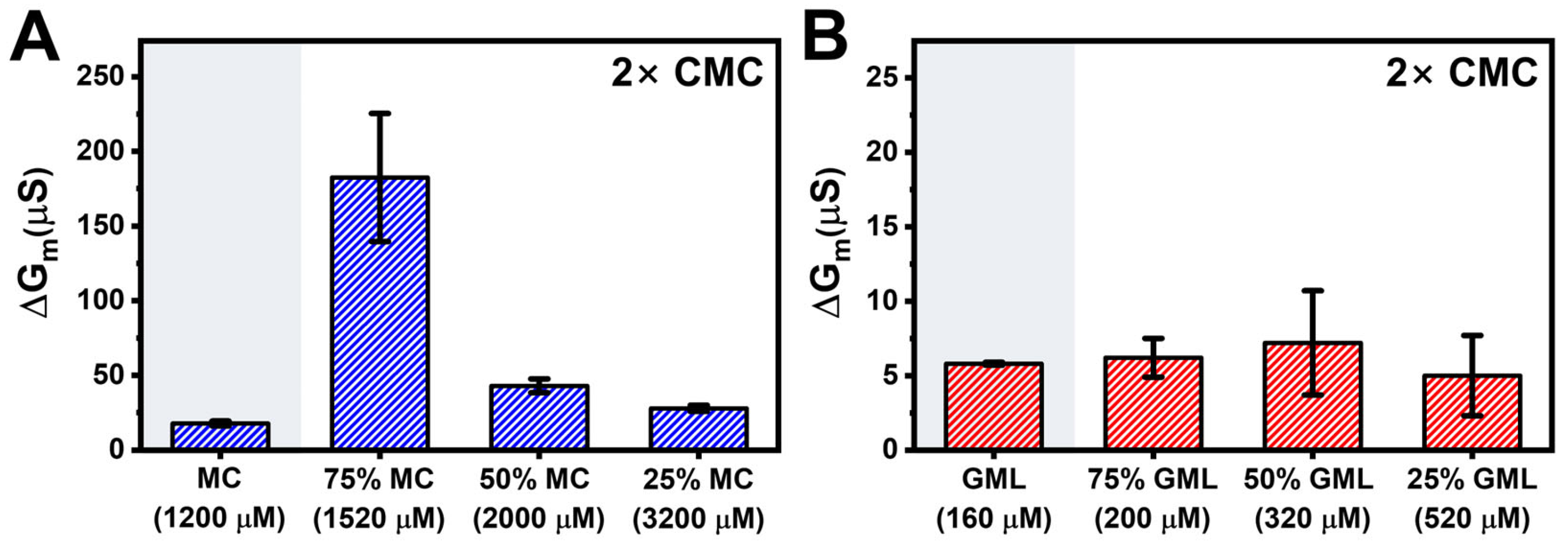
3.2. Antimicrobial Lipid Mixture Screening
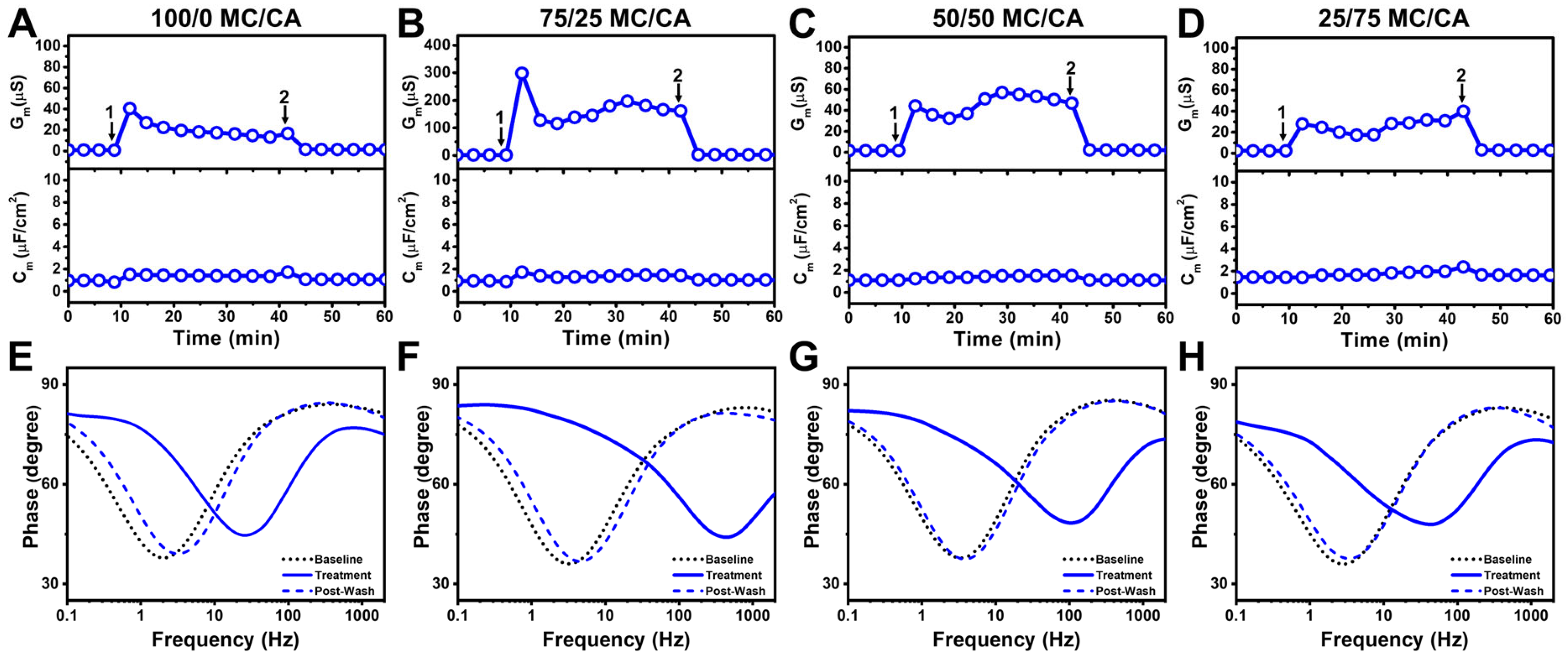
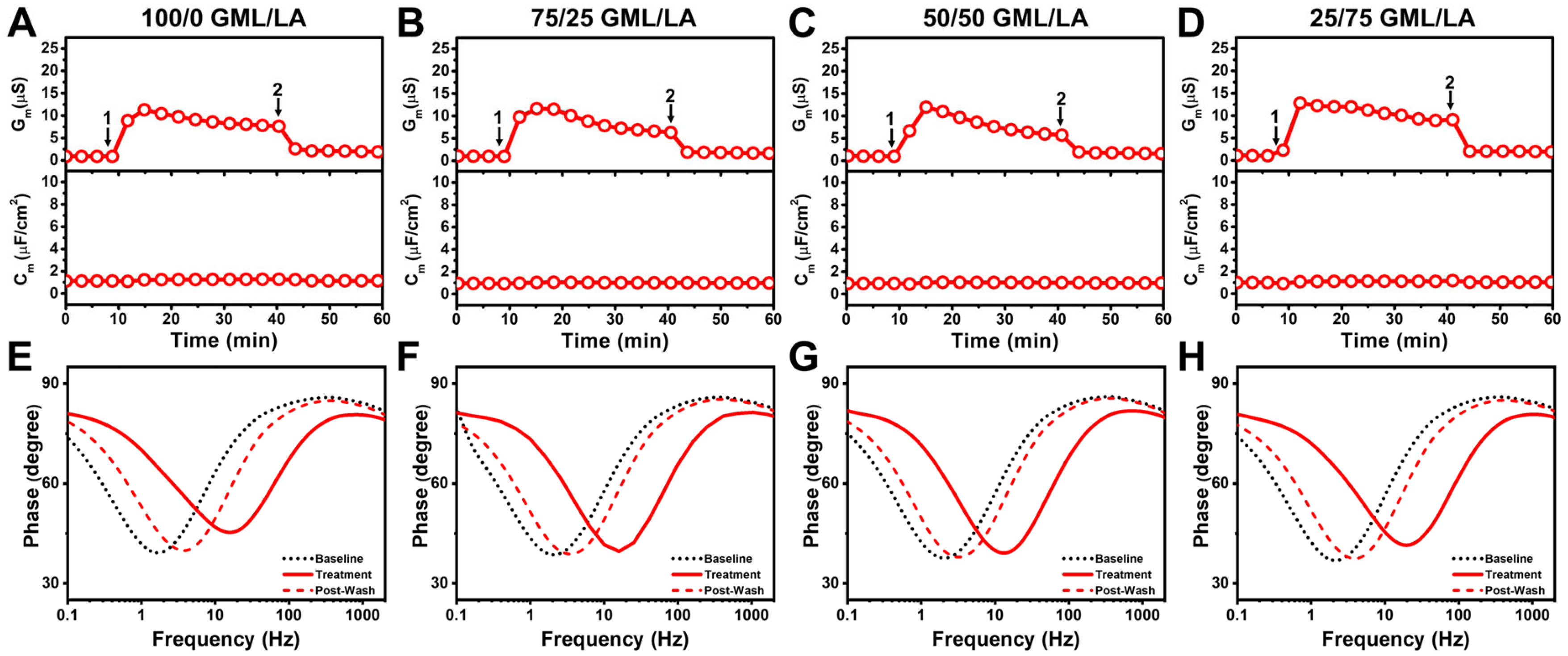
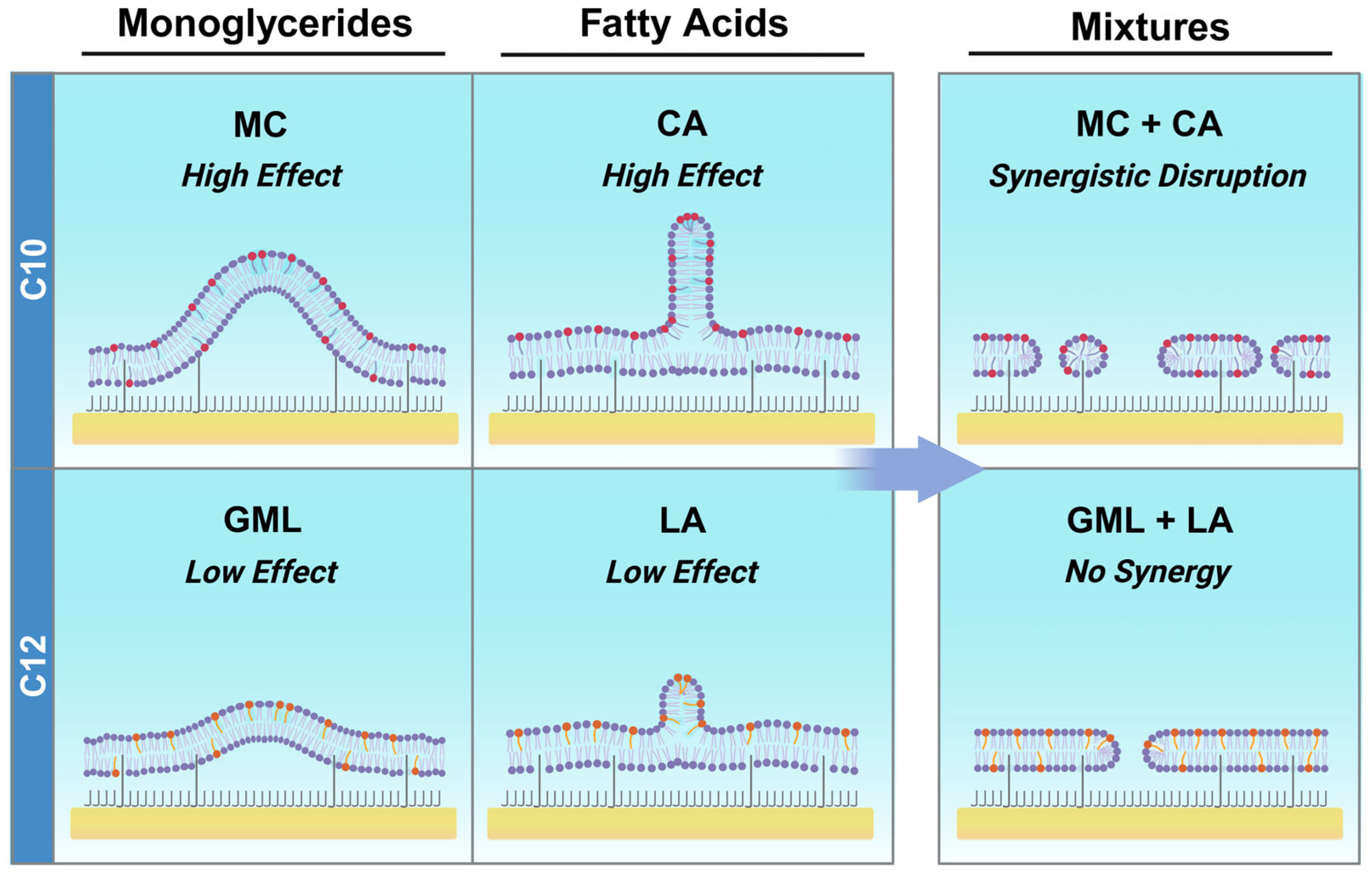
3.3. Mechanistic Comparison of Antimicrobial Lipid Mixtures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sackmann, E. Supported membranes: Scientific and practical applications. Science 1996, 271, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Sackmann, E. Polymer-supported membranes as models of the cell surface. Nature 2005, 437, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Castellana, E.T.; Cremer, P.S. Solid supported lipid bilayers: From biophysical studies to sensor design. Surf. Sci. Rep. 2006, 61, 429–444. [Google Scholar] [CrossRef]
- Richter, R.P.; Brisson, A.R. Following the formation of supported lipid bilayers on mica: A study combining AFM, QCM-D, and ellipsometry. Biophys. J. 2005, 88, 3422–3433. [Google Scholar] [CrossRef]
- Hardy, G.J.; Nayak, R.; Zauscher, S. Model cell membranes: Techniques to form complex biomimetic supported lipid bilayers via vesicle fusion. Curr. Opin. Colloid Interface Sci. 2013, 18, 448–458. [Google Scholar] [CrossRef]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; Del Valle, R.G.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial fatty acids: An update of possible mechanisms of action and implications in the development of the next-generation of antibacterial agents. Prog. Lipid Res. 2021, 82, 101093. [Google Scholar] [CrossRef]
- Arellano, H.; Nardello-Rataj, V.; Szunerits, S.; Boukherroub, R.; Fameau, A.-L. Saturated long chain fatty acids as possible natural alternative antibacterial agents: Opportunities and challenges. Adv. Colloid Interface Sci. 2023, 318, 102952. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A. Medium-chain fatty acids and monoglycerides: Nanoarchitectonics-based insights into molecular self-assembly, membrane interactions, and applications. Adv. Colloid Interface Sci. 2025, 340, 103465. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Park, S.; Mokrzecka, N.; Cho, N.-J. Characterizing the membrane-disruptive behavior of dodecylglycerol using supported lipid bilayers. Langmuir 2019, 35, 3568–3575. [Google Scholar] [CrossRef]
- Anacarso, I.; Quartieri, A.; De Leo, R.; Pulvirenti, A. Evaluation of the antimicrobial activity of a blend of monoglycerides against Escherichia coli and enterococci with multiple drug resistance. Arch. Microbiol. 2018, 200, 85–89. [Google Scholar] [CrossRef]
- Churchward, C.P.; Alany, R.G.; Snyder, L.A. Alternative antimicrobials: The properties of fatty acids and monoglycerides. Crit. Rev. Microbiol. 2018, 44, 561–570. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Kim, M.C.; Cho, N.-J. Spectrum of membrane morphological responses to antibacterial fatty acids and related surfactants. Langmuir 2015, 31, 10223–10232. [Google Scholar] [CrossRef]
- Yoon, B.K.; Park, H.; Zhdanov, V.P.; Jackman, J.A.; Cho, N.-J. Real-time nanoplasmonic sensing of three-dimensional morphological changes in a supported lipid bilayer and antimicrobial testing applications. Biosens. Bioelectron. 2021, 174, 112768. [Google Scholar] [CrossRef] [PubMed]
- Gahan, C.G.; Patel, S.J.; Chen, L.M.; Manson, D.E.; Ehmer, Z.J.; Blackwell, H.E.; Van Lehn, R.C.; Lynn, D.M. Bacterial quorum sensing signals promote large-scale remodeling of lipid membranes. Langmuir 2021, 37, 9120–9136. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Bernaldez, M.; Stamatis, S.D.; Rose, J.P.; Sun, R. Interaction between permeation enhancers and lipid bilayers. J. Phys. Chem. B 2024, 128, 1668–1679. [Google Scholar] [CrossRef] [PubMed]
- Valle-González, E.R.; Jackman, J.A.; Yoon, B.K.; Park, S.; Sut, T.N.; Cho, N.-J. Characterizing how acidic pH conditions affect the membrane-disruptive activities of lauric acid and glycerol monolaurate. Langmuir 2018, 34, 13745–13753. [Google Scholar] [CrossRef]
- Moon, S.; Yoon, B.K.; Jackman, J.A. Effect of membrane curvature nanoarchitectonics on membrane-disruptive interactions of antimicrobial lipids and surfactants. Langmuir 2022, 38, 4606–4616. [Google Scholar] [CrossRef]
- Yoon, B.K.; Park, S.; Ma, G.J.; Kolahdouzan, K.; Zhdanov, V.P.; Jackman, J.A.; Cho, N.-J. Competing interactions of fatty acids and monoglycerides trigger synergistic phospholipid membrane remodeling. J. Phys. Chem. Lett. 2020, 11, 4951–4957. [Google Scholar] [CrossRef]
- Moon, S.; Sut, T.N.; Yoon, B.K.; Jackman, J.A. Unraveling how antimicrobial lipid mixtures disrupt virus-mimicking lipid vesicles: A QCM-D study. Biomimetics 2024, 9, 67. [Google Scholar] [CrossRef]
- Tan, S.W.; Yoon, B.K.; Jackman, J.A. Membrane-disruptive effects of fatty acid and monoglyceride mitigants on E. coli bacteria-derived tethered lipid bilayers. Molecules 2024, 29, 237. [Google Scholar] [CrossRef]
- Lind, T.K.; Wacklin, H.; Schiller, J.; Moulin, M.; Haertlein, M.; Pomorski, T.G.; Cárdenas, M. Formation and characterization of supported lipid bilayers composed of hydrogenated and deuterated Escherichia coli lipids. PLoS ONE 2015, 10, e0144671. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.W.; Jeon, W.-Y.; Yoon, B.K.; Jackman, J.A. Mechanistic evaluation of antimicrobial lipid interactions with tethered lipid bilayers by electrochemical impedance spectroscopy. Sensors 2022, 22, 3712. [Google Scholar] [CrossRef] [PubMed]
- Cranfield, C.G.; Cornell, B.A.; Grage, S.L.; Duckworth, P.; Carne, S.; Ulrich, A.S.; Martinac, B. Transient potential gradients and impedance measures of tethered bilayer lipid membranes: Pore-forming peptide insertion and the effect of electroporation. Biophys. J. 2014, 106, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Cranfield, C.; Carne, S.; Martinac, B.; Cornell, B. The assembly and use of tethered bilayer lipid membranes (tBLMs). In Methods in Membrane Lipids; Owen, D.M., Ed.; Springer: New York, NY, USA, 2015; pp. 45–53. [Google Scholar]
- Alobeedallah, H.; Cornell, B.; Coster, H. The effect of benzyl alcohol on the dielectric structure of lipid bilayers. J. Membr. Biol. 2016, 249, 833–844. [Google Scholar] [CrossRef]
- Cranfield, C.G.; Henriques, S.T.; Martinac, B.; Duckworth, P.; Craik, D.J.; Cornell, B. Kalata B1 and kalata B2 have a surfactant-like activity in phosphatidylethanolomine-containing lipid membranes. Langmuir 2017, 33, 6630–6637. [Google Scholar] [CrossRef]
- Medhashree, H.; Shetty, A.N. Electrochemical investigation on the effects of sulfate ion concentration, temperature and medium pH on the corrosion behavior of Mg–Al–Zn–Mn alloy in aqueous ethylene glycol. J. Magnes. Alloys 2017, 5, 64–73. [Google Scholar] [CrossRef]
- Pizzol, P.C.M.; Portugal, M.L.; Trevizan, H.F.; Seraphim, P.M.; Teixeira, M.F.S. Impedimetric immunosensor for 5-methylcytosine detection based on a poly(o-phenylenediamine)-encapsulated gold nanoparticle platform. ACS Appl. Electron. Mater. 2025, 7, 3486–3500. [Google Scholar] [CrossRef]
- Holland, P.; Rubingh, D. Nonideal multicomponent mixed micelle model. J. Phys. Chem. 1983, 87, 1984–1990. [Google Scholar] [CrossRef]
- Stigter, D. Micelle formation by ionic surfactants. II. Specificity of head groups, micelle structure. J. Phys. Chem. 1974, 78, 2480–2485. [Google Scholar] [CrossRef]
- Andersson, J.; Kleinheinz, D.; Ramach, U.; Kiesenhofer, N.; Ashenden, A.; Valtiner, M.; Holt, S.; Koeper, I.; Schmidpeter, P.A.; Knoll, W. Native function of the bacterial ion channel SthK in a sparsely tethered lipid bilayer membrane architecture. J. Phys. Chem. B 2023, 127, 3641–3650. [Google Scholar] [CrossRef]
- Alghalayini, A.; Garcia, A.; Berry, T.; Cranfield, C.G. The use of tethered bilayer lipid membranes to identify the mechanisms of antimicrobial peptide interactions with lipid bilayers. Antibiotics 2019, 8, 12. [Google Scholar] [CrossRef]
- Berry, T.; Dutta, D.; Chen, R.; Leong, A.; Wang, H.; Donald, W.A.; Parviz, M.; Cornell, B.; Willcox, M.; Kumar, N. Lipid membrane interactions of the cationic antimicrobial peptide chimeras melimine and cys-melimine. Langmuir 2018, 34, 11586–11592. [Google Scholar] [CrossRef]
- Helenius, A.; Simons, K. Solubilization of membranes by detergents. Biochim. Biophys. Acta Rev. Biomembr. 1975, 415, 29–79. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.P.; Langer, R.; Jensen, K.F. Intracellular delivery by membrane disruption: Mechanisms, strategies, and concepts. Chem. Rev. 2018, 118, 7409–7531. [Google Scholar] [CrossRef] [PubMed]
- Brayden, D.J.; Gleeson, J.; Walsh, E.G. A head-to-head multi-parametric high content analysis of a series of medium chain fatty acid intestinal permeation enhancers in Caco-2 cells. Eur. J. Pharm. Biopharm. 2014, 88, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Brayden, D.J.; Maher, S.; Bahar, B.; Walsh, E. Sodium caprate-induced increases in intestinal permeability and epithelial damage are prevented by misoprostol. Eur. J. Pharm. Biopharm. 2015, 94, 194–206. [Google Scholar] [CrossRef]
- Sharma, P.; Vaiwala, R.; Parthasarathi, S.; Patil, N.; Verma, A.; Waskar, M.; Raut, J.S.; Basu, J.K.; Ayappa, K.G. Interactions of surfactants with the bacterial cell wall and inner membrane: Revealing the link between aggregation and antimicrobial activity. Langmuir 2022, 38, 15714–15728. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Kim, M.C.; Sut, T.N.; Cho, N.-J. Correlating membrane morphological responses with micellar aggregation behavior of capric acid and monocaprin. Langmuir 2017, 33, 2750–2759. [Google Scholar] [CrossRef]
- Kabara, J.J.; Swieczkowski, D.M.; Conley, A.J.; Truant, J.P. Fatty acids and derivatives as antimicrobial agents. Antimicrob. Agents Chemother. 1972, 2, 23–28. [Google Scholar] [CrossRef]
- Batovska, D.I.; Todorova, T.; Tsvetkova, V.; Najdenski, H.M. Antibacterial study of the medium chain fatty acids and their 1-monoglycerides: Individual effects and synergistic relationships. Pol. J. Microbiol. 2009, 58, 43–47. [Google Scholar]
- Marounek, M.; Skřivanová, E.; Rada, V. Susceptibility of Escherichia coli to C2-C18 fatty acids. Folia Microbiol. 2003, 48, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Skřivanová, E.; Marounek, M.; Benda, V.; Březina, P. Susceptibility of Escherichia coli, Salmonella sp. and Clostridium perfringens to organic acids and monolaurin. Vet. Med. 2006, 51, 81–88. [Google Scholar] [CrossRef]
- Wang, W.; Wang, R.; Zhang, G.; Chen, F.; Xu, B. In vitro antibacterial activities and mechanisms of action of fatty acid monoglycerides against four foodborne bacteria. J. Food Prot. 2020, 83, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhao, J.; Zeng, Z.; Wan, D.; Yu, P.; Cheng, D.; Gong, D.; Deng, S. Antibacterial activity and membrane-disrupting mechanism of monocaprin against Escherichia coli and its application in apple and carrot juices. LWT 2020, 131, 109794. [Google Scholar] [CrossRef]
- Schlievert, P.; Deringer, J.R.; Kim, M.H.; Projan, S.J.; Novick, R. Effect of glycerol monolaurate on bacterial growth and toxin production. Antimicrob. Agents Chemother. 1992, 36, 626–631. [Google Scholar] [CrossRef]
- Preuss, H.G.; Echard, B.; Enig, M.; Brook, I.; Elliott, T.B. Minimum inhibitory concentrations of herbal essential oils and monolaurin for gram-positive and gram-negative bacteria. Mol. Cell. Biochem. 2005, 272, 29–34. [Google Scholar] [CrossRef]
- Zare, M.A.; Razavi Rohani, S.M.; Raeisi, M.; Javadi Hosseini, S.; Hashemi, M. Antibacterial effects of monolaurin, sorbic acid and potassium sorbate on Staphylococcus aureus and Escherichia coli. J. Food Qual. Hazards Control 2014, 1, 52–55. [Google Scholar]
- Kim, S.; Rhee, M. Marked synergistic bactericidal effects and mode of action of medium-chain fatty acids in combination with organic acids against Escherichia coli O157: H7. Appl. Environ. Microbiol. 2013, 79, 6552–6560. [Google Scholar] [CrossRef]
- Yoon, B.K.; Park, S.; Jackman, J.A.; Cho, N.-J. Supported lipid bilayer platform for characterizing the optimization of mixed monoglyceride nano-micelles. Appl. Mater. Today 2020, 19, 100598. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sut, T.N.; Yoon, B.K.; Jackman, J.A. Synergistic Membrane Disruption of E. coli Tethered Lipid Bilayers by Antimicrobial Lipid Mixtures. Biomimetics 2025, 10, 739. https://doi.org/10.3390/biomimetics10110739
Sut TN, Yoon BK, Jackman JA. Synergistic Membrane Disruption of E. coli Tethered Lipid Bilayers by Antimicrobial Lipid Mixtures. Biomimetics. 2025; 10(11):739. https://doi.org/10.3390/biomimetics10110739
Chicago/Turabian StyleSut, Tun Naw, Bo Kyeong Yoon, and Joshua A. Jackman. 2025. "Synergistic Membrane Disruption of E. coli Tethered Lipid Bilayers by Antimicrobial Lipid Mixtures" Biomimetics 10, no. 11: 739. https://doi.org/10.3390/biomimetics10110739
APA StyleSut, T. N., Yoon, B. K., & Jackman, J. A. (2025). Synergistic Membrane Disruption of E. coli Tethered Lipid Bilayers by Antimicrobial Lipid Mixtures. Biomimetics, 10(11), 739. https://doi.org/10.3390/biomimetics10110739








