A Hybrid 3D-2D Image Registration Framework for Pedicle Screw Trajectory Registration between Intraoperative X-ray Image and Preoperative CT Image
Abstract
1. Introduction
2. Literature Survey
2.1. 3D-2D Registration Principled Surgical Tool Navigation Systems
2.2. ICP Based 3D-2D Registration in the Clinical Applications
2.3. Navigation Systems Based on Augmented Reality and Robotic Assistance
3. Materials and Methods
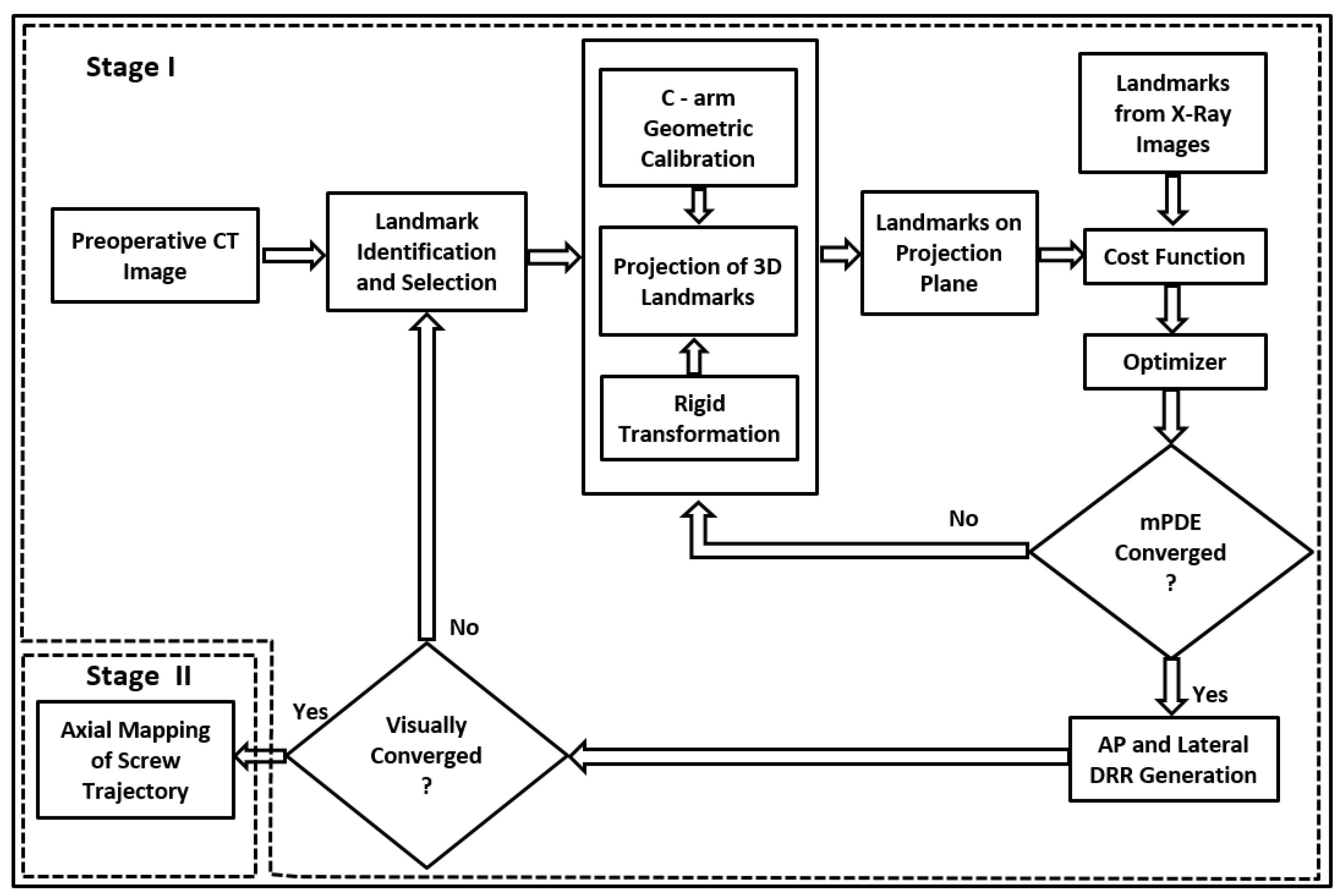
3.1. Stage I—Anatomy Landmark Registration in AP and Lateral Planes
3.1.1. Preprocessing and Trajectory Planning
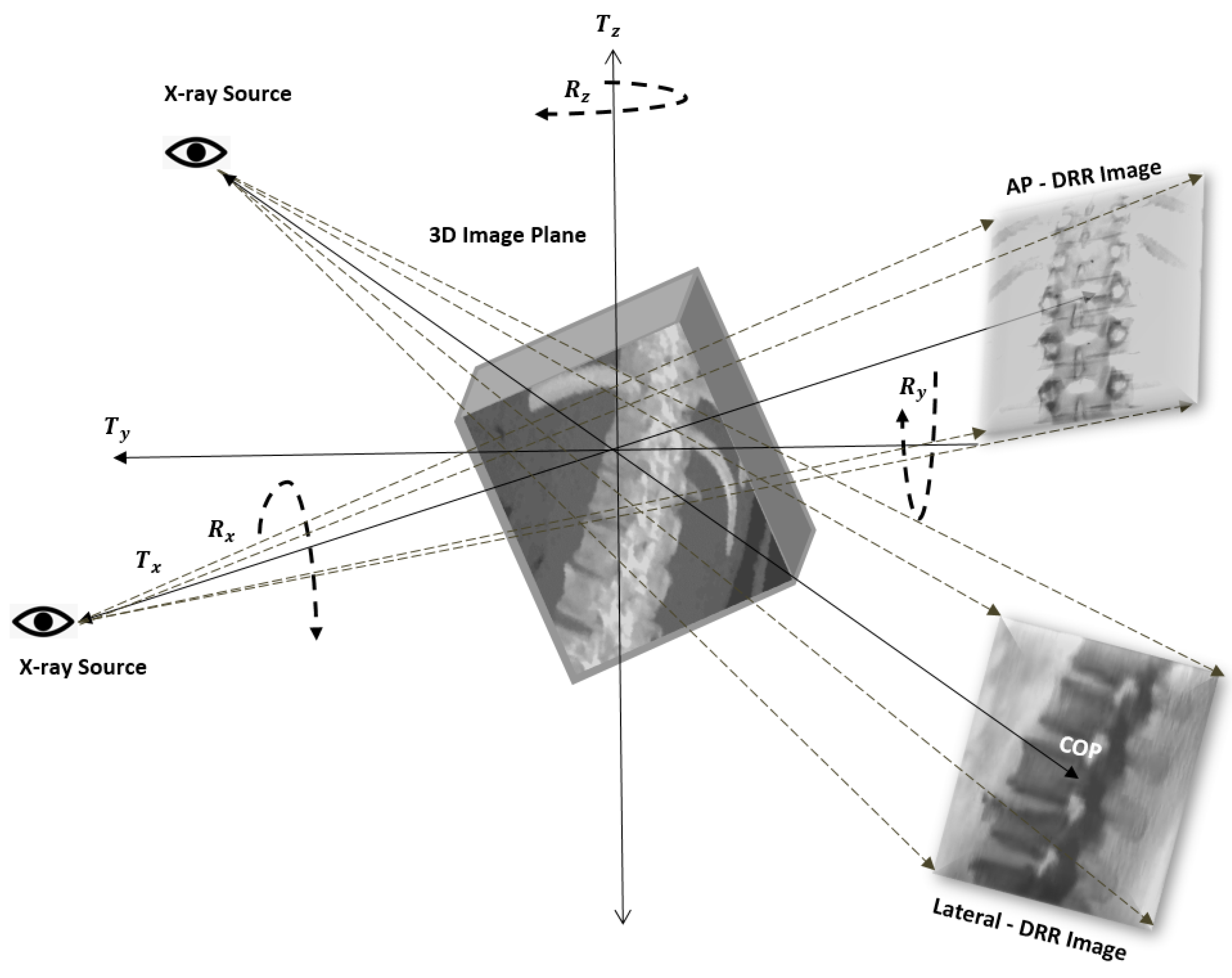
3.1.2. C-Arm Camera Model and Mathematical Preliminaries to Project a 3D Point onto a 2D Plane
3.1.3. Generation of DRR Image
3.1.4. Anatomical Landmark Identifications and Registration
3.1.5. Cost Function
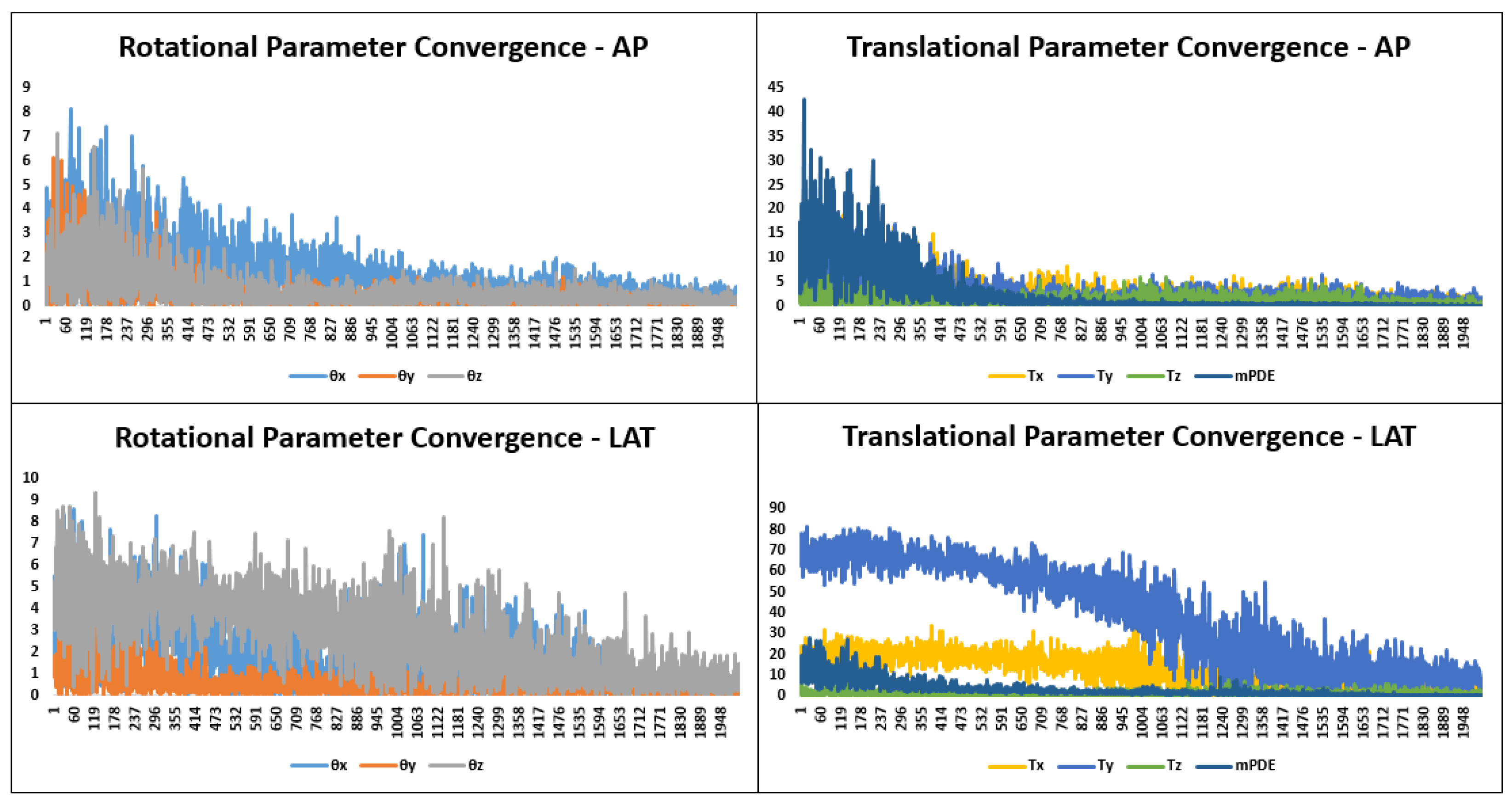
3.1.6. Optimization
3.2. Stage II—Trajectory Registration
4. Evaluation
5. Results
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Dataset Specification
| Dataset | CT Volume | CT Voxel Dimension | X-ray Image Size | X-ray Pixel Resolution |
|---|---|---|---|---|
| Patient 1 (T11 and L1) | 512 × 512 × 243 | 0.56 × 0.56 × 3 | 1024 × 1280 | 0.23 × 0.23 |
| Patient 2 (L3, L4 and L5) | 512 × 512 × 242 | 0.47 × 0.47 × 3 | 576 × 576 | 0.35 × 0.35 |
References
- Azar, F.M.; Canale, S.T.; Beaty, J.H. Campbell’s Operative Orthopaedics; E-Book; Elsevier Health Sciences: New York, NY, USA, 2020. [Google Scholar]
- Gautschi, O.P.; Schatlo, B.; Schaller, K.; Tessitore, E. Clinically relevant complications related to pedicle screw placement in thoracolumbar surgery and their management: A literature review of 35,630 pedicle screws. Neurosurg. Focus 2011, 31, E8. [Google Scholar] [CrossRef]
- Samdani, A.F.; Ranade, A.; Sciubba, D.M.; Cahill, P.J.; Antonacci, M.D.; Clements, D.H.; Betz, R.R. Accuracy of free-hand placement of thoracic pedicle screws in adolescent idiopathic scoliosis: How much of a difference does surgeon experience make? Eur. Spine J. 2010, 19, 91–95. [Google Scholar] [CrossRef]
- Gertzbein, S.D.; Robbins, S.E. Accuracy of pedicular screw placement in vivo. Spine 1990, 15, 11–14. [Google Scholar] [CrossRef]
- Puvanesarajah, V.; Liauw, J.A.; Lo, S.-F.; Lina, I.A.; Witham, T.F. Techniques and accuracy of thoracolumbar pedicle screw placement. World J. Orthop. 2014, 5, 112. [Google Scholar] [CrossRef]
- Nolte, L.-P.; Zamorano, L.J.; Jiang, Z.; Wang, Q.; Langlotz, F.; Berlemann, U. Image-guided insertion of transpedicular screws. A laboratory set-up. SAS J. 2010, 4, 129–130. [Google Scholar] [CrossRef]
- Abrah ao, G.S.; Rosa, R.C.; Okubo, R.; Shimano, A.C. Effect of the pilot hole preparation on the anchorage of pedicle screws. Acta Ortop. Bras. 2012, 20, 274–279. [Google Scholar]
- Erkan, S.; Hsu, B.; Wu, C.; Mehbod, A.A.; Perl, J.; Transfeldt, E.E. Alignment of pedicle screws with pilot holes: Can tapping improve screw trajectory in thoracic spines? Eur. Spine J. 2010, 19, 71–77. [Google Scholar] [CrossRef][Green Version]
- Silva, P.; Rosa, R.C.; Shimano, A.C.; Defino, H.L. Effect of pilot hole on biomechanical and in vivo pedicle screw–bone interface. Eur. Spine J. 2013, 22, 1829–1836. [Google Scholar] [CrossRef]
- Ito, Y.; Sugimoto, Y.; Tomioka, M.; Hasegawa, Y.; Nakago, K.; Yagata, Y. Clinical accuracy of 3D fluoroscopy–assisted cervical pedicle screw insertion. J. Neurosurg. Spine 2008, 9, 450–453. [Google Scholar] [CrossRef]
- Sembrano, J.N.; Polly, D.W.; Ledonio, C.G.T.; Santos, E.R.G. Intraoperative 3-dimensional imaging (o-arm) for assessment of pedicle screw position: Does it prevent unacceptable screw placement? Int. J. Spine Surg. 2012, 6, 49–54. [Google Scholar] [CrossRef]
- Silbermann, J.; Riese, F.; Allam, Y.; Reichert, T.; Koeppert, H.; Gutberlet, M. Computer tomography assessment of pedicle screw placement in lumbar and sacral spine: Comparison between free-hand and o-arm based navigation techniques. Eur. Spine J. 2011, 20, 875–881. [Google Scholar] [CrossRef]
- Nowitzke, A.; Wood, M.; Cooney, K. Improving accuracy and reducing errors in spinal surgery—A new technique for thoracolumbar-level localization using computer–assisted image guidance. Spine J. 2008, 8, 597–604. [Google Scholar] [CrossRef]
- Patil, S.; Lindley, E.M.; Burger, E.L.; Yoshihara, H.; Patel, V.V. Pedicle screw placement with o-arm and stealth navigation. Orthopedics 2012, 35, e61–e65. [Google Scholar] [CrossRef]
- Newell, R.; Esfandiari, H.; Anglin, C.; Bernard, R.; Street, J.; Hodgson, A.J. An intraoperative fluoroscopic method to accurately measure the post-implantation position of pedicle screws. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 1257–1267. [Google Scholar] [CrossRef]
- Manbachi, A.; Cobbold, R.S.; Ginsberg, H.J. Guided pedicle screw insertion: Techniques and training. Spine J. 2014, 14, 165–179. [Google Scholar] [CrossRef]
- Ripka, P.; Tipek, A. Modern Sensors Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Fan, Z.; Chen, G.; Wang, J.; Liao, H. Spatial position measurement system for surgical navigation using 3-d image marker-based tracking tools with compact volume. IEEE Trans. Biomed. Eng. 2017, 65, 378–389. [Google Scholar] [CrossRef]
- Fu, D.; Kuduvalli, G. A fast, accurate, and automatic 2D–3D image registration for image-guided cranial radiosurgery. Med. Phys. 2008, 35, 2180–2194. [Google Scholar] [CrossRef]
- Jin, J.-Y.; Ryu, S.; Faber, K.; Mikkelsen, T.; Chen, Q.; Li, S.; Movsas, B. 2D/3D image fusion for accurate target localization and evaluation of a mask based stereotactic system in fractionated stereotactic radiotherapy of cranial lesions. Med. Phys. 2006, 33, 4557–4566. [Google Scholar] [CrossRef]
- Markelj, P.; Tomaževič, D.; Likar, B.; Pernuš, F. A review of 3D/2D registration methods for image-guided interventions. Med. Image Anal. 2012, 16, 642–661. [Google Scholar] [CrossRef]
- de Kraats, E.B.V.; Penney, G.P.; Tomazevic, D.; Walsum, T.V.; Niessen, W.J. Standardized evaluation methodology for 2-D-3-D registration. IEEE Trans. Med. Imaging 2005, 24, 1177–1189. [Google Scholar] [CrossRef]
- Uneri, A.; Silva, T.D.; Stayman, J.; Kleinszig, G.; Vogt, S.; Khanna, A.; Gokaslan, Z.; Wolinsky, J.; Siewerdsen, J. Known-component 3D–2D registration for quality assurance of spine surgery pedicle screw placement. Phys. Med. Biol. 2015, 60, 8007. [Google Scholar] [CrossRef]
- Uneri, A.; Stayman, J.W.; Silva, T.D.; Wang, A.; Kleinszig, G.; Vogt, S.; Khanna, A.J.; Wolinsky, J.-P.; Gokaslan, Z.L.; Siewerdsen, J.H. Known-component 3D-2D registration for image guidance and quality assurance in spine surgery pedicle screw placement. In Medical Imaging 2015: Image-Guided Procedures, Robotic Interventions, and Modeling Proceedings, Orlando, FL, USA, 21–26 February 2015; International Society for Optics and Photonics: Bellingham, WA, USA, 2015; Volume 9415, p. 94151F. [Google Scholar]
- Uneri, A.; Otake, Y.; Wang, A.; Kleinszig, G.; Vogt, S.; Khanna, A.J.; Siewerdsen, J. 3D–2D registration for surgical guidance: Effect of projection view angles on registration accuracy. Phys. Med. Biol. 2013, 59, 271. [Google Scholar] [CrossRef]
- Uneri, A.; Silva, T.D.; Goerres, J.; Jacobson, M.; Ketcha, M.; Reaungamornrat, S.; Kleinszig, G.; Vogt, S.; Khanna, A.; Osgood, G.; et al. Intraoperative evaluation of device placement in spine surgery using known-component 3D–2D image registration. Phys. Med. Biol. 2017, 62, 3330. [Google Scholar] [CrossRef]
- Uneri, A.; Goerres, J.; Silva, T.D.; Jacobson, M.W.; Ketcha, M.D.; Reaungamornrat, S.; Kleinszig, G.; Vogt, S.; Khanna, A.J.; Wolinsky, J.-P.; et al. Deformable 3D-2D registration of known components for image guidance in spine surgery. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Athens, Greece, 17–21 October 2016; Springer: Berlin, Germany, 2016; pp. 124–132. [Google Scholar]
- Esfandiari, H.; Newell, R.; Anglin, C.; Street, J.; Hodgson, A.J. A deep learning framework for segmentation and pose estimation of pedicle screw implants based on c-arm fluoroscopy. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 1269–1282. [Google Scholar] [CrossRef]
- Esfandiari, H.; Anglin, C.; Guy, P.; Street, J.; Weidert, S.; Hodgson, A.J. A comparative analysis of intensity-based 2D–3D registration for intraoperative use in pedicle screw insertion surgeries. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 1725–1739. [Google Scholar] [CrossRef]
- Jaramaz, B.; Eckman, K. 2d/3d registration for measurement of implant alignment after total hip replacement. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Athens, Greece, 17–21 October 2016; Springer: Berlin, Germany, 2006; pp. 653–661. [Google Scholar]
- Goerres, J.; Uneri, A.; Jacobson, M.; Ramsay, B.; Silva, T.D.; Ketcha, M.; Han, R.; Manbachi, A.; Vogt, S.; Kleinszig, G.; et al. Planning, guidance, and quality assurance of pelvic screw placement using deformable image registration. Phys. Med. Biol. 2017, 62, 9018. [Google Scholar] [CrossRef]
- Guan, S.-Y.; Wang, T.-M.; Meng, C.; Wang, J.-C. A review of point feature based medical image registration. Chin. J. Mech. Eng. 2018, 31, 76. [Google Scholar] [CrossRef]
- Zhu, J.; Li, H.; Ai, D.; Yang, Q.; Fan, J.; Huang, Y.; Song, H.; Han, Y.; Yang, J. Iterative closest graph matching for non-rigid 3D/2D coronary arteries registration. Comput. Methods Programs Biomed. 2021, 199, 105901. [Google Scholar] [CrossRef]
- Fu, K.; Liu, Y.; Wang, M. Global registration of 3D cerebral vessels to its 2D projections by a new branch-and-bound algorithm. IEEE Trans. Med. Robot. Bionics 2021, 3, 115–124. [Google Scholar] [CrossRef]
- McLaughlin, R.A.; Hipwell, J.; Hawkes, D.J.; Noble, J.A.; Byrne, J.V.; Cox, T.C. A comparison of a similarity-based and a feature-based 2-D-3-D registration method for neurointerventional use. IEEE Trans. Med. Imaging 2005, 24, 1058–1066. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, Y.; Song, Z.; Wang, M. 2D-3D point set registration based on global rotation search. IEEE Trans. Image Process. 2018, 28, 2599–2613. [Google Scholar] [CrossRef]
- Hu, X.; Baena, F.R.Y.; Cutolo, F. Head-mounted augmented reality platform for markerless orthopaedic navigation. IEEE J. Biomed. Health Inform. 2021, 26, 910–921. [Google Scholar] [CrossRef]
- Liebmann, F.; Roner, S.; von Atzigen, M.; Scaramuzza, D.; Sutter, R.; Snedeker, J.; Farshad, M.; Fürnstahl, P. Pedicle screw navigation using surface digitization on the microsoft hololens. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 1157–1165. [Google Scholar] [CrossRef]
- Sommer, F.; Goldberg, J.L.; McGrath, L.; Kirnaz, S.; Medary, B.; Härtl, R. Image guidance in spinal surgery: A critical appraisal and future directions. Int. J. Spine Surg. 2021, 15, S74–S86. [Google Scholar] [CrossRef]
- Gsaxner, C.; Li, J.; Pepe, A.; Schmalstieg, D.; Egger, J. Inside-out instrument tracking for surgical navigation in augmented reality. In Proceedings of the 27th ACM Symposium on Virtual Reality Software and Technology, Osaka, Japan, 8–10 December 2021; pp. 1–11. [Google Scholar]
- Huang, M.; Tetreault, T.A.; Vaishnav, A.; York, P.J.; Staub, B.N. The current state of navigation in robotic spine surgery. Ann. Transl. Med. 2021, 9, 86. [Google Scholar] [CrossRef]
- Ketcha, M.; Silva, T.D.; Uneri, A.; Jacobson, M.; Goerres, J.; Kleinszig, G.; Vogt, S.; Wolinsky, J.; Siewerdsen, J. Multi-stage 3D–2D registration for correction of anatomical deformation in image-guided spine surgery. Phys. Med. Biol. 2017, 62, 4604. [Google Scholar] [CrossRef]
- Szczodry, M.; Solitro, G.F.; Amirouche, F.; Patel, P. Pedicle screw with increased cortical purchase can be inserted with same accuracy as the screw in straightforward trajectory using 3D modeling landmarks. Spine Deform. 2018, 6, 20–27. [Google Scholar] [CrossRef]
- Xu, W.-X.; Ding, W.-G.; Xu, B.; Hu, T.-H.; Sheng, H.-F.; Zhu, J.-F.; Zhu, X.-L. Appropriate insertion point for percutaneous pedicle screw placement in the lumbar spine using c-arm fluoroscopy: A cadaveric study. BMC Musculoskelet. Disord. 2020, 21, 750. [Google Scholar] [CrossRef]
- Dorgham, O.M.; Laycock, S.D.; Fisher, M.H. Gpu accelerated generation of digitally reconstructed radiographs for 2-D/3-D image registration. IEEE Trans. Biomed. Eng. 2012, 59, 2594–2603. [Google Scholar] [CrossRef]
- Hansen, N. The CMA evolution strategy: A comparing review. In Towards a New Evolutionary Computation; Springer: Berlin/Heidelberg, Germany, 2006; pp. 75–102. [Google Scholar]
- Li, Z.; Zhang, Q. A simple yet efficient rank one update for covariance matrix adaptation. arXiv 2017, arXiv:1710.03996. [Google Scholar]
- Hansen, N.; Ostermeier, A. Adapting arbitrary normal mutation distributions in evolution strategies: The covariance matrix adaptation. Proceedings of IEEE International Conference on Evolutionary Computation, Nagoya, Japan, 20–22 May 1996; pp. 312–317. [Google Scholar]
- Rampersaud, Y.R.; Simon, D.A.; Foley, K.T. Accuracy requirements for image-guided spinal pedicle screw placement. Spine 2001, 26, 352–359. [Google Scholar] [CrossRef]
- Bai, J.-Y.; Zhang, W.; An, J.-L.; Sun, Y.-P.; Ding, W.-Y.; Shen, Y. True anteroposterior view pedicle screw insertion technique. Ther. Clin. Risk Manag. 2016, 12, 1039. [Google Scholar]

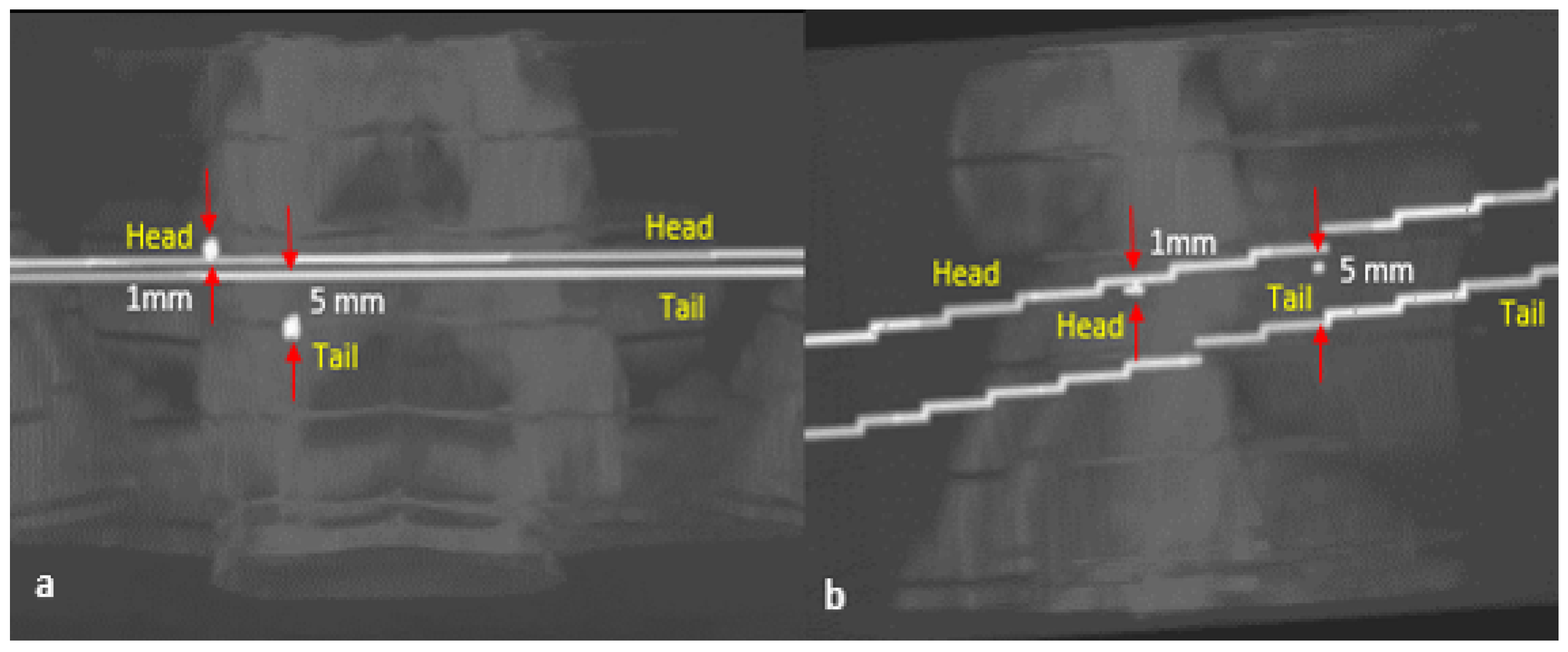

| Vertebral Level | Head | Tail |
|---|---|---|
| T11 | 2.00 mm | 1.00 mm |
| L1 | 0.00 mm | 0.00 mm |
| L3 | 0.00 mm | 1.00 mm |
| L4 | 0.00 mm | 1.00 mm |
| L5 | 1.00 mm | 5.00 mm |
| Vertebral Level | AP—Plane | Lateral—Plane | Trajectory Length Error | ||||
|---|---|---|---|---|---|---|---|
| Head | Tail | Angle | Head | Tail | Angle | ||
| T11 | 1.00 mm | 0.00 mm | 1.00 mm | 2.00 mm | 2.06 mm | ||
| L1 | 1.00 mm | 0.00 mm | 0.00 mm | 1.00 mm | 0.91 mm | ||
| L3 | 0.00 mm | 1.00 mm | 0.00 mm | 0.00 mm | 3.68 mm | ||
| L4 | 0.00 mm | 0.00 mm | 1.00 mm | 2.00 mm | 6.65 mm | ||
| L5 | 1.00 mm | 3.00 mm | 1.41 mm | 3.16 mm | 0.03 mm | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naik, R.R.; Hoblidar, A.; Bhat, S.N.; Ampar, N.; Kundangar, R. A Hybrid 3D-2D Image Registration Framework for Pedicle Screw Trajectory Registration between Intraoperative X-ray Image and Preoperative CT Image. J. Imaging 2022, 8, 185. https://doi.org/10.3390/jimaging8070185
Naik RR, Hoblidar A, Bhat SN, Ampar N, Kundangar R. A Hybrid 3D-2D Image Registration Framework for Pedicle Screw Trajectory Registration between Intraoperative X-ray Image and Preoperative CT Image. Journal of Imaging. 2022; 8(7):185. https://doi.org/10.3390/jimaging8070185
Chicago/Turabian StyleNaik, Roshan Ramakrishna, Anitha Hoblidar, Shyamasunder N. Bhat, Nishanth Ampar, and Raghuraj Kundangar. 2022. "A Hybrid 3D-2D Image Registration Framework for Pedicle Screw Trajectory Registration between Intraoperative X-ray Image and Preoperative CT Image" Journal of Imaging 8, no. 7: 185. https://doi.org/10.3390/jimaging8070185
APA StyleNaik, R. R., Hoblidar, A., Bhat, S. N., Ampar, N., & Kundangar, R. (2022). A Hybrid 3D-2D Image Registration Framework for Pedicle Screw Trajectory Registration between Intraoperative X-ray Image and Preoperative CT Image. Journal of Imaging, 8(7), 185. https://doi.org/10.3390/jimaging8070185






