When Not to Operate on Acute Cases—A Surgeon’s Perspective on Rapid Assessment of Emergency Abdominopelvic Computed Tomography
Abstract
1. Introduction
2. The Algorithm for Clinical Decisions Concerning Abdominal Pain
3. The Need for Standardization of Search Patterns in Emergency Abdominopelvic CT
4. Map Charting, Image Modulators, and Discovery of Cues
5. Merging Clinical Pathways with Radiological Cues
5.1. Acute Appendicitis
5.2. Acute Pancreatitis
5.3. Bowel Obstruction
5.4. Acute Cholecystitis
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Custers, E.J.F.M. Chapter 2: Training Clinical Reasoning: Historical and Theoretical Background. In Principles and Practice of Case-Based Clinical Reasoning Education: A Method for Preclinical Students; ten Cate, O., Custers, E.J.F.M., Durning, S.J., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Maude, J. Differential diagnosis: The key to reducing diagnosis error, measuring diagnosis and a mechanism to reduce healthcare costs. Diagnosis 2014, 1, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.A.; Stein, J.A.; Pelc, N.J. How CT happened: The early development of medical computed tomography. J. Med. Imaging 2021, 8, 052110. [Google Scholar] [CrossRef] [PubMed]
- de Burlet, K.; Lam, A.; Larsen, P.; Dennett, E. Acute abdominal pain-changes in the way we assess it over a decade. N. Zeal. Med. J. 2017, 130, 39–44. [Google Scholar] [PubMed]
- Loftus, S. Rethinking clinical reasoning: Time for a dialogical turn. Med. Educ. 2012, 46, 1174–1178. [Google Scholar] [CrossRef]
- Yazdani, S.; Hoseini Abardeh, M. Five decades of research and theorization on clinical reasoning: A critical review. Adv. Med. Educ. Pract. 2019, 10, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Elstein, A.S.; Shulman, L.S.; Sprafka, S.A. Medical problem solving a ten-year retrospective. Eval. Health Prof. 1990, 13, 5–36. [Google Scholar] [CrossRef]
- Eddy, D.M.; Clanton, C.H. The Art of Diagnosis. N. Engl. J. Med. 1982, 306, 1263–1268. [Google Scholar] [CrossRef]
- Adamo, S.H.; Cain, M.S.; Mitroff, S.R. An individual differences approach to multiple-target visual search errors: How search errors relate to different characteristics of attention. Vis. Res. 2017, 141, 258–265. [Google Scholar] [CrossRef]
- Smith, M.J. Error and Variation in Diagnostic Radiology; C.C. Thomas: Springfield, IL, USA, 1967. [Google Scholar]
- Kliewer, M.A.; Bagley, A.R. How to Read an Abdominal CT: Insights from the Visual and Cognitive Sciences Translated for Clinical Practice. Curr. Probl. Diagn. Radiol. 2022, 51, 639–647. [Google Scholar] [CrossRef]
- Wolfe, J.; Horowitz, T.; Kenner, N. Rare items often missed in visual searches. Nature 2005, 435, 439–440. [Google Scholar] [CrossRef]
- Wolfe, J.M.; Van Wert, M.J. Varying target prevalence reveals two dissociable decision criteria in visual search. Curr. Biol. 2010, 20, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Reicher, M.A.; Wolfe, J.M. Let’s Use Cognitive Science to Create Collaborative Workstations. J. Am. Coll. Radiol. 2016, 13, 571–575. [Google Scholar] [CrossRef][Green Version]
- Leslie, A.; Jones, A.J.; Goddard, P.R. The influence of clinical information on the reporting of CT by radiologists. Br. J. Radiol. 2000, 73, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Drew, T.; Vo, M.L.; Olwal, A.; Jacobson, F.; Seltzer, S.E.; Wolfe, J.M. Scanners and drillers: Characterizing expert visual search through volumetric images. J. Vis. 2013, 13, 3. [Google Scholar] [CrossRef]
- Kliewer, M.A.; Hartung, M.; Green, C.S. The Search Patterns of Abdominal Imaging Subspecialists for Abdominal Computed Tomography: Toward a Foundational Pattern for New Radiology Residents. J. Clin. Imaging Sci. 2021, 11, 1. [Google Scholar] [CrossRef]
- Van der Gijp, A.; van der Schaaf, M.F.; van der Schaaf, I.C.; Huige, J.C.B.M.; Ravesloot, C.J.; van Schaik, J.P.J.; ten Cate, T.J. Interpretation of radiological images: Towards a framework of knowledge and skills. Adv. Health Sci. Educ. 2014, 19, 565–580. [Google Scholar] [CrossRef]
- Parag, P.; Hardcastle, T.C. Interpretation of emergency CT scans in polytrauma: Trauma surgeon vs. radiologist. Afr. J. Emerg. Med. 2020, 10, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Norman, G.R.; Coblentz, C.L.; Brooks, L.R.; Babcook, C.J. Expertise in visual diagnosis: A review of the literature. Acad. Med. 1992, 67, S78–S83. [Google Scholar] [CrossRef]
- Parker, R.; Bedwell, G.J.; Hodkinson, P.; Lourens, A.; Setshedi, M. Managing acute abdominal pain in the emergency centre: Lessons from a patient’s experience. Afr. J. Emerg. Med. Rev. Afr. Med. D’urgence 2021, 11, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Kamin, R.A.; Nowicki, T.A.; Courtney, D.S.; Powers, R.D. Pearls and pitfalls in the emergency department evaluation of abdominal pain. Emerg. Med. Clin. N. Am. 2003, 21, 61–72. [Google Scholar] [CrossRef]
- Silva, H.S.; Oliveira, F.K.F.; Prado, L.O.M.; Almeida-Santos, M.; Reis, F.P. Abdominal Computed Tomography in the Emergency Room: Overuse of Medical Technologies and the Depreciation of Clinical Diagnosis. Rev. Bras. Educ. Méd. 2019, 43, 498–504. [Google Scholar] [CrossRef]
- Bhatt, A.; Yang, X.; Karnik, N.; Sill, A.; Kowdley, G. Use of Computerized Tomography in Abdominal Pain. Am. Surg. 2018, 84, 1091–1096. [Google Scholar] [CrossRef]
- De Burlet, K.J.; MacKay, M.; Larsen, P.; Dennett, E.R. Appropriateness of CT scans for patients with non-traumatic acute abdominal pain. Br. J. Radiol. 2018, 91, 20180158. [Google Scholar] [CrossRef] [PubMed]
- Koontz, N.A.; Gunderman, R.B. Gestalt theory: Implications for radiology education. Am. J. Roentgenol. 2008, 190, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Wagemans, J.; Elder, J.H.; Kubovy, M.; Palmer, S.E.; Peterson, M.A.; Singh, M.; von der Heydt, R. A century of Gestalt psychology in visual perception: I. Perceptual grouping and figure-ground organization. Psychol. Bull. 2012, 138, 1172–1217. [Google Scholar] [CrossRef] [PubMed]
- Wagemans, J.; Feldman, J.; Gepshtein, S.; Kimchi, R.; Pomerantz, J.R.; van der Helm, P.A.; van Leeuwen, C. A century of Gestalt psychology in visual perception: II. Conceptual and theoretical foundations. Psychol. Bull. 2012, 138, 1218–1252. [Google Scholar] [CrossRef]
- Jung, H. Basic physical principles and clinical applications of computed tomography. Prog. Med. Phys. 2021, 32, 1–17. [Google Scholar] [CrossRef]
- DenOtter, T.D.; Schubert, J. Hounsfield Unit. In StatPearls; Updated on 6 March 2023; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Pickhardt, P.J. Positive oral contrast material for abdominal CT: Current clinical indications and areas of controversy. Am. J. Roentgenol. 2020, 215, 69–78. [Google Scholar] [CrossRef]
- American College of Radiology. ACR Appropriateness Criteria. Available online: https://www.acr.org/Clinical-Resources/ACR-Appropriateness-Criteria (accessed on 1 September 2020).
- Radetic, M.; DeVita, R.; Haaga, J. When is contrast needed for abdominal and pelvic CT? Cleve. Clin. J. Med. 2020, 87, 595–598. [Google Scholar] [CrossRef]
- De Muzio, F.; Cutolo, C.; Granata, V.; Fusco, R.; Ravo, L.; Maggialetti, N.; Brunese, M.C.; Grassi, R.; Grassi, F.; Bruno, F.; et al. CT study protocol optimization in acute non-traumatic abdominal settings. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 860–878. [Google Scholar]
- Almushayti, Z.; Alsalhi, A.; Alsagri, M.; Alqarzaee, R.; Alsuwaidan, H.; Alolayan, F.; Alosaimi, H.T.; Alhussain, S.; Almushayqih, A.; Alolayan, S.; et al. Abnormal CT findings among patients with abdominal pain in the radiology department of a tertiary care center. F1000 Res. 2022, 11, 1113. [Google Scholar] [CrossRef]
- Güven, R.; Akça, A.H.; Çaltılı, Ç.; Sasmaz, M.I.; Kaykısız, E.K.; Baran, S.; Şahin, L.; Arı, A.; Eyüpoğlu, G.; Kırpat, V. Comparing the interpretation of emergency department computed tomography between emergency physicians and attending radiologists: A multicenter study. Niger. J. Clin. Pract. 2018, 21, 1323–1329. [Google Scholar] [CrossRef]
- Andersson, R.E.; Petzold, M.G. Nonsurgical treatment of appendiceal abscess or phlegmon: A systematic review and meta-analysis. Ann. Surg. 2007, 246, 741–748. [Google Scholar] [CrossRef]
- Al-Tarakji, M.; Zarour, A.; Singh, R.; Ghali, M.S. The Role of Alvarado Score in Predicting Acute Appendicitis and Its Severity in Correlation to Histopathology: A Retrospective Study in a Qatar Population. Cureus 2022, 14, e26902. [Google Scholar] [CrossRef]
- Brănescu, C.; Serban, D.; Dascălu, A.M.; Oprescu, S.M.; Savlovschi, C. Interleukin 6 and lipopolysaccharide binding protein—Markers of inflammation in acute appendicitis. Chirurgia 2013, 108, 206–214. [Google Scholar]
- Pelin, M.; Paquette, B.; Revel, L.; Landecy, M.; Bouveresse, S.; Delabrousse, E. Acute appendicitis: Factors associated with inconclusive ultrasound study and the need for additional computed tomography. Diagn. Interv. Imaging 2018, 99, 809–814. [Google Scholar] [CrossRef]
- Crocker, C.; Akl, M.; Abdolell, M.; Kamali, M.; Costa, A.F. Ultrasound and CT in the Diagnosis of Appendicitis: Accuracy with Consideration of Indeterminate Examinations According to STARD Guidelines. Am. J. Roentgenol. 2020, 215, 639–644. [Google Scholar] [CrossRef]
- Jones, M.W.; Lopez, R.A.; Deppen, J.G. Appendicitis. In StatPearls; Updated on 24 April 2023; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Jiang, J.; Wu, Y.; Tang, Y.; Shen, Z.; Chen, G.; Huang, Y.; Zheng, S.; Zheng, Y.; Dong, R. A novel nomogram for the differential diagnosis between advanced and early appendicitis in pediatric patients. Biomark. Med. 2019, 13, 1157–1173. [Google Scholar] [CrossRef]
- Lam, R.; Zakko, A.; Petrov, J.C.; Kumar, P.; Duffy, A.J.; Muniraj, T. Gallbladder Disorders: A Comprehensive Review. Dis. Mon. 2021, 67, 101130. [Google Scholar] [CrossRef]
- Iannuzzi, J.P.; King, J.A.; Leong, J.H.; Quan, J.; Windsor, J.W.; Tanyingoh, D.; Coward, S.; Forbes, N.; Heitman, S.J.; Shaheen, A.A.; et al. Global Incidence of Acute Pancreatitis Is Increasing Over Time: A Systematic Review and Meta-Analysis. Gastroenterology 2022, 162, 122–134. [Google Scholar] [CrossRef]
- Fonseca Sepúlveda, E.V.; Guerrero-Lozano, R. Acute pancreatitis and recurrent acute pancreatitis: An exploration of clinical and etiologic factors and outcomes. J. Pediatr. 2019, 95, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Şavlovschi, C.; Comandaşu, M.; Şerban, D. Specifics of diagnosis and treatment in synchronous colorectal cancers (SCC). Chirurgia 2013, 108, 43–45. [Google Scholar] [PubMed]
- Navadgi, S.; Pandanaboyana, S.; Windsor, J.A. Surgery for Acute Pancreatitis. Indian J. Surg. 2015, 77, 446–452. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bollen, T.L. Imaging of acute pancreatitis: Update of the revised Atlanta classification. Radiol. Clin. N. Am. 2012, 50, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Busireddy, K.K.; AlObaidy, M.; Ramalho, M.; Kalubowila, J.; Baodong, L.; Santagostino, I.; Semelka, R.C. Pancreatitis-imaging approach. World J. Gastrointest. Pathophysiol. 2014, 5, 252–270. [Google Scholar] [CrossRef] [PubMed]
- Savlovschi, C.; Serban, D.; Andreescu, C.; Dascalu, A.; Pantu, H. Economic analysis of medical management applied for left colostomy. Chirurgia 2013, 108, 666–669. [Google Scholar] [PubMed]
- Nishimori, I.; Tamakoshi, A.; Otsuki, M. Prevalence of autoimmune pancreatitis in Japan from a nationwide survey in 2002. J. Gastroenterol. 2007, 42 (Suppl. 18), 6–8. [Google Scholar] [CrossRef]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef]
- Jackson, P.G.; Raiji, M.T. Evaluation and management of intestinal obstruction. Am. Fam. Physician 2011, 83, 159–165. [Google Scholar]
- Nicolaou, S.; Kai, B.; Ho, S.; Su, J.; Ahamed, K. Imaging of acute small-bowel obstruction. Am. J. Roentgenol. 2005, 185, 1036–1044. [Google Scholar] [CrossRef]
- Smith, D.A.; Kashyap, S.; Nehring, S.M. Bowel Obstruction. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Nelms, D.W.; Kann, B.R. Imaging Modalities for Evaluation of Intestinal Obstruction. Clin. Colon Rectal Surg. 2021, 34, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, G.; Busch, R.; Dimcheff, D.; Al-Hawary, M.; Saad, R.; Seagull, F.J.; Somand, D.; Cherry-Bukowiec, J.; Wanacata, L.; Pumiglia, L. Evaluation and Management of Mechanical Small Bowel Obstruction in Adults; Michigan Medicine University of Michigan: Ann Arbor, MI, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK572336/ (accessed on 15 July 2023).
- Schuster, K.M.; O’Connor, R.; Cripps, M.; Kuhlenschmidt, K.; Taveras, L.; Kaafarani, H.M.; El Hechi, M.; Puri, R.; Schroeppel, T.J.; Enniss, T.M.; et al. Revision of the AAST grading scale for acute cholecystitis with comparison to physiologic measures of severity. J. Trauma Acute Care Surg. 2022, 92, 664–674. [Google Scholar] [CrossRef]
- Pisano, M.; Allievi, N.; Gurusamy, K.; Borzellino, G.; Cimbanassi, S.; Boerna, D.; Coccolini, F.; Tufo, A.; Di Martino, M.; Leung, J.; et al. 2020 World Society of Emergency Surgery updated guidelines for the diagnosis and treatment of acute calculus cholecystitis. World J. Emerg. Surg. WJES 2020, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Serban, D.; Popa Cherecheanu, A.; Dascalu, A.M.; Socea, B.; Vancea, G.; Stana, D.; Smarandache, G.C.; Sabau, A.D.; Costea, D.O. Hypervirulent Klebsiella pneumoniae Endogenous Endophthalmitis-A Global Emerging Disease. Life 2021, 11, 676. [Google Scholar] [CrossRef]
- Hwang, H.; Marsh, I.; Doyle, J. Does ultrasonography accurately diagnose acute cholecystitis? Improving diagnostic accuracy based on a review at a regional hospital. Can. J. Surgery. J. Can. Chir. 2014, 57, 162–168. [Google Scholar] [CrossRef]
- Ansaloni, L.; Pisano, M.; Coccolini, F.; Peitzmann, A.B.; Fingerhut, A.; Catena, F.; Agresta, F.; Allegri, A.; Bailey, I.; Balogh, Z.J.; et al. 2016 WSES guidelines on acute calculous cholecystitis. World J. Emerg. Surg. WJES 2016, 11, 25. [Google Scholar] [CrossRef]
- O’Connor, O.J.; Maher, M.M. Imaging of cholecystitis. AJR Am. J. Roentgenol. 2011, 196, W367–W374. [Google Scholar] [CrossRef]

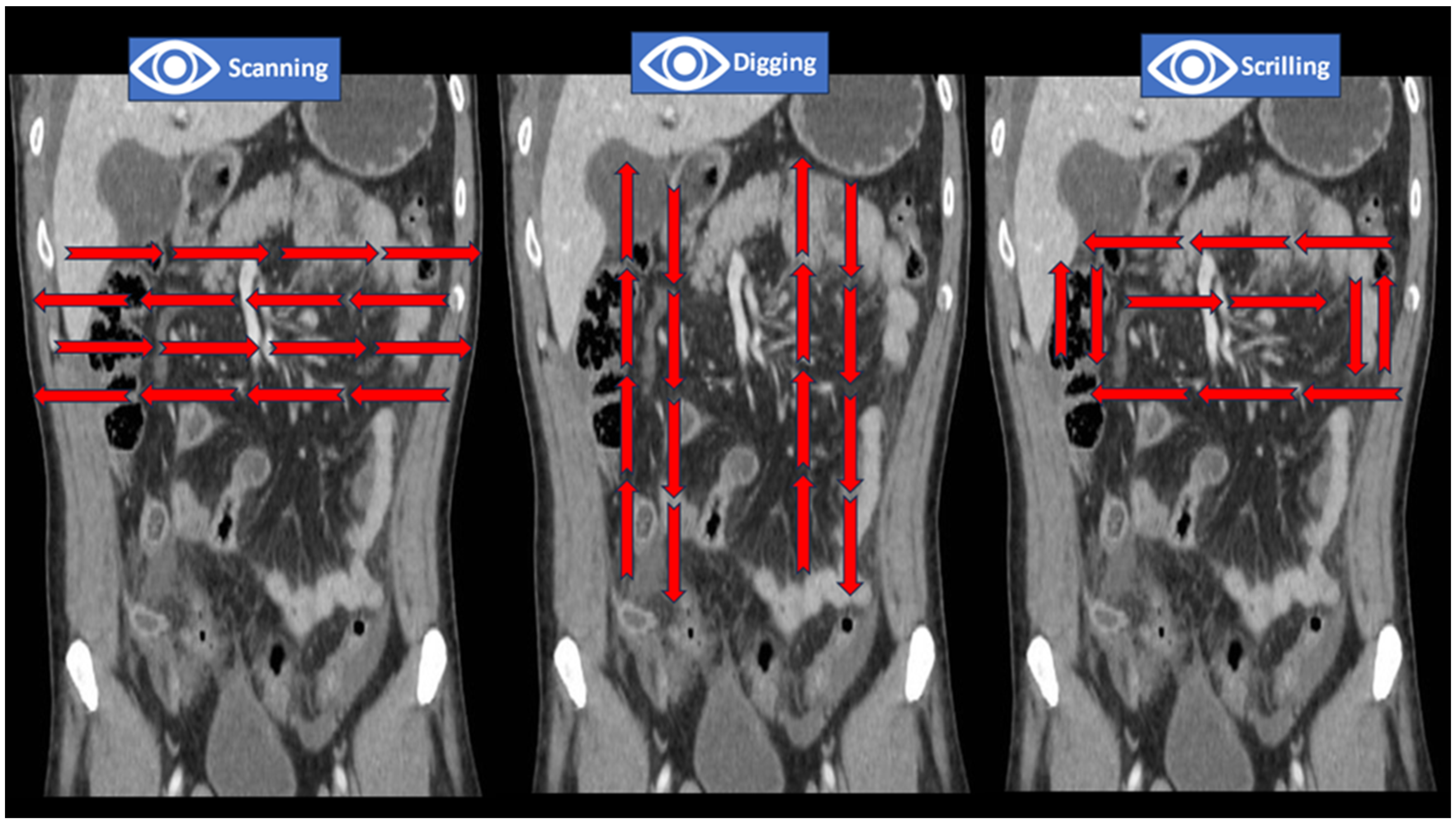
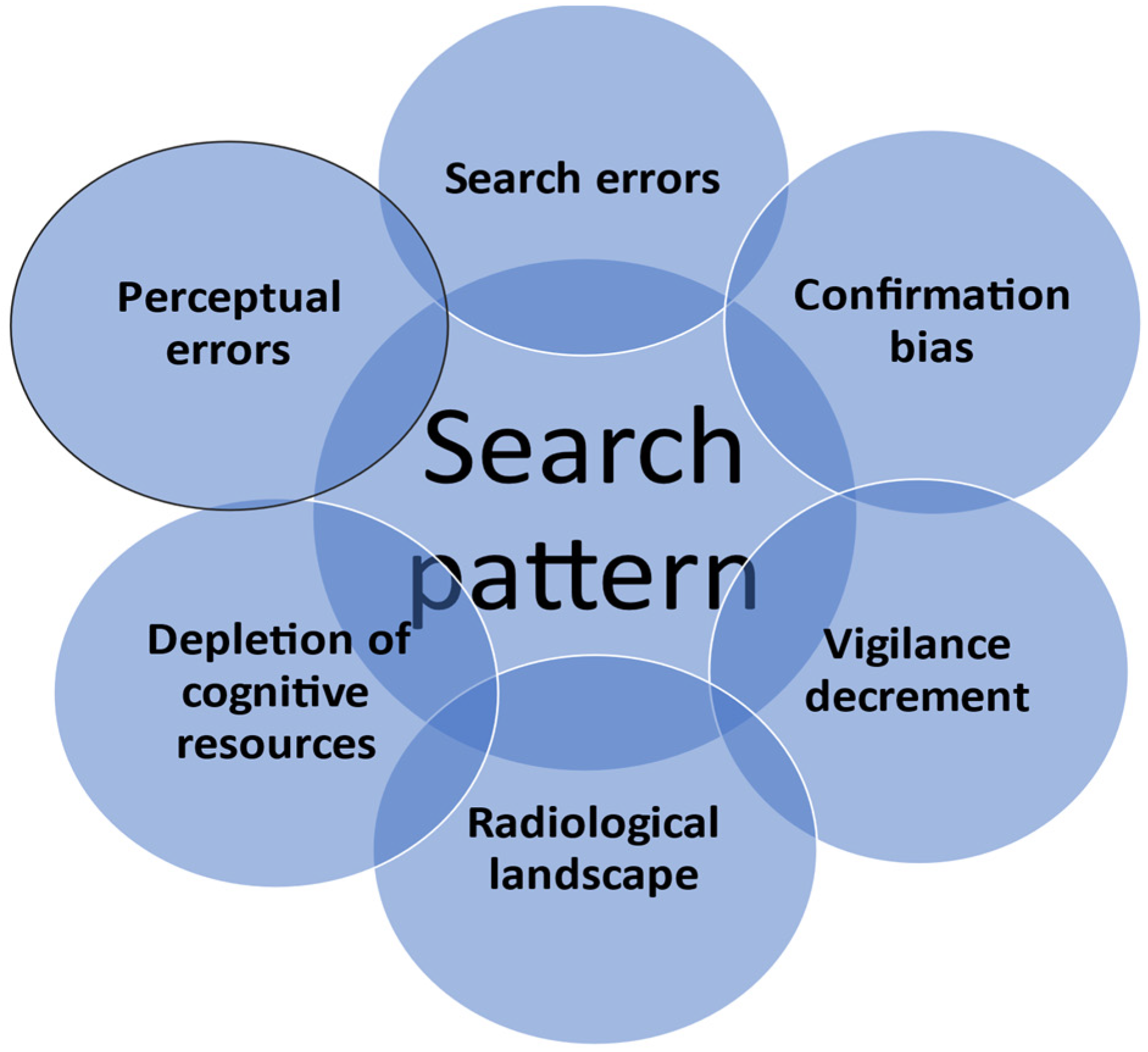



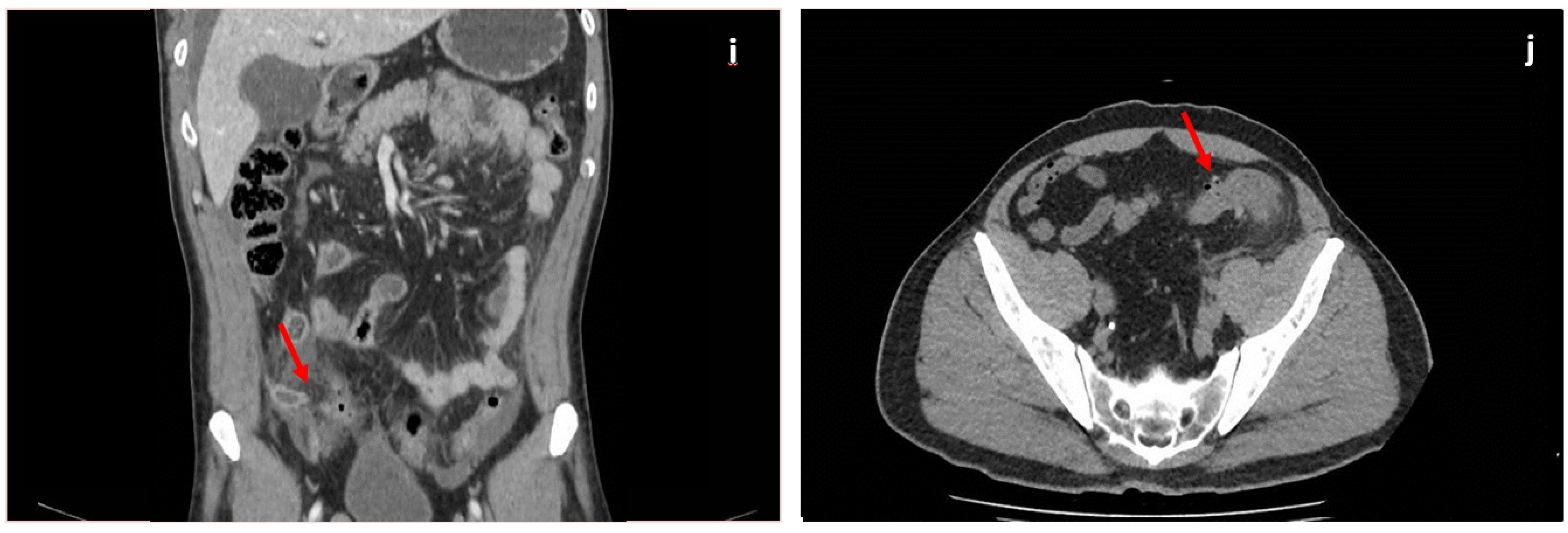

| Step | Salient Features and Pearls | Anatomical Concepts | Attenuation Values | Best Windows | Contrast Phases |
|---|---|---|---|---|---|
| Step 1: Liver | Size, shape, morphology of the parenchyma, duct dilatation, veins, and arteries | Liver segmentation, variations in the portal vein branching | Cystic lesions and hypo- and hyper-attenuating lesions | Use appropriate soft-tissue windows to enhance parenchymatous lesions and aeroportia by using the lung window | Examine during all contrast phases |
| Step 2: Porta Hepatis, Gallbladder, and CBD | Size (long and short axes), contour, and features of the gallbladder wall (edematous, discontinued, heterogeneity), presence of stones, adenomyomatosis, fluid around gallbladder | Anatomical variations, presence, and character of the dilatation of the biliary tree (choledochal cysts, distal narrowing, wall homogeneity) | Assess for the presence of air in the biliary tree and the periportal and periductal oedema | Use appropriate soft-tissue windows to enhance parenchymatous lesions. Change to lung for the detection of air | Examine during all contrast phases |
| Step 3: Spleen | Size (best in coronal), contour, and integrity of the capsule, fluid around the spleen, and proximity of lesions | Accessory spleens, splenic hilum | Cystic lesions, hypo- and hyperattenuating lesions, fluid attenuation values, and calcifications | Use appropriate soft-tissue windows to enhance parenchymatous lesions | The spleen is inhomogeneous during the arterial phase and homogenizes during the venous phase. Search for filling defects and correlate appearance with phase |
| Step 4: Pancreas | Pancreatic contour (diffuse enlargement and heterogeneous attenuation), presence of edema, peripancreatic fluid, hypo- and hyper-attenuating masses, duct dilatation/cut-off, acute peripancreatic collections, acute necrotic collections, pseudocysts, walled-off necroses, atrophy | Anatomical variations (cystic lesions, divisum, or annular pancreas) | Intra- and/or peripancreatic presence of air, reticular stranding of the surrounding fat, calcifications | Use appropriate soft-tissue windows to enhance parenchymatous lesions. Change to lung for the detection of air | Examine during all contrast phases. For suspicion of acute pancreatitis, a pancreatic arterial phase scan is required (acquired 45 s after the injection starts) |
| Step 5: Adrenals and kidneys | Size (long and short axes), shape, presence of macroscopic fat, nodules, thickening, hypo- and hyper-attenuation, atrophy, hydroureter and hydronephrosis, cysts, perinephric fluid, abscess | Linear or V-/Y-shaped; ectopic, fused/horseshoe kidney, corticomedullary differentiation | Calcifications, stones, perinephric stranding, gas | Use appropriate soft-tissue windows to enhance parenchymatous lesions | Examine during all contrast phases. NB: washout of contrast (rapid/slow) |
| Step 6: Pelvic content | Size (long and short axes), contour, presence of masses, cysts, free fluid, abscess | Anatomical variations, presence of genital organs, cysts | Fat stranding, calcifications | Use appropriate soft-tissue windows to enhance parenchymatous/intramural lesions. Change to lung for the detection of air | Examine during all contrast phases |
| Step 7: Stomach | Size (long and short axes), shape (dilation, stenosis), wall thickening, masses, previous surgeries | Anatomical variations, diverticulum, cysts | Assess for presence of free air/fluid, foreign bodies | Use appropriate soft-tissue windows to enhance parenchymatous/intramural lesions. Change to lung for the detection of air | Examine during all contrast phases |
| Step 8: Large bowel | Bowel loop diameter (dilated/decompressed), wall thickening, pneumatosis, stricture, diverticula, bowel content (fluid, solid, stool), mass lesions, free fluid | Anatomical variations | Free air, fat stranding, foreign bodies, abnormal density (hemorrhage), +/− oral contrast | Use appropriate soft-tissue windows to enhance parenchymatous/intramural lesions. Change to lung for the detection of air | Examine during all contrast phases |
| Step 9: Small bowel | Bowel loop diameter (dilated/decompressed), wall thickening, pneumatosis, strictures, diverticula, mass lesions, free fluid | Anatomical variations | Free air, fat stranding, foreign bodies, +/− oral contrast | Use appropriate soft-tissue windows to enhance parenchymatous/intramural lesions. Change to lung for the detection of air | Examine during all contrast phases |
| Step 10: Vessels | Caliber, patency, stenosis, aneurysm, dissection, atherosclerosis, intramural hematoma, thrombus | Anatomical variations, collaterals, cavernous transformation, presence/absence of vessels | Intramural hematoma, contrast extravasation (active bleeding), perivascular fat stranding, intraluminal air | Use appropriate soft-tissue windows to enhance parenchymatous/intramural lesions. Change to lung for the detection of air | Examine during all contrast phases |
| Step 11: Nodes | Size (short axis), shape (lobular, irregular), presence of necrosis, homogeneity/heterogeneity, location | Anatomical variations, morphology (fatty hilum) | Assess for presence of necrosis | Use appropriate soft-tissue windows to enhance parenchymatous lesions | Examine during all contrast phases |
| Step 12: Peritoneum and retroperitoneum | Thickness of peritoneum, free air/fluid, mass lesions, hematoma, fat stranding, fibrosis, collections | Anatomical variations | Assess for presence of fluid/air, fat stranding | Use appropriate soft-tissue windows to enhance parenchymatous lesions. Change to lung for the detection of air | Examine during all contrast phases |
| Step 13: Bones | Mineralization, fractures, lytic/mass lesions, periosteal reaction, cortical destruction | Anatomical variations, bone cyst | Calcifications | Use bone window | Examine during all contrast phases |
| Step 14: Muscles | Atrophy/hypertrophy, asymmetry, mass lesions, invasion (tumor), hematoma, edema, fatty infiltration | Anatomical variations, morphology | Calcifications | Use appropriate soft-tissue windows | Examine during all contrast phases |
| Step 15: Air and fluid | Location (intraabdominal intraperitoneal, intraabdominal extraperitoneal, pseudoperitoneum), quantity, density, defects in the wall of a hollow viscus | Anatomical variations | Air, fluid | Use appropriate soft-tissue windows. Change to lung for the detection of air | Examine during all contrast phases |
| Step 16: Miscellaneous | Edema, abscesses, soft tissue defects/infiltration, tumors | Anatomical variations | Air, fluid, calcifications | Use appropriate soft-tissue windows. Change to lung for the detection of air | Examine during all contrast phases |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alius, C.; Serban, D.; Tribus, L.C.; Costea, D.O.; Cristea, B.M.; Serboiu, C.; Motofei, I.; Dascalu, A.M.; Velescu, B.; Tudor, C.; et al. When Not to Operate on Acute Cases—A Surgeon’s Perspective on Rapid Assessment of Emergency Abdominopelvic Computed Tomography. J. Imaging 2023, 9, 200. https://doi.org/10.3390/jimaging9100200
Alius C, Serban D, Tribus LC, Costea DO, Cristea BM, Serboiu C, Motofei I, Dascalu AM, Velescu B, Tudor C, et al. When Not to Operate on Acute Cases—A Surgeon’s Perspective on Rapid Assessment of Emergency Abdominopelvic Computed Tomography. Journal of Imaging. 2023; 9(10):200. https://doi.org/10.3390/jimaging9100200
Chicago/Turabian StyleAlius, Catalin, Dragos Serban, Laura Carina Tribus, Daniel Ovidiu Costea, Bogdan Mihai Cristea, Crenguta Serboiu, Ion Motofei, Ana Maria Dascalu, Bruno Velescu, Corneliu Tudor, and et al. 2023. "When Not to Operate on Acute Cases—A Surgeon’s Perspective on Rapid Assessment of Emergency Abdominopelvic Computed Tomography" Journal of Imaging 9, no. 10: 200. https://doi.org/10.3390/jimaging9100200
APA StyleAlius, C., Serban, D., Tribus, L. C., Costea, D. O., Cristea, B. M., Serboiu, C., Motofei, I., Dascalu, A. M., Velescu, B., Tudor, C., Socea, B., Bobirca, A., Vancea, G., Tanasescu, D., & Bratu, D. G. (2023). When Not to Operate on Acute Cases—A Surgeon’s Perspective on Rapid Assessment of Emergency Abdominopelvic Computed Tomography. Journal of Imaging, 9(10), 200. https://doi.org/10.3390/jimaging9100200









