Dual-Energy CT of the Heart: A Review
Abstract
:1. Introduction
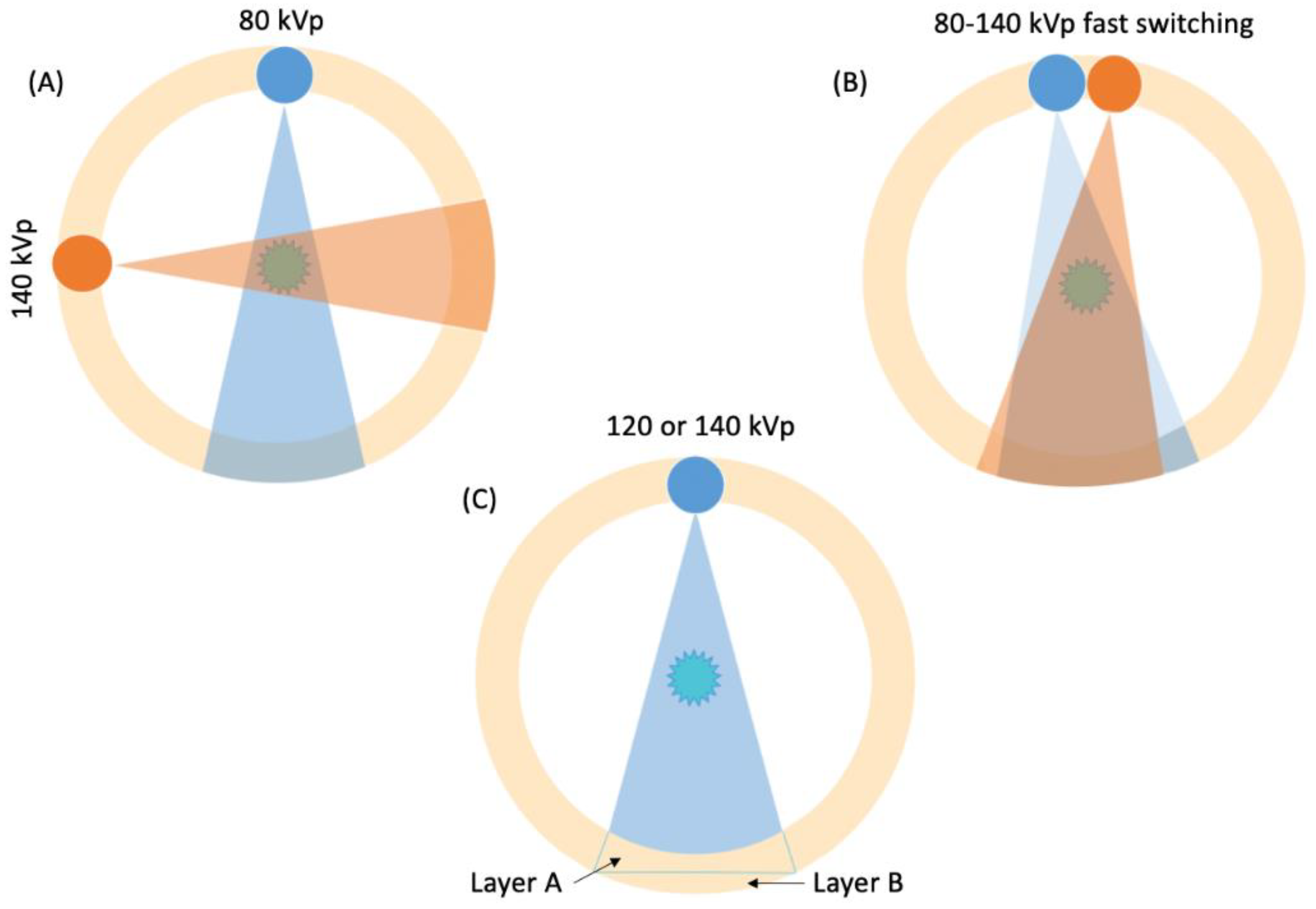
2. Basic Principles of DECT
3. Applications
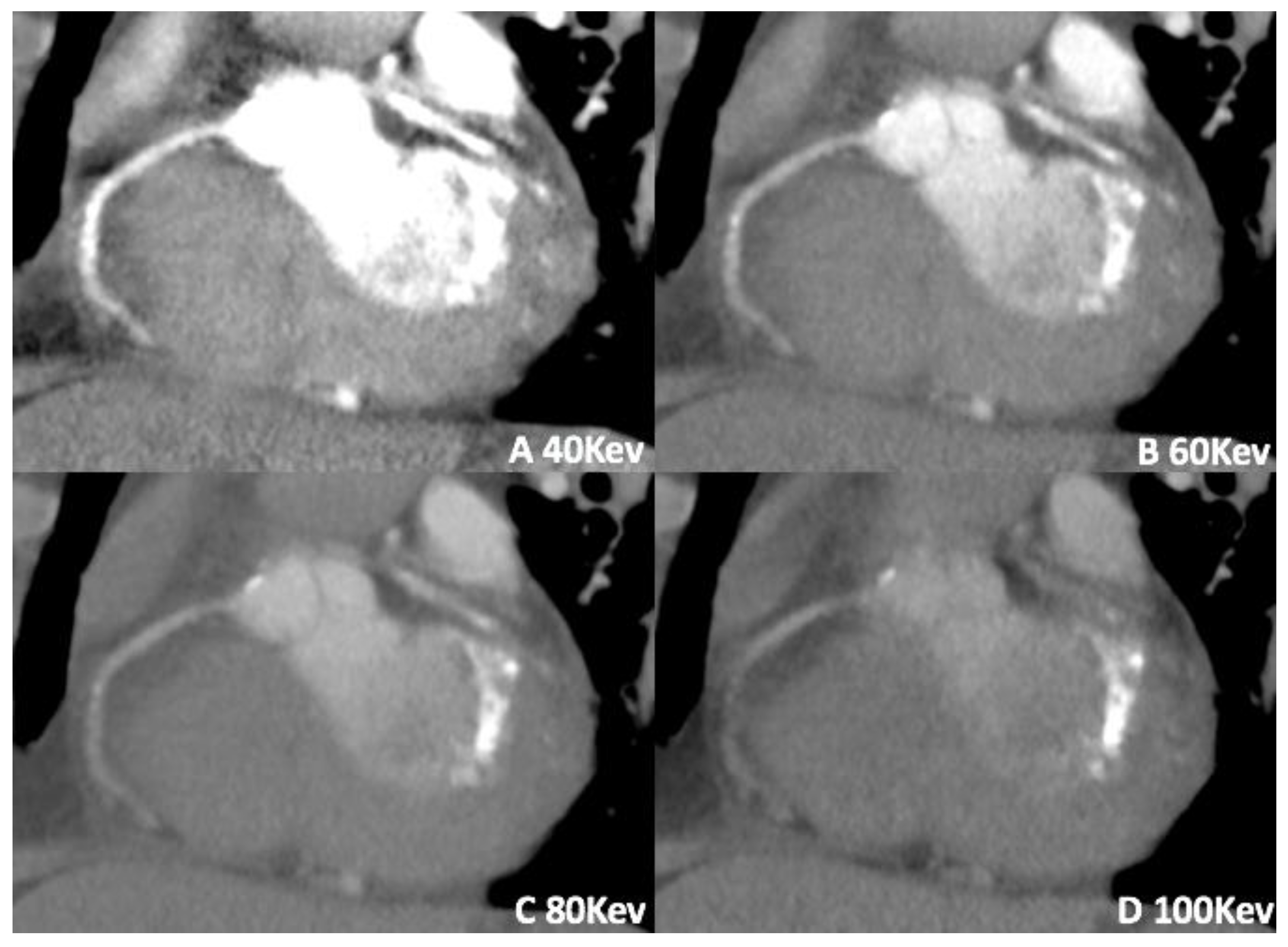
3.1. Virtual Monoenergetic Imaging (VMI)
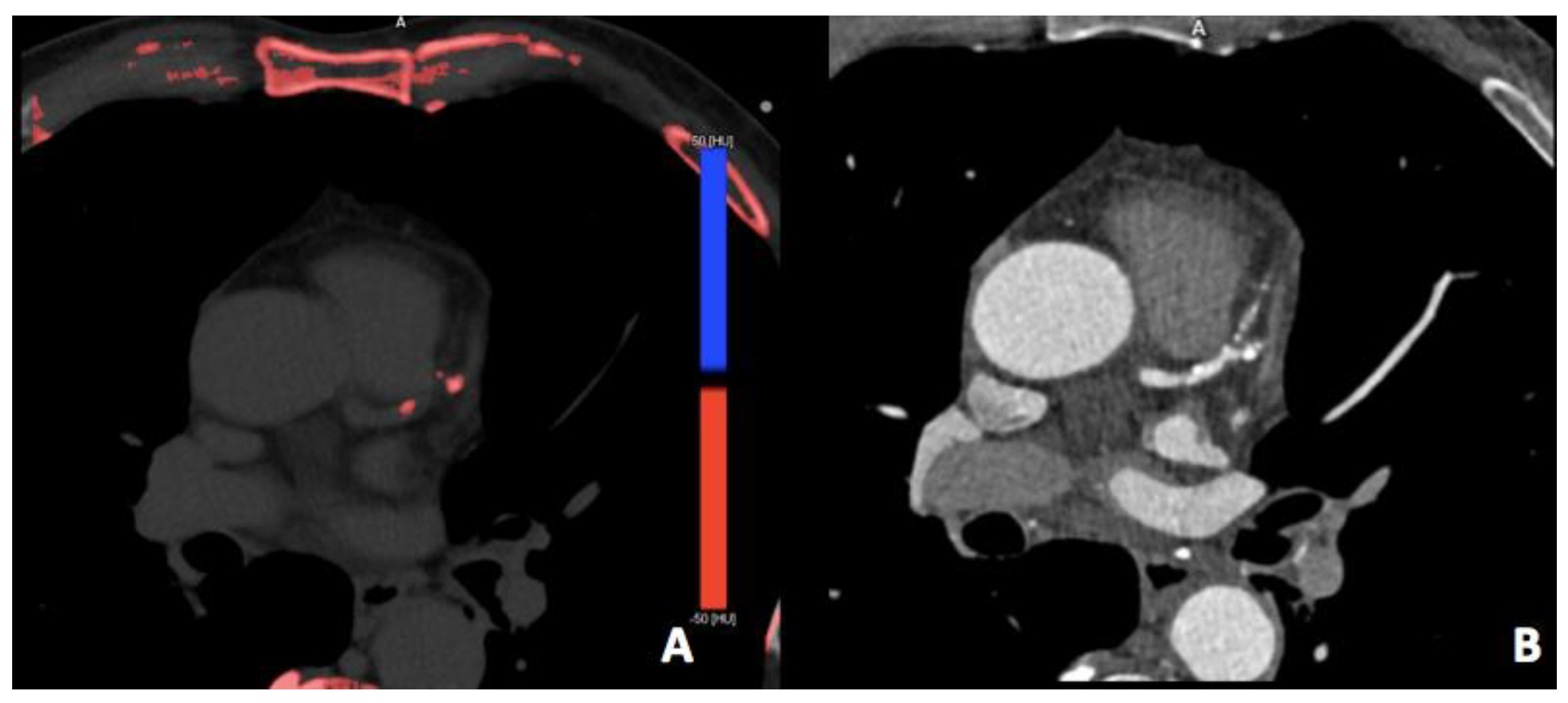
3.2. Virtual Non-Contrast Imaging
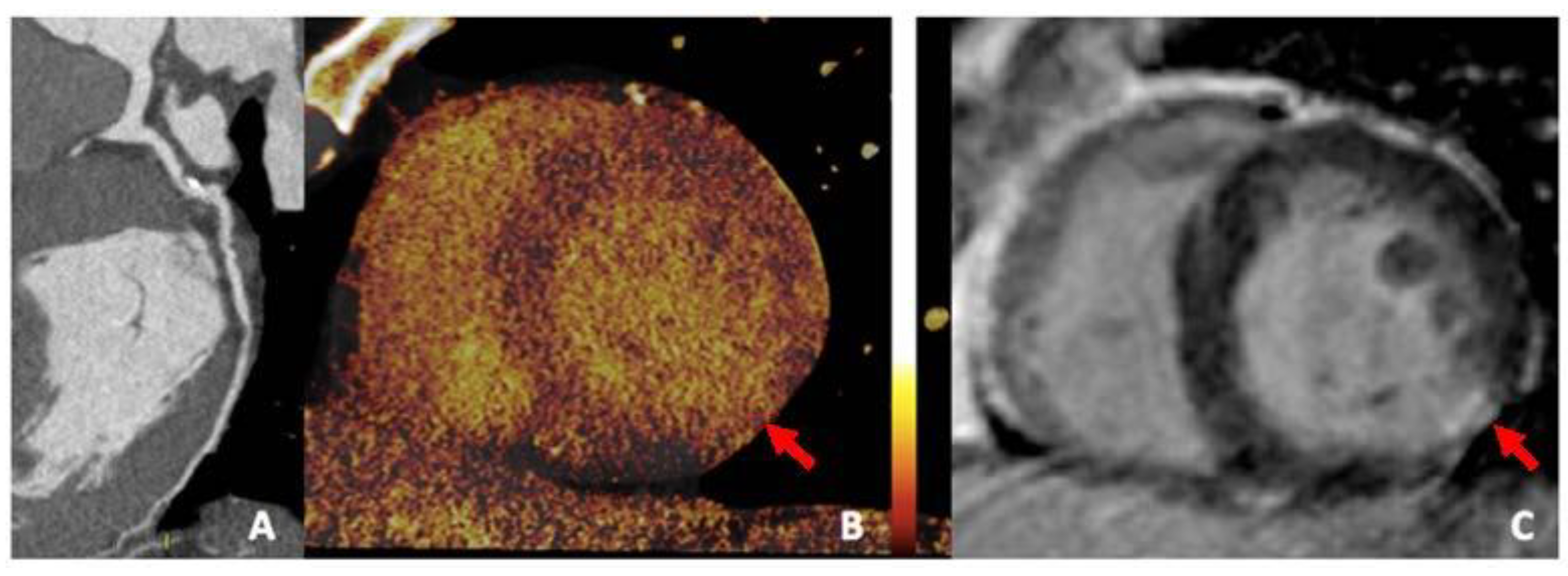
3.3. Virtual Calcium Subtraction
3.4. Iodine Perfusion Maps
3.5. Plaque Imaging and Analysis
3.6. Extracellular Volume (ECV)
4. DECT Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, M.J.; Kaza, R.K.; Bolus, D.N.; Boll, D.T.; Rofsky, N.M.; De Cecco, C.N.; Foley, W.D.; Morgan, D.E.; Schoepf, U.J.; Sahani, D.V.; et al. White Paper of the Society of Computed Body Tomography and Magnetic Resonance on Dual-Energy CT, Part 1: Technology and Terminology. J. Comput. Assist. Tomogr. 2016, 40, 841–845. [Google Scholar] [CrossRef] [PubMed]
- De Cecco, C.N.; Darnell, A.; Rengo, M.; Muscogiuri, G.; Bellini, D.; Ayuso, C.; Laghi, A. Dual-energy CT: Oncologic applications. Am. J. Roentgenol. 2012, 199, S98–S105. [Google Scholar] [CrossRef]
- Vliegenthart, R.; Pelgrim, G.J.; Ebersberger, U.; Rowe, G.W.; Oudkerk, M.; Schoepf, U.J. Dual-energy CT of the heart. Am. J. Roentgenol. 2012, 199, S54–S63. [Google Scholar] [CrossRef]
- Albrecht, M.H.; Bickford, M.W.; Nance, J.W.J.; Zhang, L.; De Cecco, C.N.; Wichmann, J.L.; Vogl, T.J.; Schoepf, U.J. State-of-the-Art Pulmonary CT Angiography for Acute Pulmonary Embolism. Am. J. Roentgenol. 2017, 208, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Ruzsics, B.; Lee, H.; Powers, E.R.; Flohr, T.G.; Costello, P.; Schoepf, U.J. Images in cardiovascular medicine. Myocardial ischemia diagnosed by dual-energy computed tomography: Correlation with single-photon emission computed tomography. Circulation 2008, 117, 1244–1245. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.H.; Trommer, J.; Wichmann, J.L.; Scholtz, J.-E.; Martin, S.S.; Lehnert, T.; Vogl, T.J.; Bodelle, B. Comprehensive Comparison of Virtual Monoenergetic and Linearly Blended Reconstruction Techniques in Third-Generation Dual-Source Dual-Energy Computed Tomography Angiography of the Thorax and Abdomen. Investig. Radiol. 2016, 51, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Min, J.K.; He, X.; Raman, S.V. Computation of Calcium Score with Dual-Energy Computed Tomography: A Phantom Study. J. Comput. Assist. Tomogr. 2017, 41, 156–158. [Google Scholar] [CrossRef]
- Varga-Szemes, A.; Meinel, F.G.; De Cecco, C.N.; Fuller, S.R.; Bayer, R.R.; Schoepf, U.J. CT myocardial perfusion imaging. Am. J. Roentgenol. 2015, 204, 487–497. [Google Scholar] [CrossRef]
- Johnson, T.R.C.; Krauß, B.; Sedlmair, M.; Grasruck, M.; Bruder, H.; Morhard, D.; Fink, C.; Weckbach, S.; Lenhard, M.; Schmidt, B.; et al. Material differentiation by dual energy CT: Initial experience. Eur. Radiol. 2007, 17, 1510–1517. [Google Scholar] [CrossRef]
- Si-Mohamed, S.A.; Restier, L.M.; Branchu, A.; Boccalini, S.; Congi, A.; Ziegler, A.; Tomasevic, D.; Bochaton, T.; Boussel, L.; Douek, P.C. Diagnostic Performance of Extracellular Volume Quantified by Dual-Layer Dual-Energy CT for Detection of Acute Myocarditis. J. Clin. Med. 2021, 10, 3286. [Google Scholar] [CrossRef]
- Qi, R.-X.; Shao, J.; Jiang, J.-S.; Ruan, X.-W.; Huang, S.; Zhang, Q.; Hu, C.-H. Myocardial extracellular volume fraction quantitation using cardiac dual-energy CT with late iodine enhancement in patients with heart failure without coronary artery disease: A single-center prospective study. Eur. J. Radiol. 2021, 140, 109743. [Google Scholar] [CrossRef] [PubMed]
- Ponsiglione, A.; Stanzione, A.; Cuocolo, R.; Ascione, R.; Gambardella, M.; De Giorgi, M.; Nappi, C.; Cuocolo, A.; Imbriaco, M. Cardiac CT and MRI radiomics: Systematic review of the literature and radiomics quality score assessment. Eur. Radiol. 2022, 32, 2629–2638. [Google Scholar] [CrossRef]
- Rajiah, P.; Parakh, A.; Kay, F.; Baruah, D.; Kambadakone, A.R.; Leng, S. Update on Multienergy CT: Physics, Principles, and Applications. RadioGraphics 2020, 40, 1284–1308. [Google Scholar] [CrossRef]
- De Cecco, C.N.; Schoepf, U.J.; Steinbach, L.; Boll, D.T.; Foley, W.D.; Kaza, R.K.; Bolus, D.N.; Morgan, D.E.; Sahani, D.V.; Shuman, W.P.; et al. White Paper of the Society of Computed Body Tomography and Magnetic Resonance on Dual-Energy CT, Part 3. J. Comput. Assist. Tomogr. 2017, 41, 1–7. [Google Scholar] [CrossRef]
- Krauss, B.; Grant, K.L.; Schmidt, B.T.; Flohr, T.G. The Importance of Spectral Separation. Investig. Radiol. 2015, 50, 114–118. [Google Scholar] [CrossRef]
- Tesche, C.; De Cecco, C.N.; Vliegenthart, R.; Albrecht, M.H.; Varga-Szemes, A.; Duguay, T.M.; Ebersberger, U.; Bayer, R.R.; Canstein, C.; Schmidt, B.; et al. Accuracy and Radiation Dose Reduction Using Low-Voltage Computed Tomography Coronary Artery Calcium Scoring with Tin Filtration. Am. J. Cardiol. 2017, 119, 675–680. [Google Scholar] [CrossRef]
- Layritz, C.; Schmid, J.; Achenbach, S.; Ulzheimer, S.; Wuest, W.; May, M.; Ropers, D.; Klinghammer, L.; Daniel, W.G.; Pflederer, T.; et al. Accuracy of prospectively ECG-triggered very low-dose coronary dual-source CT angiography using iterative reconstruction for the detection of coronary artery stenosis: Comparison with invasive catheterization. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1238–1245. [Google Scholar] [CrossRef]
- Meyer, M.; Haubenreisser, H.; Schoepf, U.J.; Vliegenthart, R.; Leidecker, C.; Allmendinger, T.; Lehmann, R.; Sudarski, S.; Borggrefe, M.; Schoenberg, S.O.; et al. Closing in on the K Edge: Coronary CT Angiography at 100, 80, and 70 kV—Initial Comparison of a Second- versus a Third-Generation Dual-Source CT System. Radiology 2014, 273, 373–382. [Google Scholar] [CrossRef]
- Hell, M.M.; Bittner, D.; Schuhbaeck, A.; Muschiol, G.; Brand, M.; Lell, M.; Uder, M.; Achenbach, S.; Marwan, M. Prospectively ECG-triggered high-pitch coronary angiography with third-generation dual-source CT at 70 kVp tube voltage: Feasibility, image quality, radiation dose, and effect of iterative reconstruction. J. Cardiovasc. Comput. Tomogr. 2014, 8, 418–425. [Google Scholar] [CrossRef]
- Grant, K.L.; Flohr, T.G.; Krauss, B.; Sedlmair, M.; Thomas, C.; Schmidt, B. Assessment of an Advanced Image-Based Technique to Calculate Virtual Monoenergetic Computed Tomographic Images from a Dual-Energy Examination to Improve Contrast-to-Noise Ratio in Examinations Using Iodinated Contrast Media. Investig. Radiol. 2014, 49, 586–592. [Google Scholar] [CrossRef]
- Sandfort, V.; Persson, M.; Pourmorteza, A.; Noël, P.B.; Fleischmann, D.; Willemink, M.J. Spectral photon-counting CT in cardiovascular imaging. J. Cardiovasc. Comput. Tomogr. 2021, 15, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.F.; Elbanna, K.Y.; Mohammed, A.M.E.; Murray, N.; Azzumea, F.; Almazied, G.; Nicolaou, S. Practical Applications of Dual-Energy Computed Tomography in the Acute Abdomen. Radiol. Clin. N. Am. 2018, 56, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.H.; De Cecco, C.N.; Schoepf, U.J.; Spandorfer, A.; Eid, M.; De Santis, D.; Varga-Szemes, A.; van Assen, M.; von Knebel-Doeberitz, P.L.; Tesche, C.; et al. Dual-energy CT of the heart current and future status. Eur. J. Radiol. 2018, 105, 110–118. [Google Scholar] [CrossRef]
- Zeng, Y.; Geng, D.; Zhang, J. Noise-optimized virtual monoenergetic imaging technology of the third-generation dual-source computed tomography and its clinical applications. Quant. Imaging Med. Surg. 2021, 11, 4627–4643. [Google Scholar] [CrossRef]
- Beeres, M.; Trommer, J.; Frellesen, C.; Nour-Eldin, N.-E.A.; Scholtz, J.E.; Herrmann, E.; Vogl, T.J.; Wichmann, J.L. Evaluation of different keV-settings in dual-energy CT angiography of the aorta using advanced image-based virtual monoenergetic imaging. Int. J. Cardiovasc. Imaging 2016, 32, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.H.; Scholtz, J.-E.; Hüsers, K.; Beeres, M.; Bucher, A.M.; Kaup, M.; Martin, S.S.; Fischer, S.; Bodelle, B.; Bauer, R.W.; et al. Advanced image-based virtual monoenergetic dual-energy CT angiography of the abdomen: Optimization of kiloelectron volt settings to improve image contrast. Eur. Radiol. 2016, 26, 1863–1870. [Google Scholar] [CrossRef]
- Albrecht, M.H.; Scholtz, J.-E.; Kraft, J.; Bauer, R.W.; Kaup, M.; Dewes, P.; Bucher, A.M.; Burck, I.; Wagenblast, J.; Lehnert, T.; et al. Assessment of an Advanced Monoenergetic Reconstruction Technique in Dual-Energy Computed Tomography of Head and Neck Cancer. Eur. Radiol. 2015, 25, 2493–2501. [Google Scholar] [CrossRef]
- Kang, H.-J.; Lee, J.M.; Lee, S.M.; Yang, H.K.; Kim, R.H.; Nam, J.G.; Karnawat, A.; Han, J.K. Value of virtual monochromatic spectral image of dual-layer spectral detector CT with noise reduction algorithm for image quality improvement in obese simulated body phantom. BMC Med. Imaging 2019, 19, 76. [Google Scholar] [CrossRef]
- De Cecco, C.N.; Caruso, D.; Schoepf, U.J.; Wichmann, J.L.; Ter Louw, J.R.; Perry, J.D.; Picard, M.M.; Schaefer, A.R.; Parker, L.W.; Hardie, A.D. Optimization of window settings for virtual monoenergetic imaging in dual-energy CT of the liver: A multi-reader evaluation of standard monoenergetic and advanced imaged-based monoenergetic datasets. Eur. J. Radiol. 2016, 85, 695–699. [Google Scholar] [CrossRef]
- Caruso, D.; Parinella, A.H.; Schoepf, U.J.; Stroebel, M.H.; Mangold, S.; Wichmann, J.L.; Varga-Szemes, A.; Ball, B.D.; De Santis, D.; Laghi, A.; et al. Optimization of window settings for standard and advanced virtual monoenergetic imaging in abdominal dual-energy CT angiography. Abdom. Radiol. 2017, 42, 772–780. [Google Scholar] [CrossRef]
- D’Angelo, T.; Bucher, A.M.; Lenga, L.; Arendt, C.T.; Peterke, J.L.; Caruso, D.; Mazziotti, S.; Blandino, A.; Ascenti, G.; Othman, A.E.; et al. Optimisation of window settings for traditional and noise-optimised virtual monoenergetic imaging in dual-energy computed tomography pulmonary angiography. Eur. Radiol. 2018, 28, 1393–1401. [Google Scholar] [CrossRef]
- Chang, S.; Han, K.; Youn, J.-C.; Im, D.J.; Kim, J.Y.; Suh, Y.J.; Hong, Y.J.; Hur, J.; Kim, Y.J.; Choi, B.W.; et al. Utility of Dual-Energy CT-based Monochromatic Imaging in the Assessment of Myocardial Delayed Enhancement in Patients with Cardiomyopathy. Radiology 2018, 287, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, J.L.; Arbaciauskaite, R.; Kerl, J.M.; Frellesen, C.; Bodelle, B.; Lehnert, T.; Monsefi, N.; Vogl, T.J.; Bauer, R.W. Evaluation of monoenergetic late iodine enhancement dual-energy computed tomography for imaging of chronic myocardial infarction. Eur. Radiol. 2014, 24, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Lenga, L.; Albrecht, M.H.; Othman, A.E.; Martin, S.S.; Leithner, D.; D’Angelo, T.; Arendt, C.; Scholtz, J.-E.; De Cecco, C.N.; Schoepf, U.J.; et al. Monoenergetic Dual-energy Computed Tomographic Imaging: Cardiothoracic Applications. J. Thorac. Imaging 2017, 32, 151–158. [Google Scholar] [CrossRef]
- Martin, S.S.; Wichmann, J.L.; Weyer, H.; Scholtz, J.-E.; Leithner, D.; Spandorfer, A.; Bodelle, B.; Jacobi, V.; Vogl, T.J.; Albrecht, M.H. Endoleaks after endovascular aortic aneurysm repair: Improved detection with noise-optimized virtual monoenergetic dual-energy CT. Eur. J. Radiol. 2017, 94, 125–132. [Google Scholar] [CrossRef]
- Eberhard, M.; Mergen, V.; Higashigaito, K.; Allmendinger, T.; Manka, R.; Flohr, T.; Schmidt, B.; Euler, A.; Alkadhi, H. Coronary Calcium Scoring with First Generation Dual-Source Photon-Counting CT—First Evidence from Phantom and In-Vivo Scans. Diagnostics 2021, 11, 1708. [Google Scholar] [CrossRef] [PubMed]
- Mergen, V.; Ried, E.; Allmendinger, T.; Sartoretti, T.; Higashigaito, K.; Manka, R.; Euler, A.; Alkadhi, H.; Eberhard, M. Epicardial Adipose Tissue Attenuation and Fat Attenuation Index: Phantom Study and In Vivo Measurements with Photon-Counting Detector CT. Am. J. Roentgenol. 2022, 218, 822–829. [Google Scholar] [CrossRef]
- Mangold, D.; Salatzki, J.; Riffel, J.; Kauczor, H.-U.; Weber, T.F. Dual-Layer Spectral CTA for TAVI Planning Using a Split-Phase Protocol and Low-keV Virtual Monoenergetic Images: Improved Image Quality in Comparison with Single-Phase Conventional CTA. RöFo Fortschr. Geb. Röntgenstrahlen Bildgeb. Verfahr. 2021, 194, 652–659. [Google Scholar] [CrossRef]
- Cavallo, A.U.; Patterson, A.J.; Thomas, R.; Alaiti, M.A.; Attizzani, G.F.; Laukamp, K.; Große Hokamp, N.; Bezerra, H.; Gilkeson, R.; Rajagopalan, S. Low dose contrast CT for transcatheter aortic valve replacement assessment: Results from the prospective SPECTACULAR study (spectral CT assessment prior to TAVR). J. Cardiovasc. Comput. Tomogr. 2020, 14, 68–74. [Google Scholar] [CrossRef]
- Delesalle, M.-A.; Pontana, F.; Duhamel, A.; Faivre, J.-B.; Flohr, T.; Tacelli, N.; Remy, J.; Remy-Jardin, M. Spectral optimization of chest CT angiography with reduced iodine load: Experience in 80 patients evaluated with dual-source, dual-energy CT. Radiology 2013, 267, 256–266. [Google Scholar] [CrossRef]
- Oda, S.; Takaoka, H.; Katahira, K.; Honda, K.; Nakaura, T.; Nagayama, Y.; Taguchi, N.; Kidoh, M.; Utsunomiya, D.; Funama, Y.; et al. Low contrast material dose coronary computed tomographic angiography using a dual-layer spectral detector system in patients at risk for contrast-induced nephropathy. Br. J. Radiol. 2019, 92, 20180215. [Google Scholar] [PubMed]
- Mangold, S.; Cannaó, P.M.; Schoepf, U.J.; Wichmann, J.L.; Canstein, C.; Fuller, S.R.; Muscogiuri, G.; Varga-Szemes, A.; Nikolaou, K.; De Cecco, C.N. Impact of an advanced image-based monoenergetic reconstruction algorithm on coronary stent visualization using third generation dual-source dual-energy CT: A phantom study. Eur. Radiol. 2016, 26, 1871–1878. [Google Scholar] [PubMed]
- Rotzinger, D.C.; Si-Mohamed, S.A.; Yerly, J.; Boccalini, S.; Becce, F.; Boussel, L.; Meuli, R.A.; Qanadli, S.D.; Douek, P.C. Reduced-iodine-dose dual-energy coronary CT angiography: Qualitative and quantitative comparison between virtual monochromatic and polychromatic CT images. Eur. Radiol. 2021, 31, 7132–7142. [Google Scholar]
- Huang, X.; Gao, S.; Ma, Y.; Lu, X.; Jia, Z.; Hou, Y. The optimal monoenergetic spectral image level of coronary computed tomography (CT) angiography on a dual-layer spectral detector CT with half-dose contrast media. Quant. Imaging Med. Surg. 2020, 10, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Bucher, A.M.; Wichmann, J.L.; Schoepf, U.J.; Wolla, C.D.; McQuiston, A.D.; Krazinski, A.W.; Canstein, C.; De Cecco, C.N.; Meinel, F.G.; Geyer, L.L.; et al. Quantitative evaluation of beam-hardening artefact correction in dual-energy CT myocardial perfusion imaging. Eur. Radiol. 2016, 26, 3215–3222. [Google Scholar] [PubMed]
- So, A.; Hsieh, J.; Narayanan, S.; Thibault, J.-B.; Imai, Y.; Dutta, S.; Leipsic, J.; Min, J.; LaBounty, T.; Lee, T.-Y. Dual-energy CT and its potential use for quantitative myocardial CT perfusion. J. Cardiovasc. Comput. Tomogr. 2012, 6, 308–317. [Google Scholar]
- De Santis, D.; Eid, M.; De Cecco, C.N.; Jacobs, B.E.; Albrecht, M.H.; Varga-Szemes, A.; Tesche, C.; Caruso, D.; Laghi, A.; Schoepf, U.J. Dual-Energy Computed Tomography in Cardiothoracic Vascular Imaging. Radiol. Clin. N. Am. 2018, 56, 521–534. [Google Scholar]
- Bamberg, F.; Dierks, A.; Nikolaou, K.; Reiser, M.F.; Becker, C.R.; Johnson, T.R.C. Metal artifact reduction by dual energy computed tomography using monoenergetic extrapolation. Eur. Radiol. 2011, 21, 1424–1429. [Google Scholar]
- Secchi, F.; De Cecco, C.N.; Spearman, J.V.; Silverman, J.R.; Ebersberger, U.; Sardanelli, F.; Schoepf, U.J. Monoenergetic extrapolation of cardiac dual energy CT for artifact reduction. Acta Radiol. 2015, 56, 413–418. [Google Scholar]
- Ohta, Y.; Kitao, S.; Watanabe, T.; Kishimoto, J.; Yamamoto, K.; Ogawa, T. Evaluation of image quality of coronary artery plaque with rapid kVp-switching dual-energy CT. Clin. Imaging 2017, 43, 42–49. [Google Scholar]
- Boiselle, P.M.; Nikolaou, K.; Schoepf, U.J.; Seo, J.B. Expert Opinion. J. Thorac. Imaging 2012, 27, 6. [Google Scholar] [CrossRef] [PubMed]
- Song, I.; Yi, J.G.; Park, J.H.; Kim, S.M.; Lee, K.S.; Chung, M.J. Virtual Non-Contrast CT Using Dual-Energy Spectral CT: Feasibility of Coronary Artery Calcium Scoring. Korean J. Radiol. 2016, 17, 321–329. [Google Scholar]
- Machida, H.; Tanaka, I.; Fukui, R.; Shen, Y.; Ishikawa, T.; Tate, E.; Ueno, E. Current and Novel Imaging Techniques in Coronary CT. RadioGraphics 2015, 35, 991–1010. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Jinzaki, M.; Okamura, T.; Yamada, M.; Tanami, Y.; Abe, T.; Kuribayashi, S. Feasibility of coronary artery calcium scoring on virtual unenhanced images derived from single-source fast kVp-switching dual-energy coronary CT angiography. J. Cardiovasc. Comput. Tomogr. 2014, 8, 391–400. [Google Scholar] [PubMed]
- Carrascosa, P.M.; Cury, R.C.; Deviggiano, A.; Capunay, C.; Campisi, R.; López de Munain, M.; Vallejos, J.; Tajer, C.; Rodriguez-Granillo, G.A. Comparison of myocardial perfusion evaluation with single versus dual-energy CT and effect of beam-hardening artifacts. Acad. Radiol. 2015, 22, 591–599. [Google Scholar]
- Allmendinger, T.; Nowak, T.; Flohr, T.; Klotz, E.; Hagenauer, J.; Alkadhi, H.; Schmidt, B. Photon-Counting Detector CT-Based Vascular Calcium Removal Algorithm: Assessment Using a Cardiac Motion Phantom. Investig. Radiol. 2022, 57, 399–405. [Google Scholar] [CrossRef]
- Sartoretti, T.; Eberhard, M.; Nowak, T.; Gutjahr, R.; Jost, G.; Pietsch, H.; Schmidt, B.; Flohr, T.; Alkadhi, H.; Euler, A. Photon-Counting Multienergy Computed Tomography with Spectrally Optimized Contrast Media for Plaque Removal and Stenosis Assessment. Investig. Radiol. 2021, 56, 563–570. [Google Scholar]
- De Santis, D.; Jin, K.N.; Schoepf, U.J.; Grant, K.L.; De Cecco, C.N.; Nance, J.W.; Vogl, T.J.; Laghi, A.; Albrecht, M.H. Heavily Calcified Coronary Arteries. Investig. Radiol. 2018, 53, 103–109. [Google Scholar]
- Foley, W.D.; Shuman, W.P.; Siegel, M.J.; Sahani, D.V.; Boll, D.T.; Bolus, D.N.; De Cecco, C.N.; Kaza, R.K.; Morgan, D.E.; Schoepf, U.J.; et al. White Paper of the Society of Computed Body Tomography and Magnetic Resonance on Dual-Energy CT, Part 2. J. Comput. Assist. Tomogr. 2016, 40, 846–850. [Google Scholar]
- Rodriguez-Granillo, G.A. Delayed enhancement cardiac computed tomography for the assessment of myocardial infarction: From bench to bedside. Cardiovasc. Diagn. Ther. 2017, 7, 159–170. [Google Scholar] [CrossRef]
- Mahnken, A.H.; Lautenschläger, S.; Fritz, D.; Koos, R.; Scheuering, M. Perfusion weighted color maps for enhanced visualization of myocardial infarction by MSCT: Preliminary experience. Int. J. Cardiovasc. Imaging 2008, 24, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.W.; Kerl, J.M.; Fischer, N.; Burkhard, T.; Larson, M.C.; Ackermann, H.; Vogl, T.J. Dual-Energy CT for the Assessment of Chronic Myocardial Infarction in Patients with Chronic Coronary Artery Disease: Comparison with 3-T MRI. Am. J. Roentgenol. 2010, 195, 639–646. [Google Scholar]
- Rubinshtein, R.; Miller, T.D.; Williamson, E.E.; Kirsch, J.; Gibbons, R.J.; Primak, A.N.; McCollough, C.H.; Araoz, P.A. Detection of myocardial infarction by dual-source coronary computed tomography angiography using quantitated myocardial scintigraphy as the reference standard. Heart 2009, 95, 1419–1422. [Google Scholar] [CrossRef]
- Nakahara, T.; Toyama, T.; Jinzaki, M.; Seki, R.; Saito, Y.; Higuchi, T.; Yamada, M.; Arai, M.; Tsushima, Y.; Kuribayashi, S.; et al. Quantitative Analysis of Iodine Image of Dual-energy Computed Tomography at Rest. J. Thorac. Imaging 2018, 33, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, P.M.; Deviggiano, A.; Capunay, C.; Campisi, R.; de Munain, M.L.; Vallejos, J.; Tajer, C.; Rodriguez-Granillo, G.A. Incremental value of myocardial perfusion over coronary angiography by spectral computed tomography in patients with intermediate to high likelihood of coronary artery disease. Eur. J. Radiol. 2015, 84, 637–642. [Google Scholar] [PubMed]
- Kido, T.; Watanabe, K.; Saeki, H.; Shigemi, S.; Matsuda, T.; Yamamoto, M.; Kurata, A.; Kanza, R.E.; Itoh, T.; Mochizuki, T. Adenosine triphosphate stress dual-source computed tomography to identify myocardial ischemia: Comparison with invasive coronary angiography. SpringerPlus 2014, 3, 75. [Google Scholar]
- De Cecco, C.N.; Harris, B.S.; Schoepf, U.J.; Silverman, J.R.; McWhite, C.B.; Krazinski, A.W.; Bayer, R.R.; Meinel, F.G. Incremental Value of Pharmacological Stress Cardiac Dual-Energy CT over Coronary CT Angiography Alone for the Assessment of Coronary Artery Disease in a High-Risk Population. Am. J. Roentgenol. 2014, 203, W70–W77. [Google Scholar] [CrossRef]
- Weininger, M.; Schoepf, U.J.; Ramachandra, A.; Fink, C.; Rowe, G.W.; Costello, P.; Henzler, T. Adenosine-stress dynamic real-time myocardial perfusion CT and adenosine-stress first-pass dual-energy myocardial perfusion CT for the assessment of acute chest pain: Initial results. Eur. J. Radiol. 2012, 81, 3703–3710. [Google Scholar] [CrossRef]
- Jin, K.N.; De Cecco, C.N.; Caruso, D.; Tesche, C.; Spandorfer, A.; Varga-Szemes, A.; Schoepf, U.J. Myocardial perfusion imaging with dual energy CT. Eur. J. Radiol. 2016, 85, 1914–1921. [Google Scholar]
- Delgado Sánchez-Gracián, C.; Oca Pernas, R.; Trinidad López, C.; Santos Armentia, E.; Vaamonde Liste, A.; Vázquez Caamaño, M.; Tardáguila de la Fuente, G. Quantitative myocardial perfusion with stress dual-energy CT: Iodine concentration differences between normal and ischemic or necrotic myocardium. Initial experience. Eur. Radiol. 2016, 26, 3199–3207. [Google Scholar]
- Ruzsics, B.; Lee, H.; Zwerner, P.L.; Gebregziabher, M.; Costello, P.; Schoepf, U.J. Dual-energy CT of the heart for diagnosing coronary artery stenosis and myocardial ischemia-initial experience. Eur. Radiol. 2008, 18, 2414–2424. [Google Scholar] [PubMed]
- Wang, R.; Yu, W.; Wang, Y.; He, Y.; Yang, L.; Bi, T.; Jiao, J.; Wang, Q.; Chi, L.; Yu, Y.; et al. Incremental value of dual-energy CT to coronary CT angiography for the detection of significant coronary stenosis: Comparison with quantitative coronary angiography and single photon emission computed tomography. Int. J. Cardiovasc. Imaging 2011, 27, 647–656. [Google Scholar]
- Deseive, S.; Bauer, R.W.; Lehmann, R.; Kettner, M.; Kaiser, C.; Korkusuz, H.; Tandi, C.; Theisen, A.; Schächinger, V.; Schoepf, U.J.; et al. Dual-energy computed tomography for the detection of late enhancement in reperfused chronic infarction: A comparison to magnetic resonance imaging and histopathology in a porcine model. Investig. Radiol. 2011, 46, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Granillo, G.A. Non-invasive assessment of vulnerable plaque. Expert Opin. Med. Diagn. 2009, 3, 53–66. [Google Scholar] [CrossRef]
- Motoyama, S.; Sarai, M.; Harigaya, H.; Anno, H.; Inoue, K.; Hara, T.; Naruse, H.; Ishii, J.; Hishida, H.; Wong, N.D.; et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J. Am. Coll. Cardiol. 2009, 54, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Maurovich-Horvat, P.; Hoffmann, U.; Vorpahl, M.; Nakano, M.; Virmani, R.; Alkadhi, H. The napkin-ring sign: CT signature of high-risk coronary plaques? JACC Cardiovasc. Imaging 2010, 3, 440–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Velzen, J.E.; de Graaf, F.R.; de Graaf, M.A.; Schuijf, J.D.; Kroft, L.J.; de Roos, A.; Reiber, J.H.C.; Bax, J.J.; Jukema, J.W.; Boersma, E.; et al. Comprehensive assessment of spotty calcifications on computed tomography angiography: Comparison to plaque characteristics on intravascular ultrasound with radiofrequency backscatter analysis. J. Nucl. Cardiol. 2011, 18, 893–903. [Google Scholar] [PubMed]
- Barreto, M.; Schoenhagen, P.; Nair, A.; Amatangelo, S.; Milite, M.; Obuchowski, N.A.; Lieber, M.L.; Halliburton, S.S. Potential of dual-energy computed tomography to characterize atherosclerotic plaque: Ex vivo assessment of human coronary arteries in comparison to histology. J. Cardiovasc. Comput. Tomogr. 2008, 2, 234–242. [Google Scholar]
- Obaid, D.R.; Calvert, P.A.; Gopalan, D.; Parker, R.A.; West, N.E.J.; Goddard, M.; Rudd, J.H.F.; Bennett, M.R. Dual-energy computed tomography imaging to determine atherosclerotic plaque composition: A prospective study with tissue validation. J. Cardiovasc. Comput. Tomogr. 2014, 8, 230–237. [Google Scholar]
- Tanami, Y.; Ikeda, E.; Jinzaki, M.; Satoh, K.; Nishiwaki, Y.; Yamada, M.; Okada, Y.; Kuribayashi, S. Computed tomographic attenuation value of coronary atherosclerotic plaques with different tube voltage: An ex vivo study. J. Comput. Assist. Tomogr. 2010, 34, 58–63. [Google Scholar] [CrossRef]
- Yamak, D.; Panse, P.; Pavlicek, W.; Boltz, T.; Akay, M. Non-calcified coronary atherosclerotic plaque characterization by dual energy computed tomography. IEEE J. Biomed. Health Inform. 2014, 18, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.Y.; Ghosn, M.G.; Khan, M.A.; Gramze, N.L.; Brunner, G.; Nabi, F.; Nambi, V.; Nagueh, S.F.; Nguyen, D.T.; Graviss, E.A.; et al. Myocardial Extracellular Volume Fraction Adds Prognostic Information Beyond Myocardial Replacement Fibrosis. Circ. Cardiovasc. Imaging 2019, 12, e009535. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.A.; Naish, J.H.; Bishop, P.; Coutts, G.; Clark, D.; Zhao, S.; Ray, S.G.; Yonan, N.; Williams, S.G.; Flett, A.S.; et al. Comprehensive Validation of Cardiovascular Magnetic Resonance Techniques for the Assessment of Myocardial Extracellular Volume. Circ. Cardiovasc. Imaging 2013, 6, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Im, D.J.; Youn, J.-C.; Chang, S.; Suh, Y.J.; Hong, Y.J.; Kim, Y.J.; Hur, J.; Choi, B.W. Myocardial Extracellular Volume Fraction with Dual-Energy Equilibrium Contrast-enhanced Cardiac CT in Nonischemic Cardiomyopathy: A Prospective Comparison with Cardiac MR Imaging. Radiology 2016, 280, 49–57. [Google Scholar] [CrossRef]
- Wang, R.; Liu, X.; Schoepf, U.J.; van Assen, M.; Alimohamed, I.; Griffith, L.P.; Luo, T.; Sun, Z.; Fan, Z.; Xu, L. Extracellular volume quantitation using dual-energy CT in patients with heart failure: Comparison with 3T cardiac MR. Int. J. Cardiol. 2018, 268, 236–240. [Google Scholar] [CrossRef]
- Ohta, Y.; Kishimoto, J.; Kitao, S.; Yunaga, H.; Mukai-Yatagai, N.; Fujii, S.; Yamamoto, K.; Fukuda, T.; Ogawa, T. Investigation of myocardial extracellular volume fraction in heart failure patients using iodine map with rapid-kV switching dual-energy CT: Segmental comparison with MRI T1 mapping. J. Cardiovasc. Comput. Tomogr. 2020, 14, 349–355. [Google Scholar] [CrossRef]
- Tarkowski, P.; Czekajska-Chehab, E. Dual-energy heart CT: Beyond better angiography—Review. J. Clin. Med. 2021, 10, 5193. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dell’Aversana, S.; Ascione, R.; De Giorgi, M.; De Lucia, D.R.; Cuocolo, R.; Boccalatte, M.; Sibilio, G.; Napolitano, G.; Muscogiuri, G.; Sironi, S.; et al. Dual-Energy CT of the Heart: A Review. J. Imaging 2022, 8, 236. https://doi.org/10.3390/jimaging8090236
Dell’Aversana S, Ascione R, De Giorgi M, De Lucia DR, Cuocolo R, Boccalatte M, Sibilio G, Napolitano G, Muscogiuri G, Sironi S, et al. Dual-Energy CT of the Heart: A Review. Journal of Imaging. 2022; 8(9):236. https://doi.org/10.3390/jimaging8090236
Chicago/Turabian StyleDell’Aversana, Serena, Raffaele Ascione, Marco De Giorgi, Davide Raffaele De Lucia, Renato Cuocolo, Marco Boccalatte, Gerolamo Sibilio, Giovanni Napolitano, Giuseppe Muscogiuri, Sandro Sironi, and et al. 2022. "Dual-Energy CT of the Heart: A Review" Journal of Imaging 8, no. 9: 236. https://doi.org/10.3390/jimaging8090236
APA StyleDell’Aversana, S., Ascione, R., De Giorgi, M., De Lucia, D. R., Cuocolo, R., Boccalatte, M., Sibilio, G., Napolitano, G., Muscogiuri, G., Sironi, S., Di Costanzo, G., Cavaglià, E., Imbriaco, M., & Ponsiglione, A. (2022). Dual-Energy CT of the Heart: A Review. Journal of Imaging, 8(9), 236. https://doi.org/10.3390/jimaging8090236








