Short-Term Precision and Repeatability of Radiofrequency Echographic Multi Spectrometry (REMS) on Lumbar Spine and Proximal Femur: An In Vivo Study
Abstract
1. Background
2. Materials and Methods
2.1. Study Design
2.2. REMS Acquisitions
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NIH Consensus Development Panel on Osteoporosis Prevention Diagnosis, Therapy—Osteoporosis Prevention, Diagnosis, and Therapy. JAMA J. Am. Med. Assoc. 2001, 285, 785–795. [CrossRef]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.-Y. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef] [PubMed]
- Hillier, T.A.; Cauley, J.A.; Rizzo, J.H.; Pedula, K.L.; Ensrud, K.E.; Bauer, D.C.; Lui, L.-Y.; Vesco, K.K.; Black, D.M.; Donaldson, M.G.; et al. WHO absolute fracture risk models (FRAX): Do clinical risk factors improve fracture prediction in older women without osteoporosis? J. Bone Miner. Res. 2011, 26, 1774–1782. [Google Scholar] [CrossRef]
- Johnell, O.; Kanis, J.A.; Oden, A.; Johansson, H.; De Laet, C.; Delmas, P.; Eisman, J.A.; Fujiwara, S.; Kroger, H.; Mellstrom, D.; et al. Predictive Value of BMD for Hip and Other Fractures. J. Bone Miner. Res. 2005, 20, 1185–1194. [Google Scholar] [CrossRef]
- de Liefde, I.I.; van der Klift, M.; de Laet, C.E.D.H.; Van Daele, P.L.A.; Hofman, A.; Pols, H.A.P. Bone mineral density and fracture risk in type-2 diabetes mellitus: The Rotterdam Study. Osteoporos. Int. 2005, 16, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Zysset, P.; Glüer, C.C.; McClung, M.; Biver, E.; Pierroz, D.; Ferrari, S.L. Perspectives on the non-invasive evaluation of femoral strength in the assessment of hip fracture risk. Osteoporos. Int. 2020, 31, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Messina, C.; Bandirali, M.; Sconfienza, L.M.; D’alonzo, N.K.; Di Leo, G.; Papini, G.D.E.; Ulivieri, F.M.; Sardanelli, F. Prevalence and type of errors in dual-energy X-ray absorptiometry. Eur. Radiol. 2015, 25, 1504–1511. [Google Scholar] [CrossRef]
- Bazzocchi, A.; Isaac, A.; Dalili, D.; Fotiadou, A.; Kariki, E.P.; Kirschke, J.S.; Krestan, C.R.; Messina, C.; Oei, E.H.; Phan, C.M.; et al. Imaging of Metabolic Bone Diseases: The Spine View, Part I. Semin. Musculoskelet. Radiol. 2022, 26, 478–490. [Google Scholar] [CrossRef]
- Diez-Perez, A.; Brandi, M.L.; Al-Daghri, N.; Branco, J.C.; Bruyère, O.; Cavalli, L.; Cooper, C.; Cortet, B.; Dawson-Hughes, B.; Dimai, H.P.; et al. Radiofrequency echographic multi-spectrometry for the in-vivo assessment of bone strength: State of the art—Outcomes of an expert consensus meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Mus. Aging Clin. Exp. Res. 2019, 31, 1375–1389. [Google Scholar] [CrossRef]
- Olszynski, W.P.; Brown, J.P.; Adachi, J.D.; Hanley, D.A.; Ioannidis, G.; Davison, K.S.; CaMos Research Group. Multisite quantitative ultrasound for the prediction of fractures over 5 years of follow-up: The Canadian Multicentre Osteoporosis Study. J. Bone Miner. Res. 2013, 28, 2027–2034. [Google Scholar] [CrossRef]
- Conversano, F.; Franchini, R.; Greco, A.; Soloperto, G.; Chiriacò, F.; Casciaro, E.; Aventaggiato, M.; Renna, M.D.; Pisani, P.; Di Paola, M.; et al. A Novel Ultrasound Methodology for Estimating Spine Mineral Density. Ultrasound Med. Biol. 2015, 41, 281–300. [Google Scholar] [CrossRef] [PubMed]
- Casciaro, S.; Peccarisi, M.; Pisani, P.; Franchini, R.; Greco, A.; De Marco, T.; Grimaldi, A.; Quarta, L.; Quarta, E.; Muratore, M.; et al. An Advanced Quantitative Echosound Methodology for Femoral Neck Densitometry. Ultrasound Med. Biol. 2016, 42, 1337–1356. [Google Scholar] [CrossRef] [PubMed]
- Pisani, P.; Greco, A.; Conversano, F.; Renna, M.D.; Casciaro, E.; Quarta, L.; Costanza, D.; Muratore, M.; Casciaro, S. A quantitative ultrasound approach to estimate bone fragility: A first comparison with dual X-ray absorptiometry. Measurement 2017, 101, 243–249. [Google Scholar] [CrossRef]
- di Paola, M.; Gatti, D.; Viapiana, O.; Cianferotti, L.; Cavalli, L.; Caffarelli, C.; Conversano, F.; Quarta, E.; Pisani, P.; Girasole, G.; et al. Radiofrequency echographic multispectrometry compared with dual X-ray absorptiometry for osteoporosis diagnosis on lumbar spine and femoral neck. Osteoporos. Int. 2019, 30, 391–402. [Google Scholar] [CrossRef]
- Caffarelli, C.; Tomai Pitinca, M.D.; Al Refaie, A.; Ceccarelli, E.; Gonnelli, S. Ability of radiofrequency echographic multispectrometry to identify osteoporosis status in elderly women with type 2 diabetes. Aging Clin. Exp. Res. 2022, 34, 121–127. [Google Scholar] [CrossRef]
- Lalli, P.; Mautino, C.; Busso, C.; Bardesono, F.; Di Monaco, M.; Lippi, L.; Invernizzi, M.; Minetto, M.A. Reproducibility and Accuracy of the Radiofrequency Echographic Multi-Spectrometry for Femoral Mineral Density Estimation and Discriminative Power of the Femoral Fragility Score in Patients with Primary and Disuse-Related Osteoporosis. J. Clin. Med. 2022, 29, 3761. [Google Scholar] [CrossRef]
- el Maghraoui, A.; Achemlal, L.; Bezza, A. Monitoring of Dual-Energy X-ray Absorptiometry Measurement in Clinical Practice. J. Clin. Densitom. 2006, 9, 281–286. [Google Scholar] [CrossRef]
- Engelke, K.; Glüer, C.-C. Quality and performance measures in bone densitometry. Osteoporos. Int. 2006, 17, 1283–1292. [Google Scholar] [CrossRef]
- Knapp, K.M.; Welsman, J.R.; Hopkins, S.J.; Fogelman, I.; Blake, G.M. Obesity Increases Precision Errors in Dual-Energy X-Ray Absorptiometry Measurements. J. Clin. Densitom. 2012, 15, 315–319. [Google Scholar] [CrossRef]
- Lewiecki, E.M.; Gordon, C.M.; Baim, S.; Leonard, M.B.; Bishop, N.J.; Bianchi, M.-L.; Kalkwarf, H.J.; Langman, C.B.; Plotkin, H.; Rauch, F.; et al. International Society for Clinical Densitometry 2007 Adult and Pediatric Official Positions. Bone 2008, 43, 1115–1121. [Google Scholar] [CrossRef]
- Bandirali, M.; Poloni, A.; Sconfienza, L.M.; Messina, C.; Papini, G.D.E.; Petrini, M.; Ulivieri, F.M.; Di Leo, G.; Sardanelli, F. Short-term precision assessment of trabecular bone score and bone mineral density using dual-energy X-ray absorptiometry with different scan modes: An in vivo study. Eur. Radiol. 2015, 25, 2194–2198. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, S.J.; Welsman, J.R.; Knapp, K.M. Short-term Precision Error in Dual Energy X-Ray Absorptiometry, Bone Mineral Density and Trabecular Bone Score Measurements; and Effects of Obesity on Precision Error. J. Biomed. Graph. Comput. 2014, 4, 8. [Google Scholar] [CrossRef]
- Messina, C.; Buonomenna, C.; Menon, G.; Magnani, S.; Albano, D.; Gitto, S.; Ulivieri, F.M.; Sconfienza, L.M. Fat Mass Does Not Increase the Precision Error of Trabecular Bone Score Measurements. J. Clin. Densitom. 2019, 22, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Lodder, M.C.; Lems, W.F.; Ader, H.J.; Marthinsen, A.E.; Van Coeverden, S.C.C.M.; Lips, P.; Netelenbos, J.C.; Dijkmans, B.A.C.; Roos, J.C. Reproducibility of bone mineral density measurement in daily practice. Ann. Rheum. Dis. 2004, 63, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Messina, C.; Acquasanta, M.; Rinaudo, L.; Tortora, S.; Arena, G.; Albano, D.; Sconfienza, L.M.; Ulivieri, F.M. Short-Term Precision Error of Bone Strain Index, a New DXA-Based Finite Element Analysis Software for Assessing Hip Strength. J. Clin. Densitom. 2021, 24, 330–337. [Google Scholar] [CrossRef]
- Leslie, W.D.; Moayyeri, A. Minimum sample size requirements for bone density precision assessment produce inconsistency in clinical monitoring. Osteoporos. Int. 2006, 17, 1673–1680. [Google Scholar] [CrossRef]
- Shepherd, J.A.; Morgan, S.L.; Lu, Y. Comparing BMD Results between Two Similar DXA Systems Using the Generalized Least Significant Change. J. Clin. Densitom. 2008, 11, 237–242. [Google Scholar] [CrossRef]
- Albano, D.; Agnollitto, P.M.; Petrini, M.; Biacca, A.; Ulivieri, F.M.; Sconfienza, L.M.; Messina, C. Operator-Related Errors and Pitfalls in Dual Energy X-Ray Absorptiometry: How to Recognize and Avoid Them. Acad. Radiol. 2021, 28, 1272–1286. [Google Scholar] [CrossRef]
- Bandirali, M.; Sconfienza, L.M.; Aliprandi, A.; Di Leo, G.; Marchelli, D.; Ulivieri, F.M.; Sardanelli, F. In vivo differences among scan modes in bone mineral density measurement at dual-energy X-ray absorptiometry. Radiol. Med. 2014, 119, 257–260. [Google Scholar] [CrossRef]
- Caffarelli, C.; Adami, G.; Arioli, G.; Bianchi, G.; Brandi, M.L.; Casciaro, S.; Cianferotti, L.; Ciardo, D.; Conversano, F.; Gatti, D.; et al. AB1082 Influence of the Variation of the Operator, Patient Position and Device on the Measurement Performance of Radiofrequency Echographic Multi Spectrometry (REMS). Ann. Rheum. Dis. 2020, 79, 1830. [Google Scholar] [CrossRef]
- el Maghraoui, A. Monitoring DXA Measurement in Clinical Practice. In Dual Energy X-ray Absorptiometry; InTech: London, UK, 2012. [Google Scholar]
- Shuhart, C.R.; Yeap, S.S.; Anderson, P.A.; Jankowski, L.G.; Lewiecki, E.M.; Morse, L.R.; Rosen, H.N.; Weber, D.R.; Zemel, B.S.; Shepherd, J.A. Executive Summary of the 2019 ISCD Position Development Conference on Monitoring Treatment, DXA Cross-calibration and Least Significant Change, Spinal Cord Injury, Peri-prosthetic and Orthopedic Bone Health, Transgender Medicine, and Pediatrics. J. Clin. Densitom. 2019, 22, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Blake, G.M.; Rymer, J.; Fogelman, I. Long-Term Precision of DXA Scanning Assessed over Seven Years in Forty Postmenopausal Women. Osteoporos. Int. 2000, 11, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Khu, A.; Sumardi, M. A REMS Scan-Based Report on Relation between Body Mass Index and Osteoporosis in Urban Population of Medan at Royal Prima Hospital. Maj. Kedokt. Bdg. 2020, 52, 22–27. [Google Scholar] [CrossRef]
- Bolotin, H.H. DXA in vivo BMD methodology: An erroneous and misleading research and clinical gauge of bone mineral status, bone fragility, and bone remodelling. Bone 2007, 41, 138–154. [Google Scholar] [CrossRef]
- el Maghraoui, A.; do Santos Zounon, A.A.; Jroundi, I.; Nouijai, A.; Ghazi, M.; Achemlal, L.; Bezza, A.; Tazi, M.A.; Abouqual, R. Reproducibility of bone mineral density measurements using dual X-ray absorptiometry in daily clinical practice. Osteoporos. Int. 2005, 16, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Cortet, B.; Dennison, E.; Diez-Perez, A.; Locquet, M.; Muratore, M.; Nogués, X.; Crespo, D.O.; Quarta, E.; Brandi, M.L. Radiofrequency Echographic Multi Spectrometry (REMS) for the diagnosis of osteoporosis in a European multicenter clinical context. Bone 2021, 143, 115786. [Google Scholar] [CrossRef] [PubMed]

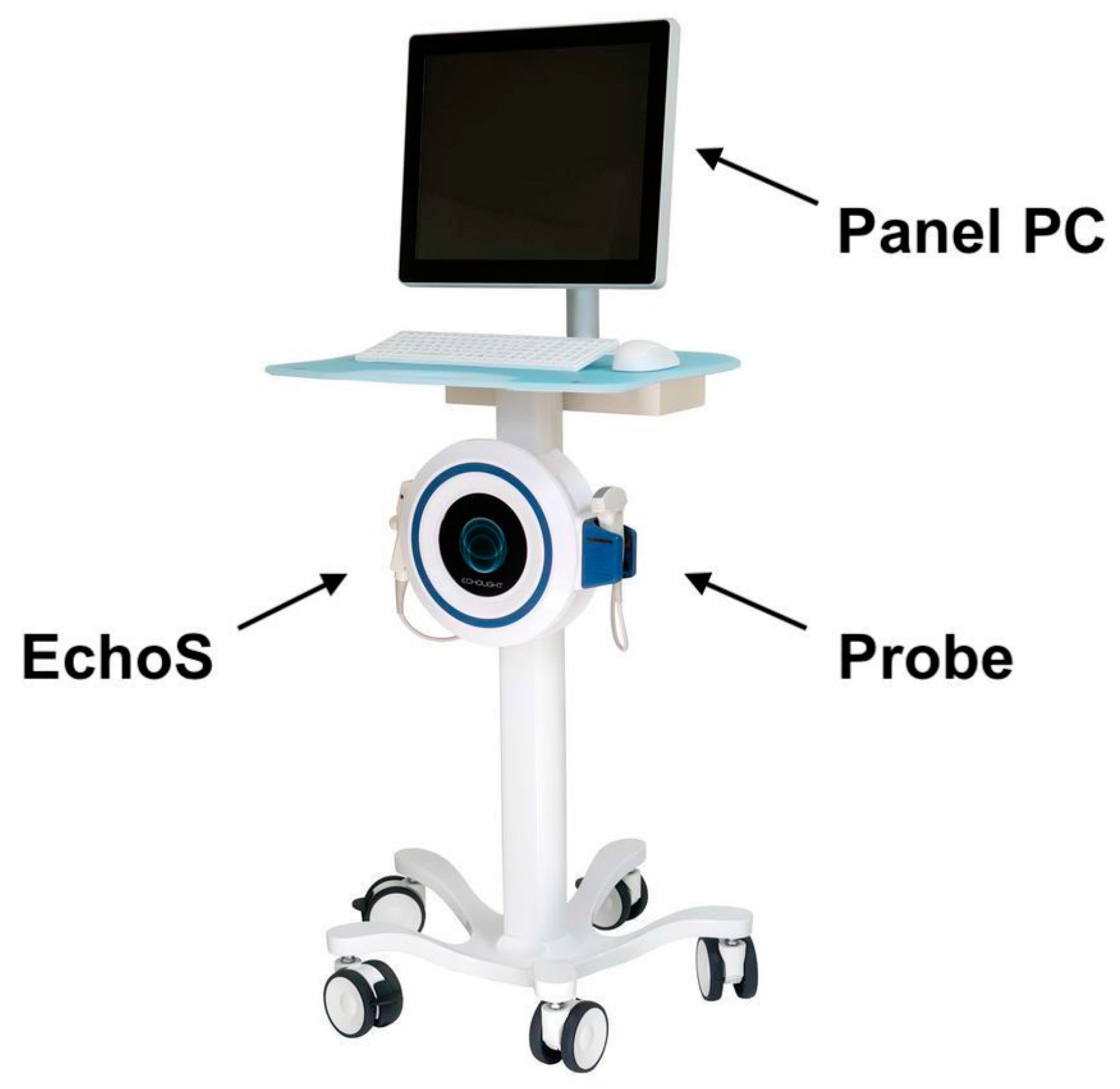
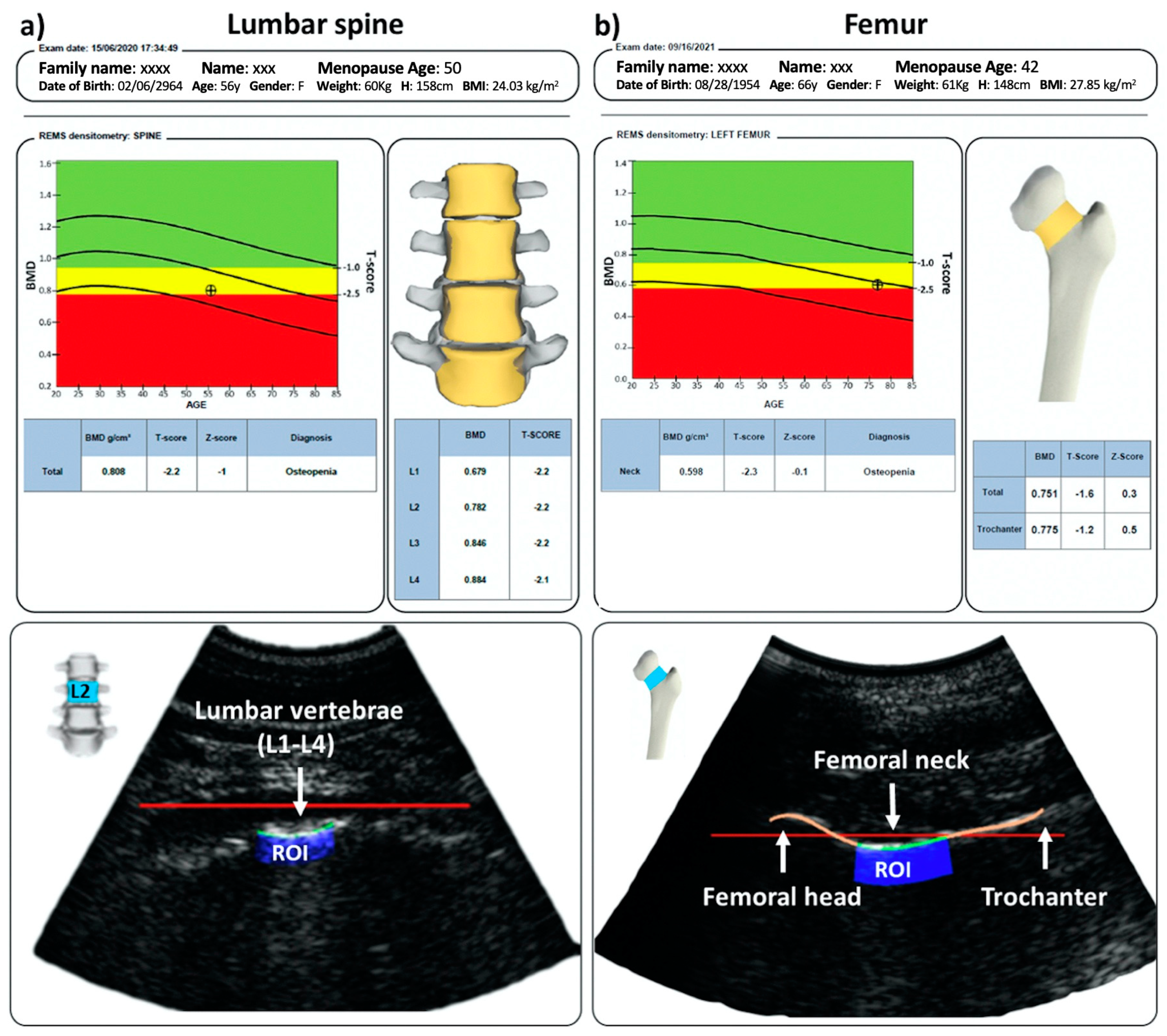

| Demographic Data | LS (42) | FEM (37) |
|---|---|---|
| Age (years) | 48.9 ± 6.8 | 48.3 ± 6.1 |
| Ethnicity | Caucasian | Caucasian |
| Diagnosis | ||
| Normal | 16 (38.1%) | 12 (32.4%) |
| Osteopenia | 22 (52.4%) | 23 (62.2%) |
| Osteoporosis | 4 (9.5%) | 2 (5.4%) |
| BMI (kg/m2) | 24.71 ± 4.21 | 25.0 ± 4.84 |
| BMD (g/cm2) | 0.914 ± 0.1 | 0.709 ± 0.1 |
| Age (years) | 48.9 ± 6.8 | 48.3 ± 6.1 |
| Short Term Precision | ||||||
|---|---|---|---|---|---|---|
| LS (42) | FEM (37) | |||||
| RMS-CV (%) | LSC (%) | SDD (g/cm2) | RMS-CV (%) | LSC (%) | SDD (g/cm2) | |
| Intra-operator precision | 0.47 | 1.29 | 0.009 | 0.32 | 0.89 | 0.004 |
| Inter-operator repeatability | 0.55 | 1.52 | 0.009 | 0.51 | 1.40 | 0.008 |
| Site | BMI Category | n | BMD (g/cm2) | p-Value | RMS-CV (%) | LSC (%) | SDD (g/cm2) |
|---|---|---|---|---|---|---|---|
| LS | Optimal $ | 26 | 0.879 ± 0.10 | p = 0.004 | 0.44 | 1.23 | 0.008 |
| Overweight/obese | 14 | 0.975 ± 0.09 | 0.50 | 1.40 | 0.011 | ||
| FEM | Optimal $ | 24 | 0.659 ± 0.07 | p < 0.0001 | 0.28 | 0.73 | 0.004 |
| Overweight/obese | 13 | 0.794 ± 0.09 | 0.39 | 1.07 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messina, C.; Gitto, S.; Colombo, R.; Fusco, S.; Guagliardo, G.; Piazza, M.; Poli, J.C.; Albano, D.; Sconfienza, L.M. Short-Term Precision and Repeatability of Radiofrequency Echographic Multi Spectrometry (REMS) on Lumbar Spine and Proximal Femur: An In Vivo Study. J. Imaging 2023, 9, 118. https://doi.org/10.3390/jimaging9060118
Messina C, Gitto S, Colombo R, Fusco S, Guagliardo G, Piazza M, Poli JC, Albano D, Sconfienza LM. Short-Term Precision and Repeatability of Radiofrequency Echographic Multi Spectrometry (REMS) on Lumbar Spine and Proximal Femur: An In Vivo Study. Journal of Imaging. 2023; 9(6):118. https://doi.org/10.3390/jimaging9060118
Chicago/Turabian StyleMessina, Carmelo, Salvatore Gitto, Roberta Colombo, Stefano Fusco, Giada Guagliardo, Mattia Piazza, Jacopo Carlo Poli, Domenico Albano, and Luca Maria Sconfienza. 2023. "Short-Term Precision and Repeatability of Radiofrequency Echographic Multi Spectrometry (REMS) on Lumbar Spine and Proximal Femur: An In Vivo Study" Journal of Imaging 9, no. 6: 118. https://doi.org/10.3390/jimaging9060118
APA StyleMessina, C., Gitto, S., Colombo, R., Fusco, S., Guagliardo, G., Piazza, M., Poli, J. C., Albano, D., & Sconfienza, L. M. (2023). Short-Term Precision and Repeatability of Radiofrequency Echographic Multi Spectrometry (REMS) on Lumbar Spine and Proximal Femur: An In Vivo Study. Journal of Imaging, 9(6), 118. https://doi.org/10.3390/jimaging9060118








