Understanding Insect Bite Hypersensitivity in Horses: A Narrative Review for Clinical Practice
Abstract
1. Introduction
2. Etiology
2.1. Genetic Predisposition and Breed Susceptibility
2.2. Immunopathogenesis of IBH
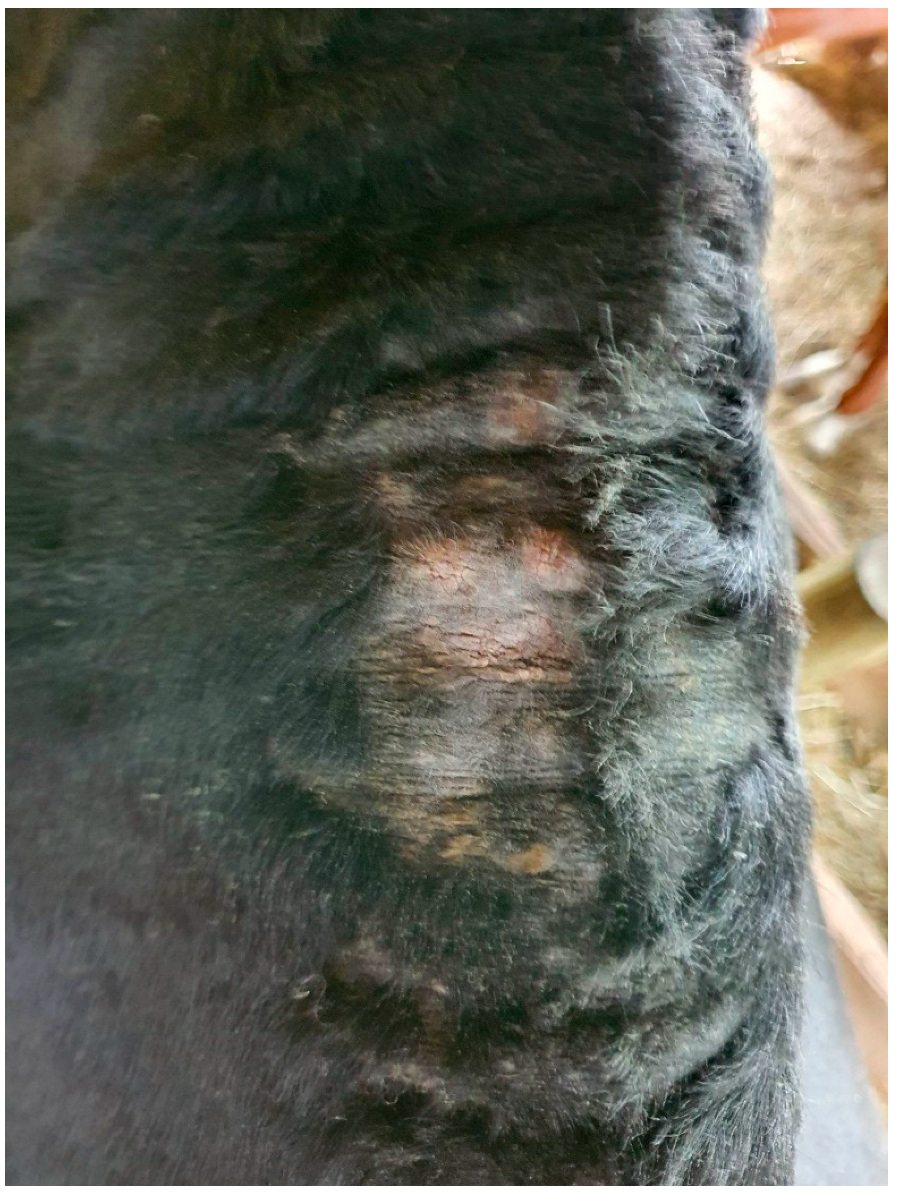
3. Clinical Presentation and Diagnosis
4. Management Approaches
5. Advances in Immunotherapy and Other Therapeutic Interventions
6. Nutritional Support
7. Comparisons to Other Species and Equids
| Treatment/ Class | Formulation and Concentration | Suggested Dose/Frequency | Evidence Level and Key Comments | Key Refs. |
|---|---|---|---|---|
| Prednisolone (systemic GC) | Oral tablets/ powder | 1.5–2 mg kg q24 h × 7–10 d → taper to 0.5 mg kg q48 h | Widely used; no prospective trials; long-term monotherapy discouraged, low risk for laminitis | [40,91,92] |
| Dexamethasone (systemic GC) | Oral solution/inj. | 0.02–0.10 mg kg q24 h (load) → 0.01–0.02 mg kg q48–72 h | Useful for prednisolone-non-responders or severe cases | [40,91,92] |
| Permethrin (pour-on) | 3.6% | 20 mL topline; repeat q14 d | Small field trial: non-sig. midge reduction; clinical value possible | [51] |
| Permethrin (spray) | 2% | Spray to coat q14 d | RCT: ↓ lesion scores vs. control | [1] |
| Cypermethrin (spray) | 0.15% | Spray; protection ≈ 2 h | Net study: brief repellency only | [1] |
| Cypermethrin (spray) | 1% | Label q24 h | No controlled data | [1] |
| Deltamethrin (spray) | 1% | Label directions | Ineffective at repelling Culicoides | [52] |
| DEET (spray/lotion) | 15% | Apply; repels ≈ 6 h | Proven midge repellency | [53] |
| Citronella + lemon-eucalyptus | 5–10% blend | Apply q2–4 h | Field study: ineffective or attractive | [55] |
| Essential-oil herbal spray | Camphor/lemongrass/ may-chang/peppermint/ patchouli | Daily × 28 d | DBPC cross-over: 95% owner-reported relief | [1] |
| Omega-3/6 topical cream | Fatty acids + humectants | Apply q24 h | Split-body RCT: ↓ lesions on treated side | [66] |
| Phytogenic ointment | Plant extracts | Apply bid × 21 d | DBPC: ↑ owner comfort vs. placebo | [93] |
| Cetirizine | Oral tablets | 0.4 mg kg−1 PO bid × 3 wks | Placebo-controlled: no benefit | [69] |
| Chlorphenamine | Oral | 0.1–0.5 mg kg−1 PO bid | Histology benefit; clinical effect untested | [1] |
| Doxepin | Oral | 0.75–1 mg kg−1 PO bid | Anecdotal only; competition restrictions | [1] |
| Diphenhydramine | Oral | 1–2 mg kg−1 PO q8–12 h | Variable response; competition restrictions | [1] |
| Linseed (flax) oil | n-3 source | 1 lb/1000 lb BW q24 h × 6 wk | RCT: no sig. change; some owner improvement | [79] |
| Flaxseed (ground) | Diet | Same as above × 42 d | ↓ intradermal reactions in AD horses | [79] |
| Evening-primrose + fish oil (80:20) | Oral | 20 g day−1 × 13 wk | 10/14 IBH horses ≥good response | [1] |
| Sunflower oil + vit/AA/peptides | Oral | 30 d course | DBPC n = 50: placebo limbs worsened; owner scores NS | [80] |
| Pentoxifylline | Oral | 10–15 mg kg−1 PO bid (may ↑ to 30 mg kg−1 day−1) | No IBH trials; empiric use | [1,94] |
| Oclacitinib (off-label) | Oral | 0.1–0.25 mg kg−1 PO q24 h | Small RCT: pruritus ↓ at 0.25 mg kg−1 | [95] |
| Hydrocortisone aceponate 0.058% spray | Topical | 2–4 sprays to focal lesions q24 h | Minimal systemic absorption; useful for mane/tail | [96] |
| Fly sheet/mask | — | Continuous wear; remove daily | Effective barrier; heat/sweat risk in humid climates | [1] |
| Stable fans | — | Airflow > 2.5 m s−1 dusk/night | ↓ Culicoides landings | [48] |
| Allergen-specific immunotherapy (SC) | Aqueous or alum-precipitated allergens | Escalating course → maintenance ~20,000 PNU mL q21 d | ~70% improve; customize mix; evaluate ≥12 mo | [1,97] |
8. Future Perspectives and Integrated Approaches
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marsella, R.; White, S.; Fadok, V.; Wilson, D.; Mueller, R.; Outerbridge, C.; Rosenkrantz, W. Equine allergic skin diseases: Clinical consensus guidelines of the World Association for Veterinary Dermatology. Vet. Dermatol. 2023, 34, 175–208. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, M.; Eriksson, S.; Schurink, A.; Andersson, L.; Sundquist, M.; Frey, R.; Lindgren, G. Genome-wide association study of insect bite hypersensitivity in Swedish-born Icelandic horses. J. Hered. 2015, 106, 366–374. [Google Scholar] [CrossRef]
- Prudhomme, J.; Bardet, C.; Rakotoarivony, I.; Garros, C.; Bouhsira, É.; Lienard, E. Local investigation into the role of Culicoides species diversity in recurrent horse dermatitis cases in south-west France. Parasites Vectors 2025, 18, 86. [Google Scholar] [CrossRef]
- Jónsdóttir, S.; Cvitaš, I.; Svansson, V.; Fettelschoss-Gabriel, A.; Torsteinsdóttir, S.; Marti, E. New strategies for prevention and treatment of insect bite hypersensitivity in horses. Curr. Dermatol. Rep. 2019, 8, 303–312. [Google Scholar] [CrossRef]
- Novotny, E.; White, S.; Wilson, A.; Stefánsdóttir, S.; Tijhaar, E.; Jónsdóttir, S.; Marti, E. Component-resolved microarray analysis of IgE sensitisation profiles to Culicoides recombinant allergens in horses with insect bite hypersensitivity. Allergy 2021, 76, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Torsteinsdóttir, S.; Scheidegger, S.; Baselgia, S.; Jónsdóttir, S.; Svansson, V.; Björnsdóttir, S.; Marti, E. A prospective study on insect bite hypersensitivity in horses exported from Iceland into Switzerland. Acta Vet. Scand. 2018, 60, 42. [Google Scholar] [CrossRef]
- Birras, J.; White, S.; Jónsdóttir, S.; Novotny, E.; Ziegler, A.; Wilson, A.; Marti, E. First clinical expression of equine insect bite hypersensitivity is associated with co-sensitisation to multiple Culicoides allergens. PLoS ONE 2021, 16, e0257819. [Google Scholar] [CrossRef]
- Ahmad, T.; Akhtar, M.; Ayaz, M.; Nazir, M.; Ahmad, E.; Hameed, M.; Hussain, M. Yerel ırk bir kısrakta böcek sokmasına bağlı aşırı duyarlılık (tatlı kaşıntı). Kafkas Univ. Vet. Fak. Derg. 2018, 25, 277–279. [Google Scholar] [CrossRef]
- Resende, C.; Santos, A.; Cook, R.; Victor, R.; Câmara, R.; Gonçalves, G.; Reis, J. Low transmission rates of equine infectious anaemia virus in foals born to seropositive feral mares inhabiting the Amazon Delta region despite high insect vector populations. BMC Vet. Res. 2022, 18, 286. [Google Scholar] [CrossRef]
- Shrestha, M.; Solé, M.; Ducro, B.; Sundquist, M.; Thomas, R.; Schurink, A.; Lindgren, G. Genome-wide association study for insect bite hypersensitivity susceptibility in horses revealed novel associated loci on chromosome 1. J. Anim. Breed. Genet. 2019, 137, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Baldacchino, F.; Puech, L.; Manon, S.; Hertzog, L.; Jay-Robert, P. Biting behaviour of Tabanidae on cattle in mountainous summer pastures, Pyrenees, France, and effects of weather variables. Bull. Entomol. Res. 2014, 104, 471–479. [Google Scholar] [CrossRef]
- Baker, T.; Carpenter, S.; Gubbins, S.; Newton, R.; Iacono, G.; Wood, J.; Harrup, L. Can insecticide-treated netting provide protection for equids from Culicoides biting midges in the United Kingdom? Parasites Vectors 2015, 8, 1182. [Google Scholar] [CrossRef]
- Lincoln, V.J.; Page, P.C.; Kopp, C.; Mathis, A.; von Niederhäusern, R.; Burger, D.; Herholz, C. Protection of horses against Culicoides biting midges in different housing systems in Switzerland. Vet. Parasitol. 2015, 210, 206–214. [Google Scholar] [CrossRef]
- Klumplerová, M.; Vychodilova, L.; Bobrova, O.; Cvanová, M.; Futas, J.; Jánová, E.; Hořín, P. Major histocompatibility complex and other allergy-related candidate genes associated with insect bite hypersensitivity in Icelandic horses. Mol. Biol. Rep. 2013, 40, 3333–3340. [Google Scholar] [CrossRef]
- Schurink, A.; Silva, V.; Velie, B.; Dibbits, B.; Crooijmans, R.; Liesbeth, F.; Ducro, B. Copy number variations in Friesian horses and genetic risk factors for insect bite hypersensitivity. BMC Genet. 2018, 19, 65. [Google Scholar] [CrossRef]
- François, L.; Hoskens, H.; Velie, B.D.; Stinckens, A.; Tinel, S.; Lamberigts, C.; Peeters, L.; Savelkoul, H.F.J.; Tijhaar, E.; Lindgren, G.; et al. Genomic regions associated with IgE levels against Culicoides spp. antigens in three horse breeds. Genes 2019, 10, 597. [Google Scholar] [CrossRef]
- Velie, B.; Shrestha, M.; Liesbeth, F.; Schurink, A.; Tesfayonas, Y.; Stinckens, A.; Lindgren, G. Using an inbred horse breed in a high-density genome-wide scan for genetic risk factors of insect bite hypersensitivity. PLoS ONE 2016, 11, e0152966. [Google Scholar] [CrossRef] [PubMed]
- Ablondi, M.; Dadousis, C.; Vasini, M.; Eriksson, S.; Mikko, S.; Sabbioni, A. Genetic diversity and signatures of selection in a native Italian horse breed based on SNP data. Animals 2020, 10, 1005. [Google Scholar] [CrossRef] [PubMed]
- Meulenbroeks, C.; Meide, N.; Willemse, T.; Rutten, V.; Tijhaar, E. Recombinant Culicoides obsoletus complex allergens stimulate antigen-specific T cells of insect bite hypersensitive Shetland ponies in vitro. Vet. Dermatol. 2015, 26, 467. [Google Scholar] [CrossRef] [PubMed]
- Meulenbroeks, C.; Meide, N.; Zaiss, D.; Oldruitenborgh-Oosterbaan, M.; Lugt, J.; Smak, J.; Willemse, T. Seasonal differences in cytokine expression in the skin of Shetland ponies suffering from insect bite hypersensitivity. Vet. Immunol. Immunopathol. 2013, 151, 147–156. [Google Scholar] [CrossRef]
- Olomski, F.; Fettelschoss, V.; Jónsdóttir, S.; Birkmann, K.; Thoms, F.; Marti, E.; Fettelschoss-Gabriel, A. Interleukin-31 in insect bite hypersensitivity—Alleviating clinical symptoms by active vaccination against itch. Allergy 2020, 75, 862–871. [Google Scholar] [CrossRef]
- Craig, N.; Munguia, N.; Trujillo, A.; Wilkes, R.; Dorr, M.; Marsella, R. Transcription of interleukin-31 and its receptor by leukocytes after Culicoides sp. stimulation is dose dependent but is not exaggerated in allergic horses or correlated with pruritus. J. Am. Vet. Med. Assoc. 2023, 261, S75–S85. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.; Stewart, A.J. Insect Bite Hypersensitivity in Horses: Causes, Diagnosis, Scoring and New Therapies. Animals 2023, 13, 2514. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jónsdóttir, S.; Stefánsdóttir, S.B.; Mirkovitch, J.; Björnsson, J.M.; Svansson, V.; Marti, E.; Torsteinsdóttir, S. Culicoides allergens expressed in insect cells induce sulphidoleukotriene release in peripheral blood leukocytes from horses affected with insect bite hypersensitivity. Front. Immunol. 2025, 16, 1597233. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A. Immune responses to ectoparasites of horses, with a focus on insect bite hypersensitivity. Parasite Immunol. 2014, 36, 560–572. [Google Scholar] [CrossRef]
- Cvitas, I.; Oberhänsli, S.; Leeb, T.; Dettwiler, M.; Müller, E.; Bruggman, R.; Marti, E. Investigating the epithelial barrier and immune signatures in the pathogenesis of equine insect bite hypersensitivity. PLoS ONE 2020, 15, e0232189. [Google Scholar] [CrossRef]
- Cvitaš, I.; Oberhaensli, S.; Leeb, T.; Marti, E. Equine keratinocytes in the pathogenesis of insect bite hypersensitivity: Just another brick in the wall? PLoS ONE 2022, 17, e0266263. [Google Scholar] [CrossRef]
- Whetstone, C.E.; Ranjbar, M.; Omer, H.; Cusack, R.P.; Gauvreau, G.M. The role of airway epithelial cell alarmins in asthma. Cells 2022, 11, 1105. [Google Scholar] [CrossRef]
- Lehiy, C.; Reister-Hendricks, L.; Ruder, M.; McVey, D.; Drolet, B. Physiological and immunological responses to Culicoides sonorensis blood-feeding: A murine model. Parasites Vectors 2018, 11, 2935. [Google Scholar] [CrossRef]
- Swiderski, C. Hypersensitivity disorders in horses. Vet. Clin. N. Am. Equine Pract. 2000, 16, 131–151. [Google Scholar] [CrossRef]
- Fadok, V.A. Update on equine allergies. Vet. Clin. N. Am. Equine Pract. 2013, 29, 541–550. [Google Scholar] [CrossRef]
- Couëtil, L.L.; Cardwell, J.M.; Gerber, V.; Lavoie, P.; Léguillette, R.; Richard, E.A. Inflammatory Airway Disease of Horses—Revised Consensus Statement. J. Vet. Intern. Med. 2016, 30, 503–515. [Google Scholar] [CrossRef]
- Lo Feudo, C.; Stucchi, L.; Alberti, E.; Conturba, B.; Zucca, E.; Ferrucci, F. Intradermal Testing Results in Horses Affected by Mild-Moderate and Severe Equine Asthma. Animals 2021, 11, 2086. [Google Scholar] [CrossRef]
- Klier, J.; Lindner, D.; Reese, S.; Mueller, R.; Gehlen, H. Comparison of Four Different Allergy Tests in Equine Asthma Affected Horses and Allergen Inhalation Provocation Test. J. Equine Vet. Sci. 2021, 102, 103433. [Google Scholar] [CrossRef]
- Karagianni, A.; Richard, E.; Toquet, M.; Hue, E.; Courouce-Malblanc, A.; McGorum, B.; Kurian, D.; Aguilar, J.; Mazeri, S.; Wishart, T.; et al. Distinct Molecular Profiles Underpin Mild-To-Moderate Equine Asthma Cytological Profiles. Cells 2024, 13, 1926. [Google Scholar] [CrossRef]
- Woodrow, J.; Hines, M.; Sommardahl, C.; Flatland, B.; Lo, Y.; Wang, Z.; Sheats, M.; Lennon, E. Initial investigation of molecular phenotypes of airway mast cells and cytokine profiles in equine asthma. Front. Vet. Sci. 2023, 9, 997139. [Google Scholar] [CrossRef]
- Lanz, S.; Brunner, A.; Graubner, C.; Marti, E.; Gerber, V. Insect Bite Hypersensitivity in Horses is Associated with Airway Hyperreactivity. J. Vet. Intern. Med. 2017, 31, 1877–1883. [Google Scholar] [CrossRef]
- Verdon, M.; Lanz, S.; Rhyner, C.; Gerber, V.; Marti, E. Allergen-specific immunoglobulin E in sera of horses affected with insect bite hypersensitivity, severe equine asthma or both conditions. J. Vet. Intern. Med. 2018, 33, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Matsuda, A.; Iwata, E.; Ono, T.; Hisaeda, K.; Ohzawa, E.; Hiasa, Y.; Kitagawa, H. Allergen-specific immunoglobulin E for dermatitis in the Japanese native Noma horses. J. Vet. Med. Sci. 2024, 86, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Marsella, R. Allergic skin diseases. In Manual of Equine Dermatology; CABI: Wallingford, UK, 2019; pp. 32–38. [Google Scholar]
- Miller, J.; Mann, S.; Fettelschoss-Gabriel, A.; Wagner, B. Comparison of three clinical scoring systems for Culicoides hypersensitivity in a herd of Icelandic horses. Vet. Dermatol. 2019, 30, 536. [Google Scholar] [CrossRef] [PubMed]
- Söderroos, D.; Ignell, R.; Haubro Andersen, P.; Bergvall, K.; Riihimäki, M. The effect of insect bite hypersensitivity on movement activity and behaviour of the horse. Animals 2023, 13, 1283. [Google Scholar] [CrossRef]
- Birkmann, K.; Jebbawi, F.; Waldern, N.; Hug, S.; Inversini, V.; Keller, G.; Holm, A.; Grest, P.; Canonica, F.; Schmid-Grendelmeier, P.; et al. Eosinophils play a surprising leading role in recurrent urticaria in horses. Vaccines 2024, 12, 562. [Google Scholar] [CrossRef]
- Peeters, L.; Janssens, S.; Coussé, A.; Buys, N. Zomereczeem bij Belgische warmbloedpaarden: Prevalentie en risicofactoren. Vlaams Diergeneeskd. Tijdschr. 2014, 83, 240–249. [Google Scholar] [CrossRef]
- Forsyth, J.; Halliwell, R.; Harrand, R. Co-reactivity between related and unrelated environmental allergens in equine allergen-specific IgE serology testing in the UK. Vet. Dermatol. 2019, 30, 544. [Google Scholar] [CrossRef] [PubMed]
- Ginel, P.; Hernández, E.; Lucena, R.; Blanco, B.; Novales, M.; Mozos, E. Allergen-specific immunotherapy in horses with insect bite hypersensitivity: A double-blind, randomised, placebo-controlled study. Vet. Dermatol. 2014, 25, 29. [Google Scholar] [CrossRef]
- Ziegler, A.; Hamza, E.; Jónsdóttir, S.; Rhyner, C.; Wagner, B.; Schüpbach, G.; Marti, E. Longitudinal analysis of allergen-specific IgE and IgG subclasses as potential predictors of insect bite hypersensitivity after first exposure to Culicoides in Icelandic horses. Vet. Dermatol. 2018, 29, 51. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.; Mellor, P.S.; Torr, S.J. Control techniques for Culicoides biting midges and their application in the U.K. and north-western Palaearctic. Med. Vet. Entomol. 2008, 22, 175–187. [Google Scholar] [CrossRef] [PubMed]
- González, M.; López, S.; Mullens, B.A.; Baldet, T.; Goldarazena, A. A survey of Culicoides developmental sites on a farm in northern Spain, with a brief review of immature habitats of European species. Vet. Parasitol. 2013, 191, 81–93. [Google Scholar] [CrossRef]
- de Raat, I.J.; van den Boom, R.; van Poppel, M.; Sloet van Oldruitenborgh-Oosterbaan, M.M. The effect of a topical insecticide containing permethrin on the number of Culicoides midges near horses with and without insect bite hypersensitivity in The Netherlands. Tijdschr. Diergeneeskd. 2008, 133, 838–842. [Google Scholar]
- Sanders, C.J.; Selby, R.; Carpenter, S.; Reynolds, D.R. High-altitude flight of Culicoides biting midges. Vet. Rec. 2011, 169, 4245. [Google Scholar] [CrossRef]
- Robin, M.; Archer, D.; McGowan, C.; Garros, C.; Gardès, L.; Baylis, M. Repellent effect of topical deltamethrin on blood-feeding by Culicoides on horses. Vet. Rec. 2015, 176, 574. [Google Scholar] [CrossRef]
- Page, P.C.; Labuschagne, K.; Nurton, J.P.; Venter, G.J.; Guthrie, A.J. Duration of repellency of N,N-diethyl-3-methylbenzamide, citronella oil and cypermethrin against Culicoides species when applied to polyester mesh. Vet. Parasitol. 2009, 163, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, A.I.; Osimitz, T.G. Analysis and interpretation of pharmacokinetic studies following DEET administration to rats, dogs and humans. Toxicol. Res. Appl. 2022, 6, 239784732211172. [Google Scholar] [CrossRef]
- Venter, G.J.; Labuschagne, K.; Boikanyo, S.N.B.; Morey, L. Assessment of the repellent effect of citronella and lemon eucalyptus oil against South African Culicoides species. J. S. Afr. Veter-Assoc. 2014, 85, e1–e5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dillmann, J.B.; Cossetin, L.F.; de Giacometi, M.; Oliveira, D.; de Matos, A.F.I.M.; Avrella, P.D.; Garlet, Q.I.; Heinzmann, B.M.; Monteiro, S.G.; Isman, M. Adulticidal activity of Melaleuca alternifolia essential oil with high 1,8-cineole content against stable flies. J. Econ. Entomol. 2020, 113, 1810–1815. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, M. New solutions using natural products. In Insect-Borne Diseases in the 21st Century; Schneider, B.S., Higgs, S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 263–351. [Google Scholar] [CrossRef]
- El Abdali, Y.; Agour, A.; Allali, A.; Bourhia, M.; El Moussaoui, A.; Eloutassi, N.; Salamatullah, A.M.; Alzahrani, A.; Ouahmane, L.; Aboul-Soud, M.A.M.; et al. Lavandula dentata L.: Phytochemical analysis, antioxidant, antifungal and insecticidal activities of its essential oil. Plants 2022, 11, 311. [Google Scholar] [CrossRef]
- Sindle, A.; Martin, K. Art of prevention: Essential oils—Natural products not necessarily safe. Int. J. Womens Dermatol. 2020, 7, 304–308. [Google Scholar] [CrossRef]
- Venail, R.; Lhoir, J.; Fall, M.; del Río, R.; Talavera, S.; Labuschagne, K.; Miranda, M.; Pagès, N.; Venter, G.; Rakotoarivony, I.; et al. How do species, population and active ingredient influence insecticide susceptibility in Culicoides biting midges? Parasites Vectors 2015, 8, 439. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, I.; Sanchez, A. Efficacy and safety of subcutaneous allergen-specific immunotherapy in horses with allergic cutaneous and respiratory diseases—A systematic review. Vet. Sci. 2023, 10, 613. [Google Scholar] [CrossRef]
- Meulenbroeks, C.; Lugt, J.; Meide, N.; Willemse, T.; Rutten, V.; Zaiss, D. Allergen-specific cytokine polarisation protects Shetland ponies against Culicoides obsoletus-induced insect bite hypersensitivity. PLoS ONE 2015, 10, e0122090. [Google Scholar] [CrossRef]
- Jónsdóttir, S.; Svansson, V.; Stefánsdóttir, S.; Schüpbach, G.; Rhyner, C.; Marti, E.; Torsteinsdóttir, S. A preventive immunisation approach against insect bite hypersensitivity: Intralymphatic injection with recombinant allergens in alum or alum + monophosphoryl lipid A. Vet. Immunol. Immunopathol. 2016, 172, 14–20. [Google Scholar] [CrossRef]
- Stefánsdóttir, S.B.; Jónsdóttir, S.; Kristjansdottir, H.; Svansson, V.; Marti, E.; Torsteinsdóttir, S. Establishment of a protocol for preventive vaccination against equine insect bite hypersensitivity. Vet. Immunol. Immunopathol. 2022, 253, 110502. [Google Scholar] [CrossRef]
- Jonsdottir, S.; Stefansdottir, S.B.; Kristinarson, S.B.; Svansson, V.; Bjornsson, J.M.; Runarsdottir, A.; Wagner, B.; Marti, E.; Torsteinsdottir, S. Barley-produced Culicoides allergens are suitable for monitoring the immune response of horses immunised with E. coli-expressed allergens. Vet. Immunol. Immunopathol. 2018, 201, 32–37. [Google Scholar] [CrossRef]
- Fettelschoss-Gabriel, A.; Fettelschoss, V.; Olomski, F.; Birkmann, K.; Thoms, F.; Bühler, M.; Kummer, M.; Zeltins, A.; Kündig, T.M.; Bachmann, M.F. Active vaccination against interleukin-5 as long-term treatment for insect-bite hypersensitivity in horses. Allergy 2019, 74, 572–582. [Google Scholar] [CrossRef]
- Jónsdóttir, S.; Fettelschoss, V.; Olomski, F.; Talker, S.; Mirkovitch, J.; Rhiner, T.; Birkmann, K.; Thoms, F.; Wagner, B.; Bachmann, M.F.; et al. Safety profile of a virus-like particle-based vaccine targeting self-protein interleukin-5 in horses. Vaccines 2020, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Langreder, N.; Schäckermann, D.; Meier, D.; Becker, M.; Schubert, M.; Dübel, S.; Reinard, T.; Figge-Wegener, S.; Roßbach, K.; Bäumer, W.; et al. Development of an inhibiting antibody against equine interleukin-5 to treat insect bite hypersensitivity of horses. Sci. Rep. 2023, 13, 4029. [Google Scholar] [CrossRef]
- Olsén, L.; Bondesson, U.; Broström, H.; Olsson, U.; Mazogi, B.; Sundqvist, M.; Tjälve, H.; Ingvast-Larsson, C. Pharmacokinetics and effects of cetirizine in horses with insect bite hypersensitivity. Vet. J. 2011, 187, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrantz, W.; White, S.D. Equine atopic disease: Symptomatic therapy and allergen-specific immunotherapy. In Veterinary Allergy; Noli, C., Foster, A., Rosenkrantz, W., Eds.; John Wiley & Sons: Chichester, UK, 2014; pp. 283–287. [Google Scholar]
- Potter, K.; Stevens, K.; Menzies-Gow, N. Prevalence of and risk factors for acute laminitis in horses treated with corticosteroids. Vet. Rec. 2019, 185, 82. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, S.B.; Wren, J.A.; Cleaver, D.M.; Martin, D.D.; Walsh, K.F.; Harfst, J.A.; Follis, S.L.; King, V.L.; Boucher, J.F.; Stegemann, M.R. Efficacy and safety of oclacitinib for the control of pruritus and associated skin lesions in dogs with canine allergic dermatitis. Vet. Dermatol. 2013, 24, 479-e114. [Google Scholar] [CrossRef]
- Hunyadi, L.; Datta, P.; Rewers-Felkins, K.; Sundman, E.; Hale, T.; Fajt, V.; Wagner, S. Pharmacokinetics of a single dose of oclacitinib maleate as a top dress in adult horses. J. Vet. Pharmacol. Ther. 2022, 45, 320–324. [Google Scholar] [CrossRef]
- Cox, A.; Wood, K.; Coleman, G.; Stewart, A.J.; Bertin, F.-R.; Owen, H.; Suen, W.; Medina-Torres, C. Essential oil spray reduces clinical signs of insect bite hypersensitivity in horses. Aust. Vet. J. 2020, 98, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Huhmann, R.; Mueller, R.S. A cream containing omega-3-fatty acids, humectants and emollients as an aid in the treatment of equine Culicoides hypersensitivity. Vet. Dermatol. 2019, 30, 155-e46. [Google Scholar] [CrossRef]
- Lairikyengbam, D.; Wetterauer, B.; Schmiech, M.; Jahraus, B.; Kirchgessner, H.; Wetterauer, P.; Berschneider, K.; Beier, V.; Niesler, B.; Balta, E.; et al. Comparative analysis of whole-plant, flower and root extracts of Chamomilla recutita L. reveals differential anti-inflammatory effects on human T cells. Front. Immunol. 2024, 15, 1388962. [Google Scholar] [CrossRef]
- Frye, C.C.; Bei, D.; Parman, J.E.; Jones, J.; Houlihan, A.J.; Rumore, A. Efficacy of tea-tree oil in the treatment of equine streptothricosis. J. Equine Vet. Sci. 2019, 79, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Hess, T.M.; Rexford, J.K.; Hansen, D.K.; Harris, M.; Schauermann, N.; Ross, T.; Engle, T.E.; Allen, K.G.; Mulligan, C.M. Effects of two different dietary sources of long-chain omega-3 highly unsaturated fatty acids on incorporation into plasma, red blood cells and skeletal muscle in horses. J. Anim. Sci. 2012, 90, 3023–3031. [Google Scholar]
- O’Neill, W.; McKee, S.; Clarke, A.F. Flaxseed (Linum usitatissimum) supplementation associated with reduced skin-test lesional area in horses with Culicoides hypersensitivity. Can. J. Vet. Res. 2002, 66, 272–277. [Google Scholar]
- van den Boom, R.; Driessen, F.; Streumer, S.J.; Sloet van Oldruitenborgh-Oosterbaan, M.M. The effect of a supplement containing sunflower oil, vitamins, amino acids and peptides on the severity of symptoms in horses suffering insect bite hypersensitivity. Tijdschr. Diergeneeskd. 2010, 135, 520–525. [Google Scholar]
- Afrifa, D.; Engelbrecht, L.; van Eijnde, B.O.; Terblanche, E. The health benefits of rooibos tea (Aspalathus linearis)—A scoping review. J. Public Health Afr. 2023, 14, 2784. [Google Scholar] [CrossRef]
- Tahir, D.; Meyer, L.N.; Lekouch, N.; Varloud, M. Aedes (Stegomyia) aegypti mosquito bite hypersensitivity in a dog: A case report. BMC Vet. Res. 2020, 16, 402. [Google Scholar] [CrossRef]
- Hellman, L. Regulation of IgE homeostasis, and the identification of potential targets for therapeutic intervention. Biomed Pharmacother 2007, 61, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Crilly, J.P.; Nuttall, T.; del Pozo, J.; Hopker, A.; Tomlinson, M.; Sargison, N. Hypersensitivity to Culicoides midges causing seasonal dermatitis in sheep. Vet. Parasitol. Reg. Stud. Rep. 2016, 3–4, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, W.; Wantke, F. Insect hypersensitivity beyond bee and wasp venom allergy. Allergol. Sel. 2020, 4, 97–104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vander Does, A.; Labib, A.; Yosipovitch, G. Update on mosquito bite reaction: Itch and hypersensitivity, pathophysiology, prevention, and treatment. Front. Immunol. 2022, 13, 1024559. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garcia-Calderin, D.; González-Díaz, S.; Arias-Cruz, A.; Macías-Weinmann, A.; Buenfil-Lopez, J.A.; Calva, M.; Mejia, K.; Dominguez, L.; Gallego, C. 465 Skeeter Syndrome, a Case Report and Literature Review. World Allergy Organ. J. 2012, 5 (Suppl. S2), S165. [Google Scholar] [CrossRef] [PubMed Central]
- Peng, Z.; Simons, F.E. Mosquito allergy: Immune mechanisms and recombinant salivary allergens. Int. Arch. Allergy Immunol. 2004, 133, 198–209. [Google Scholar] [CrossRef]
- Barbosa, J.D.; Sodré, M.H.S.; Barbosa, C.C.; da Costa, P.S.C.; Oliveira, C.M.C.; Ferreira, T.T.A.; da Silveira, J.A.S.; Lamego, E.C.; Paz, M.C.; Caldeira, R.D.; et al. Allergic Dermatitis in Pêga Breed Donkeys (Equus asinus) Caused by Culicoides Bites in the Amazon Biome, Pará, Brazil. Animals 2024, 14, 1330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- How, M.J.; Gonzales, D.; Irwin, A.; Caro, T. Zebra stripes, tabanid biting flies and the aperture effect. Proc. Biol. Sci. 2020, 287, 20201521. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cuming, R.S.; Groover, E.S.; Wooldridge, A.A.; Caldwell, F. Review of glucocorticoid therapy in horses. Part 1: Pharmacology. Equine Vet. Educ. 2018, 30, 141–150. [Google Scholar] [CrossRef]
- McGowan, C.; Cooper, D.; Ireland, J. No evidence that therapeutic systemic corticosteroid administration is associated with laminitis in adult horses without underlying endocrine or severe systemic disease. Vet. Evidence. 2016, 1. [Google Scholar] [CrossRef][Green Version]
- van den Boom, R.; Kempenaars, M.; Sloet van Oldruitenborgh-Oosterbaan, M.M. The healing effects of a topical phytogenic ointment on insect bite hypersensitivity lesions in horses. Tijdschr. Diergeneeskd. 2011, 136, 20–26. [Google Scholar][Green Version]
- Liska, D.A.; Akucewich, L.H.; Marsella, R.; Maxwell, L.K.; Barbara, J.E.; Cole, C.A. Pharmacokinetics of pentoxifylline and its 5-hydroxyhexyl metabolite after oral and intravenous administration of pentoxifylline to healthy adult horses. Am. J. Vet. Res. 2006, 67, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Cleaver, D.; Cundiff, B.; King, V.; Sture, G. Oclacitinib maleate (Apoquel) dose determination in horses with naturally occurring allergic dermatitis. 2020 ACVIM forum on demand research abstract program. J. Vet. Int. Med. 2020, 34, 2977–2978. [Google Scholar]
- Brazzini, B.; Pimpinelli, N. New and established topical corticosteroids in dermatology: Clinical pharmacology and therapeutic use. Am. J. Clin. Dermatol. 2002, 3, 47–58. [Google Scholar] [CrossRef]
- Graner, A.; Mueller, R.S.; Geisler, J.; Bogenstätter, D.; White, S.J.; Jonsdottir, S.; Marti, E. Allergen immunotherapy using recombinant Culicoides allergens improves clinical signs of equine insect bite hypersensitivity. Front. Allergy. 2024, 5, 1467245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jebbawi, F.; Chemnitzer, A.; Dietrich, M.; Pantelyushin, S.; Lam, J.; Rhiner, T.; Keller, G.; Waldern, N.; Canonica, F.; Fettelschoss-Gabriel, A. Cytokines and chemokines skin gene expression in correlation with immune cells in blood and severity in equine insect bite hypersensitivity. Front. Immunol. 2024, 15, 1414891. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mureșan, A.N.; Țăpuc, I.M.; Neagu, D.M. Understanding Insect Bite Hypersensitivity in Horses: A Narrative Review for Clinical Practice. Allergies 2025, 5, 31. https://doi.org/10.3390/allergies5030031
Mureșan AN, Țăpuc IM, Neagu DM. Understanding Insect Bite Hypersensitivity in Horses: A Narrative Review for Clinical Practice. Allergies. 2025; 5(3):31. https://doi.org/10.3390/allergies5030031
Chicago/Turabian StyleMureșan, Alexandra Nicoleta, Ilinca Maria Țăpuc, and Daniela Mihaela Neagu. 2025. "Understanding Insect Bite Hypersensitivity in Horses: A Narrative Review for Clinical Practice" Allergies 5, no. 3: 31. https://doi.org/10.3390/allergies5030031
APA StyleMureșan, A. N., Țăpuc, I. M., & Neagu, D. M. (2025). Understanding Insect Bite Hypersensitivity in Horses: A Narrative Review for Clinical Practice. Allergies, 5(3), 31. https://doi.org/10.3390/allergies5030031






