Abstract
Psoriasis is a chronic inflammatory autoimmune disease primarily affecting the skin and, in some cases, the joints, and is characterized by erythematous, scaling lesions. Building up the doses has been conventional, but many patients will not obtain good results and a new, more targeted therapeutic strategy is desired. In the past few years, immune checkpoint inhibitors have revolutionized moderate to severe psoriasis management by blocking crucial pro-inflammatory cytokines, introducing new avenues for biological therapies. This review summarizes recent developments in biological therapies, including their mechanisms of action and clinical efficacy. While bimekizumab, an IL-17A and IL-17F inhibitor, strongly suppresses inflammation, selective inhibition of the IL-12/23 pathways is targeted with the small molecule TYK2 inhibitor deucravacitinib. For example, spesolimab, an inhibitor of IL-36 signaling, is being investigated for generalized pustular psoriasis. In this respect, new therapies provide better efficacy and quality of life, target specific psoriasis subtypes, and are safer and more effective than anti-inflammatory treatments. Such therapies could radically inform the standards of care, and the long-term safety and patient-centered outcomes of these innovative strategies will be the subject of continued research.
1. Introduction
Psoriasis is a chronic inflammatory autoimmune disease that affects the skin, as well as other organs and systems, including the joints (psoriatic arthritis). It is characterized by erythematous and scaly skin lesions and has a chronic, recurrent course. The pathogenesis of psoriasis is associated with excessive activation of immune system cells, leading to keratinocyte proliferation and inflammation [1,2].
The aim of this paper is to analyze the underlying mechanisms of psoriasis pathogenesis, highlighting recent advancements in understanding immune system dysregulation and its impact on keratinocyte function. This study contributes to the field by providing an updated perspective on the interplay between immune responses and epidermal changes, emphasizing novel insights that may pave the way for improved therapeutic strategies.
1.1. Epidemiological Data
Psoriasis is a common skin disease affecting approximately 0.51% to 11.43% of the global population [3]. In Europe and North America, the prevalence ranges between 0.6% and 4.7%, while in Asia and Africa, it is lower, at around 0.1–0.3% [3,4,5,6]. In Poland, it is estimated that about 1.1 million people suffer from psoriasis, accounting for approximately 3% of the country’s population [1]. Women are more frequently affected by the disease than men [3,4,5].
1.2. Biological Treatment of Psoriasis
In cases where traditional therapies (such as topical treatment, phototherapy, or systemic drugs) prove ineffective or cause significant adverse effects, biological therapies represent a breakthrough in the treatment of moderate to severe psoriasis. Biological therapies include drugs targeting specific molecules involved in mediating inflammation in psoriasis, such as pro-inflammatory cytokines. These interventions enable effective symptom control, improvement in patients’ quality of life, and a reduction in the risk of complications, such as the development of psoriatic arthritis or cardiovascular diseases [5,6,7,8].
In recent years, the introduction of interleukin 17 (IL-17) and interleukin 23 (IL-23) inhibitors has significantly revolutionized psoriasis treatment. These drugs, including secukinumab and ixekizumab (IL-17 inhibitors) and guselkumab, risankizumab, and tildrakizumab (IL-23 inhibitors), have demonstrated high efficacy and safety profiles in both clinical trials and real-world clinical practice [7,8]. According to the EuroGuiDerm guidelines on the systemic treatment of plaque psoriasis, IL-17 and IL-23 inhibitors are recommended as effective therapeutic options for patients with moderate to severe forms of the disease [5,9].
2. Classification and Treatment of Psoriasis According to the Recommendations of the Polish Dermatological Society
The severity of psoriasis symptoms should be evaluated according to the guidelines of the Polish Dermatological Society [8] using the Psoriasis Area and Severity Index (PASI), Body Surface Area (BSA), and the Dermatology Life Quality Index (DLQI). Mild-severity psoriasis is defined as scores of <10 on all three scales. Patients with mild disease generally receive topicals, such as vitamin D derivatives (e.g., calcipotriol) and corticosteroids (e.g., betamethasone). These treatments are effective but need regular and time-consuming applications [8].
Moderate to severe plaque psoriasis or psoriatic arthritis patients who do not achieve a satisfactory response to conventional therapy are candidates for systemic therapy, including biological therapy. Standard systemic therapies include methotrexate, cyclosporine, and acitretin. These emergency therapies are usually linked with an increased risk of adverse events, requiring close monitoring [8,10]. Biological drugs represent a significant advancement in the treatment of moderate to severe psoriasis as they are targeted therapies that inhibit specific pathways involved in the disease’s pathogenesis. Table 1 presents the groups of biological drugs used in psoriasis treatment [10].

Table 1.
Groups of biological drugs used in psoriasis treatment based on [8,9].
PASI score improvement is used to evaluate the efficacy of biological therapies. Interestingly, treatment is regarded as successful if PASI (PASI-75) declines by 75% within 3–4 months after the start of treatment [8]. PASI 50–75 patients are assessed for improvements in PASI and the quality of life of the patient (DLQI ≤ 5). An inadequate response (PASI 5) requires a re-evaluation of the treatment strategy and sometimes even a switch to other therapies [8,9].
3. Etiology of Psoriasis
Psoriasis is a multifactorial chronic inflammatory disease that has been shown to involve genetic predispositions, environmental contributions, and immune dysregulation. These factors trigger immune cell activation and the production of pro-inflammatory mediators, which mediate the hallmark skin lesions and systemic inflammation of the disease [10,11].
3.1. Risk Factors (Genetic and Environmental)
Psoriasis is a heritable trait linked to specific genes, such as HLA-Cw6, that are tightly associated with disease susceptibility. Individuals with this genetic variant are at an increased risk of developing psoriasis, particularly in the presence of environmental triggers. These triggers include bacterial infections (for example, Streptococcus pyogenes), psychological stress, trauma to the skin (the Koebner phenomenon), and specific medications, including beta-blockers or lithium. How these inherited risks act together with environmental risk factors is crucial to disease initiation and progression [6,7,11].
3.2. Immunological Mechanisms
Psoriasis is an immune-mediated disease in which the key element is the activation of dendritic cells in the skin. This process initiates the recruitment and activation of T lymphocytes, which coordinate the inflammatory cascade. Among the main cytokines involved in maintaining chronic inflammation and keratinocyte hyperproliferation are IL-23, IL-17, and TNF-α, as well as IL-1, IL-1β, and IL-6 [9,12,13,14,15,16].
Chemokines play a crucial role in the pathogenesis of psoriasis by promoting immune cell migration, inflammation, and disease persistence. Among them, CCL2, CCL3, CCL5, and CCL17 have been identified as key contributors to psoriatic inflammation:
- CCL2 (MCP-1): Primarily secreted by keratinocytes and monocytes, CCL2 promotes the recruitment of monocytes, T cells, and dendritic cells to psoriatic lesions. It is upregulated in psoriatic skin and has been correlated with disease severity. Its interaction with CCR2 leads to macrophage activation, which further amplifies inflammatory cytokine release.
- CCL3 (MIP-1α): Functions as a chemoattractant for eosinophils, monocytes, and lymphocytes. It plays a role in Th1-mediated inflammation in psoriasis by enhancing T-cell recruitment and activation.
- CCL5 (RANTES): Highly expressed in psoriatic lesions, particularly by keratinocytes, CCL5 is induced by TNF-α and IFN-γ and promotes the accumulation of T cells and other immune cells in the epidermis, contributing to chronic inflammation.
- CCL17 (TARC): Primarily associated with Th2 responses but also involved in psoriasis, CCL17 recruits T cells expressing CCR4, including Th17 cells, which are central to psoriatic inflammation [17].
3.3. Dendritic Cell Activation
Dendritic cells serve as the primary sensors of skin damage in psoriasis. Their activation leads to the secretion of pro-inflammatory cytokines, including interferon-alpha (IFN-α) and interleukin 23 (IL-23). IFN-α enhances the recruitment of immune cells and intensifies the inflammatory response, while IL-23 promotes the differentiation and survival of Th17 cells [9,12].
Interleukin 1 (IL-1) and its isoform IL-1β play a crucial role in amplifying the inflammatory response, leading to increased production of IL-6 and TNF-α, which contribute to keratinocyte hyperproliferation [13,14,15].
3.4. Role of T Lymphocyte Activation
Effector T lymphocyte subsets, such as Th1 and Th17, play a fundamental role in the pathogenesis of psoriasis. Th1 lymphocytes secrete IFN-γ and TNF-α, which exacerbate inflammation and recruit additional immune cells to the inflamed skin.
Th17 cells, activated by IL-23, produce IL-17, IL-22, and IL-6, which stimulate keratinocyte proliferation and neutrophil accumulation in the skin. IL-22 and IL-6 contribute to epidermal thickening and impairment of epidermal barrier function [9,15,16].
3.5. Role of Cytokines in Keratinocyte Proliferation and Angiogenesis
In the presence of pro-inflammatory cytokines, keratinocytes exhibit abnormal hyperproliferation and impaired differentiation, leading to hyperkeratosis and the formation of psoriatic skin lesions [16] (Table 2).

Table 2.
Cytokines involved in the pathogenesis of psoriasis [9,12,13,14,15].
Interleukin 6 (IL-6) is a key regulator in the pathogenesis of psoriasis, supporting keratinocyte proliferation and the recruitment of immune cells into the skin [2]. IL-6 receptor (IL-6R) inhibitors, such as tocilizumab, can reduce inflammatory activity in psoriasis by decreasing keratinocyte proliferation and inhibiting Th17 differentiation [15].
3.6. Efficacy and Safety of Biological Therapies
Clinical studies confirm the high efficacy of biological therapies in treating moderate to severe psoriasis. IL-17 and IL-23 inhibitors achieve PASI 75–100 in 60–90% of patients and have a more favorable safety profile compared to TNF-α inhibitors [8,16].
The presence of type I IFNs promotes the maturation and differentiation of conventional dendritic cells (cDCs), which primarily secrete interleukin (IL)-23, a key cytokine in the activation of autoreactive T cells. However, IL-23 is also produced by other immune cells, including macrophages and keratinocytes, further contributing to the inflammatory milieu. As a result, psoriatic T cells become predisposed to secrete T helper 17 (Th17) cytokines, primarily IL-17 and IL-22. Notably, these cytokines enhance AMP expression in keratinocytes, suggesting the existence of a positive feedback loop that perpetuates pDC activation and sustains IFN-driven autoimmunity.
Furthermore, type I IFNs directly enhance IL-22 receptor (IL-22R) expression on keratinocytes, increasing their responsiveness to IL-22. IL-22 primarily promotes keratinocyte proliferation, indirectly affecting terminal differentiation, leading to epidermal hyperplasia, a defining feature of psoriatic lesions (Figure 1).
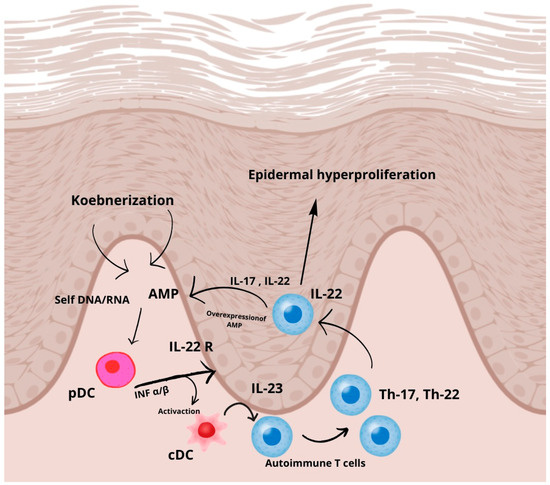
Figure 1.
Molecular pathway of psoriasis, based on [17]. In response to skin injury, antimicrobial peptides (AMPs) such as LL-37, produced by keratinocytes or deposited by infiltrating neutrophils, interact with self-DNA and RNA released from dying cells. These nucleic acid–AMP complexes are subsequently transported into intracellular compartments, where they activate plasmacytoid dendritic cells (pDCs) via endosomal toll-like receptors (TLRs) 7 and 9, leading to the early production of type I interferons (IFN-α and IFN-β) in psoriasis pathogenesis.
4. Bimekizumab
4.1. A Timeline of Clinical Trials and Registration
Bimekizumab (Skyrizi®): A humanized IgG1 monoclonal antibody, bimekizumab was evaluated in studies to determine its safety and efficacy in the treatment of moderate to severe plaque psoriasis. Athletes participated in pivotal phase III trials attempting to demonstrate effectiveness (BE VIVID and BE READY) between 2018 and 2021. It first received marketing authorization from the European Medicines Agency (EMA) in August 2021, and regulatory approval from the U.S. Food and Drug Administration (FDA) in October 2021. In August 2024, a single-dose formulation (320 mg) for subcutaneous use was approved in the European Union [18,19].
4.2. Mechanism of Action
Bimekizumab is the first biologic agent developed to target and inhibit both isoforms of interleukin 17, IL-17A and IL-17F, simultaneously. These cytokines are pivotal mediators of the inflammatory response in psoriasis. IL-17A is a strong stimulator of inflammation whilst IL-17F plays a role in maintaining chronic inflammation. Bimekizumab suppresses the inflammatory cascade more effectively than single IL-17 inhibitors through the dual inhibition of these cytokines. This dual effect both inhibits keratinocyte hyperproliferation and ameliorates epidermal thickening and inflammation [18,19].
4.3. Analogies to Drugs Previously Used
The mechanism of bimekizumab builds on the accomplishments of the earlier biologic agents targeting cytokines in the pathogenesis of psoriasis. Secukinumab and Ixekizumab are direct IL-17A inhibitors but not IL-17F inhibitors, which may restrict their ability to completely abrogate inflammation. The dual IL-17 blockade of bimekizumab results in a wider and more potent anti-inflammatory activity, reflected in better clinical outcomes [18].
4.4. Clinical Efficacy
Bimekizumab has been shown in clinical trials to be a potent treatment for moderate to severe plaque psoriasis. In patients treated with bimekizumab, significantly higher rates of skin clearance than the placebo and other IL-17 inhibitors were observed. At week 52, 72.4% of patients attained PASI 100, while 94.7% achieved a static Physician’s Global Assessment (sPGA) score of 0 or 1, and 93.3% reported a Dermatology Life Quality Index (DLQI) score of 0 or 1. These results were also seen with substantial improvements in DLQI and other quality-of-life measures, demonstrating the drug’s ability to impact both clinical and patient-reported outcomes. A rapid onset of action and long duration of efficacy underscore bimekizumab’s potential to become a leading option in the biological treatment of psoriasis [18,19,20].
4.5. Potential Adverse Effects
Bimekizumab demonstrated a consistent safety profile across all studies, with most adverse events being mild to moderate in severity. The most common side effects included upper respiratory tract infections (15.4%), injection site reactions (5.7%), mucosal candidiasis (2.3%), headaches (<2%), and diarrhea (<2%) [20,21].
5. Deukravacytynib
5.1. Timeline of Clinical Trials and Registration
The oral compound deucravacitinib, a newly approved treatment for moderate-to-severe plaque psoriasis, underwent significant evaluation in multiple clinical trials, including the pivotal phase III POETYK PSO-1 and POETYK PSO-2 studies. These trials were carried out from 2018 to 2021 to evaluate its effectiveness and safety compared to the placebo and other active treatments. Deucravacitinib was approved by the U.S. Food and Drug Administration (FDA) in 2022 as Sotyktu®. Soon thereafter, other regulatory agencies (e.g., the European Medicines Agency [EMA]) followed with their approvals [22,23].
5.2. Mechanism of Action
Deucravacitinib is an oral, highly selective allosteric inhibitor of tyrosine kinase 2 (TYK2), a member of the Janus kinase (JAK) family. It acts through selective binding to its regulatory (pseudokinase) domain, not the active catalytic domain, of TYK2. This specific binding to TYK2 inhibits TYK2 activity in various signaling transduction paths related to psoriasis pathogenesis, such as IL-12, IL-23, and type I interferon pathways [24,25].
As a highly selective inhibitor of TYK2, deucravacitinib has reduced the off-target effects seen with other broader JAK inhibitors (e.g., JAK1, JAK2, and JAK3). The agent is selective for TYK2-mediated pathways, thus minimizing the effects on hematological or metabolic parameters, which are frequently affected by other JAK inhibitors [26,27], as Figure 2 shows.
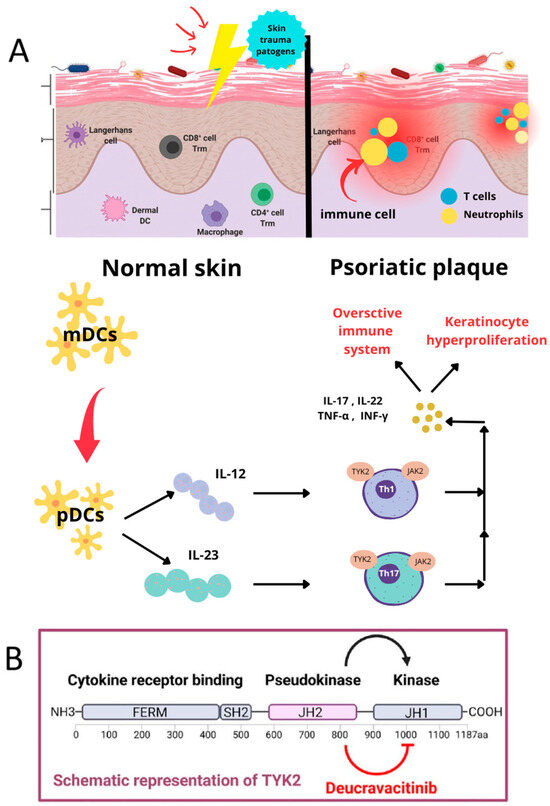
Figure 2.
The molecular mechanism of psoriasis, with an emphasis on the action of deucravacitinib, based on [28]. (A) Immunological mechanisms in healthy and psoriatic Skin. The illustration compares normal skin (left) to skin affected by psoriasis (right). Myeloid dendritic cells (mDCs) and plasmacytoid dendritic cells (pDCs) are involved in antigen surveillance and in starting immune responses in normal skin in response to pathogenic insults or skin injury. This is a balanced state of functioning for the immune system. The case of psoriatic skin shows that the immune system is overactivated. Upon activation, pDCs secrete cytokines, including IL-12 and IL-23, promoting T cell differentiation into Th1 and Th17 subpopulations. Th1 and Th17 cells secrete pro-inflammatory cytokines, such as IL-17, IL-22, TNF-α, and IFN-γ. This, in turn, results in increased inflammation and keratinocyte hyperproliferation, leading to the formation of typical psoriatic plaques. (B) The role of TYK2 and the mechanism of action of deucravacitinib. The bottom portion of the diagram shows the structure of the enzyme called tyrosine kinase 2 (TYK2) and the mechanism of action of a drug called deucravacitinib, which selectively inhibits TYK2. TYK2 comprises a cytokine receptor-binding domain, a pseudokinase domain (JH2), and an active kinase domain (JH1). TYK2 is an important component of cytokine signaling pathways mediated by IL-12 and IL-23, which are involved in the activation of Th1 and Th17 lymphocytes. Mechanistically, deucravacitinib selectively inhibits the pseudokinase domain JH2, thus leading to both TH1 and TH17 pathway signal blockades, as TYK2 has multiple signaling pathways. This leads to decreased pro-inflammatory cytokine production, regulation of pathological immune responses, and ultimately control over psoriatic symptoms [24,26,28].
5.3. Analogies to Treatments Previously Used
Deucravacitinib’s mechanism sets it apart from other oral psoriasis therapies.
Apremilast: A phosphodiesterase 4 (PDE4) inhibitor that raises intracellular cyclic adenosine monophosphate (cAMP) levels, leading to indirect modulation of inflammatory cytokines. Though effective, apremilast’s efficacy is typically less than that of deucravacitinib, as seen in comparative trials [29,30].
Conventional JAK inhibitors: Medications such as tofacitinib inhibit more than one member of the JAK family, leading to wider immunosuppression. The specificity of deucravacitinib for TYK2 offers a more selective approach with a favorable profile and has been shown to lead to a lower risk of adverse events such as anemia and lipid derangement [26,27].
5.4. Clinical Trial Results
Deucravacitinib has been shown to be efficacious in the POETYK PSO-1 and POETYK PSO-2 trials. These randomized, double-blind studies compared deucravacitinib with a placebo and apremilast. Patients treated with deucravacitinib were found to have significantly higher rates of PASI75 and PASI90 responses and significant improvement in Dermatology Life Quality Index (DLQI) scores. The noted benefits were maintained over the follow-up period, suggesting the long-term efficacy of the drug [24,25].
Besides clinical responses, patient-reported outcomes (PROs) such as rash-related itching, burning, and pain were also significantly more improved with deucravacitinib than with the placebo and apremilast. These results highlight the ability of the drug to enhance both physical and quality-of-life endpoints [29,30].
5.5. Potential Side Effects
The safety profile of deucravacitinib was consistent across studies, with most adverse events being mild to moderate in severity: upper respiratory tract infections (6.3%), rhinitis (6.3%), headaches (5.2%), diarrhea (5.2%), nausea (4.1%), acne (3.2%), folliculitis (2.1%), and herpes zoster (1.2%). Serious adverse events were rare and occurred at a comparable frequency in both the deucravacitinib and placebo groups. Notably, deucravacitinib does not appear to have a significant impact on laboratory parameters such as hemoglobin levels or lipid profiles, further supporting its selectivity for TYK2 and its long-term safety profile [26,27].
6. Spesolimab
6.1. Clinical Trials and Registration Timeline
Spesolimab, a monoclonal IgG1 antibody marketed under the brand name Spevigo®, underwent comprehensive clinical trials before its approval. The Effisayil™ clinical program included pivotal studies evaluating its safety and efficacy in patients with generalized pustular psoriasis (GPP). In September 2022, Spesolimab received FDA approval for the treatment of GPP flares in adults. It was subsequently approved in other regions, including Japan, China, and the European Union [31,32].
6.2. Differences Between GPP and Plaque Psoriasis
Generalized pustular psoriasis (GPP) is a distinct clinical entity from plaque psoriasis. GPP is driven predominantly by dysregulation of the IL-36 signaling pathway, whereas plaque psoriasis involves the IL-23/Th17 axis [30]. Clinically, GPP is characterized by widespread pustules on an erythematous background, often accompanied by systemic symptoms such as fever and malaise, while plaque psoriasis typically presents with localized, well-demarcated scaly plaques. The disease course of GPP often involves acute, life-threatening flares, while plaque psoriasis is more chronic and less severe [33,34].
6.3. Mechanism of Action
Spesolimab targets the IL-36 receptor (IL-36R), which plays a pivotal role in the pathogenesis of GPP. IL-36 cytokines (IL-36α, IL-36β, and IL-36γ) act as key mediators in the inflammatory cascade, leading to keratinocyte hyperproliferation and recruitment of neutrophils, as Figure 3 shows. Dysregulation of this pathway is a hallmark of GPP. By blocking IL-36R, spesolimab interrupts the downstream signaling cascade, effectively reducing inflammation and halting the progression of pustular lesions. This targeted approach minimizes systemic inflammation and improves patient outcomes [32,33].
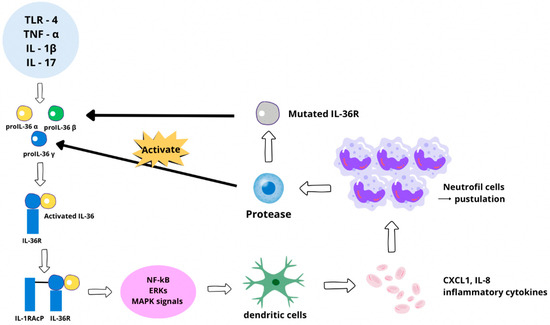
Figure 3.
IL-36 signaling pathway and its role in inflammatory processes. Schematic showing the effect of stimuli such as TLR-4, TNF-α, IL-β, and IL-17, which lead to the release of pro-IL-36 (α, β, and γ). Proteolytic cleavage of pro-IL-36 activates this cytokine, resulting in biologically active IL-36. The IL-36R receptor binds to active IL-36, followed by IL-1RAcP co-receptor binding, leading to the activation of NF-κB, ERKs, and MAPK signaling pathways. This leads to the secretion of pro-inflammatory cytokines, including CXCL1 and IL-8, which activate dendritic cells and recruit neutrophils. Overactive neutrophils cause the formation of pustules, hallmarks of inflammatory skin diseases, including pustular psoriasis. Dysregulation of this pathway can occur via a mutation in IL-36R. The mutations either increase receptor sensitivity or disrupt regulatory mechanisms, leading to excessive inflammation and exacerbation of symptoms, such as purulent skin lesions [32,35].
6.4. Comparisons with Newly Applied Therapies
Spesolimab’s specific blockade of IL-36R is a major step forward over available therapies. TNF-α inhibitors (infliximab and adalimumab) are effective in plaque psoriasis, but little is known about their efficacy in GPP due in part to discrepancies in the underlying inflammatory pathways [33]. Acute non-specific immunosuppressants (e.g., cyclosporine) have been applied but are accompanied by significant side effects and variable efficacy. Biologics directed against IL-17 and IL-23 pathways, including secukinumab and ustekinumab, concentrate on key drivers of plaque psoriasis but are not directly involved in the IL-36-related pathophysiology of GPP [28].
6.5. Clinical Trial Results
The Effisayil™ clinical program provided comprehensive evidence for spesolimab’s efficacy. In the Effisayil™1 phase II trial, patients with active GPP flares showed rapid clinical improvement. After one week, 54% of spesolimab patients achieved complete clearance of pustules compared to 6% of placebo patients. Greyed-out systemic symptoms and quality of life also improved significantly. In the Effisayil™2 phase IIb study, spesolimab achieved an 84% reduction in the recurrence of flares over 48 weeks compared to the placebo, with similar safety profiles for the treatment and placebo groups [34,35].
6.6. Potential Side Effects
The most commonly reported adverse events were upper respiratory tract infections (17.1%) and urinary tract infections (2.9%). Less frequently observed were injection site reactions (<2%), neutropenia (rare), headaches (<5%), and gastrointestinal symptoms (<5%) [34,35].
7. Discussion
Recent advances in psoriasis therapy have introduced novel mechanisms of action that enhance efficacy while addressing some limitations associated with existing biologics. While biologics targeting IL-17 or IL-23 remain foundational treatments, their effectiveness in generalized pustular psoriasis (GPP) is limited. A newer pharmacologic approach targets the IL-36 signaling pathway with spesolimab, an IL-36 receptor antagonist. Its ability to rapidly suppress systemic inflammation represents an important step forward in managing severe GPP flares [32,35].
Deucravacitinib, a selective TYK2 inhibitor, offers a new immune-modulating strategy by inhibiting IL-12, IL-23, and type I interferon pathways. Unlike broader JAK inhibitors, it appears to have fewer off-target effects, such as hematological abnormalities. Deucravacitinib provides an oral treatment alternative with efficacy comparable to biologics such as secukinumab or ustekinumab, which may benefit patients who prefer non-injectable therapies [26,27].
Bimekizumab broadens the therapeutic potential of IL-17 inhibition by targeting both IL-17A and IL-17F. By addressing residual inflammatory activity mediated by IL-17F, bimekizumab appears to have increased potency and may lead to higher rates of complete remission compared to IL-17A-specific agents such as secukinumab or ixekizumab [18,19].
A key consideration is whether these newer drugs will work equally well for all psoriasis patients. Some individuals may express low levels or even lack the target proteins necessary for these therapies, which could limit their effectiveness. Additionally, treatment adherence plays a significant role in therapeutic success. Poor adherence—whether due to side effects, patient preference, or the complexity of the treatment regimen—can lead to suboptimal outcomes in both conventional and biological therapies. Addressing this issue is essential for improving long-term treatment effectiveness.
In addition to biologics and synthetic inhibitors, naturally derived bioactive compounds are being explored as potential complementary therapies. Withaferin A, a phytochemical compound, has demonstrated anti-inflammatory and immunomodulatory properties in dermatological diseases, including psoriasis. Similarly, other bioactive natural compounds have been studied for their potential to modulate immune and inflammatory pathways relevant to psoriatic conditions. Further research is needed to clarify their role in psoriasis management [7].
These developments highlight the importance of continued research into optimizing treatment pathways, gathering real-world data, and addressing patient-centered concerns. Further studies are necessary to evaluate the long-term safety, efficacy, and best use of these newer therapies in clinical practice.
8. Summary
The development of newer psoriasis therapies has led to meaningful progress in targeting immune pathways and addressing clinical needs. These treatments provide more specific and effective options, allowing for a more personalized approach to managing the disease. Clinicians can now better tailor treatment plans to individual patients based on their disease characteristics and response to therapy.
New treatment options offer greater flexibility in managing disease severity and administration routes, such as oral versus injectable therapies. By addressing previously unmet needs, these therapies have expanded treatment options for severe and atypical psoriasis forms that were challenging to manage with older treatments.
Treatment adherence remains a crucial factor in therapeutic success. Ensuring that patients remain on therapy—by minimizing side effects, offering convenient dosing options, and addressing individual preferences—can help improve long-term outcomes. Additionally, natural bioactive compounds, such as Withaferin A, have been investigated for their potential to modulate immune and inflammatory responses in psoriasis, though further research is required to confirm their clinical relevance [35].
Ongoing research is necessary to refine treatment approaches, collect real-world data, and ensure the long-term safety and effectiveness of these therapies. As these newer treatments become integrated into clinical practice, they may help improve patient outcomes and quality of life for individuals with psoriasis.
Author Contributions
Conceptualization, M.K.O. and D.K.; methodology, M.K.O. and A.D.; writing—original draft preparation, M.K.O., A.D., D.K., S.W. and L.W.; writing—review and editing, M.K.O. and A.D.; visualization, M.K.O.; supervision, M.K.O. and A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Michalek, I.M.; Loring, B.; John, S.M. A systematic review of worldwide epidemiology of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 205–212. [Google Scholar] [CrossRef]
- Parisi, R.; Symmons, D.P.; Griffiths, C.E.; Ashcroft, D.M. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J. Investig. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef]
- World Health Organization. Global Report on Psoriasis; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Narbutt, J.; Lesiak, A.; Sysa-Jedrzejowska, A.; Wozniacka, A.; Bogaczewicz, J.; McBride, D. The impact of psoriasis on social activities and emotional functioning in Polish patients. Eur. J. Dermatol. 2014, 24, 490–495. [Google Scholar] [CrossRef]
- Nast, A.; Smith, C.; Spuls, P.I.; Avila Valle, G.; Bata-Csörgö, Z.; Boonen, H.; De Jong, E.M.; Garcia-Doval, I.; Gisondi, P.; Kaur-Knudsen, D.; et al. EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris—Part 1: Treatment and monitoring recommendations. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2461–2498. [Google Scholar] [CrossRef]
- Radu, A.; Tit, D.M.; Endres, L.M.; Radu, A.F.; Vesa, C.M.; Bungau, S.G. Naturally derived bioactive compounds as precision modulators of immune and inflammatory mechanisms in psoriatic conditions. Inflammopharmacology 2025, 33, 527–549. [Google Scholar] [CrossRef]
- Chandran, V.; Raychaudhuri, S.P. Geoepidemiology and environmental factors of psoriasis and psoriatic arthritis. J. Autoimmun. 2010, 34, J314–J321. [Google Scholar] [CrossRef]
- Reich, A.; Adamski, Z.; Chodorowska, G.; Kaszuba, A.; Krasowska, D.; Lesiak, A.; Maj, J.; Narbutt, J.; Osmola-Mańkowska, A.J.; Owczarczyk-Saczonek, A.; et al. Psoriasis. Diagnostic and therapeutic recommendations of the Polish Dermatological Society. Part 2. Dermatol. Rev. 2020, 107, 110–137. [Google Scholar] [CrossRef]
- Mosca, M.; Hong, J.; Hadeler, E.; Hakimi, M.; Liao, W.; Bhutani, T. The Role of IL-17 Cytokines in Psoriasis. Immunotargets Ther. 2021, 10, 409–418. [Google Scholar] [CrossRef]
- Sieminska, I.; Pieniawska, M.; Grzywa, T.M. The Immunology of Psoriasis—Current Concepts in Pathogenesis. Clin. Rev. Allergy Immunol. 2024, 66, 164–191. [Google Scholar] [CrossRef]
- Chen, L.; Tsai, T.-F. HLA-Cw6 and psoriasis. Br. J. Dermatol. 2018, 178, 854–862. [Google Scholar] [CrossRef]
- Fotiadou, C.; Lazaridou, E.; Sotiriou, E.; Ioannides, D. Targeting IL-23 in psoriasis: Current perspectives. Psoriasis 2018, 8, 1–5. [Google Scholar] [CrossRef]
- Seyedmirzaei, H.; Rezaei, N. Cytokine alterations in psoriasis: An updated review. Expert. Rev. Clin. Immunol. 2021, 17, 1323–1335. [Google Scholar] [CrossRef]
- Cai, Y.; Xue, F.; Quan, C.; Qu, M.; Liu, N.; Zhang, Y.; Fleming, C.; Hu, X.; Zhang, H.G.; Weichselbaum, R.; et al. A Critical Role of the IL-1β-IL-1R Signaling Pathway in Skin Inflammation and Psoriasis Pathogenesis. J. Investig. Dermatol. 2019, 139, 146–156. [Google Scholar] [CrossRef]
- Uciechowski, P.; Dempke, W.C.M. Interleukin-6: A Masterplayer in the Cytokine Network. Oncology 2020, 98, 131–137. [Google Scholar] [CrossRef]
- Sivamani, R.K.; Goodarzi, H.; Garcia, M.S.; Raychaudhuri, S.P.; Wehrli, L.N.; Ono, Y. Biologic Therapies in Psoriasis: A Systematic Review. J. Am. Acad. Dermatol. 2021, 84, 1384–1397. [Google Scholar] [CrossRef]
- Zdanowska, N.; Kasprowicz-Furmańczyk, M.; Placek, W.; Owczarczyk-Saczonek, A. The Role of Chemokines in Psoriasis—An Overview. Medicina 2021, 57, 754. [Google Scholar] [CrossRef]
- Gordon, K.B.; Foley, P.; Krueger, J.G.; Pinter, A.; Reich, K.; Vender, R.; Vanvoorden, V.; Madden, C.; White, K.; Cioffi, C.; et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): A multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet 2021, 397, 475–486. [Google Scholar] [CrossRef]
- Hagino, T.; Saeki, H.; Fujimoto, E.; Kanda, N. Effectiveness of long-term bimekizumab treatment and predictive factors for responders in moderate-to-severe psoriasis: A 52-week real-world study. J. Dermatol. 2025, 52, 317–328. [Google Scholar] [CrossRef]
- Bernardini, N.; Dattola, A.; Caldarola, G.; Orsini, D.; Assorgi, C.; D’Amore, A.; Maretti, G.; Richetta, A.G.; Tolino, E.; Skroza, N.; et al. Rapid response on facial psoriasis to bimekizumab: Case series. Drugs Context. 2024, 13, 2024-1-4. [Google Scholar] [CrossRef]
- Truong, T.M.; Pathak, G.N.; Singal, A.; Taranto, V.; Rao, B.K. Deucravacitinib: The First FDA-Approved Oral TYK2 Inhibitor for Moderate to Severe Plaque Psoriasis. Ann. Pharmacother. 2022, 58, 416–427. [Google Scholar] [CrossRef]
- Sotyktu (deucravacitinib). Package Insert; Bristol Myers Squibb: Princeton, NJ, USA, 2022; Available online: https://packageinserts.bms.com/pi/pi_sotyktu.pdf (accessed on 28 February 2025).
- Armstrong, A.W.; Augustin, M.; Beaumont, J.L.; Pham, T.P.; Hudgens, S.; Gordon, K.B.; Zhuo, J.; Becker, B.; Zhong, Y.; Kisa, R.M.; et al. Deucravacitinib Improves Patient-Reported Outcomes in Patients with Moderate to Severe Psoriasis: Results from the Phase 3 Randomized POETYK PSO-1 and PSO-2 Trials. Dermatol. Ther. 2023, 14, 2235–2248. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Lebwohl, M.; Warren, R.B.; Sofen, H.; Morita, A.; Paul, C.; Papp, K.A.; Colombo, M.J.; Scotto, J.; Vaile, J.; et al. Deucravacitinib in Plaque Psoriasis: 4-Year Safety and Efficacy Results from the Phase 3 POETYK PSO-1, PSO-2, and LTE Trials. J. Eur. Acad. Dermatol. Venereol. 2025, in press. [Google Scholar] [CrossRef]
- Gooderham, M.; Warren, R.B.; Armstrong, A.W. Deucravacitinib Versus Placebo and Apremilast in Moderate to Severe Plaque Psoriasis: Efficacy and Safety Results from the 52-Week, Randomized, Double-Blinded POETYK PSO-1 Trial. J. Am. Acad. Dermatol. 2022, 88, 29–39. [Google Scholar] [CrossRef]
- Carmona-Rocha, E.; Rusiñol, L. New and Emerging Oral/Topical Small-Molecule Treatments for Psoriasis. Pharmaceutics 2024, 16, 239. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Park, S.H.; Patel, V.; Hogan, M.; Wang, W.-J.; Davidson, D.; Chirikov, V. Matching-Adjusted Indirect Comparison of the Long-Term Efficacy of Deucravacitinib Versus Adalimumab for Moderate to Severe Plaque Psoriasis. Dermatol. Ther. 2023, 13, 2589–2603. [Google Scholar] [CrossRef]
- Granau, A.M.; Boye, T.L.; Jensen, K.B.; Nielsen, O.H. Deucravacitinib (Sotyktu™) for Plaque Psoriasis. Trends Pharmacol. Sci. 2023, 44, 252–253. [Google Scholar] [CrossRef]
- Gold, L.S.; Blauvelt, A.; Kircik, L.; Napoli, A.; Cheng, C.-Y.; Dyme, R.; Balagula, E.; Bagel, J.; Lebwohl, M. Efficacy of Deucravacitinib in Moderate to Severe Scalp Psoriasis: Analysis of Complete Clearance of Scalp Disease and Symptoms. Ski. J. Cutan. Med. 2024, 8, s430. [Google Scholar] [CrossRef]
- Orsini, D.; Malagoli, P.; Balato, A.; Bianchi, L.; Brianti, P.; Buononato, D.; Burlando, M.; Caldarola, G.; Campanati, A.; Campione, E.; et al. Bimekizumab for the Treatment of Plaque Psoriasis with Involvement of Genitalia: A 16-Week Multicenter Real-World Experience—IL PSO (Italian Landscape Psoriasis). Dermatol. Pract. Concept. 2024, 14, e2024052. [Google Scholar] [CrossRef] [PubMed]
- Morita, A.; Choon, S.E.; Bachelez, H.; Anadkat, M.J.; Marrakchi, S.; Zheng, M.; Tsai, T.F.; Turki, H.; Hua, H.; Rajeswari, S.; et al. Design of Effisayil™ 2: A Randomized, Double-Blind, Placebo-Controlled Study of Spesolimab in Preventing Flares in Patients with Generalized Pustular Psoriasis. Dermatol. Ther. Heidelb. 2023, 13, 347–359. [Google Scholar] [CrossRef]
- Burden, A.D.; Okubo, Y.; Zheng, M.; Thaçi, D.; van de Kerkhof, P.; Hu, N.; Quaresma, M.; Thoma, C.; Choon, S.E. Efficacy of Spesolimab for the Treatment of Generalized Pustular Psoriasis Flares across Pre-Specified Patient Subgroups in the Effisayil 1 Study. Exp. Dermatol. 2023, 32, 1279–1283. [Google Scholar] [CrossRef]
- Palaniappan, V.; Gopinath, H.; Murthy, A.B.; Radhakrishnan, S.; Karthikeyan, K. Spesolimab: A comprehensive review on the anti-IL-36 receptor antibody in dermatology. Int. J. Dermatol. 2024, 63, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, D.; Thaçi, D.; Torres, T. Spesolimab for the Treatment of Generalized Pustular Psoriasis. Drugs 2024, 84, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Bungau, S.; Vesa, C.M.; Abid, A.; Behl, T.; Tit, D.M.; Purza, A.L.; Pasca, B.; Todan, L.M.; Endres, L. Withaferin A-A Promising Phytochemical Compound with Multiple Results in Dermatological Diseases. Molecules 2021, 26, 2407. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).