Refining Tumor Treatment in Sinonasal Cancer Using Delta Radiomics of Multi-Parametric MRI after the First Cycle of Induction Chemotherapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Image Acquisition
2.3. Image Preprocessing and Segmentation
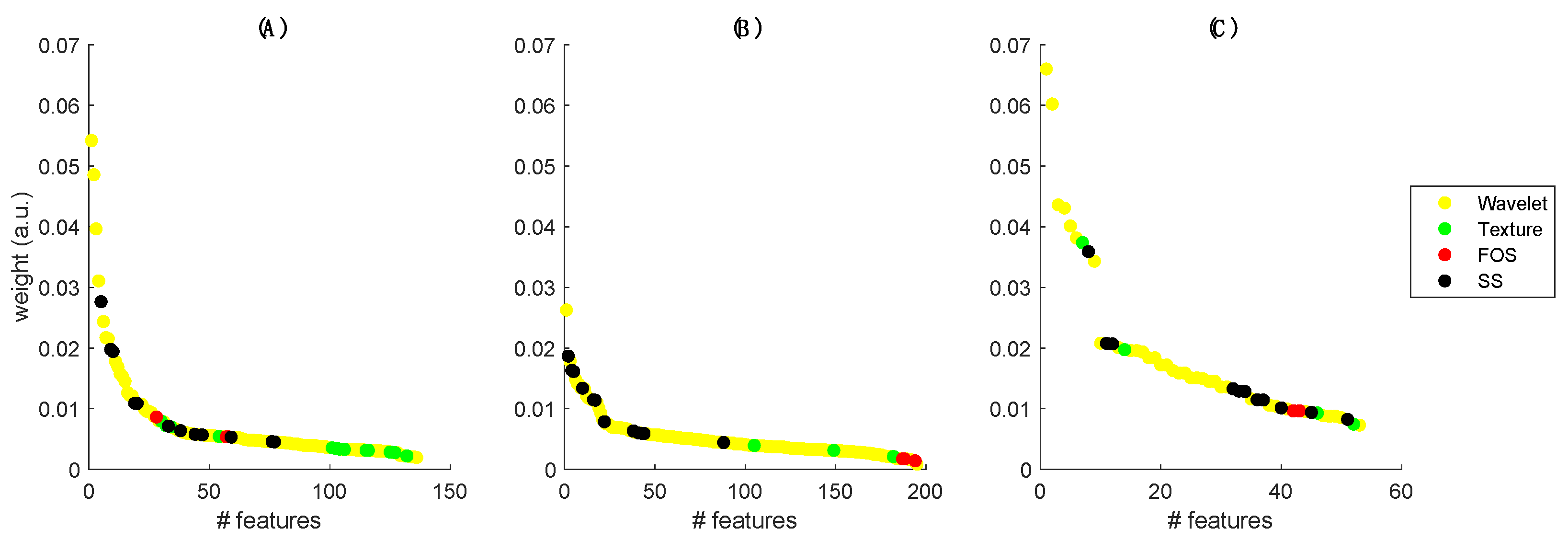
2.4. Radiomic Features Extraction
2.5. Features Selection and Classification
2.6. Comparison with Early Radiological Evaluation
2.7. Comparison with Delta-Volume Model
3. Results
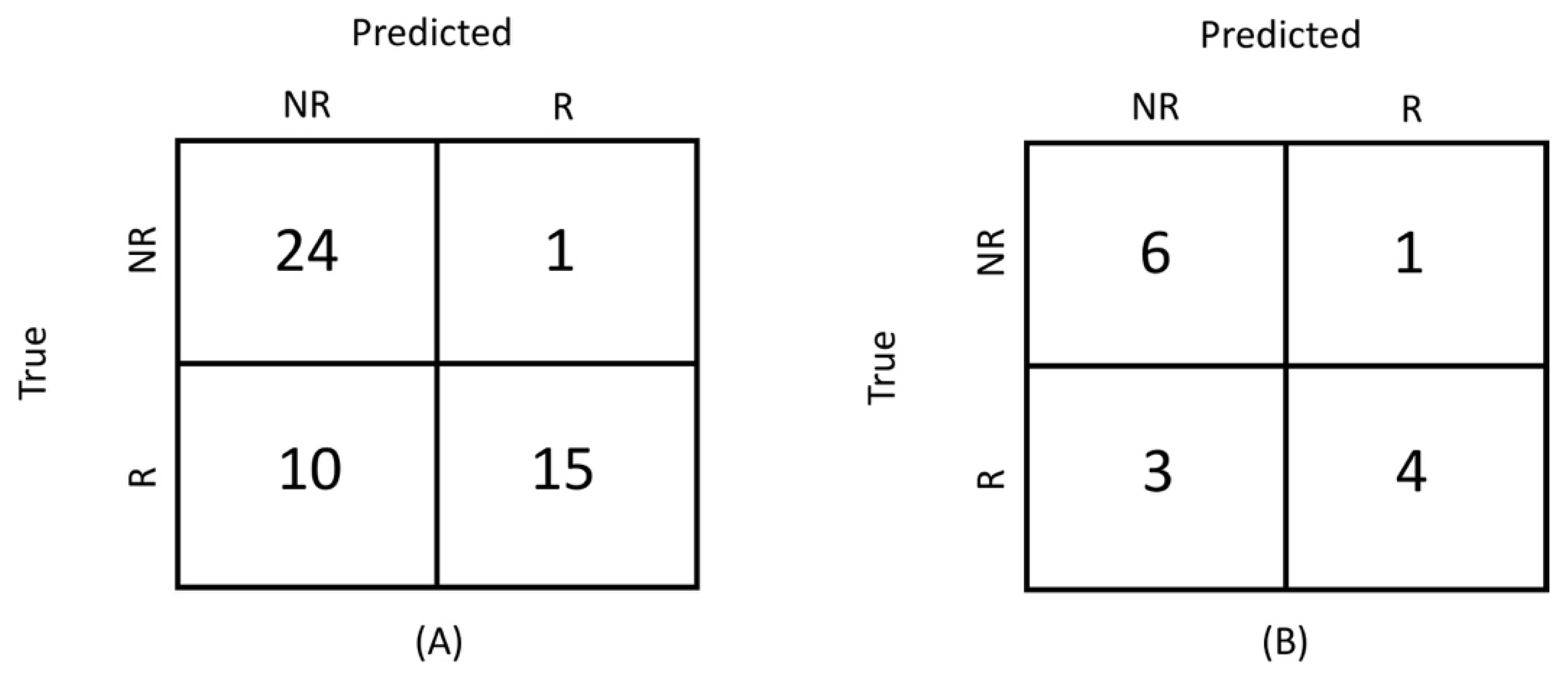
3.1. Radiomics Models
3.2. Comparison with Early Radiological Evaluation
3.3. Correlation with Delta-Volume
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dutta, R.; Dubal, P.M.; Svider, P.F.; Liu, J.K.; Baredes, S.; Eloy, J.A. Sinonasal Malignancies: A Population-Based Analysis of Site-Specific Incidence and Survival. Laryngoscope 2015, 125, 2491–2497. [Google Scholar] [CrossRef]
- Licitra, L.; Locati, L.D.; Cavina, R.; Garassino, I.; Mattevelli, F.; Pizzi, N.; Quattrone, P.; Valagussa, P.; Gianni, L.; Bonadonna, G.; et al. Primary Chemotherapy Followed by Anterior Craniofacial Resection and Radiotherapy for Paranasal Cancer. Ann. Oncol. 2003, 14, 367–372. [Google Scholar] [CrossRef]
- De Bonnecaze, G.; Verillaud, B.; Chaltiel, L.; Fierens, S.; Chapelier, M.; Rumeau, C.; Malard, O.; Gavid, M.; Dufour, X.; Righini, C.; et al. Clinical Characteristics and Prognostic Factors of Sinonasal Undifferentiated Carcinoma: A Multicenter Study. Int. Forum Allergy Rhinol. 2018, 8, 1065–1072. [Google Scholar] [CrossRef]
- Hanna, E.Y.; Cardenas, A.D.; DeMonte, F.; Roberts, D.; Kupferman, M.; Weber, R.; Rosenthal, D.; Kies, M. Induction Chemotherapy for Advanced Squamous Cell Carcinoma of the Paranasal Sinuses. Arch. Otolaryngol. Head Neck Surg. 2011, 137, 78–81. [Google Scholar] [CrossRef] [Green Version]
- Ock, C.Y.; Keam, B.; Kim, T.M.; Han, D.H.; Bin Won, T.; Lee, S.H.; Hun Hah, J.; Kwon, T.K.; Kim, D.W.; Kim, D.Y.; et al. Induction Chemotherapy in Head and Neck Squamous Cell Carcinoma of the Paranasal Sinus and Nasal Cavity: A Role in Organ Preservation. Korean J. Intern. Med. 2016, 31, 570–578. [Google Scholar] [CrossRef] [Green Version]
- Vaid, S.; Chandorkar, A.; Atre, A.; Shah, D.; Vaid, N. Differentiating Recurrent Tumours from Post-Treatment Changes in Head and Neck Cancers: Does Diffusion-Weighted MRI Solve the Eternal Dilemma? Clin. Radiol. 2017, 72, 74–83. [Google Scholar] [CrossRef]
- Brown, A.M.; Nagala, S.; Mclean, M.A.; Lu, Y.; Scoffings, D.; Apte, A.; Gonen, M.; Stambuk, H.E.; Shaha, A.R.; Tuttle, R.M.; et al. Multi-Institutional Validation of a Novel Textural Analysis Tool for Preoperative Stratification of Suspected Thyroid Tumors on Diffusion-Weighted MRI. Magn. Reson. Med. 2016, 75, 1708–1716. [Google Scholar] [CrossRef] [Green Version]
- Lambrecht, M.; Van Calster, B.; Vandecaveye, V.; De Keyzer, F.; Roebben, I.; Hermans, R.; Nuyts, S. Integrating Pretreatment Diffusion Weighted MRI into a Multivariable Prognostic Model for Head and Neck Squamous Cell Carcinoma. Radiother. Oncol. 2014, 110, 429–434. [Google Scholar] [CrossRef]
- Bologna, M.; Calareso, G.; Resteghini, C.; Sdao, S.; Montin, E.; Corino, V.; Mainardi, L.; Licitra, L.; Bossi, P. Relevance of Apparent Diffusion Coefficient Features for a Radiomics-Based Prediction of Response to Induction Chemotherapy in Sinonasal Cancer. NMR Biomed. 2020, e4265. [Google Scholar] [CrossRef]
- Lin, P.; Yang, P.F.; Chen, S.; Shao, Y.Y.; Xu, L.; Wu, Y.; Teng, W.; Zhou, X.Z.; Li, B.H.; Luo, C.; et al. A Delta-Radiomics Model for Preoperative Evaluation of Neoadjuvant Chemotherapy Response in High-Grade Osteosarcoma. Cancer Imaging 2020, 20, 7. [Google Scholar] [CrossRef] [Green Version]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Koh, D.M.; Collins, D.J. Diffusion-Weighted MRI in the Body: Applications and Challenges in Oncology. Am. J. Roentgenol. 2007, 188, 1622–1635. [Google Scholar] [CrossRef] [Green Version]
- Ye, X.; Gao, J.; Yang, Z.; Liu, Y. Apparent Diffusion Coefficient Reproducibility of the Pancreas Measured at Different MR Scanners Using Diffusion-Weighted Imaging. J. Magn. Reson. Imaging 2014, 40, 1375–1381. [Google Scholar] [CrossRef]
- Belli, G.; Busoni, S.; Ciccarone, A.; Coniglio, A.; Esposito, M.; Giannelli, M.; Mazzoni, L.N.; Nocetti, L.; Sghedoni, R.; Tarducci, R.; et al. Quality Assurance Multicenter Comparison of Different MR Scanners for Quantitative Diffusion-Weighted Imaging. J. Magn. Reson. Imaging 2016, 43, 213–219. [Google Scholar] [CrossRef]
- Corino, V.D.A.; Montin, E.; Messina, A.; Casali, P.G.; Gronchi, A.; Marchianò, A.; Mainardi, L.T. Radiomic Analysis of Soft Tissues Sarcomas Can Distinguish Intermediate from High-Grade Lesions. J. Magn. Reson. Imaging 2018, 47, 829–840. [Google Scholar] [CrossRef]
- Pyradiomics Features Description. Available online: https://pyradiomics.readthedocs.io/en/2.1.0/features.html (accessed on 20 June 2021).
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardisation Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef] [Green Version]
- Bologna, M.; Corino, V.; Mainardi, L. Technical Note: Virtual Phantom Analyses for Preprocessing Evaluation and Detection of a Robust Feature Set for MRI-Radiomics of the Brain. Med. Phys. 2019, 46, 5116–5123. [Google Scholar] [CrossRef] [Green Version]
- Bair, E.; Hastie, T.; Paul, D.; Tibshirani, R. Prediction by Supervised Principal Components. J. Am. Stat. Assoc. 2006, 101, 119–137. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Gu, Y.; Basu, S.; Berglund, A.; Eschrich, S.A.; Schabath, M.B.; Forster, K.; Aerts, H.J.W.L.; Dekker, A.; Fenstermacher, D.; et al. Radiomics: The Process and the Challenges. Magn. Reson. Imaging 2012, 30, 1234–1248. [Google Scholar] [CrossRef] [Green Version]
- Pudil, P.; Novovieova, J.; Kittler, J. Floating Search Methods in Feature Selection. Pattern Recognit. Lett. 1994, 15, 1119–1125. [Google Scholar] [CrossRef]
- Bulling, N. A Survey of Multi-Agent Decision Making. Kunstl. Intell. 2014, 28, 147–158. [Google Scholar] [CrossRef]
- Alahmari, S.S.; Cherezov, D.; Goldgof, D.B.; Hall, L.O.; Gillies, R.J.; Schabath, M.B. Delta Radiomics Improves Pulmonary Nodule Malignancy Prediction in Lung Cancer Screening. IEEE Access 2018, 6, 77796–77806. [Google Scholar] [CrossRef]
- Jeon, S.; Song, C.; Chang, W.; Kim, Y.; Kim, J. Delta-Radiomics Signature Predicts Treatment Outcomes after Preoperative Chemoradiotherapy in Rectal Cancer. Ann. Oncol. 2019, 30, iv24. [Google Scholar] [CrossRef]
- Crombé, A.; Périer, C.; Kind, M.; De Senneville, B.D.; Le Loarer, F.; Italiano, A.; Buy, X.; Saut, O. T2-Based MRI Delta-Radiomics Improve Response Prediction in Soft-Tissue Sarcomas Treated by Neoadjuvant Chemotherapy. J. Magn. Reson. Imaging 2019, 50, 497–510. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Kalbasi, A.; Hsu, W.; Ruan, D.; Fu, J.; Shao, J.; Cao, M.; Wang, C.; Eilber, F.C.; Bernthal, N.; et al. Treatment Effect Prediction for Sarcoma Patients Treated with Preoperative Radiotherapy Using Radiomics Features from Longitudinal Diffusion-Weighted MRIs. Phys. Med. Biol. 2020, 65, 175006. [Google Scholar] [CrossRef]
- Urba, S.; Wolf, G.; Eisbruch, A.; Worden, F.; Lee, J.; Bradford, C.; Teknos, T.; Chepeha, D.; Prince, M.; Hogikyan, N.; et al. Single-Cycle Induction Chemotherapy Selects Patients with Advanced Laryngeal Cancer for Combined Chemoradiation: A New Treatment Paradigm. J. Clin. Oncol. 2006, 24, 593–598. [Google Scholar] [CrossRef]
- Wolf, G.T.; Bellile, E.; Eisbruch, A.; Urba, S.; Bradford, C.R.; Peterson, L.; Prince, M.E.; Teknos, T.N.; Chepeha, D.B.; Hogikyan, N.D.; et al. Survival Rates Using Individualized Bioselection Treatment Methods in Patients with Advanced Laryngeal Cancer: The University of Michigan Experience. JAMA Otolaryngol Head Neck Surg. 2017, 143, 355–366. [Google Scholar] [CrossRef]

| Characteristics | Responders | Non-Responders | p-Value |
|---|---|---|---|
| Male:Female | 21:4 | 20:5 | 0.71 |
| T-stage | 0.357 | ||
| T3 | 2 | 1 | |
| T4a | 12 | 8 | |
| T4b | 11 | 16 | |
| Clinical trial | 0.024 | ||
| Study 1 | 17 | 9 | |
| Study 2 | 8 | 16 | |
| Histotype | 0.091 | ||
| SCC | 5 | 7 | |
| SNUC | 16 | 7 | |
| SNEC, ONB | 4 | 7 | |
| ITAC | 0 | 4 | |
| Imaging | |||
| T1-weighted | 23 | 25 | 0.977 |
| T2-weighted | 24 | 25 | |
| ADC maps | 13 | 15 |
| Characteristics | Accuracy | TPR | TNR |
|---|---|---|---|
| Validation set (radiomics) | |||
| T1w | 0.82 ± 0.10 | 0.78 ± 0.16 | 0.86 ± 0.15 |
| T2w | 0.79 ± 0.10 | 0.79 ± 0.16 | 0.80 ± 0.15 |
| ADC | 0.89 ± 0.13 | 0.89 ± 0.19 | 0.90 ± 0.18 |
| Test set (radiomics) | |||
| T1w | 0.80 ± 0.16 | 0.73 ± 0.28 | 0.86 ± 0.21 |
| T2w | 0.78 ± 0.17 | 0.75 ± 0.26 | 0.80 ± 0.25 |
| ADC | 0.87 ± 0.13 | 0.86 ± 0.19 | 0.89 ± 0.20 |
| Radiological evaluation | 0.78 | 0.6 | 0.96 |
| Volume | 0.72 ± 0.10 | 0.56 ± 0.16 | 0.89 ± 0.12 |
| Characteristics | AUC Delta Radiomics Model | AUC Baseline Model |
|---|---|---|
| Mono-modality | ||
| T1w | 0.79 (0.65–0.88) | 0.69 (0.55–0.81) |
| T2w | 0.76 (0.62–0.87) | 0.54 (0.51–0.78) |
| ADC | 0.93 (0.75–1) | 0.79 (0.63–0.91) |
| Fused signatures | ||
| T1w + T2w | 0.83 (0.70–0.92) | 0.75 (0.58–0.85) |
| T1w + ADC | 0.88 (0.74–0.95) | 0.77 (0.63–0.89) |
| T2w + ADC | 0.85 (0.73–0.95) | 0.78 (0.62–0.88) |
| T1w + T2w + ADC | 0.89 (0.75–0.95) | 0.83 (0.70–0.93) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corino, V.D.A.; Bologna, M.; Calareso, G.; Resteghini, C.; Sdao, S.; Orlandi, E.; Licitra, L.; Mainardi, L.; Bossi, P. Refining Tumor Treatment in Sinonasal Cancer Using Delta Radiomics of Multi-Parametric MRI after the First Cycle of Induction Chemotherapy. J. Imaging 2022, 8, 46. https://doi.org/10.3390/jimaging8020046
Corino VDA, Bologna M, Calareso G, Resteghini C, Sdao S, Orlandi E, Licitra L, Mainardi L, Bossi P. Refining Tumor Treatment in Sinonasal Cancer Using Delta Radiomics of Multi-Parametric MRI after the First Cycle of Induction Chemotherapy. Journal of Imaging. 2022; 8(2):46. https://doi.org/10.3390/jimaging8020046
Chicago/Turabian StyleCorino, Valentina D. A., Marco Bologna, Giuseppina Calareso, Carlo Resteghini, Silvana Sdao, Ester Orlandi, Lisa Licitra, Luca Mainardi, and Paolo Bossi. 2022. "Refining Tumor Treatment in Sinonasal Cancer Using Delta Radiomics of Multi-Parametric MRI after the First Cycle of Induction Chemotherapy" Journal of Imaging 8, no. 2: 46. https://doi.org/10.3390/jimaging8020046
APA StyleCorino, V. D. A., Bologna, M., Calareso, G., Resteghini, C., Sdao, S., Orlandi, E., Licitra, L., Mainardi, L., & Bossi, P. (2022). Refining Tumor Treatment in Sinonasal Cancer Using Delta Radiomics of Multi-Parametric MRI after the First Cycle of Induction Chemotherapy. Journal of Imaging, 8(2), 46. https://doi.org/10.3390/jimaging8020046








