Radiofrequency Echographic Multispectrometry (REMS): A New Option in the Assessment Bone Status in Adults with Osteogenesis Imperfecta
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Biochemical Parameters
2.3. Bone Mineral Density Measurement Using DXA
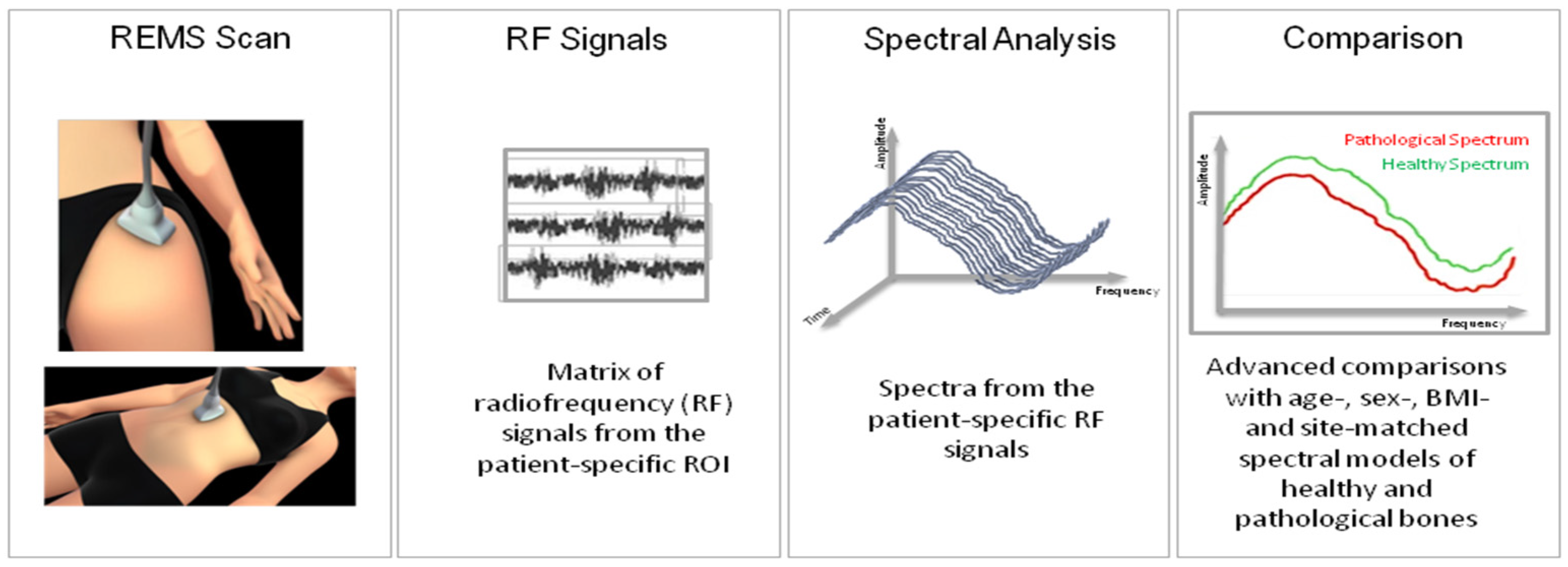
2.4. Bone Mineral Density Measurement Using REMS
2.5. Statistical Analysis
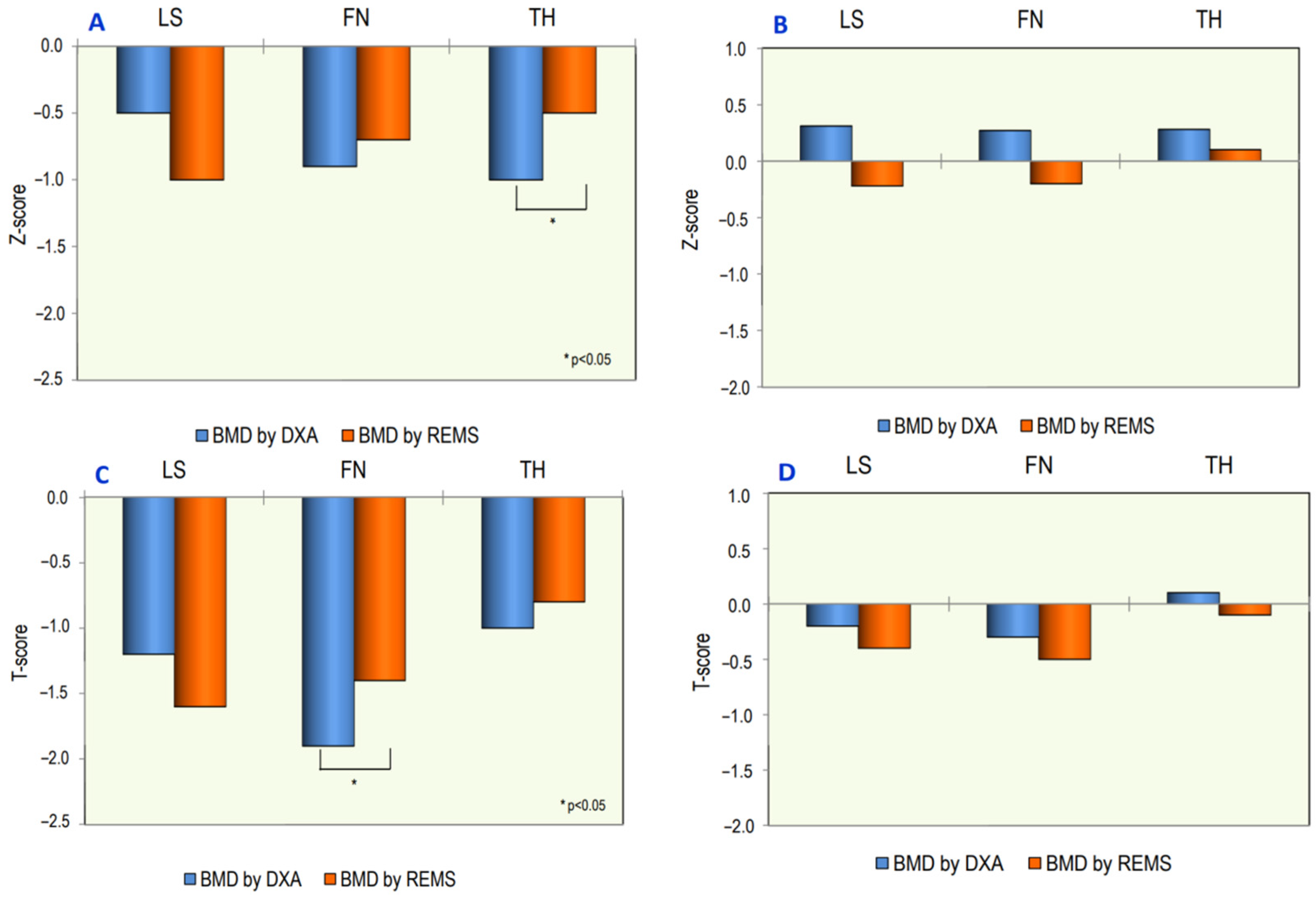
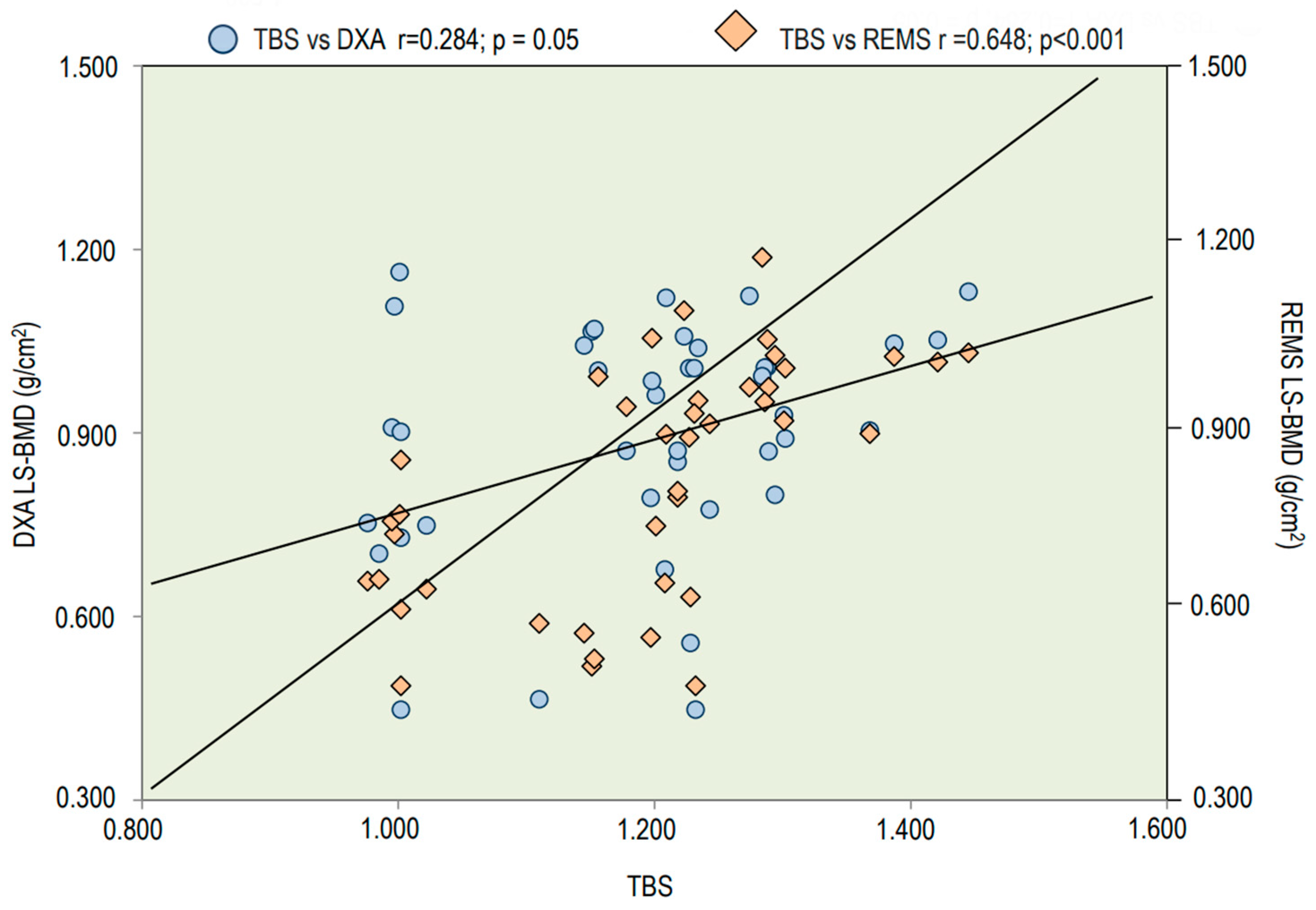
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marini, J.C.; Forlino, A.; Bächinger, H.P.; Bishop, N.J.; Byers, P.H.; Paepe, A.; Fassier, F.; Fratzl-Zelman, N.; Kozloff, K.M.; Krakow, D.; et al. Osteogenesis imperfecta. Nat. Rev. Dis. Prim. 2017, 18, 17052. [Google Scholar] [CrossRef] [PubMed]
- Besio, R.; Chow, C.W.; Tonelli, F.; Marini, J.C.; Forlino, A. Bone biology: Insights from osteogenesis imperfecta and related rare fragility syndromes. FEBS J. 2019, 286, 3033–3056. [Google Scholar] [CrossRef] [PubMed]
- Sillence, D.O.; Senn, A.; Danks, D.M. Genetic heterogeneity in osteogenesis imperfecta. J. Med. Genet. 1979, 16, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Paterson, C.R.; McAllion, S.; Stellman, J.L. Osteogenesis imperfecta after the menopause. N. Engl. J. Med. 1984, 28, 1694–1696. [Google Scholar] [CrossRef] [PubMed]
- Folkestad, L.; Hald, J.D.; Ersbøll, A.K.; Gram, J.; Hermann, A.P.; Langdahl, B.; Abrahamsen, B.; Brixen, K. Fracture Rates and Fracture Sites in Patients with Osteogenesis Imperfecta: A Nationwide Register-Based Cohort Study. J. Bone Min. Res. 2017, 32, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Gatti, D.; Colapietro, F.; Fracassi, E.; Sartori, E.; Antoniazzi, F.; Braga, V.; Rossini, M.; Adami, S. The Volumetric Bone Density and Cortical Thickness in adult patients Affected by Osteogenesis Imperfecta. J. Clin. Densitom. 2003, 6, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Vardakastani, V.; Saletti, D.; Skalli, W.; Marry, P.; Allain, J.M.; Adam, C. Increased intra-cortical porosity reduces bone stiffness and strength in pediatric patients with osteogenesis imperfecta. Bone 2014, 69, 61–67. [Google Scholar] [CrossRef]
- Nijhuis, W.H.; Eastwood, D.M.; Allgrove, J.; Hvid, I.; Weinans, H.H.; Bank, R.A.; Sakkers, R.J. Current concepts in osteogenesis imperfecta: Bone structure, biomechanics and medical management. J. Child. Orthop. 2019, 1, 1–11. [Google Scholar] [CrossRef]
- Cepollaro, C.; Gonnelli, S.; Pondrelli, C.; Montagnani, A.; Martini, S.; Bruni, D.; Gennari, C. Osteogenesis Imperfecta: Bone Turnover, Bone Density, and Ultrasound Parameters. Calcif. Tissue Int. 1999, 65, 129–132. [Google Scholar] [CrossRef]
- Braga, V.; Gatti, D.; Rossini, M.; Colapietro, F.; Battaglia, E.; Viapiana, O.; Adami, S. Bone turnover markers in patients with osteogenesis imperfecta. Bone 2004, 34, 1013–1016. [Google Scholar] [CrossRef]
- Kocijan, R.; Muschitz, C.; Haschka, J.; Hans, D.; Nia, A.; Geroldinger, A.; Ardelt, M.; Wakolbinger, R.; Resch, H. Bone structure assessed by HR-pQCT, TBS and DXL in adult patients with different types of osteogenesis imperfecta. Osteoporos. Int. 2015, 26, 2431–2440. [Google Scholar] [CrossRef] [PubMed]
- Scheres, L.J.J.; van Dijk, F.S.; Harsevoort, A.J.; van Dijk, A.T.H.; Dommisse, A.M.; Janus, G.J.M.; Franken, A.A.M. Adults with osteogenesis imperfecta: Clinical characteristics of 151 patients with a focus on bisphosphonate use and bone density measurements. Bone Rep. 2018, 25, 168–172. [Google Scholar] [CrossRef]
- Ulivieri, F.M.; Silva, B.C.; Sardanelli, F.; Hans, D.; Bilezikian, J.P.; Caudarella, R. Utility of the trabecular bone score (TBS) in secondary osteoporosis. Endocrine 2014, 47, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Conversano, F.; Franchini, R.; Greco, A.; Soloperto, G.; Chiriacò, F.; Casciaro, E.; Aventaggiato, M.; Renna, M.D.; Pisani, P.; Di Paola, M.; et al. A novel ultrasound methodology for estimating spine mineral density. Ultrasound Med. Biol. 2015, 41, 281–300. [Google Scholar] [CrossRef] [PubMed]
- Messina, C.; Gitto, S.; Colombo, R.; Fusco, S.; Guagliardo, G.; Piazza, M.; Poli, J.C.; Albano, D.; Sconfienza, L.M. Short-Term Precision and Repeatability of Radiofrequency Echographic Multi Spectrometry (REMS) on Lumbar Spine and Proximal Femur: An In Vivo Study. J. Imaging 2023, 9, 118. [Google Scholar] [CrossRef]
- Al Refaie, A.; Baldassini, L.; Mondillo, C.; Giglio, E.; De Vita, M.; Tomai Pitinca, M.D.; Gonnelli, S.; Caffarelli, C. Radiofrequency Echographic Multi Spectrometry (R.E.M.S.): New Frontiers for Ultrasound Use in the Assessment of Bone Status-A Current Picture. Diagnostics 2023, 13, 1666. [Google Scholar] [CrossRef] [PubMed]
- Pisani, P.; Conversano, F.; Muratore, M.; Adami, G.; Brandi, M.L.; Caffarelli, C.; Casciaro, E.; Di Paola, M.; Franchini, R.; Gatti, D.; et al. Fragility Score: A REMS-based indicator for the prediction of incident fragility fractures at 5 years. Aging Clin. Exp. Res. 2023, 35, 763–773. [Google Scholar] [CrossRef]
- Caffarelli, C.; Tomai Pitinca, M.D.; Al Refaie, A.; De Vita, M.; Catapano, S.; Gonnelli, S. Could radiofrequency echographic multispectrometry (REMS) overcome the overestimation in BMD by dual-energy X-ray absorptiometry (DXA) at the lumbar spine? BMC Musculoskelet. Disord. 2022, 19, 469. [Google Scholar] [CrossRef]
- Caffarelli, C.; Tomai Pitinca, M.D.; Al Refaie, A.; Ceccarelli, E.; Gonnelli, S. Ability of radiofrequency echographic multispectrometry to identify osteoporosis status in elderly women with type 2 diabetes. Aging Clin. Exp. Res. 2021, 34, 121–127. [Google Scholar] [CrossRef]
- International Society for Clinical Densitometry Official Positions—Adult. 2019. Available online: http://www.iscd.org/official-positions/2019-iscd-official-positions-adult/ (accessed on 1 December 2021).
- Di Paola, M.; Gatti, D.; Viapiana, O.; Cianferotti, L.; Cavalli, L.; Caffarelli, C.; Conversano, F.; Quarta, E.; Pisani, P.; Girasole, G.; et al. Radiofrequency echographic multispectrometry compared with dual X-ray absorptiometry for osteoporosis diagnosis on lumbar spine and femoral neck. Osteoporos. Int. 2019, 30, 391–402. [Google Scholar] [CrossRef]
- Thorby-Lister, A.; Högler, W.; Hodgson, K.; Crabtree, N.; Nightingale, P.; Shaw, N.; Saraff, V. Cumulative radiation exposure from medical imaging and associated lifetime cancer risk in children with osteogenesis imperfecta. Bone 2018, 114, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Degennaro, V.A.; Brandi, M.L.; Cagninelli, G.; Casciaro, S.; Ciardo, D.; Conversano, F.; Di Pasquo, E.; Gonnelli, S.; Lombardi, F.A.; Pisani, P.; et al. First assessment of bone mineral density in healthy pregnant women by means of Radiofrequency Echographic Multi Spectrometry (REMS) technology. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 263, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Caffarelli, C.; Al Refaie, A.; De Vita, M.; Tomai Pitinca, M.D.; Goracci, A.; Fagiolini, A.; Gonnelli, S. Radiofrequency echographic multispectrometry (REMS): An innovative technique for the assessment of bone status in young women with anorexia nervosa. Eat. Weight. Disord. 2022, 27, 3207–3213. [Google Scholar] [CrossRef] [PubMed]
- Wekre, L.L.; Eriksen, E.F.; Falch, J.A. Bone mass, bone markers and prevalence of fractures in adults with osteogenesis imperfecta. Arch. Osteoporos. 2011, 6, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Hans, D.; Barthe, N.; Boutroy, S.; Pothuaud, L.; Winzenrieth, R.; Krieg, M.A. Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: An experimental study on human cadaver vertebrae. J. Clin. Densitom. 2011, 14, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.C.; Walker, M.D.; Abraham, A.; Boutroy, S.; Zhang, C.; McMahon, D.J.; Liu, G.; Hans, D.; Bilezikian, J.P. Trabecular bone score is associated with volumetric bone density and microarchitecture as assessed by central QCT and HRpQCT in Chinese-American and White women. J. Clin. Densitom. 2013, 16, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Leslie, W.D.; Aubry-Rozier, B.; Lix, L.M.; Morin, S.N.; Majumdar, S.R.; Hans, D. Spine bone texture assessed by trabecular bone score (TBS) predicts osteoporotic fractures in men: The Manitoba Bone Density Program. Bone 2014, 67, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Kocijan, R.; Muschitz, C.; Fratzl-Zelman, N.; Haschka, J.; Dimai, H.P.; Trubrich, A.; Bittighofer, C.; Resch, H. Femoral geometric parameters and BMD measurements by DXA in adult patients with different types of osteogenesis imperfecta. Skelet. Radiol. 2013, 42, 187–194. [Google Scholar] [CrossRef]
- Hald, J.D.; Folkestad, L.; Harsløf, T.; Lund, A.M.; Duno, M.; Jensen, J.B.; Neghabat, S.; Brixen, K.; Langdahl, B. Skeletal phenotypes in adult patients with osteogenesis imperfecta-correlations with COL1A1/COL1A2 genotype and collagen structure. Osteoporos. Int. 2016, 27, 3331–3341. [Google Scholar] [CrossRef]
- Coussens, M.; Lapauw, B.; Verroken, C.; Goemaere, S.; De Wandele, I.; Malfait, F.; Banica, T.; Calders, P. Bone Mass, Density, Geometry, and Stress-Strain Index in Adults with Osteogenesis Imperfecta Type I and Their Associations with Physical Activity and Muscle Function Parameters. J. Bone Miner. Res. 2022, 37, 2456–2465. [Google Scholar] [CrossRef]
- Ben Amor, I.M.; Roughley, P.; Glorieux, F.H.; Rauch, F. Skeletal clinical characteristics of osteogenesis imperfecta caused by haploinsufficiency mutations in COL1A1. J. Bone Miner. Res. 2013, 28, 2001–2007. [Google Scholar] [CrossRef]
- Krege, J.H.; Kendler, D.; Krohn, K.; Genant, H.; Alam, J.; Berclaz, P.Y.; Coffey, B.; Loghin, C. Relationship between vertebral fracture burden, height loss, and pulmonary function in postmenopausal women with osteoporosis. J. Clin. Densitom. 2015, 18, 506–511. [Google Scholar] [CrossRef]


| Osteogenesis Imperfecta (N = 41) | Controls (N = 36) | p | |
|---|---|---|---|
| Age (years) | 40.5 ± 18.7 | 41.7 ± 16.3 | n.s. |
| Weight (kg) | 61.2 ± 16.2 | 71.5 ± 12.2 | 0.01 |
| Height (cm) | 152.6 ± 19.5 | 169.5 ± 7.6 | 0.01 |
| BMI (kg/m2) | 26.1 ± 6.9 | 24.7 ± 2.9 | n.s. |
| Creatinine (mg/dL) | 0.9 ± 0.3 | 0.9 ± 0.2 | n.s. |
| Calcium (mg/dL) | 9.4 ± 0.4 | 9.2 ± 0.5 | n.s. |
| Phosphate (mg/dL) | 3.3 ± 0.6 | 3.4 ± 0.5 | n.s. |
| 25OHD (ng/mL) | 38.0 ± 9.9 | 24.4 ± 8.9 | 0.05 |
| PTH (pg/mL) | 34.3 ± 19.7 | 36.8 ± 17.9 | n.s. |
| B-ALP (µg/L) | 13.2 ± 6.2 | 15.2 ± 8.9 | n.s. |
| β-CTX (ng/mL) | 0.377 ± 0.180 | 0.405 ± 0.142 | n.s. |
| DXA LS-BMD (g/cm2) | 0.906 ± 0.201 | 1.083 ± 0.126 | 0.001 |
| DXA FN-BMD (g/cm2) | 0.724 ± 0.168 | 0.886 ± 0.126 | 0.001 |
| DXA TH-BMD (g/cm2) | 0.789 ± 0.206 | 0.989 ± 0.112 | 0.001 |
| TBS | 1.209 ± 0.127 | 1.412 ± 0.100 | 0.001 |
| REMS LS-BMD (g/cm2) | 0.885 ± 0.105 | 1.000 ± 0.072 | 0.003 |
| REMS FN-BMD (g/cm2) | 0.714 ± 0.120 | 0.810 ± 0.093 | 0.001 |
| REMS TH-BMD (g/cm2) | 0.880 ± 0.132 | 0.955 ± 0.103 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caffarelli, C.; Al Refaie, A.; Mondillo, C.; Versienti, A.; Baldassini, L.; De Vita, M.; Tomai Pitinca, M.D.; Gonnelli, S. Radiofrequency Echographic Multispectrometry (REMS): A New Option in the Assessment Bone Status in Adults with Osteogenesis Imperfecta. J. Imaging 2023, 9, 210. https://doi.org/10.3390/jimaging9100210
Caffarelli C, Al Refaie A, Mondillo C, Versienti A, Baldassini L, De Vita M, Tomai Pitinca MD, Gonnelli S. Radiofrequency Echographic Multispectrometry (REMS): A New Option in the Assessment Bone Status in Adults with Osteogenesis Imperfecta. Journal of Imaging. 2023; 9(10):210. https://doi.org/10.3390/jimaging9100210
Chicago/Turabian StyleCaffarelli, Carla, Antonella Al Refaie, Caterina Mondillo, Alessandro Versienti, Leonardo Baldassini, Michela De Vita, Maria Dea Tomai Pitinca, and Stefano Gonnelli. 2023. "Radiofrequency Echographic Multispectrometry (REMS): A New Option in the Assessment Bone Status in Adults with Osteogenesis Imperfecta" Journal of Imaging 9, no. 10: 210. https://doi.org/10.3390/jimaging9100210
APA StyleCaffarelli, C., Al Refaie, A., Mondillo, C., Versienti, A., Baldassini, L., De Vita, M., Tomai Pitinca, M. D., & Gonnelli, S. (2023). Radiofrequency Echographic Multispectrometry (REMS): A New Option in the Assessment Bone Status in Adults with Osteogenesis Imperfecta. Journal of Imaging, 9(10), 210. https://doi.org/10.3390/jimaging9100210








