Parametric Design and Mechanical Characterization of a Selective Laser Sintering Additively Manufactured Biomimetic Ribbed Dome Inspired by the Chorion of Lepidopteran Eggs
Abstract
1. Introduction
2. Materials and Methods
2.1. Novel Biomimetic Design Strategy
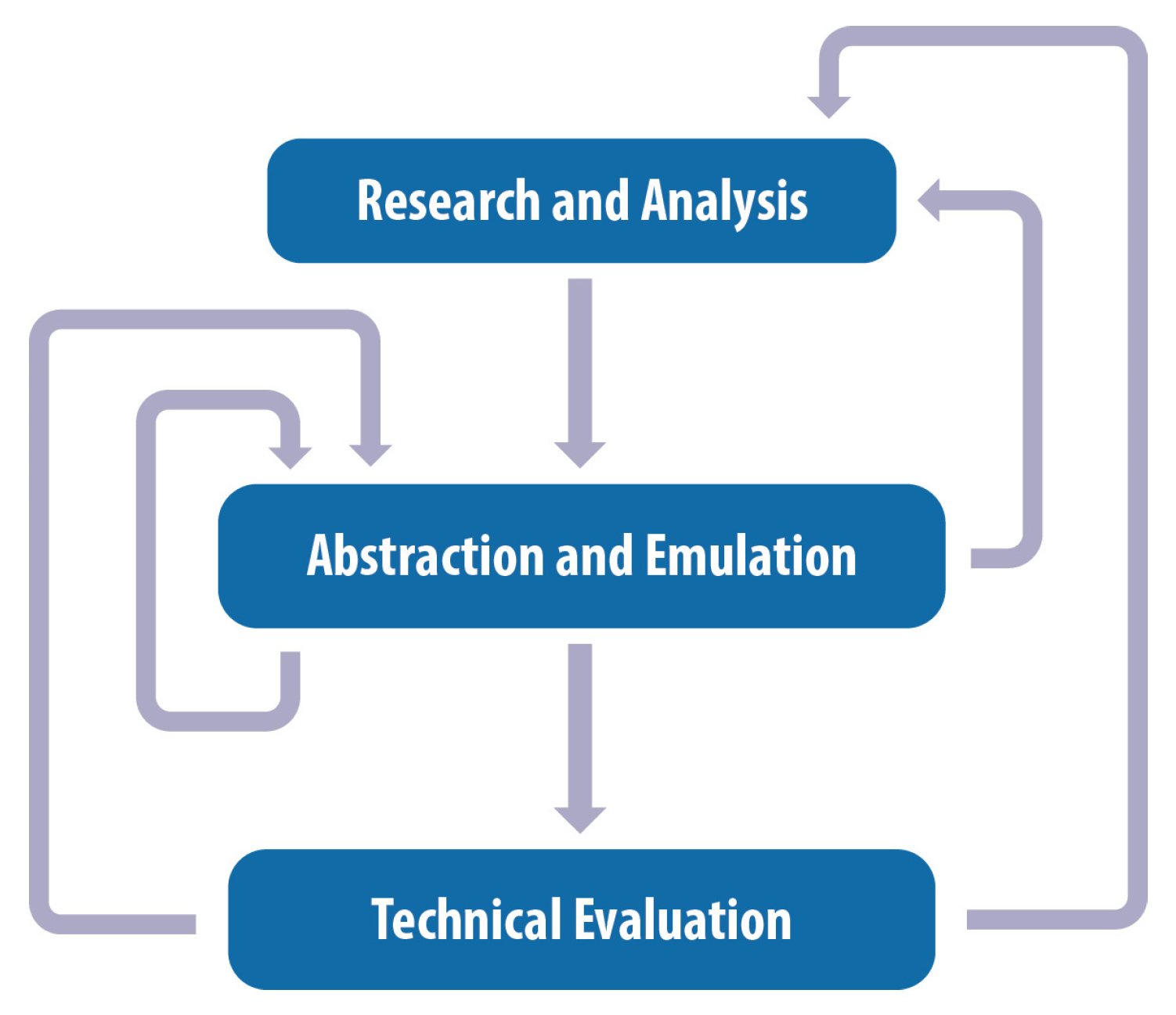
2.2. Research and Analysis
2.3. Abstraction and Emulation
2.4. Technical Evaluation
3. Biomimetic Design Process
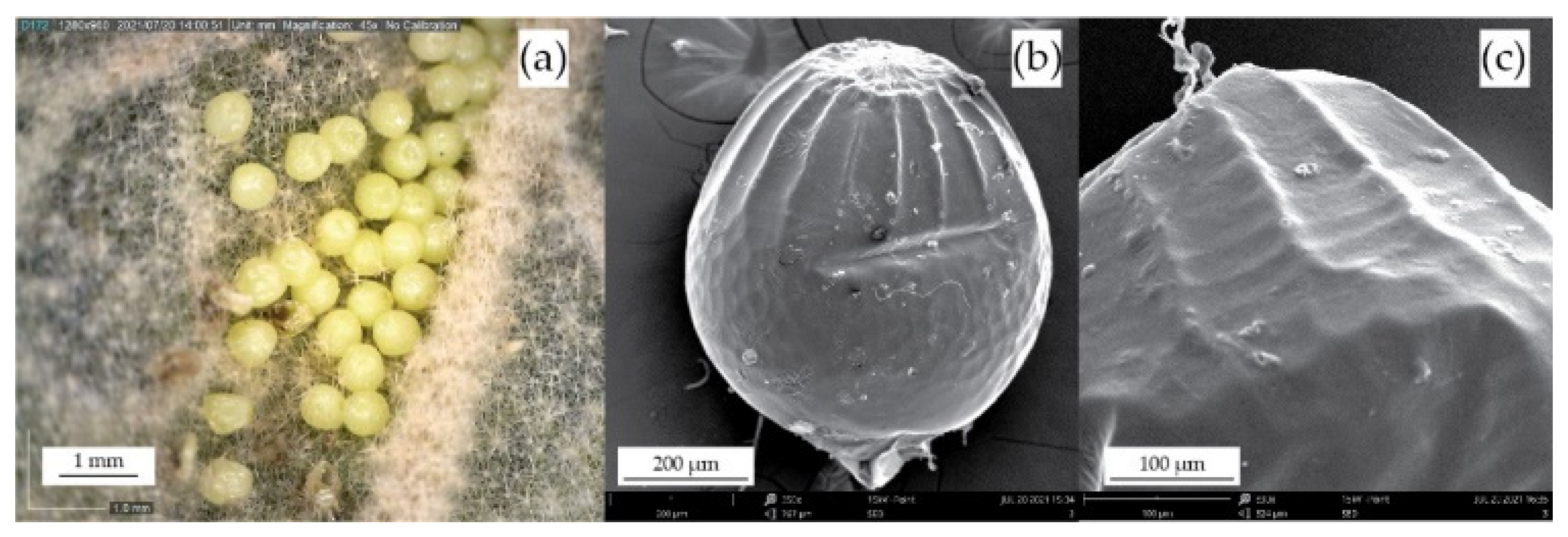
3.1. Morphological Analysis of the Biological Sample
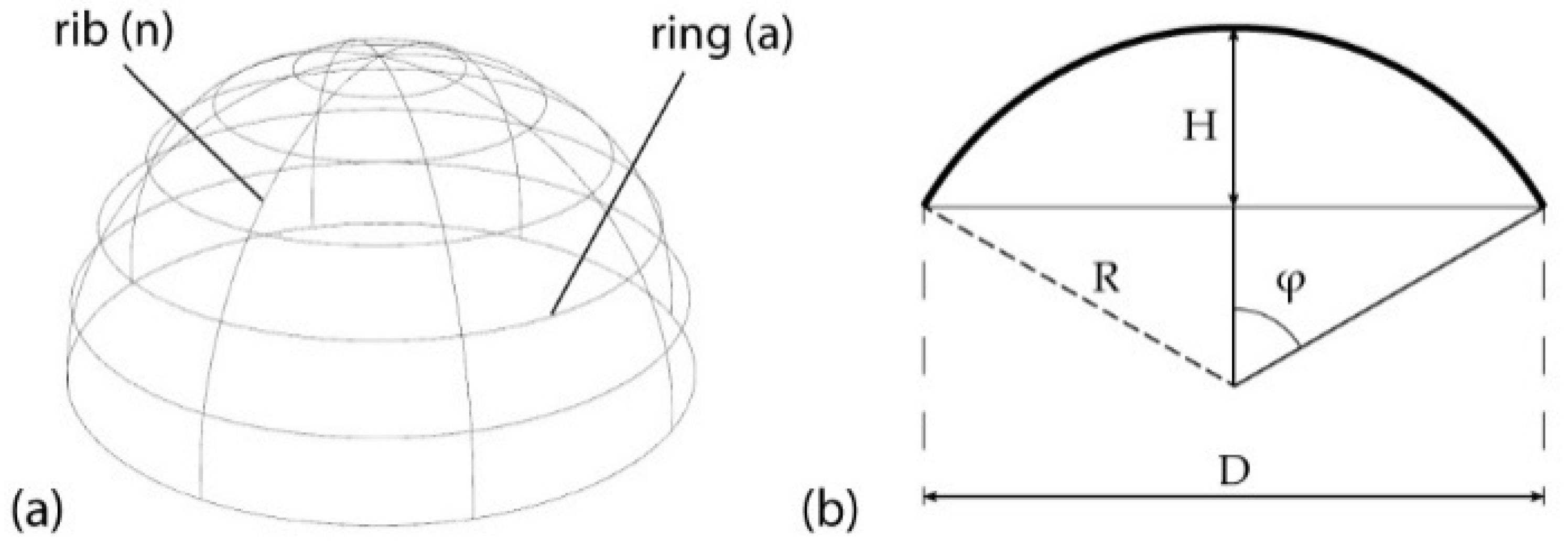
3.2. Geometric Characterization of a Ribbed Dome
- The number of ribs (n) and number of rings (a).
- The height (H) and the span (D) of the dome.
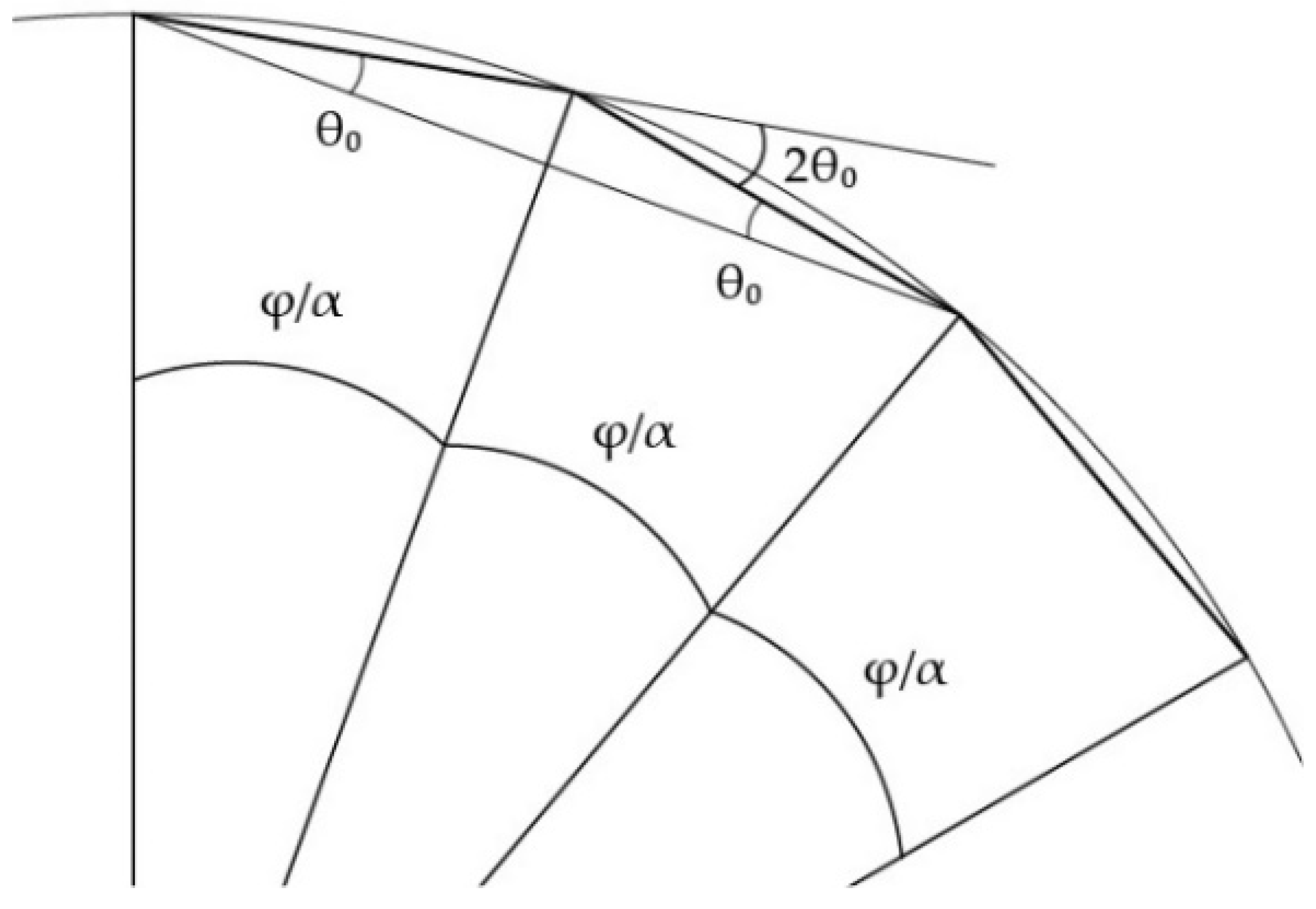
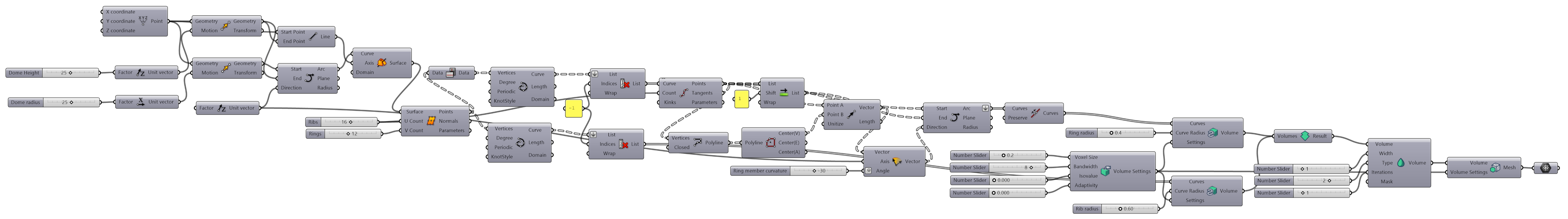
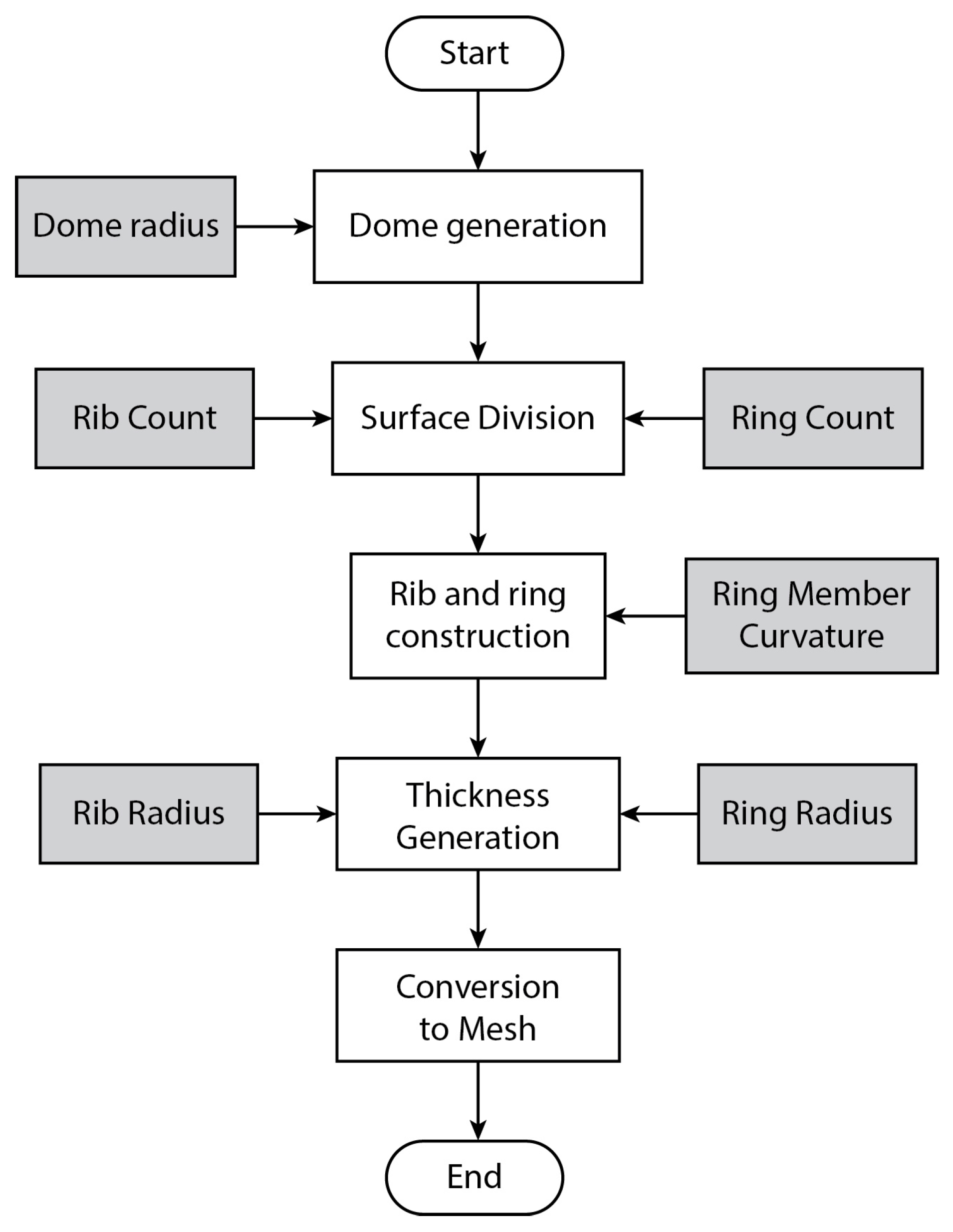
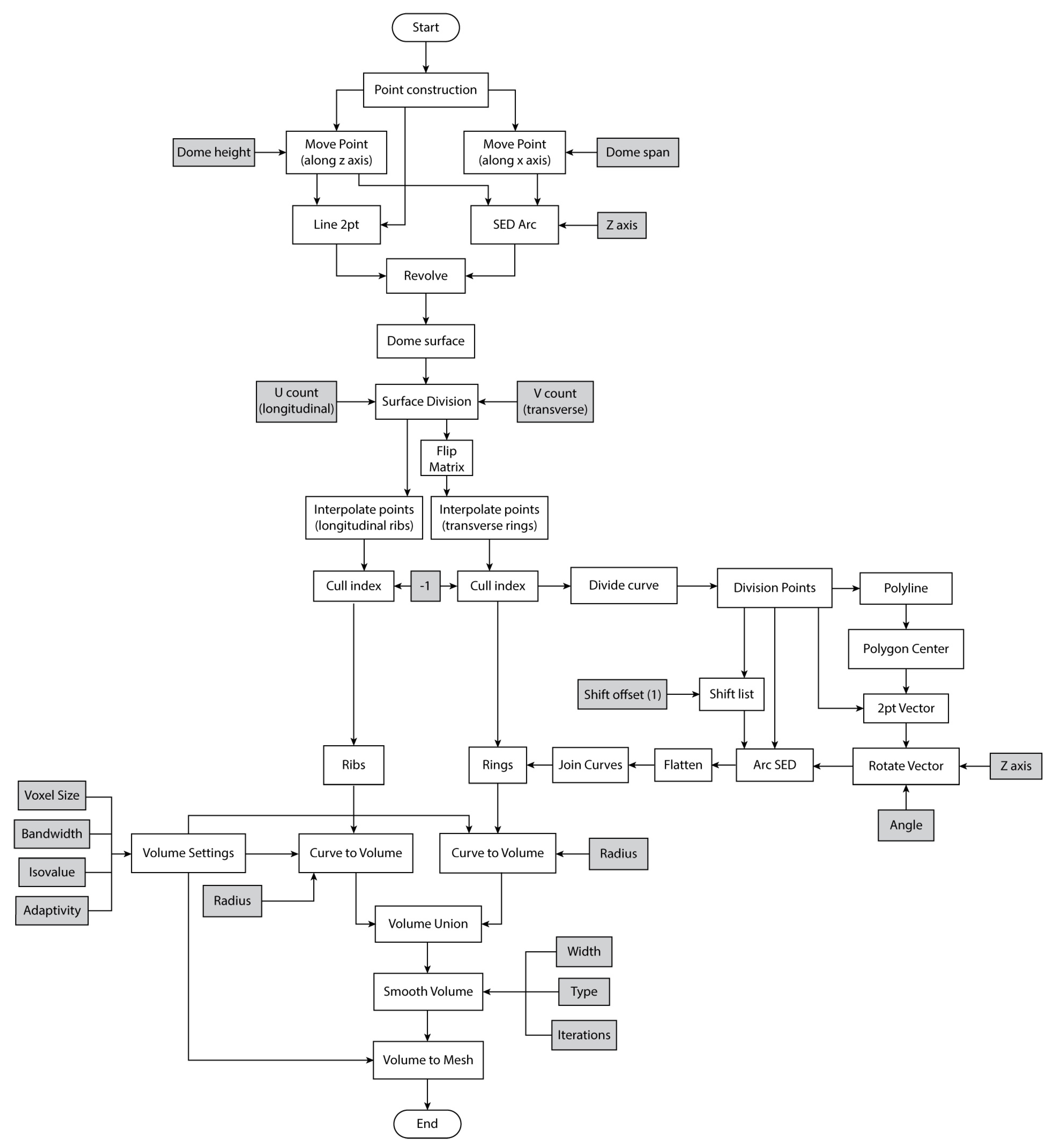
3.3. Algorithmic Design
4. Results and Discussion
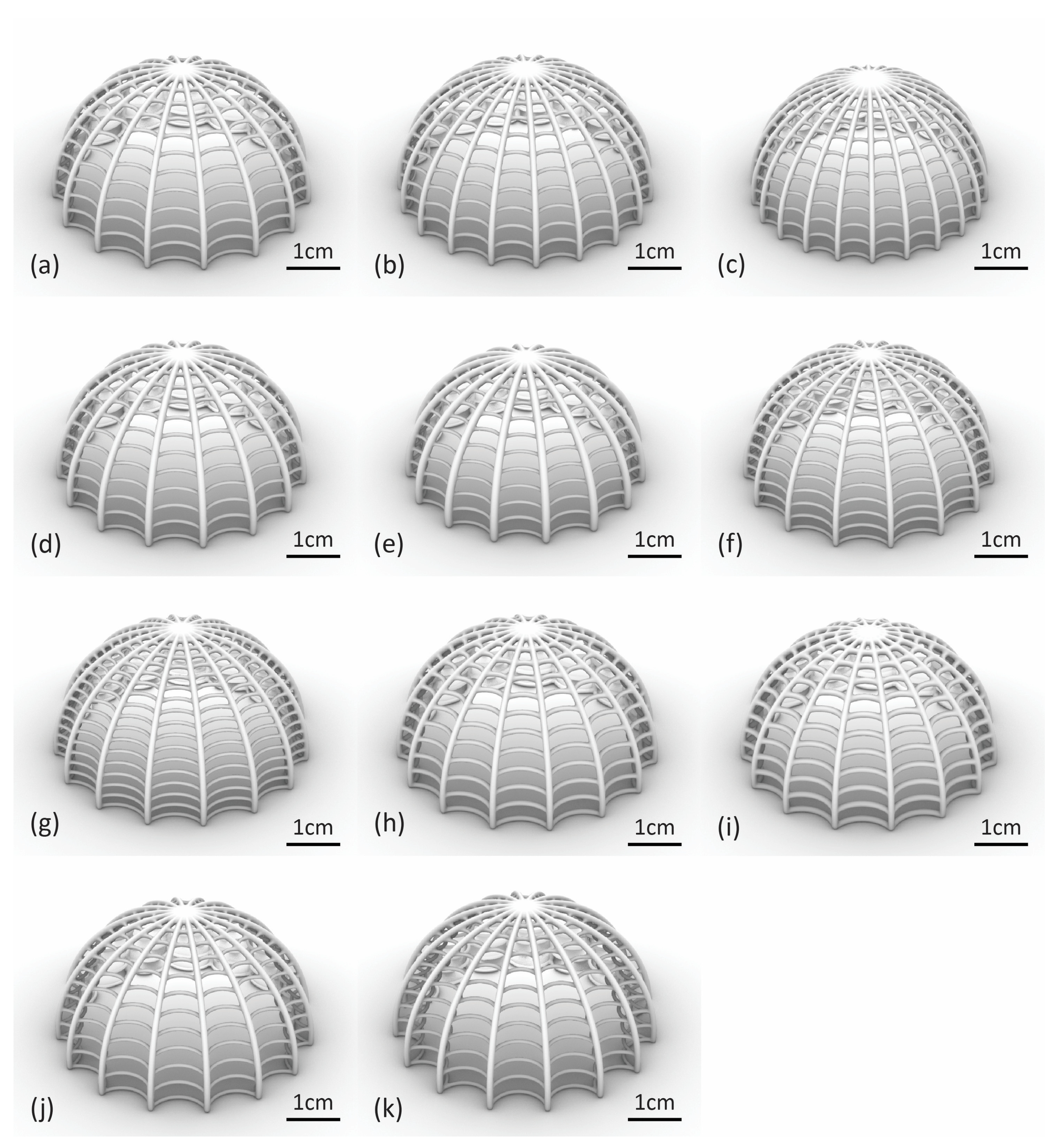
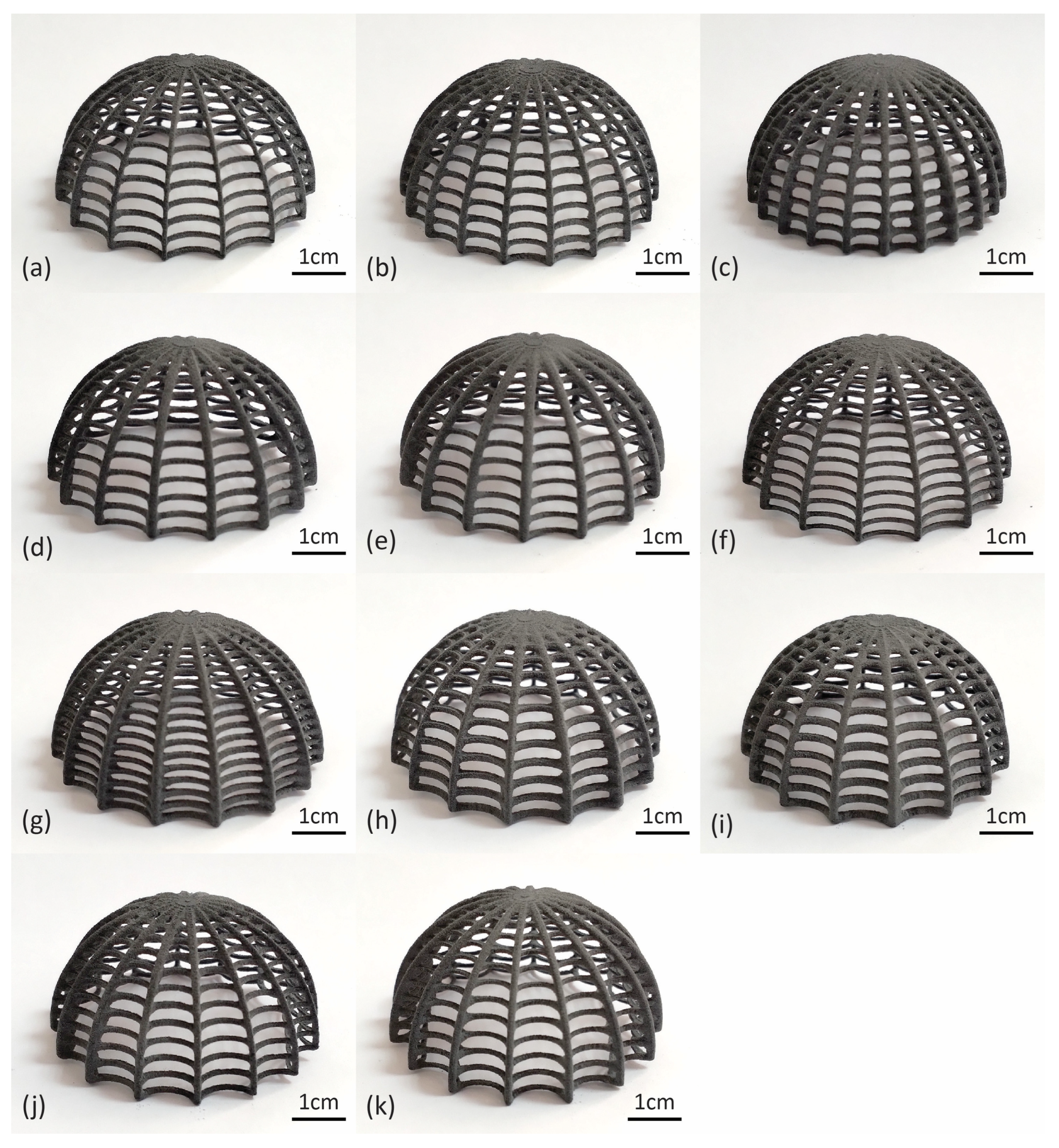
4.1. Algorithmic Design and SLS Printing Results
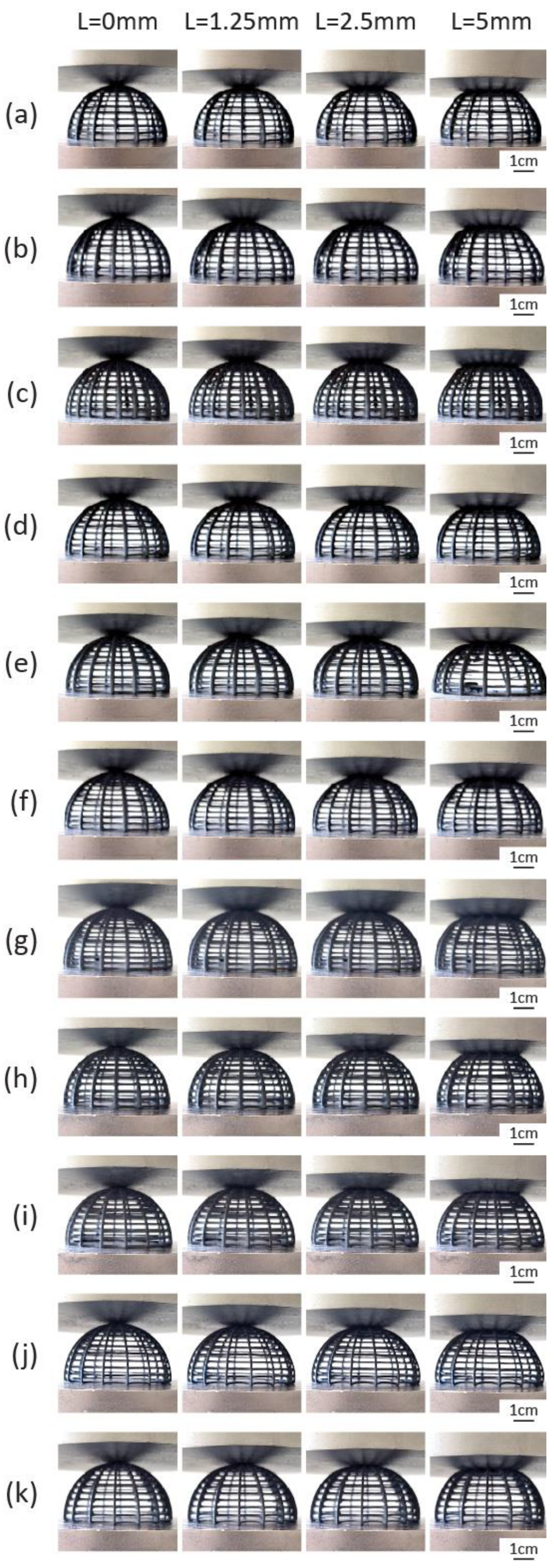
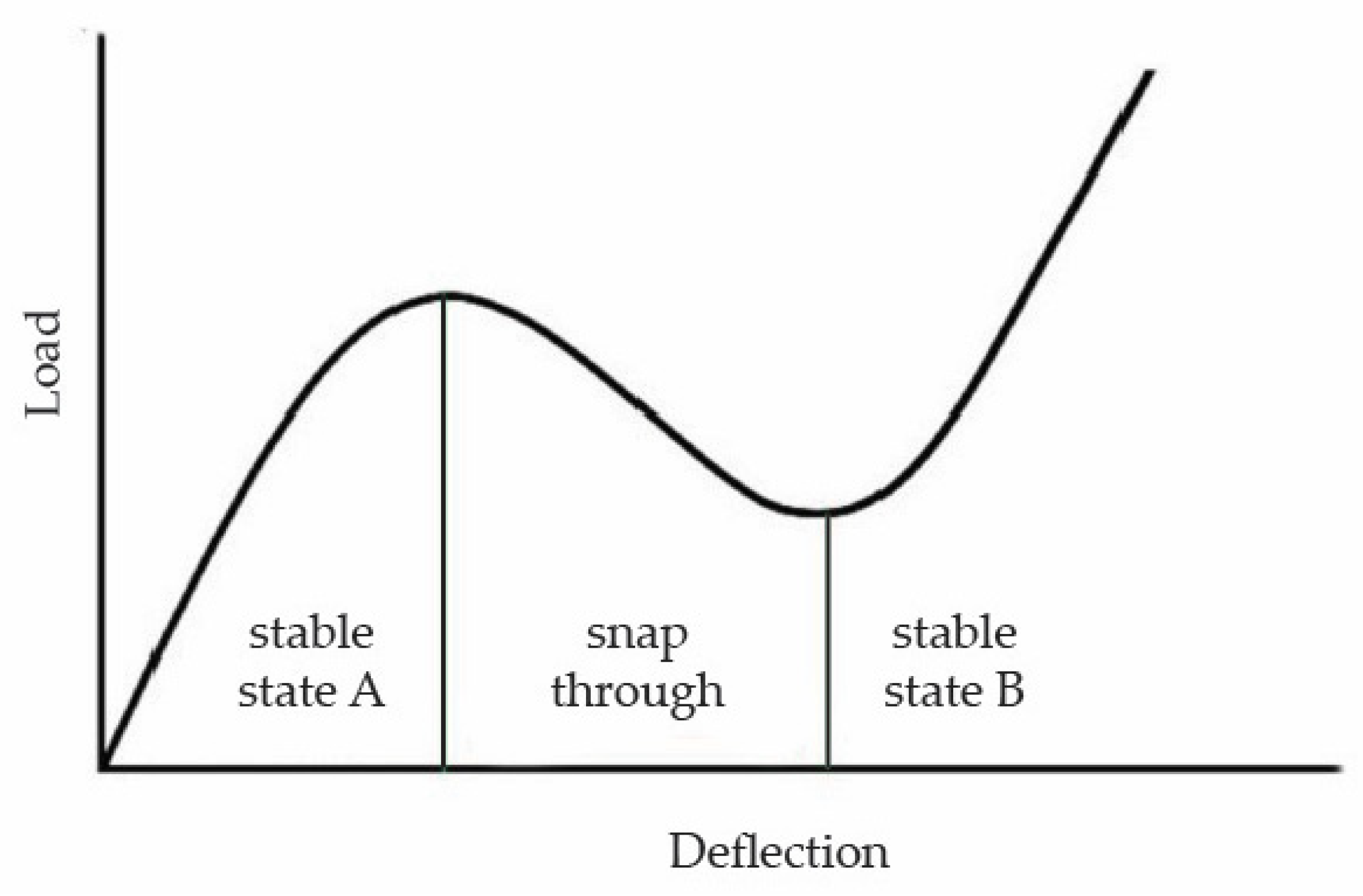
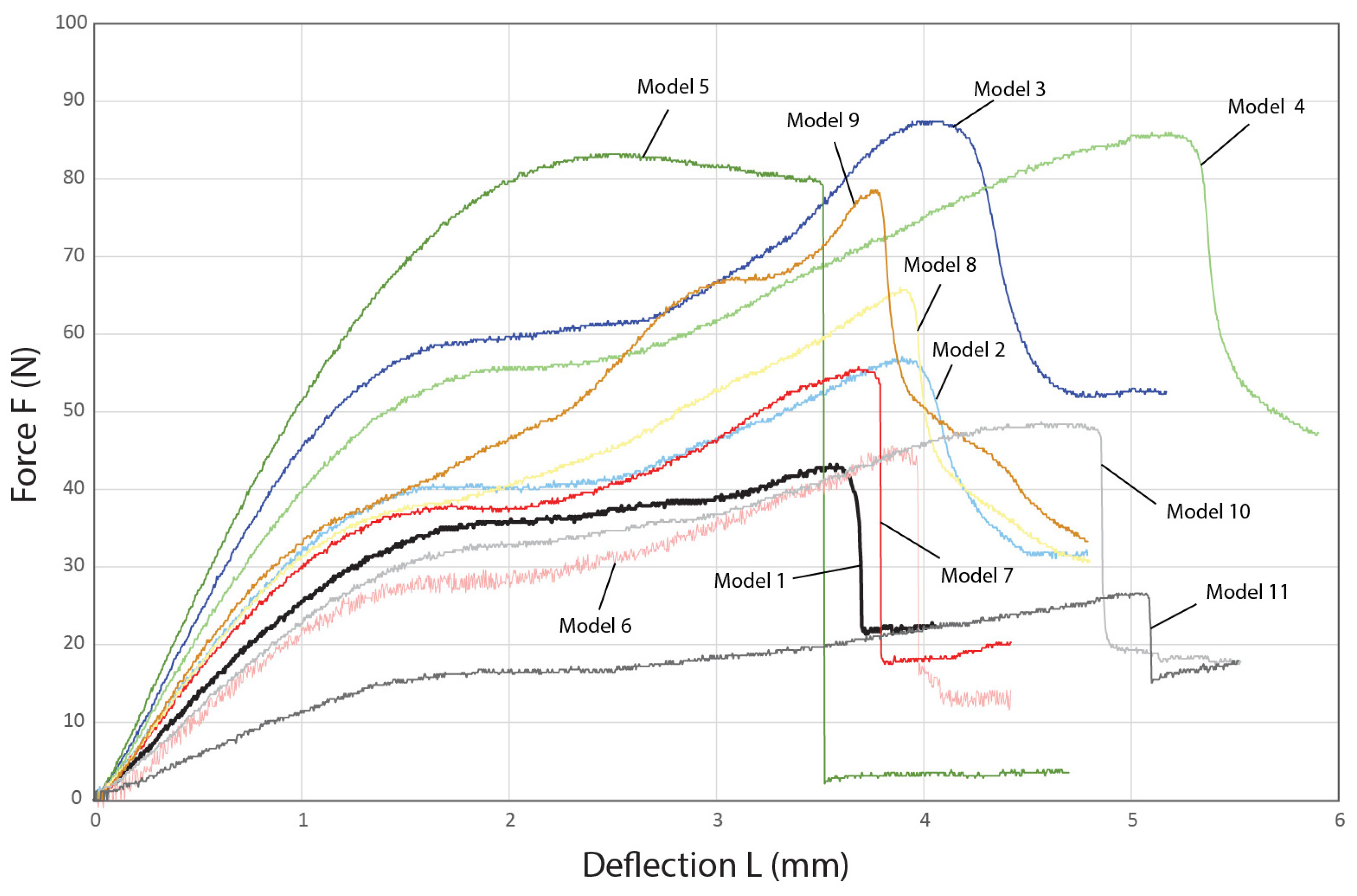
4.2. Characterization of the Compressive Behavior of the 3D-Printed Biomimetic Ribbed Dome Structure
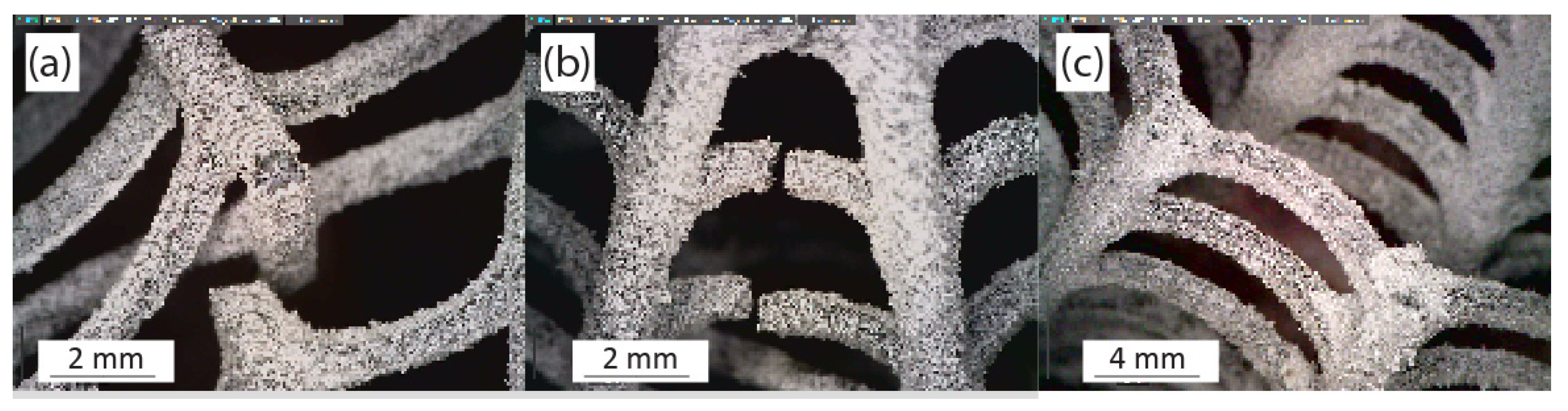
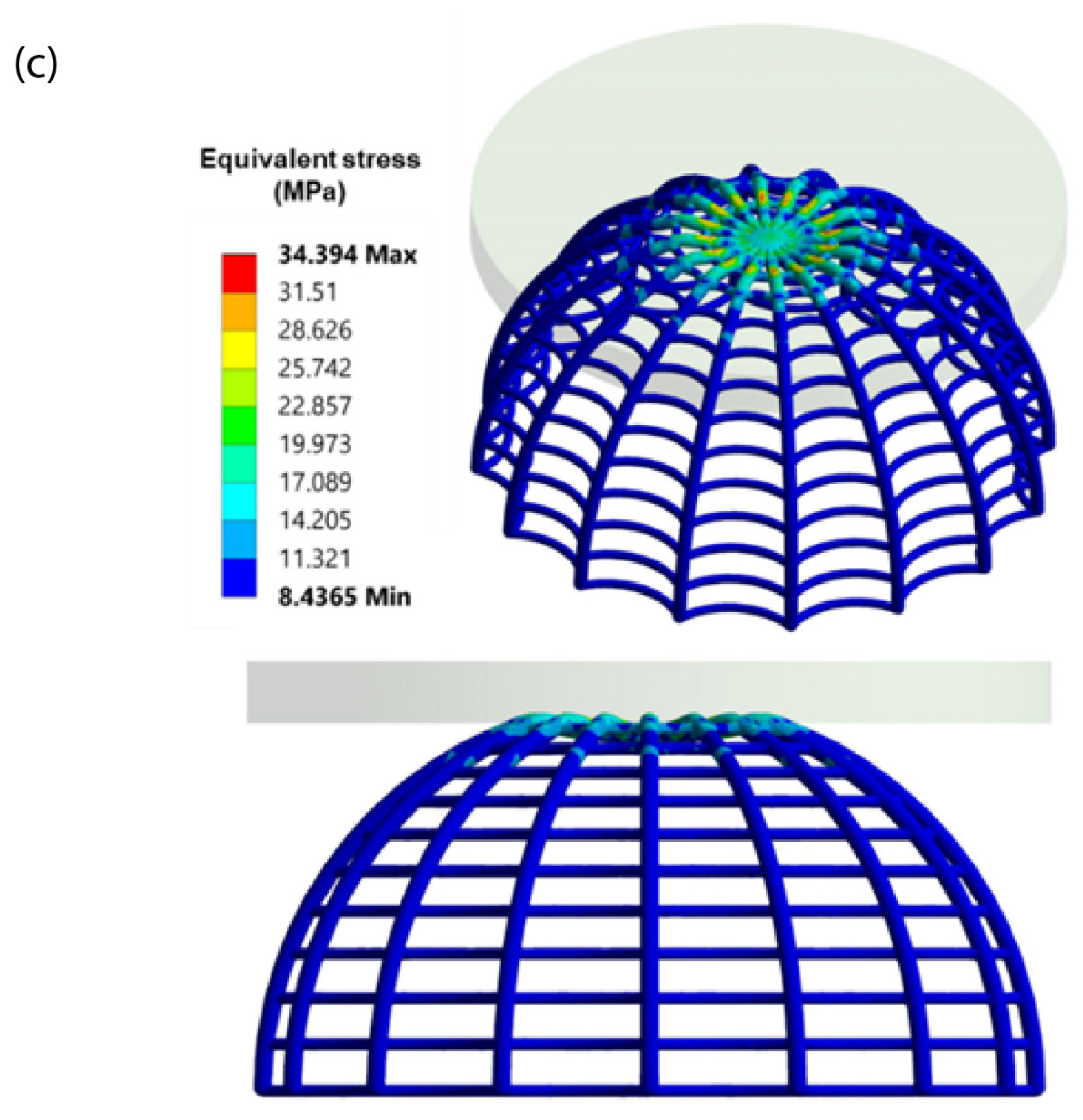
4.3. FEA Validation of Experimental Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Benyus, J.M. Biomimicry: Innovation Inspired by Nature; Harper Perennial: New York, NY, USA, 2002. [Google Scholar]
- Nagel, J.K.S.; Stone, R.B.; McAdams, D.A. An Engineering-to-Biology Thesaurus for Engineering Design. In Proceedings of the 22nd International Conference on Design Theory and Methodology; Special Conference on Mechanical Vibration and Noise, Montreal, QC, Canada, 15–18 August 2010; ASME: New York, NY, USA, 2010; Volume 5, pp. 117–128. [Google Scholar] [CrossRef]
- Hooker, G.; Smith, E. Asknature and the biomimicry taxonomy. Insight 2016, 19, 46–49. [Google Scholar] [CrossRef]
- The Biomimicry Institute. The Power of the Biomimicry Design Spiral. Biomimicry Institute. 14 June 2016. Available online: https://biomimicry.org/biomimicry-design-spiral/ (accessed on 3 October 2021).
- Symeonidou, Ι.; Efstathiadis, A. Biomimetic principles for energy efficiency in buildings. In Proceedings of the 8th International Conference on ENERGY in BUILDINGS 2019, Athens, Greece, 28 September 2019; pp. 33–42. [Google Scholar]
- Knippers, J.; Speck, T. Design and construction principles in nature and architecture. Bioinspir. Biomim. 2012, 7, 015002. [Google Scholar] [CrossRef] [PubMed]
- Knippers, J.; Nickel, K.G.; Speck, T. (Eds.) Biomimetic Research for Architecture and Building Construction: Biological Design and Integrative Structures. In Biologically-Inspired Systems, 1st ed.; No. 8; Springer International Publishing: Cham, Switzerland; Imprint: Tokyo, Japan, 2016. [Google Scholar] [CrossRef]
- Magna, R.L.; Gabler, M.; Reichert, S.; Schwinn, T.; Waimer, F.; Menges, A.; Knippers, J. From Nature to Fabrication: Biomimetic Design Principles for the Production of Complex Spatial Structures. Int. J. Space Struct. 2013, 28, 27–39. [Google Scholar] [CrossRef]
- Aish, R.; Woodbury, R. Multi-level Interaction in Parametric Design. In Smart Graphics; Butz, A., Fisher, B., Krüger, A., Olivier, P., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2005; Volume 3638, pp. 151–162. [Google Scholar] [CrossRef]
- Bertacchini, F.; Bilotta, E.; Demarco, F.; Pantano, P.; Scuro, C. Multi-objective optimization and rapid prototyping for jewelry industry: Methodologies and case studies. Int. J. Adv. Manuf. Technol. 2021, 112, 2943–2959. [Google Scholar] [CrossRef]
- Nordin, A.; Hopf, A.; Motte, D. Generative Design Systems for the Industrial Design of Functional Mass Producible Natural-Mathematical Forms. In Proceedings of the 5th International Congress of International Association of Societies of Design Research-IASDR’13, Tokyo, Japan, 26–30 August 2013; pp. 2931–2941. [Google Scholar]
- Knippers, J.; Kropp, C.; Menges, A.; Sawodny, O.; Weiskopf, D. Integrative computational design and construction: Rethinking architecture digitally. Civ. Eng. Des. 2021, 3, 123–135. [Google Scholar] [CrossRef]
- Eckert, C.; Kelly, I.; Stacey, M. Interactive generative systems for conceptual design: An empirical perspective. Artif. Intell. Eng. Des. Anal. Manuf. 1999, 13, 303–320. [Google Scholar] [CrossRef]
- Bates, S.R.G.; Farrow, I.R.; Trask, R.S. 3D printed polyurethane honeycombs for repeated tailored energy absorption. Mater. Des. 2016, 112, 172–183. [Google Scholar] [CrossRef]
- Yang, Y.; Song, X.; Li, X.; Chen, Z.; Zhou, C.; Zhou, Q.; Chen, Y. Recent Progress in Biomimetic Additive Manufacturing Technology: From Materials to Functional Structures. Adv. Mater. 2018, 30, 1706539. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Yao, H.; Zeng, Y.; Chen, J. 3D Printing of Hollow Lattice Structures of ZrO2(3Y)/Al2O3 Ceramics by Vat Photopolymerization: Process Optimization, Microstructure Evolution and Mechanical Properties. J. Manuf. Process. 2022, 83, 756–767. [Google Scholar] [CrossRef]
- Razaviye, M.K.; Tafti, R.A.; Khajehmohammadi, M. An investigation on mechanical properties of PA12 parts produced by a SLS 3D printer: An experimental approach. CIRP J. Manuf. Sci. Technol. 2022, 38, 760–768. [Google Scholar] [CrossRef]
- Goodridge, R.D.; Tuck, C.J.; Hague, R.J.M. Laser sintering of polyamides and other polymers. Prog. Mater. Sci. 2012, 57, 229–267. [Google Scholar] [CrossRef]
- Tofail, S.A.M.; Koumoulos, E.P.; Bandyopadhyay, A.; Bose, S.; O’Donoghue, L.; Charitidis, C. Additive manufacturing: Scientific and technological challenges, market uptake and opportunities. Mater. Today 2018, 21, 22–37. [Google Scholar] [CrossRef]
- Szymczyk-Ziółkowska, P.; Łabowska, M.B.; Detyna, J.; Michalak, I.; Gruber, P. A review of fabrication polymer scaffolds for biomedical applications using additive manufacturing techniques. Biocybern. Biomed. Eng. 2020, 40, 624–638. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Wu, D.; Bai, J. Current Status and Prospects of Polymer Powder 3D Printing Technologies. Materials 2020, 13, 2406. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Y.; Wu, D.; Ananth, K.P.; Bai, J. The process and performance comparison of polyamide 12 manufactured by multi jet fusion and selective laser sintering. J. Manuf. Process. 2019, 47, 419–426. [Google Scholar] [CrossRef]
- Cobian, L.; Rueda-Ruiz, M.; Fernandez-Blazquez, J.; Martinez, V.; Galvez, F.; Karayagiz, F.; Lück, T.; Segurado, J.; Monclus, M. Micromechanical characterization of the material response in a PA12-SLS fabricated lattice structure and its correlation with bulk behavior. Polym. Test. 2022, 110, 107556. [Google Scholar] [CrossRef]
- Munir, K.S.; Li, Y.; Wen, C. Metallic scaffolds manufactured by selective laser melting for biomedical applications. In Metallic Foam Bone; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–23. [Google Scholar] [CrossRef]
- Freeman, C.L.; Harding, J.H.; Quigley, D.; Rodger, P.M. Structural Control of Crystal Nuclei by an Eggshell Protein. Angew. Chem. Int. Ed. 2010, 49, 5135–5137. [Google Scholar] [CrossRef] [PubMed]
- Rezende, G.L.; Vargas, H.C.M.; Moussian, B.; Cohen, E. Composite Eggshell Matrices: Chorionic Layers and Sub-chorionic Cuticular Envelopes. In Extracellular Composite Matrices in Arthropods; Cohen, E., Moussian, B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 325–366. [Google Scholar] [CrossRef]
- Nys, Y.; Gautron, J.; Garcia-Ruiz, J.M.; Hincke, M.T. Avian eggshell mineralization: Biochemical and functional characterization of matrix proteins. C. R. Palevol 2004, 3, 549–562. [Google Scholar] [CrossRef]
- Sidorov, A.V.; Kolesnichenko, K.A.; Sviridov, A.V. Features of the Chorion Egg Structure in Nine Species of Underwing Moths Belonging to the Genus Catocala (Lepidoptera, Erebidae). Entomol. Rev. 2019, 99, 615–620. [Google Scholar] [CrossRef]
- Legendre, L.J.; Choi, S.; Clarke, J.A. The diverse terminology of reptile eggshell microstructure and its effect on phylogenetic comparative analyses. J. Anat. 2022, 241, 641–666. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Kumar, K. Ultrastructure of egg chorion of castor butterfly Ariadne merione (Crammer) (Lepidoptera: Nymphalidae). Zool. Anz.-J. Comp. Zool. 2016, 263, 1–5. [Google Scholar] [CrossRef]
- Cônsoli, F.L.; Kitajima, E.W.; Parra, J.R.P. Ultrastructure of the natural and factitious host eggs of Trichogramma galloi Zucchi and Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae). Int. J. Insect Morphol. Embryol. 1999, 28, 211–231. [Google Scholar] [CrossRef]
- Bigatti, G.; Giraud-Billoud, M.; Vega, I.A.; Penchaszadeh, P.E.; Castro-Vazquez, A. The calcareous egg capsule of the Patagonian neogastropod Odontocymbiola magellanica: Morphology, secretion and mineralogy. J. Molluscan Stud. 2010, 76, 279–288. [Google Scholar] [CrossRef]
- Nieves-Uribe, S.; Flores-Gallardo, A.; Llorente-Bousquets, J. Chorion exploration in the tribe Anthocharidini (Lepidoptera: Pieridae) and their possible importance in its systematics. Zootaxa 2020, 4868, 151–207. [Google Scholar] [CrossRef]
- Williams, M. Chapter 2. Scientific Aspects of Lepidopterology. In Practical Guide to the Study of Lepidoptera in Africa; Lepidopterist’s Society of Africa: Cascades, South Africa, 2015; 18p. [Google Scholar]
- Hincke, T.M. The eggshell: Structure, composition and mineralization. Front. Biosci. 2012, 17, 1266. [Google Scholar] [CrossRef]
- Hahn, E.N.; Sherman, V.R.; Pissarenko, A.; Rohrbach, S.D.; Fernandes, D.J.; Meyers, M.A. Nature’s technical ceramic: The avian eggshell. J. R. Soc. Interface 2017, 14, 20160804. [Google Scholar] [CrossRef]
- Efstathiadis, A.; Symeonidou, I. Developing Strategies for the Analysis and Implementation of Biomimetic Design Solutions. Presented at the eCAADe 2023: Digital Design Reconsidered, Graz, Austria, 20–22 September 2023; pp. 87–94. [Google Scholar] [CrossRef]
- Efstathiadis, A.; Symeonidou, I.; Tsongas, K.; Tzimtzimis, E.K.; Tzetzis, D. Parametric Design and Mechanical Characterization of 3D-Printed PLA Composite Biomimetic Voronoi Lattices Inspired by the Stereom of Sea Urchins. J. Compos. Sci. 2022, 7, 3. [Google Scholar] [CrossRef]
- Efstathiadis, A.; Symeonidou, I.; Tsongas, K.; Tzimtzimis, E.K.; Tzetzis, D. 3D Printed Voronoi Structures Inspired by Paracentrotus lividus Shells. Designs 2023, 7, 113. [Google Scholar] [CrossRef]
- Symeonidou, I.; Efsthathiadis, A. Digital Biomimicry: Developing an algorithmic design curriculum. Presented at the eCAADe 2023: Digital Design Reconsidered, Graz, Austria, 20–22 September 2023; pp. 109–116. [Google Scholar] [CrossRef]
- Efstathiadis, A.; Symeonidou, I. High performance biological structures. In Proceedings of the 4th International Conference for Biodigital Architecture & Genetics, Barcelona, Spain, 6 November 2020; iBAG-UIC Barcelona, Institute for Biological Architecture & Genetics, Universitat Internacional de Catalunya: Barcelona, Spain, 2020; pp. 22–33. [Google Scholar]
- García-Barros, E. The eggs of European satyrine butterflies (Nymphalidae): External morphology and its use in systematics. Zool. J. Linn. Soc. 1995, 115, 73–115. [Google Scholar] [CrossRef]
- Munguira, M.L.; Martín, J.; García-Barros, E.; Shahbazian, G.; Cancela, J.P. Morphology and morphometry of Lycaenid eggs (Lepidoptera: Lycaenidae). Zootaxa 2015, 3937, 201. [Google Scholar] [CrossRef]
- Tolman, T.; Lewington, R. Collins Butterfly Guide, New ed.; Collins: London, UK, 2008. [Google Scholar]
- Pamperis, L.N. The Butterflies of Greece; 2. Aufl; Editions Pamperis: Athens, Greece, 2009. [Google Scholar]
- Newbury, D.E. (Ed.) Advanced Scanning Electron Microscopy and X-Ray Microanalysis; Plenum Press: New York, NY, USA, 1986. [Google Scholar]
- Dendro. Food4Rhino. Available online: https://www.food4rhino.com/app/dendro (accessed on 18 March 2021).
- Llorente-Bousquets, J.; Nieves-Uribe, S.; Flores-Gallardo, A.; Hernández-Mejía, B.C.; Castro-Gerardino, J. Chorionic sculpture of eggs in the subfamily Dismorphiinae (Lepidoptera: Papilionoidea: Pieridae). Zootaxa 2018, 4429, 201. [Google Scholar] [CrossRef]
- Nieves-Uribe, S.; Llorente-Bousquets, J.; Flores-Gallardo, A. Micropylar and Perimicropylar Regions of the Egg Exochorion in Six Genera of Dismorphiinae (Lepidoptera: Pieridae). Zootaxa 2021, 4966, 251289. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Tang, T.; Yang, H.; Hao, F.; Wu, G.; Lyu, F.; Bu, Y.; Zhao, Y.; Zhao, Y.; Liu, G.; et al. The Twisting of Dome-Like Metamaterial from Brittle to Ductile. Adv. Sci. 2021, 8, 2002701. [Google Scholar] [CrossRef]
- López, A.; Puente, I.; Serna, M.A. Direct evaluation of the buckling loads of semi-rigidly jointed single-layer latticed domes under symmetric loading. Eng. Struct. 2007, 29, 101–109. [Google Scholar] [CrossRef]
- Nayak, C.B.; Jain, M.A.; Walke, S.B. Parametric Study of Dome with and Without Opening. J. Inst. Eng. India Ser. A 2020, 101, 463–475. [Google Scholar] [CrossRef]
- Timoshenko, S.; Woinowsky-Krieger, S. Theory of Plates and Shells, 2nd ed.; 13th Reprint; McGraw-Hill Education: New Delhi, India, 2015. [Google Scholar]
- Kato, S.; Mutoh, I.; Shomura, M. Collapse of semi-rigidly jointed reticulated domes with initial geometric imperfections. J. Constr. Steel Res. 1998, 48, 145–168. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, L.; Karlsson, A.M. Mechanics-based analysis of selected features of the exoskeletal microstructure of Popillia japonica. J. Mater. Res. 2009, 24, 3253–3267. [Google Scholar] [CrossRef]
- Easey, N.; Chuprynyuk, D.; Musa, W.M.S.W.; Bangs, A.; Dobah, Y.; Shterenlikht, A.; Scarpa, F. Dome-Shape Auxetic Cellular Metamaterials: Manufacturing, Modeling, and Testing. Front. Mater. 2019, 6, 86. [Google Scholar] [CrossRef]
- Leahu-Aluas, I.; Abed-Meraim, F. A proposed set of popular limit-point buckling benchmark problems. Struct. Eng. Mech. 2011, 38, 767–802. [Google Scholar] [CrossRef]
- Gioncu, V. Buckling of Reticulated Shells: State-of-the-Art. Int. J. Space Struct. 1995, 10, 1–46. [Google Scholar] [CrossRef]
- Plaut, R.H. Snap-through of shallow reticulated domes under unilateral displacement control. Int. J. Solids Struct. 2018, 148–149, 24–34. [Google Scholar] [CrossRef]
- Plaut, R.H. Snap-through of arches and buckled beams under unilateral displacement control. Int. J. Solids Struct. 2015, 63, 109–113. [Google Scholar] [CrossRef]
- Fan, F.; Yan, J.; Cao, Z. Stability of reticulated shells considering member buckling. J. Constr. Steel Res. 2012, 77, 32–42. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.L. Tuning and switching of band gap of the periodically undulated beam by the snap through buckling. AIP Adv. 2017, 7, 055210. [Google Scholar] [CrossRef]
- Hu, N.; Burgueño, R. Buckling-induced smart applications: Recent advances and trends. Smart Mater. Struct. 2015, 24, 063001. [Google Scholar] [CrossRef]
- Giarmas, E.; Tsongas, K.; Tzimtzimis, E.K.; Korlos, A.; Tzetzis, D. Mechanical and FEA-Assisted Characterization of 3D Printed Continuous Glass Fiber Reinforced Nylon Cellular Structures. J. Compos. Sci. 2021, 5, 313. [Google Scholar] [CrossRef]
- Kladovasilakis, N.; Tsongas, K.; Kostavelis, I.; Tzovaras, D.; Tzetzis, D. Effective Mechanical Properties of Additive Manufactured Strut-Lattice Structures: Experimental and Finite Element Study. Adv. Eng. Mater. 2022, 24, 2100879. [Google Scholar] [CrossRef]
- Kladovasilakis, N.; Tsongas, K.; Kostavelis, I.; Tzovaras, D.; Tzetzis, D. Effective mechanical properties of additive manufactured triply periodic minimal surfaces: Experimental and finite element study. Int. J. Adv. Manuf. Technol. 2022, 121, 7169–7189. [Google Scholar] [CrossRef]
- Zoumaki, M.; Mansour, M.T.; Tsongas, K.; Tzetzis, D.; Mansour, G. Mechanical Characterization and Finite Element Analysis of Hierarchical Sandwich Structures with PLA 3D-Printed Core and Composite Maize Starch Biodegradable Skins. J. Compos. Sci. 2022, 6, 118. [Google Scholar] [CrossRef]
- Kladovasilakis, N.; Tsongas, K.; Tzetzis, D. Finite Element Analysis of Orthopedic Hip Implant with Functionally Graded Bioinspired Lattice Structures. Biomimetics 2020, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Kladovasilakis, N.; Charalampous, P.; Boumpakis, A.; Kontodina, T.; Tsongas, K.; Tzetzis, D.; Kostavelis, I.; Givissis, P.; Tzovaras, D. Development of biodegradable customized tibial scaffold with advanced architected materials utilizing additive manufacturing. J. Mech. Behav. Biomed. Mater. 2023, 141, 105796. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.T.; Tsongas, K.; Tzetzis, D. Carbon-Fiber- and Nanodiamond-Reinforced PLA Hierarchical 3D-Printed Core Sandwich Structures. J. Compos. Sci. 2023, 7, 285. [Google Scholar] [CrossRef]
- Kladovasilakis, N.; Tsongas, K.; Karalekas, D.; Tzetzis, D. Architected Materials for Additive Manufacturing: A Comprehensive Review. Materials 2022, 15, 5919. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.T.; Tsongas, K.; Tzetzis, D. 3D Printed Hierarchical Honeycombs with Carbon Fiber and Carbon Nanotube Reinforced Acrylonitrile Butadiene Styrene. J. Compos. Sci. 2021, 5, 62. [Google Scholar] [CrossRef]

| Design Parameter | Value |
|---|---|
| Dome Span | 50 mm |
| Dome Height | 25 mm |
| U count | 16 |
| V count | 12 |
| SED angle | −30° |
| Curve Radius (ribs) | 0.6 mm |
| Curve Radius (rings) | 0.4 mm |
| Voxel Size | 0.2 |
| Bandwidth | 8 |
| Isovalue | 0 |
| Adaptivity | 0 |
| Smooth Width | 1 |
| Type | 2 (mean) |
| Iterations | 1 |
| Model | Rib Count | Rib Radius (mm) | Ring Count | Ring Radius (mm) | Ring Curvature |
|---|---|---|---|---|---|
| 1 | 16 | 0.6 | 12 | 0.4 | −30° |
| 2 | 20 | 0.6 | 12 | 0.4 | −30° |
| 3 | 24 | 0.6 | 12 | 0.4 | −30° |
| 4 | 16 | 0.7 | 12 | 0.4 | −30° |
| 5 | 16 | 0.8 | 12 | 0.4 | −30° |
| 6 | 16 | 0.6 | 15 | 0.4 | −30° |
| 7 | 16 | 0.6 | 18 | 0.4 | −30° |
| 8 | 16 | 0.6 | 12 | 0.5 | −30° |
| 9 | 16 | 0.6 | 12 | 0.6 | −30° |
| 10 | 16 | 0.6 | 12 | 0.4 | −15° |
| 11 | 16 | 0.6 | 12 | 0.4 | 0° |
| Printer Parameter | Value |
|---|---|
| Printer Model | Lisa rev.A |
| Material | Polyamide12 Smooth |
| Layer Height | 0.125 mm |
| Laser Power Ratio | 1.00 |
| Print Surface Temperature Offset | 0 °C |
| Shrink Ratio | 1.000 × 1.000 × 1.000 |
| Total Print Time (avg) | 13 h 50 min |
| Powder Needed in Feed Bed (avg) | 3.82 L |
| Refresh Powder After Print (avg) | 0.86 L |
| Feed Bed Power Height (avg) | 9.6 cm |
| Model | Loadmax (N) |
|---|---|
| 1 | 42.1 ± 2.44 |
| 2 | 58.33 ± 1.5 |
| 3 | 87.77 ± 2.27 |
| 4 | 87.2 ± 2.34 |
| 5 | 83.03 ± 5.05 |
| 6 | 45.27 ± 2.32 |
| 7 | 51.47 ± 6.97 |
| 8 | 66.47 ± 6.31 |
| 9 | 76.87 ± 11.1 |
| 10 | 47.1 ± 2.19 |
| 11 | 26.6 ± 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efstathiadis, A.; Symeonidou, I.; Tzimtzimis, E.K.; Avtzis, D.; Tsongas, K.; Tzetzis, D. Parametric Design and Mechanical Characterization of a Selective Laser Sintering Additively Manufactured Biomimetic Ribbed Dome Inspired by the Chorion of Lepidopteran Eggs. Biomimetics 2025, 10, 1. https://doi.org/10.3390/biomimetics10010001
Efstathiadis A, Symeonidou I, Tzimtzimis EK, Avtzis D, Tsongas K, Tzetzis D. Parametric Design and Mechanical Characterization of a Selective Laser Sintering Additively Manufactured Biomimetic Ribbed Dome Inspired by the Chorion of Lepidopteran Eggs. Biomimetics. 2025; 10(1):1. https://doi.org/10.3390/biomimetics10010001
Chicago/Turabian StyleEfstathiadis, Alexandros, Ioanna Symeonidou, Emmanouil K. Tzimtzimis, Dimitrios Avtzis, Konstantinos Tsongas, and Dimitrios Tzetzis. 2025. "Parametric Design and Mechanical Characterization of a Selective Laser Sintering Additively Manufactured Biomimetic Ribbed Dome Inspired by the Chorion of Lepidopteran Eggs" Biomimetics 10, no. 1: 1. https://doi.org/10.3390/biomimetics10010001
APA StyleEfstathiadis, A., Symeonidou, I., Tzimtzimis, E. K., Avtzis, D., Tsongas, K., & Tzetzis, D. (2025). Parametric Design and Mechanical Characterization of a Selective Laser Sintering Additively Manufactured Biomimetic Ribbed Dome Inspired by the Chorion of Lepidopteran Eggs. Biomimetics, 10(1), 1. https://doi.org/10.3390/biomimetics10010001












