House Dust Mite Allergy and the Der p1 Conundrum: A Literature Review and Case Series
Abstract
1. Literature Review
2. Case Series
2.1. Aim
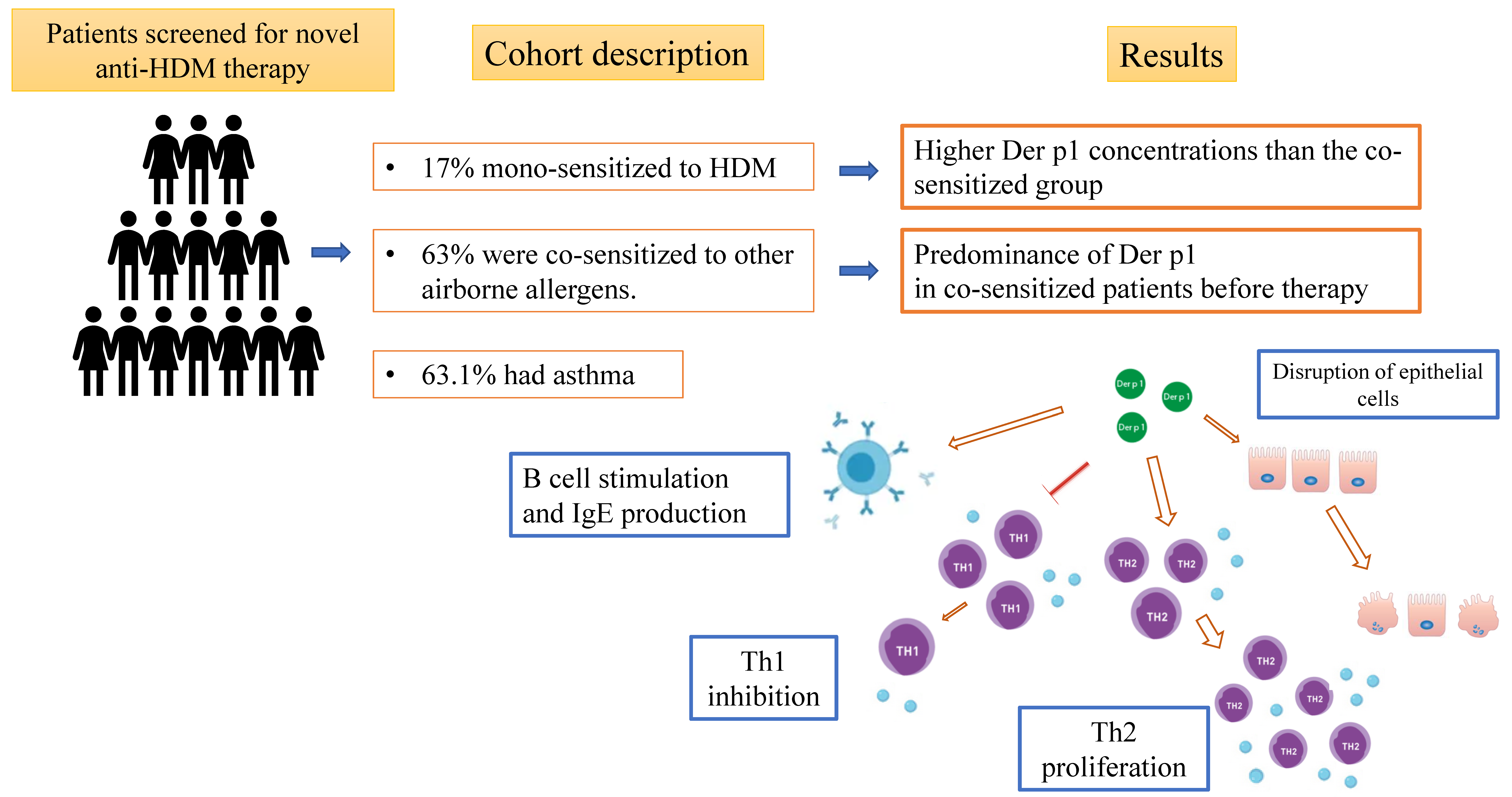
2.2. Methods
2.3. Results
2.4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frankland, A.W. Asthma and mites. Postgrad Med. J. 1971, 47, 178–180. [Google Scholar] [CrossRef][Green Version]
- Mims, J.W. Epidemiology of allergic rhinitis. Int. Forum Allergy Rhinol. 2014, 4 (Suppl S2), S18–S20. [Google Scholar] [CrossRef]
- Öçal, R.; Bayar Muluk, N.; Mullol, J. Epidemiology of Allergic Rhinitis. In All Around the Nose: Basic Science, Diseases and Surgical Management; Cingi, C., Bayar Muluk, N., Eds.; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Koyanagi, S.; Murakami, T.; Maeda, T.; Kawatsu, K.; Okamura, H.; Oda, Y.; Miyatsu, Y.; Sugawara, K.; Mizokami, H. Production-scale purification of the recombinant major house dust mite allergen Der f 2 mutant C8/119S. J. Biosci. Bioeng. 2010, 110, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Lockey, R.; Malling, H.J. Allergen immunotherapy: Therapeutic vaccines for allergic diseases. A WHO position paper. J. Allergy Clin. Immunol. 1998, 102, 558–562. [Google Scholar] [CrossRef]
- Inal, A.; Altintas, D.U.; Yilmaz, M.; Karakoc, G.B.; Kendirli, S.G.; Sertdemir, Y. Prevention of new sensitizations by specific immunotherapy in children with rhinitis and/or asthma monosensitized to house dust mite. J. Investig. Allergol. Clin. Immunol. 2007, 17, 85–91. [Google Scholar] [PubMed]
- Jacobsen, L.; Niggemann, B.; Dreborg, S.; Ferdousi, H.A.; Halken, S.; Høst, A.; Koivikko, A.; Norberg, L.A.; Valovirta, E.; Wahn, U.; et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy 2007, 62, 943–948. [Google Scholar] [CrossRef]
- Arlian, L.G.; Morgan, M.S.; Neal, J.S. Dust mite allergens: Ecology and distribution. Curr. Allergy Asthma Rep. 2002, 2, 401–411. [Google Scholar] [CrossRef]
- Aggarwal, P.; Senthilkumaran, S. Dust Mite Allergy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Thomas, W.R. Hierarchy and molecular properties of house dust mite allergens. Allergol. Int. 2015, 64, 304–311. [Google Scholar] [CrossRef]
- Bessot, J.-C.; Pauli, G. House dust mites allergens. Rev. Mal. Respir. 2011, 28, 475–495. [Google Scholar] [CrossRef]
- Cui, Y. Structural biology of mite allergens. Mol. Biol. Rep. 2013, 40, 681–686. [Google Scholar] [CrossRef]
- Celi, G.; Brusca, I.; Scala, E.; Villalta, D.; Pastorello, E.; Farioli, L.; Cortellini, G.; Deleonardi, G.; Galati, P.; Losappio, L.; et al. House dust mite allergy in Italy-Diagnostic and clinical relevance of Der p 23 (and of minor allergens): A real-life, multicenter study. Allergy 2019, 74, 1787–1789. [Google Scholar] [CrossRef]
- Weghofer, M.; Grote, M.; Resch, Y.; Casset, A.; Kneidinger, M.; Kopec, J.; Thomas, W.R.; Fernández-Caldas, E.; Kabesch, M.; Ferrara, R.; et al. Identification of Der p 23, a peritrophin-like protein, as a new major Dermatophagoides pteronyssinus allergen associated with the peritrophic matrix of mite fecal pellets. J. Immunol. 2013, 190, 3059–3067. [Google Scholar] [CrossRef]
- Yang, X.; Fan, G.; Li, J. Diagnostic value of Der p 1 and Der p 2 specific IgE in Dermatophagoides pteronyssinus IgE sensitization. Ann. Allergy. Asthma Immunol. 2016, 116, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Zhou, Y.; Zhang, W.; Cui, Y. Der p 1 and Der p 2 specific immunoglobulin E measurement for diagnosis of Dermatophagoides pteronyssinus allergy: A systematic review and meta-analysis. Allergy Asthma Proc. 2017, 38, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Chevigné, A.; Jacquet, A. Emerging roles of the protease allergen Der p 1 in house dust mite-induced airway inflammation. J. Allergy Clin. Immunol. 2018, 142, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Bessot, J.C.; Pauli, G. Mite allergens: An overview. Eur. Ann. Allergy Clin. Immunol. 2011, 43, 141–156. [Google Scholar] [PubMed]
- Mattoli, S. Allergen-induced generation of mediators in the mucosa. Environ. Health Perspect. 2001, 109 (Suppl. S4), 553–557. [Google Scholar]
- Yao, Y.; Wang, Z.-C.; Yu, D.; Liu, Z. Role of allergen-specific T-follicular helper cells in immunotherapy. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 495–501. [Google Scholar] [CrossRef]
- Bush, R.K. Indoor allergens, environmental avoidance, and allergic respiratory disease. Allergy Asthma Proc. 2008, 29, 575–579. [Google Scholar] [CrossRef]
- Bronnert, M.; Mancini, J.; Birnbaum, J.; Agabriel, C.; Liabeuf, V.; Porri, F.; Cleach, I.; Fabre, A.; Deneux, I.; Grandne, V.; et al. Component-resolved diagnosis with commercially available D. pteronyssinus Der p 1, Der p 2 and Der p 10: Relevant markers for house dust mite allergy. Clin. Exp. Allergy 2012, 42, 1406–1415. [Google Scholar] [CrossRef]
- Wilson, J.M.; Platts-Mills, T.A.E. Home Environmental Interventions for House Dust Mite. J. Allergy Clin. Immunol. Pract. 2018, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.D.; Rowntree, S.; Mitchell, E.B.; Di Prisco de Fuenmajor, M.C.; Platts-Mills, T.A. Quantitative assessments of IgG and IgE antibodies to inhalant allergens in patients with atopic dermatitis. J. Allergy Clin. Immunol. 1983, 72, 27–33. [Google Scholar] [CrossRef]
- Thomas, W.R. IgE and T-cell responses to house dust mite allergen components. Mol. Immunol. 2018, 100, 120–125. [Google Scholar] [CrossRef]
- Chapman, M.D.; Wünschmann, S.; Pomés, A. Proteases as Th2 adjuvants. Curr. Allergy Asthma Rep. 2007, 7, 363–367. [Google Scholar] [CrossRef]
- Reddy, V.B.; Lerner, E.A. Activation of mas-related G-protein-coupled receptors by the house dust mite cysteine protease Der p1 provides a new mechanism linking allergy and inflammation. J. Biol. Chem. 2017, 292, 17399–17406. [Google Scholar] [CrossRef] [PubMed]
- Dumez, M.-E.; Teller, N.; Mercier, F.; Tanaka, T.; Vandenberghe, I.; Vandenbranden, M.; Devreese, B.; Luxen, A.; Frère, J.M.; Matagne, A.; et al. Activation mechanism of recombinant Der p 3 allergen zymogen: Contribution of cysteine protease Der p 1 and effect of propeptide glycosylation. J. Biol. Chem. 2008, 283, 30606–30617. [Google Scholar] [CrossRef] [PubMed]
- López-Rodríguez, J.C.; Manosalva, J.; Cabrera-García, J.D.; Escribese, M.M.; Villalba, M.; Barber, D.; Martínez-Ruiz, A.; Batanero, E. Human glutathione-S-transferase pi potentiates the cysteine-protease activity of the Der p 1 allergen from house dust mite through a cysteine redox mechanism. Redox Biol. 2019, 26, 101256. [Google Scholar] [CrossRef] [PubMed]
- Miranda, D.O.; Silva, D.A.O.; Fernandes, J.F.C.; Queirós, M.G.J.; Chiba, H.F.; Ynoue, L.H.; Resende, R.O.; Pena, J.; Sung, S.S.J.; Segundo, G.R.; et al. Serum and salivary IgE, IgA, and IgG4 antibodies to Dermatophagoides pteronyssinus and its major allergens, Der p1 and Der p2, in allergic and nonallergic children. Clin. Dev. Immunol. 2011, 302739. [Google Scholar]
- Thomas, W.R.; Smith, W.-A.; Hales, B.J.; Mills, K.L.; O’Brien, R.M. Characterization and immunobiology of house dust mite allergens. Int. Arch. Allergy Immunol. 2002, 129, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Dumez, M.-E.; Herman, J.; Campizi, V.; Galleni, M.; Jacquet, A.; Chevigné, A. Orchestration of an Uncommon Maturation Cascade of the House Dust Mite Protease Allergen Quartet. Front. Immunol. 2014, 5, 138. [Google Scholar] [CrossRef]
- Bao, Z.-J.; Fan, Y.-M.; Cui, Y.-F.; Sheng, Y.-F.; Zhu, M. Effect of PM2.5 mediated oxidative stress on the innate immune cellular response of Der p1 treated human bronchial epithelial cells. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2907–2912. [Google Scholar] [PubMed]
- Thomas, R.G.; Rivera Reyes, B.M.; Gaston, B.M.; Rivera Acosta, N.B.; Bederman, I.R.; Smith, L.A.; Sutton, M.T.; Wang, B.; Hunt, J.F.; Bonfield, T.L. Conjugation of nitrated acetaminophen to Der p1 amplifies peripheral blood monocyte response to Der p1. PLoS ONE 2017, 12, e0188614. [Google Scholar] [CrossRef] [PubMed]
- Genç, D.; Zibandeh, N.; Nain, E.; Arığ, Ü.; Göker, K.; Aydıner, E.K.; Akkoç, T.U.N.Ç. IFN-γ stimulation of dental follicle mesenchymal stem cells modulates immune response of CD4+ T lymphocytes in Der p1+ asthmatic patients in vitro. Allergol. Immunopathol. (Madr.) 2019, 47, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Jin, L.; Che, N.; Zhang, R.; Xu, F.; Han, B. Dendritic cells modified with Der p1 antigen as a therapeutic potential for allergic rhinitis in a murine model via regulatory effects on IL-4, IL-10 and IL-13. Int. Immunopharmacol. 2019, 70, 216–224. [Google Scholar] [CrossRef]
- Deb, R.; Shakib, F.; Reid, K.; Clark, H. Major house dust mite allergens Dermatophagoides pteronyssinus 1 and Dermatophagoides farinae 1 degrade and inactivate lung surfactant proteins A and D. J. Biol. Chem. 2007, 282, 36808–36819. [Google Scholar] [CrossRef] [PubMed]
- Furmonaviciene, R.; Ghaemmaghami, A.M.; Boyd, S.E.; Jones, N.S.; Bailey, K.; Willis, A.C.; Sewell, H.F.; Mitchell, D.A.; Shakib, F. The protease allergen Der p 1 cleaves cell surface DC-SIGN and DC-SIGNR: Experimental analysis of in silico substrate identification and implications in allergic responses. Clin. Exp. Allergy 2007, 37, 231–242. [Google Scholar] [CrossRef]
- Schulz, O.; Sutton, B.J.; Beavil, R.L.; Shi, J.; Sewell, H.F.; Gould, H.J.; Laing, P.; Shakib, F. Cleavage of the low-affinity receptor for human IgE (CD23) by a mite cysteine protease: Nature of the cleaved fragment in relation to the structure and function of CD23. Eur. J. Immunol. 1997, 27, 584–588. [Google Scholar] [CrossRef]
- Schulz, O.; Sewell, H.F.; Shakib, F. Proteolytic cleavage of CD25, the alpha subunit of the human T cell interleukin 2 receptor, by Der p 1, a major mite allergen with cysteine protease activity. J. Exp. Med. 1998, 187, 271–275. [Google Scholar] [CrossRef]
- Hara, K.; Iijima, K.; Elias, M.K.; Seno, S.; Tojima, I.; Kobayashi, T.; Kita, H. Airway uric acid is a sensor of inhaled protease allergens and initiates type 2 immune responses in respiratory mucosa. J. Immunol. 2014, 192, 4032–4042. [Google Scholar] [CrossRef]
- Kato, T.; Takai, T.; Fujimura, T.; Matsuoka, H.; Ogawa, T.; Murayama, K.; Ishii, A.; Ikeda, S.; Okumura, K.; Ogawa, H. Mite serine protease activates protease-activated receptor-2 and induces cytokine release in human keratinocytes. Allergy 2009, 64, 1366–1374. [Google Scholar] [CrossRef]
- Kalsheker, N.A.; Deam, S.; Chambers, L.; Sreedharan, S.; Brocklehurst, K.; Lomas, D.A. The house dust mite allergen Der p1 catalytically inactivates alpha 1-antitrypsin by specific reactive centre loop cleavage: A mechanism that promotes airway inflammation and asthma. Biochem. Biophys. Res. Commun. 1996, 221, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, P.T.; Elliot, C.E.; Lenzo, J.C.; Jarnicki, A.G.; Larcombe, A.N.; Zosky, G.R.; Holt, P.G.; Thomas, W.R. Sensitizing and Th2 adjuvant activity of cysteine protease allergens. Int. Arch. Allergy Immunol. 2012, 158, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M. Innate and adaptive type 2 immunity in lung allergic inflammation. Immunol. Rev. 2017, 278, 162–172. [Google Scholar] [CrossRef]
- Cayrol, C.; Duval, A.; Schmitt, P.; Roga, S.; Camus, M.; Stella, A.; Burlet-Schiltz, O.; Gonzalez-de-Peredo, A.; Girard, J.P. Environmental allergens induce allergic inflammation through proteolytic maturation of IL-33. Nat. Immunol. 2018, 19, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, J.; Allen-Philbey, K.; Perera Baruhupolage, C.; Tachie-Menson, T.; Mangat, S.C.; Garrod, D.R.; Robinson, C. Innate generation of thrombin and intracellular oxidants in airway epithelium by allergen Der p 1. J. Allergy Clin. Immunol. 2016, 138, 1224–1227. [Google Scholar] [CrossRef]
- Jacquet, A.; Campisi, V.; Szpakowska, M.; Dumez, M.-E.; Galleni, M.; Chevigné, A. Profiling the Extended Cleavage Specificity of the House Dust Mite Protease Allergens Der p 1, Der p 3 and Der p 6 for the Prediction of New Cell Surface Protein Substrates. Int. J. Mol. Sci. 2017, 18, 1373. [Google Scholar] [CrossRef]
- Klimek, L.; Bachert, C.; Pfaar, O.; Becker, S.; Bieber, T.; Brehler, R.; Buhl, R.; Casper, I.; Chaker, A.; Czech, W.; et al. ARIA guideline 2019: Treatment of allergic rhinitis in the German health system. Allergol. Select 2019, 3, 22–50. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Asthma—GINA. Available online: https://ginasthma.org/ (accessed on 25 December 2020).
- D’alessandro, M.; Bergantini, L.; Perrone, A.; Beltrami, V.; Cameli, P.; Flori, L.; Saletti, M.; Vietri, L.; Sestini, P.; Bargagli, E. A real-life experience with ImmunoCAP ISAC: The advantages of a new diagnostic method. Minerva Med. 2020. [Google Scholar] [CrossRef]
- Chapman, M.D.; Platts-Mills, T.A. Purification and characterization of the major allergen from Dermatophagoides pteronyssinus-antigen P1. J. Immunol. 1980, 125, 587–592. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
d’Alessandro, M.; Bergantini, L.; Perrone, A.; Cameli, P.; Beltrami, V.; Alderighi, L.; Pini, L.; Bargagli, E.; Saletti, M. House Dust Mite Allergy and the Der p1 Conundrum: A Literature Review and Case Series. Allergies 2021, 1, 108-114. https://doi.org/10.3390/allergies1020008
d’Alessandro M, Bergantini L, Perrone A, Cameli P, Beltrami V, Alderighi L, Pini L, Bargagli E, Saletti M. House Dust Mite Allergy and the Der p1 Conundrum: A Literature Review and Case Series. Allergies. 2021; 1(2):108-114. https://doi.org/10.3390/allergies1020008
Chicago/Turabian Styled’Alessandro, Miriana, Laura Bergantini, Anna Perrone, Paolo Cameli, Valerio Beltrami, Lorenzo Alderighi, Laura Pini, Elena Bargagli, and Marco Saletti. 2021. "House Dust Mite Allergy and the Der p1 Conundrum: A Literature Review and Case Series" Allergies 1, no. 2: 108-114. https://doi.org/10.3390/allergies1020008
APA Styled’Alessandro, M., Bergantini, L., Perrone, A., Cameli, P., Beltrami, V., Alderighi, L., Pini, L., Bargagli, E., & Saletti, M. (2021). House Dust Mite Allergy and the Der p1 Conundrum: A Literature Review and Case Series. Allergies, 1(2), 108-114. https://doi.org/10.3390/allergies1020008








