The Role of Bacterial Toxins and Environmental Factors in the Development of Food Allergies
Abstract
1. Introduction
2. Prevalence and Epidemiology of FAs
2.1. Prevalence of FAs
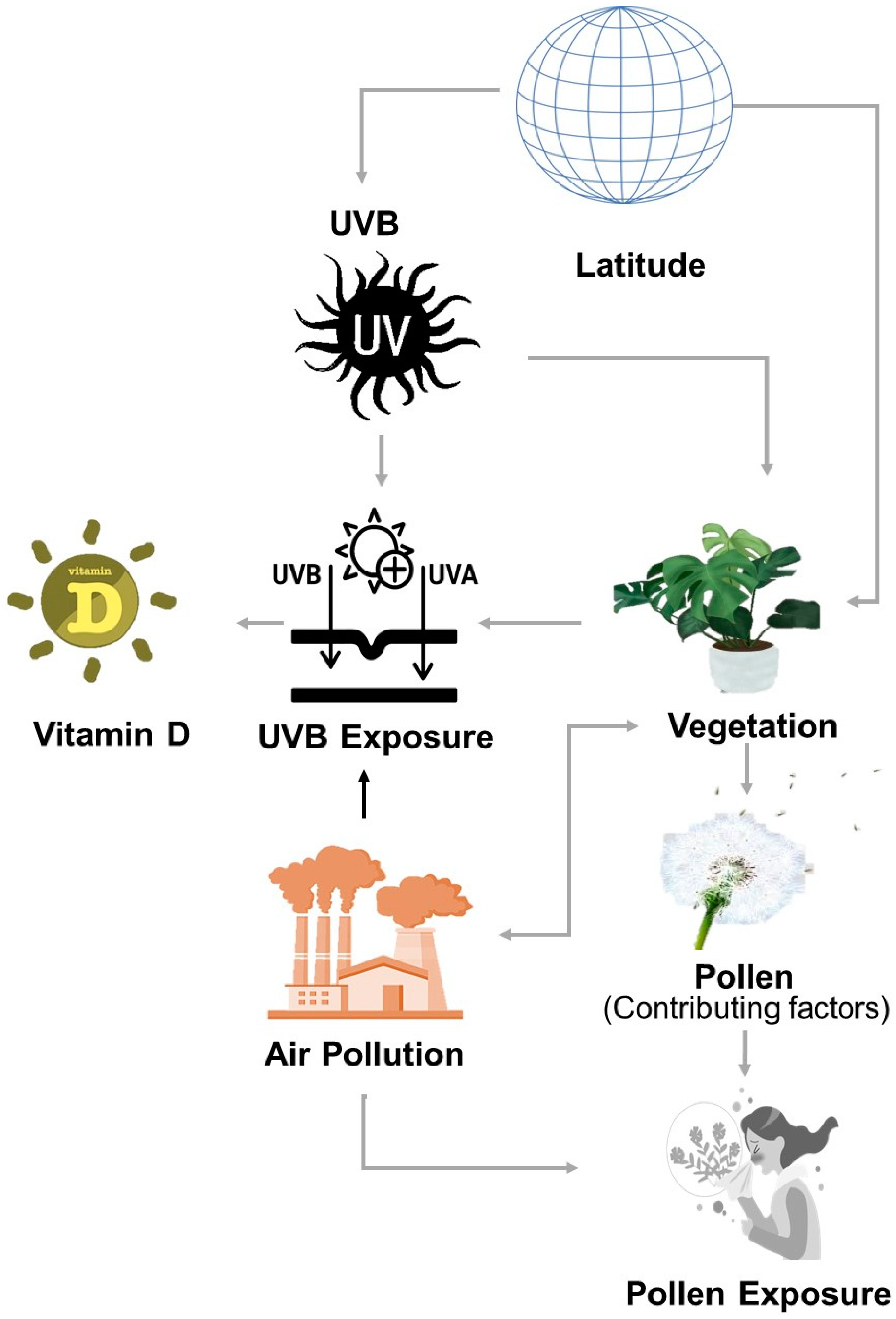
2.2. Epidemiological Factors
2.3. Global Perspective
3. Gene–Environment and Molecular Mechanisms in FA Development
3.1. The Role of Epigenetic Modifications in FAs
3.2. Mechanisms of Cross-Reactivity and Sensitization
3.3. Cross-Reactivity and Sensitization Mechanisms in Peanut Allergies
4. Bacterial Contamination in Food and Its Role in Allergy Sensitization
4.1. Contamination of Food with Staphylococcus Aureus and Its Enterotoxins
4.2. Toxicological Effects of S. aureus Enterotoxins
4.3. S. aureus Enterotoxin B Superantigenic Effects and Immune Response Mechanisms
4.4. Role of S. aureus in FA Sensitization and Immune Dysregulation
4.5. The Role of S. aureus in Atopic Dermatitis and Its Implications for FAs
5. The Role of Gut Microbiota in FA Pathogenesis
6. The Impact of Food Processing and Immune Mechanisms on Allergenicity
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Althumiri, N.A.; Basyouni, M.H.; AlMousa, N.; AlJuwaysim, M.F.; BinDhim, N.F.; Alqahtani, S.A. Prevalence of Self-Reported Food Allergies and Their Association with Other Health Conditions among Adults in Saudi Arabia. Int. J. Environ. Res. Public Health 2021, 18, 347. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, F.S.; Clarke, A.E.; Elliott, S.J. Investigating self-reported food allergy prevalence in Waterloo Region, Canada. Can. Geogr./Géographies Can. 2023, 67, 226–236. [Google Scholar] [CrossRef]
- Enroth, S.; Dahlbom, I.; Hansson, T.; Johansson, Å.; Gyllensten, U. Prevalence and sensitization of atopic allergy and coeliac disease in the Northern Sweden Population Health Study. Int. J. Circumpolar Health 2013, 72, 21403. [Google Scholar] [CrossRef] [PubMed]
- Sadighara, P.; Safta, M.; Limam, I.; Ghanati, K.; Nazari, Z.; Karami, M.; Abedini, A. Association between food additives and prevalence of allergic reactions in children: A systematic review. Rev. Environ. Health 2023, 38, 181–186. [Google Scholar] [CrossRef]
- Fogg, M.I.; Spergel, J.M. Management of food allergies. Expert Opin. Pharmacother. 2003, 4, 1025–1037. [Google Scholar] [CrossRef]
- Butkus, S.N.; Mahan, L.K. Food allergies: Immunological reactions to food. Diet. Assoc. 1986, 86, 601–608. [Google Scholar] [CrossRef]
- Perkin, M.R.; Logan, K.; Tseng, A.; Raji, B.; Ayis, S.; Peacock, J.; Brough, H.; Marrs, T.; Radulovic, S.; Craven, J.; et al. EAT Study Team Randomized Trial of Introduction of Allergenic Foods in Breast-Fed Infants. N. Engl. J. Med. 2016, 374, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Acker, W.W.; Plasek, J.M.; Blumenthal, K.G.; Lai, K.H.; Topaz, M.; Seger, D.L.; Goss, F.R.; Slight, S.P.; Bates, D.W.; Zhou, L. Prevalence of food allergies and intolerances documented in electronic health records. J. Allergy Clin. Immunol. 2017, 140, 1587–1591.e1. [Google Scholar] [CrossRef]
- Ning, J.; Akhter, T.; Sarfraz, M.; Afridi, H.I.; Albasher, G.; Unar, A. The importance of monitoring endocrine-disrupting chemicals and essential elements in biological samples of fertilizer industry workers. Environ. Res. 2023, 231, 116173. [Google Scholar] [CrossRef]
- Muraro, A.; de Silva, D.; Halken, S.; Worm, M.; Khaleva, E.; Arasi, S.; Dunn-Galvin, A.; Nwaru, B.I.; De Jong, N.W.; Rodríguez Del Río, P.; et al. GA2LEN Food Allergy Guideline Group; GALEN Food Allergy Guideline Group Managing food allergy: GA2LEN guideline 2022. World Allergy Organiz. J. 2022, 15, 100687. [Google Scholar] [CrossRef]
- Schneider, K.R.; Schneider, R.G.; Ahn, S.; Richardson, S.; Kurdmongkoltham, P.; Bertoldi, B. Food Allergies. EDIS 2017, 2017, 1–4. [Google Scholar] [CrossRef]
- Xepapadaki, P.; Fiocchi, A.; Grabenhenrich, L.; Roberts, G.; Grimshaw, K.E.C.; Fiandor, A.; Larco, J.I.; Sigurdardottir, S.; Clausen, M.; Papadopoulos, N.G.; et al. Incidence and natural history of hen’s egg allergy in the first 2 years of life-the EuroPrevall birth cohort study. Allergy 2016, 71, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Tufail, T.; Rasheed, Y.; Ain, H.B.U.; Arshad, M.U.; Hussain, M.; Akhtar, M.N.; Saewan, S.A. A review of current evidence on food allergies during pregnancy. Food Sci. Nutr. 2023, 11, 4432–4443. [Google Scholar] [CrossRef]
- Thatavarthy, S.; Fryer, K. Transgenerational development of food allergies and allergic rhinitis. J. Stud. Res. 2023, 12, 1–9. [Google Scholar] [CrossRef]
- Schäfer, T.; Breuer, K. Epidemiologie von Nahrungsmittelallergien. Hautarzt 2003, 54, 112–120. [Google Scholar] [CrossRef]
- Muraro, A.; Tropeano, A.; Giovannini, M. Allergen immunotherapy for food allergy: Evidence and outlook. Allergol. Select 2022, 6, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Jiang, J.; Blumenstock, J.A.; Davis, M.M.; Schleimer, R.P.; Nadeau, K.C. Prevalence and severity of food allergies among US adults. JAMA Netw. Open 2019, 2, e185630. [Google Scholar] [CrossRef]
- Gray, C.L.; Goddard, E.; Karabus, S.; Kriel, M. Epidemiology of IgE-mediated food allergy: Continuing medical education. South Afr. Med. 2015, 105, 68–69. [Google Scholar] [CrossRef]
- Tham, E.H.; Leung, D.Y.M. How different parts of the world provide new insights into food allergy. Allergy Asthma Immunol. Res. 2018, 10, 290–299. [Google Scholar] [CrossRef]
- Spolidoro, G.C.I.; Ali, M.M.; Amera, Y.T.; Nyassi, S.; Lisik, D.; Ioannidou, A.; Rovner, G.; Khaleva, E.; Venter, C.; van Ree, R.; et al. Prevalence estimates of eight big food allergies in Europe: Updated systematic review and meta-analysis. Allergy 2023, 78, 2361–2417. [Google Scholar] [CrossRef]
- Peters, R.L.; Koplin, J.J.; Gurrin, L.C.; Dharmage, S.C.; Wake, M.; Ponsonby, A.-L.; Tang, M.L.K.; Lowe, A.J.; Matheson, M.; Dwyer, T.; et al. HealthNuts Study The prevalence of food allergy and other allergic diseases in early childhood in a population-based study: HealthNuts age 4-year follow-up. J. Allergy Clin. Immunol. 2017, 140, 145–153.e8. [Google Scholar] [CrossRef] [PubMed]
- Munasir, Z.; Muktiarti, D. The management of food allergy in Indonesia. Asia Pac. Allergy 2013, 3, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Nwaru, B.I.; Panesar, S.S.; Hickstein, L.; Rader, T.; Werfel, T.; Muraro, A.; Hoffmann-Sommergruber, K.; Roberts, G.; Sheikh, A.; European Academy of Allergy and Clinical Immunology Food Allergy and Anaphylaxis Guidelines group. The epidemiology of food allergy in Europe: Protocol for a systematic review. Clin. Transl. Allergy 2013, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Warren, C.M.; Browning, R.L.; Ciaccio, C.E.; Gupta, R.S. Food allergy epidemiology and racial and/or ethnic differences. J. Food Allergy 2020, 2, 11–16. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef]
- Brough, H.A.; Nadeau, K.C.; Sindher, S.B.; Alkotob, S.S.; Chan, S.; Bahnson, H.T.; Leung, D.Y.M.; Lack, G. Epicutaneous sensitization in the development of food allergy: What is the evidence and how can this be prevented? Allergy 2020, 75, 2185–2205. [Google Scholar] [CrossRef] [PubMed]
- Sedghy, F.; Varasteh, A.-R.; Sankian, M.; Moghadam, M. Interaction between air pollutants and pollen grains: The role on the rising trend in allergy. Rep. Biochem. Mol. Biol. 2018, 6, 219–224. [Google Scholar]
- Lucas, J.A.; Gutierrez-Albanchez, E.; Alfaya, T.; Feo-Brito, F.; Gutiérrez-Mañero, F.J. Oxidative stress in ryegrass growing under different air pollution levels and its likely effects on pollen allergenicity. Plant Physiol. Biochem. 2019, 135, 331–340. [Google Scholar] [CrossRef]
- Sedghy, F.; Sankian, M.; Moghadam, M.; Ghasemi, Z.; Mahmoudi, M.; Varasteh, A.-R. Impact of traffic-related air pollution on the expression of Platanus orientalis pollen allergens. Int. J. Biometeorol. 2017, 61, 1–9. [Google Scholar] [CrossRef]
- Janhäll, S. Review on urban vegetation and particle air pollution—Deposition and dispersion. Atmos. Environ. 2015, 105, 130–137. [Google Scholar] [CrossRef]
- Stevens, C.J.; Bell, J.N.B.; Brimblecombe, P.; Clark, C.M.; Dise, N.B.; Fowler, D.; Lovett, G.M.; Wolseley, P.A. The impact of air pollution on terrestrial managed and natural vegetation. Philos. Transact. A Math. Phys. Eng. Sci. 2020, 378, 20190317. [Google Scholar] [CrossRef] [PubMed]
- Krempski, J.W.; Dant, C.; Nadeau, K.C. The origins of allergy from a systems approach. Ann. Allergy Asthma Immunol. 2020, 125, 507–516. [Google Scholar] [CrossRef]
- Kulis, M.D.; Smeekens, J.M.; Immormino, R.M.; Moran, T.P. The airway as a route of sensitization to peanut: An update to the dual allergen exposure hypothesis. J. Allergy Clin. Immunol. 2021, 148, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Aerts, R.; Honnay, O.; Van Nieuwenhuyse, A. Biodiversity and human health: Mechanisms and evidence of the positive health effects of diversity in nature and green spaces. Br. Med. Bull. 2018, 127, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Markevych, I.; Schoierer, J.; Hartig, T.; Chudnovsky, A.; Hystad, P.; Dzhambov, A.M.; de Vries, S.; Triguero-Mas, M.; Brauer, M.; Nieuwenhuijsen, M.J.; et al. Exploring pathways linking greenspace to health: Theoretical and methodological guidance. Environ. Res. 2017, 158, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Marselle, M.R.; Hartig, T.; Cox, D.T.C.; de Bell, S.; Knapp, S.; Lindley, S.; Triguero-Mas, M.; Böhning-Gaese, K.; Braubach, M.; Cook, P.A.; et al. Pathways linking biodiversity to human health: A conceptual framework. Environ. Int. 2021, 150, 106420. [Google Scholar] [CrossRef]
- Ray, C.; Ming, X. Climate change and human health: A review of allergies, autoimmunity and the microbiome. Int. J. Environ. Res. Public Health 2020, 17, 4814. [Google Scholar] [CrossRef]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef]
- Renz, H.; Allen, K.J.; Sicherer, S.H.; Sampson, H.A.; Lack, G.; Beyer, K.; Oettgen, H.C. Food allergy. Nat. Rev. Dis. Primers 2018, 4, 17098. [Google Scholar] [CrossRef]
- Marrs, T.; Bruce, K.D.; Logan, K.; Rivett, D.W.; Perkin, M.R.; Lack, G.; Flohr, C. Is there an association between microbial exposure and food allergy? A systematic review. Pediatr. Allergy Immunol. 2013, 24, 311–320.e8. [Google Scholar] [CrossRef]
- Kourosh, A.; Luna, R.A.; Balderas, M.; Nance, C.; Anagnostou, A.; Devaraj, S.; Davis, C.M. Fecal microbiome signatures are different in food-allergic children compared to siblings and healthy children. Pediatr. Allergy Immunol. 2018, 29, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Fazlollahi, M.; Chun, Y.; Grishin, A.; Wood, R.A.; Burks, A.W.; Dawson, P.; Jones, S.M.; Leung, D.Y.M.; Sampson, H.A.; Sicherer, S.H.; et al. Early-life gut microbiome and egg allergy. Allergy 2018, 73, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Boonpiyathad, T.; Sözener, Z.C.; Satitsuksanoa, P.; Akdis, C.A. Immunologic mechanisms in asthma. Semin. Immunol. 2019, 46, 101333. [Google Scholar] [CrossRef]
- Ruiter, B.; Smith, N.P.; Fleming, E.; Patil, S.U.; Hurlburt, B.K.; Maleki, S.J.; Shreffler, W.G. Peanut protein acts as a TH2 adjuvant by inducing RALDH2 in human antigen-presenting cells. J. Allergy Clin. Immunol. 2021, 148, 182–194.e4. [Google Scholar] [CrossRef]
- Gehring, U.; Wijga, A.H.; Brauer, M.; Fischer, P.; de Jongste, J.C.; Kerkhof, M.; Oldenwening, M.; Smit, H.A.; Brunekreef, B. Traffic-related air pollution and the development of asthma and allergies during the first 8 years of life. Am. J. Respir. Crit. Care Med. 2010, 181, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Nordling, E.; Berglind, N.; Melén, E.; Emenius, G.; Hallberg, J.; Nyberg, F.; Pershagen, G.; Svartengren, M.; Wickman, M.; Bellander, T. Traffic-related air pollution and childhood respiratory symptoms, function and allergies. Epidemiology 2008, 19, 401–408. [Google Scholar] [CrossRef]
- Blanck, H.M.; Allen, D.; Bashir, Z.; Gordon, N.; Goodman, A.; Merriam, D.; Rutt, C. Let’s go to the park today: The role of parks in obesity prevention and improving the public’s health. Child. Obes. 2012, 8, 423–428. [Google Scholar] [CrossRef]
- Oktaria, V.; Dharmage, S.C.; Burgess, J.A.; Simpson, J.A.; Morrison, S.; Giles, G.G.; Abramson, M.J.; Walters, E.H.; Matheson, M.C. Association between latitude and allergic diseases: A longitudinal study from childhood to middle-age. Ann. Allergy Asthma Immunol. 2013, 110, 80–85.e1. [Google Scholar] [CrossRef]
- Keet, C.A.; Matsui, E.C.; Savage, J.H.; Neuman-Sunshine, D.L.; Skripak, J.; Peng, R.D.; Wood, R.A. Potential mechanisms for the association between fall birth and food allergy. Allergy 2012, 67, 775–782. [Google Scholar] [CrossRef]
- Fong, K.C.; Hart, J.E.; James, P. A review of epidemiologic studies on greenness and health: Updated literature through 2017. Curr. Environ. Health Rep. 2018, 5, 77–87. [Google Scholar] [CrossRef]
- Twohig-Bennett, C.; Jones, A. The health benefits of the great outdoors: A systematic review and meta-analysis of greenspace exposure and health outcomes. Environ. Res. 2018, 166, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.S. Looking back to the future: The re-emergence of green care. BJPsych. Int. 2017, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Akaraci, S.; Feng, X.; Suesse, T.; Jalaludin, B.; Astell-Burt, T. A Systematic Review and Meta-Analysis of Associations between Green and Blue Spaces and Birth Outcomes. Int. J. Environ. Res. Public Health 2020, 17, 2949. [Google Scholar] [CrossRef]
- Markevych, I.; Ludwig, R.; Baumbach, C.; Standl, M.; Heinrich, J.; Herberth, G.; de Hoogh, K.; Pritsch, K.; Weikl, F. Residing near allergenic trees can increase risk of allergies later in life: LISA Leipzig study. Environ. Res. 2020, 191, 110132. [Google Scholar] [CrossRef]
- Bublin, M.; Breiteneder, H. Cross-reactivity of peanut allergens. Curr. Allergy Asthma Rep. 2014, 14, 426. [Google Scholar] [CrossRef]
- Popescu, F.-D. Cross-reactivity between aeroallergens and food allergens. World J. Methodol. 2015, 5, 31–50. [Google Scholar] [CrossRef]
- Peters, R.L.; Sutherland, D.; Dharmage, S.C.; Lowe, A.J.; Perrett, K.P.; Tang, M.L.K.; Lycett, K.; Knibbs, L.D.; Koplin, J.J.; Mavoa, S. The association between environmental greenness and the risk of food allergy: A population-based study in Melbourne, Australia. Pediatr. Allergy Immunol. 2022, 33, e13749. [Google Scholar] [CrossRef] [PubMed]
- Eldeirawi, K.; Kunzweiler, C.; Zenk, S.; Finn, P.; Nyenhuis, S.; Rosenberg, N.; Persky, V. Associations of urban greenness with asthma and respiratory symptoms in Mexican American children. Ann. Allergy Asthma Immunol. 2019, 122, 289–295. [Google Scholar] [CrossRef]
- Lovasi, G.S.; Quinn, J.W.; Neckerman, K.M.; Perzanowski, M.S.; Rundle, A. Children living in areas with more street trees have lower prevalence of asthma. J. Epidemiol. Community Health 2008, 62, 647–649. [Google Scholar] [CrossRef]
- Fuertes, E.; Markevych, I.; von Berg, A. Greenness and allergies: Evidence of differential associations in two areas in Germany. J. Epidemiol Community Health 2014, 68, 787–790. [Google Scholar] [CrossRef]
- Fuertes, E.; Markevych, I.; Bowatte, G.; Gruzieva, O.; Gehring, U.; Becker, A.; Berdel, D.; von Berg, A.; Bergström, A.; Brauer, M.; et al. Residential greenness is differentially associated with childhood allergic rhinitis and aeroallergen sensitization in seven birth cohorts. Allergy 2016, 71, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Dadvand, P.; Villanueva, C.M.; Font-Ribera, L.; Martinez, D.; Basagaña, X.; Belmonte, J.; Vrijheid, M.; Gražulevičienė, R.; Kogevinas, M.; Nieuwenhuijsen, M.J. Risks and benefits of green spaces for children: A cross-sectional study of associations with sedentary behavior, obesity, asthma, and allergy. Environ. Health Perspect. 2014, 122, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Andrusaityte, S.; Grazuleviciene, R.; Kudzyte, J.; Bernotiene, A.; Dedele, A.; Nieuwenhuijsen, M.J. Associations between neighbourhood greenness and asthma in preschool children in Kaunas, Lithuania: A case-control study. BMJ Open 2016, 6, e010341. [Google Scholar] [CrossRef] [PubMed]
- Gernes, R.; Brokamp, C.; Rice, G.E.; Wright, J.M.; Kondo, M.C.; Michael, Y.L.; Donovan, G.H.; Gatziolis, D.; Bernstein, D.; LeMasters, G.K.; et al. Using high-resolution residential greenspace measures in an urban environment to assess risks of allergy outcomes in children. Sci. Total Environ. 2019, 668, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Hosoki, K.; Boldogh, I.; Sur, S. Innate responses to pollen allergens. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 79–88. [Google Scholar] [CrossRef]
- Susanto, N.H.; Lowe, A.J.; Salim, A.; Koplin, J.J.; Tang, M.L.K.; Suaini, N.H.A.; Ponsonby, A.-L.; Allen, K.J.; Dharmage, S.C.; Erbas, B. Associations between grass pollen exposures in utero and in early life with food allergy in 12-month-old infants. Int. J. Environ. Health Res. 2022, 32, 712–722. [Google Scholar] [CrossRef]
- Mittag, D.; Akkerdaas, J.; Ballmer-Weber, B.K.; Vogel, L.; Wensing, M.; Becker, W.-M.; Koppelman, S.J.; Knulst, A.C.; Helbling, A.; Hefle, S.L.; et al. Ara h 8, a Bet v 1-homologous allergen from peanut, is a major allergen in patients with combined birch pollen and peanut allergy. J. Allergy Clin. Immunol. 2004, 114, 1410–1417. [Google Scholar] [CrossRef]
- Miralles, J.C.; Caravaca, F.; Guillén, F.; Lombardero, M.; Negro, J.M. Cross-reactivity between Platanus pollen and vegetables. Allergy 2002, 57, 146–149. [Google Scholar] [CrossRef]
- Berni Canani, R.; De Filippis, F.; Nocerino, R.; Paparo, L.; Di Scala, C.; Cosenza, L.; Della Gatta, G.; Calignano, A.; De Caro, C.; Laiola, M.; et al. Gut microbiota composition and butyrate production in children affected by non-IgE-mediated cow’s milk allergy. Sci. Rep. 2018, 8, 12500. [Google Scholar] [CrossRef]
- Ling, Z.; Li, Z.; Liu, X.; Cheng, Y.; Luo, Y.; Tong, X.; Yuan, L.; Wang, Y.; Sun, J.; Li, L.; et al. Altered fecal microbiota composition associated with food allergy in infants. Appl. Environ. Microbiol. 2014, 80, 2546–2554. [Google Scholar] [CrossRef]
- Moran, T.P. The external exposome and food allergy. Curr. Allergy Asthma Rep. 2020, 20, 37. [Google Scholar] [CrossRef] [PubMed]
- Moran, T.P. Impact of the exposome on food allergy development. Curr. Opin. Allergy Clin. Immunol. 2023, 23, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Skjerven, H.O.; Carlsen, K.C.L. Environmental interventions to prevent food allergy. In Encyclopedia of Food Allergy; Elsevier: Amsterdam, The Netherlands, 2024; pp. 417–421. ISBN 9780323960199. [Google Scholar]
- Wang, B.; Sun, T.; Zhao, Y.; Wang, S.; Zhang, J.; Wang, Z.; Teng, Y.-E.; Cai, L.; Yan, M.; Wang, X.; et al. A randomized phase 3 trial of Gemcitabine or Nab-paclitaxel combined with cisPlatin as first-line treatment in patients with metastatic triple-negative breast cancer. Nat. Commun. 2022, 13, 4025. [Google Scholar] [CrossRef]
- Pursey, K.M.; Stanwell, P.; Gearhardt, A.N.; Collins, C.E.; Burrows, T.L. The prevalence of food addiction as assessed by the Yale Food Addiction Scale: A systematic review. Nutrients 2014, 6, 4552–4590. [Google Scholar] [CrossRef]
- Hulin, M.; Caillaud, D.; Annesi-Maesano, I. Indoor air pollution and childhood asthma: Variations between urban and rural areas. Indoor Air 2010, 20, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Kostara, M.; Chondrou, V.; Sgourou, A.; Douros, K.; Tsabouri, S. HLA polymorphisms and food allergy predisposition. J. Pediatr. Genet. 2020, 09, 077–086. [Google Scholar] [CrossRef] [PubMed]
- Abbring, J.H.; Chiappori, P.-A.; Pinquet, J. Moral hazard and dynamic insurance data. J. Eur. Econ. Assoc. 2003, 1, 767–820. [Google Scholar] [CrossRef]
- Krajewski, D.; Polukort, S.H.; Gelzinis, J.; Rovatti, J.; Kaczenski, E.; Galinski, C.; Pantos, M.; Shah, N.N.; Schneider, S.S.; Kennedy, D.R.; et al. Protein Disulfide Isomerases Regulate IgE-Mediated Mast Cell Responses and Their Inhibition Confers Protective Effects During Food Allergy. Front. Immunol. 2020, 11, 606837. [Google Scholar] [CrossRef]
- Alashkar Alhamwe, B.; Meulenbroek, L.A.P.M.; Veening-Griffioen, D.H.; Wehkamp, T.M.D.; Alhamdan, F.; Miethe, S.; Harb, H.; Hogenkamp, A.; Knippels, L.M.J.; Pogge von Strandmann, E.; et al. Decreased Histone Acetylation Levels at Th1 and Regulatory Loci after Induction of Food Allergy. Nutrients 2020, 12, 3193. [Google Scholar] [CrossRef]
- Martino, R.; Maertens, J.; Bretagne, S.; Rovira, M.; Deconinck, E.; Ullmann, A.J.; Held, T.; Cordonnier, C. Toxoplasmosis after hematopoietic stem cell transplantation. Clin. Infect. Dis. 2000, 31, 1188–1195. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, W.; Lyu, S.-C.; Macaubas, C.; Bunning, B.; He, Z.; Mellins, E.D.; Nadeau, K.C. A positive feedback loop reinforces the allergic immune response in human peanut allergy. J. Exp. Med. 2021, 218, e20201793. [Google Scholar] [CrossRef] [PubMed]
- Neeland, M.R.; Koplin, J.J.; Dang, T.D.; Dharmage, S.C.; Tang, M.L.; Prescott, S.L.; Saffery, R.; Martino, D.J.; Allen, K.J. Early life innate immune signatures of persistent food allergy. J. Allergy Clin. Immunol. 2018, 142, 857–864.e3. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-C.; Headley, M.B.; Loo, Y.-M.; Berlin, A.; Gale, M.; Debley, J.S.; Lukacs, N.W.; Ziegler, S.F. Thymic stromal lymphopoietin is induced by respiratory syncytial virus-infected airway epithelial cells and promotes a type 2 response to infection. J. Allergy Clin. Immunol. 2012, 130, 1187–1196.e5. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Di Cagno, R.; Minervini, F.; Rizzello, C.G.; Gobbetti, M. Two-dimensional electrophoresis and IgE-mediated food allergy. Electrophoresis 2010, 31, 2126–2136. [Google Scholar] [CrossRef]
- Koshiba, R.; Oba, T.; Fuwa, A.; Arai, K.; Sasaki, N.; Kitazawa, G.; Hattori, M.; Matsuda, H.; Yoshida, T. Aggravation of food allergy by skin sensitization via systemic th2 enhancement. Int. Arch. Allergy Immunol. 2021, 182, 292–300. [Google Scholar] [CrossRef]
- Cuesta-Herranz, J.; Barber, D.; Blanco, C.; Cistero-Bahíma, A.; Crespo, J.F.; Fernández-Rivas, M.; Fernández-Sánchez, J.; Florido, J.F.; Ibáñez, M.D.; Rodríguez, R.; et al. Differences among pollen-allergic patients with and without plant food allergy. Int. Arch. Allergy Immunol. 2010, 153, 182–192. [Google Scholar] [CrossRef]
- Palacín, A.; Rivas, L.A.; Gómez-Casado, C.; Aguirre, J.; Tordesillas, L.; Bartra, J.; Blanco, C.; Carrillo, T.; Cuesta-Herranz, J.; Bonny, J.A.C.; et al. The involvement of thaumatin-like proteins in plant food cross-reactivity: A multicenter study using a specific protein microarray. PLoS ONE 2012, 7, e44088. [Google Scholar] [CrossRef] [PubMed]
- Netting, M.J.; Gold, M.S.; Palmer, D.J. Low allergen content of commercial baby foods. J. Paediatr. Child Health 2020, 56, 1613–1617. [Google Scholar] [CrossRef]
- Stefka, A.T.; Feehley, T.; Tripathi, P.; Qiu, J.; McCoy, K.; Mazmanian, S.K.; Tjota, M.Y.; Seo, G.-Y.; Cao, S.; Theriault, B.R.; et al. Commensal bacteria protect against food allergen sensitization. Proc. Natl. Acad. Sci. USA 2014, 111, 13145–13150. [Google Scholar] [CrossRef]
- Umasunthar, T.; Leonardi-Bee, J.; Turner, P.J.; Hodes, M.; Gore, C.; Warner, J.O.; Boyle, R.J. Incidence of food anaphylaxis in people with food allergy: A systematic review and meta-analysis. Clin. Exp. Allergy 2015, 45, 1621–1636. [Google Scholar] [CrossRef]
- Netting, M.J.; Allen, K.J. Advice about infant feeding for allergy prevention: A confusing picture for Australian consumers? J. Paediatr. Child Health 2017, 53, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Konya, T.; Guttman, D.S.; Field, C.J.; Sears, M.R.; HayGlass, K.T.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Becker, A.B.; et al. CHILD Study Investigators Infant gut microbiota and food sensitization: Associations in the first year of life. Clin. Exp. Allergy 2015, 45, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Futamura, M.; Movérare, R.; Tanaka, A.; Kawabe, T.; Sakamoto, T.; Borres, M.P. The usefulness of casein-specific IgE and IgG4 antibodies in cow’s milk allergic children. Clin. Mol. Allergy 2012, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Bunyavanich, S.; Shen, N.; Grishin, A.; Wood, R.; Burks, W.; Dawson, P.; Jones, S.M.; Leung, D.Y.M.; Sampson, H.; Sicherer, S.; et al. Early-life gut microbiome composition and milk allergy resolution. J. Allergy Clin. Immunol. 2016, 138, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Umasunthar, T.; Leonardi-Bee, J.; Hodes, M.; Turner, P.J.; Gore, C.; Habibi, P.; Warner, J.O.; Boyle, R.J. Incidence of fatal food anaphylaxis in people with food allergy: A systematic review and meta-analysis. Clin. Exp. Allergy 2013, 43, 1333–1341. [Google Scholar] [CrossRef]
- Muto, T.; Fukuoka, A.; Kabashima, K.; Ziegler, S.F.; Nakanishi, K.; Matsushita, K.; Yoshimoto, T. The role of basophils and proallergic cytokines, TSLP and IL-33, in cutaneously sensitized food allergy. Int. Immunol. 2014, 26, 539–549. [Google Scholar] [CrossRef]
- Mennini, M.; Dahdah, L.; Mazzina, O.; Fiocchi, A. Lupin and Other Potentially Cross-Reactive Allergens in Peanut Allergy. Curr. Allergy Asthma Rep. 2016, 16, 84. [Google Scholar] [CrossRef]
- Chan, E.S.; Greenhawt, M.J.; Fleischer, D.M.; Caubet, J.-C. Managing Cross-Reactivity in Those with Peanut Allergy. J. Allergy Clin. Immunol. Pract. 2019, 7, 381–386. [Google Scholar] [CrossRef]
- Palladino, C.; Ellinger, I.; Kalic, T.; Humeniuk, P.; Ret, D.; Mayr, V.; Hafner, C.; Hemmer, W.; Hoffmann-Sommergruber, K.; Untersmayr, E.; et al. Peanut lipids influence the response of bronchial epithelial cells to the peanut allergens Ara h 1 and Ara h 2 by decreasing barrier permeability. Front. Mol. Biosci. 2023, 10, 1126008. [Google Scholar] [CrossRef]
- D’Auria, E.; Abrahams, M.; Zuccotti, G.V.; Venter, C. Personalized nutrition approach in food allergy: Is it prime time yet? Nutrients 2019, 11, 359. [Google Scholar] [CrossRef]
- van der Valk, J.P.M.; Schreurs, M.W.J.; El Bouch, R.; Arends, N.J.T.; de Jong, N.W. Mono-sensitisation to peanut component Ara h 6: A case series of five children and literature review. Eur. J. Pediatr. 2016, 175, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, B.; Novak, N. Effects of daily food processing on allergenicity. Crit. Rev. Food Sci. Nutr. 2019, 59, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Klemans, R.J.B.; van Os-Medendorp, H.; Blankestijn, M.; Bruijnzeel-Koomen, C.A.F.M.; Knol, E.F.; Knulst, A.C. Diagnostic accuracy of specific IgE to components in diagnosing peanut allergy: A systematic review. Clin. Exp. Allergy 2015, 45, 720–730. [Google Scholar] [CrossRef] [PubMed]
- van Erp, F.C.; Klemans, R.J.B.; Meijer, Y.; van der Ent, C.K.; Knulst, A.C. Using Component-Resolved Diagnostics in the Management of Peanut-Allergic Patients. Curr. Treat. Options Allergy 2016, 3, 169–180. [Google Scholar] [CrossRef]
- Fleischer, D.M. Life after LEAP: How to implement advice on introducing peanuts in early infancy. J. Paediatr. Child Health 2017, 53, 3–9. [Google Scholar] [CrossRef]
- Foong, R.-X.; Santos, A.F. Biomarkers of diagnosis and resolution of food allergy. Pediatr. Allergy Immunol. 2021, 32, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, U.M.; Layhadi, J.A.; Golebski, K.; István Komlósi, Z.; Peng, Y.; Sekerel, B.; Durham, S.R.; Brough, H.; Morita, H.; Akdis, M.; et al. Innate lymphoid cells: The missing part of a puzzle in food allergy. Allergy 2021, 76, 2002–2016. [Google Scholar] [CrossRef]
- Mišak, Z. Infant nutrition and allergy. Proc. Nutr. Soc. 2011, 70, 465–471. [Google Scholar] [CrossRef]
- Logan, K.; Du Toit, G.; Giovannini, M.; Turcanu, V.; Lack, G. Pediatric allergic diseases, food allergy, and oral tolerance. Annu. Rev. Cell Dev. Biol. 2020, 36, 511–528. [Google Scholar] [CrossRef]
- West, C. Introduction of complementary foods to infants. Ann. Nutr. Metab. 2017, 70 (Suppl. 2), 47–54. [Google Scholar] [CrossRef]
- Parra-Padilla, D.; Zakzuk, J.; Carrasquilla, M.; Alvis-Guzmán, N.; Dennis, R.; Rojas, M.X.; Rondón, M.; Pérez, A.; Peñaranda, A.; Barragán, A.M.; et al. Cost-effectiveness of the subcutaneous house dust mite allergen immunotherapy plus pharmacotherapy for allergic asthma: A mathematical model. Allergy 2021, 76, 2229–2233. [Google Scholar] [CrossRef]
- Satitsuksanoa, P.; Jansen, K.; Głobińska, A.; van de Veen, W.; Akdis, M. Regulatory immune mechanisms in tolerance to food allergy. Front. Immunol. 2018, 9, 2939. [Google Scholar] [CrossRef]
- Damsky, W.; Peterson, D.; Ramseier, J.; Al-Bawardy, B.; Chun, H.; Proctor, D.; Strand, V.; Flavell, R.A.; King, B. The emerging role of Janus kinase inhibitors in the treatment of autoimmune and inflammatory diseases. J. Allergy Clin. Immunol. 2021, 147, 814–826. [Google Scholar] [CrossRef]
- Chong, A.C.; Chwa, W.J.; Ong, P.Y. Aeroallergens in atopic dermatitis and chronic urticaria. Curr. Allergy Asthma Rep. 2022, 22, 67–75. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.-B.; Liu, Q.; Yao, M.; Cheng, R.; Xue, H.; Zhou, H.; Yao, Z. Interactions between FLG mutations and allergens in atopic dermatitis. Arch. Dermatol. Res. 2012, 304, 787–793. [Google Scholar] [CrossRef]
- Fallon, P.G.; Sasaki, T.; Sandilands, A.; Campbell, L.E.; Saunders, S.P.; Mangan, N.E.; Callanan, J.J.; Kawasaki, H.; Shiohama, A.; Kubo, A.; et al. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat. Genet. 2009, 41, 602–608. [Google Scholar] [CrossRef]
- Bandier, J.; Ross-Hansen, K.; Carlsen, B.C.; Menné, T.; Linneberg, A.; Stender, S.; Szecsi, P.B.; Meldgaard, M.; Thyssen, J.P.; Johansen, J.D. Carriers of filaggrin gene (FLG) mutations avoid professional exposure to irritants in adulthood. Contact Derm. 2013, 69, 355–362. [Google Scholar] [CrossRef]
- Margolis, D.J.; Apter, A.J.; Gupta, J.; Hoffstad, O.; Papadopoulos, M.; Campbell, L.E.; Sandilands, A.; McLean, W.H.I.; Rebbeck, T.R.; Mitra, N. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J. Allergy Clin. Immunol. 2012, 130, 912–917. [Google Scholar] [CrossRef]
- Suther, C.; Moore, M.D.; Beigelman, A.; Zhou, Y. The gut microbiome and the big eight. Nutrients 2020, 12, 3728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Guan, X.-X.; Tang, Y.-J.; Sun, J.-F.; Wang, X.-K.; Wang, W.-D.; Fan, J.-M. Clinical effects and gut microbiota changes of using probiotics, prebiotics or synbiotics in inflammatory bowel disease: A systematic review and meta-analysis. Eur. J. Nutr. 2021, 60, 2855–2875. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; DuPont, H.L. New approaches for bacteriotherapy: Prebiotics, new-generation probiotics, and synbiotics. Clin. Infect. Dis. 2015, 60 (Suppl. 2), S108–S121. [Google Scholar] [CrossRef]
- Peterson, C.T.; Sharma, V.; Elmén, L.; Peterson, S.N. Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clin. Exp. Immunol. 2015, 179, 363–377. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Oniszczuk, T.; Gancarz, M.; Szymańska, J. Role of gut microbiota, probiotics and prebiotics in the cardiovascular diseases. Molecules 2021, 26, 1172. [Google Scholar] [CrossRef]
- Luoto, R.; Collado, M.C.; Salminen, S.; Isolauri, E. Reshaping the gut microbiota at an early age: Functional impact on obesity risk? Ann. Nutr. Metab. 2013, 63 (Suppl. 2), 17–26. [Google Scholar] [CrossRef]
- Tu, Y.; Yang, R.; Xu, X.; Zhou, X. The microbiota-gut-bone axis and bone health. J. Leukoc. Biol. 2021, 110, 525–537. [Google Scholar] [CrossRef]
- Vieira, A.T.; Teixeira, M.M.; Martins, F.S. The role of probiotics and prebiotics in inducing gut immunity. Front. Immunol. 2013, 4, 445. [Google Scholar] [CrossRef]
- Iglesia, E.G.A.; Kwan, M.; Virkud, Y.V.; Iweala, O.I. Management of Food Allergies and Food-Related Anaphylaxis. JAMA 2024, 331, 510–521. [Google Scholar] [CrossRef]
- Simpson, E.L.; Chalmers, J.R.; Hanifin, J.M.; Thomas, K.S.; Cork, M.J.; McLean, W.H.I.; Brown, S.J.; Chen, Z.; Chen, Y.; Williams, H.C. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J. Allergy Clin. Immunol. 2014, 134, 818–823. [Google Scholar] [CrossRef]
- Armstrong, J.; Rosinski, N.K.; Fial, A.; Ansah, S.; Haglund, K. Emollients to Prevent Eczema in High-Risk Infants: Integrative Review. MCN Am. J. Matern. Child Nurs. 2022, 47, 122–129. [Google Scholar] [CrossRef]
- Cipriani, F.; Dondi, A.; Ricci, G. Recent advances in epidemiology and prevention of atopic eczema. Pediatr. Allergy Immunol. 2014, 25, 630–638. [Google Scholar] [CrossRef]
- McClanahan, D.; Wong, A.; Kezic, S.; Samrao, A.; Hajar, T.; Hill, E.; Simpson, E.L. A randomized controlled trial of an emollient with ceramide and filaggrin-associated amino acids for the primary prevention of atopic dermatitis in high-risk infants. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2087–2094. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Wood, R.A.; Stablein, D.; Lindblad, R.; Burks, A.W.; Liu, A.H.; Jones, S.M.; Fleischer, D.M.; Leung, D.Y.M.; Sampson, H.A. Maternal consumption of peanut during pregnancy is associated with peanut sensitization in atopic infants. J. Allergy Clin. Immunol. 2010, 126, 1191–1197. [Google Scholar] [CrossRef]
- Greenhawt, M.; Shaker, M. Determining Levers of Cost-effectiveness for Screening Infants at High Risk for Peanut Sensitization Before Early Peanut Introduction. JAMA Netw. Open 2019, 2, e1918041. [Google Scholar] [CrossRef]
- Fu, J.; Huang, Y.; Bao, T.; Liu, C.; Liu, X.; Chen, X. The role of Th17 cells/IL-17A in AD, PD, ALS and the strategic therapy targeting on IL-17A. J. Neuroinflammation 2022, 19, 98. [Google Scholar] [CrossRef]
- Moutsoglou, D.M.; Dreskin, S.C. Prolonged Treatment of Peanut-Allergic Mice with Bortezomib Significantly Reduces Serum Anti-Peanut IgE but Does Not Affect Allergic Symptoms. Int. Arch. Allergy Immunol. 2016, 170, 257–261. [Google Scholar] [CrossRef]
- Toomer, O.T. A comprehensive review of the value-added uses of peanut (Arachis hypogaea) skins and by-products. Crit. Rev. Food Sci. Nutr. 2020, 60, 341–350. [Google Scholar] [CrossRef]
- Ma, S.; Nie, L.; Li, H.; Wang, R.; Yin, J. Component-Resolved Diagnosis of Peanut Allergy and Its Possible Origins of Sensitization in China. Int. Arch. Allergy Immunol. 2016, 169, 241–248. [Google Scholar] [CrossRef]
- Peters, R.L.; Allen, K.J.; Dharmage, S.C.; Koplin, J.J.; Dang, T.; Tilbrook, K.P.; Lowe, A.; Tang, M.L.K.; Gurrin, L.C. Natural history of peanut allergy and predictors of resolution in the first 4 years of life: A population-based assessment. J. Allergy Clin. Immunol. 2015, 135, 1257–1266.e1. [Google Scholar] [CrossRef]
- McKean, M.; Caughey, A.B.; Leong, R.E.; Wong, A.; Cabana, M.D. The timing of infant food introduction in families with a history of atopy. Clin. Pediatr. 2015, 54, 745–751. [Google Scholar] [CrossRef]
- Nowak-Węgrzyn, A.; Chatchatee, P. Mechanisms of tolerance induction. Ann. Nutr. Metab. 2017, 70 (Suppl. 2), 7–24. [Google Scholar] [CrossRef]
- Devonshire, A.L.; Fan, H.; Pujato, M.; Paranjpe, A.; Gursel, D.; Schipma, M.; Dunn, J.M.; Andorf, S.; Pongracic, J.A.; Kottyan, L.C.; et al. Whole blood transcriptomics identifies gene expression associated with peanut allergy in infants at high risk. Clin. Exp. Allergy 2021, 51, 1396–1400. [Google Scholar] [CrossRef] [PubMed]
- Strid, J.; Thomson, M.; Hourihane, J.; Kimber, I.; Strobel, S. A novel model of sensitization and oral tolerance to peanut protein. Immunology 2004, 113, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Anvari, S.; Chokshi, N.Y.; Kamili, Q.U.A.; Davis, C.M. Evolution of guidelines on peanut allergy and peanut introduction in infants: A review. JAMA Pediatr. 2017, 171, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Fisher, H.R.; Du Toit, G.; Bahnson, H.T.; Lack, G. The challenges of preventing food allergy: Lessons learned from LEAP and EAT. Ann. Allergy Asthma Immunol. 2018, 121, 313–319. [Google Scholar] [CrossRef]
- Tsilochristou, O.; du Toit, G.; Sayre, P.H.; Roberts, G.; Lawson, K.; Sever, M.L.; Bahnson, H.T.; Radulovic, S.; Basting, M.; Plaut, M.; et al. Association of Staphylococcus aureus colonization with food allergy occurs independently of eczema severity. J. Allergy Clin. Immunol. 2019, 144, 494–503. [Google Scholar] [CrossRef]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M.; et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N. Engl. J. Med. 2015, 372, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cheng, L.; Wang, J.; Hao, M.; Che, H. Antibiotic-Induced Gut Microbiota Dysbiosis Damages the Intestinal Barrier, Increasing Food Allergy in Adult Mice. Nutrients 2021, 13, 3315. [Google Scholar] [CrossRef]
- Kuehn, A.; Hilger, C. Animal allergens: Common protein characteristics featuring their allergenicity. Front. Immunol. 2015, 6, 40. [Google Scholar] [CrossRef]
- Srivastava, K.D.; Bardina, L.; Sampson, H.A.; Li, X.-M. Efficacy and immunological actions of FAHF-2 in a murine model of multiple food allergies. Ann. Allergy Asthma Immunol. 2012, 108, 351–358.e1. [Google Scholar] [CrossRef]
- Frank, L.; Marian, A.; Visser, M.; Weinberg, E.; Potter, P.C. Exposure to peanuts in utero and in infancy and the development of sensitization to peanut allergens in young children. Pediatr. Allergy Immunol. 1999, 10, 27–32. [Google Scholar] [CrossRef]
- Wennergren, G. What if it is the other way around? Early introduction of peanut and fish seems to be better than avoidance. Acta Paediatr. 2009, 98, 1085–1087. [Google Scholar] [CrossRef] [PubMed]
- Buonomo, A.; Nucera, E.; Schiavino, D. Doctor, can I desensitize my food-allergic child using directly the allergenic molecules? Curr. Opin. Allergy Clin. Immunol. 2016, 16, 278–283. [Google Scholar] [CrossRef]
- Croote, D.; Quake, S.R. Food allergen detection by mass spectrometry: The role of systems biology. NPJ Syst. Biol. Appl. 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed]
- Elmonir, W.; Abo-Remela, E.; Sobeih, A. Public health risks of Escherichia coli and Staphylococcus aureus in raw bovine milk sold in informal markets in Egypt. J. Infect. Dev. Ctries 2018, 12, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Kadariya, J.; Smith, T.C.; Thapaliya, D. Staphylococcus aureus and staphylococcal food-borne disease: An ongoing challenge in public health. Biomed Res. Int. 2014, 2014, 827965. [Google Scholar] [CrossRef]
- Dehkordi, M.K.; Shamsabadi, M.G. The occurrence of Staphylococcus aureus, enterotoxigenic and methicillin-resistant strains in Iranian food resources: A systematic review and meta-analysis. Ann. Ig. Med. Prev. Comunita 2019, 31. [Google Scholar]
- Wang, W.; Baloch, Z.; Jiang, T.; Zhang, C.; Peng, Z.; Li, F.; Fanning, S.; Ma, A.; Xu, J. Enterotoxigenicity and Antimicrobial Resistance of Staphylococcus aureus Isolated from Retail Food in China. Front. Microbiol. 2017, 8, 2256. [Google Scholar] [CrossRef]
- Bennett, S.D.; Walsh, K.A.; Gould, L.H. Foodborne disease outbreaks caused by Bacillus cereus, Clostridium perfringens, and Staphylococcus aureus—United States, 1998–2008. Clin. Infect. Dis. 2013, 57, 425–433. [Google Scholar] [CrossRef]
- Hennekinne, J.-A.; De Buyser, M.-L.; Dragacci, S. Staphylococcus aureus and its food poisoning toxins: Characterization and outbreak investigation. FEMS Microbiol. Rev. 2012, 36, 815–836. [Google Scholar] [CrossRef]
- Jorde, I.; Schreiber, J.; Stegemann-Koniszewski, S. The Role of Staphylococcus aureus and Its Toxins in the Pathogenesis of Allergic Asthma. Int. J. Mol. Sci. 2022, 24, 654. [Google Scholar] [CrossRef]
- Fluit, A.C. Livestock-associated Staphylococcus aureus. Clin. Microbiol. Infect. 2012, 18, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Adugna, F.; Pal, M.; Girmay, G. Prevalence and Antibiogram Assessment of Staphylococcus aureus in Beef at Municipal Abattoir and Butcher Shops in Addis Ababa, Ethiopia. Biomed Res. Int. 2018, 2018, 5017685. [Google Scholar] [CrossRef]
- Argaw, S.; Addis, M. A review on staphylococcal food poisoning. Food Sci. Qual. Manag. 2015, 40, 59–72. [Google Scholar]
- Thomas, D.; Chou, S.; Dauwalder, O.; Lina, G. Diversity in Staphylococcus aureus enterotoxins. Chem. Immunol. Allergy 2007, 93, 24–41. [Google Scholar] [CrossRef] [PubMed]
- Maina, E.K.; Hu, D.-L.; Tsuji, T.; Omoe, K.; Nakane, A. Staphylococcal enterotoxin A has potent superantigenic and emetic activities but not diarrheagenic activity. Int. J. Med. Microbiol. 2012, 302, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Sheahan, D.G.; Jervis, H.R.; Takeuchi, A.; Sprinz, H. The effect of staphylococcal enterotoxin on the epithelial mucosubstances of the small intestine of rhesus monkeys. Am. J. Pathol. 1970, 60, 1–18. [Google Scholar]
- Fraser, J.D.; Proft, T. The bacterial superantigen and superantigen-like proteins. Immunol. Rev. 2008, 225, 226–243. [Google Scholar] [CrossRef] [PubMed]
- Grumann, D.; Nübel, U.; Bröker, B.M. Staphylococcus aureus toxins--their functions and genetics. Infect. Genet. Evol. 2014, 21, 583–592. [Google Scholar] [CrossRef]
- Oliveira, D.; Borges, A.; Simões, M. Staphylococcus aureus Toxins and Their Molecular Activity in Infectious Diseases. Toxins 2018, 10, 252. [Google Scholar] [CrossRef]
- Jabara, H.H.; Geha, R.S. The superantigen toxic shock syndrome toxin-1 induces CD40 ligand expression and modulates lgE isotype switching. Int. Immunol. 1996, 8, 1503–1510. [Google Scholar] [CrossRef]
- Tiedemann, R.E.; Fraser, J.D. Cross-linking of MHC class II molecules by staphylococcal enterotoxin A is essential for antigen-presenting cell and T cell activation. J. Immunol. 1996, 157, 3958–3966. [Google Scholar] [CrossRef] [PubMed]
- Arad, G.; Levy, R.; Nasie, I.; Hillman, D.; Rotfogel, Z.; Barash, U.; Supper, E.; Shpilka, T.; Minis, A.; Kaempfer, R. Binding of superantigen toxins into the CD28 homodimer interface is essential for induction of cytokine genes that mediate lethal shock. PLoS Biol. 2011, 9, e1001149. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.Q.; Ho, A.W.S.; Tang, Y.; Wong, K.H.S.; Chua, B.Y.L.; Gasser, S.; Kemeny, D.M. NK cells regulate CD8+ T cell priming and dendritic cell migration during influenza A infection by IFN-γ and perforin-dependent mechanisms. J. Immunol. 2012, 189, 2099–2109. [Google Scholar] [CrossRef] [PubMed]
- Chintagumpala, M.M.; Mollick, J.A.; Rich, R.R. Staphylococcal toxins bind to different sites on HLA-DR. J. Immunol. 1991, 147, 3876–3881. [Google Scholar] [CrossRef]
- Mehindate, K.; Thibodeau, J.; Dohlsten, M.; Kalland, T.; Sékaly, R.P.; Mourad, W. Cross-linking of major histocompatibility complex class II molecules by staphylococcal enterotoxin A superantigen is a requirement for inflammatory cytokine gene expression. J. Exp. Med. 1995, 182, 1573–1577. [Google Scholar] [CrossRef]
- Krakauer, T. Costimulatory receptors for the superantigen staphylococcal enterotoxin B on human vascular endothelial cells and T cells. J. Leukoc. Biol. 1994, 56, 458–463. [Google Scholar] [CrossRef]
- Smith-Garvin, J.E.; Koretzky, G.A.; Jordan, M.S. T cell activation. Annu. Rev. Immunol. 2009, 27, 591–619. [Google Scholar] [CrossRef]
- van Leeuwen, J.E.; Samelson, L.E. T cell antigen-receptor signal transduction. Curr. Opin. Immunol. 1999, 11, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.; Rotfogel, Z.; Hillman, D.; Popugailo, A.; Arad, G.; Supper, E.; Osman, F.; Kaempfer, R. Superantigens hyperinduce inflammatory cytokines by enhancing the B7-2/CD28 costimulatory receptor interaction. Proc. Natl. Acad. Sci. USA 2016, 113, E6437–E6446. [Google Scholar] [CrossRef]
- Krakauer, T.; Pradhan, K.; Stiles, B.G. Staphylococcal Superantigens Spark Host-Mediated Danger Signals. Front. Immunol. 2016, 7, 23. [Google Scholar] [CrossRef]
- Park, H.-Y.; Kim, C.-R.; Huh, I.-S.; Jung, M.-Y.; Seo, E.-Y.; Park, J.-H.; Lee, D.-Y.; Yang, J.-M. Staphylococcus aureus Colonization in Acute and Chronic Skin Lesions of Patients with Atopic Dermatitis. Ann. Dermatol. 2013, 25, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Sangaphunchai, P.; Kritsanaviparkporn, C.; Treesirichod, A. Association Between Staphylococcus Aureus Colonization and Pediatric Atopic Dermatitis: A Systematic Review and Meta-Analysis. Indian J. Dermatol. 2023, 68, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.E.; Yung, A.; Rademaker, M. Prevalence of Staphylococcus aureus and antibiotic resistance in children with atopic dermatitis: A New Zealand experience. Australas. J. Dermatol. 2011, 52, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Glatz, M.; Horiuchi, K.; Kawasaki, H.; Akiyama, H.; Kaplan, D.H.; Kong, H.H.; Amagai, M.; Nagao, K. Dysbiosis and Staphylococcus aureus Colonization Drives Inflammation in Atopic Dermatitis. Immunity 2015, 42, 756–766. [Google Scholar] [CrossRef]
- Tomczak, H.; Wróbel, J.; Jenerowicz, D.; Sadowska-Przytocka, A.; Wachal, M.; Adamski, Z.; Czarnecka-Operacz, M.M. The role of Staphylococcus aureus in atopic dermatitis: Microbiological and immunological implications. Postepy Dermatol. Alergol. 2019, 36, 485–491. [Google Scholar] [CrossRef]
- Ay, M.; Doğan, M. Investigation of the Effects of Kitchen Hygiene Training on Reducing Personnel-Associated Microbial Contamination. Istanb. Gelisim Univ. J. Health Sci. 2020, 11, 161–177. [Google Scholar] [CrossRef]
- Regasa, S.; Mengistu, S.; Abraha, A. Milk Safety Assessment, Isolation, and Antimicrobial Susceptibility Profile of Staphylococcus aureus in Selected Dairy Farms of Mukaturi and Sululta Town, Oromia Region, Ethiopia. Vet. Med. Int. 2019, 2019, 3063185. [Google Scholar] [CrossRef]
- Yulianto, N.S.; Armiyanti, Y.; Agustina, D.; Hermansyah, B.; Utami, W.S. Analysis of milking hygiene and its association to staphylococcus aureus contamination in fresh cow milk. JKL 2023, 15, 275–282. [Google Scholar] [CrossRef]
- Totté, J.E.E.; van der Feltz, W.T.; Hennekam, M.; van Belkum, A.; van Zuuren, E.J.; Pasmans, S.G.M.A. Prevalence and odds of Staphylococcus aureus carriage in atopic dermatitis: A systematic review and meta-analysis. Br. J. Dermatol. 2016, 175, 687–695. [Google Scholar] [CrossRef]
- Ramadhan, E.L.; Retnowati, W.; Dewanti, L.; Wahyunitisari, M.R. Cavendish banana peel extract’s antibacterial activities potential as disinfectant. Univ. Airlangga 2023, 14, 100–104. [Google Scholar] [CrossRef]
- Bonso, M.; Bedada, D.; Dires, S. Bacterial contamination and antimicrobial resistance in drinking water from food and drinking establishments in shashemane town, ethiopia. Environ. Health Insights 2023, 17, 11786302231216864. [Google Scholar] [CrossRef] [PubMed]
- Daka, D.; G/Silassie, S.; Yihdego, D. Antibiotic-resistance Staphylococcus aureus isolated from cow’s milk in the Hawassa area, South Ethiopia. Ann. Clin. Microbiol. Antimicrob. 2012, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Elshiekh, N.; Mohammed, G.; Abdalla, M.; Elkhair, O.; Altayeb, H. Isolation and molecular identification of staphylococcus species in cow’s milk distributed in khartoum state. Egypt. J. Vet. Sci. 2020, 51, 271–281. [Google Scholar] [CrossRef]
- Bender, J.B.; Schiffman, E.; Hiber, L.; Gerads, L.; Olsen, K. Recovery of staphylococci from computer keyboards in a veterinary medical centre and the effect of routine cleaning. Vet. Rec. 2012, 170, 414. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Wang, C.; Huang, C.; Chen, S.; Tseng, M.; Lo, W. Antimicrobial susceptibility of Staphylococcus aureus in children with atopic dermatitis. Pediatr. Int. 2011, 53, 363–367. [Google Scholar] [CrossRef]
- Afridayani, M.; Prastiwi, Y.I.; Aulawi, K.; Rahmat, I.; Nirwati, H.; Haryani, H. Relationship between hand hygiene behavior and Staphylococcus aureus colonization on cell phones of nurses in the intensive care unit. Belitung Nurs. J. 2021, 7, 24–30. [Google Scholar] [CrossRef]
- Mohamed Eltahlawy, A.; Morshdy, A.; Hafez, A.E.-S.; Mahmoud, A.F.; El Bayomi, R.; Hussein, M. Evaluation of the hygienic status at El-Qurein abattoir. Zagazig Vet. J. 2022, 50, 101–115. [Google Scholar] [CrossRef]
- van Belkum, A.; Rochas, O. Laboratory-Based and Point-of-Care Testing for MSSA/MRSA Detection in the Age of Whole Genome Sequencing. Front. Microbiol. 2018, 9, 1437. [Google Scholar] [CrossRef]
- Graves, N.; Page, K.; Martin, E.; Brain, D.; Hall, L.; Campbell, M.; Fulop, N.; Jimmeison, N.; White, K.; Paterson, D.; et al. Cost-Effectiveness of a National Initiative to Improve Hand Hygiene Compliance Using the Outcome of Healthcare Associated Staphylococcus aureus Bacteraemia. PLoS ONE 2016, 11, e0148190. [Google Scholar] [CrossRef]
- Majewski, S.; Bhattacharya, T.; Asztalos, M.; Bohaty, B.; Durham, K.C.; West, D.P.; Hebert, A.A.; Paller, A.S. Sodium hypochlorite body wash in the management of Staphylococcus aureus-colonized moderate-to-severe atopic dermatitis in infants, children, and adolescents. Pediatr. Dermatol. 2019, 36, 442–447. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.E.; Ahn, K.; Leung, D.Y.M. Interactions Between Atopic Dermatitis and Staphylococcus aureus Infection: Clinical Implications. Allergy Asthma Immunol. Res. 2019, 11, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-W.; Kim, S.M.; Kim, J.M.; Oh, B.M.; Kim, J.Y.; Jung, H.J.; Lim, H.J.; Kim, B.S.; Lee, W.J.; Lee, S.-J.; et al. Potential Immunoinflammatory Role of Staphylococcal Enterotoxin A in Atopic Dermatitis: Immunohistopathological Analysis and in vitro Assay. Ann. Dermatol. 2013, 25, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Cavallo, I.; Bordignon, V.; Prignano, G.; Sperduti, I.; Gurtner, A.; Trento, E.; Toma, L.; Pimpinelli, F.; Capitanio, B.; et al. Inflammatory cytokines and biofilm production sustain Staphylococcus aureus outgrowth and persistence: A pivotal interplay in the pathogenesis of Atopic Dermatitis. Sci. Rep. 2018, 8, 9573. [Google Scholar] [CrossRef]
- Callewaert, C.; Nakatsuji, T.; Knight, R.; Kosciolek, T.; Vrbanac, A.; Kotol, P.; Ardeleanu, M.; Hultsch, T.; Guttman-Yassky, E.; Bissonnette, R.; et al. IL-4Rα Blockade by Dupilumab Decreases Staphylococcus aureus Colonization and Increases Microbial Diversity in Atopic Dermatitis. J. Investig. Dermatol. 2020, 140, 191–202.e7. [Google Scholar] [CrossRef]
- Park, S.M.; Choi, W.S.; Yoon, Y.; Jung, G.H.; Lee, C.K.; Ahn, S.H.; Wonsuck, Y.; Yoo, Y. Breast abscess caused by Staphylococcus aureus in 2 adolescent girls with atopic dermatitis. Korean J. Pediatr. 2018, 61, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Gui, H.C.; Bo, A.K.; Chan, I.P.; You, Y.K. The effect of phytosphingosine isolated from Asterina pectinifera on cell damage induced by mite antigen in HaCaT cell and antibacterial activity against Staphylococcus aureus. Afr. J. Biotechnol. 2010, 9, 920–926. [Google Scholar] [CrossRef]
- Moran, M.C.; Cahill, M.P.; Brewer, M.G.; Yoshida, T.; Knowlden, S.; Perez-Nazario, N.; Schlievert, P.M.; Beck, L.A. Staphylococcal virulence factors on the skin of atopic dermatitis patients. mSphere 2019, 4, 10–128. [Google Scholar] [CrossRef]
- Kou, K.; Okawa, T.; Yamaguchi, Y.; Ono, J.; Inoue, Y.; Kohno, M.; Matsukura, S.; Kambara, T.; Ohta, S.; Izuhara, K.; et al. Periostin levels correlate with disease severity and chronicity in patients with atopic dermatitis. Br. J. Dermatol. 2014, 171, 283–291. [Google Scholar] [CrossRef]
- Jin, B.-Y.; Li, Z.; Xia, Y.-N.; Li, L.-X.; Zhao, Z.-X.; Li, X.-Y.; Li, Y.; Li, B.; Zhou, R.-C.; Fu, S.-C.; et al. Probiotic interventions alleviate food allergy symptoms correlated with cesarean section: A murine model. Front. Immunol. 2021, 12, 741371. [Google Scholar] [CrossRef]
- Chernikova, D.A.; Zhao, M.Y.; Jacobs, J.P. Microbiome therapeutics for food allergy. Nutrients 2022, 14, 5155. [Google Scholar] [CrossRef]
- Zhao, W.; Ho, H.-E.; Bunyavanich, S. The gut microbiome in food allergy. Ann. Allergy Asthma Immunol. 2019, 122, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Song, Y.; Wu, W.; Yu, K.; Zhang, G. The gut microbiota, environmental factors, and links to the development of food allergy. Clin. Mol. Allergy 2020, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, M.; Rudi, K.; Angell, I.L.; Dirven, H.; Nygaard, U.C. Allergen Immunization Induces Major Changes in Microbiota Composition and Short-Chain Fatty Acid Production in Different Gut Segments in a Mouse Model of Lupine Food Allergy. Int. Arch. Allergy Immunol. 2018, 177, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Lei, Z.; Rui, B.; Li, Y.; Li, M. Gut microbiota promotes immune tolerance by regulating rorγt+ treg cells in food allergy. Adv. Gut Microbiome Res. 2022, 2022, 8529578. [Google Scholar] [CrossRef]
- Parrish, A.; Boudaud, M.; Kuehn, A.; Ollert, M.; Desai, M.S. Intestinal mucus barrier: A missing piece of the puzzle in food allergy. Trends Mol. Med. 2022, 28, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Qu, Y.; Gao, Y.; Sun, S.; Wu, R.; Wu, J. Research Progress on the Correlation between the Intestinal Microbiota and Food Allergy. Foods 2022, 11, 2913. [Google Scholar] [CrossRef]
- Aizawa, S.; Uebanso, T.; Shimohata, T.; Mawatari, K.; Takahashi, A. Effects of the loss of maternal gut microbiota before pregnancy on gut microbiota, food allergy susceptibility, and epigenetic modification on subsequent generations. Biosci. Microbiota Food Health 2023, 42, 203–212. [Google Scholar] [CrossRef]
- Yan, X.; Yan, J.; Xiang, Q.; Dai, H.; Wang, Y.; Fang, L.; Huang, K.; Zhang, W. Early-life gut microbiota in food allergic children and its impact on the development of allergic disease. Ital. J. Pediatr. 2023, 49, 148. [Google Scholar] [CrossRef]
- De Martinis, M.; Sirufo, M.M.; Suppa, M.; Ginaldi, L. New perspectives in food allergy. Int. J. Mol. Sci. 2020, 21, 1474. [Google Scholar] [CrossRef]
- Berni Canani, R.; Paparo, L.; Nocerino, R.; Di Scala, C.; Della Gatta, G.; Maddalena, Y.; Buono, A.; Bruno, C.; Voto, L.; Ercolini, D. Gut microbiome as target for innovative strategies against food allergy. Front. Immunol. 2019, 10, 191. [Google Scholar] [CrossRef]
- Poole, A.; Song, Y.; Brown, H.; Hart, P.H.; Zhang, G.B. Cellular and molecular mechanisms of vitamin D in food allergy. J. Cell. Mol. Med. 2018, 22, 3270–3277. [Google Scholar] [CrossRef] [PubMed]
- Eiwegger, T.; Hung, L.; San Diego, K.E.; O’Mahony, L.; Upton, J. Recent developments and highlights in food allergy. Allergy 2019, 74, 2355–2367. [Google Scholar] [CrossRef] [PubMed]
- van Ginkel, C.D.; Pettersson, M.E.; Dubois, A.E.J.; Koppelman, G.H. Association of STAT6 gene variants with food allergy diagnosed by double-blind placebo-controlled food challenges. Allergy 2018, 73, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, N.; Berin, M.C. Pathogenesis of IgE-mediated food allergy and implications for future immunotherapeutics. Pediatr. Allergy Immunol. 2021, 32, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, A.; Lo Presti, E.; Chini, R.; Gammeri, L.; Inchingolo, R.; Lohmeyer, F.M.; Nucera, E.; Gangemi, S. Emerging role of alarmins in food allergy: An update on pathophysiological insights, potential use as disease biomarkers, and therapeutic implications. J. Clin. Med. 2023, 12, 2699. [Google Scholar] [CrossRef]
- Abdel-Gadir, A.; Schneider, L.; Casini, A.; Charbonnier, L.M.; Little, S.V.; Harrington, T.; Umetsu, D.T.; Rachid, R.; Chatila, T.A. Oral immunotherapy with omalizumab reverses the Th2 cell-like programme of regulatory T cells and restores their function. Clin. Exp. Allergy 2018, 48, 825–836. [Google Scholar] [CrossRef]
- Sahiner, U.M.; Giovannini, M.; Escribese, M.M.; Paoletti, G.; Heffler, E.; Alvaro Lozano, M.; Barber, D.; Canonica, G.W.; Pfaar, O. Mechanisms of allergen immunotherapy and potential biomarkers for clinical evaluation. J. Pers. Med. 2023, 13, 845. [Google Scholar] [CrossRef]
- Feehley, T.; Plunkett, C.H.; Bao, R.; Choi Hong, S.M.; Culleen, E.; Belda-Ferre, P.; Campbell, E.; Aitoro, R.; Nocerino, R.; Paparo, L.; et al. Healthy infants harbor intestinal bacteria that protect against food allergy. Nat. Med. 2019, 25, 448–453. [Google Scholar] [CrossRef]
- Imran, S.; Neeland, M.R.; Koplin, J.; Dharmage, S.; Tang, M.L.; Sawyer, S.; Dang, T.; McWilliam, V.; Peters, R.; Perrett, K.P.; et al. Epigenetic programming underpins B-cell dysfunction in peanut and multi-food allergy. Clin. Transl. Immunol. 2021, 10, e1324. [Google Scholar] [CrossRef]
- Potaczek, D.P.; Bazan-Socha, S.; Wypasek, E.; Wygrecka, M.; Garn, H. Recent developments in the role of histone acetylation in asthma. Int. Arch. Allergy Immunol. 2024, 185, 641–651. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of the MDPI and/or the editor(s). The MDPI and/or the editor(s) disclose responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
| Immunological Process | Key Mechanistic Components | Mechanistic Overview | Description |
|---|---|---|---|
| Induction of Allergic Immune Responses | Food Allergen Processing | Breakdown and recognition of food allergens by hydrolytic enzymes in the gastrointestinal tract. Processed by antigen-presenting cells (APCs) and recognized by antigen-specific T cells. | Upon ingestion, food allergens encounter digestive enzymes like proteases and lipases that break them down into smaller peptides. These peptides are processed by dendritic cells (DCs) and macrophages, which present them to T cells, initiating an immune response. |
| Th2 Cell Differentiation | Naïve T helper cells differentiate into Th2 cells under the influence of IL-4, promoting IL-4, IL-5, IL-10, and IL-13 secretion, leading to IgE production by B cells. | IL-4 plays a crucial role in shifting immune responses toward a Th2 phenotype. The secretion of IL-4 and IL-13 promotes class switching in B cells, resulting in the production of allergen-specific IgE, which is central to allergic reactions. | |
| Mast Cell Activation | IgE binds to FcεRI receptors on mast cells and basophils, triggering degranulation and mediator release (e.g., histamine) during antigen re-exposure. | Upon re-exposure to the allergen, cross-linking of IgE molecules on mast cells and basophils leads to the release of histamine, leukotrienes, and prostaglandins, causing symptoms like vasodilation, bronchoconstriction, and mucus production in allergic individuals. | |
| Influence of Cooking on Allergenicity | Cooking can reduce (e.g., peanut allergens Ara h 1 in fried or boiled peanuts) or increase allergenicity (e.g., shellfish allergens increase post-heating). | Cooking methods, such as boiling, can denature food proteins, making them less recognizable by the immune system and thus reducing allergenicity. However, in some cases like with shellfish, heat can enhance allergenicity by exposing IgE-binding epitopes that are normally hidden. | |
| Oral Tolerance | Development of Tolerance | Occurs in the gut-associated lymphoid tissue (GALT), where a breakdown leads to allergic responses. | Oral tolerance involves the immune system becoming desensitized to food antigens that are consistently encountered in the diet. When oral tolerance is lost, the immune system instead mounts an allergic response, characterized by the production of allergen-specific IgE. |
| Mechanisms | Involves antigen recognition by dendritic cells, followed by the induction of T regulatory cells (Tregs) and B regulatory cells (Bregs). The gut environment, such as metabolites, modulates responses. | The gut microbiome and its metabolites (e.g., SCFAs) play a crucial role in maintaining immune homeostasis. Tregs induced in response to dietary antigens help suppress aberrant Th2 responses, maintaining tolerance. Disruptions to this environment lead to allergy. | |
| Dendritic Cells (DCs) | Antigen Uptake and Migration | Antigens are taken up by M cells and GAPs, transferred to dendritic cells. Tolerogenic CD103 + CX3CR1− DCs promote Treg development, while CX3CR1 + DCs are inflammatory. | DCs in the gut sample food antigens through specialized epithelial cells like M cells or goblet cell-associated passages (GAPs). Tolerogenic DCs promote immune tolerance, whereas inflammatory DCs contribute to sensitization and allergic inflammation. |
| Treg Induction | Tolerogenic CD103+ DCs migrate to mesenteric lymph nodes, promoting Treg induction via TGF-β and RALDH2. | Tolerogenic DCs express the enzyme RALDH2, which converts vitamin A into retinoic acid, crucial for the differentiation of Tregs. These cells produce immunosuppressive cytokines like IL-10, which are essential for the suppression of allergic responses. | |
| T Regulatory Cells (Tregs) | Role in Allergy and Tolerance | Th2 cells drive allergic inflammation, while Tregs (FOXP3+ and Th3 cells) regulate immune tolerance, maintaining the balance between allergy and tolerance. | Tregs are central to maintaining peripheral tolerance to food antigens. They inhibit effector Th2 responses through the production of IL-10 and TGF-β, thereby preventing the activation of the allergic cascade. The disruption of Treg function can lead to allergic diseases. |
| Mechanisms of Treg Function | Tregs secrete IL-10 and TGF-β to inhibit APCs and suppress effector T cell proliferation, crucial for maintaining tolerance. Dysfunction results in allergic inflammation. | Tregs exert their regulatory effects by directly suppressing antigen presentation by DCs and macrophages and by dampening the activity of effector T cells. This prevents excessive immune responses, helping maintain immune homeostasis in the gut and peripheral tissues. | |
| B Regulatory Cells (Bregs) | Suppression of Allergic Responses | Bregs suppress effector T cells by producing IL-10, TGF-β, and IL-35. They contribute to tolerance by producing IgG4, which inhibits the IgE-mediated degranulation of mast cells and basophils. | Bregs support immune tolerance by secreting anti-inflammatory cytokines and producing IgG4 antibodies that block allergen–IgE interactions. Their regulatory role extends to suppressing T cell proliferation and dampening DC activation, further preventing allergic responses. |
| Mucosal Tolerance | Bregs maintain mucosal tolerance, involving IL-10-producing CD5+ Bregs and interaction with CD40L+ ILC3s. | Bregs are essential for mucosal tolerance, particularly in the gut. Their production of IL-10 and interaction with innate lymphoid cells (ILC3s) help preserve barrier integrity and prevent excessive immune activation against food antigens. | |
| Epithelial Barrier Dysfunction | Impaired Barrier Integrity | The disruption of epithelial tight junctions by pro-inflammatory cytokines (e.g., IL-4, IL-13) increases permeability, allowing allergen penetration and immune system exposure. | Epithelial cells form a physical barrier that prevents allergens from entering the systemic circulation. Th2 cytokines like IL-4 and IL-13 weaken this barrier by disrupting tight junctions, increasing the likelihood of allergen translocation and subsequent immune activation. |
| Molecular Mechanisms of Barrier Loss | IL-33, TSLP, and IL-25 released by epithelial cells activate ILC2s and Th2 cells, enhancing allergic inflammation and contributing to barrier breakdown. | Epithelial damage leads to the release of danger signals like IL-33 and TSLP, which activate innate and adaptive immune responses. This further weakens the barrier, perpetuating inflammation and increasing allergen penetration, aggravating the allergic response. | |
| Molecular Pathways | JAK-STAT Signaling Pathways | IL-4/IL-13-mediated signaling through JAK-STAT pathways promotes Th2 differentiation, IgE class switching in B cells, and epithelial barrier dysfunction. | The JAK-STAT pathway is a critical signaling mechanism for Th2 cytokines. In allergic individuals, this pathway is hyperactive, promoting the overproduction of IgE and impairing epithelial function. Targeting this pathway is a therapeutic strategy in allergic diseases. |
| MAPK and NF-κB Pathways | These pathways drive the inflammatory response in allergic reactions, regulating cytokine production (e.g., TNF-α, IL-6) and promoting epithelial barrier dysfunction. | MAPK and NF-κB are key transcriptional pathways that regulate the expression of pro-inflammatory cytokines during allergic responses. Their activation contributes to tissue inflammation, airway remodeling, and the breakdown of the epithelial barrier. |
| Factor | Mechanism of Sensitization | Clinical and Research Implications | References |
|---|---|---|---|
| Immune System Immaturity | Immature neonatal immune responses skew toward Th2 dominance, promoting IgE production. Deficient regulatory T cell (Treg) activity fails to induce oral tolerance. Dendritic cell function remains suboptimal, reducing antigen presentation efficiency. Immaturity of the mucosal immune system, including limited secretory IgA, further hampers tolerance induction. | Exploring early immunomodulatory interventions, such as Treg-boosting therapies, could support the development of oral tolerance. Early introduction of allergens should be evaluated in high-risk infants, especially those with family histories of atopy. | [25,111,112,113] |
| Genetic Susceptibility | Mutations in FLG (filaggrin) impair skin barrier function, promoting allergen penetration and sensitization. HLA class II alleles (HLA-DQ2/DQ8) are strongly associated with FAs. Epigenetic mechanisms, including DNA methylation changes, may further modulate immune responses in genetically predisposed infants. | Genetic screening in early infancy can identify high-risk groups. Targeted interventions, such as barrier-enhancing treatments or early allergen exposure, may be particularly effective for infants with FLG mutations. Gene–environment interactions should be a focus of future research. | [112,114,115,116,117,118,119] |
| Gut Microbiome Alterations | Reduced diversity in gut microbiota, especially the loss of Bifidobacterium and Lactobacillus species, impairs oral tolerance by altering regulatory cytokine production (e.g., IL-10, TGF-β). Changes in the gut-associated lymphoid tissue (GALT) and microbial metabolites like short-chain fatty acids (SCFAs) disrupt immune homeostasis. | Probiotic and prebiotic interventions in early infancy could modulate gut microbiota to restore immune homeostasis. Clinical trials should evaluate the role of specific microbial strains in preventing sensitization. | [120,121,122,123,124,125,126,127] |
| Environmental Exposures | Epicutaneous exposure to peanut allergens, particularly in infants with impaired skin barriers (e.g., eczema), sensitizes via Langerhans cells, promoting Th2-driven IgE responses. Household and environmental allergens, such as dust mites, may further exacerbate this process by acting as adjuvants. | Research should define the threshold levels of environmental allergen exposure required for sensitization. Topical interventions, such as emollients or barrier creams, may prevent sensitization in infants with atopic dermatitis (AD) or eczema. Studies should also assess combined exposures to multiple allergens. | [128,129,130,131,132] |
| Atopic Dermatitis (AD) | AD leads to chronic skin inflammation and impaired epidermal barrier function, facilitating allergen entry and sensitization. Skin immune cells, particularly epidermal dendritic cells and Th2 cytokines (e.g., IL-4, IL-13), drive IgE-mediated responses. The filaggrin deficiency associated with AD further exacerbates this barrier dysfunction. | Preventive strategies focusing on early skin care, including the regular use of emollients and topical anti-inflammatory agents, may reduce allergen penetration and sensitization. Emerging therapies targeting the Th2 cytokine axis (e.g., anti-IL-4/IL-13 agents) could be evaluated for their role in reducing peanut sensitization in infants with AD. | [133,134,135,136,137,138] |
| Impact of Early Feeding Practices | Early introduction of peanut proteins via oral routes (by 4–6 months) promotes oral tolerance through Treg activation and reduced Th2 cytokine responses. Delayed introduction, especially in infants with eczema, increases the risk of sensitization due to a lack of early immune priming. | Current guidelines recommending early peanut introduction should be rigorously followed, particularly in infants with AD or a family history of atopy. Further research should explore dose–response relationships for oral tolerance induction, particularly in high-risk populations. | [106,139,140,141,142,143,144,145,146,147] |
| Prenatal and Perinatal Factors | Maternal diet during pregnancy and breastfeeding, as well as in utero allergen exposure, can influence infant immune responses. Epigenetic modifications, such as changes in DNA methylation and histone acetylation, may impact Th1/Th2 balance and immune programming in the fetus. | Maternal dietary interventions during pregnancy, such as controlled exposure to allergens, may modulate fetal immune responses. Epigenetic biomarkers could help identify infants at risk for sensitization and guide preventive strategies. | [114,148] |
| Animal Models in Peanut Sensitization | Murine models highlight key sensitization pathways, including disrupted Treg function and gut epithelial barrier dysfunction. These models also show that environmental and oral exposures play critical roles in the loss of oral tolerance. The role of specific microbial communities in sensitization pathways is increasingly studied. | Advances in murine models that mimic human peanut sensitization should continue to inform therapeutic development, including allergen immunotherapy and oral tolerance strategies. Translational research focusing on the microbiome’s role in sensitization is critical for future interventions. | [149,150,151,152,153,154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unar, A.; Qureshi, M.; Afridi, H.I.; Wassan, S. The Role of Bacterial Toxins and Environmental Factors in the Development of Food Allergies. Allergies 2024, 4, 192-217. https://doi.org/10.3390/allergies4040014
Unar A, Qureshi M, Afridi HI, Wassan S. The Role of Bacterial Toxins and Environmental Factors in the Development of Food Allergies. Allergies. 2024; 4(4):192-217. https://doi.org/10.3390/allergies4040014
Chicago/Turabian StyleUnar, Ahsanullah, Muqaddas Qureshi, Hassan Imran Afridi, and Shafkatullah Wassan. 2024. "The Role of Bacterial Toxins and Environmental Factors in the Development of Food Allergies" Allergies 4, no. 4: 192-217. https://doi.org/10.3390/allergies4040014
APA StyleUnar, A., Qureshi, M., Afridi, H. I., & Wassan, S. (2024). The Role of Bacterial Toxins and Environmental Factors in the Development of Food Allergies. Allergies, 4(4), 192-217. https://doi.org/10.3390/allergies4040014







