NeuroActivityToolkit—Toolbox for Quantitative Analysis of Miniature Fluorescent Microscopy Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Implantation of GRIN-Lens and Baseplate for Miniscope Recordings
2.3. Miniscope Recording Acquisition and Preprocessing
2.4. Neuron-Active-State Determination
2.5. Neuronal Network Description
2.6. Correlation Analysis for Co-Active Neurons
2.7. Neuronal-Activity Random Shuffling
2.8. PCA Analysis
3. Results
3.1. Calcium-Indicator-Signal Active-Phase Determination
3.2. Neuronal network Activity Properties
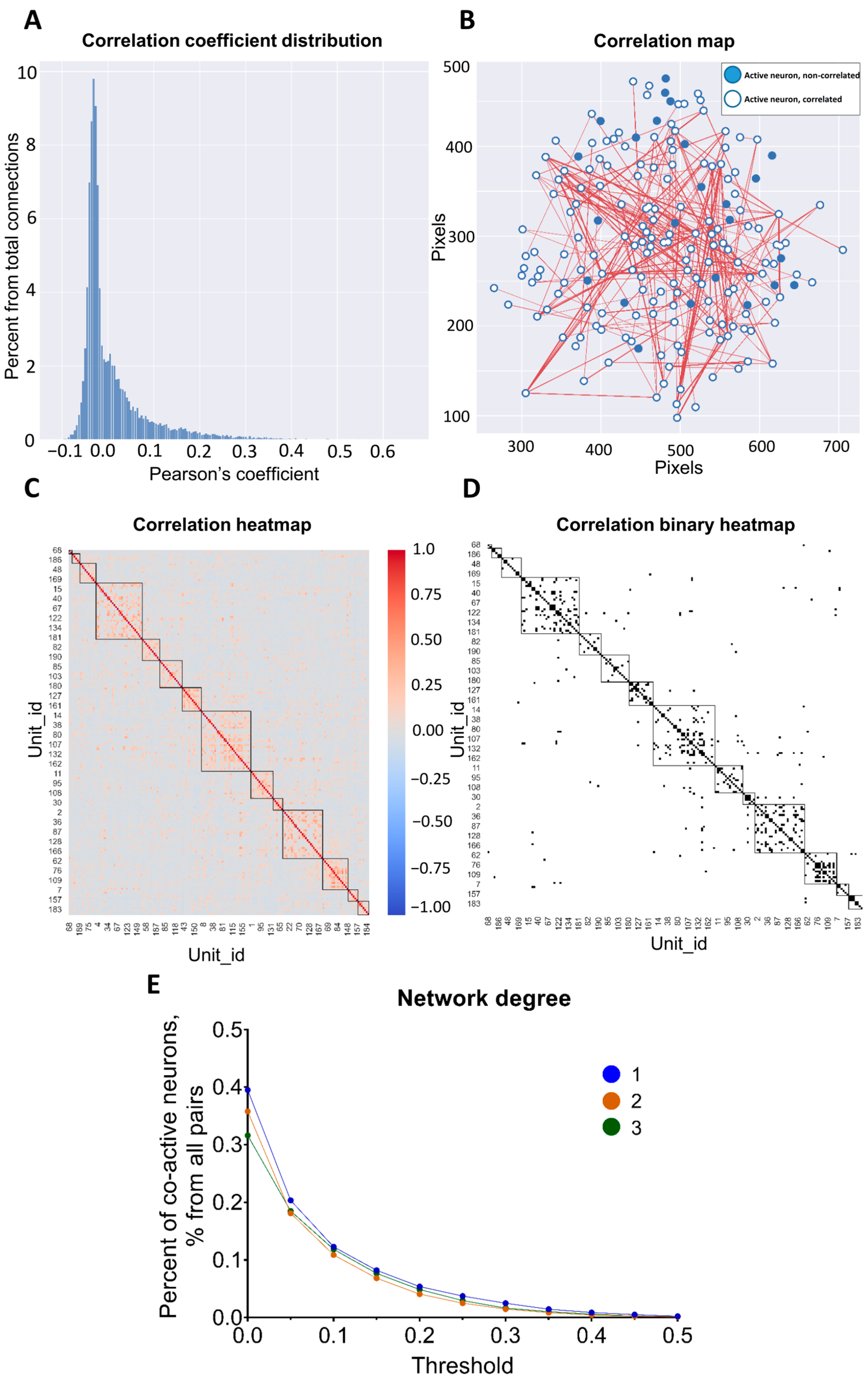
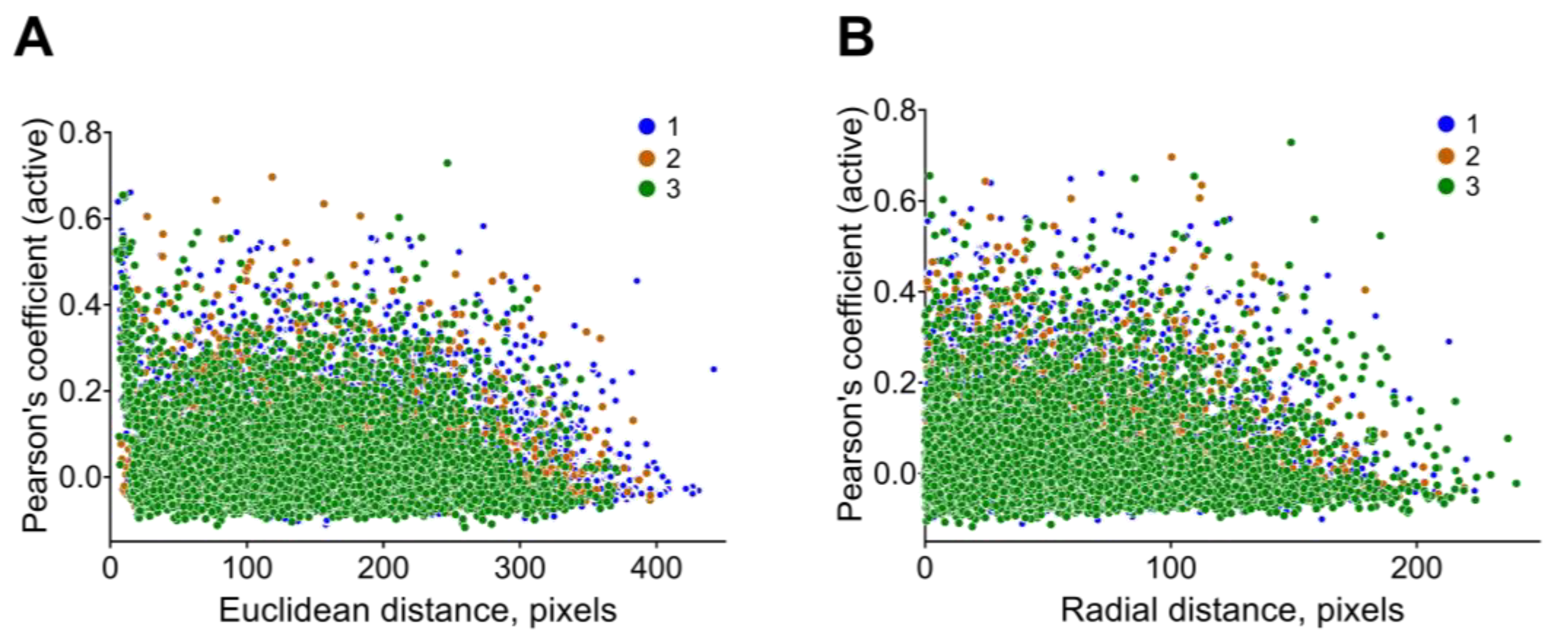
3.3. Pairwise Neuronal-Activity Correlation in the Neuronal Network
3.4. Random Neuronal-Activity Shuffling
3.5. Principal Component Analysis of Statistical Metrics
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rubinov, M.; Sporns, O. Complex Network Measures of Brain Connectivity: Uses and Interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef]
- Lütcke, H.; Margolis, D.J.; Helmchen, F. Steady or Changing? Long-Term Monitoring of Neuronal Population Activity. Trends Neurosci. 2013, 36, 375–384. [Google Scholar] [CrossRef]
- Pologruto, T.A.; Yasuda, R.; Svoboda, K. Monitoring Neural Activity and [Ca2+] with Genetically Encoded Ca2+ Indicators. J. Neurosci. 2004, 24, 9572–9579. [Google Scholar] [CrossRef]
- Sawinski, J.; Wallace, D.J.; Greenberg, D.S.; Grossmann, S.; Denk, W.; Kerr, J.N.D. Visually Evoked Activity in Cortical Cells Imaged in Freely Moving Animals. Proc. Natl. Acad. Sci. USA 2009, 106, 19557–19562. [Google Scholar] [CrossRef]
- Zhang, T.; Hernandez, O.; Chrapkiewicz, R.; Shai, A.; Wagner, M.J.; Zhang, Y.; Wu, C.H.; Li, J.Z.; Inoue, M.; Gong, Y.; et al. Kilohertz Two-Photon Brain Imaging in Awake Mice. Nat. Methods 2019, 16, 1119–1122. [Google Scholar] [CrossRef]
- Sofroniew, N.J.; Flickinger, D.; King, J.; Svoboda, K. A Large Field of View Two-Photon Mesoscope with Subcellular Resolution for in Vivo Imaging. Elife 2016, 5, e14472. [Google Scholar] [CrossRef]
- Fujishiro, A.; Kaneko, H.; Kawashima, T.; Ishida, M.; Kawano, T. In Vivo Neuronal Action Potential Recordings via Three-Dimensional Microscale Needle-Electrode Arrays. Sci. Rep. 2014, 4, 4868. [Google Scholar] [CrossRef]
- Erofeev, A.; Kazakov, D.; Makarevich, N.; Bolshakova, A.; Gerasimov, E.; Nekrasov, A.; Kazakin, A.; Komarevtsev, I.; Bolsunovskaja, M.; Bezprozvanny, I.; et al. An Open-Source Wireless Electrophysiological Complex for in Vivo Recording Neuronal Activity in the Rodent’s Brain. Sensors 2021, 21, 7189. [Google Scholar] [CrossRef]
- Adams, C.; Mathieson, K.; Gunning, D.; Cunningham, W.; Rahman, M.; Morrison, J.D.; Prydderch, M.L. Development of Flexible Arrays for in Vivo Neuronal Recording and Stimulation. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2005, 546, 154–159. [Google Scholar] [CrossRef]
- Jang, J.W.; Kang, Y.N.; Seo, H.W.; Kim, B.; Choe, H.K.; Park, S.H.; Lee, M.G.; Kim, S. Long-Term in-Vivo Recording Performance of Flexible Penetrating Microelectrode Arrays. J. Neural Eng. 2021, 18, 066018. [Google Scholar] [CrossRef]
- Ghosh, K.K.; Burns, L.D.; Cocker, E.D.; Nimmerjahn, A.; Ziv, Y.; El Gamal, A.; Schnitzer, M.J. Miniaturized Integration of a Fluorescence Microscope. Nat. Methods 2011, 8, 871–878. [Google Scholar] [CrossRef]
- Aharoni, D.; Khakh, B.S.; Silva, A.J.; Golshani, P. All the Light That We Can See: A New Era in Miniaturized Microscopy. Nat. Methods 2019, 16, 11–13. [Google Scholar] [CrossRef]
- Aharoni, D.; Hoogland, T.M. Circuit Investigations with Open-Source Miniaturized Microscopes: Past, Present and Future. Front. Cell. Neurosci. 2019, 13, 341. [Google Scholar] [CrossRef]
- Liberti, W.A.; Schmid, T.A.; Forli, A.; Snyder, M.; Yartsev, M.M. A Stable Hippocampal Code in Freely Flying Bats. Nature 2022, 604, 98–103. [Google Scholar] [CrossRef]
- Oh, J.; Lee, C.; Kaang, B.K. Imaging and Analysis of Genetically Encoded Calcium Indicators Linking Neural Circuits and Behaviors. Korean J. Physiol. Pharmacol. 2019, 23, 237–249. [Google Scholar] [CrossRef]
- Pachitariu, M.; Stringer, C.; Harris, K.D. Robustness of Spike Deconvolution for Neuronal Calcium Imaging. J. Neurosci. 2018, 38, 7976–7985. [Google Scholar] [CrossRef]
- Stringer, C.; Wang, T.; Michaelos, M.; Pachitariu, M. Cellpose: A Generalist Algorithm for Cellular Segmentation. Nat. Methods 2021, 18, 100–106. [Google Scholar] [CrossRef]
- Pachitariu, M.; Packer, A.; Pettit, N.; Dagleish, H.; Hausser, M.; Sahani, M. Extracting Regions of Interest from Biological Images with Convolutional Sparse Block Coding. In Proceedings of the 26th International Conference on Neural Information Processing Systems, Red Hook, NY, USA, 5–10 December 2013; Volume 2, pp. 1745–1753. [Google Scholar]
- Dong, Z.; Mau, W.; Feng, Y.; Pennington, Z.T.; Chen, L.; Zaki, Y.; Rajan, K.; Shuman, T.; Aharoni, D.; Cai, D.J. Minian, an Open-Source Miniscope Analysis Pipeline. Elife 2022, 11, e70661. [Google Scholar] [CrossRef]
- Zhou, P.; Resendez, S.L.; Rodriguez-Romaguera, J.; Jimenez, J.C.; Neufeld, S.Q.; Giovannucci, A.; Friedrich, J.; Pnevmatikakis, E.A.; Stuber, G.D.; Hen, R.; et al. Efficient and Accurate Extraction of in Vivo Calcium Signals from Microendoscopic Video Data. Elife 2018, 7, e28728. [Google Scholar] [CrossRef]
- Lu, J.; Li, C.; Singh-Alvarado, J.; Zhou, Z.C.; Fröhlich, F.; Mooney, R.; Wang, F. MIN1PIPE: A Miniscope 1-Photon-Based Calcium Imaging Signal Extraction Pipeline. Cell Rep. 2018, 23, 3673–3684. [Google Scholar] [CrossRef]
- Kolar, K.; Dondorp, D.; Zwiggelaar, J.C.; Høyer, J.; Chatzigeorgiou, M. Mesmerize Is a Dynamically Adaptable User-Friendly Analysis Platform for 2D and 3D Calcium Imaging Data. Nat. Commun. 2021, 12, 6569. [Google Scholar] [CrossRef]
- Cantu, D.A.; Wang, B.; Gongwer, M.W.; He, C.X.; Goel, A.; Suresh, A.; Kourdougli, N.; Arroyo, E.D.; Zeiger, W.; Portera-Cailliau, C. EZcalcium: Open-Source Toolbox for Analysis of Calcium Imaging Data. Front. Neural Circuits 2020, 14, 25. [Google Scholar] [CrossRef]
- Osten, P.; Cetin, A.; Komai, S.; Eliava, M.; Seeburg, P.H. Stereotaxic Gene Delivery in the Rodent Brain. Nat. Protoc. 2007, 1, 3166–3173. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, B.; Barbera, G.; Hawes, S.; Zhang, Y.; Stump, K.; Baum, I.; Yang, Y.; Li, Y.; Lin, D.-T. Miniscope GRIN Lens System for Calcium Imaging of Neuronal Activity from Deep Brain Structures in Behaving Animals. Curr. Protoc. Neurosci. 2019, 86, e56. [Google Scholar] [CrossRef]
- Cotterill, E.; Hall, D.; Wallace, K.; Mundy, W.R.; Eglen, S.J.; Shafer, T.J. Characterization of Early Cortical Neural Network Development in Multiwell Microelectrode Array Plates. J. Biomol. Screen. 2016, 21, 510–519. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Erofeev, A.I.; Barinov, D.S.; Gerasimov, E.I.; Pchitskaya, E.I.; Bolsunovskaja, M.V.; Vlasova, O.L.; Bezprozvanny, I.B. NeuroInfoViewer: A Software Package for Analysis of Miniscope Data. Neurosci. Behav. Physiol. 2021, 51, 1199–1205. [Google Scholar] [CrossRef]
- Resendez, S.L.; Jennings, J.H.; Ung, R.L.; Namboodiri, V.M.K.; Zhou, Z.C.; Otis, J.M.; Nomura, H.; Mchenry, J.A.; Kosyk, O.; Stuber, G.D. Visualization of Cortical, Subcortical and Deep Brain Neural Circuit Dynamics during Naturalistic Mammalian Behavior with Head-Mounted Microscopes and Chronically Implanted Lenses. Nat. Protoc. 2016, 11, 566–597. [Google Scholar] [CrossRef]
- Jennings, J.H.; Ung, R.L.; Resendez, S.L.; Stamatakis, A.M.; Taylor, J.G.; Huang, J.; Veleta, K.; Kantak, P.A.; Aita, M.; Shilling-Scrivo, K.; et al. Visualizing Hypothalamic Network Dynamics for Appetitive and Consummatory Behaviors. Cell 2015, 160, 516–527. [Google Scholar] [CrossRef]
- Ingiosi, A.M.; Hayworth, C.R.; Harvey, D.O.; Singletary, K.G.; Rempe, M.J.; Wisor, J.P.; Frank, M.G. A Role for Astroglial Calcium in Mammalian Sleep and Sleep Regulation. Curr. Biol. 2020, 30, 4373–4383.e7. [Google Scholar] [CrossRef]
- Gerasimov, E.I.; Erofeev, A.I.; Pushkareva, S.A.; Barinov, D.S.; Bolsunovskaja, M.V.; Yang, X.; Yang, H.; Zhou, C.; Vlasova, O.L.; Li, W.; et al. Miniature Fluorescent Microscope: History, Application, and Data Processing. Zhurnal Vyss. Nervn. Deyatelnosti Im. I.P. Pavlov. 2020, 70, 852–864. [Google Scholar] [CrossRef]
- Giovannucci, A.; Friedrich, J.; Gunn, P.; Kalfon, J.; Brown, B.L.; Koay, S.A.; Taxidis, J.; Najafi, F.; Gauthier, J.L.; Zhou, P.; et al. Caiman an Open Source Tool for Scalable Calcium Imaging Data Analysis. Elife 2019, 8, e38173. [Google Scholar] [CrossRef]
- Kuznetsova, T.; Antos, K.; Malinina, E.; Papaioannou, S.; Medini, P. Visual Stimulation with Blue Wavelength Light Drives V1 Effectively Eliminating Stray Light Contamination during Two-Photon Calcium Imaging. J. Neurosci. Methods 2021, 362, 109287. [Google Scholar] [CrossRef]
- Murakami, T.; Yoshida, T.; Matsui, T.; Ohki, K. Wide-Field Ca2+ Imaging Reveals Visually Evoked Activity in the Retrosplenial Area. Front. Mol. Neurosci. 2015, 8, 20. [Google Scholar] [CrossRef]
- Barry, J.; Oikonomou, K.D.; Peng, A.; Yu, D.; Yang, C.; Golshani, P.; Evans, C.J.; Levine, M.S.; Cepeda, C. Dissociable Effects of Oxycodone on Behavior, Calcium Transient Activity, and Excitability of Dorsolateral Striatal Neurons. Front. Neural Circuits 2022, 16, 983323. [Google Scholar] [CrossRef]
- Bråthen, A.C.S.; Sørensen, Ø.; de Lange, A.M.G.; Mowinckel, A.M.; Fjell, A.M.; Walhovd, K.B. Cognitive and Hippocampal Changes Weeks and Years after Memory Training. Sci. Rep. 2022, 12, 7877. [Google Scholar] [CrossRef]
- Zhou, X.; Tien, R.N.; Ravikumar, S.; Chase, S.M. Distinct Types of Neural Reorganization during Long-Term Learning. J. Neurophysiol. 2019, 121, 1329–1341. [Google Scholar] [CrossRef]
- Kobayashi, K.S.; Matsuo, N. Persistent Representation of the Environment in the Hippocampus. Cell Rep. 2023, 42, 111989. [Google Scholar] [CrossRef]
- Beacher, N.J.; Washington, K.A.; Werner, C.T.; Zhang, Y.; Barbera, G.; Li, Y.; Lin, D.T. Circuit Investigation of Social Interaction and Substance Use Disorder Using Miniscopes. Front. Neural Circuits 2021, 15, 762441. [Google Scholar] [CrossRef]
- Kingsbury, L.; Huang, S.; Wang, J.; Gu, K.; Golshani, P.; Wu, Y.E.; Hong, W. Correlated Neural Activity and Encoding of Behavior across Brains of Socially Interacting Animals. Cell 2019, 178, 429–446.e16. [Google Scholar] [CrossRef]
- Ren, X.; Brodovskaya, A.; Hudson, J.L.; Kapur, J. Connectivity and Neuronal Synchrony during Seizures. J. Neurosci. 2021, 41, 7623–7635. [Google Scholar] [CrossRef]
- Hagemann, A.; Wilting, J.; Samimizad, B.; Mormann, F.; Priesemann, V. Assessing Criticality in Pre-Seizure Single-Neuron Activity of Human Epileptic Cortex. PLoS Comput. Biol. 2021, 17, e1008773. [Google Scholar] [CrossRef]
- Vico Varela, E.; Etter, G.; Williams, S. Excitatory-Inhibitory Imbalance in Alzheimer’s Disease and Therapeutic Significance. Neurobiol. Dis. 2019, 127, 605–615. [Google Scholar] [CrossRef]
- Shuman, T.; Aharoni, D.; Cai, D.J.; Lee, C.R.; Chavlis, S.; Page-Harley, L.; Vetere, L.M.; Feng, Y.; Yang, C.Y.; Mollinedo-Gajate, I.; et al. Breakdown of Spatial Coding and Interneuron Synchronization in Epileptic Mice. Nat. Neurosci. 2020, 23, 229–238. [Google Scholar] [CrossRef]
- Barry, J.; Peng, A.; Levine, M.S.; Cepeda, C. Calcium Imaging: A Versatile Tool to Examine Huntington’s Disease Mechanisms and Progression. Front. Neurosci. 2022, 16, 1040113. [Google Scholar] [CrossRef]
- Harris, S.S.; Wolf, F.; De Strooper, B.; Busche, M.A. Tipping the Scales: Peptide-Dependent Dysregulation of Neural Circuit Dynamics in Alzheimer’s Disease. J. Clean. Prod. 2020, 107, 417–435. [Google Scholar] [CrossRef]
- Radhiyanti, P.T.; Konno, A.; Matsuzaki, Y.; Hirai, H. Comparative Study of Neuron-Specific Promoters in Mouse Brain Transduced by Intravenously Administered AAV-PHP.EB. Neurosci. Lett. 2021, 756, 135956. [Google Scholar] [CrossRef]
- Finneran, D.J.; Njoku, I.P.; Flores-Pazarin, D.; Ranabothu, M.R.; Nash, K.R.; Morgan, D.; Gordon, M.N. Toward Development of Neuron Specific Transduction After Systemic Delivery of Viral Vectors. Front. Neurol. 2021, 12, 685802. [Google Scholar] [CrossRef]
- Hoshino, C.; Konno, A.; Hosoi, N.; Kaneko, R.; Mukai, R.; Nakai, J.; Hirai, H. GABAergic Neuron-Specific Whole-Brain Transduction by AAV-PHP.B Incorporated with a New GAD65 Promoter. Mol. Brain 2021, 14, 33. [Google Scholar] [CrossRef]
- Dimidschstein, J.; Chen, Q.; Tremblay, R.; Rogers, S.L.; Saldi, G.-A.; Guo, L.; Xu, Q.; Liu, R.; Lu, C.; Chu, J.; et al. A Viral Strategy for Targeting and Manipulating Interneurons across Vertebrate Species. Nat. Neurosci. 2016, 19, 1743–1749. [Google Scholar] [CrossRef]
- Tchumatchenko, T.; Geisel, T.; Volgushev, M.; Wolf, F. Spike Correlations—What Can They Tell about Synchrony? Front. Neurosci. 2011, 5, 68. [Google Scholar] [CrossRef]
- Cohen, M.R.; Kohn, A. Measuring and Interpreting Neuronal Correlations. Nat. Neurosci. 2011, 14, 811–819. [Google Scholar] [CrossRef]
- Frost, N.A.; Haggart, A.; Sohal, V.S. Dynamic Patterns of Correlated Activity in the Prefrontal Cortex Encode Information about Social Behavior. PLoS Biol. 2021, 19, e3001235. [Google Scholar]
- Narayanan, N.S.; Laubach, M. Methods for Studying Functional Interactions among Neuronal Populations. Methods Mol. Biol. 2009, 489, 135–165. [Google Scholar]






| No. | Parameter | Meaning | Module Location |
|---|---|---|---|
| 1 | spike | Method for the active-phase detection as a sharply rising stage of calcium-indicator intensity | ActiveStateAnalyzer |
| 2 | full | Method for the active-phase detection as a part above calculated threshold value | ActiveStateAnalyzer |
| 3 | signal | Method for the detection of the active phase as a whole initial signal of intensity (applicable to Pearson’s coefficient calculations and other connected metrics and distance analysis) | ActiveStateAnalyzer, Distance analysis |
| 4 | warm | Minimal duration of the fluorescence-signal passive phase (can be varied from 0 to 100), number of frames | ActiveStateAnalyzer |
| 5 | cold | Minimal duration of the fluorescence-signal active phase (can be varied from 0 to 100), number of frames | ActiveStateAnalyzer |
| 6 | window | Configurable value for fluorescent signal smoothing, number of pixels | ActiveStateAnalyzer |
| 7 | burst rate | Number of “cell activations” for a set period of time, percent of neurons with the given number of activations | ActiveStateAnalyzer |
| 8 | single neuron “activations” | Number of single neuron activations per minute (can be obtained via saving the Burst rate as a “.xlsx”), number of activations per minute | ActiveStateAnalyzer |
| 9 | network spike rate | Percent of active neurons over a certain period of time, % | ActiveStateAnalyzer |
| 10 | network spike peak | Maximal number of simultaneously active cells for a certain period of time, % | ActiveStateAnalyzer |
| 11 | network spike duration | Time length in which the number of simultaneously active cells is higher than the preset threshold value, percent of total time | ActiveStateAnalyzer |
| 12 | Pearson’s coefficient of correlation | Calculated for the intensity of the original signal(signal), the intensity derivative (diff), binary results of active-phase segmentation (active or full) method, coefficient of linear pairwise correlation, and connection of intersection (active_acc, relation of the simultaneously active states duration to the sum of the both of neurons activity time) | ActiveStateAnalyzer, Distance analysis |
| 13 | lag | Maximal delay value between neuronal activations, frames | ActiveStateAnalyzer |
| 14 | network degree | Percent of co-active neuronal pairs above the threshold level, % | ActiveStateAnalyzer |
| 15 | connectivity | Distribution of the connectivity shares for each neuron, % | ActiveStateAnalyzer |
| 16 | mean correlation range | Difference between the maximal and minimal value of correlation | MultipleShuffling |
| 17 | rho | Distance to neurons from the center of their mass in polar coordinates, pixels | Distance analysis |
| 18 | Euclidean | Distance between co-active neuronal pairs, pixels | Distance analysis |
| 19 | radial | Difference in distances between co-active pairs of neurons from the mass center of all neurons for recording, pixels | Distance analysis |
| 20 | transfer entropy | The entropy of transfer from neuron X to another neuron Y is the amount of uncertainty reduced in future values of Y by knowing the past values of X, providing the corresponding past values of Y (metric to apply or not for PCA analysis (step 2)) | Dimensionality reduction |
| No. | Statistical Metric | Possible Biological Interpretation |
|---|---|---|
| 1 | single neuron “activations” and burst rate | Describes a total number of neuronal activations at the single-cell level and as a total activity of the whole network. It can be used for the comparison of the neuronal network state, in particular conditions or pathological states, for validation of the hypo- or hyperactivation profile of the brain region. Also, it can be used as a trivial marker of agonist/antagonist action on neuronal excitation levels. |
| 2 | network spike rate | Neuronal network excitation levels could be described by these metrics. Analyzing shifts in the distributions might provide complex information about changes in the firing rate of all neurons that are part of the network. It is a more sophisticated and informative way to validate differences in activation profiles observed in the distinct area of the brain, which is often affected by various pathologies. |
| 3 | network spike peak | |
| 4 | network spike duration | Time duration in which more than a set percent of neurons was active in the neuronal network. This metric is tightly bound to the ones mentioned above; nevertheless, it explicitly reflects an elongation/reduction in the total neuronal activity duration, which might indicate changes in the excitation or elevation/decrease in the synchronically firing pattern shifts of the distinct brain region. |
| 5 | Pearson’s coefficient of correlation | The similarity in the activation patterns between neurons can be reflected as a correlation coefficient. On the one hand, the disruption of the synaptic plasticity processes is a hallmark of various neuropathologies, for example, neurodegenerative diseases. Correlation coefficient evaluation with changing levels of strictness might be a promising way to determine early changes in the prodromal stage of diseases. On the other hand, processes of learning, adaptation, etc., are also connected with pairwise neuronal correlations as new pairs appear and others vanish. Such reorganization might be possibly expressed in the elevation or decrease in the mean value of Pearson’s coefficient with a set threshold value. |
| 6 | network degree | |
| 7 | shuffled neuronal activity | This module is performed to determine the regularity of the statistics obtained (they have a biological/physiological nature) or if they are random variables. In this module, the number of activations is kept constant for each neuron, while the duration of active states and the duration between them are determined randomly. |
| 8 | distance between coactive neurons (Euclidian or radial) | The evaluation of the reorganization of the neuronal network during applied stimuli or specific conditions. Investigation of the architecture of neuronal coactive pairs and its regularity for defined areas of the brain. |
| 9 | principal component analysis applied to calculated metrics | PCA method for obtained statistical-metric clustering for determining differences in the total neuronal network state as a response to external shifts, processes of learning, etc. Might be a powerful tool for early-stage estimations of changes during pathological processes at the total neuronal network level. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerasimov, E.; Mitenev, A.; Pchitskaya, E.; Chukanov, V.; Bezprozvanny, I. NeuroActivityToolkit—Toolbox for Quantitative Analysis of Miniature Fluorescent Microscopy Data. J. Imaging 2023, 9, 243. https://doi.org/10.3390/jimaging9110243
Gerasimov E, Mitenev A, Pchitskaya E, Chukanov V, Bezprozvanny I. NeuroActivityToolkit—Toolbox for Quantitative Analysis of Miniature Fluorescent Microscopy Data. Journal of Imaging. 2023; 9(11):243. https://doi.org/10.3390/jimaging9110243
Chicago/Turabian StyleGerasimov, Evgenii, Alexander Mitenev, Ekaterina Pchitskaya, Viacheslav Chukanov, and Ilya Bezprozvanny. 2023. "NeuroActivityToolkit—Toolbox for Quantitative Analysis of Miniature Fluorescent Microscopy Data" Journal of Imaging 9, no. 11: 243. https://doi.org/10.3390/jimaging9110243
APA StyleGerasimov, E., Mitenev, A., Pchitskaya, E., Chukanov, V., & Bezprozvanny, I. (2023). NeuroActivityToolkit—Toolbox for Quantitative Analysis of Miniature Fluorescent Microscopy Data. Journal of Imaging, 9(11), 243. https://doi.org/10.3390/jimaging9110243






