In Situ Formation of Silver Nanoparticles-Containing Gallic Acid-Conjugated Chitosan Hydrogels as Antimicrobial Tissue Adhesive Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
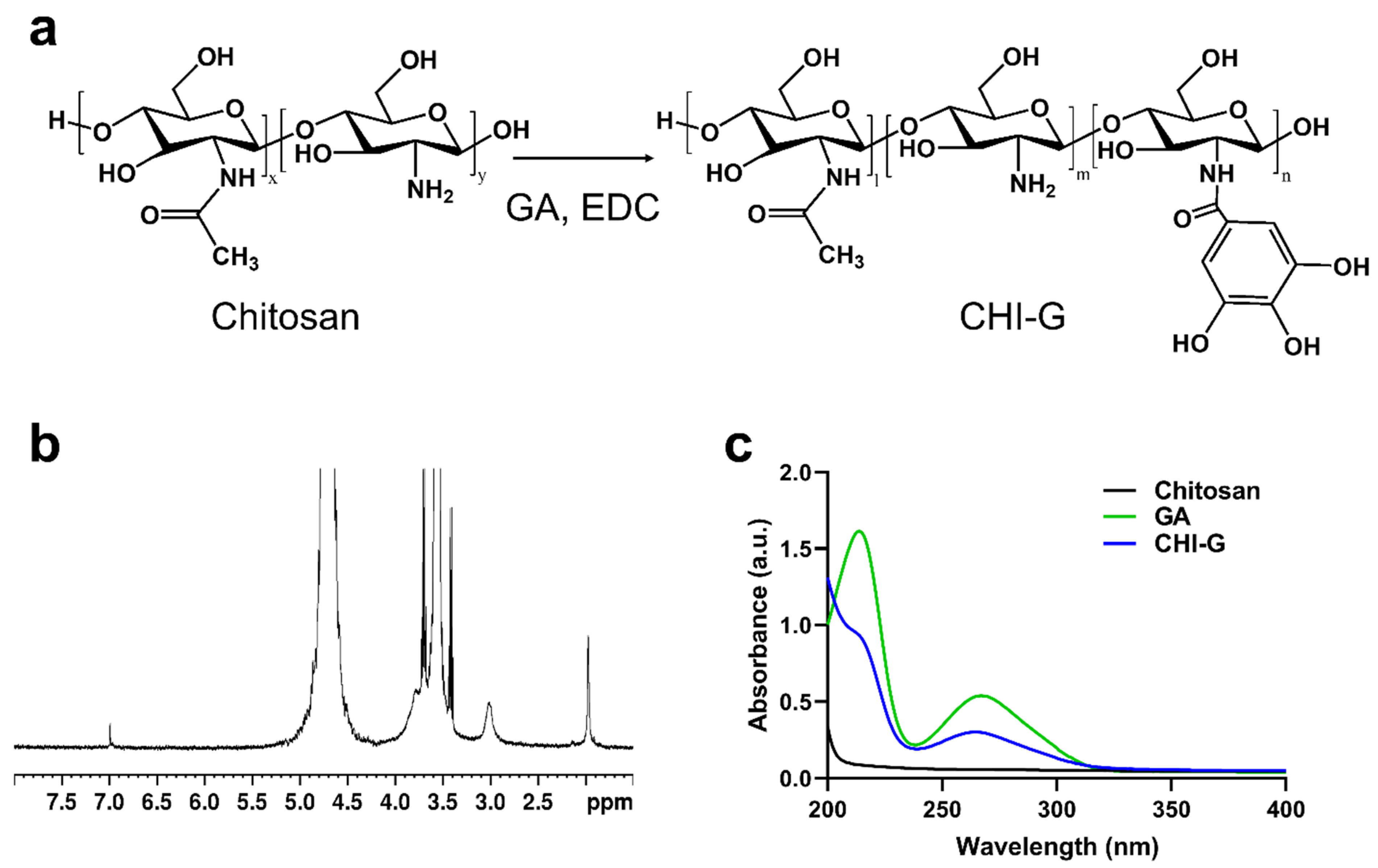
2.2. Synthesis of Gallic Acid-Conjugated Chitosan
2.3. Characterizations of CHI-G
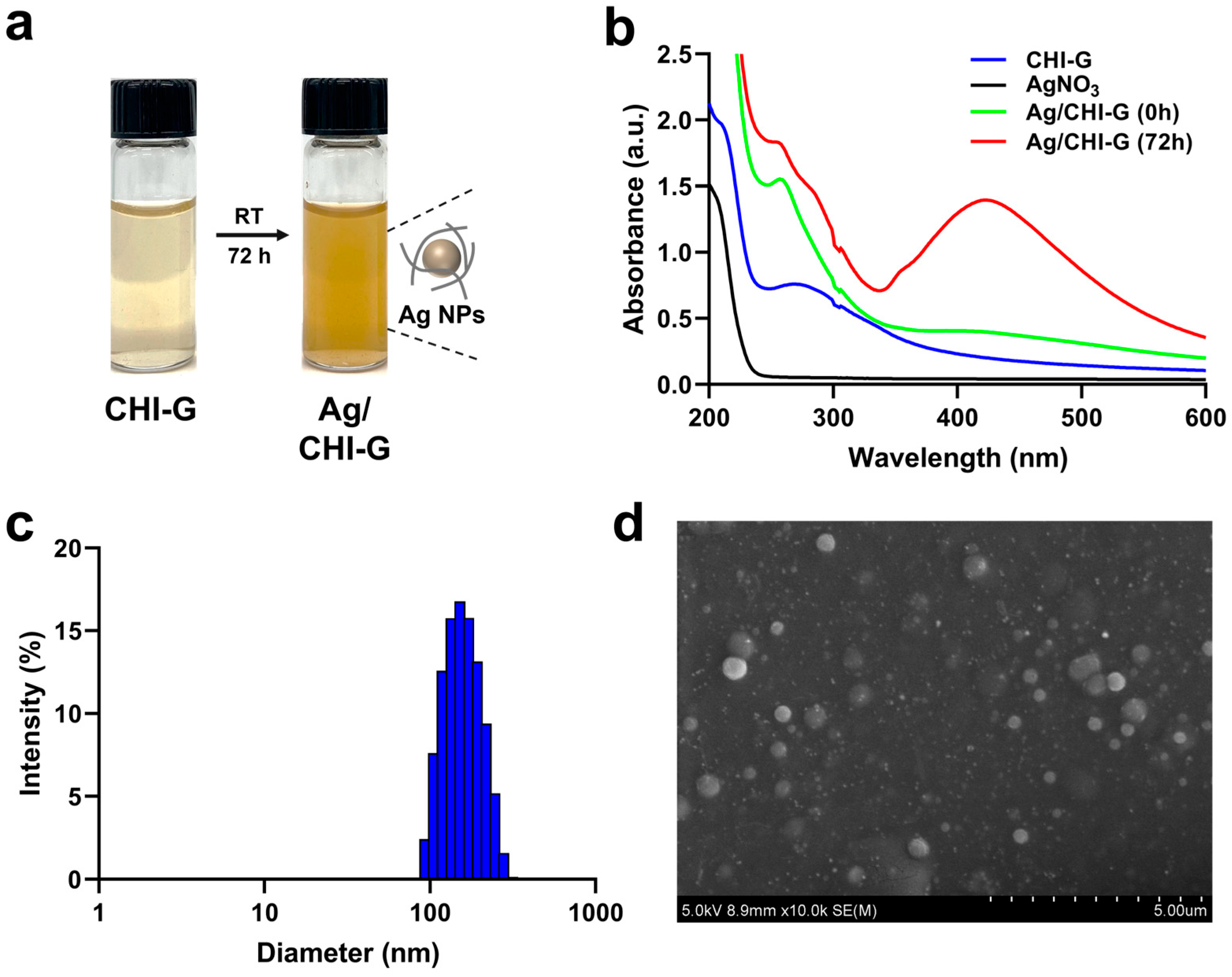
2.4. Study of Formation of Ag NPs Using CHI-G
2.5. Particle Size Distributions of Ag NPs Using CHI-G
2.6. Preparation of Ag/CHI-G Hydrogels
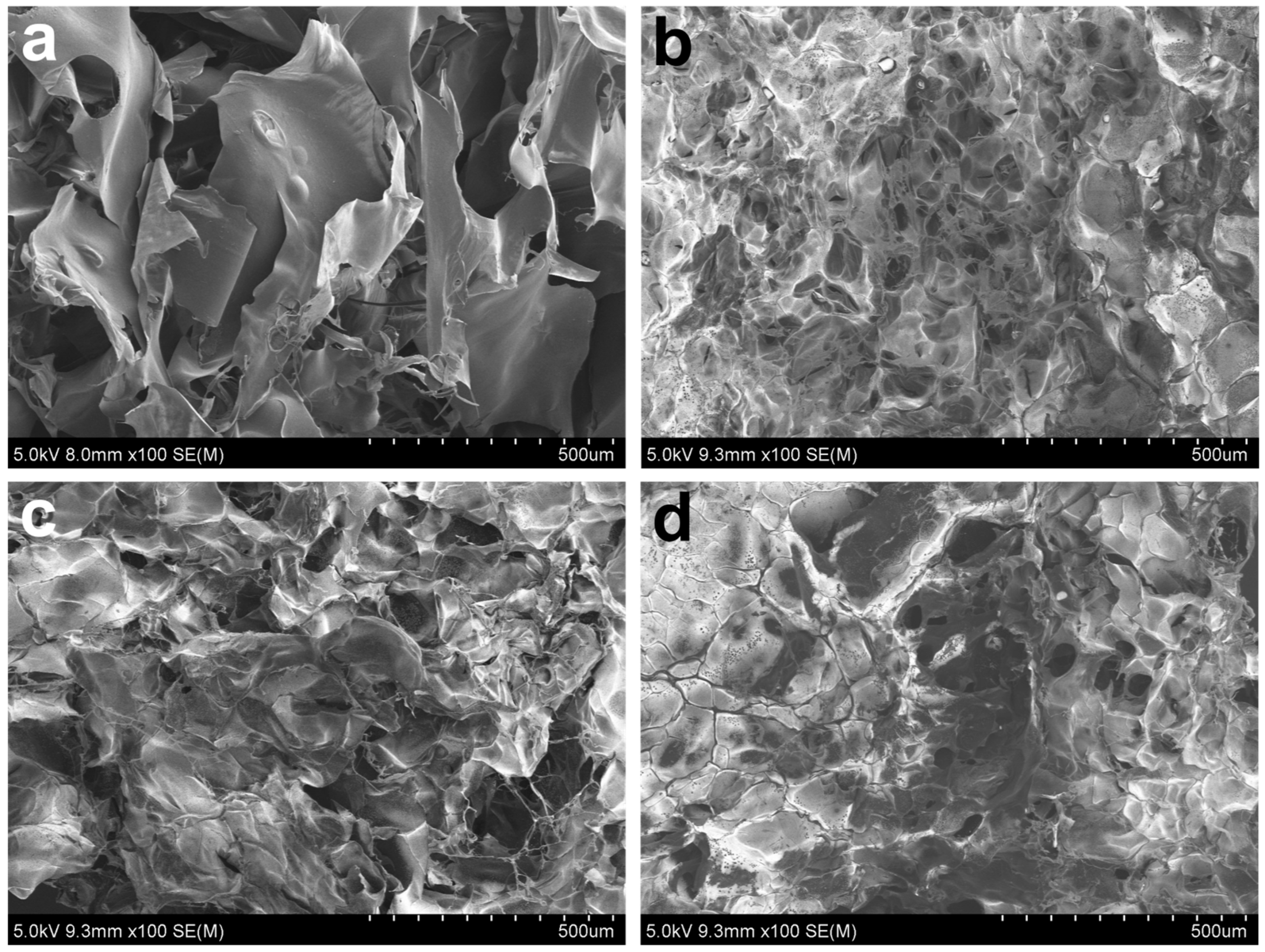
2.7. Morphological Analysis of Ag/CHI-G Hydrogels
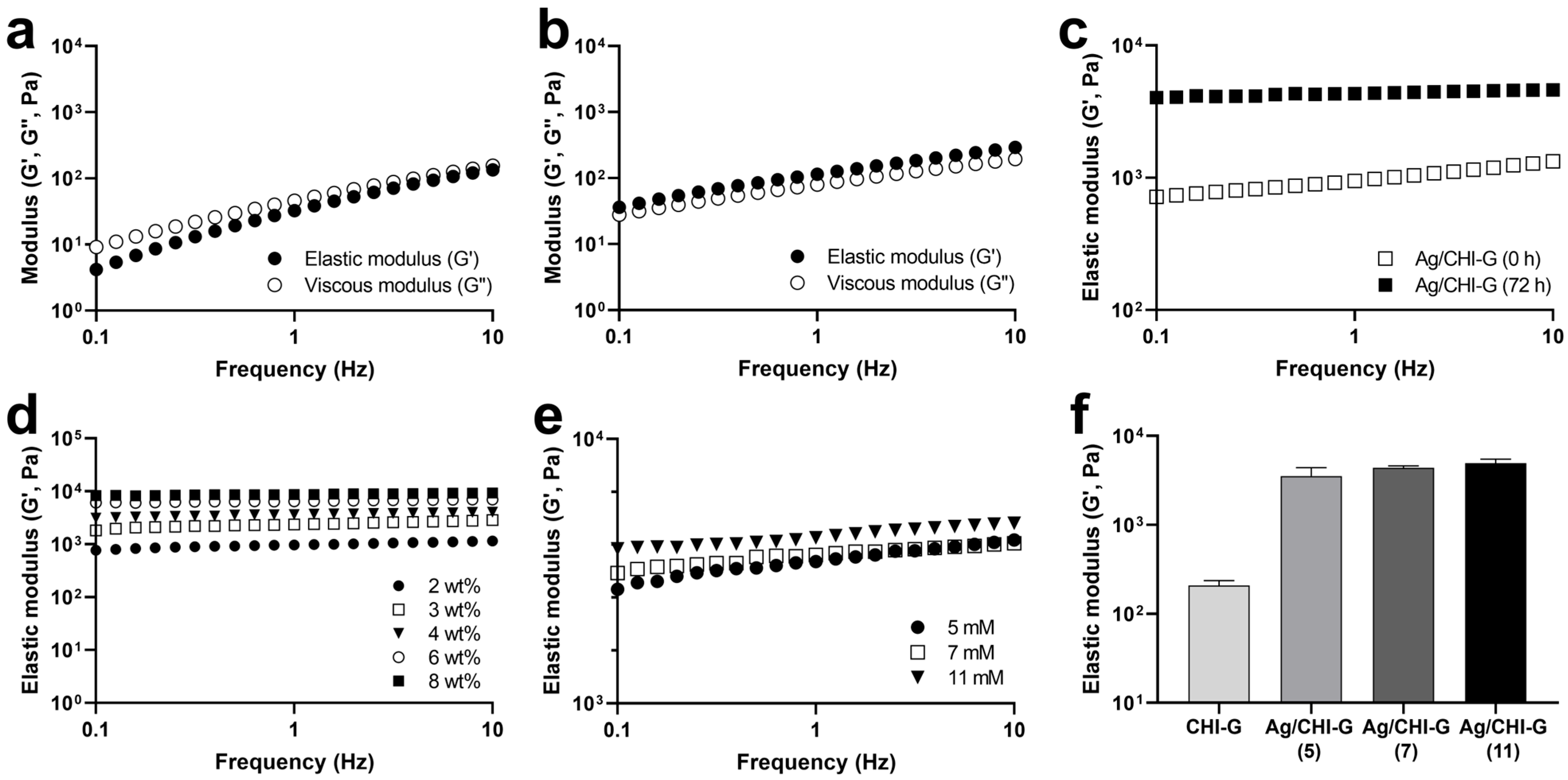
2.8. Rheological Analysis of Ag/CHI-G Hydrogels
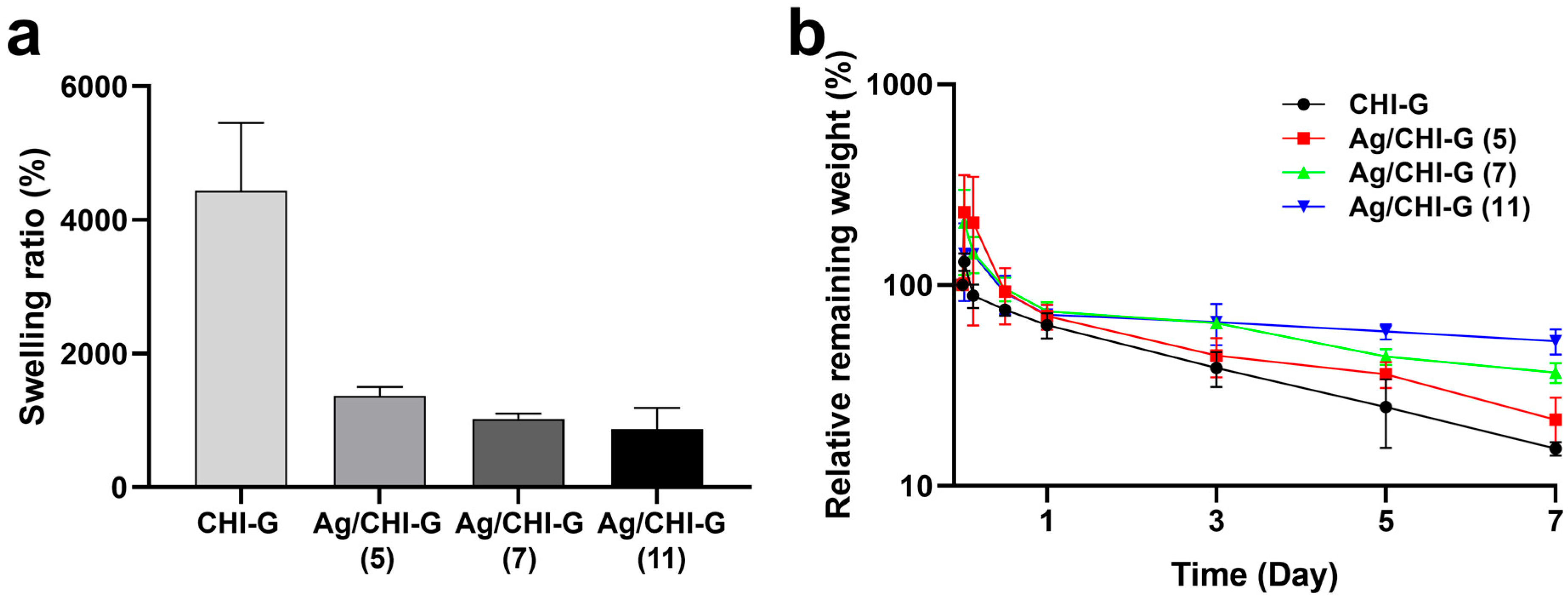
2.9. Swelling Ratios and Relative Remaining Weights of Ag/CHI-G Hydrogels
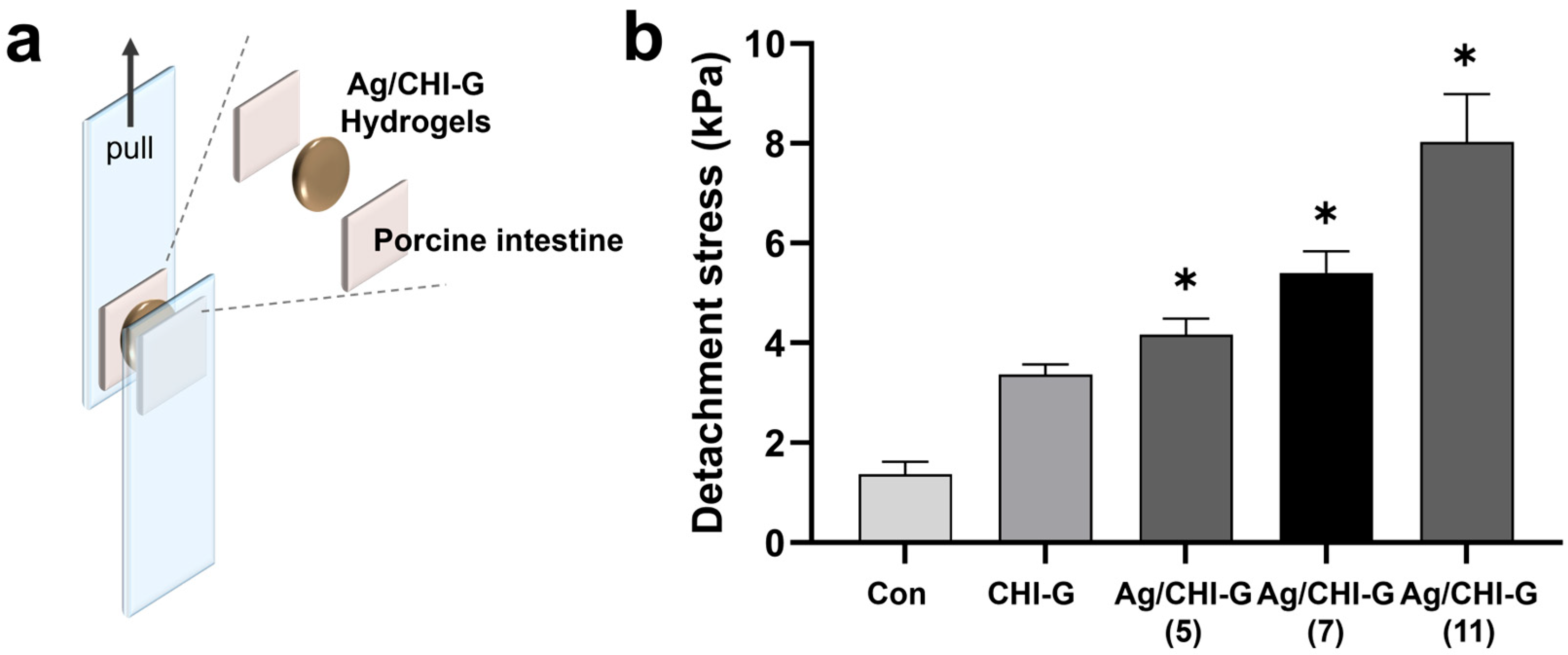
2.10. Tissue Adhesive Properties of Ag/CHI-G Hydrogels
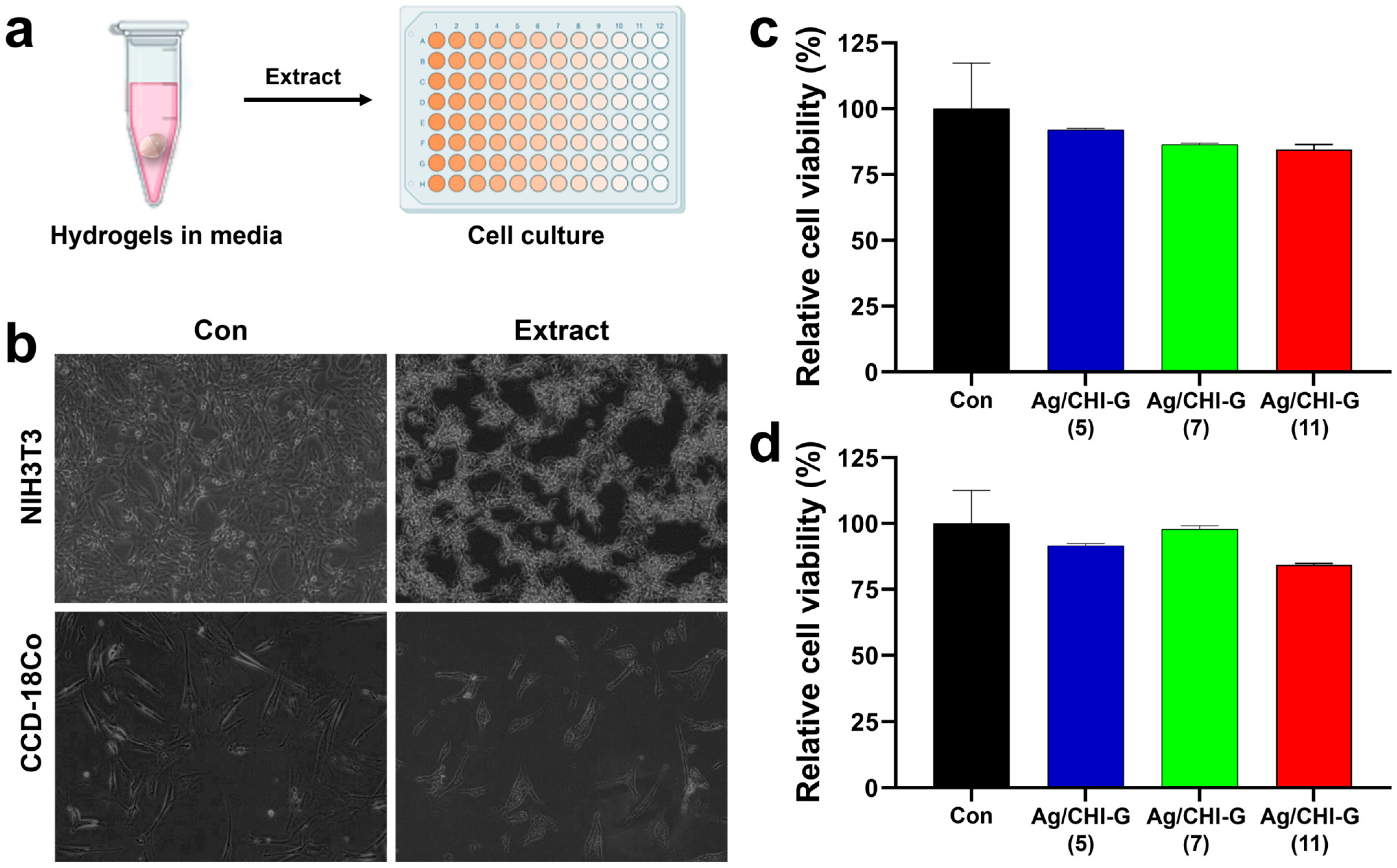
2.11. Cell Viability Assay of Ag/CHI-G Hydrogels
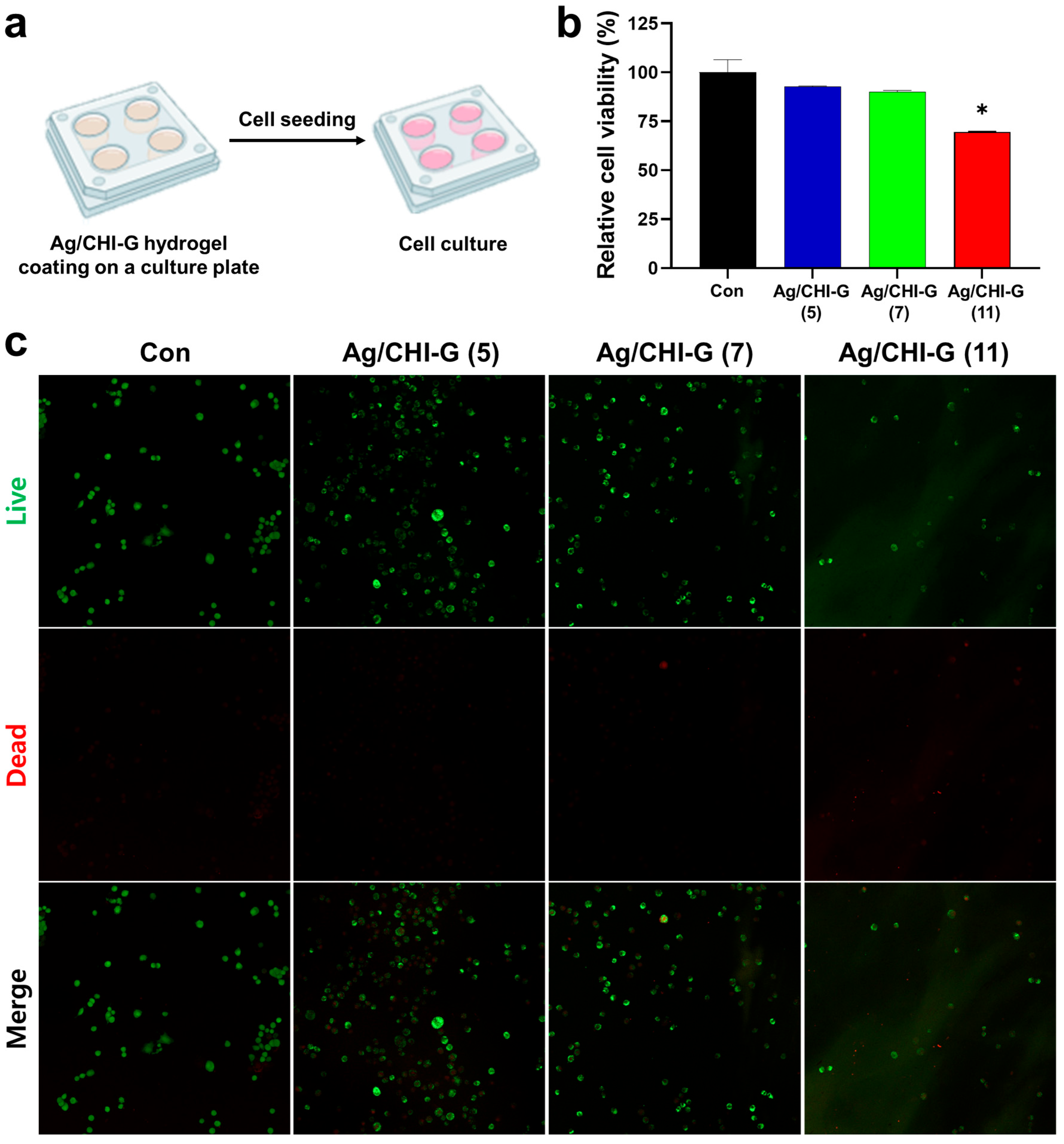
2.12. Live/Dead Assay on Ag/CHI-G Hydrogel-Coated Surfaces
2.13. Zone of Inhibition Assay of Ag/CHI-G Hydrogels
2.14. Evaluation of Antibacterial Activity in Liquid Culture
2.15. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterizations of CHI-G
3.2. Synthesis and Characterizations of Ag NPs Using CHI-G
3.3. Preparation and Characterization of Ag NPs-Containing CHI-G Hydrogels
3.4. Tissue Adhesiveness of Ag NPs-Containing CHI-G Hydrogels
3.5. Cell Viability of Ag/CHI-G Hydrogels
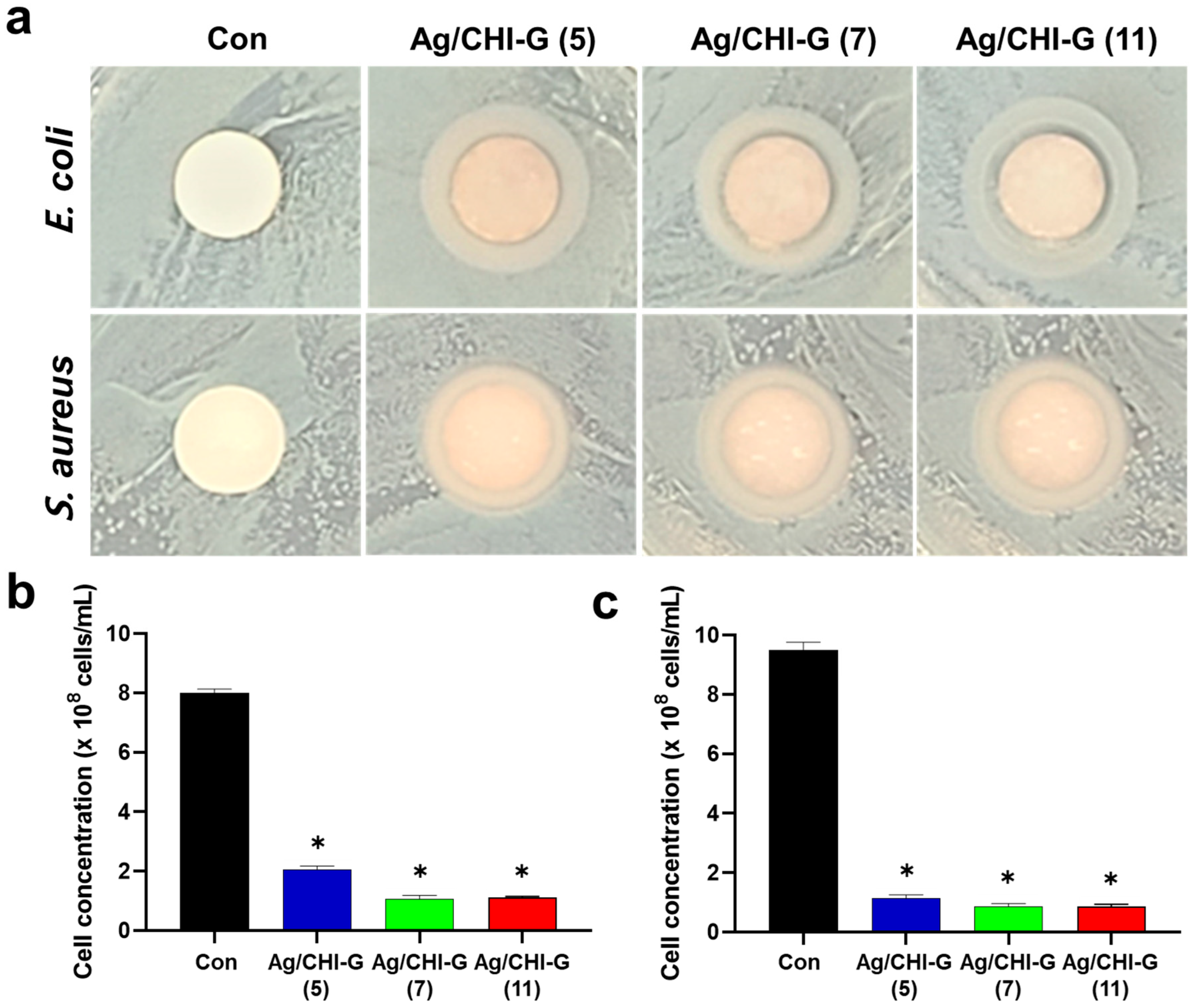
3.6. Antibacterial Activity of Ag/CHI-G Hydrogels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CHI-G | Gallic acid-conjugated chitosan |
| Ag NPs | Silver nanoparticles |
| Ag/CHI-G | Ag NP-containing CHI-G |
| EDC | 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| NHS | N-Hydroxysuccinimide |
| DDW | Distilled and deionized water |
| NMR | Nuclear magnetic resonance |
| UV-Vis | Ultraviolet-visible |
| SEM | Scanning electron microscopy |
| G′ | Elastic modulus |
| G″ | Viscous modulus |
| ZOI | Zone of inhibition |
| RCV | Relative cell viability |
References
- Uberoi, A.; McCready-Vangi, A.; Grice, E.A. The wound microbiota: Microbial mechanisms of impaired wound healing and infection. Nat. Rev. Microbiol. 2024, 22, 507–521. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic wound healing: A review of current management and treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the treatment of chronic wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Sisay, M.; Worku, T.; Edessa, D. Microbial epidemiology and antimicrobial resistance patterns of wound infection in Ethiopia: A meta-analysis of laboratory-based cross-sectional studies. BMC Pharmacol. Toxicol. 2019, 20, 35. [Google Scholar] [CrossRef]
- Pfalzgraff, A.; Brandenburg, K.; Weindl, G. Antimicrobial peptides and their therapeutic potential for bacterial skin infections and wounds. Front. Pharmacol. 2018, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Emanuel, C.; Cutting, K.F.; Williams, D.W. Microbiology of the skin and the role of biofilms in infection. Int. Wound J. 2012, 9, 14–32. [Google Scholar] [CrossRef]
- Nakipoglu, M.; Tezcaner, A.; Contag, C.H.; Annabi, N.; Ashammakhi, N. Bioadhesives with antimicrobial properties. Adv. Mater. 2023, 35, e2300840. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.; Zhu, S.; Chen, D.; Zhang, H.; Zou, L.; Zeng, Y. Highly adhesive antibacterial bioactive composite hydrogels with controllable flexibility and swelling as wound dressing for full-thickness skin healing. Front. Bioeng. Biotechnol. 2021, 9, 785302. [Google Scholar] [CrossRef] [PubMed]
- Wallace, L.A.; Gwynne, L.; Jenkins, T. Challenges and opportunities of pH in chronic wounds. Ther. Deliv. 2019, 10, 719–735. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, R.; Zheng, B.; Guo, W.; Li, C.; He, W.; Wei, Y.; Du, Y.; Wang, H.; Wu, D.; et al. Highly stretchable, adhesive, biocompatible, and antibacterial hydrogel dressings for wound healing. Adv. Sci. 2021, 8, 2003627. [Google Scholar] [CrossRef]
- Ren, Z.; Duan, Z.; Zhang, Z.; Fu, R.; Zhu, C.; Fan, D. Instantaneous self-healing and strongly adhesive self-adaptive hyaluronic acid-based hydrogel for controlled drug release to promote tendon wound healing. Int. J. Biol. Macromol. 2023, 242, 125001. [Google Scholar] [CrossRef]
- Guo, H.; Huang, S.; Xu, A.; Xue, W. Injectable adhesive self-healing multiple-dynamic-bond crosslinked hydrogel with photothermal antibacterial activity for infected wound healing. Chem. Mater. 2022, 34, 2655–2671. [Google Scholar] [CrossRef]
- Jin, X.; Wei, C.; Li, K.; Yin, P.; Wu, C.; Zhang, W. Polyphenol-mediated hyaluronic acid/tannic acid hydrogel with short gelation time and high adhesion strength for accelerating wound healing. Carbohydr. Polym. 2024, 342, 122372. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Han, X.; Zou, D.; Luo, X.; Liu, L.; Wang, J.; Maitz, M.F.; Yang, P.; Huang, N.; Zhao, A. Catechol-chitosan/polyacrylamide hydrogel wound dressing for regulating local inflammation. Mater. Today Bio. 2022, 16, 100392. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Y.; Chen, G.; Gao, H.; Peng, Q. Chitosan-based multifunctional biomaterials as active agents or delivery systems for antibacterial therapy. Bioengineering 2024, 11, 1278. [Google Scholar] [CrossRef] [PubMed]
- Harugade, A.; Sherje, A.P.; Pethe, A. Chitosan: A review on properties, biological activities and recent progress in biomedical applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- Ryu, J.H.; Lee, Y.; Kong, W.H.; Kim, T.G.; Park, T.G.; Lee, H. Catechol-functionalized chitosan/pluronic hydrogels for tissue adhesives and hemostatic materials. Biomacromolecules 2011, 12, 2653–2659. [Google Scholar] [CrossRef]
- Lu, Y.; Lou, X.; Jiang, J.; Wang, J.; Peng, X.; Yao, H.; Wu, J. Antioxidative, anti-inflammatory, antibacterial, photo-cross-linkable hydrogel of gallic acid-chitosan methacrylate: Synthesis, in vitro, and in vivo assessments. Biomacromolecules 2024, 25, 4358–4373. [Google Scholar] [CrossRef]
- Khainskaya, K.; Hileuskaya, K.; Nikalaichuk, V.; Ladutska, A.; Akhmedov, O.; Abrekova, N.; You, L.; Shao, P.; Odonchimeg, M. Chitosan-gallic acid conjugate with enhanced functional properties and synergistic wound healing effect. Carbohydr. Res. 2025, 553, 109496. [Google Scholar] [CrossRef]
- Kim, K.; Kim, K.; Ryu, J.H.; Lee, H. Chitosan-catechol: A polymer with long-lasting mucoadhesive properties. Biomaterials 2015, 52, 161–170. [Google Scholar] [CrossRef]
- Hyun, D.H.; Shin, H.H.; Seog, D.J.H.; Jang, H.; Choi, J.; Yoon, G.; Jin, E.J.; Park, J.H.; Ryu, J.H. Gallol-containing chitosan/hyaluronic acid composite hydrogel patches as wound sealing and dressing materials. Int. J. Biol. Macromol. 2025, 306, 141115. [Google Scholar] [CrossRef]
- Hino, Y.; Ejima, H. Tissue adhesive properties of functionalized chitosan: A comparative study of phenol, catechol and gallol. J. Photopolym. Sci. Technol. 2020, 33, 123–127. [Google Scholar] [CrossRef]
- Kaparekar, P.S.; Pathmanapan, S.; Anandasadagopan, S.K. Polymeric scaffold of gallic acid loaded chitosan nanoparticles infused with collagen-fibrin for wound dressing application. Int. J. Biol. Macromol. 2020, 165, 930–947. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Guan, L.; Zhu, Y.; Niu, R.; Zhu, S.; Lin, Q. Gallic acid-grafted chitosan antibacterial hydrogel incorporated with polydopamine-modified hydroxyapatite for enhancing bone healing. Front. Bioeng. Biotechnol. 2023, 11, 1162202. [Google Scholar] [CrossRef] [PubMed]
- Dallas, P.; Sharma, V.K.; Zboril, R. Silver polymeric nanocomposites as advanced antimicrobial agents: Classification, synthetic paths, applications, and perspectives. Adv. Colloid Interface Sci. 2011, 166, 119–135. [Google Scholar] [CrossRef]
- Rojas, M.A.; Amalraj, J.; Santos, L.S. Biopolymer-based composite hydrogels embedding small silver nanoparticles for advanced antimicrobial applications: Experimental and theoretical insights. Polymers 2023, 15, 3370. [Google Scholar] [CrossRef]
- Lara, H.H.; Garza-Treviño, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- Vazquez-Munoz, R.; Lopez, F.D.; Lopez-Ribot, J.L. Silver nanoantibiotics display strong antifungal activity against the emergent multidrug-resistant yeast Candida auris under both planktonic and biofilm growing conditions. Front. Microbiol. 2020, 11, 1673. [Google Scholar] [CrossRef]
- El-Sherbiny, G.M.; Lila, M.K.; Shetaia, Y.M.; F Elswify, M.M.; Mohamed, S.S. Antimicrobial activity of biosynthesised silver nanoparticles against multidrug-resistant microbes isolated from cancer patients with bacteraemia and candidaemia. Indian J. Med. Microbiol. 2020, 38, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Seong, M.; Lee, D.G. Silver nanoparticles against Salmonella enterica serotype Typhimurium: Role of inner membrane dysfunction. Curr. Microbiol. 2017, 74, 661–670. [Google Scholar] [CrossRef]
- Bondarenko, O.M.; Sihtmäe, M.; Kuzmičiova, J.; Ragelienė, L.; Kahru, A.; Daugelavičius, R. Plasma membrane is the target of rapid antibacterial action of silver nanoparticles in Escherichia coli and Pseudomonas aeruginosa. Int. J. Nanomed. 2018, 13, 6779–6790. [Google Scholar] [CrossRef]
- You, C.; Han, C.; Wang, X.; Zheng, Y.; Li, Q.; Hu, X.; Sun, H. The progress of silver nanoparticles in the antibacterial mechanism, clinical application and cytotoxicity. Mol. Biol. Rep. 2012, 39, 9193–9201. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Silver nanoparticles as an antimicrobial agent: A case study on Staphylococcus aureus and Escherichia coli as models for Gram-positive and Gram-negative bacteria. J. Gen. Appl. Microbiol. 2017, 63, 36–43. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Green silver nanoparticles: An antibacterial mechanism. Antibiotics 2024, 14, 5. [Google Scholar] [CrossRef]
- Ali, H.M.; Karam, K.; Khan, T.; Wahab, S.; Ullah, S.; Sadiq, M. Reactive oxygen species induced oxidative damage to DNA, lipids, and proteins of antibiotic-resistant bacteria by plant-based silver nanoparticles. 3 Biotech 2023, 13, 414. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Tikoo, K. Evaluating cell specific cytotoxicity of differentially charged silver nanoparticles. Food Chem. Toxicol. 2013, 51, 1–14. [Google Scholar] [CrossRef]
- Wang, X.-H.; Wang, Z.; Zhang, J.; Qi, H.-X.; Chen, J.; Xu, M. Cytotoxicity of AgNPs/CS composite films: AgNPs immobilized in chitosan matrix contributes a higher inhibition rate to cell proliferation. Bioengineered 2016, 7, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, V.; Riabov, S.; Kobylinskyi, S.; Goncharenko, L.; Rybalchenko, N.; Kruk, A.; Moskalenko, O.; Shut, M. Effect of the type of reducing agents of silver ions in interpolyelectrolyte-metal complexes on the structure, morphology and properties of silver-containing nanocomposites. Sci. Rep. 2020, 10, 7126. [Google Scholar] [CrossRef]
- Leopold, N.; Stefancu, A.; Herman, K.; Tódor, I.S.; Iancu, S.D.; Moisoiu, V.; Leopold, L.F. The role of adatoms in chloride-activated colloidal silver nanoparticles for surface-enhanced Raman scattering enhancement. Beilstein J. Nanotechnol. 2018, 9, 2236–2247. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-F.; Tsao, K.-T. Preparation and properties of nanocomposite hydrogels containing silver nanoparticles by ex situ polymerization. J. Appl. Polym. Sci. 2006, 100, 3653–3661. [Google Scholar] [CrossRef]
- Fahim, M.; Shahzaib, A.; Nishat, N.; Jahan, A.; Bhat, T.A.; Inam, A. Green synthesis of silver nanoparticles: A comprehensive review of methods, influencing factors, and applications. JCIS Open 2024, 16, 100125. [Google Scholar] [CrossRef]
- Duman, H.; Eker, F.; Akdaşçi, E.; Witkowska, A.M.; Bechelany, M.; Karav, S. Silver nanoparticles: A comprehensive review of synthesis methods and chemical and physical properties. Nanomaterials 2024, 14, 1527. [Google Scholar] [CrossRef] [PubMed]
- Bélteky, P.; Rónavári, A.; Igaz, N.; Szerencsés, B.; Tóth, I.Y.; Pfeiffer, I.; Kiricsi, M.; Kónya, Z. Silver nanoparticles: Aggregation behavior in biorelevant conditions and its impact on biological activity. Int. J. Nanomed. 2019, 14, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Popescu, I.; Constantin, M.; Pelin, I.M.; Suflet, D.M.; Ichim, D.L.; Daraba, O.M.; Fundueanu, G. Eco-friendly synthesized PVA/Chitosan/Oxalic acid nanocomposite hydrogels embedding silver nanoparticles as antibacterial materials. Gels 2022, 8, 268. [Google Scholar] [CrossRef]
- Aldakheel, F.M.; Mohsen, D.; El Sayed, M.M.; Alawam, K.A.; Binshaya, A.S.; Alduraywish, S.A. Silver nanoparticles loaded on chitosan-g-PVA hydrogel for the wound-healing applications. Molecules 2023, 28, 3241. [Google Scholar] [CrossRef]
- Lunkov, A.; Shagdarova, B.; Konovalova, M.; Zhuikova, Y.; Drozd, N.; Il’ina, A.; Varlamov, V. Synthesis of silver nanoparticles using gallic acid-conjugated chitosan derivatives. Carbohydr. Polym. 2020, 234, 115916. [Google Scholar] [CrossRef]
- Mohamady Hussein, M.A.; Olmos, J.M.; Pierański, M.K.; Grinholc, M.; Buhl, E.M.; Gunduz, O.; Youssef, A.M.; Pereira, C.M.; El-Sherbiny, I.M.; Megahed, M. Post grafted gallic acid to chitosan-Ag hybrid nanoparticles via free radical-induced grafting reactions. Int. J. Biol. Macromol. 2023, 233, 123395. [Google Scholar] [CrossRef]
- Sanandiya, N.D.; Lee, S.; Rho, S.; Lee, H.; Kim, I.S.; Hwang, D.S. Tunichrome-inspired pyrogallol functionalized chitosan for tissue adhesion and hemostasis. Carbohydr. Polym. 2019, 208, 77–85. [Google Scholar] [CrossRef]
- Pasanphan, W.; Chirachanchai, S. Conjugation of gallic acid onto chitosan: An approach for green and water-based antioxidant. Carbohydr. Polym. 2008, 72, 169–177. [Google Scholar] [CrossRef]
- Guo, P.; Anderson, J.D.; Bozell, J.J.; Zivanovic, S. The effect of solvent composition on grafting gallic acid onto chitosan via carbodiimide. Carbohydr. Polym. 2016, 140, 171–180. [Google Scholar] [CrossRef]
- Suflet, D.M.; Popescu, I.; Pelin, I.M.; Ichim, D.L.; Daraba, O.M.; Constantin, M.; Fundueanu, G. Dual cross-linked chitosan/PVA hydrogels containing silver nanoparticles with antimicrobial properties. Pharmaceutics 2021, 13, 1461. [Google Scholar] [CrossRef]
- Harun-Ur-Rashid, M.; Foyez, T.; Krishna, S.B.N.; Poda, S.; Imran, A.B. Recent advances of silver nanoparticle-based polymer nanocomposites for biomedical applications. RSC Adv. 2025, 15, 8480–8505. [Google Scholar] [CrossRef]
- Zhan, K.; Kim, C.; Sung, K.; Ejima, H.; Yoshie, N. Tunicate-inspired gallol polymers for underwater adhesive: A comparative study of catechol and gallol. Biomacromolecules 2017, 18, 2959–2966. [Google Scholar] [CrossRef] [PubMed]
- Moulay, S. Gallol-containing polymers: Synthesis and applications. Chem. Afr. 2023, 6, 2769–2815. [Google Scholar] [CrossRef]
- Andjelković, M.; Vancamp, J.; Demeulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006, 98, 23–31. [Google Scholar] [CrossRef]
- Tarrés, Q.; Aguado, R.; Zoppe, J.O.; Mutjé, P.; Fiol, N.; Delgado-Aguilar, M. Dynamic light scattering plus scanning electron microscopy: Usefulness and limitations of a simplified estimation of nanocellulose dimensions. Nanomaterials 2022, 12, 4288. [Google Scholar] [CrossRef]
- Filippov, S.K.; Khusnutdinov, R.; Murmiliuk, A.; Inam, W.; Zakharova, L.Y.; Zhang, H.; Khutoryanskiy, V.V. Dynamic light scattering and transmission electron microscopy in drug delivery: A roadmap for correct characterization of nanoparticles and interpretation of results. Mater. Horiz. 2023, 10, 5354–5370. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kang, B.; Vales, T.P.; Yang, S.K.; Lee, J.; Kim, H.J. Polyphenol-functionalized hydrogels using an interpenetrating chitosan network and investigation of their antioxidant activity. Macromol. Res. 2018, 26, 35–39. [Google Scholar] [CrossRef]
- Luo, Y.L.; Xu, F.; Chen, Y.S.; Jia, C.Y. Assembly, characterization of Ag nanoparticles in P(AAm-co-NVP)/CS semi-IPN, and swelling of the resulting composite hydrogels. Polym. Bull. 2010, 65, 181–199. [Google Scholar] [CrossRef]
- Nicolae-Maranciuc, A.; Chicea, D. Polymeric systems as hydrogels and membranes containing silver nanoparticles for biomedical and food applications: Recent approaches and perspectives. Gels 2025, 11, 699. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Seo, D.; Park, J.; Lee, S.H.; Jeon, J.; Kim, W.; Kim, J.; Yang, H.S.; Lee, J.Y. Wet tissue adhesive polymeric powder hydrogels for skeletal muscle regeneration. Bioact. Mater. 2024, 40, 334–344. [Google Scholar] [CrossRef]
- Gan, D.; Xing, W.; Jiang, L.; Fang, J.; Zhao, C.; Ren, F.; Fang, L.; Wang, K.; Lu, X. Plant-inspired adhesive and tough hydrogel based on Ag-lignin nanoparticles-triggered dynamic redox catechol chemistry. Nat. Commun. 2019, 10, 1487. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential mechanisms of action. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Mechanistic aspects of biosynthesized silver nanoparticles against Gram-positive and Gram-negative bacteria. J. Nanobiotechnol. 2016, 14, 86. [Google Scholar] [CrossRef]

| Samples | Diameter of ZOI (mm) | |
|---|---|---|
| E. coli | S. aureus | |
| Ag/CHI-G (5) | 11.32 ± 0.06 | 10.80 ± 0.02 |
| Ag/CHI-G (7) | 11.70 ± 0.12 | 11.06 ± 0.12 |
| Ag/CHI-G (11) | 12.46 ± 0.16 | 11.18 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-a.; Hyun, D.H.; Ryu, J.H. In Situ Formation of Silver Nanoparticles-Containing Gallic Acid-Conjugated Chitosan Hydrogels as Antimicrobial Tissue Adhesive Materials. Biomimetics 2025, 10, 720. https://doi.org/10.3390/biomimetics10110720
Kim S-a, Hyun DH, Ryu JH. In Situ Formation of Silver Nanoparticles-Containing Gallic Acid-Conjugated Chitosan Hydrogels as Antimicrobial Tissue Adhesive Materials. Biomimetics. 2025; 10(11):720. https://doi.org/10.3390/biomimetics10110720
Chicago/Turabian StyleKim, Se-ah, Da Han Hyun, and Ji Hyun Ryu. 2025. "In Situ Formation of Silver Nanoparticles-Containing Gallic Acid-Conjugated Chitosan Hydrogels as Antimicrobial Tissue Adhesive Materials" Biomimetics 10, no. 11: 720. https://doi.org/10.3390/biomimetics10110720
APA StyleKim, S.-a., Hyun, D. H., & Ryu, J. H. (2025). In Situ Formation of Silver Nanoparticles-Containing Gallic Acid-Conjugated Chitosan Hydrogels as Antimicrobial Tissue Adhesive Materials. Biomimetics, 10(11), 720. https://doi.org/10.3390/biomimetics10110720








