Automatic 3D Postoperative Evaluation of Complex Orthopaedic Interventions
Abstract
:1. Introduction
- The first fully automatic 3D measurement method of bone cut accuracy is presented.
- Thanks to our cut detection method, our combined segmentation and registration approach measures anatomy manipulation and repositioning automatically and accurately, even in the presence of bone in-growth and callus.
- Lastly, an accurate and fully automatic 3D screw placement quantification method is presented.
2. Materials and Methods
2.1. Patient Selection and Imaging
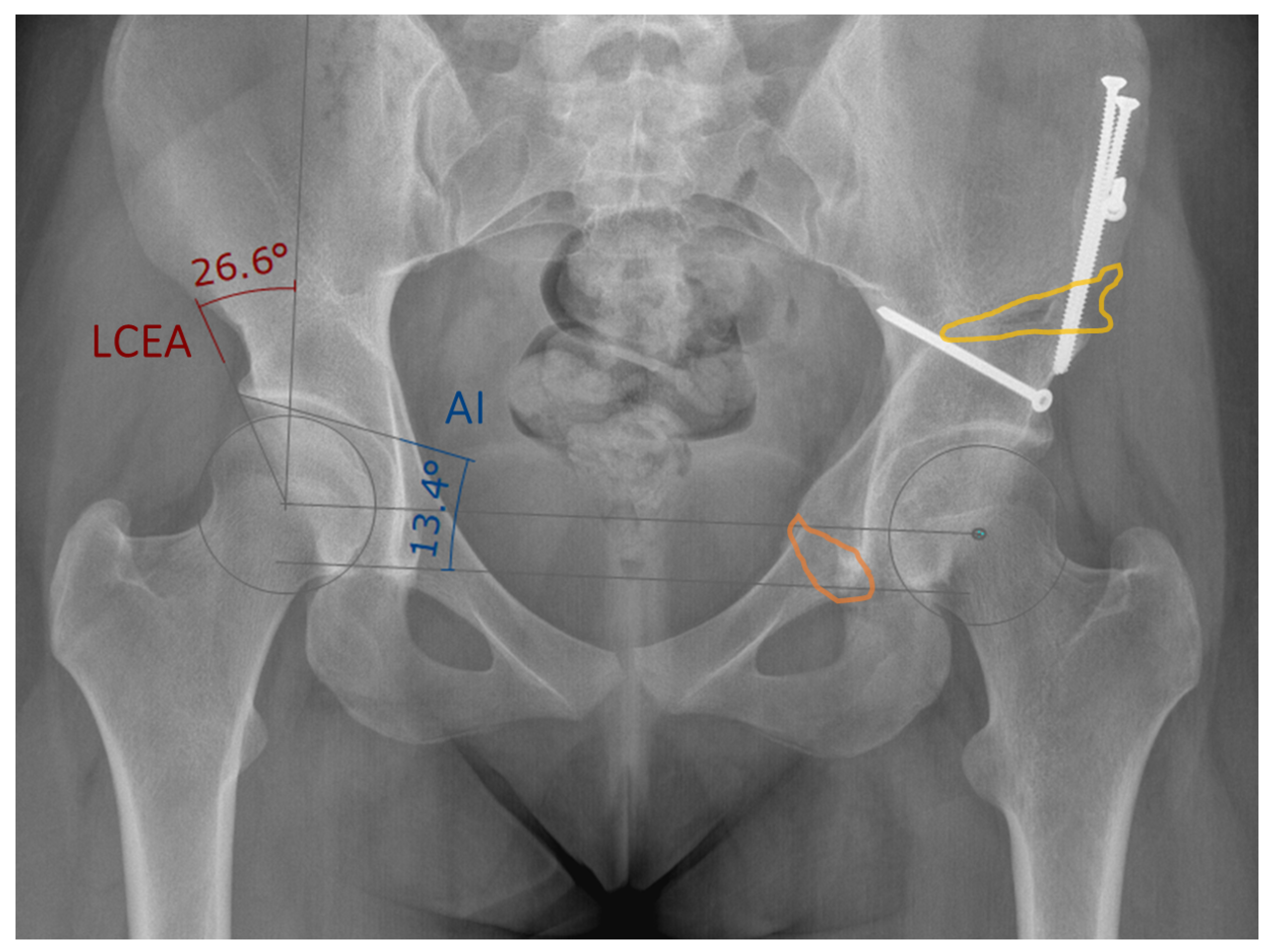
2.2. Manual 3D Postoperative Evaluation
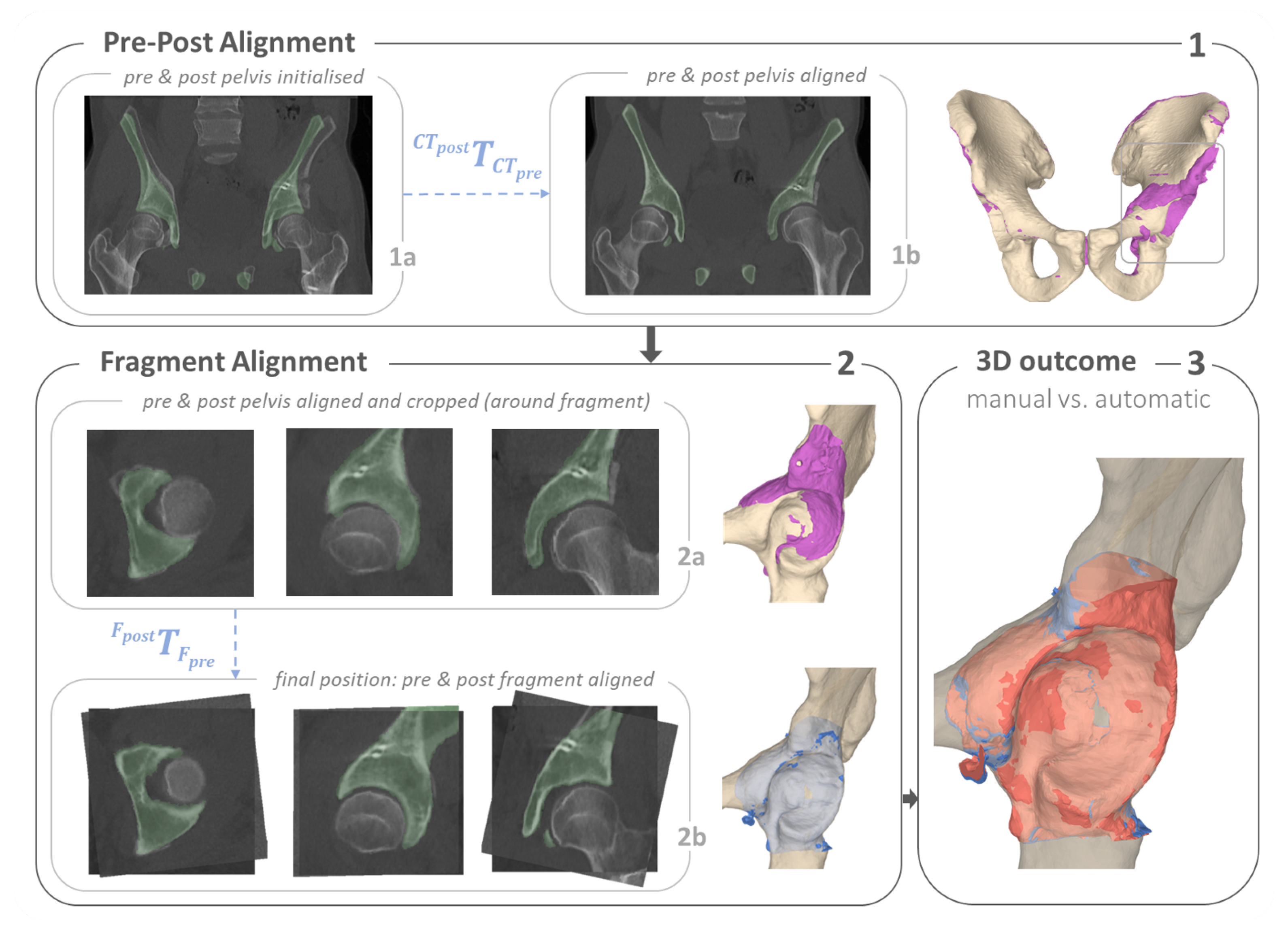
2.3. Computer-Assisted 3D Postoperative Evaluation
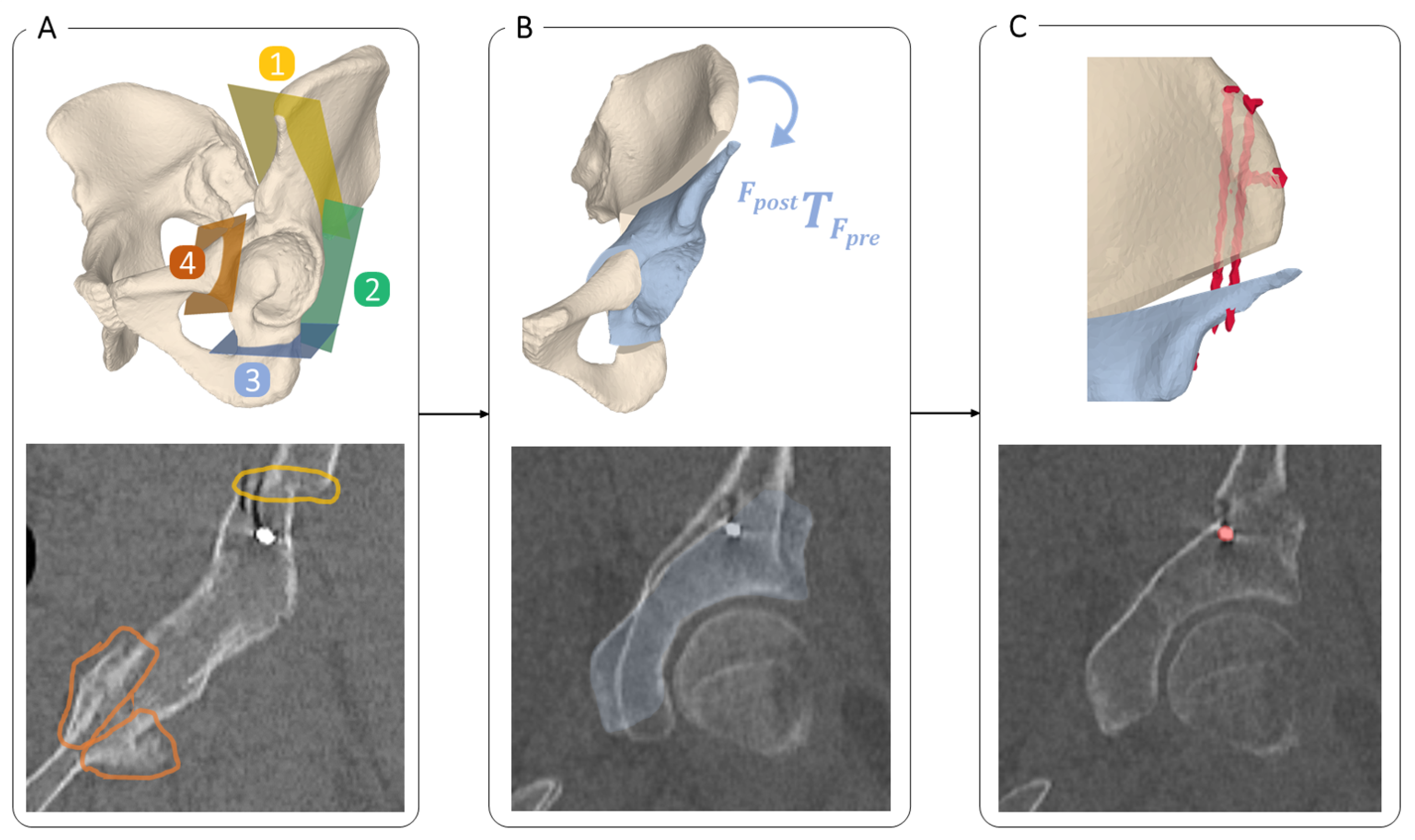
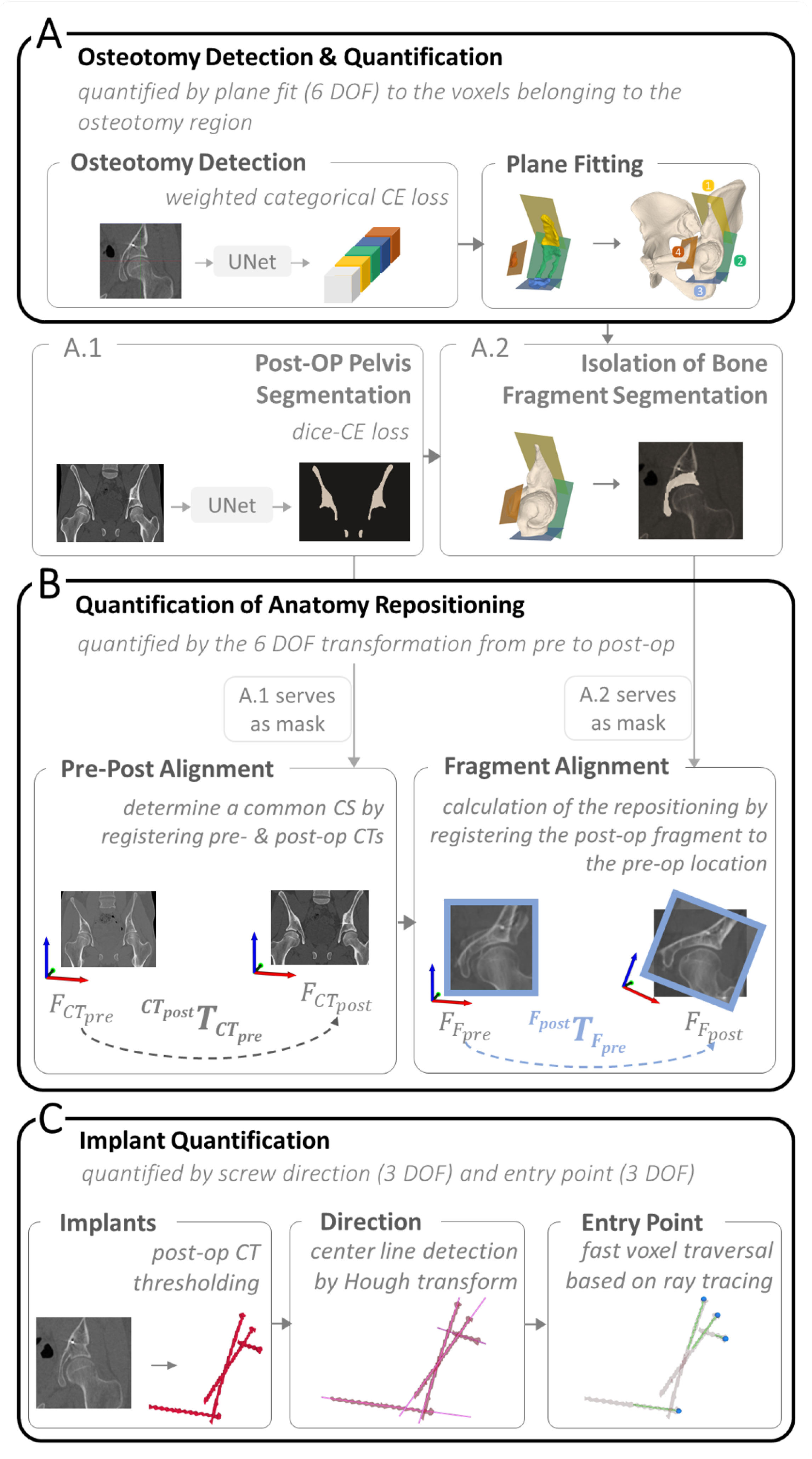
2.3.1. Osteotomy Detection and Quantification
2.3.2. Quantification of Anatomy Repositioning
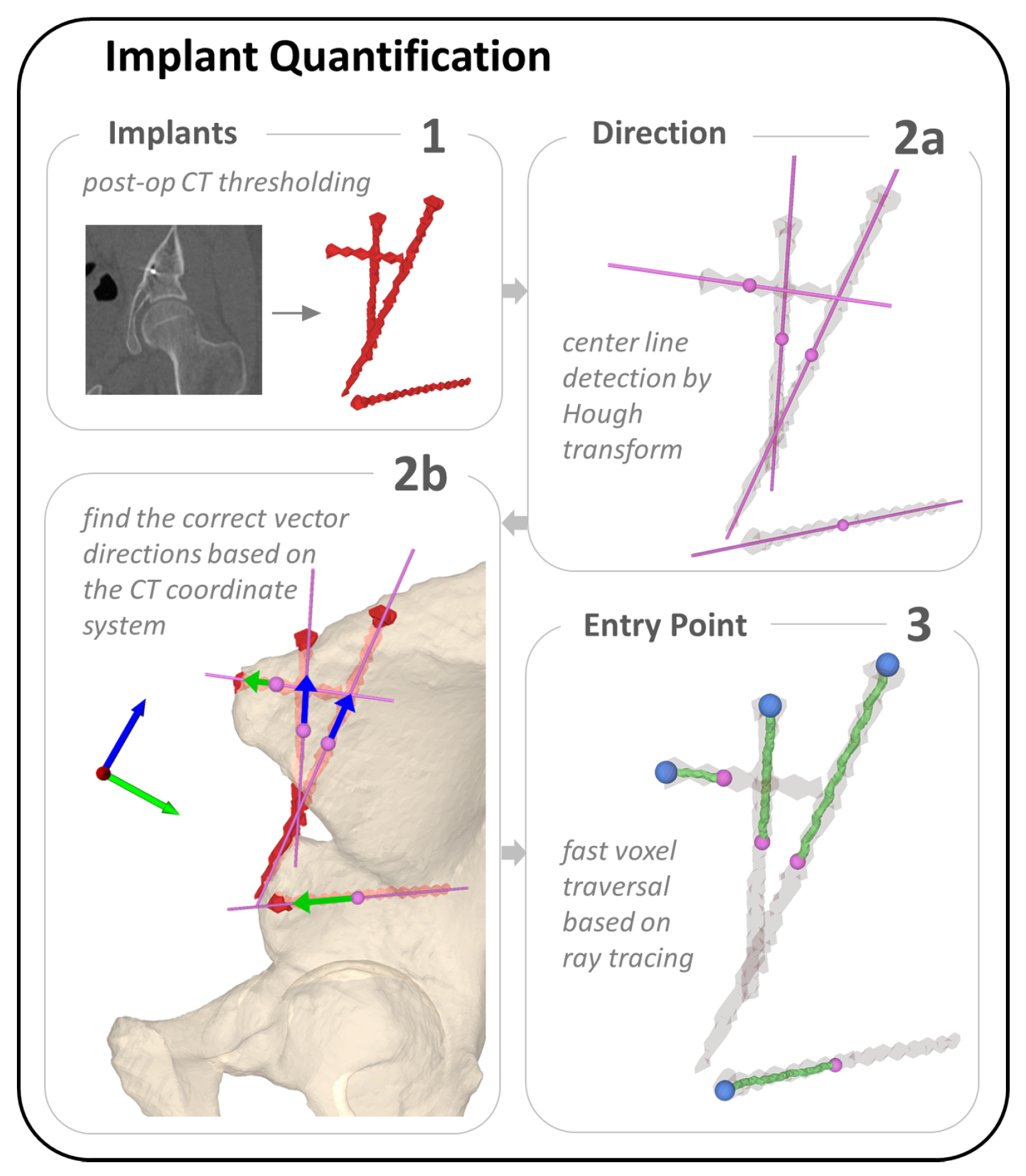
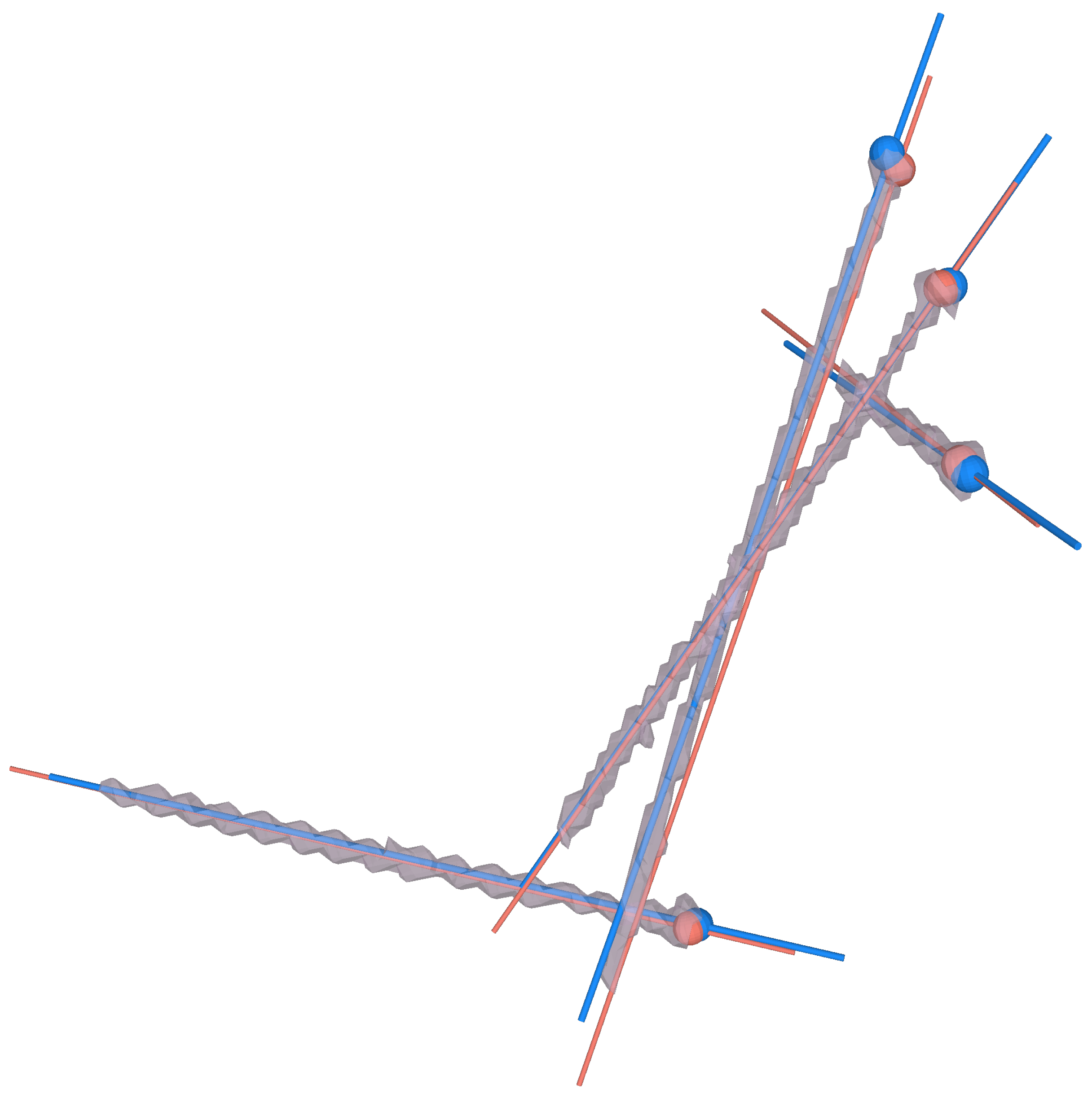
2.3.3. Implant Quantification
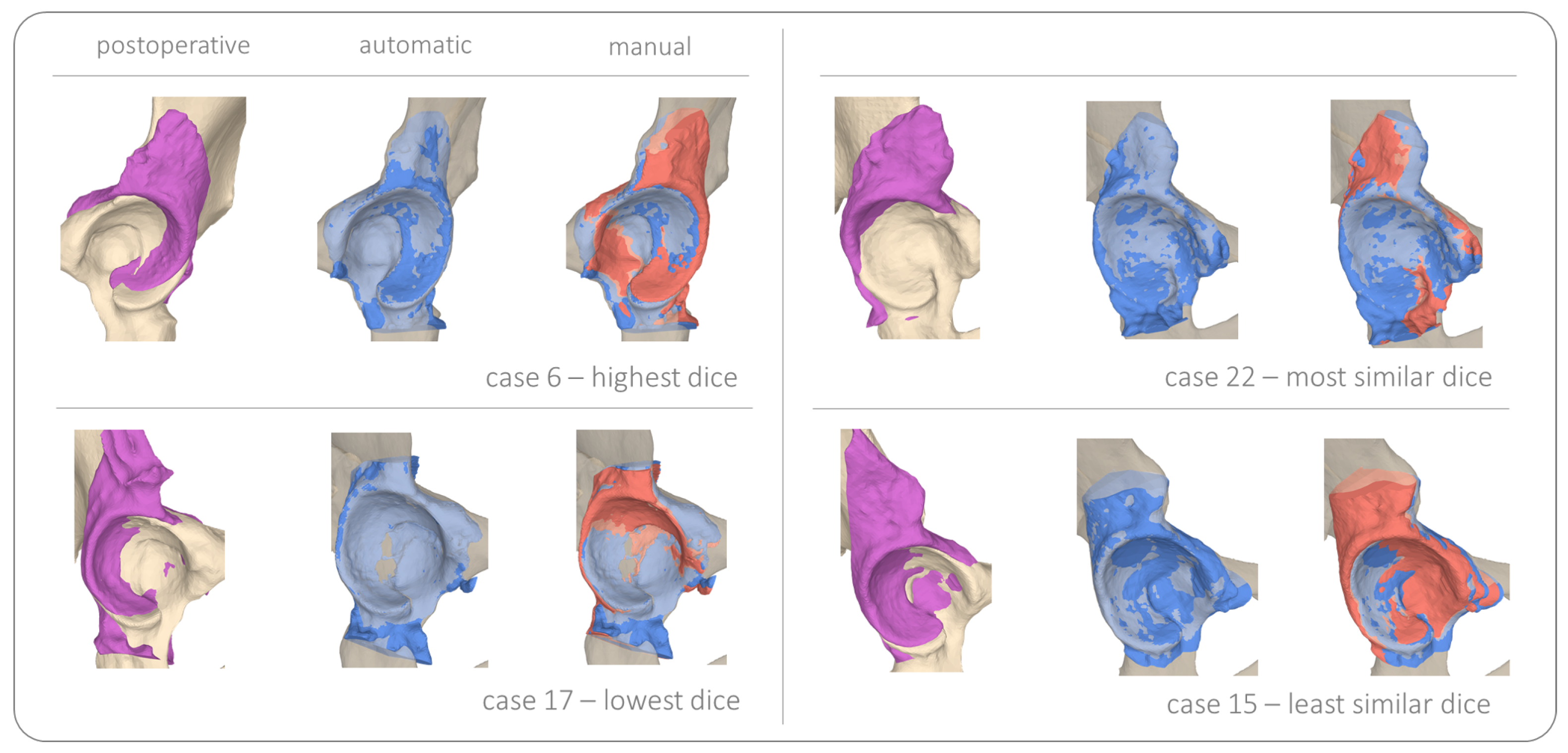
3. Results
3.1. Osteotomy Location
- : most superior point on intersection between supraacetabular osteotomy plane and the pelvic 3D model
- : most medial point on intersecting line between supraacetabular and retroacetabular osteotomy planes
- : most medial point on intersecting line between retroacetabular and ischial osteotomy planes
- : most anterior point on intersection between ischial osteotomy plane and the pelvic 3D model
- : most posterior point on intersection between pubic osteotomy plane and the pelvic 3D model
3.2. Fragment Reorientation
3.3. Implant Placement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Patient | 3D Distance [mm] | 2D Angle [°] | abs. 2D Angle | 3D Angle [°] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Deviation [°] | |||||||||||
| Manual | |||||||||||
| Automatic | |||||||||||
| Mean | 17.0 | 12.8 | 15.0 | 7.3 | 8.7 | 7.0 | 6.9 | 21.9 | 9.9 | 20.0 | 29.2 |
| 13.0 | 7.4 | 12.1 | 6.3 | 6.7 | 5.4 | 5.4 | 17.7 | 8.5 | 15.2 | 11.2 | |
| 0 | 32.4 | 25.8 | 9.9 | 6.4 | 2.0 | 11.6 | 10.5 | 0.6 | 1.1 | 11.1 | 29.5 |
| 1 | 3.3 | 5.7 | 10.9 | 8.3 | 5.1 | 2.2 | 8.9 | 1.0 | 11.1 | 9.9 | 21.6 |
| 2 | 11.2 | 7.3 | 9.4 | 2.1 | 6.2 | 8.8 | 3.5 | 3.2 | 5.2 | 0.3 | 31.2 |
| 3 | 20.6 | 10.2 | 6.7 | 1.2 | 8.2 | 12.1 | 2.3 | 8.5 | 9.8 | 6.2 | 20.0 |
| 4 | 12.4 | 8.2 | 14.2 | 8.7 | 17.7 | 0.4 | 2.0 | 14.0 | 1.6 | 16.0 | 48.5 |
| 5 | 6.6 | 10.5 | 15.0 | 5.9 | 21.2 | 2.9 | 2.9 | 14.7 | 0.0 | 11.8 | 49.1 |
| 6 | 26.7 | 7.8 | 17.3 | 7.4 | 1.3 | 2.4 | 2.8 | 39.7 | 0.4 | 36.9 | 19.2 |
| 7 | 14.5 | 27.8 | 13.1 | 5.6 | 3.4 | 3.0 | 18.9 | 19.1 | 15.9 | 0.3 | 25.3 |
| 8 | 3.3 | 18.8 | 7.0 | 2.6 | 16.9 | 7.8 | 1.9 | 17.6 | 5.9 | 15.7 | 54.4 |
| 9 | 10.9 | 16.5 | 11.8 | 25.5 | 21.1 | 1.2 | 7.0 | 43.1 | 8.2 | 36.0 | 49.3 |
| 10 | 14.6 | 2.6 | 11.4 | 5.3 | 3.6 | 5.1 | 3.9 | 26.3 | 9.0 | 22.4 | 28.5 |
| 11 | 16.8 | 26.1 | 16.5 | 6.7 | 10.1 | 7.6 | 17.1 | 15.9 | 9.5 | 1.2 | 36.9 |
| 12 | 28.2 | 3.2 | 20.0 | 4.2 | 4.3 | 7.6 | 8.6 | 39.5 | 1.1 | 30.9 | 18.4 |
| 13 | 14.0 | 10.8 | 6.0 | 1.2 | 15.4 | 9.4 | 5.1 | 12.0 | 14.5 | 17.1 | 28.3 |
| 14: worst | 54.9 | 10.9 | 71.4 | 17.3 | 23.5 | 13.1 | 23.4 | 73.3 | 36.5 | 49.9 | 32.9 |
| 15 | 2.9 | 1.8 | 15.7 | 6.5 | 14.5 | 1.6 | 5.7 | 45.8 | 7.3 | 40.1 | 38.2 |
| 16: best | 6.1 | 5.9 | 4.3 | 2.4 | 1.5 | 10.3 | 1.6 | 5.1 | 11.9 | 6.8 | 9.6 |
| 17 | 4.8 | 12.2 | 12.1 | 10.6 | 4.0 | 10.5 | 7.1 | 6.7 | 3.4 | 0.4 | 21.0 |
| 18 | 14.9 | 11.1 | 13.1 | 2.9 | 2.3 | 0.3 | 7.9 | 8.9 | 8.2 | 16.8 | 27.9 |
| 19 | 5.8 | 10.8 | 13.3 | 24.0 | 11.4 | 1.7 | 1.7 | 20.4 | 3.4 | 18.7 | 35.5 |
| 20 | 29.6 | 16.1 | 14.8 | 1.0 | 5.9 | 1.8 | 9.4 | 11.5 | 11.2 | 20.9 | 17.6 |
| 21 | 5.5 | 6.8 | 20.6 | 6.5 | 6.8 | 10.9 | 3.7 | 43.8 | 7.3 | 47.5 | 20.6 |
| 22 | 30.9 | 11.9 | 7.8 | 5.2 | 3.1 | 11.2 | 4.4 | 6.6 | 15.6 | 11.0 | 22.0 |
| 23 | 42.9 | 18.3 | 10.3 | 13.1 | 8.6 | 22.2 | 8.5 | 14.3 | 30.7 | 5.8 | 33.2 |
| 24 | 23.4 | 22.2 | 16.9 | 9.0 | 5.7 | 0.8 | 8.2 | 20.8 | 7.4 | 29.0 | 21.4 |
| 25 | 15.6 | 12.8 | 19.4 | 3.7 | 7.3 | 13.0 | 3.6 | 39.8 | 16.6 | 43.4 | 22.1 |
| 26 | 6.0 | 23.3 | 16.9 | 4.2 | 2.4 | 10.1 | 4.7 | 38.2 | 14.8 | 33.6 | 25.8 |
| Case | [mm] | [mm] | |||
|---|---|---|---|---|---|
| 1.01 | 2.10 | 0.60 | 0.62 | 0.02 | |
| 0.46 | 0.97 | 0.07 | 0.07 | 0.02 | |
| 0 | 0.38 | 1.4 | 0.48 | 0.48 | 0.003 |
| 1 | 0.42 | 2.48 | 0.67 | 0.7 | 0.036 |
| 2 | 1.47 | 1.61 | 0.71 | 0.71 | 0.002 |
| 3 | 1.43 | 2.07 | 0.71 | 0.74 | 0.027 |
| 4 | 0.79 | 1.86 | 0.62 | 0.66 | 0.039 |
| 5 | 0.51 | 2.46 | 0.58 | 0.59 | 0.016 |
| 6: | 0.71 | 5.84 | 0.68 | 0.74 | 0.059 |
| 7 | 1.83 | 2.25 | 0.61 | 0.64 | 0.028 |
| 8 | 1.25 | 1.76 | 0.62 | 0.67 | 0.048 |
| 9 | 0.86 | 1.64 | 0.62 | 0.64 | 0.017 |
| 10 | 1.57 | 1.92 | 0.63 | 0.64 | 0.003 |
| 11 | 0.66 | 1.24 | 0.49 | 0.51 | 0.011 |
| 12 | 0.41 | 0.55 | 0.62 | 0.64 | 0.025 |
| 13 | 1.2 | 2.27 | 0.56 | 0.62 | 0.052 |
| 14 | 0.54 | 1.72 | 0.57 | 0.6 | 0.034 |
| 15: | 1.72 | 2.73 | 0.58 | 0.65 | 0.068 |
| 16 | 1.69 | 3.27 | 0.71 | 0.7 | 0.004 |
| 17: | 0.27 | 0.33 | 0.44 | 0.47 | 0.023 |
| 18 | 1.31 | 2.45 | 0.69 | 0.69 | 0.004 |
| 19: | 0.88 | 1.6 | 0.53 | 0.55 | 0.017 |
| 20 | 0.74 | 2.25 | 0.53 | 0.54 | 0.008 |
| 21 | 0.96 | 2 | 0.64 | 0.65 | 0.015 |
| 22: | 1.19 | 2.04 | 0.57 | 0.57 | 0.001 |
| 23 | 1.61 | 3 | 0.53 | 0.54 | 0.007 |
| 24 | 1.3 | 2.44 | 0.6 | 0.61 | 0.003 |
| 25 | 1.2 | 1.41 | 0.68 | 0.69 | 0.01 |
| 26 | 0.48 | 2.02 | 0.6 | 0.6 | 0.002 |
References
- Pham, D.L.; Xu, C.; Prince, J.L. Current methods in medical image segmentation. Annu. Rev. Biomed. Eng. 2000, 2, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Aggarwal, L.M. Automated medical image segmentation techniques. J. Med. Phys./Assoc. Med. Phys. India 2010, 35, 3. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, D.; Garimella, R.; Eltorai, A.E.; Daniels, A.H. Computer-assisted orthopaedic surgery. Orthop. Surg. 2017, 9, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.J.; Armiger, R.S.; Lepistö, J.; Armand, M. Clinical evaluation of a biomechanical guidance system for periacetabular osteotomy. J. Orthop. Surg. Res. 2016, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Navab, N.; Blum, T.; Wang, L.; Okur, A.; Wendler, T. First deployments of augmented reality in operating rooms. Computer 2012, 45, 48–55. [Google Scholar] [CrossRef]
- Kok, E.N.; Eppenga, R.; Kuhlmann, K.F.; Groen, H.C.; van Veen, R.; van Dieren, J.M.; de Wijkerslooth, T.R.; van Leerdam, M.; Lambregts, D.M.; Heerink, W.J.; et al. Accurate surgical navigation with real-time tumor tracking in cancer surgery. NPJ Precis. Oncol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Liebmann, F.; Roner, S.; von Atzigen, M.; Scaramuzza, D.; Sutter, R.; Snedeker, J.; Farshad, M.; Fürnstahl, P. Pedicle screw navigation using surface digitization on the Microsoft HoloLens. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 1157–1165. [Google Scholar]
- Ackermann, J.; Liebmann, F.; Hoch, A.; Snedeker, J.G.; Farshad, M.; Rahm, S.; Zingg, P.O.; Fürnstahl, P. Augmented reality based surgical navigation of complex pelvic osteotomies—A feasibility study on cadavers. Appl. Sci. 2021, 11, 1228. [Google Scholar] [CrossRef]
- Vlachopoulos, L.; Schweizer, A.; Graf, M.; Nagy, L.; Fürnstahl, P. Three-dimensional postoperative accuracy of extra-articular forearm osteotomies using CT-scan based patient-specific surgical guides. BMC Musculoskelet. Disord. 2015, 16, 336. [Google Scholar] [CrossRef]
- Akiyama, H.; Goto, K.; So, K.; Nakamura, T. Computed tomography-based navigation for curved periacetabular osteotomy. J. Orthop. Sci. 2010, 15, 829. [Google Scholar] [CrossRef]
- Hsieh, P.H.; Chang, Y.H.; Shih, C.H. Image-guided periacetabular osteotomy: Computer-assisted navigation compared with the conventional technique: A randomized study of 36 patients followed for 2 years. Acta Orthop. 2006, 77, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Langlotz, F.; Bächler, R.; Berlemann, U.; Nolte, L.P.; Ganz, R. Computer assistance for pelvic osteotomies. Clin. Orthop. Relat. Res. (1976–2007) 1998, 354, 92–102. [Google Scholar] [CrossRef]
- Jecklin, S.; Jancik, C.; Farshad, M.; Fürnstahl, P.; Esfandiari, H. X23D—Intraoperative 3D Lumbar Spine Shape Reconstruction Based on Sparse Multi-View X-ray Data. J. Imaging 2022, 8, 271. [Google Scholar] [CrossRef] [PubMed]
- Langlotz, F.; Stucki, M.; Bächler, R.; Scheer, C.; Ganz, R.; Berlemann, U.; Nolte, L.P. The first twelve cases of computer assisted periacetabular osteotomy. Comput. Aided Surg. Off. J. Int. Soc. Comput. Aided Surg. (ISCAS) 1997, 2, 317–326. [Google Scholar] [CrossRef]
- Kulyk, P.; Vlachopoulos, L.; Fürnstahl, P.; Zheng, G. Fully automatic planning of total shoulder arthroplasty without segmentation: A deep learning based approach. In Proceedings of the Computational Methods and Clinical Applications in Musculoskeletal Imaging: 6th International Workshop, MSKI 2018, Held in Conjunction with MICCAI 2018, Granada, Spain, 16 September 2018; Revised Selected Papers 6. Springer: Berlin/Heidelberg, Germany, 2019; pp. 22–34. [Google Scholar]
- Ackermann, J.; Wieland, M.; Hoch, A.; Ganz, R.; Snedeker, J.G.; Oswald, M.R.; Pollefeys, M.; Zingg, P.O.; Esfandiari, H.; Fürnstahl, P. A new approach to orthopedic surgery planning using deep reinforcement learning and simulation. In Proceedings of the Medical Image Computing and Computer Assisted Intervention–MICCAI 2021: 24th International Conference, Strasbourg, France, 27 September–1 October 2021; Proceedings, Part IV 24. Springer: Berlin/Heidelberg, Germany, 2021; pp. 540–549. [Google Scholar]
- Shelton, T.J.; Shafagh, M.; Calafi, A.; Leshikar, H.B.; Haus, B.M. Preoperative 3D modeling and printing for guiding periacetabular osteotomy. Orthop. J. Sport. Med. 2021, 9, 2325967121S00026. [Google Scholar] [CrossRef]
- Vaishya, R.; Patralekh, M.K.; Vaish, A.; Agarwal, A.K.; Vijay, V. Publication trends and knowledge mapping in 3D printing in orthopaedics. J. Clin. Orthop. Trauma 2018, 9, 194–201. [Google Scholar] [PubMed]
- Belei, P.; Schkommodau, E.; Frenkel, A.; Mumme, T.; Radermacher, K. Computer-assisted single-or double-cut oblique osteotomies for the correction of lower limb deformities. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2007, 221, 787–800. [Google Scholar] [CrossRef]
- Carrillo, F.; Vlachopoulos, L.; Schweizer, A.; Nagy, L.; Snedeker, J.; Fürnstahl, P. A time saver: Optimization approach for the fully automatic 3D planning of forearm osteotomies. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention—MICCAI 2017: 20th International Conference, Quebec City, QC, Canada, 11–13 September 2017; Proceedings, Part II 20. Springer: Berlin/Heidelberg, Germany, 2017; pp. 488–496. [Google Scholar]
- Schkommodau, E.; Frenkel, A.; Belei, P.; Recknagel, B.; Wirtz, D.C.; Radermacher, K. Computer-assisted optimization of correction osteotomies on lower extremities. Comput. Aided Surg. 2005, 10, 345–350. [Google Scholar] [CrossRef]
- Tschannen, M.; Vlachopoulos, L.; Gerber, C.; Székely, G.; Fürnstahl, P. Regression forest-based automatic estimation of the articular margin plane for shoulder prosthesis planning. Med. Image Anal. 2016, 31, 88–97. [Google Scholar] [CrossRef]
- Hoch, A.; Grossenbacher, G.; Jungwirth-Weinberger, A.; Götschi, T.; Fürnstahl, P.; Zingg, P.O. The periacetabular osteotomy: Angulation of the supraacetabular osteotomy for quantification of correction. Hip Int. 2022, 11207000221103079. [Google Scholar] [CrossRef]
- Ganz, R.; Klaue, K.; Vinh, T.S.; Mast, J.W. A New Periacetabular Osteotomy for the Treatment of Hip Dysplasias Technique and Preliminary Results. Clin. Orthop. Relat. Res. (1976–2007) 1988, 232, 26–36. [Google Scholar] [CrossRef]
- Tönnis, D. Congenital Dysplasia and Dislocation of the Hip in Children and Adults; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Tannast, M.; Hanke, M.S.; Zheng, G.; Steppacher, S.D.; Siebenrock, K.A. What are the radiographic reference values for acetabular under-and overcoverage? Clin. Orthop. Relat. Res. 2015, 473, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M.; Smit, K. Anatomical description and classification of hip dysplasia. Hip Dysplasia Underst. Treat. Instab. Nativ. Hip 2020, 23–37. [Google Scholar]
- Schweizer, A.; Mauler, F.; Vlachopoulos, L.; Nagy, L.; Fürnstahl, P. Computer-assisted 3-dimensional reconstructions of scaphoid fractures and nonunions with and without the use of patient-specific guides: Early clinical outcomes and postoperative assessments of reconstruction accuracy. J. Hand Surg. 2016, 41, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Hirsiger, S.; Schweizer, A.; Miyake, J.; Nagy, L.; Fürnstahl, P. Corrective osteotomies of phalangeal and metacarpal malunions using patient-specific guides: CT-based evaluation of the reduction accuracy. Hand 2018, 13, 627–636. [Google Scholar] [CrossRef]
- Roner, S.; Schweizer, A.; Da Silva, Y.; Carrillo, F.; Nagy, L.; Fürnstahl, P. Accuracy and early clinical outcome after 3-dimensional correction of distal radius intra-articular malunions using patient-specific instruments. J. Hand Surg. 2020, 45, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Roner, S.; Vlachopoulos, L.; Nagy, L.; Schweizer, A.; Fürnstahl, P. Accuracy and early clinical outcome of 3-dimensional planned and guided single-cut osteotomies of malunited forearm bones. J. Hand Surg. 2017, 42, 1031.e1–1031.e8. [Google Scholar] [CrossRef]
- Miyake, J.; Murase, T.; Oka, K.; Moritomo, H.; Sugamoto, K.; Yoshikawa, H. Computer-assisted corrective osteotomy for malunited diaphyseal forearm fractures. JBJS 2012, 94, e150. [Google Scholar] [CrossRef]
- Vlachopoulos, L.; Schweizer, A.; Meyer, D.C.; Gerber, C.; Fürnstahl, P. Computer-assisted planning and patient-specific guides for the treatment of midshaft clavicle malunions. J. Shoulder Elb. Surg. 2017, 26, 1367–1373. [Google Scholar] [CrossRef]
- Vlachopoulos, L.; Schweizer, A.; Meyer, D.C.; Gerber, C.; Fürnstahl, P. Three-dimensional corrective osteotomies of complex malunited humeral fractures using patient-specific guides. J. Shoulder Elb. Surg. 2016, 25, 2040–2047. [Google Scholar] [CrossRef]
- Fürnstahl, P.; Vlachopoulos, L.; Schweizer, A.; Fucentese, S.F.; Koch, P.P. Complex osteotomies of tibial plateau malunions using computer-assisted planning and patient-specific surgical guides. J. Orthop. Trauma 2015, 29, e270–e276. [Google Scholar] [CrossRef] [PubMed]
- Fucentese, S.F.; Meier, P.; Jud, L.; Köchli, G.L.; Aichmair, A.; Vlachopoulos, L.; Fürnstahl, P. Accuracy of 3D-planned patient specific instrumentation in high tibial open wedge valgisation osteotomy. J. Exp. Orthop. 2020, 7, 7. [Google Scholar] [CrossRef]
- Victor, J.; Premanathan, A. Virtual 3D planning and patient specific surgical guides for osteotomies around the knee: A feasibility and proof-of-concept study. Bone Jt. J. 2013, 95, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Munier, M.; Donnez, M.; Ollivier, M.; Flecher, X.; Chabrand, P.; Argenson, J.N.; Parratte, S. Can three-dimensional patient-specific cutting guides be used to achieve optimal correction for high tibial osteotomy? Pilot study. Orthop. Traumatol. Surg. Res. 2017, 103, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Chaouche, S.; Jacquet, C.; Fabre-Aubrespy, M.; Sharma, A.; Argenson, J.N.; Parratte, S.; Ollivier, M. Patient-specific cutting guides for open-wedge high tibial osteotomy: Safety and accuracy analysis of a hundred patients continuous cohort. Int. Orthop. 2019, 43, 2757–2765. [Google Scholar] [CrossRef] [PubMed]
- Viehöfer, A.F.; Wirth, S.H.; Zimmermann, S.M.; Jaberg, L.; Dennler, C.; Fürnstahl, P.; Farshad, M. Augmented reality guided osteotomy in hallux Valgus correction. BMC Musculoskelet. Disord. 2020, 21, 438. [Google Scholar] [CrossRef]
- Weigelt, L.; Fürnstahl, P.; Hirsiger, S.; Vlachopoulos, L.; Espinosa, N.; Wirth, S.H. Three-dimensional correction of complex ankle deformities with computer-assisted planning and patient-specific surgical guides. J. Foot Ankle Surg. 2017, 56, 1158–1164. [Google Scholar] [CrossRef]
- Wirth, S.; Viehöfer, A.; Laurenz, J.; Zimmermann, S.; Dennler, C.; Fürnstahl, P.; Farshad, M. Augmented Reality Guided Osteotomy in Hallux Valgus Surgery. Foot Ankle Orthop. 2018, 3, 2473011418S00518. [Google Scholar] [CrossRef]
- Kyo, T.; Nakahara, I.; Kuroda, Y.; Miki, H. Effects of coordinate-system construction methods on postoperative computed tomography evaluation of implant orientation after total hip arthroplasty. Comput. Aided Surg. 2015, 20, 52–60. [Google Scholar] [CrossRef]
- Gubian, A.; Kausch, L.; Neumann, J.O.; Kiening, K.; Ishak, B.; Maier-Hein, K.; Unterberg, A.; Scherer, M. CT-Navigated Spinal Instrumentations–Three-Dimensional Evaluation of Screw Placement Accuracy in Relation to a Screw Trajectory Plan. Medicina 2022, 58, 1200. [Google Scholar] [CrossRef]
- Uozumi, Y.; Nagamune, K.; Nishizawa, Y.; Araki, D.; Hoshino, Y.; Matsushita, T.; Kuroda, R.; Kurosaka, M. An automatic three-dimensional evaluation of screw placement after anterior cruciate ligament reconstruction using mdct images. J. Adv. Comput. Intell. Intell. Inform. 2013, 17, 818–827. [Google Scholar] [CrossRef]
- Hotelling, H. Analysis of a complex of statistical variables into principal components. J. Educ. Psychol. 1933, 24, 417. [Google Scholar] [CrossRef]
- Esfandiari, H.; Newell, R.; Anglin, C.; Street, J.; Hodgson, A.J. A deep learning framework for segmentation and pose estimation of pedicle screw implants based on C-arm fluoroscopy. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.; Singh, T.P.; Joshi, A.K. Deep learning-based localization and segmentation of wrist fractures on X-ray radiographs. Neural Comput. Appl. 2022, 34, 19061–19077. [Google Scholar] [CrossRef]
- Guan, B.; Yao, J.; Wang, S.; Zhang, G.; Zhang, Y.; Wang, X.; Wang, M. Automatic detection and localization of thighbone fractures in X-ray based on improved deep learning method. Comput. Vis. Image Underst. 2022, 216, 103345. [Google Scholar] [CrossRef]
- Wei, J.; Yao, J.; Zhanga, G.; Guan, B.; Zhang, Y.; Wang, S. Semi-supervised object detection based on single-stage detector for thighbone fracture localization. arXiv 2022, arXiv:2210.10998. [Google Scholar]
- Dupuis, M.; Delbos, L.; Veil, R.; Adamsbaum, C. External validation of a commercially available deep learning algorithm for fracture detection in children. Diagn. Interv. Imaging 2022, 103, 151–159. [Google Scholar] [CrossRef]
- Hendrix, N.; Hendrix, W.; van Dijke, K.; Maresch, B.; Maas, M.; Bollen, S.; Scholtens, A.; de Jonge, M.; Ong, L.L.S.; van Ginneken, B.; et al. Musculoskeletal radiologist-level performance by using deep learning for detection of scaphoid fractures on conventional multi-view radiographs of hand and wrist. Eur. Radiol. 2023, 33, 1575–1588. [Google Scholar] [CrossRef]
- Yu, J.; Yu, S.; Erdal, B.; Demirer, M.; Gupta, V.; Bigelow, M.; Salvador, A.; Rink, T.; Lenobel, S.; Prevedello, L.; et al. Detection and localisation of hip fractures on anteroposterior radiographs with artificial intelligence: Proof of concept. Clin. Radiol. 2020, 75, 237.e1–237.e9. [Google Scholar] [CrossRef]
- Blüthgen, C.; Becker, A.S.; de Martini, I.V.; Meier, A.; Martini, K.; Frauenfelder, T. Detection and localization of distal radius fractures: Deep learning system versus radiologists. Eur. J. Radiol. 2020, 126, 108925. [Google Scholar] [CrossRef]
- Brett, A.; Miller, C.G.; Hayes, C.W.; Krasnow, J.; Ozanian, T.; Abrams, K.; Block, J.E.; van Kuijk, C. Development of a clinical workflow tool to enhance the detection of vertebral fractures: Accuracy and precision evaluation. Spine 2009, 34, 2437–2443. [Google Scholar] [CrossRef] [PubMed]
- Olczak, J.; Fahlberg, N.; Maki, A.; Razavian, A.S.; Jilert, A.; Stark, A.; Sköldenberg, O.; Gordon, M. Artificial intelligence for analyzing orthopedic trauma radiographs: Deep learning algorithms—Are they on par with humans for diagnosing fractures? Acta Orthop. 2017, 88, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W.; Han, S.S.; Lee, J.W.; Oh, K.S.; Kim, N.R.; Yoon, J.P.; Kim, J.Y.; Moon, S.H.; Kwon, J.; Lee, H.J.; et al. Automated detection and classification of the proximal humerus fracture by using deep learning algorithm. Acta Orthop. 2018, 89, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; MacKinnon, T. Artificial intelligence in fracture detection: Transfer learning from deep convolutional neural networks. Clin. Radiol. 2018, 73, 439–445. [Google Scholar] [CrossRef]
- Lindsey, R.; Daluiski, A.; Chopra, S.; Lachapelle, A.; Mozer, M.; Sicular, S.; Hanel, D.; Gardner, M.; Gupta, A.; Hotchkiss, R.; et al. Deep neural network improves fracture detection by clinicians. Proc. Natl. Acad. Sci. USA 2018, 115, 11591–11596. [Google Scholar] [CrossRef]
- Adams, M.; Chen, W.; Holcdorf, D.; McCusker, M.W.; Howe, P.D.; Gaillard, F. Computer vs. human: Deep learning versus perceptual training for the detection of neck of femur fractures. J. Med. Imaging Radiat. Oncol. 2019, 63, 27–32. [Google Scholar] [CrossRef]
- Urakawa, T.; Tanaka, Y.; Goto, S.; Matsuzawa, H.; Watanabe, K.; Endo, N. Detecting intertrochanteric hip fractures with orthopedist-level accuracy using a deep convolutional neural network. Skelet. Radiol. 2019, 48, 239–244. [Google Scholar] [CrossRef]
- Tomita, N.; Cheung, Y.Y.; Hassanpour, S. Deep neural networks for automatic detection of osteoporotic vertebral fractures on CT scans. Comput. Biol. Med. 2018, 98, 8–15. [Google Scholar] [CrossRef]
- Kolanu, N.; Silverstone, E.J.; Ho, B.H.; Pham, H.; Hansen, A.; Pauley, E.; Quirk, A.R.; Sweeney, S.C.; Center, J.R.; Pocock, N.A. Clinical utility of computer-aided diagnosis of vertebral fractures from computed tomography images. J. Bone Miner. Res. 2020, 35, 2307–2312. [Google Scholar] [CrossRef]
- Zhou, Q.Q.; Tang, W.; Wang, J.; Hu, Z.C.; Xia, Z.Y.; Zhang, R.; Fan, X.; Yong, W.; Yin, X.; Zhang, B.; et al. Automatic detection and classification of rib fractures based on patients’ CT images and clinical information via convolutional neural network. Eur. Radiol. 2021, 31, 3815–3825. [Google Scholar] [CrossRef]
- Warin, K.; Limprasert, W.; Suebnukarn, S.; Paipongna, T.; Jantana, P.; Vicharueang, S. Maxillofacial fracture detection and classification in computed tomography images using convolutional neural network-based models. Sci. Rep. 2023, 13, 3434. [Google Scholar] [CrossRef]
- He, K.; Gkioxari, G.; Dollár, P.; Girshick, R. Mask r-cnn. In Proceedings of the IEEE International Conference on Computer Vision, Venice, Italy, 22–29 October 2017; pp. 2961–2969. [Google Scholar]
- Lewis, M.; Reid, K.; Toms, A.P. Reducing the effects of metal artefact using high keV monoenergetic reconstruction of dual energy CT (DECT) in hip replacements. Skelet. Radiol. 2013, 42, 275–282. [Google Scholar] [CrossRef]
- BESL, P. A Method for Registration of 3-D Shapes. Trans. PAMI 1992, 1611, 586–606. [Google Scholar] [CrossRef]
- Dalitz, C.; Schramke, T.; Jeltsch, M. Iterative Hough transform for line detection in 3D point clouds. Image Process. Line 2017, 7, 184–196. [Google Scholar] [CrossRef]
- Amanatides, J.; Woo, A. A fast voxel traversal algorithm for ray tracing. Eurographics 1987, 87, 3–10. [Google Scholar]
- Çiçek, Ö.; Abdulkadir, A.; Lienkamp, S.S.; Brox, T.; Ronneberger, O. 3D U-Net: Learning dense volumetric segmentation from sparse annotation. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention–MICCAI 2016: 19th International Conference, Athens, Greece, 17–21 October 2016; Proceedings, Part II 19. Springer: Berlin/Heidelberg, Germany, 2016; pp. 424–432. [Google Scholar]
- Kingma, D.P.; Ba, J. Adam: A method for stochastic optimization. arXiv 2014, arXiv:1412.6980. [Google Scholar]
- Milletari, F.; Navab, N.; Ahmadi, S. V-net: Fully convolutional neural networks for volumetric medical image segmentation. In Proceedings of the 2016 Fourth International Conference on 3D Vision (3DV), Stanford, CA, USA, 25–28 October 2016; pp. 565–571. [Google Scholar]
- Zhang, Z.; Sabuncu, M. Generalized cross entropy loss for training deep neural networks with noisy labels. Adv. Neural Inf. Process. Syst. 2018, 31. [Google Scholar]
- Yoo, T.S.; Ackerman, M.J.; Lorensen, W.E.; Schroeder, W.; Chalana, V.; Aylward, S.; Metaxas, D.; Whitaker, R. Engineering and algorithm design for an image processing API: A technical report on ITK-the insight toolkit. In Medicine Meets Virtual Reality 02/10; IOS Press: Amsterdam, The Netherlands, 2002; pp. 586–592. [Google Scholar]
- Johnson, H.J.; McCormick, M.; Ibanez, L. The ITK Software Guide: Design and Functionality; Kitware Inc.: Clifton Park, NY, USA, 2015. [Google Scholar]
- Rao, Y.R.; Prathapani, N.; Nagabhooshanam, E. Application of normalized cross correlation to image registration. Int. J. Res. Eng. Technol. 2014, 3, 12–16. [Google Scholar]
- Mambo, S. Optimisation and Performance Evaluation in Image Registration Technique. Ph.D. Thesis, Université Paris-Est, Tshwane University of Technology, Pretoria, South Africa, 2018. [Google Scholar]
- Mukhopadhyay, P.; Chaudhuri, B.B. A survey of Hough Transform. Pattern Recognit. 2015, 48, 993–1010. [Google Scholar] [CrossRef]
- Burger, W.; Burge, M. Principles of Digital Image Processing: Core Algorithms; Springer: London, UK, 2010. [Google Scholar]
- Murphy, K.; Van Ginneken, B.; Reinhardt, J.M.; Kabus, S.; Ding, K.; Deng, X.; Cao, K.; Du, K.; Christensen, G.E.; Garcia, V.; et al. Evaluation of registration methods on thoracic CT: The EMPIRE10 challenge. IEEE Trans. Med Imaging 2011, 30, 1901–1920. [Google Scholar] [CrossRef]
- Jiang, B.; Pennington, Z.; Zhu, A.; Matsoukas, S.; Ahmed, A.K.; Ehresman, J.; Mahapatra, S.; Cottrill, E.; Sheppell, H.; Manbachi, A.; et al. Three-dimensional assessment of robot-assisted pedicle screw placement accuracy and instrumentation reliability based on a preplanned trajectory. J. Neurosurg. Spine 2020, 33, 519–528. [Google Scholar] [CrossRef]
- Stern, C.; Sommer, S.; Germann, C.; Galley, J.; Pfirrmann, C.W.; Fritz, B.; Sutter, R. Pelvic bone CT: Can tin-filtered ultra-low-dose CT and virtual radiographs be used as alternative for standard CT and digital radiographs? Eur. Radiol. 2021, 31, 6793–6801. [Google Scholar] [CrossRef]
- Kasten, Y.; Doktofsky, D.; Kovler, I. End-to-end convolutional neural network for 3D reconstruction of knee bones from bi-planar X-ray images. In Proceedings of the Machine Learning for Medical Image Reconstruction: Third International Workshop, MLMIR 2020, Held in Conjunction with MICCAI 2020, Lima, Peru, 8 October 2020; Proceedings 3. Springer: Berlin/Heidelberg, Germany, 2020; pp. 123–133. [Google Scholar]


| Measure | Manual | Automatic | Mean | Min | Max | |
|---|---|---|---|---|---|---|
| 3D distance [mm] | 17.0 | 13.0 | 2.9 | 54.9 | ||
| 12.8 | 7.4 | 1.8 | 27.8 | |||
| 15.0 | 12.1 | 4.3 | 71.4 | |||
| 7.3 | 6.3 | 1.0 | 25.5 | |||
| 8.7 | 6.7 | 1.3 | 23.5 | |||
| 2D angle [°] | 7.0 | 5.4 | 0.3 | 22.2 | ||
| 6.9 | 5.4 | 1.6 | 23.4 | |||
| 21.9 | 17.7 | 0.6 | 73.3 | |||
| Abs. 2D angle deviation [°] | 9.9 | 8.5 | 0.0 | 36.5 | ||
| 20.0 | 15.2 | 0.3 | 49.9 | |||
| 3D angle [°] | 29.2 | 11.2 | 9.6 | 54.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ackermann, J.; Hoch, A.; Snedeker, J.G.; Zingg, P.O.; Esfandiari, H.; Fürnstahl, P. Automatic 3D Postoperative Evaluation of Complex Orthopaedic Interventions. J. Imaging 2023, 9, 180. https://doi.org/10.3390/jimaging9090180
Ackermann J, Hoch A, Snedeker JG, Zingg PO, Esfandiari H, Fürnstahl P. Automatic 3D Postoperative Evaluation of Complex Orthopaedic Interventions. Journal of Imaging. 2023; 9(9):180. https://doi.org/10.3390/jimaging9090180
Chicago/Turabian StyleAckermann, Joëlle, Armando Hoch, Jess Gerrit Snedeker, Patrick Oliver Zingg, Hooman Esfandiari, and Philipp Fürnstahl. 2023. "Automatic 3D Postoperative Evaluation of Complex Orthopaedic Interventions" Journal of Imaging 9, no. 9: 180. https://doi.org/10.3390/jimaging9090180
APA StyleAckermann, J., Hoch, A., Snedeker, J. G., Zingg, P. O., Esfandiari, H., & Fürnstahl, P. (2023). Automatic 3D Postoperative Evaluation of Complex Orthopaedic Interventions. Journal of Imaging, 9(9), 180. https://doi.org/10.3390/jimaging9090180







