Biomimetic Silk Fibroin Scaffolds Functionalized with Hydroxyapatite and Platelet Growth Factors for Bone Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Silk Fibroin Scaffolds
2.2. Hemopoietic Stem Cells
2.3. Platelet Growth Factor Collection
2.4. Biocompatibility Evaluation of Silk Fibroin-Based Scaffolds and Platelet Growth Factors
2.4.1. MTT Assay
2.4.2. Live/Dead Assay
2.4.3. Cytoskeleton Structure Analysis
2.5. Osteoinductivity Propriety Evaluation of Silk Fibroin-Based Scaffolds and Platelet Growth Factors
2.6. Statistical Analysis
3. Results
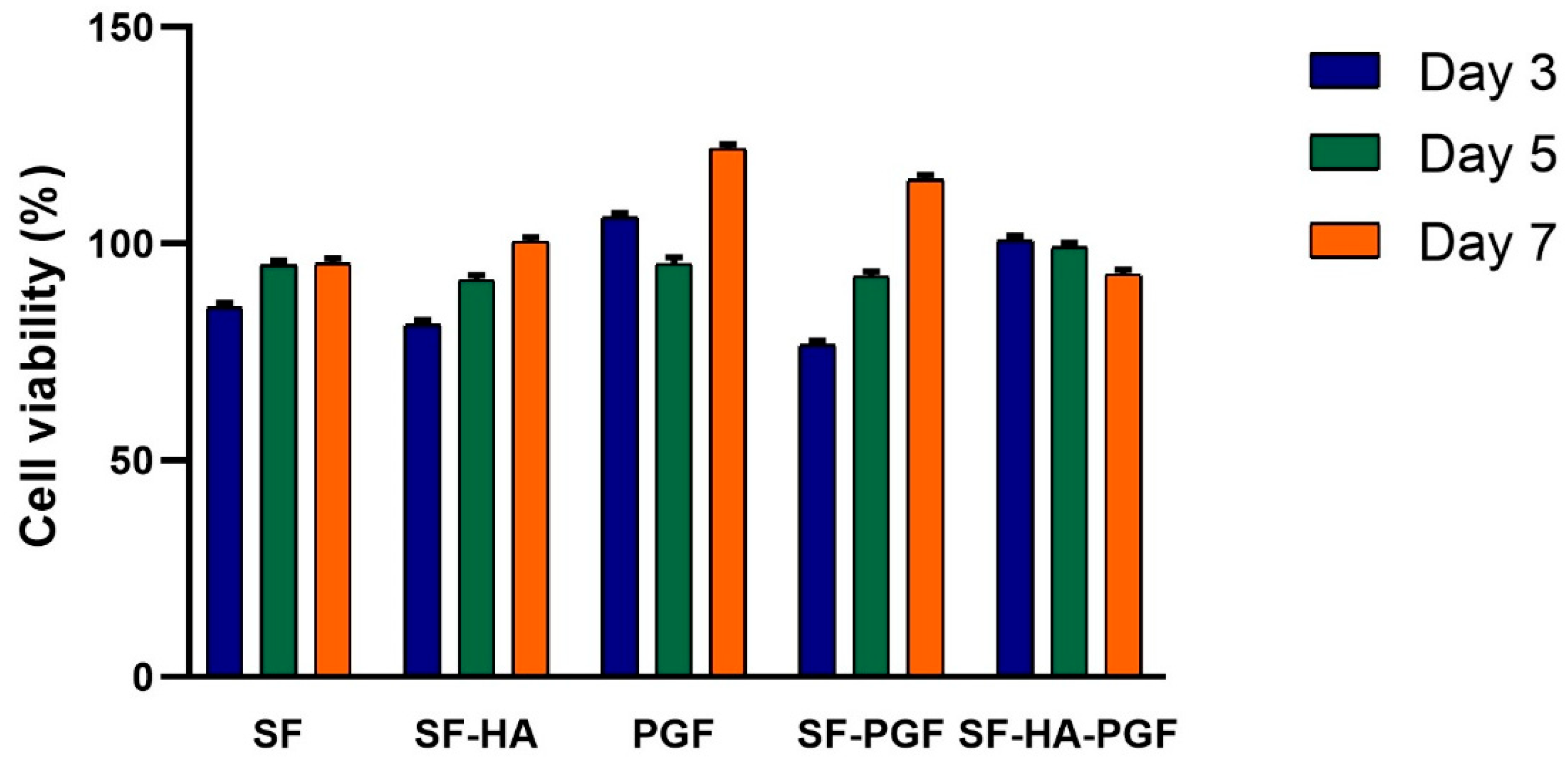
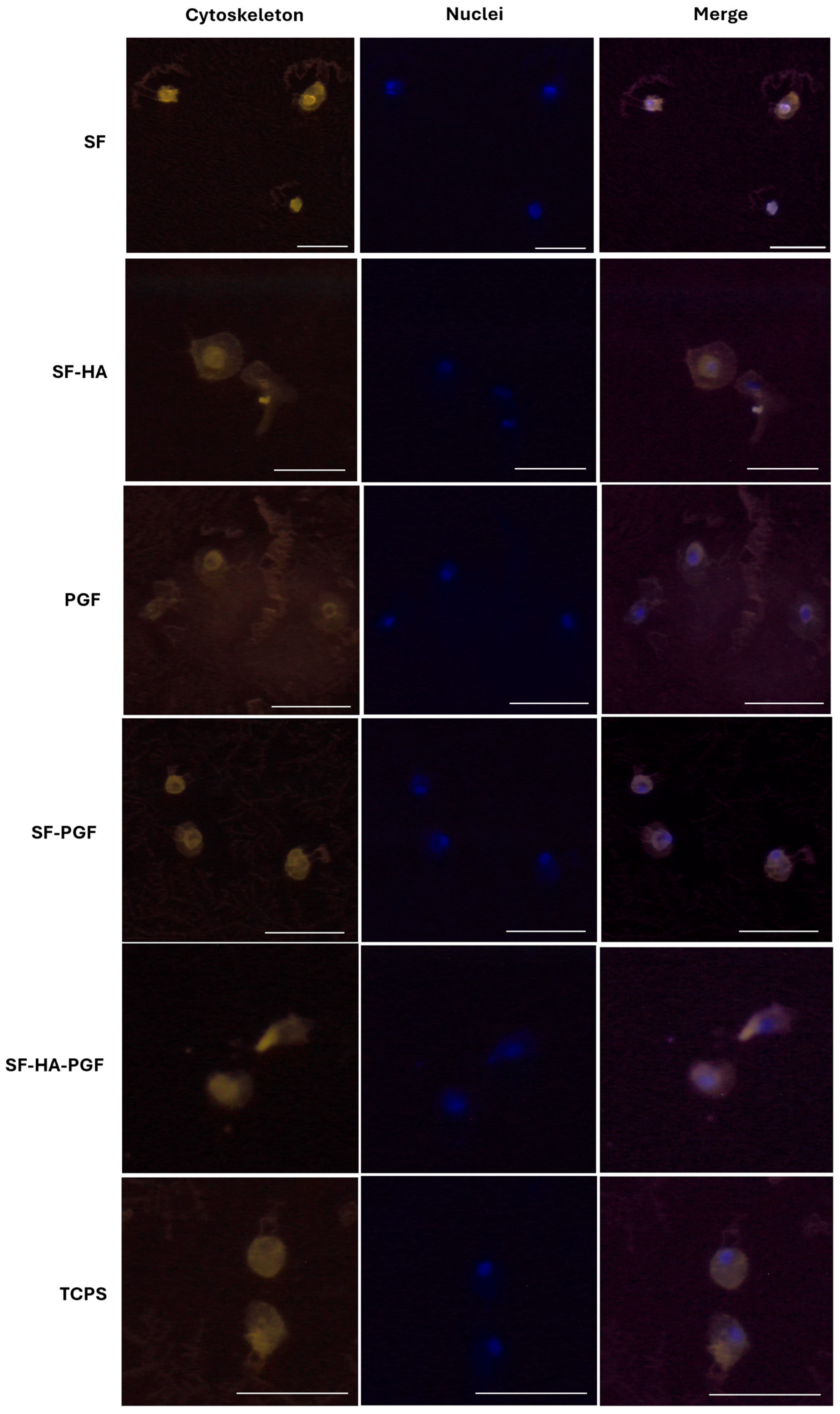
3.1. Biocompatibility of Silk Fibroin-Based Scaffolds and Platelet Growth Factors
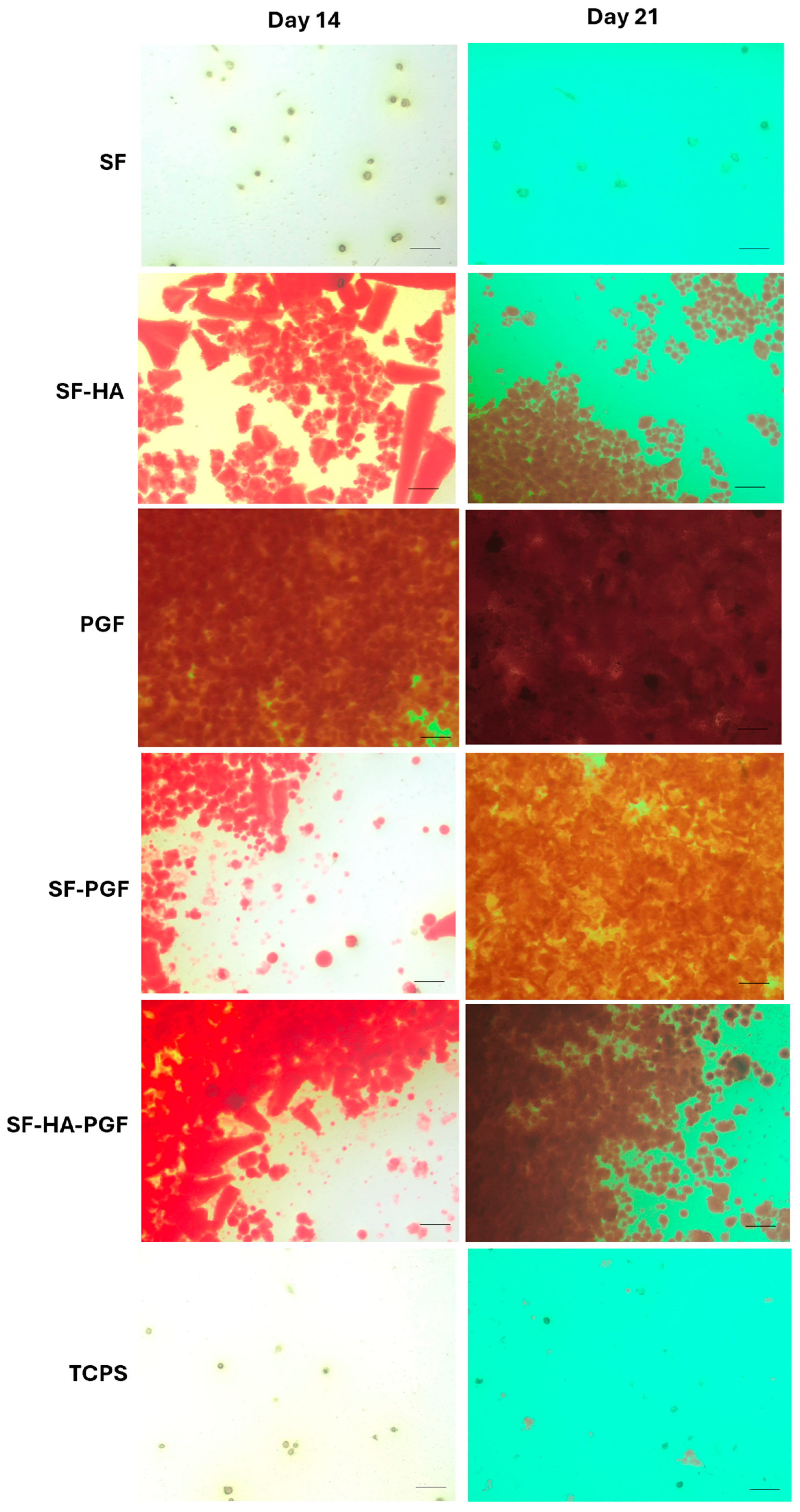
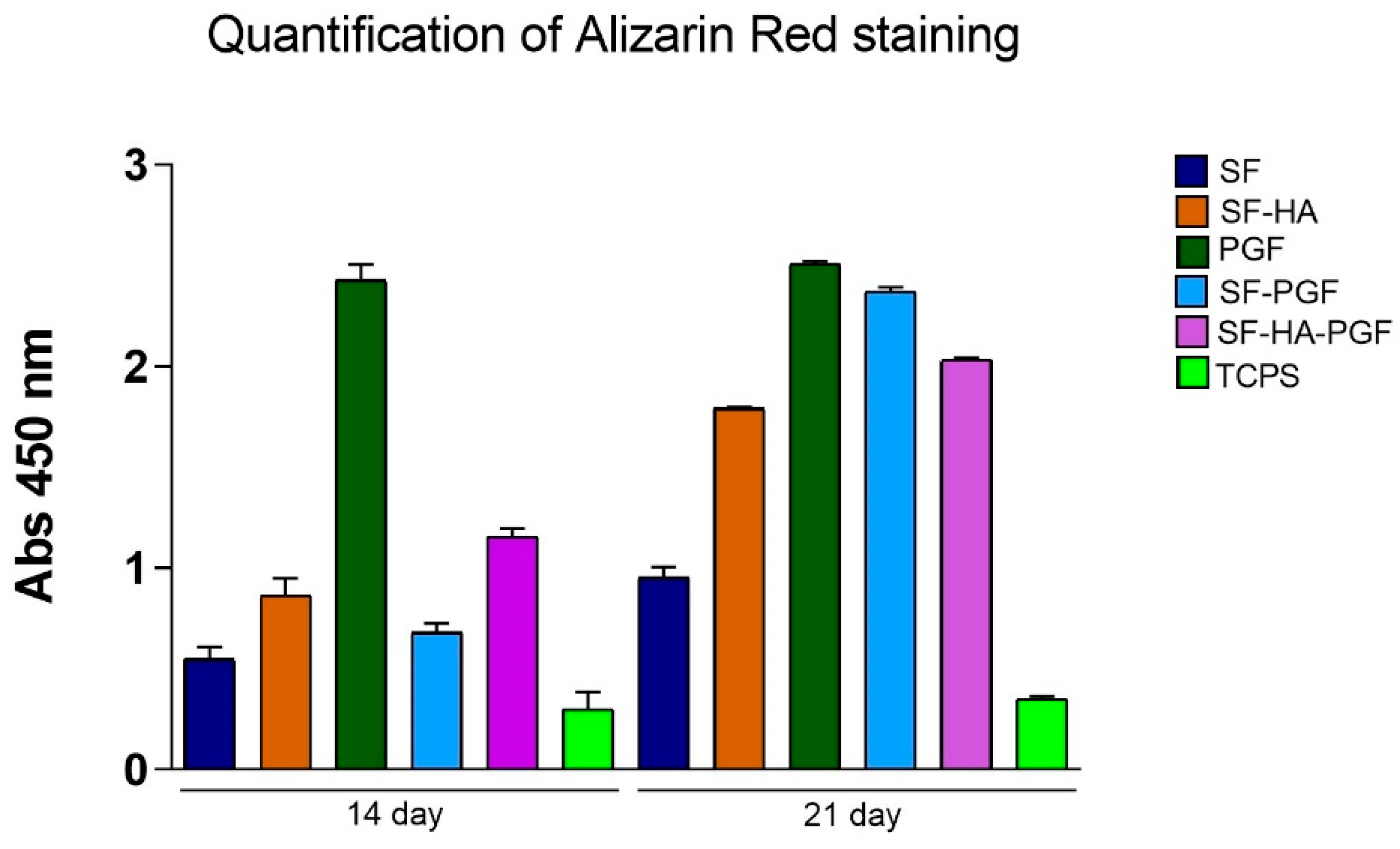
3.2. Osteoinductivity Propriety of Silk Fibroin-Based Scaffolds and Platelet Growth Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwartzman, J.D.; McCall, M.; Ghattas, Y.; Pugazhendhi, A.S.; Wei, F.; Ngo, C.; Ruiz, J.; Seal, S.; Coathup, M.J. Multifunctional scaffolds for bone repair following age-related biological decline: Promising prospects for smart biomaterial-driven technologies. Biomaterials 2024, 311, 122683. [Google Scholar] [CrossRef]
- Ehlen, Q.T.; Costello, J.P.I.; Mirsky, N.A.; Slavin, B.V.; Parra, M.; Ptashnik, A.; Nayak, V.V.; Coelho, P.G.; Witek, L. Treatment of Bone Defects and Nonunion via Novel Delivery Mechanisms, Growth Factors, and Stem Cells: A Review. ACS Biomater. Sci. Eng. 2024, 10, 7314–7336. [Google Scholar] [CrossRef]
- Thomas, J.D.; Kehoe, J.L. Bone Nonunion. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK554385/ (accessed on 29 July 2025).
- Wu, N.; Lee, Y.; Segina, D.; Murray, H.; Wilcox, T.; Boulanger, L. Economic burden of illness among US patients experiencing fracture nonunion. ORR 2013, 5, 21–33. [Google Scholar] [CrossRef]
- Vanderkarr, M.F.; Ruppenkamp, J.W.; Vanderkarr, M.; Holy, C.E.; Blauth, M. Risk factors and healthcare costs associated with long bone fracture non-union: A retrospective US claims database analysis. J. Orthop. Surg. Res. 2023, 18, 745. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef]
- Palanisamy, P.; Alam, M.; Li, S.; Chow, S.K.H.; Zheng, Y. Low-Intensity Pulsed Ultrasound Stimulation for Bone Fractures Healing. J. Ultrasound. Med. 2022, 41, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52, S18–S22. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, X.; Liu, H.; Li, J.; Gholipourmalekabadi, M.; Lin, K.; Yuan, C.; Wang, P. Strategies of functionalized GelMA-based bioinks for bone regeneration: Recent advances and future perspectives. Bioact. Mater. 2024, 38, 346–373. [Google Scholar] [CrossRef] [PubMed]
- Percival, K.M.; Paul, V.; Husseini, G.A. Recent Advancements in Bone Tissue Engineering: Integrating Smart Scaffold Technologies and Bio-Responsive Systems for Enhanced Regeneration. Int. J. Mol. Sci. 2024, 25, 6012. [Google Scholar] [CrossRef] [PubMed]
- Paladini, F.; Pollini, M. Novel Approaches and Biomaterials for Bone Tissue Engineering: A Focus on Silk Fibroin. Materials 2022, 15, 6952. [Google Scholar] [CrossRef]
- Zhou, K.; Yuan, T.; Wang, S.; Hu, F.; Luo, L.; Chen, L.; Yang, L. Beyond natural silk: Bioengineered silk fibroin for bone regeneration. Mater. Today Bio 2025, 33, 102014. [Google Scholar] [CrossRef]
- Paladini, F.; Russo, F.; Masi, A.; Lanzillotti, C.; Sannino, A.; Pollini, M. Silver-Treated Silk Fibroin Scaffolds for Prevention of Critical Wound Infections. Biomimetics 2024, 9, 659. [Google Scholar] [CrossRef]
- Pollini, M.; Paladini, F. The Emerging Role of Silk Fibroin for the Development of Novel Drug Delivery Systems. Biomimetics 2024, 9, 295. [Google Scholar] [CrossRef]
- Liu, W.; Cheong, N.; He, Z.; Zhang, T. Application of Hydroxyapatite Composites in Bone Tissue Engineering: A Review. J. Funct. Biomater. 2025, 16, 127. [Google Scholar] [CrossRef]
- Bhumiratana, S.; Grayson, W.L.; Castaneda, A.; Rockwood, D.N.; Gil, E.S.; Kaplan, D.L.; Vunjak-Novakovic, G. Nucleation and growth of mineralized bone matrix on silk-hydroxyapatite composite scaffolds. Biomaterials 2011, 32, 2812–2820. [Google Scholar] [CrossRef]
- Wu, H.; Lin, K.; Zhao, C.; Wang, X. Silk fibroin scaffolds: A promising candidate for bone regeneration. Front. Bioeng. Biotechnol. 2022, 10, 1054379. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Liu, X.; Li, R.; Malekmohammadi, S.; Feng, Y.; Song, J.; Gong, R.H.; Li, J. 3D Poly (L-lactic acid) fibrous sponge with interconnected porous structure for bone tissue scaffold. Int. J. Biol. Macromol. 2024, 268, 131688. [Google Scholar] [CrossRef]
- Meng, C.; Liu, X.; Li, J. Hierarchical porous PLLA/ACP fibrous membrane towards bone tissue scaffold. J. Mech. Behav. Biomed. Mater. 2024, 152, 106455. [Google Scholar] [CrossRef]
- Ezati, M.; Safavipour, H.; Houshmand, B.; Faghihi, S. Development of a PCL/gelatin/chitosan/β-TCP electrospun composite for guided bone regeneration. Prog. Biomater. 2018, 7, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Feng, J.; Liang, M.; Zhou, F.; Xia, Y.; Dong, Z.; Xue, Q.; Li, Z.; Pu, F.; Xia, P. Enhancing Bone Repair with β-TCP-Based Composite Scaffolds: A Review of Design Strategies and Biological Mechanisms. Orthop. Res. Rev. 2025, 17, 313–340. [Google Scholar] [CrossRef] [PubMed]
- Heydari, S.F.; Shahgholi, M.; Karimipour, A.; Salehi, M.; Galehdari, S.A. The effects of graphene oxide nanoparticles on the mechanical and thermal properties of polyurethane/polycaprolactone nanocomposites; a molecular dynamics approach. Results Eng. 2024, 24, 102933. [Google Scholar] [CrossRef]
- Zamani, M.; Yaghoubi, Y.; Movassaghpour, A.; Shakouri, K.; Mehdizadeh, A.; Pishgahi, A.; Yousefi, M. Novel therapeutic approaches in utilizing platelet lysate in regenerative medicine: Are we ready for clinical use? J. Cell. Physiol. 2019, 234, 17172–17186. [Google Scholar] [CrossRef]
- Li, T.; Lu, H.; Zhou, L.; Jia, M.; Zhang, L.; Wu, H.; Shan, L. Growth factors-based platelet lysate rejuvenates skin against ageing through NF-κB signalling pathway: In vitro and in vivo mechanistic and clinical studies. Cell Prolif. 2022, 55, e13212. [Google Scholar] [CrossRef]
- Rodriguez, I.A.; Kalaf, E.A.G.; Bowlin, G.L.; Sell, S.A. Platelet-Rich Plasma in Bone Regeneration: Engineering the Delivery for Improved Clinical Efficacy. Biomed. Res. Int. 2014, 2014, 392398. [Google Scholar] [CrossRef]
- Bacevich, B.M.; Smith, R.D.J.; Reihl, A.M.; Mazzocca, A.D.; Hutchinson, I.D. Advances with Platelet-Rich Plasma for Bone Healing. Biologics 2024, 18, 29–59. [Google Scholar] [CrossRef]
- Chotinantakul, K.; Leeanansaksiri, W. Hematopoietic stem cell development, niches, and signaling pathways. Bone Marrow Res. 2012, 2012, 270425. [Google Scholar] [CrossRef]
- Mehrotra, M.; Rosol, M.; Ogawa, M.; Larue, A.C. Amelioration of a mouse model of osteogenesis imperfecta with hematopoietic stem cell transplantation: Microcomputed tomography studies. Exp. Hematol. 2010, 38, 593–602. [Google Scholar] [CrossRef]
- Abbruzzese, L.; Nescis, E.; Turco, E.; Amoroso, P.; Carluccio, G. Efficacy of allogeneic platelet growth factors in actinic cystitis: The resolution of trouble? Transfus. Apher. Sci. 2023, 62, 103732. [Google Scholar] [CrossRef]
- Chong, P.-P.; Selvaratnam, L.; Abbas, A.A.; Kamarul, T. Human peripheral blood derived mesenchymal stem cells demonstrate similar characteristics and chondrogenic differentiation potential to bone marrow derived mesenchymal stem cells. J. Orthop. Res. 2012, 30, 634–642. [Google Scholar] [CrossRef]
- Paladini, F.; Lanzillotti, C.; Panico, A.; Pollini, M. Biological Evaluation of Silver-Treated Silk Fibroin Scaffolds for Application as Antibacterial and Regenerative Wound Dressings. Nanomaterials 2025, 15, 919. [Google Scholar] [CrossRef] [PubMed]
- Nozhat, Z.; Khalaji, M.S.; Hedayati, M.; Kia, S.K. Different Methods for Cell Viability and Proliferation Assay: Essential Tools in Pharmaceutical Studies. Anti-Cancer Agents Med. Chem. 2022, 22, 703–712. [Google Scholar] [CrossRef]
- Gallo, A.L.; Pollini, M.; Paladini, F. A combined approach for the development of novel sutures with antibacterial and regenerative properties: The role of silver and silk sericin functionalization. J. Mater. Sci. Mater. Med. 2018, 29, 133. [Google Scholar] [CrossRef]
- Cao, H.; Yue, L.; Shao, J.; Kong, F.; Liu, S.; Huai, H.; He, Z.; Mao, Z.; Yang, Y.; Tan, Y.; et al. Small extracellular vesicles derived from umbilical cord mesenchymal stem cells alleviate radiation-induced cardiac organoid injury. Stem Cell Res. Ther. 2024, 15, 493. [Google Scholar] [CrossRef]
- Lanzillotti, C.; Iaquinta, M.R.; De Pace, R.; Mosaico, M.; Patergnani, S.; Giorgi, C.; Tavoni, M.; Dapporto, M.; Sprio, S.; Tampieri, A.; et al. Osteosarcoma cell death induced by innovative scaffolds doped with chemotherapeutics. J. Cell. Physiol. 2024, 239, e31256. [Google Scholar] [CrossRef]
- Corazza, M.; Oton-Gonzalez, L.; Scuderi, V.; Rotondo, J.C.; Lanzillotti, C.; Di Mauro, G.; Tognon, M.; Martini, F.; Borghi, A. Tissue cytokine/chemokine profile in vulvar lichen sclerosus: An observational study on keratinocyte and fibroblast cultures. J. Dermatol. Sci. 2020, 100, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, C.; Cervellera, C.F.; Lanzillotti, C.; Touzé, A.; Gaboriaud, P.; Tognon, M.; Martini, F.; Rotondo, J.C. MicroRNA dysregulations in Merkel cell carcinoma: Molecular mechanisms and clinical applications. J. Med. Virol. 2023, 95, e28375. [Google Scholar] [CrossRef] [PubMed]
- Arvidson, K.; Abdallah, B.M.; Applegate, L.A.; Baldini, N.; Cenni, E.; Gomez-Barrena, E.; Granchi, D.; Kassem, M.; Konttinen, Y.T.; Mustafa, K.; et al. Bone regeneration and stem cells. J. Cell. Mol. Med. 2011, 15, 718–746. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.; Liu, Y.; Yu, J.; Sun, X.; Wang, L.; Zhou, Y. Effects of platelet-rich fibrin on osteogenic differentiation of Schneiderian membrane derived mesenchymal stem cells and bone formation in maxillary sinus. Cell Commun. Signal. 2022, 20, 88. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.; Kale, V.P. TGF-β signaling and its role in the regulation of hematopoietic stem cells. Syst. Synth. Biol. 2015, 9, 1–10. [Google Scholar] [CrossRef]
- Yokota, J.; Chosa, N.; Sawada, S.; Okubo, N.; Takahashi, N.; Hasegawa, T.; Kondo, H.; Ishisaki, A. PDGF-induced PI3K-mediated signaling enhances the TGF-β-induced osteogenic differentiation of human mesenchymal stem cells in a TGF-β-activated MEK-dependent manner. Int. J. Mol. Med. 2014, 33, 534–542. [Google Scholar] [CrossRef]
- Wang, Y.; Rudym, D.D.; Walsh, A.; Abrahamsen, L.; Kim, H.-J.; Kim, H.S.; Kirker-Head, C.; Kaplan, D.L. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials 2008, 29, 3415–3428. [Google Scholar] [CrossRef] [PubMed]
- Tajvar, S.; Hadjizadeh, A.; Samandari, S.S. Scaffold degradation in bone tissue engineering: An overview. Int. Biodeterior. Biodegrad. 2023, 180, 105599. [Google Scholar] [CrossRef]
- Sheen, J.R.; Mabrouk, A.; Garla, V.V. Fracture Healing Overview. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK551678/ (accessed on 4 September 2025).
- Lubojański, A.; Zakrzewski, W.; Samól, K.; Bieszczad-Czaja, M.; Świtała, M.; Wiglusz, R.; Watras, A.; Mielan, B.; Dobrzyński, M. Application of Nanohydroxyapatite in Medicine—A Narrative Review. Molecules 2024, 29, 5628. [Google Scholar] [CrossRef] [PubMed]
- Iaquinta, M.R.; Martini, F.; D’Agostino, A.; Trevisiol, L.; Bersani, M.; Torreggiani, E.; Tognon, M.; Rotondo, J.C.; Mazzoni, E. Stem Cell Fate and Immunomodulation Promote Bone Regeneration via Composite Bio-Oss®/AviteneTM Biomaterial. Front. Bioeng. Biotechnol. 2022, 10, 873814. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pollini, M.; Lanzillotti, C.; De Sangro, M.A.; Cazzato, M.R.; Abbruzzese, L.; Paladini, F. Biomimetic Silk Fibroin Scaffolds Functionalized with Hydroxyapatite and Platelet Growth Factors for Bone Tissue Engineering. Biomimetics 2025, 10, 703. https://doi.org/10.3390/biomimetics10100703
Pollini M, Lanzillotti C, De Sangro MA, Cazzato MR, Abbruzzese L, Paladini F. Biomimetic Silk Fibroin Scaffolds Functionalized with Hydroxyapatite and Platelet Growth Factors for Bone Tissue Engineering. Biomimetics. 2025; 10(10):703. https://doi.org/10.3390/biomimetics10100703
Chicago/Turabian StylePollini, Mauro, Carmen Lanzillotti, Maria Antonietta De Sangro, Maria Rosaria Cazzato, Luciano Abbruzzese, and Federica Paladini. 2025. "Biomimetic Silk Fibroin Scaffolds Functionalized with Hydroxyapatite and Platelet Growth Factors for Bone Tissue Engineering" Biomimetics 10, no. 10: 703. https://doi.org/10.3390/biomimetics10100703
APA StylePollini, M., Lanzillotti, C., De Sangro, M. A., Cazzato, M. R., Abbruzzese, L., & Paladini, F. (2025). Biomimetic Silk Fibroin Scaffolds Functionalized with Hydroxyapatite and Platelet Growth Factors for Bone Tissue Engineering. Biomimetics, 10(10), 703. https://doi.org/10.3390/biomimetics10100703







