Development of a Visualisation Approach for Analysing Incipient and Clinically Unrecorded Enamel Fissure Caries Using Laser-Induced Contrast Imaging, MicroRaman Spectroscopy and Biomimetic Composites: A Pilot Study
Abstract
:1. Introduction
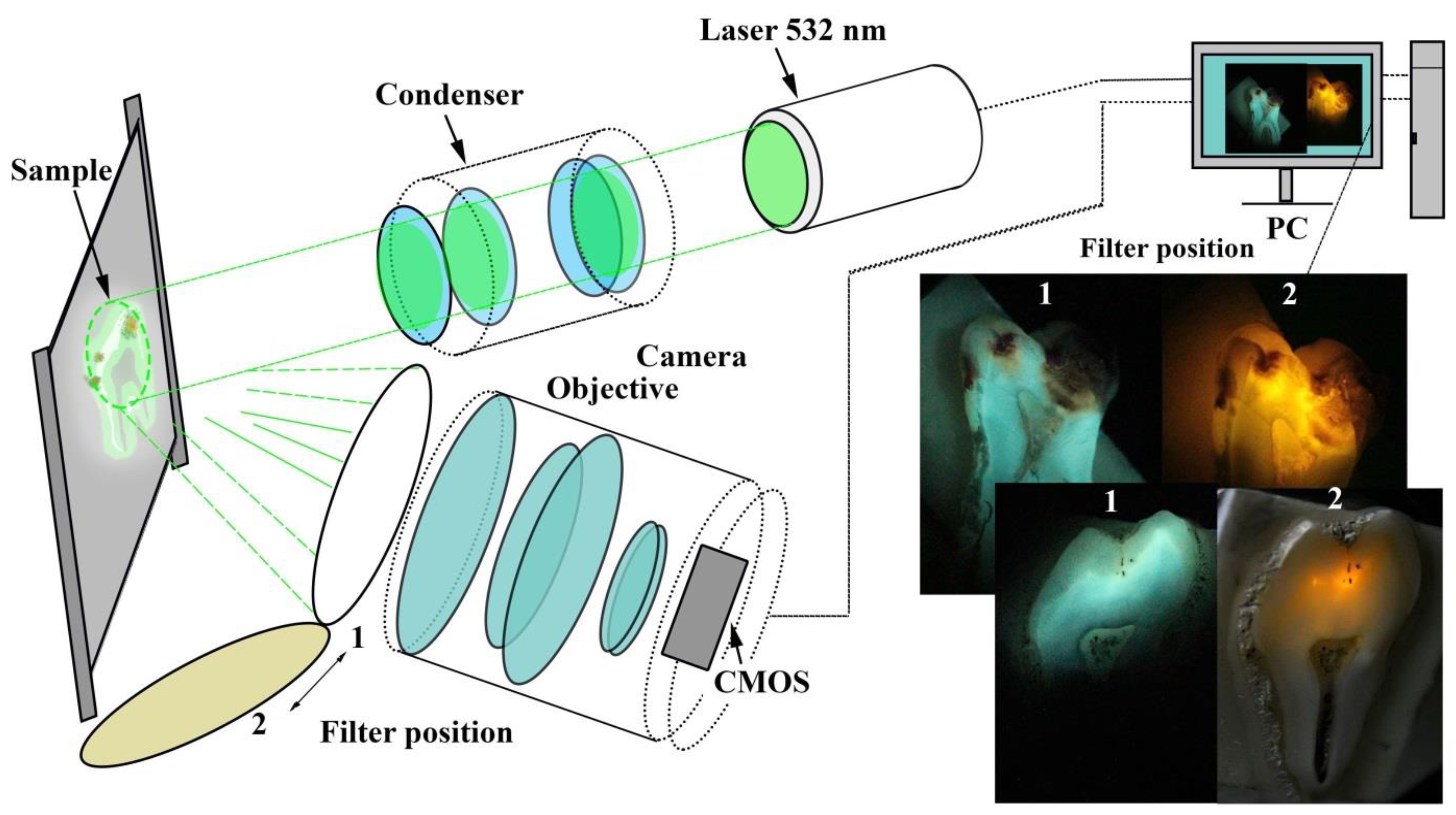
2. Materials and Methods of Investigation
3. Results
3.1. Laser-Induced Fluorescence
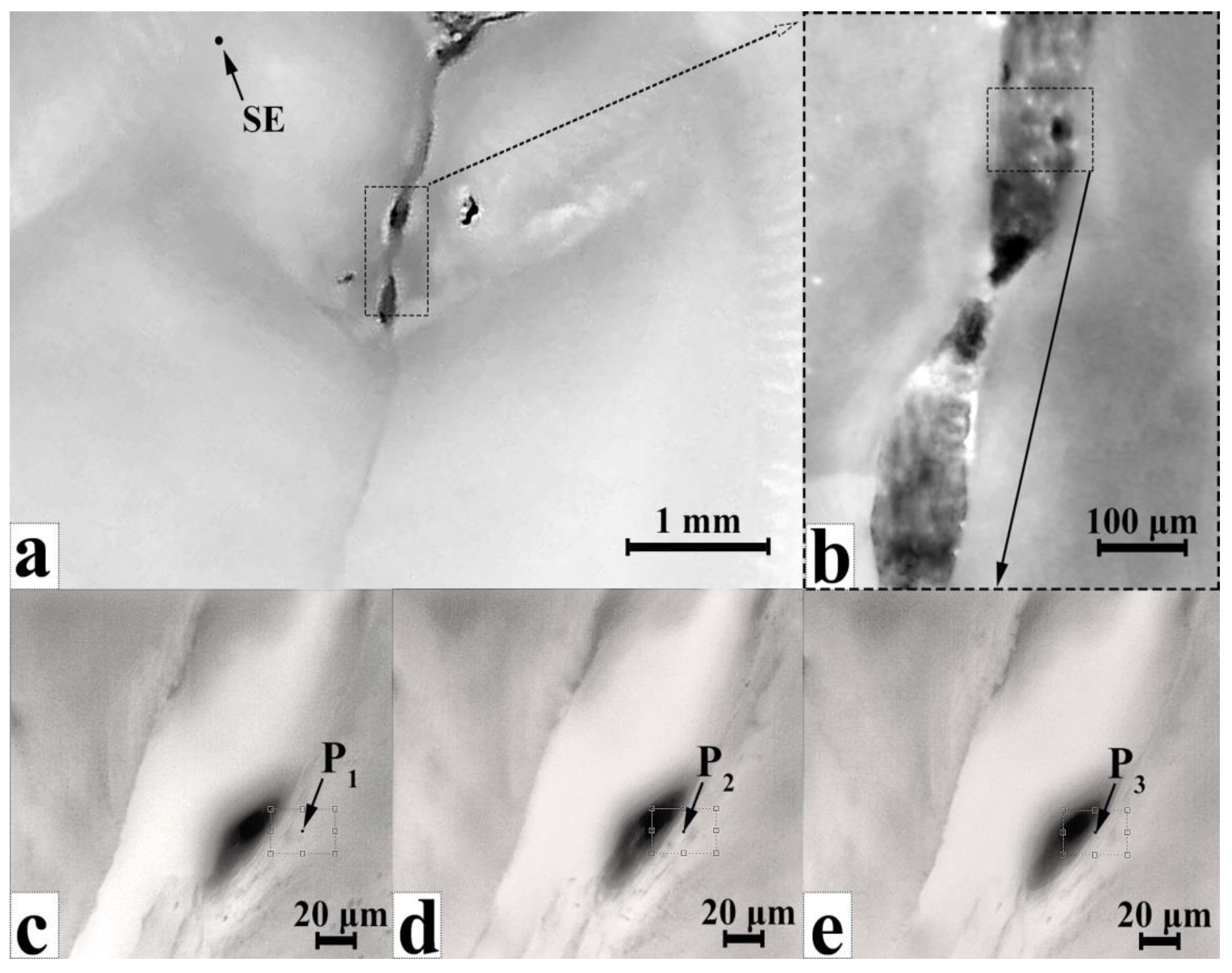
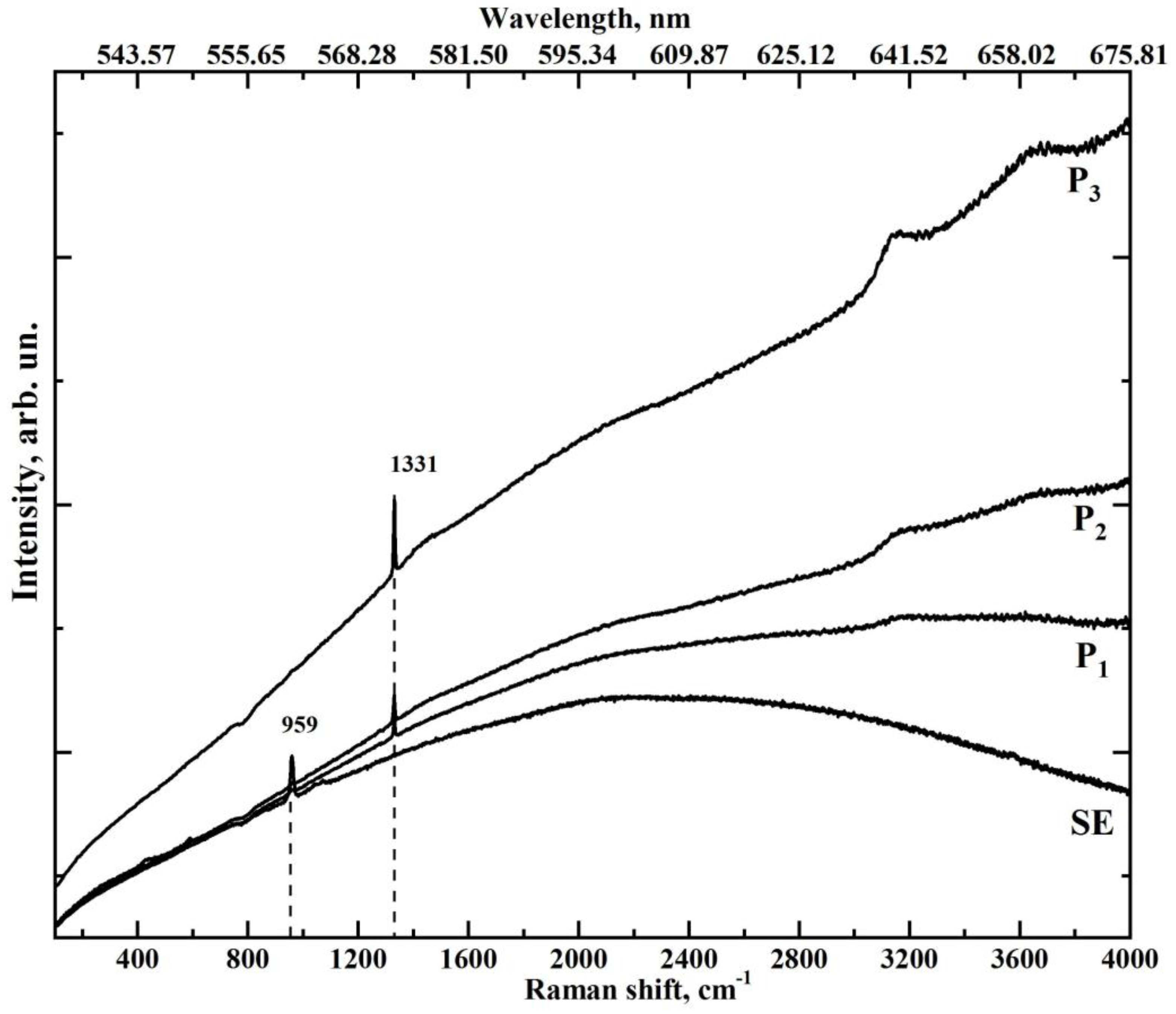
3.2. MicroRaman Spectroscopy
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethical Permission
References
- Zhang, Y.Y.; Li, Q.L.; Wong, H.M. Cell-Free Biomimetic Mineralization Strategies to Regenerate the Enamel Microstructure. Crystals 2021, 11, 1385. [Google Scholar] [CrossRef]
- Pandya, M.; Diekwisch, T.G.H. Enamel Biomimetics—Fiction or Future of Dentistry. Int. J. Oral Sci. 2019, 11, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seredin, P.; Goloshchapov, D.; Ippolitov, Y.; Vongsvivut, J. Engineering of a Biomimetic Interface between a Native Dental Tissue and Restorative Composite and Its Study Using Synchrotron FTIR Microscopic Mapping. Int. J. Mol. Sci. 2021, 22, 6510. [Google Scholar] [CrossRef] [PubMed]
- Goloshchapov, D.; Kashkarov, V.; Nikitkov, K.; Seredin, P. Investigation of the Effect of Nanocrystalline Calcium Carbonate-Substituted Hydroxyapatite and L-Lysine and L-Arginine Surface Interactions on the Molecular Properties of Dental Biomimetic Composites. Biomimetics 2021, 6, 70. [Google Scholar] [CrossRef]
- Bomfim, D.I.; Rahim, N.M.; Austin, R.S. Biomechanical Planning for Minimally Invasive Indirect Restorations. Br. Dent. J. 2020, 229, 425–429. [Google Scholar] [CrossRef]
- Diniz, M.B.; Rodrigues, J.A.; Lussi, A. Traditional and Novel Caries Detection Methods. In Contemporary Approach to Dental Caries; Li, M.-Y., Ed.; InTech: London, UK, 2012; pp. 105–128. ISBN 978-953-51-0305-9. [Google Scholar]
- Patil, S.; Alkahtani, A.; Bhandi, S.; Mashyakhy, M.; Alvarez, M.; Alroomy, R.; Hendi, A.; Varadarajan, S.; Reda, R.; Raj, A.T.; et al. Ultrasound Imaging versus Radiographs in Differentiating Periapical Lesions: A Systematic Review. Diagnostics 2021, 11, 1208. [Google Scholar] [CrossRef]
- Shimada, Y.; Sadr, A.; Sumi, Y.; Tagami, J. Application of Optical Coherence Tomography (OCT) for Diagnosis of Caries, Cracks, and Defects of Restorations. Curr. Oral Health Rep. 2015, 2, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Reda, R.; Zanza, A.; Mazzoni, A.; Cicconetti, A.; Testarelli, L.; Di Nardo, D. An Update of the Possible Applications of Magnetic Resonance Imaging (MRI) in Dentistry: A Literature Review. J. Imaging 2021, 7, 75. [Google Scholar] [CrossRef]
- Contaldo, M.; Di Stasio, D.; Santoro, R.; Laino, L.; Perillo, L.; Petruzzi, M.; Lauritano, D.; Serpico, R.; Lucchese, A. Non-Invasive In Vivo Visualization of Enamel Defects by Reflectance Confocal Microscopy (RCM). Odontology 2015, 103, 177–184. [Google Scholar] [CrossRef]
- Contaldo, M.; Serpico, R.; Lucchese, A. In Vivo Imaging of Enamel by Reflectance Confocal Microscopy (RCM): Non-Invasive Analysis of Dental Surface. Odontology 2014, 102, 325–329. [Google Scholar] [CrossRef]
- Chen, Q.G.; Zhu, H.H.; Xu, Y.; Lin, B.; Chen, H. Quantitative Method to Assess Caries via Fluorescence Imaging from the Perspective of Autofluorescence Spectral Analysis. Laser Phys. 2015, 25, 085601. [Google Scholar] [CrossRef]
- Contaldo, M.; Di Stasio, D.; della Vella, F.; Lauritano, D.; Serpico, R.; Santoro, R.; Lucchese, A. Real Time In Vivo Confocal Microscopic Analysis of the Enamel Remineralization by Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP): A Clinical Proof-of-Concept Study. Appl. Sci. 2020, 10, 4155. [Google Scholar] [CrossRef]
- Tassery, H.; Levallois, B.; Terrer, E.; Manton, D.; Otsuki, M.; Koubi, S.; Gugnani, N.; Panayotov, I.; Jacquot, B.; Cuisinier, F.; et al. Use of New Minimum Intervention Dentistry Technologies in Caries Management. Aust. Dent. J. 2013, 58, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Haapasalo, M.; Imazato, S.; Lee, J.I.; Momoi, Y.; Murakami, S.; Whelton, H.; Wilson, N. Dentistry in the 21st Century: Challenges of a Globalising World. Int. Dent. J. 2014, 64, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Besnard, C.; Harper, R.A.; Moxham, T.E.J.; James, J.D.; Storm, M.; Salvati, E.; Landini, G.; Shelton, R.M.; Korsunsky, A.M. 3D Analysis of Enamel Demineralisation in Human Dental Caries Using High-Resolution, Large Field of View Synchrotron X-Ray Micro-Computed Tomography. Mater. Today Commun. 2021, 27, 102418. [Google Scholar] [CrossRef]
- Paolone, G. Direct Composites in Anteriors: A Matter of Substrate. Int. J. Esthet. Dent. 2017, 12, 468–481. [Google Scholar]
- Scolavino, S.; Paolone, G.; Orsini, G.; Devoto, W.; Putignano, A. The Simultaneous Modeling Technique: Closing Gaps in Posteriors. Int. J. Esthet. Dent. 2016, 11, 58–81. [Google Scholar]
- Wang, P.; Anderson, E.J.D.; Muller, E.A.; Gao, F.; Zhong, Y.; Raschke, M.B. Hyper-Spectral Raman Imaging Correlating Chemical Substitution and Crystallinity in Biogenic Hydroxyapatite: Dentin and Enamel in Normal and Hypoplastic Human Teeth. J. Raman Spectrosc. 2018, 49, 1559–1567. [Google Scholar] [CrossRef]
- Desoutter, A.; Slimani, A.; Al-Obaidi, R.; Barthélemi, S.; Cuisinier, F.; Tassery, H.; Salehi, H. Cross Striation in Human Permanent and Deciduous Enamel Measured with Confocal Raman Microscopy. J. Raman Spectrosc. 2019, 50, 548–556. [Google Scholar] [CrossRef]
- Bērziņš, K.; Sutton, J.J.; Loch, C.; Beckett, D.; Wheeler, B.J.; Drummond, B.K.; Fraser-Miller, S.J.; Gordon, K.C. Application of Low-Wavenumber Raman Spectroscopy to the Analysis of Human Teeth. J. Raman Spectrosc. 2019, 50, 1375–1387. [Google Scholar] [CrossRef]
- Shah, F.A. Micro-Raman Spectroscopy Reveals the Presence of Octacalcium Phosphate and Whitlockite in Association with Bacteria-Free Zones Within the Mineralized Dental Biofilm. Microsc. Microanal. 2019, 25, 129–134. [Google Scholar] [CrossRef] [PubMed]
- de Barros Pinto, L.; Lira, M.L.L.A.; Cavalcanti, Y.W.; Dantas, E.L.d.A.; Vieira, M.L.O.; de Carvalho, G.G.; de Sousa, F.B. Natural Enamel Caries, Dentine Reactions, Dentinal Fluid and Biofilm. Sci. Rep. 2019, 9, 2841. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.-J.; Wang, B.-W.; Yang, C.-M.; Wu, C.-Y.; Ou-Yang, M. Autofluorescence Detection Method for Dental Plaque Bacteria Detection and Classification: Example of Porphyromonas Gingivalis, Aggregatibacter Actinomycetemcomitans, and Streptococcus Mutans. Dent. J. 2021, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Slimani, A.; Nouioua, F.; Panayotov, I.; Giraudeau, N.; Chiaki, K.; Shinji, Y.; Cloitre, T.; Levallois, B.; Gergely, C.; Cuisinier, F.; et al. Porphyrin and Pentosidine Involvement in the Red Fluorescence of Enamel and Dentin Caries. Int. J. Exp. Dent. Sci. 2016, 5, 1–10. [Google Scholar] [CrossRef]
- Moriyama, C.M.; Rodrigues, J.A.; Lussi, A.; Diniz, M.B. Effectiveness of Fluorescence-Based Methods to Detect In Situ Demineralization and Remineralization on Smooth Surfaces. Caries Res. 2014, 48, 507–514. [Google Scholar] [CrossRef]
- Akarslan, Z. Introductory Chapter: Diagnosis of Dental Caries. In Dental Caries—Diagnosis, Prevention and Management; InTech Open: London, UK, 2018. [Google Scholar]
- Iranzo-Cortés, J.E.; Montiel-Company, J.M.; Almerich-Torres, T.; Bellot-Arcís, C.; Almerich-Silla, J.M. Use of DIAGNOdent and VistaProof in Diagnostic of Pre-Cavitated Caries Lesions—A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 20. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.H.; Lee, S.R.; Choi, J.Y.; Choi, Y.S.; Kim, S.H.; Yoon, H.C.; Nelson, G. Detection of Dental Caries and Cracks with Quantitative Light-Induced Fluorescence in Comparison to Radiographic and Visual Examination: A Retrospective Case Study. Sensors 2021, 21, 1741. [Google Scholar] [CrossRef]
- Slimani, A.; Terrer, E.; Manton, D.J.; Tassery, H. Carious Lesion Detection Technologies: Factual Clinical Approaches. Br. Dent. J. 2020, 229, 432–442. [Google Scholar] [CrossRef]
- Panayotov, I.; Terrer, E.; Salehi, H.; Tassery, H.; Yachouh, J.; Cuisinier, F.J.G.; Levallois, B. In Vitro Investigation of Fluorescence of Carious Dentin Observed with a Soprolife® Camera. Clin. Oral Investig. 2012, 17, 757–763. [Google Scholar] [CrossRef]
- Muruppel, A.M. Laser-Assisted Diagnostics. In Lasers in Dentistry—Current Concepts; Coluzzi, D.J., Parker, S.P.A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 107–130. ISBN 978-3-319-51943-2. [Google Scholar]
- Thanh, M.T.G.; Van Toan, N.; Toan, D.T.T.; Thang, N.P.; Dong, N.Q.; Dung, N.T.; Hang, P.T.T.; Anh, L.Q.; Tra, N.T.; Ngoc, V.T.N. Diagnostic Value of Fluorescence Methods, Visual Inspection and Photographic Visual Examination in Initial Caries Lesion: A Systematic Review and Meta-Analysis. Dent. J. 2021, 9, 30. [Google Scholar] [CrossRef]
- Miyamoto, N.; Adachi, T.; Boschetto, F.; Zanocco, M.; Yamamoto, T.; Marin, E.; Somekawa, S.; Ashida, R.; Zhu, W.; Kanamura, N.; et al. Molecular Fingerprint Imaging to Identify Dental Caries Using Raman Spectroscopy. Materials 2020, 13, 4900. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, T.; Okulus, Z.; Szybowicz, M. Raman Spectroscopy as a Tool of Early Dental Caries Detection—New Insights. J. Raman Spectrosc. 2017, 48, 1094–1102. [Google Scholar] [CrossRef]
- Alturki, M.; Koller, G.; Warburton, F.; Almhöjd, U.; Banerjee, A. Biochemical Characterisation of Carious Dentine Zones Using Raman Spectroscopy. J. Dent. 2021, 105, 103558. [Google Scholar] [CrossRef] [PubMed]
- Almahdy, A.; Downey, F.C.; Sauro, S.; Cook, R.J.; Sherriff, M.; Richards, D.; Watson, T.F.; Banerjee, A.; Festy, F. Microbiochemical Analysis of Carious Dentine Using Raman and Fluorescence Spectroscopy. Caries Res. 2012, 46, 432–440. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Prutskij, T.; Ippolitov, Y. Phase Transformations in a Human Tooth Tissue at the Initial Stage of Caries. PLoS ONE 2015, 10, e0124008. [Google Scholar]
- Ko, A.C.; Hewko, M.; Sowa, M.G.; Dong, C.C.; Cleghorn, B.; Choo-Smith, L.-P. Early Dental Caries Detection Using a Fibre-Optic Coupled Polarization-Resolved Raman Spectroscopic System. Opt. Express 2008, 16, 6274. [Google Scholar] [CrossRef] [Green Version]
- Goloshchapov, D.L.; Kashkarov, V.M.; Ippolitov, Y.A.; Prutskij, T.; Seredin, P.V. Early Screening of Dentin Caries Using the Methods of Micro-Raman and Laser-Induced Fluorescence Spectroscopy. Results Phys. 2018, 10, 346–347. [Google Scholar] [CrossRef]
- Timchenko, E.V.; Bazhutova, I.V.; Frolov, O.O.; Volova, L.T.; Timchenko, P.E. Raman Spectroscopy for Assessment of Hard Dental Tissues in Periodontitis Treatment. Diagnostics 2021, 11, 1595. [Google Scholar] [CrossRef]
- Spizzirri, P.G.; Cochrane, N.J.; Prawer, S.; Reynolds, E.C. A Comparative Study of Carbonate Determination in Human Teeth Using Raman Spectroscopy. Caries Res. 2012, 46, 353–360. [Google Scholar] [CrossRef]
- Adachi, T.; Pezzotti, G.; Yamamoto, T.; Ichioka, H.; Boffelli, M.; Zhu, W.; Kanamura, N. Vibrational Algorithms for Quantitative Crystallographic Analyses of Hydroxyapatite-Based Biomaterials: II, Application to Decayed Human Teeth. Anal. Bioanal. Chem. 2015, 407, 3343–3356. [Google Scholar] [CrossRef]
- Al-Obaidi, R.; Salehi, H.; Desoutter, A.; Bonnet, L.; Etienne, P.; Terrer, E.; Jacquot, B.; Levallois, B.; Tassery, H.; Cuisinier, F. Chemical & Nano-Mechanical Study of Artificial Human Enamel Subsurface Lesions. Sci. Rep. 2018, 8, 4047. [Google Scholar] [PubMed] [Green Version]
- Kinoshita, H.; Miyoshi, N.; Fukunaga, Y.; Ogawa, T.; Ogasawara, T.; Sano, K. Functional Mapping of Carious Enamel in Human Teeth with Raman Microspectroscopy. J. Raman Spectrosc. 2008, 39, 655–660. [Google Scholar] [CrossRef]
- Al-Obaidi, R.; Salehi, H.; Desoutter, A.; Tassery, H.; Cuisinier, F. Formation and Assessment of Enamel Subsurface Lesions In Vitro. J. Oral Sci. 2019, 61, 454–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchwald, T.; Buchwald, Z. Assessment of the Raman Spectroscopy Effectiveness in Determining the Early Changes in Human Enamel Caused by Artificial Caries. Analyst 2019, 144, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.P.; Gomes-Silva, J.M.; Menezes-Oliveira, M.A.H.; Soares, L.E.S.; Palma-Dibb, R.G.; Borsatto, M.C. FT-Raman Spectroscopy, Μ-EDXRF Spectrometry, and Microhardness Analysis of the Dentin of Primary and Permanent Teeth. Microsc. Res. Tech. 2018, 81, 509–514. [Google Scholar] [CrossRef]
- Coello, B.; López-Álvarez, M.; Rodríguez-Domínguez, M.; Serra, J.; González, P. Quantitative Evaluation of the Mineralization Level of Dental Tissues by Raman Spectroscopy. Biomed. Phys. Eng. Express 2015, 1, 045204. [Google Scholar] [CrossRef]
- Bergholt, M.S.; Zheng, W.; Huang, Z. Characterizing Variability in In Vivo Raman Spectroscopic Properties of Different Anatomical Sites of Normal Tissue in the Oral Cavity. J. Raman Spectrosc. 2012, 43, 255–262. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, W.; Hsu, S.C.-Y.; Huang, Z. Optical Diagnosis and Characterization of Dental Caries with Polarization-Resolved Hyperspectral Stimulated Raman Scattering Microscopy. Biomed. Opt. Express 2016, 7, 1284–1293. [Google Scholar] [CrossRef] [Green Version]
- Al-Obaidi, R.; Salehi, H.; Collart-Dutilleul, P.-Y.; Jacquot, B.; Tassery, H.; Cuisinier, F.J.G.; Gergely, C.; Cloitre, T. Relationship between Changes in Chemical Composition of Enamel Subsurface Lesions and the Emitted Nonlinear Optical Signals: An In Vitro Study. CRE 2020, 54, 144–153. [Google Scholar] [CrossRef]
- Pitts, N.; Ekstrand, K.; The ICDAS Foundation. International Caries Detection and Assessment System (ICDAS) and Its International Caries Classification and Management System (ICCMS)—Methods for Staging of the Caries Process and Enabling Dentists to Manage Caries. Community Dent. Oral Epidemiol 2013, 41, e41–e52. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Ippolitov, Y.; Vongsvivut, J. Development of a New Approach to Diagnosis of the Early Fluorosis Forms by Means of FTIR and Raman Microspectroscopy. Sci. Rep. 2020, 10, 20891. [Google Scholar] [CrossRef] [PubMed]
- Goloshchapov, D.L.; Lenshin, A.S.; Savchenko, D.V.; Seredin, P.V. Importance of Defect Nanocrystalline Calcium Hydroxyapatite Characteristics for Developing the Dental Biomimetic Composites. Results Phys. 2019, 13, 102158. [Google Scholar] [CrossRef]
- Salehi, H.; Terrer, E.; Panayotov, I.; Levallois, B.; Jacquot, B.; Tassery, H.; Cuisinier, F. Functional Mapping of Human Sound and Carious Enamel and Dentin with Raman Spectroscopy. J. Biophotonics 2012, 6, 765–774. [Google Scholar] [CrossRef]
- Matošević, D.; Tarle, Z.; Miljanić, S.; Meić, Z.; Pichler, L.; Pichler, G. Laser Induced Fluorescence of Carious Lesion Porphyrins. Acta Stomatol. Croat. 2010, 44, 82–89. [Google Scholar]
- Shakibaie, F.; George, R.; Walsh, L.J. Applications of Laser Induced Fluorescence in Dentistry. Int. J. Dent. Clin. 2011, 3, 38–44. [Google Scholar]
- Zelic, K.; Milovanovic, P.; Rakocevic, Z.; Askrabic, S.; Potocnik, J.; Popovic, M.; Djuric, M. Nano-Structural and Compositional Basis of Devitalized Tooth Fragility. Dent. Mater. 2014, 30, 476–486. [Google Scholar] [CrossRef]
- Goloshchapov, D.; Buylov, N.; Emelyanova, A.; Ippolitov, I.; Ippolitov, Y.; Kashkarov, V.; Khudyakov, Y.; Nikitkov, K.; Seredin, P. Raman and XANES Spectroscopic Study of the Influence of Coordination Atomic and Molecular Environments in Biomimetic Composite Materials Integrated with Dental Tissue. Nanomaterials 2021, 11, 3099. [Google Scholar] [CrossRef]
- Robin, M.; Euw, S.V.; Renaudin, G.; Gomes, S.; Krafft, J.-M.; Nassif, N.; Azaïs, T.; Costentin, G. Insights into OCP Identification and Quantification in the Context of Apatite Biomineralization. CrystEngComm 2020, 22, 2728–2742. [Google Scholar] [CrossRef]
- Crane, N.J.; Popescu, V.; Morris, M.D.; Steenhuis, P.; Ignelzi, M.A., Jr. Raman Spectroscopic Evidence for Octacalcium Phosphate and Other Transient Mineral Species Deposited during Intramembranous Mineralization. Bone 2006, 39, 434–442. [Google Scholar] [CrossRef]
- Pezzotti, G.; Zhu, W.; Boffelli, M.; Adachi, T.; Ichioka, H.; Yamamoto, T.; Marunaka, Y.; Kanamura, N. Vibrational Algorithms for Quantitative Crystallographic Analyses of Hydroxyapatite-Based Biomaterials: I, Theoretical Foundations. Anal. Bioanal. Chem. 2015, 407, 3325–3342. [Google Scholar] [CrossRef]
- Xu, C.; Karan, K.; Yao, X.; Wang, Y. Molecular Structural Analysis of Noncarious Cervical Sclerotic Dentin Using Raman Spectroscopy. J. Raman Spectrosc. 2009, 40, 1780–1785. [Google Scholar] [CrossRef]
- Gieroba, B.; Krysa, M.; Wojtowicz, K.; Wiater, A.; Pleszczyńska, M.; Tomczyk, M.; Sroka-Bartnicka, A. The FT-IR and Raman Spectroscopies as Tools for Biofilm Characterization Created by Cariogenic Streptococci. Int. J. Mol. Sci. 2020, 21, 3811. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, L.; Zezell, D.M.; Ribeiro, A.d.C.; Gomes, L.; Ito, A.S. Fluorescence Spectroscopy of Biological Tissues—A Review. Appl. Spectrosc. Rev. 2006, 41, 575–590. [Google Scholar] [CrossRef]
- Thoms, M. Detection of Intaoral Lesions Using a Fluorescence Camera; International Society for Optics and Photonics: Bellingham, WA, USA, 2006; Volume 6137, pp. 613705–613707. [Google Scholar]
- Figueiredo, A.C.R.; Kurachi, C.; Bagnato, V.S. Comparison of Fluorescence Detection of Carious Dentin for Different Excitation Wavelengths. Caries Res. 2005, 39, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Borisova, E.; Uzunov, T.; Avramov, L. Laser-Induced Autofluorescence Study of Caries Model In Vitro. Lasers Med. Sci. 2006, 21, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Gatin, E.G.; Nagy, P.; Iordache, S.-M.; Iordache, A.-M.; Luculescu, C.R. Raman Spectroscopy: In Vivo Application for Bone Evaluation in Oral Reconstructive (Regenerative) Surgery. Diagnostics 2022, 12, 723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, L.; Wang, Q.; Wen, Z.; Liu, C.; Ding, Y. Raman Spectroscopy: A Potential Diagnostic Tool for Oral Diseases. Front. Cell Infect. Microbiol. 2022, 12, 775236. [Google Scholar] [CrossRef]
- Otel, I.; Dias, K.; Pereira, R.; Fonseca, M.; Jesus, A.P.; Mata, A.; Vassilenko, V.; Silveira, J.M.; Pessanha, S. Investigation of the Protective Suitability of a Dental Fluorinated Varnish by Means of X Ray Fluorescence and Raman Spectroscopy. J. Trace Elem. Med. Biol. 2022, 71, 126938. [Google Scholar] [CrossRef]




| Sample Group/Number of Typical Samples, n | Description | ICDAS [53] |
|---|---|---|
| A n = 5 | First molar tooth in the lower jaw: apparent carious lesion of dentin with destruction of dentin and enamel | 6 |
| B n = 5 | Second molar in the lower jaw: carious lesion of enamel and dentin with destruction | 5 |
| C n = 5 | Third molar in the upper jaw: destructive carious lesion of enamel and carious infection of dentin | 4–3 |
| D n = 5 | Third molar in the upper jaw: demineralisation of the lateral wall of the fissure, developing caries of dentin, not detectable by visual examination | 2 |
| E n = 5 | Third molar in the upper jaw: demineralisation of the lateral wall of the fissure, incipient caries in the fissure, not detectable by visual examination | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Emelyanova, A.; Buylov, N.; Ippolitov, Y.; Prutskij, T. Development of a Visualisation Approach for Analysing Incipient and Clinically Unrecorded Enamel Fissure Caries Using Laser-Induced Contrast Imaging, MicroRaman Spectroscopy and Biomimetic Composites: A Pilot Study. J. Imaging 2022, 8, 137. https://doi.org/10.3390/jimaging8050137
Seredin P, Goloshchapov D, Kashkarov V, Emelyanova A, Buylov N, Ippolitov Y, Prutskij T. Development of a Visualisation Approach for Analysing Incipient and Clinically Unrecorded Enamel Fissure Caries Using Laser-Induced Contrast Imaging, MicroRaman Spectroscopy and Biomimetic Composites: A Pilot Study. Journal of Imaging. 2022; 8(5):137. https://doi.org/10.3390/jimaging8050137
Chicago/Turabian StyleSeredin, Pavel, Dmitry Goloshchapov, Vladimir Kashkarov, Anna Emelyanova, Nikita Buylov, Yuri Ippolitov, and Tatiana Prutskij. 2022. "Development of a Visualisation Approach for Analysing Incipient and Clinically Unrecorded Enamel Fissure Caries Using Laser-Induced Contrast Imaging, MicroRaman Spectroscopy and Biomimetic Composites: A Pilot Study" Journal of Imaging 8, no. 5: 137. https://doi.org/10.3390/jimaging8050137
APA StyleSeredin, P., Goloshchapov, D., Kashkarov, V., Emelyanova, A., Buylov, N., Ippolitov, Y., & Prutskij, T. (2022). Development of a Visualisation Approach for Analysing Incipient and Clinically Unrecorded Enamel Fissure Caries Using Laser-Induced Contrast Imaging, MicroRaman Spectroscopy and Biomimetic Composites: A Pilot Study. Journal of Imaging, 8(5), 137. https://doi.org/10.3390/jimaging8050137








